Abstract
BACKGROUND:
The outcome of medically refractory patients with obstructive hypertrophic cardiomyopathy treated according to the American College of Cardiology/American Heart Association consensus guideline recommendations is not known. The objectives of this study were to define the short- and long-term outcomes of medically refractory obstructive hypertrophic cardiomyopathy patients undergoing alcohol septal ablation (ASA) and surgical septal myectomy (SM) with patient management in accordance with these consensus guidelines, as well as to quantify procedural risk and burden of comorbid conditions at the time of treatment.
METHODS AND RESULTS:
Patients with obstructive hypertrophic cardiomyopathy referred for either ASA or SM from 2004 to 2015 were followed for the primary end point of short- and long-term mortality and compared with respective age- and sex-matched US populations. Of 477 consecutive severely symptomatic patients, 99 underwent ASA and 378 SM. Compared with SM, ASA patients were older (P<0.001), had a higher burden of comorbid conditions (P<0.01), and significantly higher predicted surgical mortality (P<0.005). Procedure-related mortality was 0.3% and similarly low in both groups (0% in ASA and 0.8% in SM). Over 4.0±2.9 years of follow-up, 95% of patients had substantial improvement in heart failure symptoms to New York Heart Association class I/II (96% in SM and 90% in ASA). Long-term mortality was similar between the 2 groups with no difference compared with age- and sex-matched US populations.
CONCLUSIONS:
Guideline-based referral for ASA and SM leads to excellent outcomes with low procedural mortality, excellent long-term survival, and improvement in symptoms. These outcomes occur in ASA patients despite being an older cohort with significantly more comorbidities.
VISUAL OVERVIEW:
A visual overview is available for this article.
Keywords: alcohol septal ablation, heart failure, hypertrophic cardiomyopathy, surgical myectomy
Current American College of Cardiology (ACC)/American Heart Association (AHA) expert guidelines recommend septal myectomy (SM) for obstructive hypertrophic cardiomyopathy (HCM) patients with medically refractory symptoms, reserving alcohol septal ablation (ASA) as a treatment strategy for patients in whom surgery is contraindicated or considered prohibitive risk, or those who decline surgery.1 Although the outcomes of septal reduction therapy (SRT) in cohorts of patients undergoing either ASA or SM are established, outcomes remain unknown for consecutive patients selected for SRT according to guideline recommendations. Specifically, while reported outcomes from large cohorts of SM patients are likely reflective of this guideline approach (given the availability of ASA in the majority of major SM centers2–7), the majority of reported ASA cohorts are from institutions that predominantly performed ASA (with minimal SM expertise8–11), potentially leading to a younger, healthier cohort of ASA patients not reflective of these consensus guidelines. Since its inception, The Hypertrophic Cardiomyopathy Center at Tufts Medical Center has followed guideline-based therapy for the treatment of their medically refractory obstructive HCM patients. The purpose of this study is to define the short- and long-term outcomes of patients undergoing either SM or ASA in a large HCM referral center where management recommendations have been based on 2003 ACC/European Society of Cardiology12 and 2011 ACC/AHA1 consensus guidelines.
METHODS
Study Design and Patient Population
This was a single-center retrospective observational study. The population consisted of 477 consecutive HCM patients referred for SRT at Tufts Medical Center between 2004 and 2015 because of medically refractory symptoms in the setting of left ventricular outflow tract (LVOT) obstruction. This study was reviewed and approved by the Institutional Review Board at Tufts Medical Center allowing retrospective review of medical records and granted a waiver of informed consent. The authors declare that all supporting data are available within the article and its Data Supplement.
Since the inception of the Tufts HCM clinic in 2004, expertise in both SM and ASA were available, with all SMs performed by a single cardiothoracic surgeon (H.R.), and all ASAs performed by a single interventional cardiologist (C.K.). The decision to pursue SM or ASA was based on guideline recommendations and a process of shared decision-making between the patient and experienced HCM clinicians (M.S.M., E.J.R.).1,12 Surgical myectomy risk was assessed by a highly experienced cardiothoracic surgeon (H.R.) incorporating the individual patient’s comprehensive clinical profile, including age, comorbidities, and patient preference, and in concert with the recommendations of the 2011 ACC/AHA HCM expert consensus guidelines. SM was the initial consideration for patients with low or intermediate surgical risk (n=332), as well as those higher risk patients with anatomy felt to be unsuitable for ASA (n=46). ASA was initially considered in patients with suitable anatomy based on a comprehensive assessment with imaging and evaluation by a multidisciplinary HCM team with the greatest emphasis on basal septal thickness <30 mm, absence of concomitant cardiac disease independently requiring surgical correction (eg, intrinsic mitral valve disease or coronary artery disease), and excluding specific abnormalities of the mitral and submitral apparatus such as excessively elongated mitral valve leaflets, excessive and abnormally displaced papillary muscles, and anomalous papillary muscle insertion directly into the anterior mitral leaflet (in the absence of chordae tendineae). Patients with suitable anatomy and perceived high risk due to comorbidities or advanced age ≥75 years (n=75) constituted the majority of our ASA cohort. In a select group of 24 patients (and representing just 7% of all low or intermediate surgical risk patients electing for SRT), ASA was performed after a balanced and thorough discussion of risks and benefits of both procedures with patient expressing strong preferred for ASA due to either concerns regarding longer recovery (n=14) or fear of general anesthesia/invasive surgery (n=10) with SM.
Baseline Clinical Information
Retrospective analysis was performed by review of the electronic medical record and HCM Center database. Demographic and baseline clinical information was captured.
Risk Scores and Comorbidity Burden
In an effort to retrospectively characterize patient disability and compare the predicted operative mortality and the severity of comorbid conditions among SM and ASA patients, risk scores were calculated for each patient. For each patient, 2 procedural risk scores were calculated: the Society for Thoracic Surgeons Mortality Score for Isolated Aortic Valve Replacement13 and EuroSCORE II.14 Two comorbidity indices were evaluated: the Modified Charlson Score15 and the Goodman Health and Human Service Criteria Score.16 The Society of Thoracic Surgeons risk models predict the risk of operative mortality and morbidity after adult cardiac surgery on the basis of patient demographic and clinical variables including heart failure, renal failure, cardiac symptoms, prior myocardial infarction, cardiac arrhythmia, chronic lung disease, cerebrovascular disease, peripheral artery disease, diabetes mellitus, hypertension, immunocompromised state, cardiogenic shock, and valve disease; EuroSCORE II includes parameters of age, sex, renal impairment, previous cardiac surgery, chronic lung disease, diabetes mellitus, New York Heart Association (NYHA), angina at rest, left ventricular (LV) function, recent myocardial infarction, pulmonary hypertension, critical preoperative state, and urgency of procedure14; the Modified Charlson Score includes parameters of age, myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, liver disease, diabetes mellitus, renal disease, cancer, and AIDS15; and the Goodman Health and Human Service Criteria Score includes hypertension, stroke, diabetes mellitus, atrial fibrillation, chronic obstructive pulmonary disease, coronary artery disease, prior myocardial infarction, renal impairment, hyperlipidemia, liver disease, cancer, AIDS, dementia, arthritis, asthma, depression, osteoporosis, and substance abuse disorder.16
Follow-Up and End Points
Follow-up in the 477 patients was obtained by telephone contact or clinic visit, with a mean follow-up time of 4.0±2.9 years (range to 12.0 years); 24 patients (5%) were lost to clinical follow-up. Echocardiographic follow-up was obtained from the most recent echocardiographic data performed at least 12 months after the initial procedure.
The objective of this study was to define long-term outcomes in HCM patients undergoing either SM or ASA in a large HCM referral center where management has been guided by 2003 ACC/European Society of Cardiology and 2011 ACC/AHA consensus guidelines. The primary end point was short- and long-term mortality of the individual procedures in comparison to their respective age- and sex-matched US populations. For comparison with ASA and SM patients, an expected survival curve for the general population was generated from US Health Statistics, which incorporate all-cause mortality. Each ASA and SM patient was matched to the US population by age, sex, and the year of study entry. Secondary end points included symptom improvement, gradient reduction, and arrhythmias after SRT.
Statistical Analyses
Continuous variables are presented as mean±SD, and categorical variables are expressed as percentages. Baseline characteristics and procedural outcomes were compared with unpaired Student t tests, χ2 test, or Fisher exact tests where appropriate for continuous and categorical data. All statistical tests were 2 sided, and P<0.05 was set a priori to be statistically significant. All of these statistical analyses were conducted using GraphPad Prism 7.
For patients with known survival status, the fraction at each follow-up interval was estimated by Kaplan-Meier method. Patients lost to follow-up were censored at the time of the last clinical encounter. Expected fraction surviving at each time after the initial visit was computed by assigning probability of surviving, appropriate to age and sex, based on US Census data. Actual and expected surviving fractions were compared using log-rank tests. Multivariable Cox proportional hazard models were used to identify independent predictors of all-cause mortality. Proportional hazards assumption was assessed graphically before proceeding. To assess internal model validation, bootstrapping (1000 repetitions) was performed with the point estimates and 95% CIs shown in Table I in the Data Supplement. Computations used version 3.4.3 of R software systems (R Core Team 2017).
RESULTS
Clinical Characteristics
Baseline demographic, clinical, and echocardiographic parameters are shown in Table 1. There were 477 patients studied, of whom 378 (79.2%) underwent SM and 99 (20.8%) underwent ASA (Table 1). The mean patient age was 55.5±15.2 years; range, 12.9 to 85.9 years; and 46.3% of the patients were women. All patients met criteria for invasive SRT with NYHA functional class (FC) or CCS class III or IV symptoms, LVOT gradient ≥50 mm Hg, and a maximal basal septal thickness ≥15 mm. Patients undergoing ASA were significantly older than those undergoing SM (66.3±11.9 versus 52.7±14.7; P<0.001), and a significantly higher proportion of ASA patients were women (63.6% versus 41.8%; P<0.001). There were no significant differences between groups in NYHA FC before SRT (94.9% versus 93.2% NYHA FC III/IV ASA versus SM patients, respectively; P=0.72) or in β-blocker, calcium channel blocker, or disopyramide use.
Table 1.
Baseline Characteristics
| Myectomy | Alcohol Ablation | P Value* | |
|---|---|---|---|
| Patients, n (%) | 378 (79.2) | 99 (20.8) | |
| Age, y | 52.7±14.7 | 66.3±11.9 | <0.001 |
| Women, n (%) | 158 (41.8) | 63 (63.6) | <0.001 |
| BMI, kg/m2 | 31.1±6.7 | 31.9±10.2 | 0.385 |
| Resting heart rate | 64±10 | 62±10 | |
| NYHA class (study entry), n (%) | |||
| I | 1 (0.3) | 0 (0) | |
| II | 25 (6.6) | 5 (5.1) | |
| III | 348 (92.1) | 93 (93.9) | |
| IV | 4 (1.1) | 1 (1) | 0.715 |
| Maximum LV thickness, mm | 20.3±4.8 | 19.6±2.9 | 0.155 |
| LV wall thickness ≥30 mm | 15 (4.0) | 0 (0) | <0.001 |
| LV wall thickness ≤16 mm | 66 (17.5) | 9 (9.1) | <0.05 |
| LVOT gradient at rest, mm Hg | 58.0±41.8 | 65.7±40.7 | 0.105 |
| LVOT gradient at rest ≥30 mm Hg, n (%) | 280 (74.1) | 76 (76.8) | 0.583 |
| Moderate or severe mitral regurgitation, n (%) | 127 (34) | 37 (37) | 0.481 |
| LVEF, % | 65±5 | 65±5 | 0.931 |
| History of atrial fibrillation, n (%) | 116 (30.7) | 16 (16.2) | <0.005 |
| Syncope, n (%) | 49 (13.0) | 4 (4.0) | <0.05 |
| NSVT on ambulatory Holter | 44 (11.6) | 16 (16.2) | 0.281 |
| Family history of SCD | 35 (9.3) | 2 (2.0) | <0.05 |
| Medications, n (%) | |||
| β-Blocker | 290 (76.7) | 81 (81.8) | 0.381 |
| Calcium channel blocker | 143 (37.8) | 36 (36.4) | 0.765 |
| Disopyramide | 33 (8.7) | 11 (11.1) | 0.607 |
| Amiodarone | 8 (2.1) | 8 (8.1) | <0.005 |
| ACE inhibitors/ARB | 47 (12.4) | 27 (27.3) | <0.001 |
| Diuretic | 66 (17.5) | 28 (28.3) | <0.05 |
| Coumadin | 35 (9.3) | 9 (9.1) | 0.959 |
| History, n (%) | |||
| COPD | 33 (8.7) | 8 (8.1) | 0.838 |
| CVA | 19 (5.0) | 7 (7.1) | 0.412 |
| Diabetes mellitus | 42 (11.1) | 16 (16.2) | 0.171 |
| CKD | 5 (1.3) | 11 (11.1) | <0.001 |
| Liver disease | 8 (2.1) | 7 (7.1) | <0.05 |
| Cancer | 27 (7.1) | 2 (2.0) | 0.058 |
| Cardiac surgery | 3 (0.8) | 2 (2.0) | 0.287 |
| Comorbidity indices | |||
| Modified Charlson Score | 2.6±1.5 | 3.7±1.7 | <0.001 |
| EuroSCORE II | 1.0±0.6 | 1.2±0.9 | <0.005 |
| Goodman HHS Criteria Score | 2.9±1.6 | 3.4±1.9 | <0.01 |
| STS mortality score, % | 1.0±0.6 | 2.0±1.5 | <0.001 |
Values are mean±SD or n (%). ACE indicates angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; HHS, Health and Human Service; LV, left ventricle; LVEF, left ventricular ejection fraction; LVOT, left ventricular outflow tract; NSVT, nonsustained ventricular tachycardia; NYHA, New York Heart Association; SCD, sudden cardiac death; and STS, Society of Thoracic Surgeons.
P value is for comparison of myectomy vs alcohol ablation.
SM patients more frequently had a history of atrial fibrillation (30.7% SM versus 16.2% ASA; P<0.005), syncope (13.0% SM versus 4.0% ASA; P<0.05), and family history of sudden death (9.3% SM versus 2.0% ASA; P<0.05). There were no significant between-group differences in a history of nonsustained ventricular tachycardia (11.6% SM versus 16.2% ASA; P=0.28). Adjunctive surgical procedures performed in the SM group appear in Table II in the Data Supplement.
Baseline Echocardiographic Parameters
Echocardiographic parameters of patients referred for ASA and SM were not significantly different, including maximal LV wall thickness, LV ejection fraction, presence of resting obstruction (LVOT gradient >30 mm Hg), and LVOT gradient. However, more patients with a maximal LV septal thickness >30 mm were referred for SM as opposed to ASA (4.0% SM versus 0% ASA; P<0.001).
Risk Scores and Comorbidity Burden
In an effort to compare baseline differences in procedural risk and overall health status between patients undergoing SM and ASA, patient-level data were used to evaluate 2 different operative risk scores and 2 different comorbidity indices (Table 1; Figure 1). The ASA patients had significantly higher predicted procedural mortality (STS PROM: 2.0±1.5 versus 1.0±0.6, P<0.001; EuroSCORE II: 1.2±0.9 versus 1.0±0.6, P<0.005) and a higher burden of comorbid conditions than SM patients (Modified Charlson Score: 3.7±1.7 versus 2.6±1.5, P<0.001; Goodman Health and Human Service Criteria Score: 3.4±1.9 versus 2.9±1.6; P<0.01). Patients who were referred for ASA because of high surgical risk or age >75 years had significantly higher comorbidity indices when compared with patients seeking to avoid cardiac surgery (Modified Charlson Score: 4.1±1.7 versus 2.5±1.0, P<0.001; EuroSCORE II: 1.4±0.9 versus 0.7±0.5, P<0.001; Goodman Health and Human Service Criteria Score: 3.8±2.0 versus 2.4±0.9, P<0.005; STS PROM: 2.3±1.6% versus 1.1±0.5, P<0.001; high comorbidity population versus patient preference, respectively).
Figure 1. Comorbidity indices.
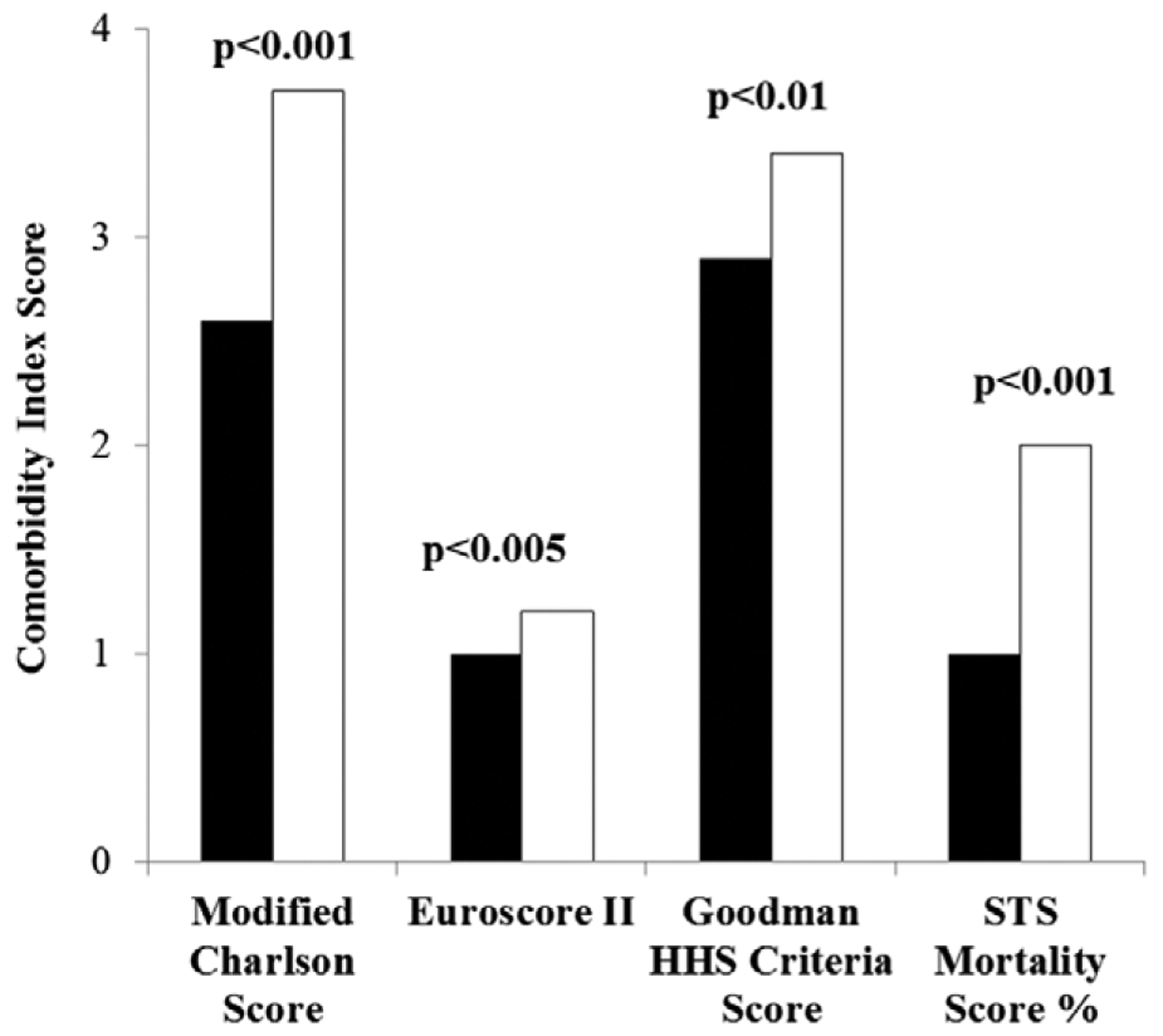
Four different comorbidity indices for alcohol septal ablation (ASA) and surgical septal myectomy (SM) patients. For each index, ASA patients were at significantly elevated risk as compared with SM patients. HHS indicates Health and Human Service; and STS, Society of Thoracic Surgeons.
Alcohol Volumes
In an effort to determine whether volumes of alcohol used during ASA decreased over time, we grouped patients in cohorts of 20 moving forward from 2004 (Figure 2). Alcohol volumes were the highest in the earliest cases and the lowest in the most recent procedures (1.9±0.4 versus 1.4±0.4 mL; P<0.001 for trend over time).
Figure 2. Alcohol volumes.
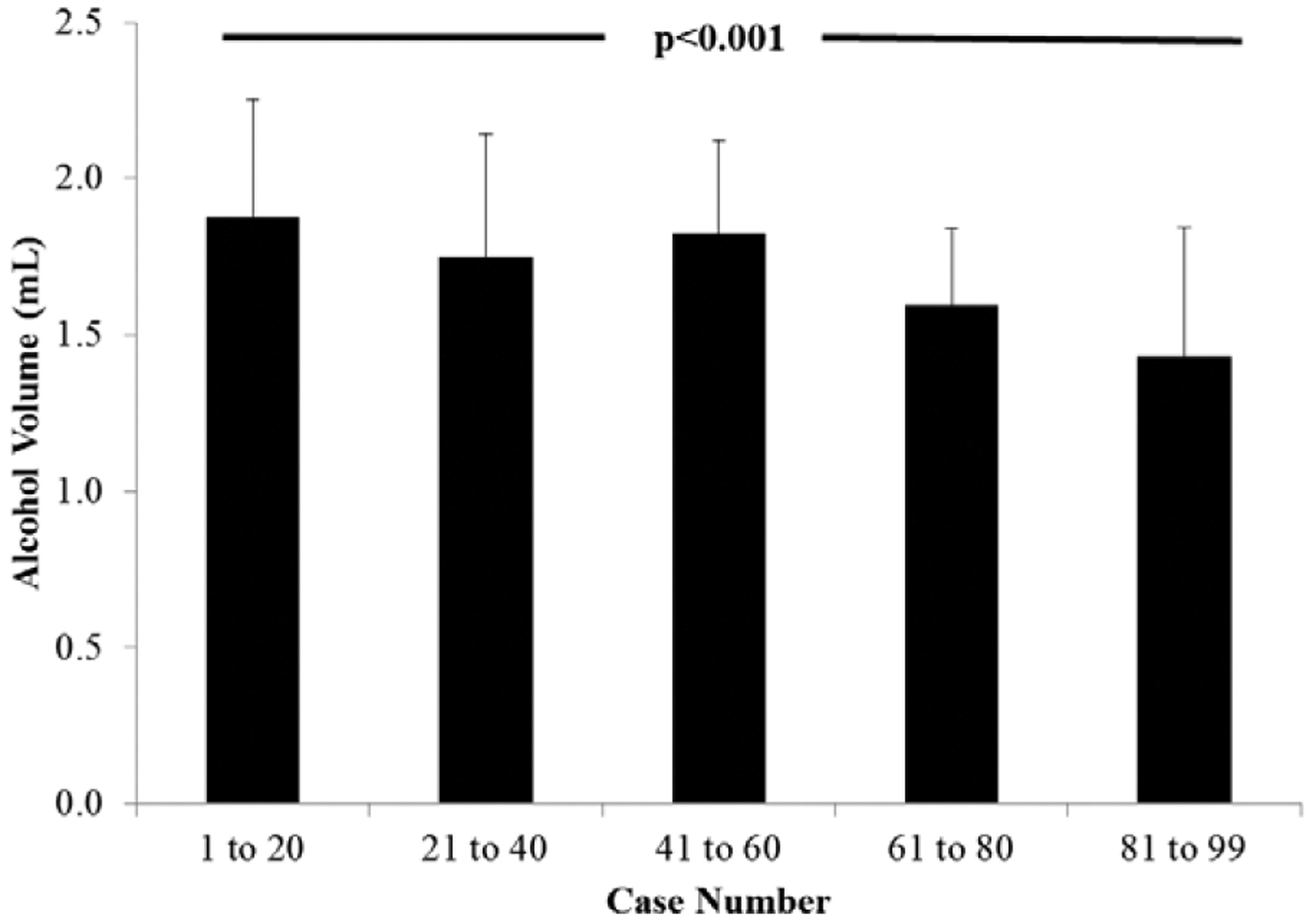
Volumes of alcohol used in consecutive cases grouped by quintiles. Despite historical use of low alcohol volumes, over time, there was a significant reduction in the volume of alcohol used.
Procedural Outcomes
There were no significant differences in procedural outcomes between the 2 groups (Table 2). Average length of stay was 5.8±5.6 days in the ASA group versus 6.4±3.3 days in SM patients, P=0.18. Thirty-day mortality for the entire population was 0.3% and was similarly low in both groups (0% in ASA and 0.8% in SM). The rate of periprocedural heart block was higher in the ASA group than in SM group (26.3±2.6% and 14.0±0.7%, respectively, P<0.01). When excluding patients with the preexisting conduction abnormalities (for the ASA group: first-degree atrioventricular block, left anterior fascicular block, left bundle branch block; for the SM group: right bundle branch block and conditions/surgical procedures increasing the risk for developing complete heart block—mild hypertrophy of the basal septum <15 mm and Cox-Maze procedure), there was no significant difference in the need for postprocedural pacemaker implantation (6.1% ASA patients versus 5.0% SM patients; P=0.62).
Table 2.
Procedural Outcomes
| Myectomy | Alcohol Ablation | P Value* | |
|---|---|---|---|
| Length of stay, d | 6.4±3.3 | 5.8±5.6 | 0.18 |
| Nonfatal procedural complications | |||
| Pacemaker before discharge without predisposing conditions | 19 (5.0) | 6 (6.1) | 0.62 |
| CVA, n (%) | 5 (1.3) | 0 (0) | 0.59 |
| AKI requiring dialysis, n (%) | 3 (0.8) | 0 (0) | 1.0 |
| Tamponade, n (%) | 6 (1.6) | 1 (1.0) | 0.67 |
| VSD, n (%) | 3 (0.8) | 0 (0) | |
| Nonfatal cardiac arrest, n (%) | 7 (1.9) | 1 (1.0) | 0.56 |
| 30-d mortality | 3 (0.8) | 0 (0) | 1.0 |
Values are mean±SD or n (%). AKI indicates acute kidney injury; CVA, cerebrovascular accident; and VSD, ventricular septal defect.
P value is for comparison of myectomy vs alcohol ablation.
Long-Term Clinical and Echocardiographic Outcomes
SRT patients were followed on average for 4.0±2.9 years with a similar duration of follow-up in both treatment groups. On long-term follow-up, 95% of patients undergoing SRT had substantial improvement in heart failure symptoms to NYHA class I/II (96% of SM patients versus 90% of ASA patients; P<0.001). ASA patients were more likely to have a residual LVOT gradient >30 mm Hg as measured by echocardiography (26.3% ASA group versus 2.1% SM group; P<0.001; Table 3). A significantly higher proportion of ASA patients reported NYHA FC III and IV symptoms at the latest follow-up (10.3% ASA group versus 4.2% SM group; P<0.001; Table 3). Repeat SRT occurred more frequently in ASA patients (7.1% ASA versus 0% in SM; P<0.001). LV ejection fraction was higher following ASA than SM on long-term follow-up, although LV ejection fraction remained normal in both groups (64±5% versus 58±7% ASA versus SM, respectively; P<0.001). There were no differences in the rates of heart transplantation, resuscitation from cardiac arrest, or appropriate implantable cardioverter defibrillator interventions (Table 3).
Table 3.
Long-Term Clinical and Echocardiographic Outcomes
| Myectomy | Alcohol Ablation | P Value* | |
|---|---|---|---|
| Duration of follow-up, y | 4.0±2.8 | 4.1±3.1 | 0.59 |
| NYHA class, n (%) | |||
| I | 192 (61.5) | 29 (33.3) | |
| II | 107 (34.3) | 49 (56.3) | |
| III/IV | 13 (4.2) | 9 (10.3) | <0.001 |
| LVOT gradient ≥30 mm Hg, n (%) | 8 (2.1) | 26 (26.3) | <0.001 |
| LVEF, % | 58±7 | 64±5 | <0.001 |
| Resting moderate mitral regurgitation | 24 (6) | 21 (21) | <0.001 |
| Major interventions | |||
| Repeat invasive septal therapy, n (%) | 0 (0) | 7 (7.1) | <0.001 |
| Mitral valve intervention, n (%) | 2 (0.5) | 0 (0) | 1.0 |
| Heart transplantation, n (%) | 0 (0) | 1 (1.0) | 1.0 |
| Resuscitated cardiac arrest, n (%) | 1 (0.3) | 0 (0) | 1.0 |
| Appropriate ICD interventions, n (%) | 8(2.1) | 0 (0) | 0.21 |
| Mortality, n (%) | 11 (2.9) | 2 (2.0) | 0.63 |
Values are mean±SD or n (%). ICD indicates implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; LVOT, left ventricular outflow tract; and NYHA, New York Heart Association.
P value is for comparison of myectomy vs alcohol ablation.
Mortality on long-term follow-up was 2.0% in the ASA group and 2.9% in SM patients (P=0.63), with no difference in mortality for either group as compared with age- and sex-matched US populations (Figure 3). Survival for the 2 treatment groups adjusted for medians of age and Charlson Score is not significantly different between ASA and SM patients (P=0.15; Figure I in the Data Supplement). In addition, using a multivariable Cox proportional hazard model, Modified Charlson Score was an independent predictor of all-cause mortality (hazard ratio, 1.37; 95% CI, 1.04–1.81; P=0.03); however, neither ASA/SM (P=0.15) nor age (P=0.55) predicted mortality (Table III in the Data Supplement).
Figure 3. Mortality of surgical septal myectomy (SM) and alcohol septal ablation (ASA) patients compared with controls.
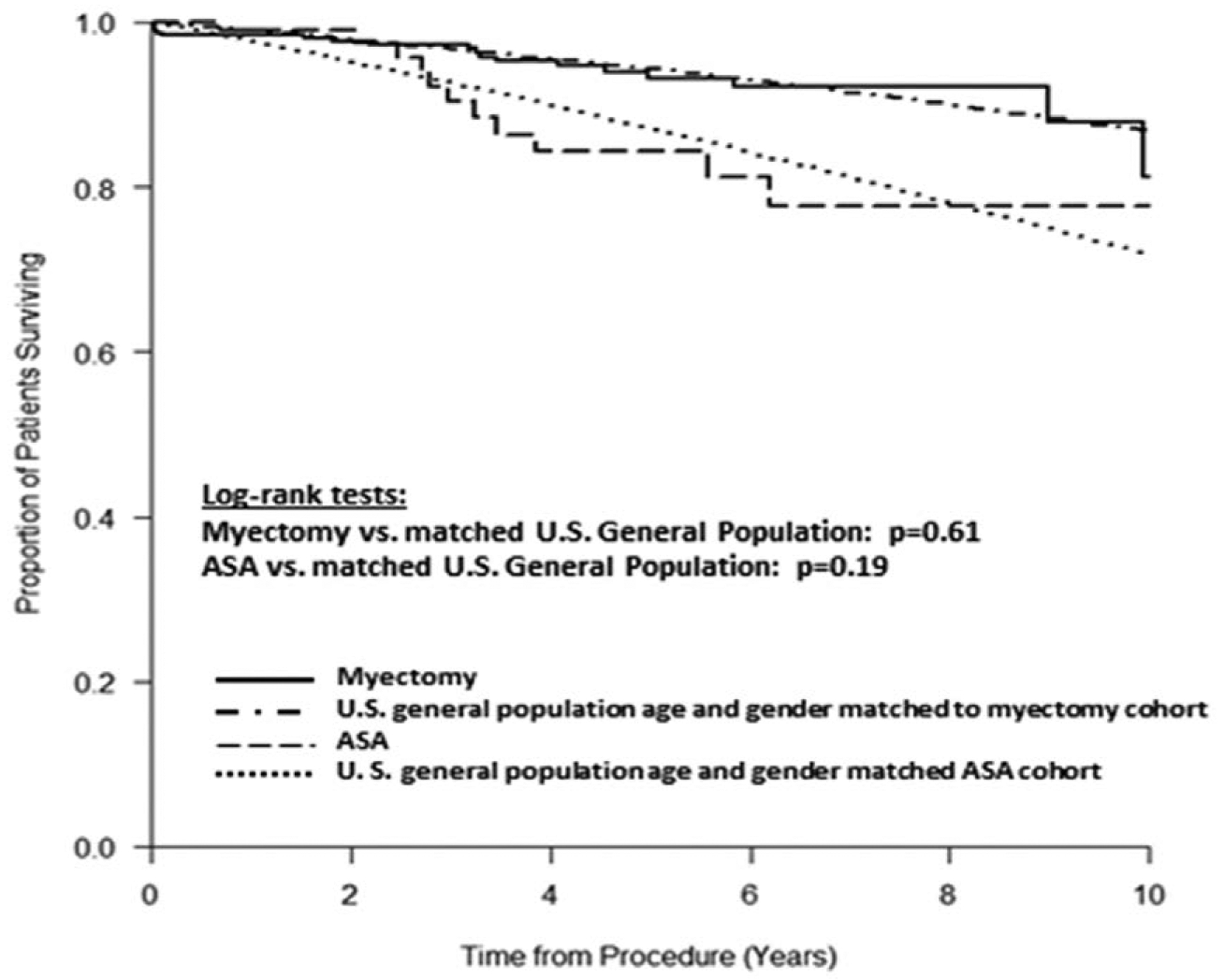
The survival of SM and ASA patients over follow-up was compared with age- and sex-matched US general population. The survival of both SM and ASA patients did not differ from their respective control population.
Clinical Nonresponders and Patients Requiring Repeat SRT
A total of 16 patients failed to have symptom improvement after ASA (16% of ASA cohort) including 7 with residual obstruction who underwent repeat SRT (ASA in 1 and SM in 6) 2.7±2.0 years after initial SRT (range, 0.3–5.5 years). Each of these 7 patients had relief of obstruction with associated symptom improvement after repeat intervention to NYHA class I or II. In the remaining 9 patients, 4 had residual outflow obstruction likely contributing to persistent symptoms, each declined repeat SRT. In contrast, outflow obstruction was successfully relieved in the remaining 5, but advanced heart failure symptoms persisted. Those patients who did not have symptomatic improvement despite relief of obstruction had significantly higher comorbidity indices than the remaining 94 ASA patients with symptom improvement (Table IV in the Data Supplement).
A total of 13 patients failed to have symptom improvement after SM despite relief of obstruction in each patient (3% of SM cohort). Two additional patients who responded clinically to SM developed severe mitral regurgitation 0.8±0.7 years after the initial intervention requiring MV repair. All 13 had persistent heart failure symptoms, including 2 with the development of systolic dysfunction (ejection fraction<50%) and 11 with preserved ejection fraction. Comorbidity indices did not differ significantly in patients with and without symptomatic improvement after surgical myectomy.
DISCUSSION
Choosing Between ASA and SM
The optimal treatment strategy for patients with medication-refractory obstructive HCM remains controversial. No randomized trials have compared therapeutic options in this patient population, and recommendations are based on expert opinion. Several large patient cohorts have demonstrated that both SM and ASA improve patient functional status with low periprocedural mortality.17–19 In this regard, current guidelines recommend ASA in those patients when surgery is contraindicated or the risk is considered unacceptable because of comorbid medical conditions, advanced age, or patient preference1; however, outcomes in consecutive patients using this approach remain unknown. Additionally, while outcomes in large cohorts of SM patients are likely reflective of these guidelines, reported outcomes in the majority of ASA cohorts are from institutions in which experience is predominantly ASA, with minimal SM performed, potentially leading to a younger cohort of patients with less comorbidities, and not reflective of these consensus guideline recommendations.8–11 We, therefore, felt it timely to examine our ASA and SM experience, with patient selection based on these guidelines.
The Hypertrophic Cardiomyopathy Center at Tufts Medical Center is a large referral center, which has extensive experience with both SM and ASA and has, since its inception, followed this guideline-based approach for treatment of drug-refractory obstructive HCM. The major finding of this study is that following these guideline-based recommendations leads to excellent outcomes for all HCM patients undergoing SRT for refractory symptoms. In fact, mortality following guideline-recommended SRT with either ASA or SM does not differ significantly from age- and sex-matched respective populations. This stands in contrast to prior data reported on younger patients undergoing ASA, a strategy that appears to be at odds with the ACC/AHA guidelines, and in whom post-ASA mortality rates were higher than an age- and sex-matched population.20 Additionally, after guideline-based SRT, almost 95% of patients had substantial improvement in previously limiting heart failure symptoms (to NYHA FC I/II).
Quantifying Comorbidities in Obstructive HCM Patients
For the first time in the ASA literature, we have formally quantified procedural risk estimates and the burden of comorbid conditions present at the time of treatment using this guideline-based approach, demonstrating ASA patients are significantly older, with increased comorbidities as compared with patients undergoing SM. Although surgical risk estimates with EuroSCORE and Society for Thoracic Surgeons have not been directly validated for myectomy, we consider these scores to provide reasonable estimates of overall patient surgical risk and, when combined with the other indexes we studied, allow advising clinicians to generate a more robust comparison of differences in individual patient comorbidities. Indeed, we found the average age of individuals undergoing ASA in our institution was 66 years of age and over 10 years older than reported in previous large cohorts and meta-analyses of ASA patients and with potentially increased comorbidities.8,17,18 However, despite ASA patients having elevated risk estimates and a higher burden of prognostically significant comorbidities, there was no increase in short- or long-term mortality associated with the ASA when compared with SM, with low rates of procedural complications. Indeed, this procedure allowed extended longevity, with survival post-ASA no different than the age- and sex-matched US population. Further, ASA allowed for improved quality of life, with almost 90% of these patients experiencing significant postprocedural symptom reduction.
In our cohort of patients, the excellent procedural outcomes associated with SM, including low operative mortality and low rates of major procedural complications, is, in large part, secondary to the high-volume surgical expertise4,5; however, further contributing to low surgical mortality is likely the availability and efficacy of ASA. Specifically, ASA allowed for an alternative treatment option for higher risk patients with suitable anatomy, enabling surgical mortality rates of <1% in our cohort (and in other high-volume SM centers), mortality rates that likely would not be possible if these higher risk patients had SM as only procedural option. Additionally, these data were derived from an HCM center with long-standing expertise in performing both SM and ASA with high-volume expert operators. Based on recent data demonstrating substantially increased mortality and morbidity for both SM and ASA when performed in lower volume less-experienced centers,20 we support the recommendations suggested in the 2011 ACC/AHA HCM guidelines that greater weight be given to selecting appropriate patients for referral to established clinical programs of excellence staffed by cardiologists and cardiac surgeons familiar with the contemporary management of HCM.
Alcohol Volumes in ASA
As has been reported previously,11,21 we documented a reduction in the volume of alcohol used per case over time. However, unlike prior experience, our center has historically used a limited dose of alcohol. In our earliest procedures, the volume of alcohol averaged <2.0 mL. The low dose of alcohol used at our center might, in part, explain the salutary outcome data with no periprocedural mortalities and a low rate of permanent pacemaker implantation (6%) in patients without preexisting conduction system disease.
Clinical Nonresponders
On examination of the 9 patients who did not have a salutary symptomatic result after ASA, and remaining in NYHA FC III/IV, 5 individuals had excellent relief of dynamic LVOT obstruction with an average gradient reduction of 92% and 58% at rest and following provocation, respectively. It, therefore, seems likely that failure to relieve symptoms in patients following guideline-directed ASA may be because of the comorbid conditions that initially led to ASA referral as opposed to SM. Indeed, on multivariable analysis, the only independent predictor for nonresponse in this subset of patients was substantially elevated comorbidity indexes.
ASA Complications
Consistent with prior reports,8,19,22–25 there was no signal in our ASA group of a postprocedural increased risk of ventricular tachyarrhythmias despite the theoretical risk of myocardial scar, which is associated with ASA because of alcohol-mediated myocardial necrosis. Additionally, when accounting for preexisting conduction system disease in our older, sicker ASA population, we found a low rate of permanent pacemaker implantation (≈5%) with no significant difference between the rate of implantation in the ASA and SM groups before hospital discharge.
Limitations
This was a single-center, retrospective analysis; consequently, the comorbidity indices were not prospectively assessed, nor were they specifically designed to assess procedural risk in patients with obstructive HCM. However, we attempted to overcome this limitation by using multiple, disparate indices. In addition, although it might appear that this single-center study with single dedicated operators performing ASA and SM could limit the generalizability of the results, it should be noted that this approach is in line with the ACC/AHA guidelines that medication-refractory obstructive HCM patients in need of SRT be cared for at centers of excellence where appropriate therapies can be prescribed by expert multidisciplinary teams.
Conclusions
In summary, by following guideline-based referral for SRT, outcomes for both ASA and SM are excellent with low procedural mortality, extended survival, and substantial improvement in heart failure symptoms. Although ASA patients are older with a significant increase in comorbid illness, nevertheless they have similar excellent outcomes to those reported in younger and potentially healthier populations. ASA as an alternative treatment option for high-risk patients may contribute to low procedural SM morbidity and mortality. Thereby, these guidelines allow all obstructive HCM patients the opportunity for extended longevity and good quality of life. These data support patient referral based on these consensus guideline recommendations. Whether these excellent outcomes influence an expanded role for ASA for younger patients with less comorbidities in future guideline recommendations remains to be seen.
Supplementary Material
WHAT IS KNOWN.
Optimal treatment strategy for medication-resistant obstructive hypertrophic cardiomyopathy patients is controversial, but, current expert guidelines recommend alcohol septal ablation (ASA) when septal myectomy (SM) is contraindicated, considered prohibitive risk or for those who decline surgery.
In contrast to these guideline recommendations, the majority of reported outcome data for ASA are derived from institutions that preferentially perform ASA over SM. Such cohorts are comprised often of obstructive hypertrophic cardiomyopathy patients who are young without significant medical comorbidities. These data demonstrate that both SM and ASA are effective in reducing left ventricular outflow tract gradients and improving symptoms, with comparably low periprocedural mortality.
It is unknown whether the outcome of medically refractory patients with obstructive hypertrophic cardiomyopathy, treated according to American College of Cardiology/American Heart Association consensus guideline recommendations, is similar between ASA and SM.
WHAT THE STUDY ADDS.
Following guideline-based recommendations for procedure selection, hypertrophic cardiomyopathy patients undergoing ASA have similar reductions in left ventricular outflow tract gradients and improvement in heart failure symptoms compared with SM patients, leading to excellent clinical outcomes.
For the first time, using 4 separate comorbidity indices, patients referred for ASA following guideline recommendations are documented to be significantly older and have significantly higher comorbid risk scores when compared with patients referred for SM.
When following guideline recommendations, survival after SM and ASA is no different than age- and sex-matched populations.
Footnotes
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCINTERVENTIONS.118.007673.
REFERENCES
- 1.Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Failure Society of America; Heart Rhythm Society; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:e783–e831. doi: 10.1161/CIR.0b013e318223e2bd [DOI] [PubMed] [Google Scholar]
- 2.Patel P, Dhillon A, Popovic ZB, Smedira NG, Rizzo J, Thamilarasan M, Agler D, Lytle BW, Lever HM, Desai MY. Left ventricular outflow tract obstruction in hypertrophic cardiomyopathy patients without severe septal hypertrophy: implications of mitral valve and papillary muscle abnormalities assessed using cardiac magnetic resonance and echocardiography. Circ Cardiovasc Imaging. 2015;8:e003132. doi: 10.1161/CIRCIMAGING.115.003132 [DOI] [PubMed] [Google Scholar]
- 3.Ommen SR, Maron BJ, Olivotto I, Maron MS, Cecchi F, Betocchi S, Gersh BJ, Ackerman MJ, McCully RB, Dearani JA, Schaff HV, Danielson GK, Tajik AJ, Nishimura RA. Long-term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46:470–476. doi: 10.1016/j.jacc.2005.02.090 [DOI] [PubMed] [Google Scholar]
- 4.Maron BJ, Dearani JA, Ommen SR, Maron MS, Schaff HV, Nishimura RA, Ralph-Edwards A, Rakowski H, Sherrid MV, Swistel DG, Balaram S, Rastegar H, Rowin EJ, Smedira NG, Lytle BW, Desai MY, Lever HM. Low operative mortality achieved with surgical septal myectomy: role of dedicated hypertrophic cardiomyopathy centers in the management of dynamic subaortic obstruction. J Am Coll Cardiol. 2015;66:1307–1308. doi: 10.1016/j.jacc.2015.06.1333 [DOI] [PubMed] [Google Scholar]
- 5.Rastegar H, Boll G, Rowin EJ, Dolan N, Carroll C, Udelson JE, Wang W, Carpino P, Maron BJ, Maron MS, Chen FY. Results of surgical septal myectomy for obstructive hypertrophic cardiomyopathy: the Tufts experience. Ann Cardiothorac Surg. 2017;6:353–363. doi: 10.21037/acs.2017.07.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorajja P, Ommen SR, Holmes DR Jr, Dearani JA, Rihal CS, Gersh BJ, Lennon RJ, Nishimura RA. Survival after alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Circulation. 2012;126:2374–2380. doi: 10.1161/CIRCULATIONAHA.111.076257 [DOI] [PubMed] [Google Scholar]
- 7.Kwon DH, Kapadia SR, Tuzcu EM, Halley CM, Gorodeski EZ, Curtin RJ, Thamilarasan M, Smedira NG, Lytle BW, Lever HM, Desai MY. Long-term outcomes in high-risk symptomatic patients with hypertrophic cardiomyopathy undergoing alcohol septal ablation. JACC Cardiovasc Interv. 2008;1:432–438. doi: 10.1016/j.jcin.2008.05.009 [DOI] [PubMed] [Google Scholar]
- 8.Veselka J, Jensen MK, Liebregts M, Januska J, Krejci J, Bartel T, Dabrowski M, Hansen PR, Almaas VM, Seggewiss H, Horstkotte D, Tomasov P, Adlova R, Bundgaard H, Steggerda R, Ten Berg J, Faber L. Long-term clinical outcome after alcohol septal ablation for obstructive hypertrophic cardiomyopathy: results from the Euro-ASA registry. Eur Heart J. 2016;37:1517–1523. doi: 10.1093/eurheartj/ehv693 [DOI] [PubMed] [Google Scholar]
- 9.Nagueh SF, Groves BM, Schwartz L, Smith KM, Wang A, Bach RG, Nielsen C, Leya F, Buergler JM, Rowe SK, Woo A, Maldonado YM, Spencer WH III. Alcohol septal ablation for the treatment of hypertrophic obstructive cardiomyopathy. A multicenter North American registry. J Am Coll Cardiol. 2011;58:2322–2328. doi: 10.1016/j.jacc.2011.06.073 [DOI] [PubMed] [Google Scholar]
- 10.Noseworthy PA, Rosenberg MA, Fifer MA, Palacios IF, Lowry PA, Ruskin JN, Sanborn DM, Picard MH, Vlahakes GJ, Mela T, Das S. Ventricular arrhythmia following alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Am J Cardiol. 2009;104:128–132. doi: 10.1016/j.amjcard.2009.02.056 [DOI] [PubMed] [Google Scholar]
- 11.Liebregts M, Faber L, Jensen MK, Vriesendorp PA, Januska J, Krejci J, Hansen PR, Seggewiss H, Horstkotte D, Adlova R, Bundgaard H, Ten Berg JM, Veselka J. Outcomes of alcohol septal ablation in younger patients with obstructive hypertrophic cardiomyopathy. JACC Cardiovasc Interv. 2017;10:1134–1143. doi: 10.1016/j.jcin.2017.03.030 [DOI] [PubMed] [Google Scholar]
- 12.Maron BJ, McKenna WJ, Danielson GK, Kappenberger LJ, Kuhn HJ, Seidman CE, Shah PM, Spencer WH III, Spirito P, Ten Cate FJ, Wigle ED. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. J Am Coll Cardiol. 2003;42:1687–1713. [DOI] [PubMed] [Google Scholar]
- 13.Shahian DM, O’Brien SM, Filardo G, Ferraris VA, Haan CK, Rich JB, Normand SL, DeLong ER, Shewan CM, Dokholyan RS, Peterson ED, Edwards FH, Anderson RP. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: Part 1- coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88:S2–S22. doi: 10.1016/j.athoracsur.2009.05.053 [DOI] [PubMed] [Google Scholar]
- 14.Di Dedda U, Pelissero G, Agnelli B, De Vincentiis C, Castelvecchio S, Ranucci M. Accuracy, calibration and clinical performance of the new EuroSCORE II risk stratification system. Eur J Cardiothorac Surg. 2013;43:27–32. doi: 10.1093/ejcts/ezs196 [DOI] [PubMed] [Google Scholar]
- 15.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. [DOI] [PubMed] [Google Scholar]
- 16.Goodman RA, Posner SF, Huang ES, Parekh AK, Koh HK. Defining and measuring chronic conditions: imperatives for research, policy, program, and practice. Prev Chronic Dis. 2013;10:120239. doi: 10.5888/pcd10.120239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal S, Tuzcu EM, Desai MY, Smedira N, Lever HM, Lytle BW, Kapadia SR. Updated meta-analysis of septal alcohol ablation versus myectomy for hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;55:823–834. doi: 10.1016/j.jacc.2009.09.047 [DOI] [PubMed] [Google Scholar]
- 18.Vriesendorp PA, Liebregts M, Steggerda RC, Schinkel AF, Willems R, Ten Cate FJ, van Cleemput J, Ten Berg JM, Michels M. Long-term outcomes after medical and invasive treatment in patients with hypertrophic cardiomyopathy. JACC Heart Fail. 2014;2:630–636. doi: 10.1016/j.jchf.2014.06.012 [DOI] [PubMed] [Google Scholar]
- 19.Liebregts M, Vriesendorp PA, Mahmoodi BK, Schinkel AF, Michels M, ten Berg JM. A systematic review and meta-analysis of long-term outcomes after septal reduction therapy in patients with hypertrophic cardiomyopathy. JACC Heart Fail. 2015;3:896–905. doi: 10.1016/j.jchf.2015.06.011 [DOI] [PubMed] [Google Scholar]
- 20.Kim LK, Swaminathan RV, Looser P, Minutello RM, Wong SC, Bergman G, Naidu SS, Gade CL, Charitakis K, Singh HS, Feldman DN. Hospital volume outcomes after septal myectomy and alcohol septal ablation for treatment of obstructive hypertrophic cardiomyopathy: US nationwide inpatient database, 2003–2011. JAMA Cardiol. 2016;1:324–332. doi: 10.1001/jamacardio.2016.0252 [DOI] [PubMed] [Google Scholar]
- 21.Kuhn H, Lawrenz T, Lieder F, Leuner C, Strunk-Mueller C, Obergassel L, Bartelsmeier M, Stellbrink C. Survival after transcoronary ablation of septal hypertrophy in hypertrophic obstructive cardiomyopathy (TASH): a 10 year experience. Clin Res Cardiol. 2008;97:234–243. doi: 10.1007/s00392-007-0616-7 [DOI] [PubMed] [Google Scholar]
- 22.Cuoco FA, Spencer WH III, Fernandes VL, Nielsen CD, Nagueh S, Sturdivant JL, Leman RB, Wharton JM, Gold MR. Implantable cardioverter-defibrillator therapy for primary prevention of sudden death after alcohol septal ablation of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2008;52:1718–1723. doi: 10.1016/j.jacc.2008.07.061 [DOI] [PubMed] [Google Scholar]
- 23.Jensen MK, Prinz C, Horstkotte D, van Buuren F, Bitter T, Faber L, Bundgaard H. Alcohol septal ablation in patients with hypertrophic obstructive cardiomyopathy: low incidence of sudden cardiac death and reduced risk profile. Heart. 2013;99:1012–1017. doi: 10.1136/heartjnl-2012-303339 [DOI] [PubMed] [Google Scholar]
- 24.Leonardi RA, Kransdorf EP, Simel DL, Wang A. Meta-analyses of septal reduction therapies for obstructive hypertrophic cardiomyopathy: comparative rates of overall mortality and sudden cardiac death after treatment. Circ Cardiovasc Interv. 2010;3:97–104. doi: 10.1161/CIRCINTERVENTIONS.109.916676 [DOI] [PubMed] [Google Scholar]
- 25.Liebregts M, Vriesendorp PA, Ten Berg JM. Alcohol septal ablation for obstructive hypertrophic cardiomyopathy: a word of endorsement. J Am Coll Cardiol. 2017;70:481–488. doi: 10.1016/j.jacc.2017.02.080 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


