Abstract
Throughout our lifespan, new sensory experiences and learning continually shape our neuronal circuits to form new memories. Plasticity at the level of synapses has been recognized and studied for decades, but recent work has revealed an additional form of plasticity — affecting oligodendrocytes and the myelin sheaths they produce — that plays a crucial role in learning and memory. In this Review, we summarize recent work characterizing plasticity in the oligodendrocyte lineage following sensory experience and learning, the physiological and behavioural consequences of manipulating that plasticity, and the evidence for oligodendrocyte and myelin dysfunction in neurodevelopmental disorders with cognitive symptoms. We also discuss the limitations of existing approaches and the conceptual and technical advances that are needed to move forward this rapidly developing field.
The brain’s ability to adapt to experience has long been considered the key to learning and memory. Recent reports of experience-induced changes in myelination have introduced the concept of myelination as an additional form of plasticity that could mediate long-lasting changes in neural circuit function. Though myelin has historically been considered a structurally permanent feature that serves to increase axonal conductance, genetic fate mapping and in vivo imaging have revealed that oligodendrocyte lineage cells and the myelin sheaths they produce continually change throughout an animal’s lifespan1–9. Combined with the observation that many axons in the mammalian neocortex remain partially myelinated in adulthood10, these studies indicate that, rather than reaching a pre-programmed point of saturation, there exists a large window within which external factors could influence the rate and pattern of myelination in the brain. Indeed, there is now considerable evidence that manipulating sensory experience2,11–13 or neuronal activity14–16 can impact the proliferation and differentiation of oligodendrocyte lineage cells in rodents (Fig. 1). Furthermore, functional changes in the oligodendrocyte lineage appear to be required for several types of learning and memory17–19. These findings suggest that oligodendrocytes and myelin play critical roles during and/or following learning, but exactly how they influence the underlying neural circuits remains an active and expanding area of investigation.
Fig. 1 |. Forms of plasticity within the oligodendrocyte lineage.
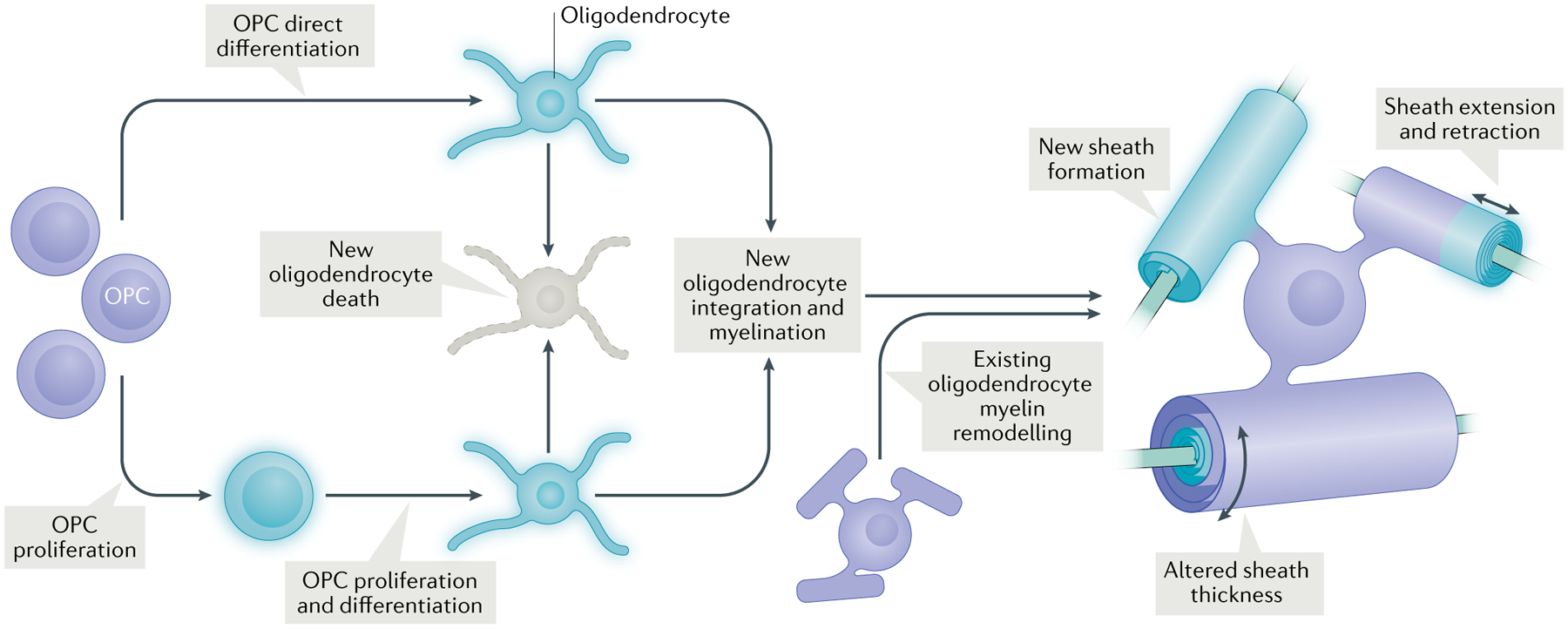
Oligodendrocyte precursor cells (OPCs) proliferate and a subset of these newly formed OPCs differentiate into new oligodendrocytes17,19. Other existing OPCs directly differentiate into new oligodendrocytes38,39. A subset of these new oligodendrocytes undergo cell death, whereas others stably integrate into the circuit and form new sheaths on previously unmyelinated axon segments2. Existing oligodendrocytes can also undergo plasticity in the form of altered sheath length and thickness2,33,34,39 (and, under specific conditions, new sheath generation)39.
Oligodendrocytes and myelin may be uniquely suited to modulate long-term changes in circuit function. Across species, mature oligodendrocytes and myelin sheaths are remarkably stable once formed2,4,20,21. They are also the only cell type for which a pool of committed precursor cells, which can respond to environmental cues by rapidly dividing and differentiating as needed1, are maintained throughout the brain. Once mature, a single oligodendrocyte can make upwards of 100 myelin sheaths2,9,22. By contrast, Schwann cells — the myelinating cells present in the peripheral nervous system — form myelin around single axons. This divergence in the evolution of myelinating cells may hint at the potential of oligodendrocyte precursor cells (OPCs) to act as integrators of nearby neuronal activity and of mature oligodendrocytes to act as maestros of synchrony in neuronal circuits. Oligodendrocytes can also influence multiple aspects of neuronal function, including (but not limited to) axonal conductance, synaptic efficacy23,24, excitability25,26 and structural plasticity3,27,28 (Fig. 2).
Fig. 2 |. Modulation of neuronal function and plasticity by oligodendroglia and myelin.
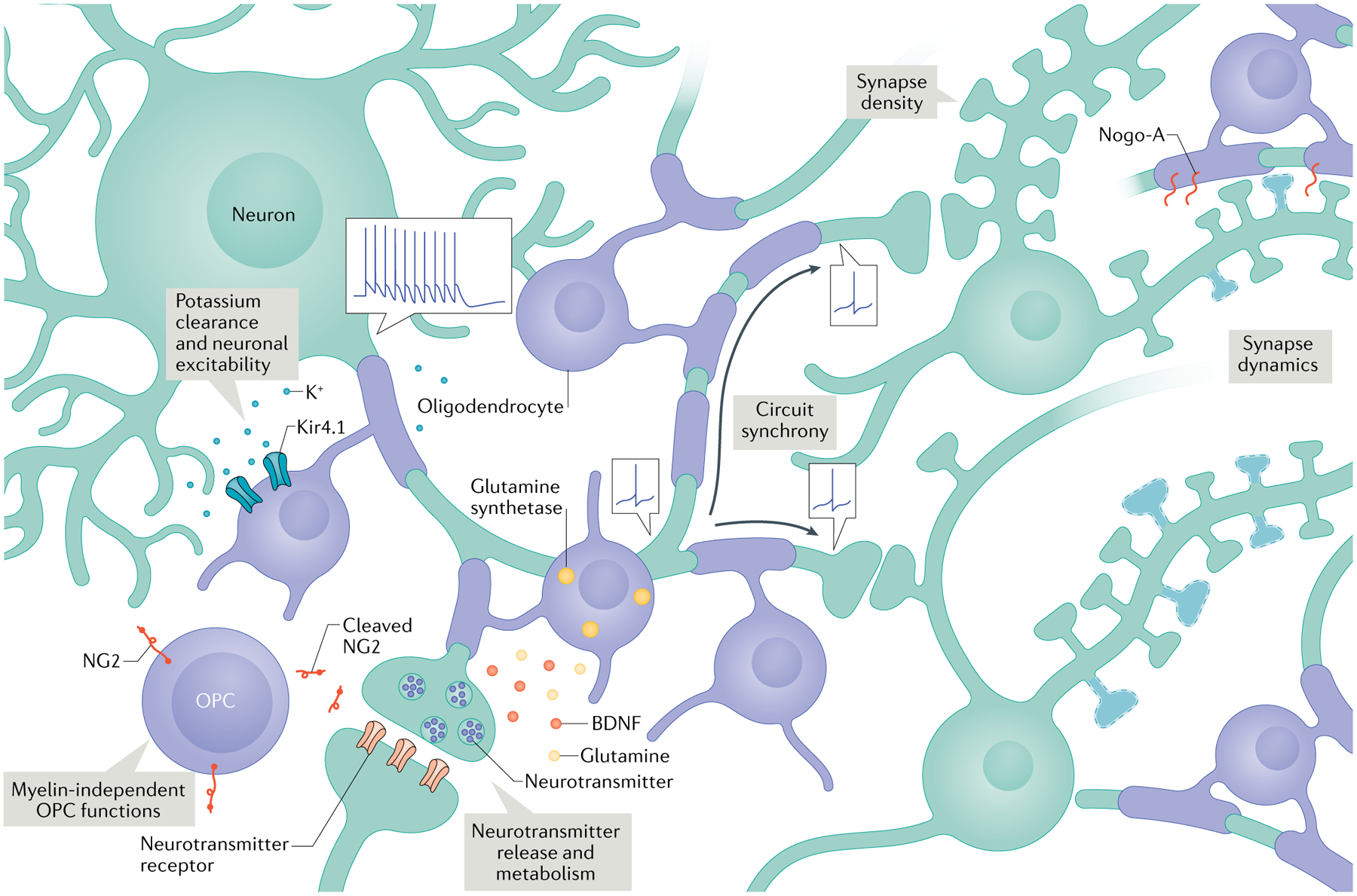
Oligodendrocytes express the inward rectifying potassium channel Kir4.1 and participate in extracellular potassium clearance26, which can affect neuronal excitability. They also regulate neurotransmitter release and metabolism through the expression of glutamine synthetase23 and the release of molecules like glutamine and brain-derived neurotrophic factor (BDNF)24. Differential (and adjustable) myelination patterns can regulate the precise timing of action potential arrival in various circuits46,57,59,60. Manipulating levels of myelination also changes synapse density in development and in ageing3,28. Oligodendrocytes and myelin may regulate structural synapse plasticity through Nogo-A27. Finally, oligodendrocyte precursor cells (OPCs) may influence circuit activity in myelin-independent ways, such as through cleavage and secretion of NG2 (REF.40).
As cell biologists and systems neuroscientists converge upon the question of how myelin shapes neuronal plasticity to influence learning and memory, greater dialogue between us is needed to uphold a high standard of rigour in our collective investigation. In service of that discourse, we summarize below the recent findings implicating oligodendrocyte lineage cells and myelin in the acquisition and maintenance of learning and memory and discuss the tools and conceptual advances that are still needed.
Experience-induced plasticity
Sensory experience and oligodendrocyte plasticity.
Several rodent studies have examined the effect of sensory deprivation on myelination. In mice, whisker trimming from birth led to increased proliferation and a more uniform distribution of OPCs in the barrel cortex at postnatal day 3 (P3) and P6 (REF.29). Whisker trimming from birth, from P0 to P30, or from P30 to P60 resulted in significant reductions in the extent of myelination in the barrel cortex at P60 (REF.11). A shorter period of sensory deprivation, from to P6 to P12, also led to fewer mature oligodendrocytes at P12 and more OPCs undergoing apoptosis30. Intriguingly, whisker trimming from P60 to P90 induced a paradoxical increase in myelination in the barrel walls of the barrel cortex11, which suggests that the rules governing input-driven myelination may vary between development and adulthood. In the visual cortex, adult rats deprived of input from one eye between P91 and P98 had increased levels of myelin basic protein (MBP) — indicating more myelination — in the non-deprived (ipsilateral) visual cortex and decreased MBP in the deprived (contralateral) cortex relative to control rats31. Thus, cortical myelination seems to be sensitive to sensory deprivation in both development and adulthood.
Other studies have investigated the effects of increased sensory stimulation, for example, by exposing animals to an enriched environment. Mice reared for 10 days in an enriched environment had greater numbers of newly formed OPCs (identified with bromodeoxyuridine (BrdU), which labels newly divided cells; TAble 1) and differentiated oligodendrocytes in the sensorimotor cortex than control animals housed in normal conditions32. However, there were fewer new oligodendrocytes 32 days after removal from the enriched environment, indicating that not all of the newly formed oligodendrocytes stably integrated into the circuit (Fig. 1). This interpretation is in line with findings from longitudinal in vivo imaging where, in middle-aged mice (8–14 months), only 22% of newly formed oligodendrocytes became stabilized; the remainder died within 1.8 days of downregulating the expression of NG2, a chondroitin sulfate proteoglycan that is expressed by OPCs but not by differentiated oligodendrocytes2. Placing the animals in an enriched environment for 20 days increased the number of successfully integrated new oligodendrocytes by fivefold in the sensory cortex, though these cells still only accounted for 1% of the total population of oligodendrocytes2. Nevertheless, these newly formed oligodendrocytes generated hundreds of additional sheaths in the imaged region, in line with previous work showing that each oligodendrocyte can form dozens of sheaths22. Studies using histology to quantify gross levels of myelin in rats also found increased myelination in middle-aged (12 months) and old (24 months) animals after being housed in enriched environments for 4 months just prior to histology12,13. Together, these studies demonstrate a large capacity for sensory experience to shape myelination well into adulthood.
Table 1 |.
Tools used to study oligodendrocyte and myelin plasticity in rodents
| Approach | Advantages | Disadvantages |
|---|---|---|
| Tools to impair or prevent new oligodendrocyte differentiation | ||
| OPC-specific Cre drivers (such as NG2-CreER or PDGFRα-CreER) to conditionally knock out key genes involved in myelination (creating Myrf cKOs or Olig2 cKOs, for example) | Allows cell type-specific manipulation; onset of impaired differentiation can be controlled by tamoxifen administration | Requires an OPC Cre driver; Myrf or Olig2 may have additional functions beyond regulation of differentiation; effect is not region-specific; does not differentiate between ongoing and experience-induced myelination |
| Tools to enhance myelination | ||
| Clemastine or bazedoxifene | Does not require Cre driver; simple to administer (systemic injection) | Not cell type or region-specific; may have unknown off-target effects |
| OPC-specific Cre drivers (such as NG2-CreER or PDGFRα-CreER) to conditionally knock out M1R, a negative regulator of myelination (Chrm1 cKO) | Cell type-specific manipulation; onset of enhanced differentiation can be controlled by tamoxifen administration | Requires OPC Cre driver; M1R may have other effects beyond regulating differentiation; effect is not region-specific |
| Methods to label new oligodendrocyte or myelin formation | ||
| Use EdU or BrdU, together with immunostaining for PDGFRa or NG2 (OPCs), CC1 or ASPA (mature oligodendrocytes), or OLIG2 or SOX10 (oligodendrocyte lineage cells) | Does not require Cre driver; identifies OPCs, oligodendrocytes or oligodendrocyte lineage cells that arose from OPCs that proliferated after EdU or BrdU administration | Does not capture oligodendrocytes that directly differentiated without proliferating first; cannot visualize myelin sheaths |
| OPC-specific Cre drivers (such as NG2-CreER or PDGFRα-CreER) to drive the expression of tau-mGFP | Captures newly formed oligodendrocytes and sheaths that arose from direct differentiation and from OPCs that first proliferated; allows for visualization of myelin sheaths | Requires OPC Cre driver; does not demonstrate the presence of bona fide compact myelin |
| Expression of fluorescent proteins under the control of OPC-specific promoters (such as NG2-mEGFPorMOBP-EGFP) | Allows for in vivo visualization and tracking of OPCs and differentiated oligodendrocytes; allows for visualization of fine processes in both cell types | Requires in vivo longitudinal imaging to differentiate between existing and newly formed cells; does not demonstrate the presence of bona fide compact myelin |
ASPA, aspartoacylase; BrdU, 5-bromo-2′-deoxyuridine; cKO, conditional knockout; EdU, 5-ethynyl-2′-deoxyuridine; OPC, oligodendrocyte precursor cell.
Social interaction and oligodendrocyte maturation.
Social isolation is another form of ‘deprivation’ that impacts oligodendrocyte maturation and myelination33–35. Juvenile mice (P21) singly housed for 2 weeks had thinner myelin in the prefrontal cortex than group-housed controls as well as decreased levels of myelin-associated transcripts such as Mog and Mbp33. A separate study also found that mice socially isolated for 2 weeks after weaning had reduced Mbp and Mag transcripts, fewer myelin sheaths, and thinner myelin in the prefrontal cortex at P65 (REF.34). The number of oligodendrocytes was unchanged, indicating that social isolation may interfere with the progression of myelination rather than with OPC differentiation (Fig. 1). Re-integration following social isolation between P21 and P35 did not normalize myelin transcript levels by P65 (REF.34), indicating the existence of a critical period during which social interaction is required for proper myelination in the prefrontal cortex. In adult animals (P60), one study found that 2 weeks of social isolation was sufficient to reduce myelin thickness33. However, another study34 found no difference in myelin transcripts in mice isolated from P35 to P65. Thus, although early social isolation (before P35) seems to robustly impair myelination in the prefrontal cortex, further work is needed to clarify the influence of adult social isolation on myelination.
Motor learning-induced oligodendrocyte plasticity.
Motor activity engages the motor cortex, which exhibits robust structural and functional neuronal plasticity following repeated motor training36,37. The same appears to be true for oligodendrocyte lineage cells; one study found that training rats for 10 days on a skilled reaching task increased the numbers of newly generated OPCs and oligodendrocytes in the sensorimotor cortex32. In a different motor learning task, in which mice learn to run on a ‘complex wheel’ containing irregularly spaced rungs, the numbers of newly divided OPCs in the corpus callosum increased after 4 days of running17. The overall density of OPCs increased by day 6 and, after 3 weeks of running, the number of EdU-labelled (EdU+) mature oligodendrocytes was increased, indicating that some of the newly proliferated OPCs had differentiated into new oligodendrocytes. A follow-up study looking more closely at the timeline of new oligodendrocyte generation found that the number of EdU+ oligodendrocytes was already increased in the motor cortex and underlying white matter after 4 days of running38. The density of new oligodendrocytes was unchanged in optic nerve, suggesting that learning-induced increases in oligodendrogenesis were restricted to task-related regions. The same study identified a small population (<5%) of Mbp-expressing and Olig2-expressing oligodendrocytes that also expressed high levels of the transcript Enpp6 (Enpp6high oligodendrocytes)38. Based on the morphology and low level of MBP protein in Enpp6high oligodendrocytes as well as on their absence in mice that cannot form new oligodendrocytes, the authors concluded that Enpp6 labels newly differentiated oligodendrocytes, which can appear as early as 2 h after motor learning38. The number of Enpp6high oligodendrocytes increased in the callosal white matter beneath the motor cortex after only 2.5 h of complex wheel running and remained high after 8 days of running.
These findings raised the important consideration that not all newly formed oligodendrocytes necessarily come from newly divided OPCs (Fig. 1). Indeed, a study using in vivo imaging to track OPCs and oligodendrocytes over the course of motor learning on a forelimb reaching task found that, within the 4-week imaging period, nearly 90% of OPCs in the motor cortex differentiated without proliferating first39. Differentiation actually decreased during the week of training, then increased in the 2 weeks post-learning. The rate of increased oligodendrogenesis was significantly correlated with the animal’s performance on the reaching task. OPC proliferation also decreased in the days following training, before returning to pre-learning rates. Furthermore, learning increased the number of retractions of existing myelin sheaths, and sheaths that were extending prior to learning stopped growing at the onset of motor training. The retraction of existing sheaths (as well as the transient suppression of OPC differentiation) may permit greater structural plasticity of neurons post-learning, allowing, for example, new sprouting of axon terminals in previously ensheathed segments.
There are some discrepancies in the studies described above regarding the timing and dynamics of OPC proliferation and differentiation during motor learning, which may be due to differences in the paradigm used. However, one finding that emerges across these studies is the existence of both oligodendrocytes that differentiate following recent OPC proliferation and oligodendrocytes that directly differentiate from existing OPCs (Fig. 1). Studies that treat animals with EdU or BrdU just prior to a behavioural manipulation may not capture the latter population, which appears to account for the majority of newly differentiated oligodendrocytes1,39. These studies further suggest that the rapid and robust increase in OPC proliferation following certain types of learning17–19 is a homeostatic response to replace the OPCs that directly differentiated. This large increase in the number of dividing OPCs may also have consequences beyond their subsequent differentiation; for example, transiently altered levels of NG2 in the extracellular environment could modulate certain forms of synaptic plasticity40.
Oligodendrocyte plasticity in other forms of learning.
In a study on spatial learning, mice were administered EdU immediately following training in a water maze containing a hidden platform for escape18; 28 days after training, the number of EdU+ OPCs was increased in the prefrontal cortex and corpus callosum of trained mice relative to untrained controls. The number of EdU+ oligodendrocytes also increased in the prefrontal cortex, corpus callosum and anterior cingulate cortex. The increase in EdU+ oligodendrocytes and the unchanged number of EdU+ OPCs in the anterior cingulate suggests that learning-induced proliferation and differentiation may proceed separately and at different rates, depending on the circuit or region. Unexpectedly, EdU+ oligodendrocytes did not increase in other regions associated with spatial learning, including hippocampal CA1 or the alveus. Thus, oligodendrocyte plasticity following spatial learning may be more pronounced in regions associated with long-term memory consolidation41,42.
Fear learning engages similar neural circuits to those involved in spatial learning. In one study that investigated changes to the oligodendrocyte lineage following fear conditioning, EdU administered just prior to contextual fear conditioning (CFC) revealed a significant increase in the number of EdU+ OLIG2-expressing (OLIG2+) oligodendrocyte lineage cells 24 h later in the prefrontal cortex19. After 30 days, animals that had undergone CFC had significantly more EdU+ oligodendrocytes and myelinated axons in the prefrontal cortex than home cage controls. In the basolateral amygdala, the number of EdU+ OLIG2+ cells was also elevated 24 h after conditioning, but the number of EdU+ oligodendrocytes was unchanged after 30 days — again highlighting a dissociation between initial learning-induced OPC proliferation and the eventual stabilization of newly formed oligodendrocytes. The same study also used an oligodendrocyte lineage-specific Cre driver (NG2-CreER) to drive the expression of tau-mGFP and label newly formed oligodendrocytes (tau is expressed in oligodendrocytes but not OPCs; thus, mGFP labels only newly differentiated cells; TAble 2) by administering tamoxifen just prior to CFC. Compared to home cage controls, the number of GFP-labelled (GFP+) MBP-expressing (MBP+) cells in the prefrontal cortex of animals that had undergone CFC was increased at 14 and 30 days post-conditioning. It is worth noting that, at 7 days post-conditioning, no GFP+ MBP+ cells were detected, indicating that it takes between 7 and 14 days for new myelin to form in the prefrontal cortex of fear-conditioned animals. This time course would suggest that myelination per se may only come into play later in memory consolidation rather than having an impact on acute learning.
Table 2 |.
Evidence of a requirement for oligodendrocyte and myelin plasticity in learning and memory
| Learning paradigm | Age | Manipulation | Behavioural outcome and timeline | conclusion | Refs |
|---|---|---|---|---|---|
| Motor learning (complex wheel with irregularly spaced rungs) | P60–90 | Conditional Myrf deletion in OPCs, preventing OPC differentiation | Impaired running speed on complex wheel (2 h after wheel exposure and throughout 8 days of training) | Oligodendrogenesis is required for motor learning | 17,38 |
| Motor learning (pull and hold lever) | P60 | Expression of extra copies of PLP1, producing thinner myelin and abnormal oligodendrocyte processes | Impaired success rate (9 days after training and throughout 1 additional week of training) | Normal myelin architecture is required for motor learning | 46 |
| Spatial learning (water maze) | P70–84 | Conditional Myrf deletion in OPCs, preventing OPC differentiation | Less time spent in platform zone (24 h, 28 days after training) | Oligodendrogenesis is required for recent and remote recall but not initial spatial learning | 18 |
| Spatial learning (water maze) | P120 | Conditional Olig2 deletion in OPCs, impairing OPC differentiation | Less time spent in platform zone (24 h after training) | Oligodendrogenesis is required for recent recall but not initial spatial learning | 3 |
| Spatial learning (water maze) | P540 | Conditional Chrm1 deletion in OPCs, increasing OPC differentiation | More time spent in platform zone (24 h after training) | Increasing oligodendrogenesis in aged mice improves recent recall without altering initial spatial learning | 3 |
| Fear learning (contextual fear conditioning) | P56 | Conditional Myrf deletion in OPCs, preventing OPC differentiation | Less time freezing in conditioned context (30 days after conditioning) | Oligodendrogenesis is required for remote recall but not initial fear learning or recent recall | 19 |
| Spatial learning (T-maze) | P56 | Conditional Cdk5 deletion from oligodendrocyte lineage cells, impairing myelination from P14 onwards (possibly earlier) | Impaired performance (after 3 h in maze), contextual/cue fear conditioning and rotarod test | Normal myelination is required for a wide range of learning and memory tasks | 52 |
| Fear learning (passive avoidance and contextual and cued fear conditioning) | Impaired performance (after 24 h) | ||||
| Motor learning (rotarod) | Impaired performance (after 3 trials) | ||||
| Motor learning (rotarod) | ‘Adult’; specific age not specified | Conditional expression of constitutively active MEK1 in mature oligodendrocytes, increasing myelin thickness in optic nerve, corpus callosum and spinal cord | No alteration in 5 days of rotarod test | Inducing hypermyelination in existing oligodendrocytes improves recent recall of a contextual fear memory but not motor learning or recent recall of a cue-related fear memory | 107 |
| Fear learning (contextual fear conditioning and cued fear conditioning) | More freezing in conditioned context but no change in freezing to conditioned cue after 24 h) |
This table lists studies demonstrating a requirement for oligodendroglia-related processes in learning and memory but not studies only describing experience-induced or learning-induced oligodendroglial plasticity. OPC, oligodendrocyte precursor cell; P, postnatal day.
Learning-induced versus experience-induced oligodendrocyte plasticity.
One question that emerges from this body of work is whether it is possible or relevant to separate the influence of passive experience from that of learning a non-innate association or skill on the oligodendrocyte lineage. One study17 did attempt to make this distinction by training animals on a complex wheel and subsequently re-exposing them to the same wheel; only the first exposure induced an increase in motor cortex oligodendrogenesis. Other studies have not typically included a similar behavioural control. Another conceptual question to consider is whether exercise-induced or stress-induced oligodendrogenesis affects the underlying circuits in the same way that motor learning-induced or fear learning-induced oligodendrogenesis does. One can imagine that learning-induced changes may have a more specific role in preserving the circuit activity required to form or maintain memories, whereas general sensory experiences might enhance oligodendrogenesis more globally. Though challenging to address experimentally, this distinction should nevertheless be considered when interpreting results in learning paradigms, which invariably involve some components of exercise, stress, or sensory enrichment (all of which are sufficient to induce oligodendrocyte plasticity on their own).
Developmental versus experience-induced myelination.
In the growing dialogue around plasticity within the oligodendrocyte lineage, there is a tendency to distinguish between ‘developmentally programmed’ and experience-induced myelination. However, there is no clear age after which new myelin is no longer formed, even in the absence of any overt manipulation2–4. Furthermore, myelination at very early postnatal ages also seems to be shaped by differences in the availability of external stimuli11,29,30. These studies suggest that progression of myelination, both in development and in adulthood, is modulated by experience. Therefore, rather than framing developmental and experience-induced myelination as distinct processes, it may be more accurate to consider cell-autonomous and environmental cues as factors that interact with one another throughout life to shape the function of oligodendrocyte lineage cells.
Overall, the data from animal models as well as from human imaging studies (box 1) broadly support the same general conclusions: white matter and/or oligodendrocyte plasticity occurs in response to sensory experience and learning, and is mostly restricted to task-related brain regions; the extent of plasticity is correlated with subsequent behavioural outcomes; and, though plasticity diminishes with age, repeated training can produce changes in white matter and/or oligodendrocyte lineage cells even in older adults43.
Box 1 | White matter plasticity in human imaging studies.
Diffusion tensor imaging, and specifically the measurement of fractional anisotropy, has been used to quantify changes in human white matter108. Fractional anisotropy, a parameter that is based on modelling the diffusivity of water molecules in the brain, decreases and becomes more directionally dependent — or anisotropic — in highly membranous regions such as myelinated axon tracts. although changes in fractional anisotropy are associated with109 and typically interpreted as changes in myelination110, the possibility that changes in other cell types (astrocytes, oligodendrocyte precursors) or structures (axons, blood vessels) are also at least partially responsible for altered fractional anisotropy measurements cannot be ruled out111. Nevertheless, studies using fractional anisotropy as a measure have shown that the general white matter structure in humans can be significantly altered by experience.
There is compelling evidence that skilled motor learning can induce structural changes in specific white matter tracts. in a study comparing professional concert pianists with age-matched non-musicians, diffusion tensor imaging revealed significantly higher fractional anisotropy in the internal capsule of the pianist group112. Furthermore, in pianists, the number of childhood piano practice hours correlated with fractional anisotropy in the corpus callosum and fibre tracts in the frontal lobe. the regions that correlated with childhood practice were distinct from the regions that correlated with adulthood practice, which suggests that extensive practicing in adulthood can still lead to changes in some fibre tracts, albeit to a lesser extent than training in childhood. indeed, a study that trained adult participants to juggle over 6 weeks found that fractional anisotropy in white matter underlying the intraparietal sulcus was significantly higher in the trained group than in an untrained control group113. this increase was still detectable 4 weeks after the end of the training period, indicating that the adult training-induced changes may also be long lasting.
Studies have also reported a relationship between adaptations in reading ability and white matter structure in both children and adults114–118. One study paired intensive remedial reading instruction with diffusion tensor imaging in children characterized as having poor reading abilities116. Participants were scanned prior to receiving 100 h of remedial instruction and then scanned a second time 6 months later. at the initial scan, poor readers had significantly lower fractional anisotropy in the anterior left centrum semiovale region relative to a control group of good readers. at the post-instruction scan, poor readers showed significantly increased fractional anisotropy in the same region. taken together, these studies demonstrate region-specific changes in human white matter following multiple forms of learning.
A role for oligodendrocyte plasticity
As discussed above, there have been numerous reports of experience-induced or learning-induced changes in the oligodendrocyte lineage. However, these studies cannot tell us what the functional relevance of these changes may be. Below, we summarize evidence suggesting that oligodendrocyte plasticity may play vital roles in learning and memory.
Motor learning.
Much of the work implicating oligodendrocyte lineage cells in learning and memory was made possible by the identification of myelin regulatory factor (Myrf)44,45, a transcription factor required for OPC differentiation. In a landmark study, Myrf deletion was used to prevent oligodendrogenesis in adulthood17. Mice carrying a floxed Myrf gene were bred with an inducible Cre line that targets OPCs (generating Myrf conditional knockout mice (Myrf cKOs); TAble 1), allowing for the elimination of new OPC differentiation following tamoxifen administration. To investigate the role of newly generated oligodendrocytes in motor learning, the authors gave animals tamoxifen 3 weeks before exposing them to the complex running wheel. Myrf cKOs began showing deficits in running speed after 2 days of running and did not catch up to Myrf fl/+ littermates over 8 days of testing. In a follow-up study38, the same group examined the early dynamics of motor learning and found that divergent running speeds between Myrf cKOs and control animals appeared as early as 2 h following complex wheel exposure. Training Myrf-cKO mice prior to tamoxifen administration allowed them to run at the same level as heterozygous littermates, indicating that there was no general motor impairment in Myrf cKOs and that performing a pre-learned motor skill does not require new oligodendrogenesis.
Further evidence for the involvement of oligodendrocytes in motor learning comes from a study in which mice were trained to pull and hold a lever for 600 ms in order to obtain a reward46. In this case, the authors tested a mouse model in which subtle impairments in myelination (thinner myelin and abnormal oligodendro cyte processes) were induced by the introduction of extra copies of the myelin proteolipid protein 1 (PLP-tg mice; TAble 2). Compared to wild-type mice, PLP-tg mice began to show deficits in their success rate after 9 days of training on the lever task, which persisted after a week of extended training. The later onset of learning deficits in this model may be due to the milder impairment in myelination and the lack of impairment in OPC function. Nevertheless, the findings lend additional support to the idea that oligodendrocytes and myelin are involved in motor learning.
Spatial memory.
A similar strategy was used to determine whether new oligodendrogenesis is required for spatial learning and memory18. Myrf cKOs were trained to find a hidden platform in a water maze and tamoxifen was administered during training to induce Myrf deletion in OPCs. The time required to reach the platform steadily decreased over the training period and was similar in both Myrf cKOs and CreER negative control animals, indicating no impairment in spatial learning. However, in a recent recall test (taking place 24 h after training) and a remote recall test (taking place 28 days later), Myrf cKOs spent significantly less time than control animals in the platform zone, suggesting impaired recall of the platform location. Similarly, another study disrupted new oligodendrogenesis by conditionally deleting the transcription factor Olig2 (REF.47) from OPCs3. Like Myrf cKOs, Olig2 cKOs had fewer newly generated oligodendrocytes than Olig2fl/+ littermates 4 weeks after tamoxifen administration. After multiple days of training in the water maze, both groups showed similar declines in time to find the hidden platform. However, Olig2 cKOs showed a deficit in recent memory recall as evidenced by less time spent and fewer crossings into the platform zone. These results indicate that new oligodendrogenesis is required for the consolidation and/or maintenance of spatial memory, but not for initial spatial learning.
The experiments described above were conducted in relatively young adult mice (between P60 and 4 months), in which baseline myelination in the absence of any learning or novel sensory experience remains quite high3. To examine whether diminished oligodendrogenesis contributes to ageing-related deficits in spatial memory, one study genetically enhanced oligodendrogenesis through the conditional deletion of Chrm1 (encoding the muscarinic M1 receptor, a negative regulator of OPC differentiation48) from OPCs (Chrm1 cKO) at 13 months of age3. By 18 months, Chrm1 cKOs had significantly higher levels of newly formed myelin than Chrm1+/+ controls. Furthermore, 18-month-old Chrm1-cKO animals trained on the water maze performed significantly better in a recent recall test than control animals. Wild-type animals treated with the pro-myelinating compound clemastine for 4 months, beginning at 12 months of age, also displayed improved spatial memory in the water maze relative to vehicle-treated controls3. These results suggest that enhancing myelination in ageing could be beneficial for combatting age-related impairment in memory retention.
Fear memory.
Myrf-cKO mice have also been used to study the role of new oligodendrogenesis in fear learning19. In this study, animals were treated with tamoxifen 3 weeks prior to a single session of CFC, in which they received foot shocks in a novel context. When re-exposed to the context 24 h later, Myrf cKOs froze for the same amount of time as their CreER-negative littermates, indicating that initial fear learning and recent memory were unimpaired. However, when re-exposed to the same context 30 days later, Myrf cKOs froze significantly less than the control animals. Furthermore, fewer cFOS-expressing neurons were detected in the prefrontal cortex, basolateral amygdala, and hippocampus of Myrf cKOs following re-exposure to the context, indicating impaired retention or reactivation of fear-related engrams in these regions. The number of cFOS-expressing neurons in the somatosensory cortex or in animals exposed only to their home cages did not differ between Myrf cKOs and controls; thus, only the activation of neurons related to the fear memory was impaired by Myrf deletion. The same study also took a gain-of-function approach to ask what would happen to remote fear memory recall if myelination was enhanced by giving the animals the pro-myelinating drug clemastine49,50 (beginning 3 days before fear conditioning and ending 21 days after conditioning). Clemastine-treated animals re-exposed to the context 30 days later froze significantly more than vehicle-treated animals. Importantly, clemastine did not influence freezing in Myrf-cKO animals, indicating that its effect on freezing behaviour is likely to occur through the modulation of OPC differentiation. Thus, fear memory retention seems to be dependent on new oligodendrogenesis and can be enhanced by a pro-myelinating drug.
To explore the impact of Myrf deletion on neuronal plasticity in fear learning, fibre photometry was used to record bulk neuronal calcium activity in the prefrontal cortex of control and Myrf-cKO animals during and following fear learning19. Previous studies have found a suppression of neuronal activity in the infralimbic subregion of the medial prefrontal cortex in response to conditioned cues 24 h after conditioning51. Although both Myrf cKOs and control mice showed suppression of infralimbic activity 1 day after training, this cue-induced suppression of activity dynamically changed with time in control mice but not in Myrf cKOs, suggesting that the normal temporal evolution of PFC neuronal responses during the consolidation period had not occurred. The initial similarity in both behavioural and neurophysiological responses between the two groups suggests that Myrf deletion specifically impairs the consolidation, but not the acquisition, of the fear memory.
There is some variance among these studies in the timing of behavioural deficits (see TAble 2 for summary), but it is clear that disrupting adult oligodendrogenesis impairs multiple forms of learning and memory. Surprisingly, disrupting developmental myelination also seems to selectively impair learning and memory-related tasks over more instinctive behaviours. Mice lacking the gene Cdk5 in oligodendrocyte lineage cells (Cdk5 cKOs)52 display hypomyelination as early as 14 days postnatally, which does not return to normal levels in early adulthood. 2-month-old Cdk5 cKOs, relative to controls, showed significant deficits in the T-maze test (testing short-term spatial memory), the passive avoidance test (testing recent contextual fear memory), contextual and cued fear conditioning (testing recent contextual and cued fear memory), and the rotarod test (testing motor learning), whereas anxiety-related behaviours were unchanged. Thus, normal myelination is required for a wide range of learning and memory tasks.
Disentangling the relative contributions of OPCs, oligodendrocytes and myelin.
Though there is some evidence that existing mature oligodendrocytes can contribute to circuit plasticity and learning (box 2), most studies have focused on the role of newly formed oligodendrocytes in learning and memory (TAble 2). It is currently unclear why some studies implicate oligodendrocytes and myelin in learning acquisition and recent memory3,17,18,38, whereas others only find a role for oligodendrogenesis in preserving remote memory19. One caveat to the existing evidence is the heavy reliance on one model for disrupting oligodendrogenesis, that is, the conditional deletion of Myrf from OPCs in early adulthood to prevent new OPC differentiation (TAble 1). This powerful approach has unquestionably advanced the study of oligodendrocyte-neuron interactions in adulthood. It is easy to conclude from these studies that adaptive myelination — that is, the formation of new myelin induced by new experiences and/or learning — underlies the behavioural deficits observed in Myrf-cKO animals; however, does the evidence truly support this narrow interpretation? These studies were conducted at an age when myelination is actively proceeding in the absence of any manipulation and Myrf deletion impacts oligodendrogenesis throughout the brain. For example, although spatial learning did not increase oligodendrogenesis in the alveus of wild-type animals, knocking out Myrf decreased the numbers of EdU+ mature oligodendrocytes in the alveus18. Given that Myrf cKOs have globally disrupted oligodendrogenesis, including in regions not affected by learning, we cannot be certain that adaptive myelination is solely responsible for the behavioural deficits observed.
Box 2 | A role for plasticity in mature oligodendrocytes?
Although most of the focus in myelin plasticity-related studies has been on the generation of new oligodendrocytes, studies using carbon dating to birth date oligodendrocytes in post-mortem tissue suggest that oligodendrocytes turn over at relatively low rates in humans20,119. in mice, extensions and retractions of existing sheaths have also been observed in vivo2,4 and can be regulated by learning39. thus, it is possible that existing oligodendrocytes may also influence learning and memory by forming new sheaths or remodelling existing sheaths, which could significantly impact axon conductance by altering sodium channel clustering at nodes of ranvier57,58. One study107 took a gain-of-function approach to examine this possibility by expressing a constitutively active form of the erK1/2 kinase MeK1 specifically in mature oligodendrocytes, which led to thicker myelin in the optic nerve, corpus callosum and spinal cord in adult mice. relative to wild-type mice, mutant mice performed similarly in the open field test and rotarod test but froze more in a recall session 24 h after contextual fear conditioning. interestingly, although the mutants seemed to better recall a fear-conditioned context, their response to a fear-conditioned cue (a tone) was the same as that of wild-type animals. therefore, inducing hypermyelination in existing oligodendrocytes can improve memory retention in some tasks. Of course, the question of whether mature oligodendrocytes normally participate in learning-induced adaptations will still need to be addressed through loss-of-function studies.
The rapid onset of deficits observed in Myrf cKOs during motor learning38 also raises the question of whether decreased myelination is truly the culprit. One study found that mature oligodendrocytes generated after fear conditioning were only detectable after 14 days in the prefrontal cortex19. In vivo imaging revealed increased OPC differentiation rates after 1 week but not in the immediate days following motor training39, suggesting that OPC differentiation is not immediately required for motor learning to proceed. Perhaps other functions fulfilled by newly formed oligodendrocytes, such as potassium buffering26, neurotransmitter recycling23 or metabolic support53–55, are more rapidly impacted by Myrf deletion, which results in impaired learning within hours of motor training38.
Furthermore, Myrf is an unusual transcription factor in that it is a transmembrane protein and must be cleaved to free an N-terminal region that translocates to the nucleus to activate the expression of myelin-associated genes44. Could the transmembrane domain or the uncleaved MYRF protein have other functions within OPCs in addition to regulating differentiation? This possibility could account for some results that are unlikely to be caused by impaired adaptive myelination. As previously mentioned, during motor learning, Myrf cKOs exhibit deficits in performance within the first 2 h of training38, which seems to preclude the involvement of newly formed compact myelin19. In the study on spatial learning18, inducing Myrf deletion during the recent memory probe test actually led to improved performance over control animals. It seems unlikely that impaired adaptive myelination could underlie this improved performance; however, it may reflect some acute adaptation in OPCs in response to Myrf deletion that temporarily heightens memory retention. One possibility is that, in the absence of MYRF, OPCs that would otherwise have differentiated instead underwent cell death and triggered local inflammation, though there are no signs of astrocyte reactivity, blood–brain barrier breakdown, or an increase in microglia or phagocytic cells in the brains of Myrf cKOs at the timepoint when they were tested (3 weeks following tamoxifen administration)17,19. Nevertheless, low-grade inflammation could be present, or Myrf deletion could alter OPC function in other ways at a shorter timescale. To bolster the conclusion that adaptive myelination is the key cellular adaptation required in these learning and memory tasks, it may be time to validate these findings with additional complementary models, such as by conditionally deleting other transcription factors required for oligodendrocyte differentiation3,56.
Oligodendrocytes and circuit function
The terms ‘learning’ and ‘memory’ cover a broad spectrum of behaviours and associated changes in brain function. The overall changes in a neural circuit that lead to long-lasting alterations in behaviour are still being defined. Adaptations are also likely to be distinct between different circuits and behaviours and to occur at multiple levels (molecular, cellular and network). A similar picture is beginning to emerge in the field of myelin plasticity. Changes in axon conductance are an obvious candidate mechanism underlying circuit modification by myelination, but we now recognize that oligodendrocytes and myelin can shape neuronal function in multiple ways that are potentially relevant to circuit plasticity (Fig. 2).
A frequently proposed role of adaptive myelination is to tune the conduction velocity of individual axons (by altering sodium channel clustering at nodes of Ranvier, for example)57,58 to optimize the timing of spike arrivals in specific circuits. In the avian auditory system, myelin sheath lengths vary greatly along axon bifurcations of a single neuron, allowing action potentials to arrive at both ipsilateral and contralateral target regions within microseconds of one another, despite large differences in axon segment length59. Similarly, action potentials in mouse thalamic neurons can reach a wide range of cortical projection targets at the same time despite considerable differences in distance, which seems to be achieved via partial myelination that can produce a near tenfold difference in conduction velocity along different parts of the same tract60. In mice with constitutive mild hypomyelination, the latency of evoked antidromic spikes from thalamocortical axons was significantly more spread out than in control animals with normal myelination46. A similar loss of synchrony was observed in inferior olivary inputs to the cerebellar cortex in myelin-deficient rats61, suggesting that myelination is used across brain regions and species to achieve optimal spike timing within circuits. In fear learning18, the correlation coefficient between hippocampal CA1 sharp wave ripples and anterior cingulate cortex spindles, which typically increases following fear conditioning, was unchanged in animals with impaired adult oligodendrogenesis. However, since tamoxifen (and consequent Myrf deletion from OPCs) was administered up to a day before the recordings were performed, the disruption in ripple-spindle synchrony is unlikely to be caused by a deficit in the formation of compact myelin, which may not yet have occurred19,30. Nevertheless, this general function of oligodendrocytes and myelin is most likely an important cellular mechanism for shaping circuit function after learning.
Other functions ascribed to oligodendrocytes may affect the overall excitability of neurons. Oligodendrocytes express high levels of inward-rectifying potassium channels, particularly Kir4.1 (REF.62). Deletion of Kir4.1 from oligodendrocyte lineage cells did not impact OPC differentiation or myelination but significantly reduced extracellular potassium clearance by oligodendrocytes and slowed the recovery of optic nerve axonal firing patterns after high frequency stimulation26. A similar study found that Kir4.1 knockout using Olig2-Cre resulted in the progressive loss of axon integrity63, though this result is slightly more difficult to interpret given that both OLIG2 and Kir4.1 are also expressed by astrocytes62. Still, it seems likely that oligodendrocytes can participate in regulating extracellular potassium levels, particularly during periods of high frequency neuronal firing. In support of this possibility, one study reported that cortical pyramidal neurons with oligodendrocytes tightly apposed to their cell bodies were less likely to exhibit burst firing upon bath application of extracellular potassium than those without25. This result is in line with the finding that oligodendrocyte-specific Kir4.1-knockout animals develop spontaneous seizures by early adulthood26. Thus, the addition of new oligodendrocytes or alterations in the expression levels of oligodendrocyte potassium channels may regulate neuronal excitability or support higher frequency firing in circuits during or following learning.
Oligodendrocytes may also influence neuronal plasticity at the level of synapses. Knockout of Rtn4, encoding neurite outgrowth inhibitor A (Nogo-A; also known as reticulon 4, a myelin-associated inhibitor of neurite outgrowth) from oligodendrocyte lineage cells increased dendritic branching complexity and spine turnover in adult cortical pyramidal neurons27. It is not clear whether the neuronal adaptations are directly due to the loss of interactions with oligodendrocyte Nogo-A or due to a change in overall myelination, as Nogo-A also influences the myelinogenic potential of oligodendrocytes22. Either way, it suggests that oligodendrocytes and myelin may restrict dendritic branching and spine turnover in the cortex. Furthermore, Olig2 deletion from oligodendrocytes results in hypomyelination and reduced synapse density in the developing motor cortex28. This interaction seems to persist throughout life, as genetically enhancing myelination in adulthood increased synapse density in hippocampal CA1 of aged animals3. Together, these studies demonstrate the capacity for oligodendrocytes and myelin to regulate structural synapse plasticity, which could be an important cellular mechanism through which they influence learning and memory.
Neurotransmitter recycling and release probability may also be tied to oligodendrocyte function. Oligodendrocyte-specific deletion of glutamine synthetase, an enzyme that converts glutamate into glutamine, disrupted glutamatergic synaptic transmission in the midbrain23. Decreasing myelination overall or disrupting myelin tight junctions also led to aberrantly increased glutamate levels in the adolescent cortex64 and adult brainstem65. Apart from regulating neurotransmitter homeostasis, oligodendrocytes may have additional ways of influencing neurotransmitter release. Deleting the neurotrophin brain-derived neurotrophic factor (BDNF) from oligodendrocyte lineage cells significantly reduced the exocytosis of vesicular glutamate from calyx terminals in the auditory brainstem24. We do not currently know whether oligodendrocyte expression of glutamate-related enzymes or of BDNF is regulated by experience, but these molecules may be important for supporting circuit activity on a shorter timescale than the formation of new compact myelin and may potentially account for the early deficits that Myrf and Olig2 cKOs exhibit in some learning paradigms3,38.
There is some evidence that OPCs themselves can regulate neuronal signalling. Mice lacking the chondroitin sulfate proteoglycan NG2, which is highly expressed in OPCs, have reduced glutamatergic post-synaptic currents and impaired induction of NMDA receptor-dependent long-term potentiation in cortical pyramidal neurons40. In vitro and in slices, NG2 can be cleaved in an activity-dependent manner to release an ectodomain that, when applied to brain slices derived from NG2-knockout mice, increased the number of cFOS-expressing cortical neurons. The use of a constitutive knockout limits our ability to specifically assign these phenotypes to OPCs but certainly suggests that we should further investigate their myelin-independent functions.
We have discussed several potential ways through which oligodendrocytes can influence neuronal function, but it should be noted that other mechanisms not directly related to these functions have also been implicated in learning and memory. For example, adult neurogenesis appears to be critical for certain types of hippocampus-dependent learning66. Other cell types, namely astrocytes67,68 and microglia69,70, also have important roles in regulating memory formation and, in the case of microglia, memory elimination71. These other cellular mechanisms may act in parallel to direct circuit function or may interact with oligodendrocyte lineage cells and myelin in ways that we have yet to uncover. Despite the recent surge of ground-breaking studies on myelin and cognition, the uncharted waters remain excitingly vast.
Oligodendrocytes in neuropathology
In light of the numerous rodent studies showing an important role for oligodendrocytes and myelin in learning and memory, it may come as no surprise that studies have also identified oligodendrocyte abnormalities in individuals with neurological and psychiatric disorders that present with cognitive symptoms72–74. A full discussion of all brain disorders that involve white matter pathology is beyond the scope of this review; however, we highlight below two neurodevelopmental disorders in which recent studies have identified myelin dysfunction as a central feature of the disease phenotype.
Williams syndrome is a genetic neurodevelopmental disorder characterized by hypersociability, intellectual disability and elevated anxiety75. In a mouse model of Williams syndrome in which the gene Gtf2i is selectively deleted in excitatory forebrain neurons (Gtf2i cKOs)76, 70% of downregulated transcripts detected by RNA sequencing were associated with myelination. The numbers of mature oligodendrocytes were reduced in cortex and corpus callosum of 1-month-old Gtf2i cKOs relative to Cre-negative controls, whereas OPC density was unchanged, indicating a deficit in oligodendrocyte maturation. At the ultrastructural level in the corpus callosum, the percentage of myelinated axons was lower in Gtf2i-cKO animals. Reduced expression of myelin-related genes and decreased mature oligodendrocyte density were also observed in frontal cortical regions of post-mortem tissue obtained from individuals with Williams syndrome. Encouragingly, treatment with the pro-myelinating drug clemastine rescued the hypersociability phenotype of Gtf2i-cKO animals, indicating that targeting myelination postnatally may be beneficial for people with Williams syndrome. It remains to be seen whether the cognitive deficits experienced by these individuals can also be alleviated by enhancing myelination.
Pitt–Hopkins syndrome is another neurodevelopmental disorder presenting with intellectual disability, along with failure to acquire language and other behavioural phenotypes shared with autism spectrum disorders (ASDs)77. Transcriptomic analysis of brain tissue from multiple genetic mouse models of Pitt–Hopkins syndrome and ASDs, as well as datasets from post-mortem patients with ASDs, revealed a common signature of downregulated oligodendrocyte and myelin-associated genes78. To validate these results, the same group examined one Pitt–Hopkins model (Tcf4- mutant mice) and found increased numbers of OPCs, reduced numbers of mature oligodendrocytes, and reduced levels of myelin proteins in the prefrontal cortex, indicating a deficit in oligodendrocyte maturation. This deficit appears to arise from a cell-autonomous requirement for TCF4 in OPCs to differentiate78,79. Studies in other genetic mouse models of ASDs have also reported deficits in myelination80–82. The prevalence of myelin dysfunction across these models raises the question of whether abnormal myelination is a key driver of the shared phenotypes across these disparate genetic models and whether, as in models of Williams syndrome76, treatment with clemastine or other pro-myelinating drugs may reverse some of the behavioural symptoms associated with idiopathic ASDs.
Alterations in oligodendrocyte and myelin function have now been identified in many other neuro-pathological conditions with cognitive symptoms72–74. We have known for some time now that white matter atrophies considerably in normal ageing83,84. Combined with the growing evidence for myelin and oligodendrocyte involvement in learning and memory3,18,19, there is good reason to suspect that some of the cognitive decline we experience with ageing is related to myelin loss. Myelin dysfunction has also been reported in post-traumatic stress disorder85, Alzheimer disease86–88 and depression89–91. These results should be interpreted with caution — oligodendrocyte and myelin-associated genes may be preferentially picked up in large-scale transcriptomic analyses because they are so abundant62,92. Furthermore, OPCs are the most proliferative cell type in the adult brain17,93 and respond strongly to perturbations in the local environment1. It is not yet clear whether these changes are truly relevant to the aetiology and progression of these diseases or simply reflect the high intrinsic plasticity of the oligodendrocyte lineage. However, examples like the Williams syndrome model, in which behavioural deficits were reversed by clemastine treatment76, suggest that aberrant myelination may indeed contribute significantly to behavioural phenotypes in some of these neuropathologies.
Myelin plasticity — the next 10 years
Like neuronal plasticity, plasticity within the oligodendrocyte lineage can manifest in multiple ways. Dissociations between experience-induced changes in OPC proliferation, differentiation, myelin sheath remodelling, and myelin thickness18,19,34 tell us that these forms of plasticity do not necessarily proceed in concert. Furthermore, the time course of oligodendrocyte plasticity varies significantly between different forms of learning. For example, whereas fear conditioning induced a rapid increase in OPC proliferation 24 h post conditioning19, motor learning suppressed proliferation in the days immediately following training39. Thus, we may not be able to identify a general organizing principle for oligodendrocyte plasticity in learning and memory and instead recognize that, like synaptic plasticity, oligodendrocyte and myelin plasticity will be custom tailored to each circuit and/or behaviour.
Our ability to form a mechanistic understanding of how oligodendrocytes interact with neuronal circuits to influence behaviour is limited by our current tools, which cannot tease apart the relative contributions of OPCs, oligodendrocytes and myelination in various contexts. To do so would require manipulations that separate OPC differentiation from subsequent myelination. This has been challenging to date, though there is some evidence that these processes can be independently regulated94. We will also need tools to selectively disrupt OPC proliferation, though they would inevitably prevent the formation of new oligodendrocytes and myelin that would have been generated from newly proliferated OPCs. Nevertheless, it would be informative to compare the short-term effects of disrupting OPC proliferation versus differentiation, as OPCs themselves may play distinct roles in shaping neuronal function89,91. There is also evidence for some degree of heterogeneity among OPCs in their physiological and molecular properties95,96. One could imagine that a subset of OPCs is primed to differentiate, whereas others interact with and regulate circuit activity in myelin-independent ways. Whether these differences reflect or produce differential OPC-neuron interactions will require additional studies that disrupt specific aspects of OPC physiology.
Another limitation of our current models is the global nature of our manipulations. We can now prevent the progression of myelination at any age using inducible CreER lines that target OPCs and we can enhance myelination with systemic drug delivery, yet these methods affect myelination throughout the entire CNS. From these experiments, it is impossible to determine the specific locus of myelin deposition that is relevant for a given aspect of learning or memory. Perhaps global changes are indeed required; however, this seems unlikely given the region-specific changes in OPC proliferation and differentiation observed in wild-type animals that have undergone learning18,19. Even within one region, myelination of different types of axons could alter circuits in different ways. In the cortex, both excitatory and inhibitory neurons are myelinated97–99. We currently do not know whether the altered patterns of neuronal activity in Myrf cKOs18,19 are due to cell-autonomous interactions or network-level changes caused by, for example, the dysregulation of parvalbumin interneuron myelination. To answer these questions, we will need to develop tools that allow region-specific, input-specific and cell type-specific control of myelination. The former will require viral tools to selectively manipulate OPCs and oligodendrocytes, whereas the latter two may be achieved by manipulating neurons rather than OPCs, perhaps by overexpressing negative regulators of myelination100 along subsets of axons. Cre-independent methods to alter myelination will also be of significant use, as many disease models require the presence of a neuronal or neural progenitor population-specific Cre. Although we can perform some studies with drugs to enhance48,49,101 or disrupt102 myelination, these pharmacological manipulations can have off-target effects and, in the case of disrupting myelination, can directly damage axons as well as other cell types103. Finally, just as neuronal manipulation has become more sophisticated with the development of tools like FosTrap104 to specifically target activated neurons, we would ideally find a way to specifically inhibit learning-induced OPC differentiation or myelination.
Though we speculated above on the myriad ways in which oligodendrocytes could influence neuronal function, we do not yet know when or if these various interactions are engaged and relevant in the context of learning and memory. It is clear, however, that oligodendrocytes and myelin plasticity can influence neuronal function in multiple ways beyond changing axonal conductance. What are the overall effects of increased myelination and are the same processes always engaged, or can specific oligodendrocyte/myelin-associated functions be altered independently of one another? Is it useful to manipulate individual molecular pathways within these cells or is it more informative to consider the net effect of OPC differentiation and myelination as it occurs in vivo? Many mechanistic questions remain, some of which will require conceptual advances in both systems neuroscience and cell biology to answer, including a better understanding of the neuronal adaptations that establish a memory engram and the identification of signals that drive learning-induced oligodendrocyte plasticity105,106. We have broken ground on characterizing the involvement of oligodendrocytes in learning and memory, but the road ahead is still long and paved with exciting cellular mysteries to unravel.
Genetic fate mapping.
A technique that uses genetic methods, such as Cre recombinase, to selectively express a reporter gene in a subset of cells and follow their developmental fate over time.
Barrel cortex.
Whisker-related primary somatosensory cortex.
Enriched environment.
An environment designed to provide enhanced multisensory stimulation.
Memory consolidation.
A process during which recent learned experiences are stabilized into long-term memory.
Contextual fear conditioning.
(CFC). A behavioural paradigm in which a novel context is paired with an aversive stimulus (e.g. a foot shock), thereby inducing a learned association between the previously neutral context and the aversive experience.
Cre driver.
A genetically engineered rodent line in which Cre recombinase is expressed under a cell type-specific promoter.
Engrams.
Enduring physical changes in a neural circuit that underlie specific memories.
Carbon dating.
A technique for inferring the age of organic matter (for example, cells) based on the proportion of carbon-14 it contains relative to other carbon isotopes and historical atmospheric proportions of carbon-14.
Acknowledgements
We are grateful to M. Kheirbek, R. Roth and members of the Chan lab for their valuable input on the manuscript. We apologize for the omission of many important studies due to space constraints. This work was supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke (grants R01NS097428 & R01NS095889 to JRC and grant F32NS116214 to WX), the Adelson Medical Research Foundation (ANDP grant A130141 to JRC), and the Rachleff family endowment.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Hughes EG, Kang SH, Fukaya M & Bergles DE Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat. Neurosci 16, 668–676 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes EG, Orthmann-Murphy JL, Langseth AJ & Bergles DE Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat. Neurosci 21, 696–706 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang F et al. Myelin degeneration and diminished myelin renewal contribute to age-related deficits in memory. Nat. Neurosci 23, 481–486 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill RA, Li AM & Grutzendler J Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain. Nat. Neurosci 21, 683–695 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rivers LE et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat. Neurosci 11, 1392–1401 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimou L, Simon C, Kirchhoff F, Takebayashi H & Götz M Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J. Neurosci 28, 10434–10442 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang SH, Fukaya M, Yang JK, Rothstein JD & Bergles DE NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 68, 668–681 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu X et al. Age-dependent fate and lineage restriction of single NG2 cells. Development 138, 745–753 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young KM et al. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 77, 873–885 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomassy GS et al. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science 344, 319–324 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrera K et al. Organization of myelin in the mouse somatosensory barrel cortex and the effects of sensory deprivation. Dev. Neurobiol 73, 297–314 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Y-Y et al. Enriched environment increases the total number of CNPase positive cells in the corpus callosum of middle-aged rats. Acta Neurobiol. Exp 71, 322–330 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Yang S et al. Effects of an enriched environment on myelin sheaths in the white matter of rats during normal aging: a stereological study. Neuroscience 234, 13–21 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Gibson EM et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitew S et al. Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nat. Commun 9, 306 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Q, Brus-Ramer M, Martin JH & McDonald JW Electrical stimulation of the medullary pyramid promotes proliferation and differentiation of oligodendrocyte progenitor cells in the corticospinal tract of the adult rat. Neurosci. Lett 479, 128–133 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKenzie IA et al. Motor skill learning requires active central myelination. Science 346, 318–322 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steadman PE et al. Disruption of oligodendrogenesis impairs memory consolidation in adult mice. Neuron 105, 150–164. e6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan S, Mayoral SR, Choi HS, Chan JR & Kheirbek MA Preservation of a remote fear memory requires new myelin formation. Nat. Neurosci 23, 487–499 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeung MSY et al. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell 159, 766–774 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Tripathi RB et al. Remarkable stability of myelinating oligodendrocytes in mice. Cell Rep 21, 316–323 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chong SYC et al. Neurite outgrowth inhibitor Nogo-A establishes spatial segregation and extent of oligodendrocyte myelination. Proc. Natl Acad. Sci. USA 109, 1299–1304 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xin W et al. Oligodendrocytes support neuronal glutamatergic transmission via expression of glutamine synthetase. Cell Rep 27, 2262–2271 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jang M, Gould E, Xu J, Kim EJ & Kim JH Oligodendrocytes regulate presynaptic properties and neurotransmission through BDNF signaling in the mouse brainstem. eLife 8, e42156 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Battefeld A, Klooster J & Kole MHP Myelinating satellite oligodendrocytes are integrated in a glial syncytium constraining neuronal high-frequency activity. Nat. Commun 7, 11298 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larson VA et al. Oligodendrocytes control potassium accumulation in white matter and seizure susceptibility. eLife 7, e34829 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zemmar A et al. Oligodendrocyte-and neuron-specific nogo-a restrict dendritic branching and spine density in the adult mouse motor cortex. Cereb. Cortex 28, 2109–2117 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang F et al. Enhancing oligodendrocyte myelination rescues synaptic loss and improves functional recovery after chronic hypoxia. Neuron 99, 689–701 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mangin J-M, Li P, Scafidi J & Gallo V Experience-dependent regulation of NG2 progenitors in the developing barrel cortex. Nat. Neurosci 15, 1192–1194 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill RA, Patel KD, Goncalves CM, Grutzendler J & Nishiyama A Modulation of oligodendrocyte generation during a critical temporal window after NG2 cell division. Nat. Neurosci 17, 1518–1527 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy KM, Mancini SJ, Clayworth KV, Arbabi K & Beshara S Experience-dependent changes in myelin basic protein expression in adult visual and somatosensory cortex. Front. Cell. Neurosci 14, 56 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keiner S et al. Effect of skilled reaching training and enriched environment on generation of oligodendrocytes in the adult sensorimotor cortex and corpus callosum. BMC Neurosci 18, 31 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci 15, 1621–1623 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Makinodan M, Rosen KM, Ito S & Corfas G A critical period for social experience–dependent oligodendrocyte maturation and myelination. Science 337, 1357–1360 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swire M, Kotelevtsev Y, Webb DJ, Lyons DA & ffrench-Constant C Endothelin signalling mediates experience-dependent myelination in the CNS. eLife 8, e49493 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu T et al. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 462, 915–919 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Komiyama T et al. Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature 464, 1182–1186 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Xiao L et al. Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat. Neurosci 19, 1210–1217 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bacmeister CM et al. Motor learning promotes remyelination via new and surviving oligodendrocytes. Nat. Neurosci 23, 819–831 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakry D et al. Oligodendrocyte precursor cells modulate the neuronal network by activity-dependent ectodomain cleavage of glial NG2. PLoS Biol 12, e1001993 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kitamura T et al. Engrams and circuits crucial for systems consolidation of a memory. Science 356, 73–78 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teixeira CM, Pomedli SR, Maei HR, Kee N & Frankland PW Involvement of the anterior cingulate cortex in the expression of remote spatial memory. J. Neurosci 26, 7555–7564 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Engvig A et al. Memory training impacts short-term changes in aging white matter: a longitudinal diffusion tensor imaging study. Hum. Brain Mapp 33, 2390–2406 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bujalka H et al. MYRF is a membrane-associated transcription factor that autoproteolytically cleaves to directly activate Myelin genes. PLoS Biol 11, e1001625 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Emery B et al. Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell 138, 172–185 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kato D et al. Motor learning requires myelination to reduce asynchrony and spontaneity in neural activity. Glia 68, 193–210 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu Y et al. Olig2 targets chromatin remodelers to enhancers to initiate oligodendrocyte differentiation. Cell 152, 248–261 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mei F et al. Accelerated remyelination during inflammatory demyelination prevents axonal loss and improves functional recovery. eLife 5, e18246 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mei F et al. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat. Med 20, 954–960 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Green AJ et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Lancet 390, 2481–2489 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Giustino TF, Fitzgerald PJ & Maren S Fear expression suppresses medial prefrontal cortical firing in rats. PLoS ONE 11, e0165256 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo F et al. Oligodendrocyte-specific loss of Cdk5 disrupts the architecture of nodes of Ranvier as well as learning and memory. Exp. Neurol 306, 92–104 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee Y et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487, 443–448 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fünfschilling U et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485, 517–521 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saab AS et al. Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron 91, 119–132 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Howng SYB et al. ZFP191 is required by oligodendrocytes for CNS myelination. Genes Dev 24, 301–311 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ford MC et al. Tuning of Ranvier node and internode properties in myelinated axons to adjust action potential timing. Nat. Commun 6, 8073 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arancibia-Cárcamo IL et al. Node of Ranvier length as a potential regulator of myelinated axon conduction speed. eLife 6, e23329 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seidl AH, Rubel EW & Harris DM Mechanisms for adjusting interaural time differences to achieve binaural coincidence detection. J. Neurosci 30, 70–80 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salami M, Itami C, Tsumoto T & Kimura F Change of conduction velocity by regional myelination yields constant latency irrespective of distance between thalamus and cortex. Proc. Natl Acad. Sci. USA 100, 6174–6179 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lang EJ & Rosenbluth J Role of myelination in the development of a uniform olivocerebellar conduction time. J. Neurophysiol 89, 2259–2270 (2003). [DOI] [PubMed] [Google Scholar]
- 62.Zhang Y et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci 34, 11929–11947 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schirmer L et al. Oligodendrocyte-encoded Kir4.1 function is required for axonal integrity. eLife 7, e36428 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen X et al. Impairment of oligodendroglia maturation leads to aberrantly increased cortical glutamate and anxiety-like behaviors in Juvenile mice. Front. Cell. Neurosci 9, 467 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maheras KJ et al. Absence of claudin 11 in CNS myelin perturbs behavior and neurotransmitter levels in mice. Sci. Rep 8, 3798 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gonçalves JT, Schafer ST & Gage FH Adult neurogenesis in the hippocampus: from stem cell behavior. Cell 167, 897–914 (2016). [DOI] [PubMed] [Google Scholar]
- 67.Adamsky A et al. Astrocytic activation generates de novo neuronal potentiation and memory enhancement. Cell 174, 59–71 (2018). [DOI] [PubMed] [Google Scholar]
- 68.Santello M, Toni N & Volterra A Astrocyte function from information processing to cognition and cognitive impairment. Nat. Neurosci 22, 154–166 (2019). [DOI] [PubMed] [Google Scholar]
- 69.Parkhurst CN et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155, 1596–1609 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nguyen PT et al. Microglial remodeling of the extracellular matrix promotes synapse plasticity. Cell 182, 388–403 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang C et al. Microglia mediate forgetting via complement-dependent synaptic elimination. Science 367, 688–694 (2020). [DOI] [PubMed] [Google Scholar]
- 72.Gibson EM, Geraghty AC & Monje M Bad wrap: myelin and myelin plasticity in health and disease. Dev. Neurobiol 78, 123–135 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mot AI, Depp C & Nave K-A An emerging role of dysfunctional axon-oligodendrocyte coupling in neurodegenerative diseases. Dialogues Clin. Neurosci 20, 283–292 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ettle B, Schlachetzki JCM & Winkler J Oligodendroglia and Myelin in neurodegenerative diseases: more than just bystanders? Mol. Neurobiol 53, 3046–3062 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barak B & Feng G Neurobiology of social behavior abnormalities in autism and Williams syndrome. Nat. Neurosci 19, 647–655 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barak B et al. Neuronal deletion of Gtf2i, associated with Williams syndrome, causes behavioral and myelin alterations rescuable by a remyelinating drug. Nat. Neurosci 22, 700–708 (2019). [DOI] [PubMed] [Google Scholar]
- 77.Goodspeed K et al. Pitt-Hopkins syndrome: a review of current literature, clinical approach, and 23-patient case series. J. Child Neurol 33, 233–244 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Phan BN et al. A myelin-related transcriptomic profile is shared by Pitt–Hopkins syndrome models and human autism spectrum disorder. Nat. Neurosci 23, 375–385 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wedel M et al. Transcription factor Tcf4 is the preferred heterodimerization partner for Olig2 in oligodendrocytes and required for differentiation. Nucleic Acids Res 48, 4839–4857 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scott R et al. Loss of Cntnap2 causes axonal excitability deficits, developmental delay in cortical myelination, and abnormal stereotyped motor behavior. Cereb. Cortex 29, 586–597 (2019). [DOI] [PubMed] [Google Scholar]
- 81.Pacey LKK et al. Delayed myelination in a mouse model of fragile X syndrome. Hum. Mol. Genet 22, 3920–3930 (2013). [DOI] [PubMed] [Google Scholar]
- 82.Ercan E et al. Neuronal CTGF/CCN2 negatively regulates myelination in a mouse model of tuberous sclerosis complex. J. Exp. Med 214, 681–697 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marner L, Nyengaard JR, Tang Y & Pakkenberg B Marked loss of myelinated nerve fibers in the human brain with age. J. Comp. Neurol 462, 144–152 (2003). [DOI] [PubMed] [Google Scholar]
- 84.Salat DH et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol. Aging 26, 1215–1227 (2005). [DOI] [PubMed] [Google Scholar]
- 85.Chao LL, Tosun D, Woodward SH, Kaufer D & Neylan TC Preliminary evidence of increased hippocampal myelin content in veterans with posttraumatic stress disorder. Front. Behav. Neurosci 9, 333 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mathys H et al. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 570, 332–337 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Behrendt G et al. Dynamic changes in myelin aberrations and oligodendrocyte generation in chronic amyloidosis in mice and men. Glia 61, 273–286 (2013). [DOI] [PubMed] [Google Scholar]
- 88.Pak K, Chan SL & Mattson MP Presenilin-1 mutation sensitizes oligodendrocytes to glutamate and amyloid toxicities, and exacerbates white matter damage and memory impairment in mice. Neuromol. Med 3, 53–64 (2003). [DOI] [PubMed] [Google Scholar]
- 89.Nagy C et al. Single-nucleus transcriptomics of the prefrontal cortex in major depressive disorder implicates oligodendrocyte precursor cells and excitatory neurons. Nat. Neurosci 23, 771–781 (2020). [DOI] [PubMed] [Google Scholar]
- 90.Boda E Myelin and oligodendrocyte lineage cell dysfunctions: new players in the etiology and treatment of depression and stress-related disorders. Eur. J. Neurosci 10.1111/ejn.14621 (2019). [DOI] [PubMed] [Google Scholar]
- 91.Birey F et al. Genetic and stress-induced loss of NG2 glia triggers emergence of depressive-like behaviors through reduced secretion of FGF2. Neuron 88, 941–956 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nishida Y, Yoshioka M & St-Amand J The top 10 most abundant transcripts are sufficient to characterize the organs functional specificity: evidences from the cortex, hypothalamus and pituitary gland. Gene 344, 133–141 (2005). [DOI] [PubMed] [Google Scholar]
- 93.Simon C, Götz M & Dimou L Progenitors in the adult cerebral cortex: cell cycle properties and regulation by physiological stimuli and injury. Glia 59, 869–881 (2011). [DOI] [PubMed] [Google Scholar]
- 94.Zhou X et al. Mature myelin maintenance requires Qki to coactivate PPARβ-RXRα–mediated lipid metabolism. J. Clin. Invest 130, 2220–2236 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marisca R et al. Functionally distinct subgroups of oligodendrocyte precursor cells integrate neural activity and execute myelin formation. Nat. Neurosci 23, 363–374 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Spitzer SO et al. Oligodendrocyte progenitor cells become regionally diverse and heterogeneous with age. Neuron 101, 459–471. e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stedehouder J et al. Local axonal morphology guides the topography of interneuron myelination in mouse and human neocortex. eLife 8, e48615 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stedehouder J et al. Fast-spiking parvalbumin interneurons are frequently myelinated in the cerebral cortex of mice and humans. Cereb. Cortex 27, 5001–5013 (2017). [DOI] [PubMed] [Google Scholar]
- 99.Micheva KD et al. A large fraction of neocortical myelin ensheathes axons of local inhibitory neurons. eLife 5, e15784 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Redmond SA et al. Somatodendritic expression of JAM2 inhibits oligodendrocyte myelination. Neuron 91, 824–836 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rankin KA et al. Selective estrogen receptor modulators enhance CNS remyelination independent of estrogen receptors. J. Neurosci 39, 2184–2194 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Keough MB, Jensen SK & Yong VW Experimental demyelination and remyelination of murine spinal cord by focal injection of lysolecithin. J. Vis. Exp 97, 52679 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mitchell J & Caren CA Degeneration of non-myelinated axons in the rat sciatic nerve following lysolecithin injection. Acta Neuropathol 56, 187–193 (1982). [DOI] [PubMed] [Google Scholar]
- 104.DeNardo L & Luo L Genetic strategies to access activated neurons. Curr. Opin. Neurobiol 45, 121–129 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mount CW & Monje M Wrapped to adapt: experience-dependent myelination. Neuron 95, 743–756 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Foster AY, Bujalka H & Emery B Axoglial interactions in myelin plasticity: evaluating the relationship between neuronal activity and oligodendrocyte dynamics. Glia 67, 2038–2049 (2019). [DOI] [PubMed] [Google Scholar]
- 107.Jeffries MA et al. ERK1/2 activation in preexisting oligodendrocytes of adult mice drives new myelin synthesis and enhanced CNS function. J. Neurosci 36, 9186–9200 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Le Bihan D Looking into the functional architecture of the brain with diffusion MRI. Nat. Rev. Neurosci 4, 469–480 (2003). [DOI] [PubMed] [Google Scholar]
- 109.Sampaio-Baptista C et al. Motor skill learning induces changes in white matter microstructure and myelination. J. Neurosci 33, 19499–19503 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sampaio-Baptista C et al. White matter structure and myelin-related gene expression alterations with experience in adult rats. Prog. Neurobiol 187, 101770 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sampaio-Baptista C & Johansen-Berg H White matter plasticity in the adult brain. Neuron 96, 1239–1251 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bengtsson SL et al. Extensive piano practicing has regionally specific effects on white matter development. Nat. Neurosci 8, 1148–1150 (2005). [DOI] [PubMed] [Google Scholar]
- 113.Scholz J, Klein MC, Behrens TEJ & Johansen-Berg H Training induces changes in white-matter architecture. Nat. Neurosci 12, 1370–1371 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hofstetter S, Friedmann N & Assaf Y Rapid language-related plasticity: microstructural changes in the cortex after a short session of new word learning. Brain Struct. Funct 222, 1231–1241 (2017). [DOI] [PubMed] [Google Scholar]
- 115.Ekerdt CEM, Kühn C, Anwander A, Brauer J & Friederici AD Word learning reveals white matter plasticity in preschool children. Brain Struct. Funct 225, 607–619 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Keller TA & Just MA Altering cortical connectivity: remediation-induced changes in the white matter of poor readers. Neuron 64, 624–631 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Carreiras M et al. An anatomical signature for literacy. Nature 461, 983–986 (2009). [DOI] [PubMed] [Google Scholar]
- 118.Schlegel AA, Rudelson JJ & Tse PU White matter structure changes as adults learn a second language. J. Cogn. Neurosci 24, 1664–1670 (2012). [DOI] [PubMed] [Google Scholar]
- 119.Yeung MSY et al. Dynamics of oligodendrocyte generation in multiple sclerosis. Nature 566, 538–542 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


