Abstract
The increase in life expectancy and the migration of individuals with Chagas disease (ChD) from rural to urban centers exposes them to the development of chronic-degenerative abnormalities that may increase the prevalence of metabolic syndrome (MetS). The present study aimed to identify the prevalence of MetS and its components in individuals with chronic ChD. This is a cross-sectional study with 361 patients of both sexes, aging >18 years, followed at a national reference center (Rio de Janeiro, Brazil). MetS diagnosis followed the International Diabetes Federation 2005 criteria. The association between the variables was determined through logistic regression models. The mean age was and 60.7±10.8 years. About half (56.2%) were female and the majority self-reported their race as mulatto (59.8%). The percentage of individuals with MetS was 40.4%. The variables independently associated with MetS were age (OR 1.06; 95%CI 1.04–1.09), high education levels (OR 0.36; 95%CI 0.17–0.79) and cardiac form with heart failure (OR 0.34; 95%CI 0.17–0.68). Therefore, a high prevalence of MetS was found in this Brazilian chronic ChD cohort. The identification of the associated factors can facilitate the development of effective approaches for preventing and managing MetS in ChD patients.
Introduction
Chagas disease (ChD) is a neglected tropical disease caused by the protozoan Trypanosoma cruzi with 8 million people estimated to be infected worldwide [1–3]. Initially restricted to Latin America, it is currently widespread over several countries in almost all continents, being considered a global epidemic [1, 4]. The increase in life expectancy together with migration of large part of the ChD population from rural to urban areas increased the exposure of these patients to inadequate lifestyle, facilitating the development of non-infectious chronic conditions such as obesity, insulin resistance, hypertension and dyslipidemia [5]. Together, these factors exponentially increase the risk of cardiovascular events and death, leading to the development of an important clinical condition known as metabolic syndrome (MetS) [6].
MetS was initially described by Reaven as a cluster of clinical and metabolic abnormalities including glucose intolerance, hypertension, dyslipidemia, and insulin resistance that have in the visceral fat accumulation as a common pathway [7, 8]. Currently, MetS is defined by the World Health Organization (WHO) as a pathological condition characterized by central obesity, insulin resistance, hypertension and hyperlipidemia [9].
The prevalence of MetS has been growing at an alarming rate over the past decades, reaching up to 40% of the entire population, depending on the diagnostic criteria and the studied population [10]. This high prevalence is particularly concerning since individuals with MetS have two to threefold risk of developing cardiovascular disease and a fivefold risk of developing diabetes [11]. However, the percentage of patients with ChD presenting MetS is still unknown and the deleterious effects of MetS together with the clinical ChD-related abnormalities may further decrease quality of life and increase health-related costs, morbidity and mortality rates [12, 13]. Thus, studies aiming to identify the factors associated to MetS in ChD patients are of paramount importance and could facilitate the development of effective approaches for preventing and managing MetS in ChD patients.
Therefore, the present study aimed to investigate the prevalence of MetS in individuals with chronic ChD as well as to identify the main associated factors related to this clinical syndrome in this population.
Methods
Study design, period and population
This is an observational cross-sectional study, conducted from March 2014 to March 2017, including residents in the city of Rio de Janeiro of both sexes, aging >18 years, with diagnosis of ChD confirmed by two simultaneously positive serological tests (enzyme-linked immunosorbent assay and indirect immunofluorescence). All patients were under follow-up at the outpatient center of the national reference center for treatment and research in infectious and tropical diseases in Rio de Janeiro, Brazil (Evandro Chagas National Institute of Infectious Disease/ Oswaldo Cruz Foundation). Participants were excluded if they presented autoimmune disorders, cancer, other infectious diseases at the time of study recruitment, non-Chagasic heart diseases, severe cognitive impairments that precluded the completion of the questionnaires, current use of chronic anti-inflammatory or corticosteroids, or pregnant.
Sample size
The sample size was calculated based on a previous study conducted in a Brazilian urban center that achieved a 30% prevalence of MetS [14]. Considering a precision of 5% and 95% confidence interval, 323 individuals were necessary to perform this study. The sample size was further increased by 20% to account for refusals, totalizing a sample of 400 individuals.
Ethical considerations
All participants received information about the goals and procedures of the study and agreed to participate by signing an informed consent form. The study was approved by the Institutional Review Board of the Evandro Chagas National Institute of Infectious Disease (CAAE: 58273916.0.000.5262).
Study procedures
Patients were invited to participate during their regular clinic visits and were submitted to the study procedures in two visits within a period of no more than two months. In the first visit, patients signed the informed consent, completed all the questionnaires, and performed anthropometric and blood pressure measurements whereas in the second visit they underwent a clinical evaluation and blood tests. Trained staffs administered the questionnaires and performed the anthropometric and blood pressure measurements. The same physician performed the clinical evaluation in all participants. Blood samples were draw in the morning after a 12-hour fasting.
Metabolic syndrome
MetS was defined following the criteria established by the International Diabetes Federation in 2005 as the presence of central obesity, measured as ethnic-specific increased waist circumference (for South American population ≥ 90 cm in men and ≥ 80 cm in women), plus at least two of the following components: 1) raised triglycerides (≥150 mg/dL or specific treatment for this lipid abnormality); 2) reduced HDL-cholesterol (<40 mg/dL in males and <50 mg/dL in females or specific treatment for this lipid abnormality); 3) raised resting blood pressure (systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85mmHg or treatment of previously diagnosed hypertension); 4) raised fasting plasma glucose (glucose≥100mg/dL or previously diagnosed type 2 diabetes mellitus) [15].
Clinical form of ChD
Patients were classified using clinical, electrocardiographic, echocardiographic, and digestive exams according to the presence of ChD related abnormalities into indeterminate, cardiac without heart failure, cardiac with heart failure or digestive forms following the Brazilian Consensus on Chagas Disease [16].
Comorbidities
Comorbidities (hypertension, diabetes, dyslipidemia, and obesity) were obtained using information from medical records and anthropometric measures during the clinical evaluation. Obesity was diagnosed if body mass index [BMI = weight (kg)/squared height (m2)] was ≥ 30 kg/m2. Blood pressure measurements were taken twice with participants seated in a quiet room after 5 minutes rest using an Omron® digital sphygmomanometer and the mean value was considered.
Socioeconomic data and lifestyle
Information on age, sex, schooling, race, residents by domicile and income per capita were obtained during the interviews. Age was calculated subtracting date of interview from date of birth and considered as a continuous variable. Schooling was categorized based on the formal years of study into <9 years, 9 to 12 years and >12 years. Race was self-reported and classified as white, black, mulatto and others. The income per capita was obtained summing up all income from each resident in the domicile and dividing by the number of residents [17]. Smoking, alcohol consumption, sleep duration, physical activity level and food intake were evaluated during the interviews. Smoking was classified as current (regular use of tobacco, regardless of how long), former (past occasional use of tobacco for at least 3 months or daily use for a period of at least 1 month) or non-smoker (currently does not use any tobacco product that emits smoke, even occasionally, even if have experienced) [18]. Alcohol consumption was categorized into none (never ingested alcohol during life), former (did not consume any amount of alcohol in the last 30 days, having ingested in the past) or current (consumed any amount of alcohol in the last 30 days). Sleep hours was determined through a direct question and treated as a continuous variable. Physical activity levels were determined using the validated Brazilian short version of the International Physical Activity Questionnaire (IPAQ-short) [19, 20]. This instrument comprises eight questions regarding the duration and frequency of vigorous, moderate, and light physical activity, allowing individuals to be classified into three different categories: mild, moderate and high. Food consumption was assessed using a 24-hour recall that consists on the identification and quantification of all food and beverages consumed in the day before the interview [21]. Macronutrients were calculated using DietWin Professional Version 2008 software.
Data management and statistical analysis
Exploratory data analysis was performed calculating means (standard deviations) and frequency (percentages) of the variables of interest. The association between MetS and exposure variables was determined using logistic regression models. A univariate logistic regression was performed to determine the variables that should be included in the multivariate model, that included only those with p<0.20 in the univariate analysis. The backwards method was used to sequentially remove variables with p-values greater than 0.05 in the multivariate analysis, until the final model that maintained only those with p<0.05. The Research Electronic Data Capture (REDCap) web application was used for data management and the data analysis was conducted using Stata 13.0 software. Statistical significance was set at p≤0.05 for all analyses.
Results
From 397 included patients, 36 were excluded due to the following reasons: 6 with other infectious diseases, 3 with auto-immune diseases, 6 with cancer, 8 with non-chagasic cardiomyopathy, 5 in use of anti-inflammatory or corticosteroids, and 8 did not return to the second visit (losses to follow-up). Therefore, the final sample consisted of 361 individuals. The overall mean age was 60.7 (±10.8) years, with 56.2% (n = 203) women. There was a predominance of mulatto race (59.8%; n = 216) and most participants had less than 9 years of schooling (67.3%; n = 243). The percentage of individuals diagnosed with MetS was 40.4% (n = 146). The prevalence of hypertension, dyslipidemia, obesity and diabetes were 67.3% (n = 243), 53.5% (n = 193), 25.8% (n = 93) and 21.6% (n = 78), respectively (Fig 1).
Fig 1.
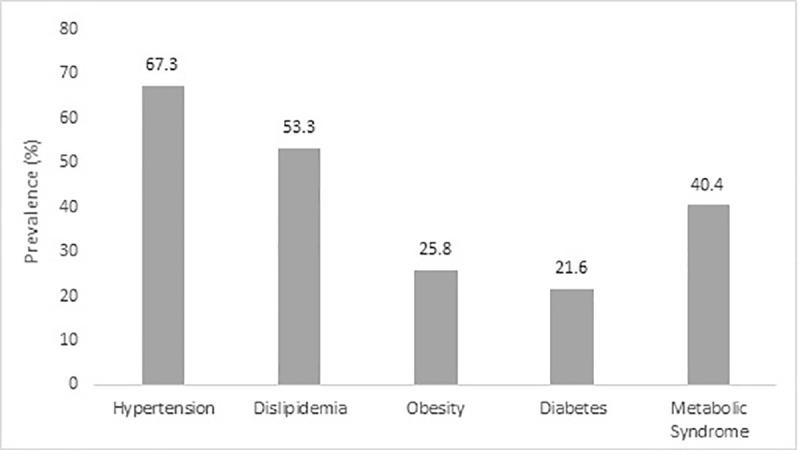
The description of the variables stratified by the presence of MetS is shown in Table 1. Overall, MetS patients were older (64.6 vs 58.1; p<0.001), with a predominance of females (63.7% vs 51.2%; p = 0.02) and less than 9 years of education (74.7% vs 62.3%; p = 0.004). The minority of MetS patients presented cardiac form with heart failure (8.2% vs 20.5%; p = 0.002) but had a greater prevalence of comorbidities (2.7 vs 0.99; p<0.001), and higher levels of triglycerides (127.4 vs 105.5; p<0.001), very low-density lipoprotein (25.4 vs 20.5; p<0.001), glucose (108.1 vs 97.4; p<0.001), and systolic blood pressure (139.2 vs 129.1; p<0.001). The consumption of carbohydrates (181.1 vs 204.7; p = 0.009) and lipids (36.3 vs 41.2; p = 0.020) was lower among MetS patients.
Table 1. Characteristics of participants included in the study (n = 361).
| Variables | Metabolic Syndrome | p-value* | |
|---|---|---|---|
| No | Yes | ||
| (60.6%; n = 215) | (40.4%; n = 146) | ||
| Age (years) | 58.1 (±11.7) | 64.6 (±7.9) | <0.001 |
| Residents by domicile (persons) | 2.8 (±1.35) | 2.8 (±1.60) | 0.91 |
| Income per capita (per R$1000.00) | 905.7 (±1013.5) | 954.9 (±7745) | 0.62 |
| Sex (%) | |||
| Male | 48.8 (105) | 36.3 (53) | 0.02 |
| Female | 51.2 (110) | 63.7 (93) | |
| Race (%) | |||
| White | 25.1 (54) | 18.5 (27) | 0.21 |
| Black | 12.1 (26) | 16.4 (24) | |
| Mulatto | 60.0 (129) | 59.6 (87) | |
| Others | 2.8 (6) | 5.5 (8) | |
| Schooling (%) | |||
| < 9 years | 62.3 (134) | 74.7 (109) | 0.004 |
| 9–12 years | 18.6 (40) | 18.5 (27) | |
| >12 years | 19.1 (41) | 6.9 (10) | |
| Sleep duration (hours) | 6.5 (±1.57) | 6.8 (±1.62) | 0.08 |
| SBP (mmHg) | 129.1 (±22.4) | 139.2 (±19.8) | <0.001 |
| DBP (mmHg) | 75.7 (±13.4) | 77.8 (±10.4) | 0.11 |
| Comorbidities (%) | |||
| Hypertension | 48.4 (104) | 95.2 (139) | <0.001 |
| Diabetes Mellitus | 8.8 (19) | 40.4 (59) | <0.001 |
| Dyslipidemia | 29.8 (64) | 88.4 (129) | <0.001 |
| Obesity | 12.1 (26) | 45.9 (67) | <0.001 |
| Medication | |||
| Antihypertensive | 69.8 (150) | 94.5 (138) | <0.001 |
| Hypoglycemic | 7.4 (16) | 21.2 (31) | <0.001 |
| Hipolipemic | 23.7 (51) | 61.6 (90) | <0.001 |
| Number of comorbidities (%) | 0.99 (± 0.78) | 2.7 (± 0.72) | <0.001 |
| Biomarkers | |||
| Total Cholesterol (mg/dL) (n = 355) | 182.9 (±35.5) | 187.2 (±37.4) | 0.267 |
| Triglycerides (mg/dL) (n = 354) | 105.5 (±65.2) | 127.4 (±56.4) | <0.001 |
| HDL-cholesterol (mg/dL) (n = 304) | 51.7 (±14.5) | 50.0 (±14.7) | 0.538 |
| LDL-cholesterol (mg/dL) (n = 303) | 113.3 (±30.0) | 113.1 (±35.9) | 0.969 |
| VLDL-cholesterol (mg/dL) (n = 352) | 20.5 (±11.2) | 25.4 (±11.3) | <0.001 |
| Glucose (mg/dL) (n = 360) | 97.4 (±18.4) | 108.1 (±37.0) | <0.001 |
| Glycated Hemoglobin (%) (n = 296) | 6.1 (±1.0) | 6.3 (±1.0) | 0.052 |
| C-reactive protein (mg/L) (n = 275) | 0.44 (±1.4) | 0.55 (±1.6) | 0.574 |
| Smoking (%) | |||
| Non-smoker | 53.9 (116) | 52.1 (76) | 0.929 |
| Former | 40.5 (87) | 41.8 (61) | |
| Current | 5.6 (12) | 6.2 (9) | |
| Alcohol consumption (%) | |||
| None | 61.9 (133) | 58.2 (85) | 0.776 |
| Former | 14.9 (32) | 15.8 (23) | |
| Current | 23.3 (50) | 26.0 (38) | |
| Physical activity level (%) | |||
| Low | 25.6 (55) | 26.0 (38) | 0.834 |
| Moderate | 47.0 (101) | 49.3 (72) | |
| High | 27.4 (59) | 24.7 (36) | |
| Indeterminate form | 26.6 (56) | 28.1 (41) | 0.67 |
| Cardiac form without heart failure | 50.7 (109) | 58.9 (86) | 0.13 |
| Cardiac form with heart failure | 20.5 (44) | 8.2 (12) | 0.002 |
| Digestive form | 16.3 (35) | 15.8 (23) | 0.89 |
| Caloric consumption (Kcal) | 1279.3 (±722.8) | 1161.4 (±643.8) | 0.101 |
| Macronutrients (g) | |||
| Carbohydrate | 204.7 (±89.4) | 181.1 (±84.1) | 0.009 |
| Protein | 69.8 (±34.3) | 64.2 (±30.3) | 0.123 |
| Lipid | 41.2 (±22.2) | 36.3 (±18.2) | 0.020 |
| Fibers | 18.9 (±10.5) | 17.6 (±12.1) | 0.214 |
* Unpaired t-test for continuous and chi-squared test for categorical variables.
Means (standard deviation) for continuous and percentage (absolute frequency) for categorical variables.
NYHA- New York Heart Association; SBP- systolic blood pressure; DBP- diastolic blood pressure; HDL- high-density lipoprotein; LDL- low-density lipoprotein; VLDL- very low-density lipoprotein.
The univariate analysis showed a significant association between MetS and age (OR 1.06; 95% CI 1.04 to 1.09), female sex (OR 1.67; 95% CI 1.09 to 2.58), education levels > 12 years (OR 0.30; 95% CI 0.14 to 0.63), cardiac form with heart failure (OR 0.35; 95% CI 0.18 to 0.69), and the consumption of carbohydrate (OR 0.99; 95% CI 0.99 to 0.99) and lipids (OR 0.99; 95% CI 0.98 to 1.00) (Table 2). In the multivariate model, the variables that were independently associated with MetS were age (OR 1.06; 95% CI 1.04 to 1.09), education levels > 12 years (OR 0.36; 95% CI 0.17 to 0.79) and cardiac form with heart failure (OR 0.34; 95% CI 0.17 to 0.68) (Table 3).
Table 2. Univariate logistic regression for the association between MetS and exposure variables in patients with chronic Chagas disease (n = 361).
| Variables | Odds Ratio | 95%CI | p-value |
|---|---|---|---|
| Age (years) | 1.06 | 1.04–1.09 | <0.001 |
| Sex (female) | 1.67 | 1.09–2.58 | 0.02 |
| Residents by domicilie (persons) | 0.99 | 0.86–1.15 | 0.91 |
| Income per capita (per R$ 1000.00) | 1.00 | 1.00–1.00 | 0.62 |
| Race | |||
| White | Reference | Reference | Reference |
| Black | 1.85 | 0.90–3.80 | 0.09 |
| Mulatto | 1.35 | 0.79–2.31 | 0.27 |
| Others | 2.67 | 0.84–8.46 | 0.09 |
| Schooling | |||
| <9 years | Reference | Reference | Reference |
| 9–12 years | 0.83 | 0.48–1.44 | 0.51 |
| >12 years | 0.30 | 0.14–0.63 | <0.001 |
| Sleep duration (hours) | 1.13 | 0.99–1.29 | 0.08 |
| Smoking (%) | |||
| Non-smoker | Reference | Reference | Reference |
| Former | 1.07 | 0.69–1.66 | 0.76 |
| Current | 1.14 | 0.46–2.85 | 0.77 |
| Alcohol consumption (%) | |||
| None | Reference | Reference | Reference |
| Former | 1.12 | 0.62–2.05 | 0.70 |
| Current | 1.19 | 0.72–1.96 | 0.50 |
| Physical activity level (%) | |||
| Low | Reference | Reference | Reference |
| Moderate | 1.03 | 0.62–1.72 | 0.90 |
| High | 0.88 | 0.49–1.59 | 0.68 |
| Indeterminate form | 1.11 | 0.69–1.78 | 0.67 |
| Cardiac form without heart failure | 1.39 | 0.91–2.13 | 0.13 |
| Cardiac form with heart failure | 0.35 | 0.18–0.69 | 0.002 |
| Digestive form | 0.96 | 0.54–1.71 | 0.89 |
| Carbohydrates (g) | 0.99 | 0.99–0.99 | 0.01 |
| Protein (g) | 0.99 | 0.99–1.00 | 0.11 |
| Lipids (g) | 0.99 | 0.98–1.00 | 0.03 |
| Fibers (g) | 0.99 | 0.97–1.01 | 0.23 |
| Caloric consumption (kcal) | 1.00 | 1.00–1.00 | 0.12 |
Table 3. Multivariate logistic regression for the association between MetS and exposure variables in patients with Chagas disease (n = 361).
| Variables | Odds Ratio | 95%CI | p-value |
|---|---|---|---|
| Age (years) | 1.06 | 1.04–1.09 | <0.001 |
| Schooling | |||
| <9 years | Reference | Reference | Reference |
| 9–12 years | 0.91 | 0.51–1.62 | 0.75 |
| >12 years | 0.36 | 0.17–0.79 | 0.01 |
| Cardiac form with heart failure | 0.34 | 0.17–0.68 | 0.003 |
Discussion
The main finding of the present study was a high prevalence (about 40%) of MetS in patients with chronic ChD that was greater than in the general population in other studies conducted in Brazil and worldwide [22, 23]. For instance, a study including 1.663 individuals from a random sample of an overall Brazilian urban adult population found a lower MetS prevalence (about 30%) in comparison to our study [14]. Moreover, a study including 137 Latin American migrants that were diagnosed with ChD at the Geneva University Hospitals found a MetS prevalence of 16.8% [24]. In Europe and United States, the MetS prevalence widely ranged from 20 to 60% depending on the classification criteria and the characteristics of the studied population (e.g, age, sex, and race) [25–27]. In this context, a cross-sectional analysis including 243 older patients (> 60 years) found a high MetS prevalence (more than 60%) using the same criteria than our study (IDF 2005), suggesting that age could be considered an important risk factor for MetS [28]. Similarly, a study including data from the National Health and Nutrition Survey also found an increased prevalence of MetS with aging, that varied from 18% in the 2nd decade of life to 50% after age 60 [27]. Therefore, the elevated prevalence of MetS found in the present study could be attributed to the aging of Chagas disease patients over the last decades, as previously demonstrated by others [12, 29]. In our outpatient cohort, the mean age raised from 45 to 61 years over the last two decades, reinforcing the aging pattern of this population, especially for those that live in urban areas where the disease transmission is quite low and the access and quality of healthcare services related to ChD improved over the last years [13].
The prevalence of comorbidities in our study was high, confirming previous findings in ChD patients of our group that described similar frequencies of hypertension (56%), dyslipidemia (42%), and diabetes (30%) in 619 ChD patients, with most of them (more than 70%) presenting at least one of the aforementioned comorbidities [30]. Others also showed an elevated prevalence of comorbidities in Brazilians (57% of hypertension, 20% of dyslipidemia, 10% of diabetes mellitus) [12] and Bolivians (64% of hypertension, 67% of obese or overweight) [31] patients with ChD. The alarming high prevalence of comorbidities among ChD patients could be explained by the migration from rural to urban areas, that improved life expectancy but also increased the exposure to inadequate lifestyles, such as unhealthy eating habits and decreased physical activity levels.
Three variables were independently associated with MetS as follows: age, educational level, and clinical form of ChD. As previously discussed, the prevalence of MetS increases with age, with prevalence of 10% in individuals aged 20 to 29 years, 20% in individuals between 40 and 49 years and 45% in individuals between 60 and 69 years [32]. Nonetheless, the high prevalence of individuals with MetS may indicate the changes in the characteristics of the ChD population over the last decades and that non-ChD comorbidities could potentially impact their health, deserving more attention.
Higher educational levels (>12 years) were associated with 64% lower odds of having MetS in comparison to < 9 years of education. Similarly, a cohort study conducted with 1.915 Korean adults found an inverse association between educational level and MetS, suggesting that socioeconomic disparities may increase the risk of MetS [33]. To our knowledge, no previous study examined the influence of socioeconomic variables on the risk of MetS among patients ChD. People with high educational level tend to be healthier than those with low educational level [34], as they usually present a better socioeconomic status, an important health determinant, and have more general knowledge and practice of healthy lifestyles (dietary habits, physical activity, nonsmoking, mental health), that contribute to a better control of the comorbidities that characterize MetS [35, 36].
In the present study, individuals that presented cardiac form with heart failure had a lower prevalence of MetS. A possible explanation for this finding is that advanced heart failure is associated with significant weight loss, due to the high state of catabolism and cachexia, decreasing body fat deposition [37]. Moreover, individuals with heart failure are encouraged to closely self-manage their illness, monitoring signs and symptoms (e.g, weight gain), and better complying with medical regimens and lifestyle recommendations to optimize health outcomes and quality of life [38, 39].
Surprisingly, carbohydrate consumption was a protective factor for the development of MetS in the univariate analysis (OR 0.99; 95% CI 0.99 to 1.00; p < 0.01), although not reaching statistical significance in the multivariate model (OR 0.99; 95%CI 0.99 to 1.00; p = 0.06). Studies evaluating food consumption in patients with ChD are scarce. In a case-control study including 81 patients with ChD and 81 controls, Castilhos et al. [40] evaluated food and nutrients intake among ChD patients followed in a tertiary hospital. Similar to our results, the prevalence of obesity and increased waist circumference was lower among ChD patients, but with a higher intake of carbohydrates. The lack of information about the quality of the carbohydrate consumed in the present study could explain this unexpected finding in which a greater consumption of high-quality carbohydrates (not only the quantity) is associated with a lower risk of MetS [41]. Moreover, since patients included in the present study are followed in a national reference center and received a comprehensive care treatment including a multidisciplinary approach, it is possible that those patients had been previously identified with metabolic abnormalities during their routine clinic visits and had initiated nutritional assistance before the study procedures, a classical example of reverse causation in cross-sectional studies. In this case, longitudinal studies are necessary to better elucidate the influence of macronutrients consumption on the risk of MetS in patients with ChD.
Another unexpected result was the lack of association between physical activity levels and MetS. Although largely used in epidemiological studies, questionnaire is not the golden standard measure of physical activity which may have increased measurement error, especially when applied in a sample characterized by very low educational levels, leading to nondifferential misclassification [42, 43].
The present study has some limitations. Our sample consisted of patients regularly monitored at a national reference center which may represent a selection bias, limiting the external validity. Moreover, these results should not be extrapolated to general population, since participants with ChD has specific characteristics. Moreover, the cross-sectional design prevents us from making conclusions about the causal relationship between MetS and the variables investigated. On the other hand, this is the first study evaluating the prevalence of MetS and its main associated factors that included a relatively high sample size of patients with chronic ChD. Finally, multiple comparison tests from different linear regression models can increase the probability of type 1 error, even though our results were consistent after decreasing the probability of type 1 error to 1% (all variables had p-values <0.01 in the multivariate model).
To conclude, in the present study we found a high prevalence of MetS in patients with chronic ChD while hypertension was the most prevalent comorbidity. The variables independently associated with MetS were age, education level, and clinical form of ChD (more specifically, heart failure). In this setting, the identification of patients`characteristics associated to MetS can facilitate the development of effective approaches (e.g lifestyle modifications such as nutritional counseling and physical exercise) for preventing and managing this syndrome in ChD patients.
Acknowledgments
The authors thank the Evandro Chagas National Institute of Infectious Disease for the clinical and logistical support.
Data Availability
All data files are available from the Open Science Framework database (https://osf.io/9ev8b/).
Funding Statement
This study was funded by Fundação de Amparo a Pesquisa Carlos Chagas (grant number 111.133/2014) and by the Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES) - Finance Code 001.
References
- 1.Pinazo M-J, Gascon J. The importance of the multidisciplinary approach to deal with the new epidemiological scenario of Chagas disease (global health). Acta Tropica 2015; 151:16–20. 10.1016/j.actatropica.2015.06.013 [DOI] [PubMed] [Google Scholar]
- 2.Chagas Disease. World Health Organization. [Internet]. 2020 [cited Nov 11, 2020]. Available at: https://www.who.int/en/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis).
- 3.Chagas Disease. Medecins Sans Frontieres. [Internet]. 2020 [cited Nov 11, 2020]. Available at: https://www.msf.org.br/o-que-fazemos/atividades-medicas/doenca-de-chagas.
- 4.Coura JR, Dias JCP. Epidemiology, control and surveillance of Chagas disease: 100 years after its discovery. Mem Inst Oswaldo Cruz 2009;104 (suppl 1):31–40. [DOI] [PubMed] [Google Scholar]
- 5.Santos JP, Lima-Costa MF, Peixoto SV. Nutritional aspects associated with chronic Trypanosoma cruzi (Chagas 1909) infection among older adults: Bambuí Project. Cad Saúde Pública 2013;29(6):1141–1148. [PubMed] [Google Scholar]
- 6.Honda T, Chen S, Yonemoto K, Kishimoto H, Chen T, Narazaki K, et al. Sedentary bout durations and metabolic syndrome among working adults: a prospective cohort study. BMC Public Health 2016; 16:888. 10.1186/s12889-016-3570-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reaven GM. Role of insulin resistance in human disease. Diabetes 1988; 37(12):1595–1607. 10.2337/diab.37.12.1595 [DOI] [PubMed] [Google Scholar]
- 8.Reaven GM. Pathophysiology of insulin resistance in human disease. Physiol Rev 1995; 75: 473–486. 10.1152/physrev.1995.75.3.473 [DOI] [PubMed] [Google Scholar]
- 9.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120(16):1640–1645. 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 10.Saklayen MG. The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep 2018;20(2):12. 10.1007/s11906-018-0812-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibrahim MS, Pang D, Randhawa G, Pappas Y. Risk models and scores for metabolic syndrome: systematic review protocol. BMJ Open 2019; 9(9): e027326 10.1136/bmjopen-2018-027326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alves RM de A, Thomaz RP, Almeida EA de, Wanderley J da S, Guariento ME. Chagas disease and ageing: the coexistence of other chronic diseases with Chagas disease in elderly patients. Rev Soc Bras Med Trop 2009;42(6):622–628. 10.1590/s0037-86822009000600002 [DOI] [PubMed] [Google Scholar]
- 13.Martins-Melo FR, Alencar CH, Ramos AN, Heukelbach J. Epidemiology of Mortality Related to Chagas Disease in Brazil, 1999–2007. Santiago H da C, organizador. PLoS Negl Trop Dis 2012;6(2):e1508. 10.1371/journal.pntd.0001508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salaroli LB, Barbosa GC, Mill JG, Molina MCB. Prevalence of metabolic syndrome in population-based study, Vitória, ES–Brazil. Arq Bras Endocrinol Metabol 2007;51(7):1143–1152. 10.1590/s0004-27302007000700018 [DOI] [PubMed] [Google Scholar]
- 15.Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 2006;23(5):469–480. 10.1111/j.1464-5491.2006.01858.x [DOI] [PubMed] [Google Scholar]
- 16.Dias JCP, Ramos-Junior AN, Gontijo ED, Luquetti A, Shikanai-Yasuda MA, Coura JR, et al. 2nd Brazilian Consensus on Chagas Disease, 2015. Rev Soc Bras Med Trop 2016;46:Suppl 1. [DOI] [PubMed] [Google Scholar]
- 17.Instituto Brasileiro de Geografia e Estatística (IBGE). Censo demográfico 2010: Características da população e dos domicílios [Internet]. 2011 [cites September 1, 2016]. Available at: https://biblioteca.ibge.gov.br/visualizacao/periodicos/93/cd_2010_caracteristicas_populacao_domicilios.pdf.
- 18.Instituto Brasileiro de Geografia e Estatística (IBGE). Pesquisa especial de tabagismo—PETab: relatório Brasil. Rio de Janeiro, RJ: Brasília, DF, Brasil: Instituto Nacional do Câncer, Ministério da Saúde; Organização Pan-Americana da Saúde—Representação Brasil; 2011.
- 19.Craig CL, Marshall AL, Sjõstrõm M, Bauman AE, Booth ML, Ainsworth BE, et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med Sci Sports Exerc 2003;35(8):1381–1395. 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 20.Pardini R, Matsudo S. Validation of the International Physical Activity Questionnaire (IPAQ version 6): pilot study in Brazilian young adults. Rev Bras Cien Mov 2001;9(3):45–51. [Google Scholar]
- 21.Monteiro CA, Mondini L, Costa RB. Secular changes in dietary patterns in the metropolitan areas of Brazil (1988–1996). Rev Saude Publica 2000;34(3):251–258. 10.1590/s0034-89102000000300007 [DOI] [PubMed] [Google Scholar]
- 22.Ramires EKNM, Menezes RCED, Longo-Silva G, Santos TGD, Marinho PDM, Silveira JACD. Prevalence and factors associated with Metabolic Syndrome in the Brazilian adult population: national health survey– 2013. Arq Bras Cardiol 2018;110(5):455–466. 10.5935/abc.20180072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sigit FS, Tahapary DL, Trompet S, Sartono E, van Dijk KW, Rosendaal FR, et al. The prevalence of metabolic syndrome and its association with body fat distribution in middle-aged individuals from Indonesia and the Netherlands: a cross-sectional analysis of two population-based studies. Diabetol Metab Syndr 2020; 12:2. 10.1186/s13098-019-0503-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson Y, Castillo S, Hammond P, Besson M, Brawand-Bron A, Urzola D, et al. Metabolic, mental health, behavioural and socioeconomic characteristics of migrants with Chagas disease in a non-endemic country. Trop Med Int Health 2012;17(5):595–603. 10.1111/j.1365-3156.2012.02965.x [DOI] [PubMed] [Google Scholar]
- 25.van Vliet-Ostaptchouk JV, Nuotio M-L, Slagter SN, Doiron D, Fischer K, Foco L, et al. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: a collaborative analysis of ten large cohort studies. BMC Endocr Disord 2014;14(1):9. 10.1186/1472-6823-14-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin D, Kongpakpaisarn K, Bohra C. Trends in the prevalence of metabolic syndrome and its components in the United States 2007–2014. Int J Cardiol 2018; 259:216–219. 10.1016/j.ijcard.2018.01.139 [DOI] [PubMed] [Google Scholar]
- 27.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the Metabolic Syndrome in the United States, 2003–2012. J Am Med Assoc 2015;313(19):1973–1974. 10.1001/jama.2015.4260 [DOI] [PubMed] [Google Scholar]
- 28.Saad MAN, Cardoso GP, Martins W de A, Velarde LGC, Cruz Filho RA. Prevalence of Metabolic Syndrome in Elderly and Agreement among Four Diagnostic Criteria. Arq Bras Cardiol 2014;102(3):263–269. 10.5935/abc.20140013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lidani KCF, Andrade FA, Bavia L, Damasceno FS, Beltrame MH, Messias-Reason IJ, et al. Chagas Disease: From Discovery to a Worldwide Health Problem. Front Public Health 2019; 7. 10.3389/fpubh.2019.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vizzoni AG, Varela MC, Sangenis LHC, Hasslocher-Moreno AM, do Brasil PEAA, Saraiva RM. Ageing with Chagas disease: an overview of an urban Brazilian cohort in Rio de Janeiro. Parasit Vectors 2018;11(1):354. 10.1186/s13071-018-2929-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hidron AI, Gilman RH, Justiniano J, Blackstock AJ, LaFuente C, Selum W, et al. Chagas Cardiomyopathy in the Context of the Chronic Disease Transition. PLoS Negl Trop Dis 2010;4(5): e688. 10.1371/journal.pntd.0000688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ford ES, Giles WH, Dietz WH. Prevalence of the Metabolic Syndrome Among US Adults: Findings From the Third National Health and Nutrition Examination Survey. J Am Med Assoc 2002;287(3):356–359. 10.1001/jama.287.3.356 [DOI] [PubMed] [Google Scholar]
- 33.Kim I, Song Y-M, Ko H, Sung J, Lee K, Shin J, et al. Educational Disparities in Risk for Metabolic Syndrome. Metab Syndr Relat Disord 2018;16(8):416–424. 10.1089/met.2017.0170 [DOI] [PubMed] [Google Scholar]
- 34.Baker DP, Leon J, Smith Greenaway EG, Collins J, Movit M. The Education Effect on Population Health: A Reassessment. Popul Dev Rev 2011;37(2):307–332. 10.1111/j.1728-4457.2011.00412.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dalstra JA, Kunst AE, Borrell C, Breeze E, Cambois E, Costa G, et al. Socioeconomic differences in the prevalence of common chronic diseases: an overview of eight European countries. Int J Epidemiol 2005;34(2):316–326. 10.1093/ije/dyh386 [DOI] [PubMed] [Google Scholar]
- 36.Kivimäki M, Batty GD, Pentti J, Shipley MJ, Sipilä PN, Nyberg ST, et al. Association between socioeconomic status and the development of mental and physical health conditions in adulthood: a multi-cohort study. Lancet Public Health 2020;5(3): e140–149. 10.1016/S2468-2667(19)30248-8 [DOI] [PubMed] [Google Scholar]
- 37.Forman DE, Santanasto AJ, Boudreau R, Harris T, Kanaya AM, Satterfield S, et al. Impact of incident heart failure on body composition over time in the health, aging, and body composition study population. Circ Heart Fail 2017;10(9): e003915. 10.1161/CIRCHEARTFAILURE.117.003915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toukhsati SR, Driscoll A, Hare DL. Patient Self-management in Chronic Heart Failure–Establishing Concordance Between Guidelines and Practice. Card Fail Rev 2015;1(2):128–131. 10.15420/cfr.2015.1.2.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agvall B, Alehagen U, Dahlström U. The benefits of using a heart failure management programme in Swedish primary healthcare. Eur J Heart Fail 2013;15(2):228–236. 10.1093/eurjhf/hfs159 [DOI] [PubMed] [Google Scholar]
- 40.Castilhos MP, Huguenin GVB, Rodrigues PRM, Nascimento EM, Pereira BB, Pedrosa RC. Diet Quality of patients with chronic Chagas disease in a tertiary hospital: a case-control study. Rev Soc Bras Med Trop 2017; 50(6):795–804. 10.1590/0037-8682-0237-2017 [DOI] [PubMed] [Google Scholar]
- 41.Schulze MB, Hu FB. Dietary Approaches to Prevent the Metabolic Syndrome: Quality versus quantity of carbohydrates. Diab Care 2004; 27(2):613–614. [DOI] [PubMed] [Google Scholar]
- 42.Steene-Johannessen J, Anderssen SA, van der Ploeg HP, Hendriksen IJM, Donnelly AE, Brage S, et al. Are Self-report Measures Able to Define Individuals as Physically Active or Inactive? Med Sci Sports Exerc 2016; 48(2):235–244. 10.1249/MSS.0000000000000760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winckers ANE, Mackenbach JD, Compernolle S, Nicolaou M, van der Ploeg HP, De Bourdeaudhuij I, et al. Educational differences in the validity of self-reported physical activity. BMC Public Health 2015;15(1):1299. 10.1186/s12889-015-2656-7 [DOI] [PMC free article] [PubMed] [Google Scholar]


