Abstract
Working memory capacity (WMC) measures the amount of information that can be maintained online in the face of distraction. Past work has shown that the efficiency with which the frontostriatal circuit filters out task-irrelevant distracting information is positively correlated with WMC. Recent work has demonstrated a role of posterior alpha oscillations (8–13 Hz) in providing a sensory gating mechanism. We investigated the relationship between memory load modulation of alpha power and WMC in two verbal working memory experiments. In both experiments, we found that posterior alpha power increased with memory load during memory, in agreement with previous reports. Across individuals, the degree of alpha power modulation by memory load was negatively associated with WMC, namely, the higher the WMC, the less alpha power was modulated by memory load. After the administration of topiramate, a drug known to affect alpha oscillations and have a negative impact on working memory function, the negative correlation between memory load modulation of alpha power and WMC was no longer statistically significant but still somewhat detectable. These results suggest that (1) individuals with low WMC demonstrate stronger alpha power modulation by memory load, reflecting possibly an increased reliance on sensory gating to suppress task-irrelevant information in these individuals, in contrast to their high WMC counterparts who rely more on frontal areas to perform this function and (2) this negative association between memory load modulation of alpha oscillations and WMC is vulnerable to drug-related cognitive disruption.
INTRODUCTION
Individual variability in the amount of information that can be stored in working memory with high fidelity can be quantified by working memory capacity (WMC; Cowan, 2012; Engle, Kane, & Tuholski, 1999). Higher WMC is linked to better performance on a variety of cognitive tasks, including attention, reading comprehension, planning, and problem solving (Barrett, Tugade, & Engle, 2004; Adams & Hitch, 1997; Daneman & Carpenter, 1980). It has even been demonstrated that higher WMC individuals can better overcome cognitive impairments resulting from aging and other brain disorders (Otto, Raio, Chiang, Phelps, & Daw, 2013; Grenard et al., 2008).
As a measure of executive attention, WMC measures not only the capacity of the amount of information that can be stored but also the capacity for sustaining attention to the stored information in the face of interference or distraction (Engle, 2002). Behaviorally, there is extensive evidence suggesting that the ability to inhibit task-irrelevant information is significantly higher in individuals with high WMC (Unsworth, Schrock, & Engle, 2004; Conway, Cowan, & Bunting, 2001; Kane & Engle, 2000; Conway & Engle, 1994). Neuroscientifically, fMRI studies have shown that high WMC individuals, compared with their low capacity counterparts, have greater modulation of BOLD responses in the lateral pFC when performing working memory tasks that require a high degree of attentional control, including n-back tasks (Burgess, Gray, Conway, & Braver, 2011), Sternberg tasks with distraction (Minamoto, Osaka, & Osaka, 2010), and complex span tests (Kondo, Morishita, et al., 2004; Kondo, Osaka, & Osaka, 2004; Osaka et al., 2003). Such working memory demand-dependent modulation of the lateral pFC is consistent with the notion that greater modulation of neural activation in higher WMC individuals reflects a higher capability at resolving interference from a competing task or task-irrelevant external/internal distractors to maintain goal-related information (D'Esposito & Postle, 2015; Conway, Kane, & Engle, 2003). Moreover, McNab and Klingberg (2008) showed that distractor filtering activities in frontal cortex and BG were associated with individual differences in WMC and proposed that the frontostriatal circuit is a key neural system that performs the function of excluding task-irrelevant information in high WMC individuals. Specifically, the BG helps accomplish this objective via a dynamic gating mechanism in which an inhibitory or disinhibitory signal is transiently transmitted to the pFC to either suppress the processing of task-irrelevant information or enhance the processing of task-relevant information (Hazy, Frank, & O'Reilly, 2007).
In addition to the frontal filtering of distractor information, recent work has explored sensory gating mechanisms in the posterior sensory cortex, indexed by alpha oscillation (8–13 Hz) and its goal-oriented modulation. A frequently replicated finding is that, during verbal working memory retention, alpha power increases as a function of working memory load (Sauseng et al., 2009; Klimesch, Sauseng, & Hanslmayr, 2007; Jensen, Gelfand, Kounios, & Lisman, 2002), and such increases in alpha power, argued to reflect reduced sensory gain and excitability, serve to protect the information maintained online from external interference (Mathewson et al., 2011; Haegens, Osipova, Oostenveld, & Jensen, 2010; Jensen & Mazaheri, 2010; Sauseng et al., 2009; Jokisch & Jensen, 2007; Kaiser, Heidegger, Wibral, Altmann, & Lutzenberger, 2007; Klimesch et al., 2007; Medendorp et al., 2007); the higher the memory load, the stronger the need for such sensory gating. In contrast, in the case of visual working memory tasks, in which participants maintained visual items online after they were removed from the environment, alpha power decreases with increase in visual working memory load, reflecting increased visual cortex activation necessary for maintaining the representations of the increased amount of visual information. Importantly, Fukuda, Mance, and Vogel (2015) showed that alpha power modulation by visual working memory load is associated with WMC; specifically, the larger the WMC, the more alpha power decreases as visual memory load increases. Is alpha power modulation by verbal working memory load related to WMC? This question has not been addressed. Addressing this question will help us better understand the relation between the frontal mechanisms of distractor suppression and the posterior mechanisms of sensory gating.
Many CNS drugs can affect cognition as well as alter rhythmic brain activities. Topiramate (TPM), a second-generation antiepileptic drug with formal indications for partial and generalized seizures and migraine prophylaxis, has repeatedly been shown to have a pronounced negative impact on a wide range of cognitive functions (Javed et al., 2015), including verbal fluency (Marino et al., 2012; Thompson, Baxendale, Duncan, & Sander, 2000), language comprehension (Fritz et al., 2005), attention (de Araujo Filho, Pascalicchio, Lin, Sousa, & Yacubian, 2006), and short-term (Gomer et al., 2007) and working memory (Marino et al., 2012; Jung et al., 2010). Although our previous work has examined the adverse behavioral effect of TPM on verbal working memory (Marino et al., 2012) and demonstrated a relationship between TPM-related behavioral detriments and WMC (Barkley et al., 2018), the neural correlates of TPM-mediated reduction in task performance remains to be understood. TPM is known to affect alpha oscillations (Neufeld, Kogan, Chistik, & Korczyn, 1999). How does TPM affect the task-dependent modulation of alpha oscillations? Does TPM disrupt the relationship between memory load modulation of alpha and WMC? Addressing these questions will help us better understand how alpha-mediated cognitive functions are affected by CNS drugs.
We analyzed high-density EEG data (128 channels) from two separate experiments in which two groups of healthy human volunteers performed verbal working memory tasks. In the first experiment, conducted at the University of Florida (UF), the participants were shown a set of distinct digits (zero to nine) on a CRT monitor for 1 sec. Following a 3-sec retention period, a probe digit was presented, and a “yes” or “no” button press was required to indicate whether the probe digit belonged to the cue set. Memory load was controlled by the number of digits presented in the cue set (one, three, or five digits). In the second experiment, conducted at the University of Minnesota (UM), the participants were presented a string of one, three, or five pronounceable syllables (Load 1, Load 3, and Load 5) on a monitor for 1.5 sec. This was followed by a 5-sec retention period, during which participants were instructed to retain the syllable string in memory. At the end of the retention period, a probe string was presented, and participants were instructed to press a “yes” or “no” button to indicate whether or not the probe matched the cue string. In addition to the baseline session, the participants participated in a follow-up session in which they were given different doses of TPM and had their brain responses measured while completing the same verbal working memory task.
METHODS
Overview
Two separate experiments employing similar verbal working memory paradigms were conducted at the UF (UF data set) and at the UM (UM data set). The UM data set contained one additional manipulation in which the participants, on a follow-up visit, performed the same paradigm after taking the antiepilepsy drug TPM, which is known to adversely impact cognition (Marino et al., 2012) and alter alpha oscillations (Neufeld et al., 1999). Analyses focusing on different aspects of the two data sets have been published (Barkley et al., 2018; Wang, Rajagovindan, Han, & Ding, 2016). This study is a reanalysis of the previously published data. The main purpose of including two experiments here is to test the replicability of the main findings and the robustness of the findings against drug-related cognitive impairments.
Participants and Experimental Paradigm
UF Data Set
The experimental protocol was approved by the UF institutional review board. Twenty-one healthy volunteers (ages 20–34 years, three women) gave written informed consent and participated in the study. All participants were right-handed, had normal or corrected-to-normal vision, and reported no history of psychiatric or neurological disorders. EEG was recorded from the participants while they performed a Sternberg working memory task (see below). One participant was excluded due to poor performance (accuracy less than 60%). The data from the remaining 20 individuals were analyzed and reported here.
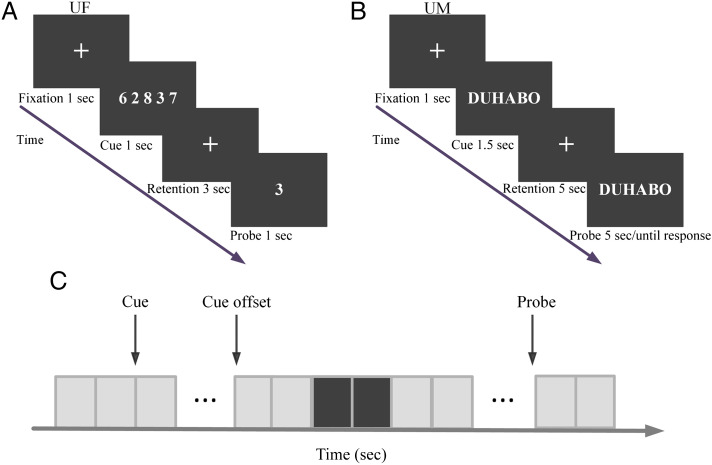
The working memory paradigm is shown in Figure 1A. In each trial, a cue set of numerical digits (zero to nine) was displayed for 1 sec (encoding), followed by a 3-sec memory retention period. At the end of the retention period, a probe digit was presented, and the participant was instructed to indicate whether the probe digit was part of the cue set by a “yes” or “no” button press. Working memory load was determined by the number of digits in the cue set, which in this case was either one, three, and five digits (Load 1, Load 3, and Load 5; Figure 1A depicts a Load 5 trial in which “yes” is the correct response). The entire experiment consisted of six blocks with 60 trials in each block. The three memory loads were equally likely to occur. Breaks were given between blocks. Participants received a practice session before the experiment to familiarize themselves with the task and to minimize the effect of learning.
Figure 1. .
Paradigms for the two experiments. (A) Timeline of the verbal working memory task used at UF. (B) Timeline of the verbal working memory task used at UM. (C) Time period of interest during retention (black). Each rectangle represents 0.5 sec.
UM Data Set
The experimental protocol was approved by the UM institutional review board before the commencement of the study. Forty-six healthy right-handed volunteers with no history of psychiatric or neurological disorders gave written informed consent and participated in the study. Seventeen participants were excluded because of missing data resulting from technical issues with data acquisition or storage. Six participants were excluded from further analyses due to (1) an overall accuracy rate below 60% or (2) excessive head or body movements. Data from the remaining 23 participants (mean age = 25.60 ± 8.04 years; 14 women) were included in the analyses.
The experimental paradigm is shown in Figure 1B. At the beginning of each trial, a cue string of one, three, or five pronounceable syllables (Load 1, Load 3, and Load 5) was displayed on a monitor for 1.5 sec (encoding). This was followed by a 5-sec retention period, during which participants were instructed to retain the syllable string in working memory. At the end of the retention period, a probe string was presented, and participants were instructed to press a “yes” or “no” button to indicate whether or not the probe matched the cue (Figure 1B depicts a Load 3 trial in which “yes” is the correct response). The next trial was triggered by either a response or the absence of one within 5 sec of probe onset. The entire task consisted of 10 blocks of 36 trials each. There were an equal number of trials for each memory load (one, three, or five syllables); trials were randomized as a function of memory load.
There were two sessions in the UM experiment. In addition to the baseline visit (BAS) in which the participants completed the task in Figure 1B while their EEG was recorded, in a follow-up visit separated by a 2-week interval, the participants were randomized to receive either 100 mg (eight participants), 150 mg (eight participants), or 200 mg (seven participants) of TPM to induce a wide range of TPM concentrations across individuals. Four hours after drug administration, the participants' EEG was recorded while they completed the same verbal working memory task. The study design was double-blind, randomized, and placebo-controlled with the drug being dispensed by the UM Investigational Drug Services pharmacy.
Data Acquisition
University of Florida
High-density EEG data were recorded using a 128-channel BioSemi System at a sampling rate of 1 kHz. Eye movements and eye blinks were monitored using additional electrodes. Stimuli were presented using the BeriSoft Experimental Run-Time System, and the participant's responses were recorded with an EXKEY microprocessor logic pad (www.berisoft.com).
University of Minnesota
High-density EEG data were recorded using a 128-channel EGI System (Electrical Geodesics, Inc.) at a sampling rate of 1 kHz. Impedances were kept below 50 KΩ per manufacturer's recommendation. Eye movements and eye blinks were monitored. Stimulus presentation and behavioral response recording were controlled with E-Prime software (Psychology Software Tools, Inc.).
Data Analysis
Working Memory Capacity
The participants' individual WMC was quantified by Cowan's K (Cowan, 2001; Pashler, 1988), which is defined as
where S is the size of the cue set, H is the hit rate, and F is the false alarm rate. To obtain a single WMC measure for each individual, K values for Load 3 and Load 5 were averaged (Fukuda et al., 2015; Vogel & Machizawa, 2004). To facilitate comparison across data sets, the WMC values so obtained within each data set were transformed into z scores.
EEG Data Preprocessing
EEG data preprocessing was performed using BESA 6.0 (www.besa.de), EEGLAB (sccn.ucsd.edu/eeglab/index.html), and custom MATLAB (The MathWorks) scripts. The continuous EEG data were bandpassed between 0.1 and 30 Hz, downsampled to 250 Hz, and re-referenced against the average reference. For the UF data, each trial was epoched from −500 to 4000 msec, with 0 msec denoting the onset of the cue and −500 to 0 msec being the precue period. For each memory load, the data from the retention time period (2000–3000 msec), defined as the time period of interest, were analyzed (Figure 1C). Here, the segment of 1000–2000 msec was excluded to avoid the negative impact of cue-offset evoked activities on the spectral analysis of ongoing neural oscillations, and the segment of the 3000–4000 msec was excluded to avoid the negative impact of the anticipation of probe processing on alpha oscillations. For the UM data set, each trial was epoched from −200 to 6500 msec, with 0 msec denoting cue onset and −200 to 0 msec being the precue period. For similar reasons as above, the time period of interest was chosen to be 2500–3500 msec. For both data sets, trials with incorrect responses or contaminated by large movement-related artifacts were excluded from further analysis. For the remaining trials, independent components analysis (Delorme & Makeig, 2004) was applied to remove artifacts due to eye movements, eye blinks, and other sources of noise that were not related to brain activity. To minimize the negative effects of volume conduction and common reference, the artifact-corrected scalp voltage data were converted to reference-free current source density by calculating 2-D surface Laplacian algorithm (Kayser & Tenke, 2006). All subsequent analyses were performed on the current source density data.
Power Spectral Density Estimation
For both data sets, fast Fourier transforms were applied to the data in the time period of interest to estimate the power spectra. Normalization by power in the precue baseline period was done on a participant-by-participant basis (1–30 Hz; Jensen et al., 2002). This normalization procedure removed the influence of amplitude variability from participant to participant and allowed more straightforward averaging across participants. Alpha frequency bands were defined to be from 8 to 13 Hz.
Alpha Modulation Index
To quantify the modulation of alpha power by memory load (L), we constructed a linear regression model
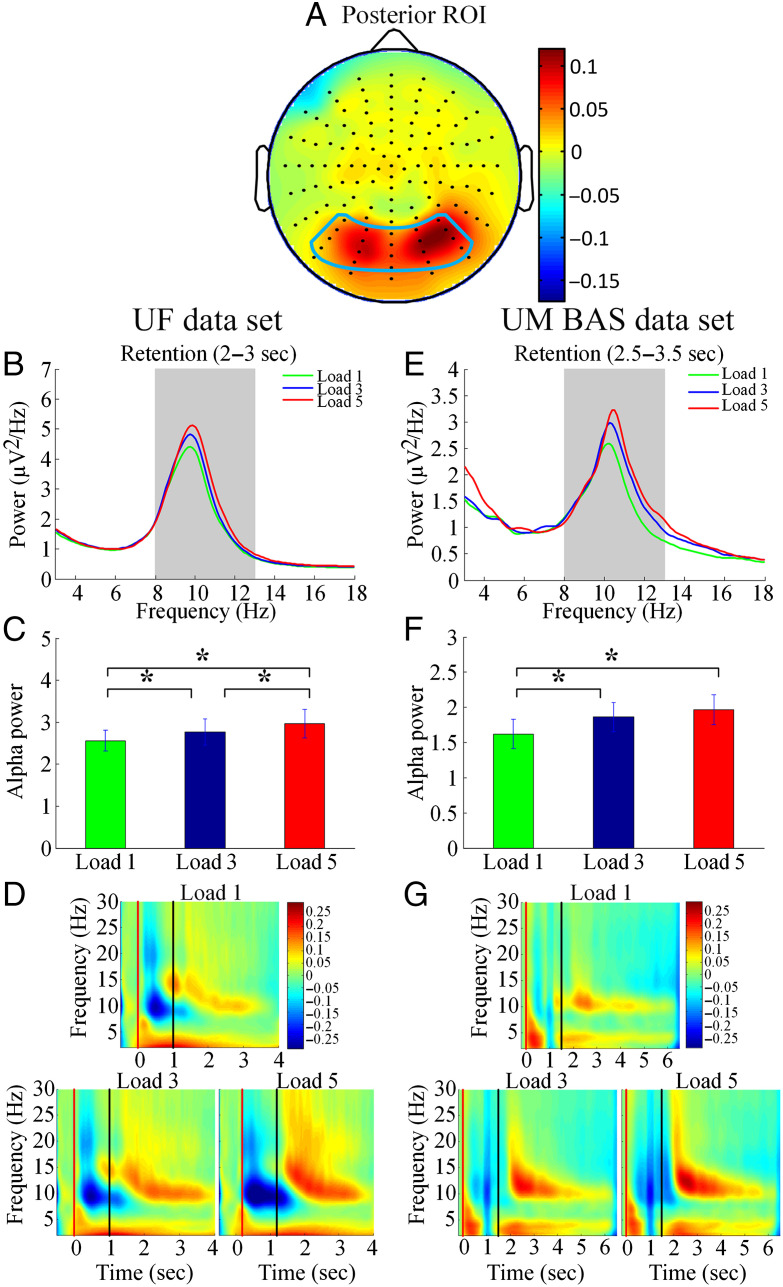
where Y is the alpha power and β is the fitted regression coefficient. Larger β values reflect stronger alpha modulation by memory load. A schematic illustration of large β versus small β was given in Figure 3A and B. L equals to 1, 3, and 5 for both UF and UM data sets. The posterior ROI used for alpha modulation analysis was defined according to our previous paper on memory load modulation of alpha in verbal working memory (Wang et al., 2016). The channels included in the ROI, such as PO7, P5, P3, P1, P03, O1, Oz, POz, Pz, P2, O2, P4, PO4, P6, and PO8, are similar to channels used in previous EEG alpha studies of verbal working memory (Klimesch, 2012; Mathewson et al., 2011; Haegens et al., 2010; Jensen & Mazaheri, 2010; Sauseng et al., 2009; Jokisch & Jensen, 2007; Medendorp et al., 2007; Jensen et al., 2002), which facilitate comparison across studies.
Figure 3. .
Alpha modulation by memory load. (A) Posterior ROI for the analysis of alpha activity was superimposed on the topographical map of alpha power modulation by working memory load (averaged across the UF and UM data sets). (B) Power spectra for different memory load and (C) modulation of alpha power (8–13 Hz) by memory load during retention for UF data set. (E) Power spectra for different memory load and (F) modulation of alpha power by memory load during retention for UM BAS data set. Time–frequency analysis for (D) UF data set and (G) UM BAS data set. *p < .05.
Time–Frequency Analysis
Time–frequency analysis was also performed to examine the evolution of neural activities under different working memory loads and across different stages of the working memory task. The time–frequency power changes were computed by calculating the percentage change in power from the precue baseline for different frequencies and different times using a complex Morlet wavelet transform (Herrmann, Mecklinger, & Pfeifer, 1999; Tallon-Baudry, Bertrand, Wienbruch, Ross, & Pantev, 1997). The number of cycles was selected according to the frequency (scale) and was increased from 0.5 at 1 Hz to 13.8 at 30 Hz. It has been suggested that this approach provides better frequency resolution at higher frequencies than a conventional wavelet approach that uses constant cycle length (Delorme & Makeig, 2004). The same wavelet transform as the one used for the whole-trial analysis was applied to the two data sets. All the time–frequency calculations for a given working memory load were performed after subtracting the ensemble mean (ERP) of that load condition from all trials to minimize the influence of stimulus-evoked response on spectral estimation (Kalcher & Pfurtscheller, 1995). This was done for each electrode, each condition, and each participant separately.
RESULTS
Behavioral Results
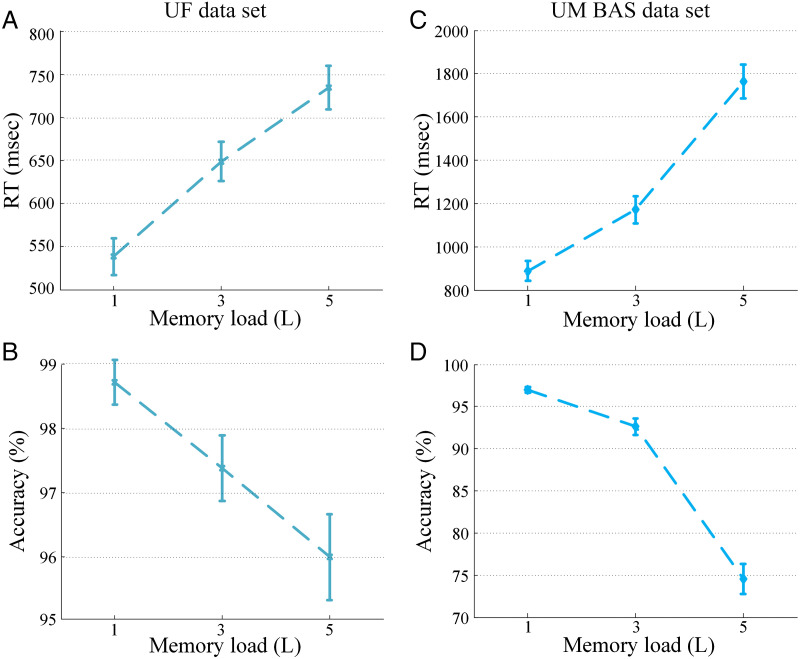
As shown in Figure 2, RT increased as a function of memory load: UF data set, F(2, 57) = 17.22, p < .001 (Figure 2A), and UM BAS data set, F(2, 66) = 42.63, p < .001 (Figure 2C), and accuracy decreased with increasing memory load: UF data set, F(2, 57) = 6.35, p < .005 (Figure 2B) and UM BAS data set, F(2, 66) = 86.39, p < .001 (Figure 2D). These results are in line with previous studies using similar paradigms (Stokić, Milovanović, Ljubisavljević, Nenadović, & Čukić, 2015; Bashivan, Bidelman, & Yeasin, 2014; Nenert, Viswanathan, Dubuc, & Visscher, 2012; Michels, Moazami-Goudarzi, Jeanmonod, & Sarnthein, 2008; Tuladhar et al., 2007; Leiberg, Lutzenberger, & Kaiser, 2006; Jensen et al., 2002).
Figure 2. .
Behavioral results. (A) RT and (B) accuracy as a function of memory load for the UF data set. (C) RT and (D) accuracy as a function of memory load for the UM BAS data set. Error bars are SEM.
Alpha Power Modulation by Memory Load during Retention
Posterior ROI for the analysis of alpha activity, superimposed on the topographical map of alpha power modulation by working memory load, was shown in Figure 3A. For the UF data, during the retention period, alpha power increased as a function of memory load (Load 5 > Load 1, t(19) = −2.67, p = .015; Load 3 > Load 1, t(19) = −2.21, p = .039; Load 5 > Load 3, t(19) = −2.15, p = .048; Figure 3B–C); a similar pattern was also observed for the UM BAS data (Load 5 > Load 1, t(22) = −2.14, p = .043; Load 3 > Load 1, t(22) = −2.58, p = .017; Load 5 > Load 3, t(22) = −0.92, p = .37; Figure 3E–F). A time–frequency analysis was carried out to examine the evolution of oscillatory activities across different stages of the working memory task under different memory loads. As shown in Figure 3D and G, in both data sets, the increase of alpha power during retention with increase in working memory load is clearly visible.
Memory Load Modulation of Alpha Power and WMC
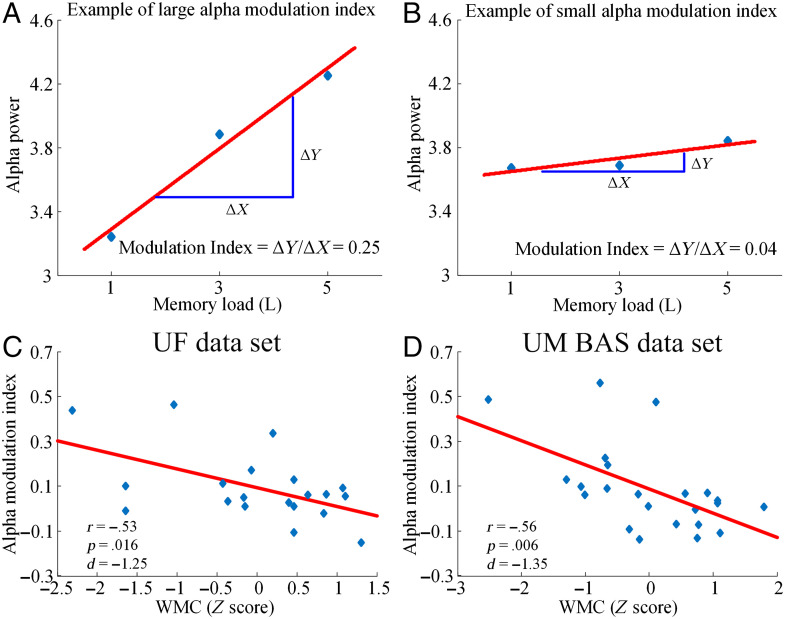
Load-dependent modulation of alpha power at the individual participant level can be quantified using the alpha modulation index (see Methods). Examples of large alpha power modulation and small alpha power modulation were schematically shown in Figure 4A and B. The relationships between the alpha modulation index and individual WMC for both data sets were shown in Figure 4C and D. For the UF data set, there was a significant negative correlation between the alpha modulation index and WMC (r = −.53, p = .016, d = −1.25; Figure 4C); in other words, the higher the WMC, the lower the alpha modulation index. Here, in addition to calculating p values, effect sizes were also reported where appropriate in terms of Cohen's d. According to established conventions, effect size was considered small if 0.2 < |d| < 0.5, medium if 0.5 < |d| < 0.8, and large if |d| > 0.8 (Rosenthal, 1984). For the UM BAS data set, the same negative correlation between alpha modulation index and WMC was observed (r = −.56, p = .006, d = −1.35; Figure 4D). In addition to WMC, we also considered behavioral accuracy. There was a significant positive correlation between accuracy and WMC in both UF (r = .99, p < .001, d = 15.72) and UM BAS data set (r = .97, p < .001, d = 9.16), suggesting that the averaged K values can explain most of the variance of the working memory task performance in both data sets. Moreover, alpha modulation index and accuracy were significantly negatively correlated in both UF (r = −.55, p < .012, d = −1.32) and UM BAS data set (r = −.59, p < .003, d = −1.44).
Figure 4. .
Alpha modulation index and WMC. Schematic examples of a large (A) and a small (B) alpha modulation index. Correlation between WMC and alpha modulation index for (C) the UF data set and (D) the UM BAS data set. Each point in the scatter plots represents an individual participant.
Alpha Power and WMC
In visual working memory, Fukuda et al. (2015) found that only alpha power modulation by memory load, not alpha power per se, was correlated with WMC. In the present verbal working memory paradigms, we examined the relationship between alpha power and WMC as well as task performance and presented the results in Table 1. Consistent with the findings in visual working memory, there were no significant correlation between alpha power and WMC for any of the memory load conditions, suggesting that it is the magnitude of task demand-based alpha power modulation rather than the magnitude of alpha power that is characteristic of an individual's working memory function.
Table 1. .
Correlation Coefficient between Alpha Power and Accuracy, RT, and Cowan's K for Each Memory Load
| Loads | Accuracy | RT | Cowan's K | |
|---|---|---|---|---|
| UF data set | Load 1 | .183 (.44) | .203 (.39) | .182 (.45) |
| Load 3 | −.091 (.70) | .042 (.86) | −.091 (.70) | |
| Load 5 | −.194 (.41) | .171 (.47) | −.193 (.42) | |
| UM BAS data set | Load 1 | −.105 (.63) | .003 (.99) | −.106 (.63) |
| Load 3 | −.066 (.76) | .081 (.71) | −.065 (.76) | |
| Load 5 | −.246 (.26) | −.189 (.39) | −.247 (.25) |
p Values are in parentheses.
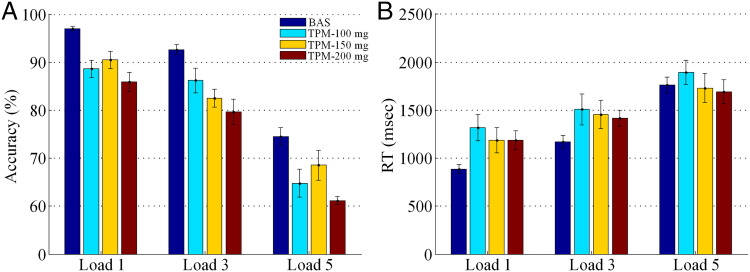
Effects of TPM on Task Performance
Effects of memory load (levels: Load 1, Load 3, or Load 5) and treatment (BAS, TPM-100 mg, TPM-150 mg, or TPM-200 mg) on accuracy (Figure 5A) and RT (Figure 5B) were analyzed using 3 × 4 repeated-measures ANOVAs. Regarding accuracy, there were significant main effects of both Memory Load, F(2, 126) = 129.30, p < .001, and Treatment, F(3, 126) = 25.89, p < .001; the Memory Load × Treatment interaction was not significant, F(6, 126) = 0.54, p = .76. Regarding RT, there were significant main effects of both Memory Load, F(2, 126) = 27.66, p < .001, and Treatment, F(3, 126) = 4.74, p = .0036; the Memory Load × Treatment interaction was not significant, F(6, 126) = 0.97, p = .45.
Figure 5. .
Effects of TPM on working memory task performance (UM TPM data set). Data were shown separately for the three doses of TPM on Load 1, Load 3, and Load 5 conditions. Error bars represent SEM.
Effects of TPM on Relation between Memory Load Modulation of Alpha and WMC
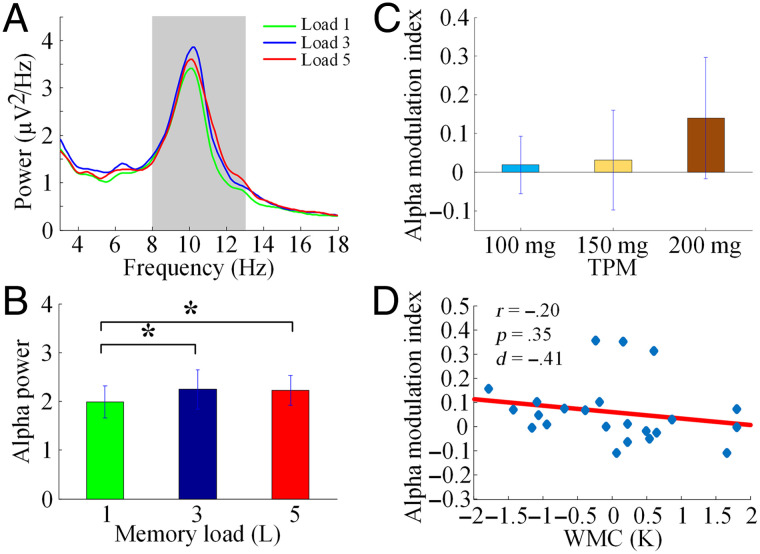
Under TPM, alpha power increased from Load 1 to Load 3 and from Load 1 to Load 5, but it was not significantly different between Load 3 and Load 5 (Load 5 > Load 1, t(22) = −2.24, p = .035; Load 3 > Load 1, t(22) = −2.29, p = .032; Load 5 < Load 3, t(22) = −0.12, p = .91; Figure 6A and B). Alpha modulation index under different doses of TPM was not significantly different (p > .1; Figure 6C), and the data from different dose groups were therefore combined for the next analysis. After the administration of TPM, the alpha modulation index and WMC were no longer significantly correlated (r = −.20, p = .35, d = −0.41; Figure 6D), although at d = −0.41, it could be said that the negative correlation is still somewhat preserved with a small effect size.
Figure 6. .
Effects of TPM on modulation of alpha power by memory load for UM TPM data set. (A) Power spectra over the posterior ROI. (B) Alpha power for each memory load condition (*p < .05). (C) Alpha modulation index under the three doses of TPM. (D) The correlation between WMC and alpha modulation index.
DISCUSSION
Two high-density EEG data sets recorded from healthy human volunteers performing verbal working memory tasks were analyzed to examine the relationship between memory load modulation of posterior alpha power and WMC. The following results were found. First, in agreement with previous reports, posterior alpha power increased with memory load during working memory retention, irrespective of whether the remembered verbal information was numerical digits or pronounceable syllables. Second, across individuals, the degree of alpha power modulation by memory load was negatively associated with WMC, namely, the higher the WMC, the less the alpha power modulation by memory load. These two results were consistent across the two data sets. Third, in the UM data set, after the administration of TPM, a drug known to adversely impact working memory and alter brain's rhythmic activities, the negative correlation between memory load modulation of alpha power and WMC became weaker (no longer statistically significant) but was still somewhat detectable with a small effect size (d = 0.41).
Increase of alpha power as a function of verbal working memory load during memory retention is a widely replicated finding. In light of the inverse relationship between alpha power and cortical excitability, this finding is thought to reflect a GABA-mediated increase in functional inhibition of visual cortex, which could serve as a sensory gating mechanism to protect information held online from sensory interference (Wang et al., 2016; Jensen & Mazaheri, 2010; Lőrincz, Kékesi, Juhász, Crunelli, & Hughes, 2009; Klimesch et al., 2007; Jones, Pinto, Kaper, & Kopell, 2000). One line of corroborating evidence supporting the alpha sensory gating hypothesis comes from simultaneous recordings of EEG and fMRI during working memory tasks (Michels et al., 2010; Scheeringa et al., 2009), which showed that alpha band power in posterior regions is negatively correlated with BOLD signal in the visual cortex during working memory retention. Furthermore, several studies have found that when to-be-remembered and to-be-ignored items are displayed simultaneously in separate hemifields, alpha activity increases over the hemisphere ipsilateral to the relevant hemifield and that this effect increases with memory load (Vissers, van Driel, & Slagter, 2016; Sauseng et al., 2009). Using a Sternberg working memory task in which distractors were presented in the retention interval, whose strength and exact timing could be anticipated, Bonnefond and Jensen found a stronger alpha power increase in occipitotemporal areas before strong compare to weak distractor onsets and observed a significant negative correlation between alpha power modulation and RT difference between weak and strong distractors. In other words, the stronger the difference in alpha power between anticipated distractor types, the smaller the difference in RT. These data suggest that alpha oscillations are modulated according to task conditions, and the degree of alpha power modulation, rather than the alpha power itself, is linked to the effectiveness of the sensory suppression of distracting information. In visual working memory, Fukuda et al. (2015) reported similar finding, namely, it is the alpha power modulation by memory load (i.e., degree of alpha decrease with increase in memory load) that predicts WMC rather than alpha power per se. Our findings that there were no significant correlation between alpha power and WMC extend their finding to the verbal working memory domain where the alpha is modulated in the opposite direction (i.e., alpha increases with memory load).
As a measure of executive attention, WMC is not simply a measure of the capacity for storing information but also a measure of the ability to sustain attention to the stored information in the face of interference or distraction (executive attention). Higher WMC individuals may be more efficient at filtering out task-irrelevant distractors in addition to having higher capacity to store more information. Indeed, studies have suggested that individuals with low WMC tend to be more easily distracted by task-irrelevant information, whereas high WMC individuals tend to excel at focusing attention on task-relevant information (Vogel, McCollough, & Machizawa, 2005). Neurophysiologically, it has been suggested that distractor filtering in working memory may be carried out by the frontostriatal circuit, principally consisting of the pFC (Vogel et al., 2005) and the BG (Alexander, DeLong, & Strick, 1986). In this circuit, the BG provides a dynamic gating mechanism by transiently providing either an inhibitory or disinhibitory signal to the pFC (Hazy et al., 2007), which suppresses the processing of task-irrelevant information or enhances task-relevant information. This role for the BG in working memory is thought to be highly similar to its involvement in gating the selection of actions in motor regions of the pFC (Mink, 1996). In addition, the involvement of the BG in selecting items to be remembered is consistent with the evidence that this structure is important for a person's ability to shift between task sets (Hayes, Davidson, Keele, & Rafal, 1998), a process in which the active inhibition of irrelevant task sets is important (Mayr & Keele, 2000). Together, the frontostriatal circuit may determine what information is actively kept online for the current task, which then determines which items will gain admittance to the limited working memory. A recent study provides direct evidence supporting a role of the BG and pFC in controlling the flow of task-relevant information into working memory (McNab & Klingberg, 2008). Consistent with the theory that an individual's WMC is determined by their ability to selectively filter task-irrelevant distractors (Vogel et al., 2005), BG and prefrontal cortical activity was a significant predictor of WMC, manifesting a positive association between WMC and the ability of the frontostriatal circuit to filter distracting information.
The observed negative correlation between alpha power modulation by memory load and WMC over two experiments adds a new dimension to the current literature: individuals with low WMC demonstrate stronger alpha power modulation by memory load. We speculate that this may reflect the fact that individuals with a weaker ability to filter out distraction in the frontostriatal circuit relies more on sensory gating to achieve this function. High WMC individuals, on the other hand, having stronger frontal mechanisms for distractor suppression, is not as dependent on sensory gating. This phenomenon is reminiscent of the neural compensation phenomenon observed in cognitive aging. Neuroimaging studies have shown that in older adults, compared with young adults, neural activity increases in a variety of brain areas, including the pFC and posterior parietal cortex (Lighthall, Huettel, & Cabeza, 2014; Park & Reuter-Lorenz, 2009; Steffener, Brickman, Rakitin, Gazes, & Stern, 2009; Greenwood, 2007; Rajah & D'Esposito, 2005). These increased activities, reflecting reorganized brain functioning, are thought to be compensatory, whose function is to counteract neural decline and to maintain task performance (Chanraud & Sullivan, 2014).
In visuospatial working memory tasks, in which participants were given a set of visual items to remember, alpha power decreases with increase in memory load. This is in contrast to verbal working memory tasks, in which alpha increases with increase in memory load (Klimesch, 2012; Mathewson et al., 2011; Haegens et al., 2010; Jensen & Mazaheri, 2010; Sauseng et al., 2009; Jokisch & Jensen, 2007; Medendorp et al., 2007; Jensen et al., 2002). Functionally, alpha decreases in visual working memory are thought to reflect increased activation of visual cortex necessary for maintaining the neural representations of remembered visual items (Fukuda et al., 2015). Recent studies on phase-coding models of visual working memory suggest that items in visual working memory are represented by synchronous low-frequency activities, and these synchronous activities coding remembered items are phase-shifted to avoid accidental synchronizations that could lead to misrepresentation or contamination of remembered information (Siegel, Warden, & Miller, 2009; Raffone & Wolters, 2001). As a result, the measured scalp EEG data as the sum of the phase-shifted signals in the cortex show a set size-dependent decrease in alpha power. Although in visual working memory, WMC also predicts alpha power modulation by memory load (Fukuda et al., 2015), the underlying neural basis may be different from what we reported in this work.
What alternative hypotheses might explain the findings reported in this study? Regarding the fact that high WMC individuals exhibited smaller alpha modulation by memory load, one may argue that these individuals utilized visual working memory to represent the verbal stimuli. In the classic Sternberg paradigm, the verbal items are presented sequentially, and sequential presentation of memory items is thought to minimize the use of visual working memory for information retention. Previous work has shown that regardless of whether memory items are presented sequentially or presented on a single screen, alpha power increases with memory load during memory retention (Tuladhar et al., 2007; Hwang et al., 2005; Jensen et al., 2002). No studies, however, have directly compared the amount of alpha power modulation by memory load under the two different ways of presenting verbal stimuli. It is thus not possible to rule out that visual working memory may have played a role in the representation of verbal information. Regarding the fact that low WMC individuals exhibited more increase with increase in memory load, one may argue that this reflects a disengagement from the task. We reason that if the participant is disengaged from the task, then task performance will suffer as the result. Closer examination of task performance data in low WMC individuals reveals that accuracy remained quite high at about 94% for Load 5 in the UF data set. Moreover, we did a within-participant analysis in which single-trial alpha power within a given memory load was estimated and sorted into high and low alpha trials, and the task performance between the high and low alpha groups was compared. The accuracy and RT were not different between the high alpha group and the low alpha group; in fact, the accuracy was actually a little higher in the high alpha group under the high memory load conditions, but the effects did not reach significant level of p < .05 (results not shown). Thus, the disengagement hypothesis does not appear to provide an adequate explanation of the data.
In both data sets, we used Cowan's K to estimate WMC (Cowan, 2001). If the performance accuracy is high, as is the case for the UF data set, the values of K may underestimate the true WMC (Rouder, Morey, Morey, & Cowan, 2011). In the UM data set, for comparable working memory loads, the performance accuracy is lower, and the underestimation of WMC is less of a concern. Yet, in both data sets, we observed the same relationship between WMC and load-dependent modulation of alpha, suggesting that the potential underestimation of WMC did not hamper our ability to uncover the relationship. In addition, we correlated alpha modulation index with accuracy instead of K values and found that there was a significant negative correlation between accuracy and alpha modulation index in both UF (r = −.55, p < .012, d = −1.32) and UM BAS data set (r = −.59, p < .003, d = −1.44), meaning that the higher the accuracy, the less the alpha power modulation by memory load, in agreement with our WMC results. Thus, whether using K values as estimation of WMC or behavioral accuracy, the same relation with alpha power modulation was observed.
In summary, in this study, we considered the relationship between memory load modulation of alpha power and WMC in two verbal working memory experiments. Consistent between the two experiments, individuals with low WMC were found to have a stronger alpha power modulation by memory load, indicating possibly an increased dependence on sensory gating for coping with task-irrelevant information in these individuals. The negative association between memory load modulation of alpha oscillations and WMC is vulnerable to drug-related cognitive and physiological disruption. These findings contribute to our understanding of the neural substrate of WMC, an important measure of executive functioning of the brain; point to the need to further study the relation between the frontal mechanisms of distractor suppression and the posterior mechanisms of sensory gating; and shed light on how CNS drugs can disrupt important neural mechanisms, resulting in impaired cognition.
Acknowledgments
This work was supported by National Institute of Health grants MH112206 and NS076665.
Reprint requests should be sent to Mingzhou Ding, J. Crayton Pruitt Family Department of Biomedical Engineering, University of Florida, Gainesville, FL 32611, or via e-mail: MDing@bme.ufl.edu.
Contributor Information
Zhenhong Hu, University of Florida.
Christopher M. Barkley, University of Minnesota
Susan E. Marino, University of Minnesota
Chao Wang, University of Florida.
Abhijit Rajan, University of Florida.
Ke Bo, University of Florida.
Immanuel Babu Henry Samuel, University of Florida.
Mingzhou Ding, University of Florida.
REFERENCES
- Adams, J. W., & Hitch, G. J. (1997). Working memory and children's mental addition. Journal of Experimental Child Psychology, 67, 21–38. [DOI] [PubMed] [Google Scholar]
- Alexander, G. E., DeLong, M. R., & Strick, P. L. (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience, 9, 357–381. [DOI] [PubMed] [Google Scholar]
- Barkley, C. M., Hu, Z., Fieberg, A. M., Eberly, L. E., Birnbaum, A. K., Leppik, I. E., et al. (2018). Individual differences in working memory capacity predict topiramate-related cognitive deficits. Journal of Clinical Psychopharmacology, 38, 481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett, L. F., Tugade, M. M., & Engle, R. W. (2004). Individual differences in working memory capacity and dual-process theories of the mind. Psychological Bulletin, 130, 553–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashivan, P., Bidelman, G. M., & Yeasin, M. (2014). Spectrotemporal dynamics of the EEG during working memory encoding and maintenance predicts individual behavioral capacity. European Journal of Neuroscience, 40, 3774–3784. [DOI] [PubMed] [Google Scholar]
- Burgess, G. C., Gray, J. R., Conway, A. R. A., & Braver, T. S. (2011). Neural mechanisms of interference control underlie the relationship between fluid intelligence and working memory span. Journal of Experimental Psychology: General, 140, 674–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud, S., & Sullivan, E. V. (2014). Compensatory recruitment of neural resources in chronic alcoholism. Handbook of Clinical Neurology, 125, 369–380. [DOI] [PubMed] [Google Scholar]
- Conway, A. R. A., Cowan, N., & Bunting, M. F. (2001). The cocktail party phenomenon revisited: The importance of working memory capacity. Psychonomic Bulletin & Review, 8, 331–335. [DOI] [PubMed] [Google Scholar]
- Conway, A. R. A., & Engle, R. W. (1994). Working memory and retrieval: A resource-dependent inhibition model. Journal of Experimental Psychology: General, 123, 354–373. [DOI] [PubMed] [Google Scholar]
- Conway, A. R. A., Kane, M. J., & Engle, R. W. (2003). Working memory capacity and its relation to general intelligence. Trends in Cognitive Sciences, 7, 547–552. [DOI] [PubMed] [Google Scholar]
- Cowan, N. (2001). The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Sciences, 24, 87–114. [DOI] [PubMed] [Google Scholar]
- Cowan, N. (2012). Working memory capacity. New York: Psychology Press. [Google Scholar]
- Daneman, M., & Carpenter, P. A. (1980). Individual differences in working memory and reading. Journal of Verbal Learning and Verbal Behavior, 19, 450–466. [Google Scholar]
- de Araujo Filho, G. M., Pascalicchio, T. F., Lin, K., Sousa, P. S., & Yacubian, E. M. T. (2006). Neuropsychiatric profiles of patients with juvenile myoclonic epilepsy treated with valproate or topiramate. Epilepsy & Behavior, 8, 606–609. [DOI] [PubMed] [Google Scholar]
- Delorme, A., & Makeig, S. (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134, 9–21. [DOI] [PubMed] [Google Scholar]
- D'Esposito, M., & Postle, B. R. (2015). The cognitive neuroscience of working memory. Annual Review of Psychology, 66, 115–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle, R. W. (2002). Working memory capacity as executive attention. Current Directions in Psychological Science, 11, 19–23. [Google Scholar]
- Engle, R. W., Kane, M. J., & Tuholski, S. W. (1999). Individual differences in working memory capacity and what they tell us about controlled attention, general fluid intelligence, and functions of the prefrontal cortex. In Miyake A. & Shah P. (Eds.), Models of working memory: Mechanisms of active maintenance and executive control (pp. 102–134). New York: Cambridge University Press. [Google Scholar]
- Fritz, N., Glogau, S., Hoffmann, J., Rademacher, M., Elger, C. E., & Helmstaedter, C. (2005). Efficacy and cognitive side effects of tiagabine and topiramate in patients with epilepsy. Epilepsy & Behavior, 6, 373–381. [DOI] [PubMed] [Google Scholar]
- Fukuda, K., Mance, I., & Vogel, E. K. (2015). α power modulation and event-related slow wave provide dissociable correlates of visual working memory. Journal of Neuroscience, 35, 14009–14016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomer, B., Wagner, K., Frings, L., Saar, J., Carius, A., Härle, M., et al. (2007). The influence of antiepileptic drugs on cognition: A comparison of levetiracetam with topiramate. Epilepsy & Behavior, 10, 486–494. [DOI] [PubMed] [Google Scholar]
- Greenwood, P. M. (2007). Functional plasticity in cognitive aging: Review and hypothesis. Neuropsychology, 21, 657–673. [DOI] [PubMed] [Google Scholar]
- Grenard, J. L., Ames, S. L., Wiers, R. W., Thush, C., Sussman, S., & Stacy, A. W. (2008). Working memory capacity moderates the predictive effects of drug-related associations on substance use. Psychology of Addictive Behaviors, 22, 426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens, S., Osipova, D., Oostenveld, R., & Jensen, O. (2010). Somatosensory working memory performance in humans depends on both engagement and disengagement of regions in a distributed network. Human Brain Mapping, 31, 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, A. E., Davidson, M. C., Keele, S. W., & Rafal, R. D. (1998). Toward a functional analysis of the basal ganglia. Journal of Cognitive Neuroscience, 10, 178–198. [DOI] [PubMed] [Google Scholar]
- Hazy, T. E., Frank, M. J., & O'Reilly, R. C. (2007). Towards an executive without a homunculus: Computational models of the prefrontal cortex/basal ganglia system. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences, 362, 1601–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann, C. S., Mecklinger, A., & Pfeifer, E. (1999). Gamma responses and ERPs in a visual classification task. Clinical Neurophysiology, 110, 636–642. [DOI] [PubMed] [Google Scholar]
- Hwang, G., Jacobs, J., Geller, A., Danker, J., Sekuler, R., & Kahana, M. J. (2005). EEG correlates of verbal and nonverbal working memory. Behavioral and Brain Functions, 1, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed, A., Cohen, B., Detyniecki, K., Hirsch, L. J., Legge, A., Chen, B., et al. (2015). Rates and predictors of patient-reported cognitive side effects of antiepileptic drugs: An extended follow-up. Seizure, 29, 34–40. [DOI] [PubMed] [Google Scholar]
- Jensen, O., Gelfand, J., Kounios, J., & Lisman, J. E. (2002). Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cerebral Cortex, 12, 877–882. [DOI] [PubMed] [Google Scholar]
- Jensen, O., & Mazaheri, A. (2010). Shaping functional architecture by oscillatory alpha activity: Gating by inhibition. Frontiers in Human Neuroscience, 4, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokisch, D., & Jensen, O. (2007). Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. Journal of Neuroscience, 27, 3244–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, S. R., Pinto, D. J., Kaper, T. J., & Kopell, N. (2000). Alpha-frequency rhythms desynchronize over long cortical distances: A modeling study. Journal of Computational Neuroscience, 9, 271–291. [DOI] [PubMed] [Google Scholar]
- Jung, K.-Y., Cho, J.-W., Joo, E. Y., Kim, S. H., Choi, K. M., Chin, J., et al. (2010). Cognitive effects of topiramate revealed by standardised low-resolution brain electromagnetic tomography (sLORETA) of event-related potentials. Clinical Neurophysiology, 121, 1494–1501. [DOI] [PubMed] [Google Scholar]
- Kaiser, J., Heidegger, T., Wibral, M., Altmann, C. F., & Lutzenberger, W. (2007). Alpha synchronization during auditory spatial short-term memory. NeuroReport, 18, 1129–1132. [DOI] [PubMed] [Google Scholar]
- Kalcher, J., & Pfurtscheller, G. (1995). Discrimination between phase-locked and non-phase-locked event-related EEG activity. Electroencephalography and Clinical Neurophysiology, 94, 381–384. [DOI] [PubMed] [Google Scholar]
- Kane, M. J., & Engle, R. W. (2000). Working-memory capacity, proactive interference, and divided attention: Limits on long-term memory retrieval. Journal of Experimental Psychology: Learning, Memory, and Cognition, 26, 336–358. [DOI] [PubMed] [Google Scholar]
- Kayser, J., & Tenke, C. E. (2006). Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clinical Neurophysiology, 117, 348–368. [DOI] [PubMed] [Google Scholar]
- Klimesch, W. (2012). Alpha-band oscillations, attention, and controlled access to stored information. Trends in Cognitive Sciences, 16, 606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch, W., Sauseng, P., & Hanslmayr, S. (2007). EEG alpha oscillations: The inhibition–timing hypothesis. Brain Research Reviews, 53, 63–88. [DOI] [PubMed] [Google Scholar]
- Kondo, H., Morishita, M., Osaka, N., Osaka, M., Fukuyama, H., & Shibasaki, H. (2004). Functional roles of the cingulo-frontal network in performance on working memory. Neuroimage, 21, 2–14. [DOI] [PubMed] [Google Scholar]
- Kondo, H., Osaka, N., & Osaka, M. (2004). Cooperation of the anterior cingulate cortex and dorsolateral prefrontal cortex for attention shifting. Neuroimage, 23, 670–679. [DOI] [PubMed] [Google Scholar]
- Leiberg, S., Lutzenberger, W., & Kaiser, J. (2006). Effects of memory load on cortical oscillatory activity during auditory pattern working memory. Brain Research, 1120, 131–140. [DOI] [PubMed] [Google Scholar]
- Lighthall, N. R., Huettel, S. A., & Cabeza, R. (2014). Functional compensation in the ventromedial prefrontal cortex improves memory-dependent decisions in older adults. Journal of Neuroscience, 34, 15648–15657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lőrincz, M. L., Kékesi, K. A., Juhász, G., Crunelli, V., & Hughes, S. W. (2009). Temporal framing of thalamic relay-mode firing by phasic inhibition during the alpha rhythm. Neuron, 63, 683–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino, S. E., Pakhomov, S. V. S., Han, S., Anderson, K. L., Ding, M., Eberly, L. E., et al. (2012). The effect of topiramate plasma concentration on linguistic behavior, verbal recall and working memory. Epilepsy & Behavior, 24, 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson, K. E., Lleras, A., Beck, D. M., Fabiani, M., Ro, T., & Gratton, G. (2011). Pulsed out of awareness: EEG alpha oscillations represent a pulsed-inhibition of ongoing cortical processing. Frontiers in Psychology, 2, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr, U., & Keele, S. W. (2000). Changing internal constraints on action: The role of backward inhibition. Journal of Experimental Psychology: General, 129, 4–26. [DOI] [PubMed] [Google Scholar]
- McNab, F., & Klingberg, T. (2008). Prefrontal cortex and basal ganglia control access to working memory. Nature Neuroscience, 11, 103–107. [DOI] [PubMed] [Google Scholar]
- Medendorp, W. P., Kramer, G. F. I., Jensen, O., Oostenveld, R., Schoffelen, J.-M., & Fries, P. (2007). Oscillatory activity in human parietal and occipital cortex shows hemispheric lateralization and memory effects in a delayed double-step saccade task. Cerebral Cortex, 17, 2364–2374. [DOI] [PubMed] [Google Scholar]
- Michels, L., Bucher, K., Lüchinger, R., Klaver, P., Martin, E., Jeanmonod, D., et al. (2010). Simultaneous EEG-fMRI during a working memory task: Modulations in low and high frequency bands. PLoS One, 5, e10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels, L., Moazami-Goudarzi, M., Jeanmonod, D., & Sarnthein, J. (2008). EEG alpha distinguishes between cuneal and precuneal activation in working memory. Neuroimage, 40, 1296–1310. [DOI] [PubMed] [Google Scholar]
- Minamoto, T., Osaka, M., & Osaka, N. (2010). Individual differences in working memory capacity and distractor processing: Possible contribution of top–down inhibitory control. Brain Research, 1335, 63–73. [DOI] [PubMed] [Google Scholar]
- Mink, J. W. (1996). The basal ganglia: Focused selection and inhibition of competing motor programs. Progress in Neurobiology, 50, 381–425. [DOI] [PubMed] [Google Scholar]
- Nenert, R., Viswanathan, S., Dubuc, D. M., & Visscher, K. M. (2012). Modulations of ongoing alpha oscillations predict successful short-term visual memory encoding. Frontiers in Human Neuroscience, 6, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld, M. Y., Kogan, E., Chistik, V., & Korczyn, A. D. (1999). Comparison of the effects of vigabatrin, lamotrigine, and topiramate on quantitative EEGs in patients with epilepsy. Clinical Neuropharmacology, 22, 80–86. [DOI] [PubMed] [Google Scholar]
- Osaka, M., Osaka, N., Kondo, H., Morishita, M., Fukuyama, H., Aso, T., et al. (2003). The neural basis of individual differences in working memory capacity: An fMRI study. Neuroimage, 18, 789–797. [DOI] [PubMed] [Google Scholar]
- Otto, A. R., Raio, C. M., Chiang, A., Phelps, E. A., & Daw, N. D. (2013). Working-memory capacity protects model-based learning from stress. Proceedings of the National Academy of Sciences, U.S.A., 110, 20941–20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, D. C., & Reuter-Lorenz, P. (2009). The adaptive brain: Aging and neurocognitive scaffolding. Annual Review of Psychology, 60, 173–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashler, H. (1988). Familiarity and visual change detection. Perception & Psychophysics, 44, 369–378. [DOI] [PubMed] [Google Scholar]
- Raffone, A., & Wolters, G. (2001). A cortical mechanism for binding in visual working memory. Journal of Cognitive Neuroscience, 13, 766–785. [DOI] [PubMed] [Google Scholar]
- Rajah, M. N., & D'Esposito, M. (2005). Region-specific changes in prefrontal function with age: A review of PET and fMRI studies on working and episodic memory. Brain, 128, 1964–1983. [DOI] [PubMed] [Google Scholar]
- Rosenthal, R. (1984). Essentials of behavioral research: Methods and data analysis. New York: McGraw-Hill Higher Education. [Google Scholar]
- Rouder, J. N., Morey, R. D., Morey, C. C., & Cowan, N. (2011). How to measure working memory capacity in the change detection paradigm. Psychonomic Bulletin & Review, 18, 324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng, P., Klimesch, W., Heise, K. F., Gruber, W. R., Holz, E., Karim, A. A., et al. (2009). Brain oscillatory substrates of visual short-term memory capacity. Current Biology, 19, 1846–1852. [DOI] [PubMed] [Google Scholar]
- Scheeringa, R., Petersson, K. M., Oostenveld, R., Norris, D. G., Hagoort, P., & Bastiaansen, M. C. M. (2009). Trial-by-trial coupling between EEG and BOLD identifies networks related to alpha and theta EEG power increases during working memory maintenance. Neuroimage, 44, 1224–1238. [DOI] [PubMed] [Google Scholar]
- Siegel, M., Warden, M. R., & Miller, E. K. (2009). Phase-dependent neuronal coding of objects in short-term memory. Proceedings of the National Academy of Sciences, U.S.A., 106, 21341–21346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffener, J., Brickman, A. M., Rakitin, B. C., Gazes, Y., & Stern, Y. (2009). The impact of age-related changes on working memory functional activity. Brain Imaging and Behavior, 3, 142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokić, M., Milovanović, D., Ljubisavljević, M. R., Nenadović, V., & Čukić, M. (2015). Memory load effect in auditory–verbal short-term memory task: EEG fractal and spectral analysis. Experimental Brain Research, 233, 3023–3038. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry, C., Bertrand, O., Wienbruch, C., Ross, B., & Pantev, C. (1997). Combined EEG and MEG recordings of visual 40 Hz responses to illusory triangles in human. NeuroReport, 8, 1103–1107. [DOI] [PubMed] [Google Scholar]
- Thompson, P. J., Baxendale, S. A., Duncan, J. S., & Sander, J. W. A. S. (2000). Effects of topiramate on cognitive function. Journal of Neurology, Neurosurgery & Psychiatry, 69, 636–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuladhar, A. M., ter Huurne, N., Schoffelen, J.-M., Maris, E., Oostenveld, R., & Jensen, O. (2007). Parieto-occipital sources account for the increase in alpha activity with working memory load. Human Brain Mapping, 28, 785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth, N., Schrock, J. C., & Engle, R. W. (2004). Working memory capacity and the antisaccade task: Individual differences in voluntary saccade control. Journal of Experimental Psychology: Learning, Memory, and Cognition, 30, 1302–1321. [DOI] [PubMed] [Google Scholar]
- Vissers, M. E., van Driel, J., & Slagter, H. A. (2016). Proactive, but not reactive, distractor filtering relies on local modulation of alpha oscillatory activity. Journal of Cognitive Neuroscience, 28, 1964–1979. [DOI] [PubMed] [Google Scholar]
- Vogel, E. K., & Machizawa, M. G. (2004). Neural activity predicts individual differences in visual working memory capacity. Nature, 428, 748–751. [DOI] [PubMed] [Google Scholar]
- Vogel, E. K., McCollough, A. W., & Machizawa, M. G. (2005). Neural measures reveal individual differences in controlling access to working memory. Nature, 438, 500–503. [DOI] [PubMed] [Google Scholar]
- Wang, C., Rajagovindan, R., Han, S.-M., & Ding, M. (2016). Top–down control of visual alpha oscillations: Sources of control signals and their mechanisms of action. Frontiers in Human Neuroscience, 10, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]