Abstract
Infectious arthritis is difficult to treat in both human and veterinary clinical practice. Recent literature reports Staphylococcus aureus as well as other gram-positive and gram-negative isolates forming free-floating biofilms in both human and equine synovial fluid that are tolerant to traditional antimicrobial therapy. Using an in vitro equine model, we investigated the ability of platelet-rich plasma (PRP) formulations to combat synovial fluid biofilm aggregates. Synovial fluid was infected, and biofilm aggregates allowed to form over a 2-hour period. PRP was collected and processed into different formulations by platelet concentration, leukocyte presence, and activation or lysis. Infected synovial fluid was treated with different PRP formulations with or without aminoglycoside cotreatment. Bacterial load (colony-forming unit/mL) was determined by serial dilutions and plate counting at 8 hours posttreatment. All PRP formulations displayed antimicrobial properties; however, formulations containing higher concentrations of platelets without leukocytes had increased antimicrobial activity. Lysis of PRP and pooling of the PRP lysate (PRP-L) from multiple horses as compared to individual horses further increased antimicrobial activity. This activity was lost with the removal of the plasma component or inhibition of the proteolytic activity within the plasma. Fractionation of pooled PRP-L identified the bioactive components to be cationic and low-molecular weight (<10 kDa). Overall, PRP-L exhibited synergism with amikacin against aminoglycoside tolerant biofilm aggregates with greater activity against gram-positive bacteria. In conclusion, the use of PRP-L has the potential to augment current antimicrobial treatment regimens which could lead to a decrease in morbidity and mortality associated with infectious arthritis.
Keywords: biofilms, equine model, infectious arthritis, platelet-rich plasma, Staphylococcus aureus
1 |. INTRODUCTION
Infectious, or septic, arthritis, caused by the invasion of a microorganism into a joint, is a potentially life-threatening condition in human and veterinary clinical practice.1,2 Rapid diagnosis and prompt treatment in the form of systemic and local antimicrobial therapy combined with surgical debridement and lavage of the joint are currently the most effective means of resolving infection.3 However, despite this aggressive treatment, the infection can persist, resulting in systemic illness, and/or permanent damage to the joint in the form of degenerative joint disease.1,3
Staphylococcus aureus is the most common bacterial isolate in infectious arthritis and periprosthetic joint infection (PJI) and is also associated with the highest treatment failure rates.3–5 Mortality rates for adults diagnosed with infectious arthritis are 6% to 15%.4,6 Of those that survive, greater than 30% will suffer long-term consequences of joint damage such as persistent pain and impaired mobility.3,4 For patients diagnosed with PJI, a two-stage exchange arthroplasty is often recommended, but results remain unpredictable with 5-year mortality rates as high as 21%.5,7 S. aureus is more commonly associated with chronic PJI cases and results in treatment failure rates as high as 72%.7,8 An explanation for the high treatment failure rates is linked to the ability of Staphylococci to form robust biofilms.9 Traditional biofilms are defined as a community of bacteria encased in a polymeric matrix attached to an abiotic or biotic surface.10 Recently researchers have described unattached biofilms in cystic fibrosis, otitis media, and within wounds that form free-floating biofilm aggregates not requiring surface attachment.11 S. aureus and Staphylococcus epidermidis form free-floating bacterial biofilms when grown in human and bovine synovial fluid.12–14 Additionally, bacteria within these biofilms have been shown to be tolerant to treatment with cefazolin and vancomycin.12,13
We have recently expanded upon that body of literature to show the formation of biofilm aggregates in equine synovial fluid from healthy horses as a surrogate to the more difficult to source synovial fluid from healthy human individuals.15 We proved that S. aureus forms biofilm aggregates in equine synovial fluid that are very similar to those formed in human synovial fluid in vitro. We also showed that the most frequent non-Staphylococcal species associated with infectious arthritis, Streptococcus zooepidemicus, Escherichia coli, and Pseudomonas aeruginosa, form biofilm aggregates in synovial fluid as well.15 Importantly, we then demonstrated that biofilm aggregate formation offers significant protection from a variety of antimicrobial agents even when used at high concentrations in both equine and human synovial fluid.15 Consequently, the horse is a valuable model to study the development of antimicrobial tolerance in synovial fluid biofilms.
Biofilms occur in approximately 65% of all human infections accounting for disease in over 12 million individuals/year, death in greater than 400 000 individuals/year and an economic burden of $1 billion/year in the United States.16,17 For this reason, it is imperative to understand the host-pathogen interactions associated with infectious arthritis and to explore alternative therapies to combat biofilms. Due to the high treatment failure rates of biofilm-related infections despite aggressive antimicrobial therapy, we set out to explore alternative therapies that could be antibiofilm or serve as adjunctive therapy to the traditional antimicrobial regime.
Platelet-rich plasma (PRP) is an autologous biologic made from a patient’s whole blood that is most commonly used in regenerative medicine to treat musculoskeletal conditions such as posttraumatic osteoarthritis, tendon and ligament injuries.18 Recent reports using in vitro conditions suggest that PRP also has antimicrobial properties.19–21 Platelets are important players in innate immunity and, when activated, degranulate and release low-molecular weight, cationic antimicrobial peptides (AMPs), such as defensins.22 Platelets have also been shown to be antibacterial against S. aureus biofilms in vitro.23 Most recently, PRP lysate (PRP-L) has been gaining traction as an alternative to PRP.24 PRP-L has distinct advantages over PRP as it is acellular, making allogeneic use an option, and rich in platelet bioactive factors.25 For antimicrobial purposes, removal of the cellular component could also mitigate the ability of bacteria such as S. aureus to exploit platelets as a virulence factor.26 In addition, PRP-L can be pooled from multiple healthy donors to decrease variability in the bioactive factors released by lysis.27–29
In this set of in vitro investigations, we explored the antimicrobial activity of different formulations of equine PRP alone and in combination with conventional antimicrobials against bacteria grown in equine synovial fluid. We hypothesized that PRP-L would display superior antimicrobial properties over PRP and that pooling PRP-L amongst multiple individuals would create a potent antimicrobial biologic to be used against biofilm aggregates that form in synovial fluid.
2 |. METHODS
2.1 |. Animals
Healthy horses from our closed research herd at North Carolina State University College of Veterinary Medicine (n = 8) free of systemic and orthopedic disease and not on any medications were used for synovial fluid and blood collection. These horses are regularly evaluated by veterinarians and have full physical examinations and lameness examinations performed monthly as well as complete blood counts, chemistry panels, and fecal egg counts performed every 6 months. They included four geldings (three Thoroughbreds, one Appendix Quarter Horse) and four nonparous mares (all Thoroughbreds) between the ages of 6 and 19 years. The Institutional Animal Care and Use Committee of North Carolina State University (16-189-O) approved the use of horses in these studies.
2.2 |. Bacterial strains and growth conditions
The laboratory strain of S. aureus, ATCC 25923, was used to initially screen the different PRP formulations. Significant findings were then validated using clinical isolates derived from cases of equine septic arthritis collected by the Clinical Microbiology Laboratory at the University of Pennsylvania School of Veterinary Medicine New Bolton Center and processed in accordance with their Standard Operating Procedures. In vitro antimicrobial susceptibility testing and microbial identification was performed using the ARIS Sensititre system, using the equine (EQUIN1F) antimicrobial susceptibility panel for veterinary organisms. Bacteria were saved in frozen stocks on glycerol at −80°C. Blood agar plates were streaked from frozen stocks and used for in vitro experiments for a maximum of 1 month. Overnight cultures were made from the blood agar plates by taking one colony and adding it to 30 mL of tryptic soy broth (TSB); these cultures were made fresh for each experiment. On the day of an experiment, 100 μL of an overnight culture was inoculated into 10 mL of fresh TSB and grown to 0.5 McFarland (~3 hours) to ensure the bacteria is in the exponential phase of growth. Concentrations of cultures were confirmed using serial plate dilutions.
2.3 |. Synovial fluid collection
Both carpi were clipped and aseptically prepared along the dorsal aspect of the radiocarpal and intercarpal joints. A total of 3 to 4 mL of synovial fluid was extracted from each joint. Synovial fluid from both the right and left carpi was pooled among each individual horse. Synovial fluid that was visually cloudy or contaminated with blood was discarded. Synovial fluid was centrifuged at 1500g for 15 minutes to remove the cellular component and passed through a 40 μM cell strainer to remove any large protein aggregates. The samples were stored at −20°C until use in the described experiments.
2.4 |. PRP formulations
Whole blood was collected from horses (n = 6 to 8) via jugular venipuncture into 60 mL syringes containing 6 mL of acid citrate dextrose. A minimum of six horses was needed to achieve a power of 0.80 as calculated with a significance level of 0.05 using our preliminary data. Horses were fasted approximately 8 hours overnight prior to collection in order to reduce glucose concentrations in the blood, which could potentially affect bacterial growth. All processing steps, including centrifugations, were performed at room temperature. Whole blood in syringes was left undisturbed for 30 minutes to allow erythrocytes to settle. Thereafter, the layer above the erythrocytes containing the leukocytes, platelets and plasma, also called leukocyte-rich PRP or L-PRP, was gently transferred to a 50 mL conical tube. The authors would like to note that this method is only applicable to horses whose erythrocytes will settle without centrifugation; other species will require an additional centrifugation step to remove the erythrocytes. The L-PRP was centrifuged at 250g for 15 minutes to generate 1× PRP which was the layer containing platelets and plasma above the leukocyte pellet. The 1× PRP was transferred to a new 50 mL conical tube and centrifuged at 1500g for 15 minutes to pellet the majority of platelets. The supernatant above the platelet-pellet, or platelet-poor plasma (PPP), was stored. The remaining platelet-pellet was then resuspended at varying concentrations of PPP to generate higher concentrations of PRP (2×, 4×, 10×, and 50×). For example, if 50 mL of 1× PRP was centrifuged, the platelet-pellet was resuspended in 25 mL of PPP to generate 2× PRP, 12.5 mL of PPP to generate 4× PRP, 5 mL of PPP to generate 10× PRP, and 1 mL of PPP to generate 50× PRP. Leukocyte, erythrocyte and platelet concentrations in each formulation were determined by staining platelets with 1 μM Calcein-AM (Invitrogen Molecular Probes; Thermo Fisher Scientific, Waltham, MA), incubating for 20 minutes, and then counting the number of fluorescent cells using a Cellometer Auto 2000 (Nexcelom Bioscience LLC, Lawrence, MA). White blood cell (WBC) counts in PPP and PRP samples were determined using a Cellometer Auto 2000 and ViaStain AOPI Staining Solution (Nexcelom Bioscience LLC). PPP samples were defined as containing less than 10 000 platelets/μL and 10 WBC/μL. 1× PRP samples were defined as containing approximately 350 000 platelet/μL and less than 200 WBC/μL. L-PRP contained the same amount of platelets as 1×PRP but with greater than 10 000 WBC/μL. To generate activated PRP (A-PRP) and PRP-L, concentrated PRP was activated with 22 mM CaCl2 for 1 hour or subjected to five freeze/thaw cycles at −80°C for 1 hour followed by 37°C for 15 minutes. The majority of cell debris was removed from the A-PRP and PRP-L by centrifugation at 20 000g for 20 minutes at room temperature. To generate formulations of PRP-L with different concentrations of plasma, dilutions of plasma were made with sterile phosphate-buffered saline (PBS) and platelets were resuspended in each dilution prior to lysis (ie, 10% plasma was one part plasma to nine parts PBS). Similarly, for formulations made with proteinase inhibitors or heat-inactivated plasma, plasma was treated and platelets were resuspended in the respective plasma prior to lysis. As a common first step in protein fractionation, ion-exchange chromatography was performed. The capture of charged components was performed by incubation with cation and anion exchange resins, UNOsphere S and Q resin (Bio-Rad Laboratories, Hercules, CA), respectively, in equilibrium binding mode. Further fractionation by molecular weight was performed with molecular weight cutoff filters (Amicon Ultra 15 mL Centrifugal Filters, 50 and 10 kDa; Millipore Sigma, Burlington, MA).
2.5 |. Experimental design
Synovial fluid pooled from three horses (n = 3) was infected at 1 × 105 colony-forming unit (CFU)/mL for S. aureus (ATCC 25923) or for each clinical isolate (S. aureus, S. zooepidemicus, P. aeruginosa, and E. coli) and incubated for 2 hours at 37°C in a microaerophilic chamber on a shaker at 120 rpm to allow for biofilm aggregate formation. Infected synovial fluid was treated with the aminoglycoside amikacin at 40 μg/mL (10× minimum inhibitory concentration, or MIC, for all pathogens in this study and within the pharmacokinetic range for this antimicrobial) and/or designated PRP treatments under the same growth conditions as during the infective period for 8 hours. Amikacin was chosen as all isolates used in this study were susceptible based on microbroth dilution. Amikacin is also the most commonly used intra-articular antimicrobial in clinical equine practice1 and would be the antimicrobial of choice for a future in vivo study. After treatment, the infected and/or treated synovial fluid was centrifuged at 8000g for 5 minutes and the supernatant was removed. The bacterial pellet was washed three times with PBS and resuspended in 1 mL of PBS with 20 μg/mL proteinase K to disperse any aggregated bacteria. Bacterial load was measured using serial dilutions and plate counting of CFU/mL. The PRP formulation that resulted in the lowest CFU/mL in each experiment was carried into the next experiment.
2.6 |. Statistical analysis
Data were analyzed using a one-way analysis of covariance (ANCOVA), with the individual horse as the covariate, with Tukey’s post hoc test for all experiments evaluating S. aureus. Data were analyzed using a two-way ANCOVA, with bacterial isolate as the covariate, with Tukey’s post hoc test for all experiments evaluating multiple isolates. An unpaired t test was performed to evaluate the antibiofilm activity of pooled versus individual PRP-L. Correlations were calculated using the Spearman correlation coefficient. The analysis was performed using JMP Pro 11.0 software (SAS Institute Inc, Cary, NC). For all comparisons, P < .05 was considered statistically significant. All graphs were generated using GraphPad Prism (GraphPad Software Inc; La Jolla, CA).
3 |. RESULTS
3.1 |. PRP displays platelet-dependent antibiofilm activity against biofilm aggregates in synovial fluid
We set out to determine whether PRP combats free-floating S. aureus biofilms that form in synovial fluid. We used a double centrifugation method to isolate L-PRP, PRP, and PPP. All three formulations displayed antimicrobial properties compared to the untreated control (P < .05); however, L-PRP and PRP were more active than PPP (P < .01) and PRP was more active than L-PRP (P < .002) (Figure 1A). A negative correlation was found between platelet concentration and bacterial load (P < .004), wherein higher platelet concentrations resulted in lower bacterial loads. Additionally, no correlation was found between leukocyte concentration and bacterial load, suggesting that the antibiofilm activity was platelet-dependent not leukocyte dependent. To further investigate this, we concentrated PRP 2×, 4×, 10×, and 50× by pelleting the platelets in the PRP formulation presented in Figure 1A and suspending the platelets in less plasma. We again found a negative correlation between platelet concentration and bacterial load (P < .003) with a dose-dependent response (Figure 1B). Therefore, we concluded that the antibiofilm properties were dependent on platelets and not on leukocytes, and resolved to use 50× PRP in all following experiments.
FIGURE 1.
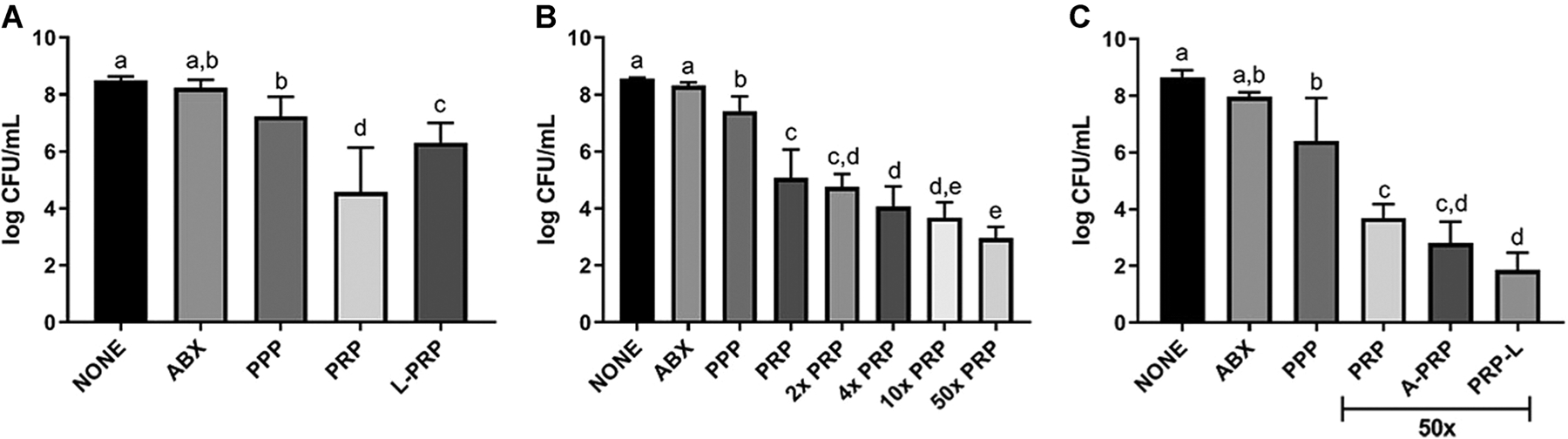
Antibiofilm properties of platelet-rich plasma (PRP) formulations against Staphylococcus aureus synovial fluid biofilms. Synovial fluid was infected with S. aureus ATCC 25923. The untreated control (NONE) was treated vol/vol with phosphate-buffered saline (PBS) while the antimicrobial control (ABX) was treated with 40 μg/mL or 10× MIC amikacin in the same volume of PBS. A, Infected synovial fluid was treated with platelet-poor plasma (PPP), leukocyte-reduced platelet-rich plasma (PRP) or leukocyte-rich platelet-rich plasma (L-PRP). B, Infected synovial fluid was treated with varying concentrations (1×, 2×, 4×, 10×, and 50×) of PRP. C, 50× PRP was activated with 20 mM CaCl2 for 1 hour (A-PRP) or lysed by five consecutive freeze/thaw cycles (PRP-L) before centrifugation to remove any cellular debris. Platelet formulations were generated from individual horses (n = 8). Bars are means and standard deviations. Differing letters indicate statistical significance of P < .05
3.2 |. Activation and lysis of PRP increases antibiofilm activity by increasing the release of platelet-derived proteins
We hypothesized that activation and/or lysis of PRP release bioactive factors present in the platelets, thereby increasing the antibiofilm properties of the 50× concentrated PRP formulation we identified in Figure 1B. We, therefore, treated this formulation with 20 mM CaCl2, incubated the PRP for 1 hour at 37°C to activate platelets and cause clot formation, and collected the supernatant after centrifugation (A-PRP). For lysis, we subjected the concentrated PRP to five freeze/thaw cycles and removed the lysed cellular debris by centrifugation (PRP-L). Only PRP-L displayed increased antibiofilm properties compared to PRP (P < .008) (Figure 1C).
3.3 |. PRP-L is more active against biofilm aggregates formed in synovial fluid by gram-positive than gram-negative organisms
We further explored whether PRP-L combats other bacterial species that form biofilm aggregates in synovial fluid besides S. aureus. To this end, we infected synovial fluid with the following isolates collected from clinical cases of equine infectious arthritis: S. aureus, S. zooepidemicus, E. coli, and P. aeruginosa. Each isolate was grown as biofilm aggregates in synovial fluid (same method as Figure 1) and subsequently treated with PPP, PRP, and PRP-L.PRP-L showed antibiofilm properties and maintained greater activity than PRP and PPP against all isolates evaluated (P < .001) (Figure 2). However, all platelet formulations were less effective against gram-negative strains compared to gram-positive strains (P < .003).
FIGURE 2.
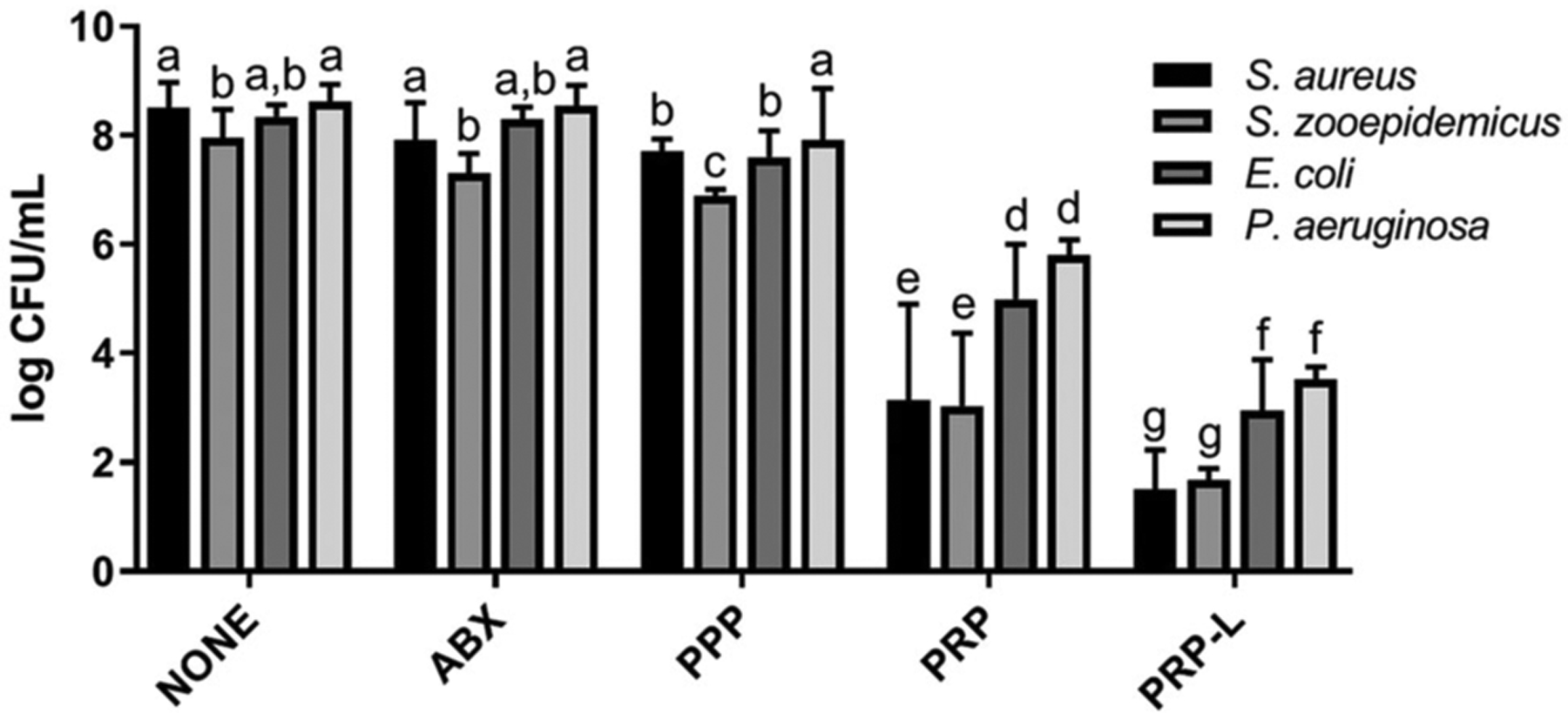
Efficacy of PRP and PRP-L against gram-positive and gram-negative clinical isolates that form biofilms in synovial fluid. Four clinical isolates from equine infectious arthritis cases were used to infect synovial fluid. Treatments were as follows: PBS (untreated control; NONE), antimicrobial treatment with amikacin at 40 μg/mL or 10× MIC (ABX), platelet-poor plasma (PPP), 50× platelet-rich plasma (PRP) or 50× platelet-rich plasma lysate (PRP-L). Platelet formulations were generated from individual horses (n = 6). Bars are means and standard deviations. Differing letters indicate statistical significance of P < .05. PBS, phosphate-buffered saline
3.4 |. PRP-L increases the sensitivity of previously antimicrobial tolerant biofilm aggregates to aminoglycosides
We tested the ability of PRP-L to help restore the activity of aminoglycosides against tolerant biofilm aggregates in synovial fluid. PRP-L displayed synergism with the aminoglycoside amikacin against biofilm aggregates formed by the laboratory S. aureus strain, ATCC 25923 (P < .0001) (Figure 3A). In addition, PRP-L showed a synergistic effect with amikacin against all four clinical isolates (P < .0001) (Figure 3B). However, as seen in Figure 2, gram-positive organisms were more susceptible to the combination of PRP-L and amikacin than gram-negative organisms (P < .0003).
FIGURE 3.

Synergism of PRP-L with aminoglycosides against antimicrobial tolerant synovial fluid biofilms. Synovial fluid infected with S. aureus ATCC 25923 (A) or the four clinical isolates shown in Figure 2 (B) left untreated (NONE) or treated with 50× platelet-rich plasma lysate (PRP-L) with or without the addition of 40 μg/mL or 10× MIC amikacin (ABX). Platelet formulations were generated from individual horses (n = 6). Bars are means and standard deviations. Differing letters indicate statistical significance of P < .05
3.5 |. Observed antibiofilm properties of PRP are dependent upon a synergism between platelets and plasma
We wanted to determine if the isolated plasma component was necessary for the antimicrobial activity of PRP and PRP-L. We challenged bacteria in synovial fluid with platelets in plasma (PRP) or platelets in buffer (PLT) and platelets lysed in plasma (PRP-L) or platelet lysed in buffer (PLT-L). Both PLT and PLT-L were antimicrobial compared to PPP, amikacin treatment, or untreated biofilm aggregates (P < .001) (Figure 4A). However, PRP and PRP-L were more active than PLT and PLT-L (P < .002 and P < .001, respectively). We then measured the effect of different ratios of plasma and platelets within the PRP-L formulation and found that 10% plasma was more efficacious compared to 100% or 0% plasma (P < .0001; Figure 4B). We concluded that the combination of platelets and plasma during platelet lysis is critical to achieve antibiofilm activity and used 10% plasma in all following preparations.
FIGURE 4.
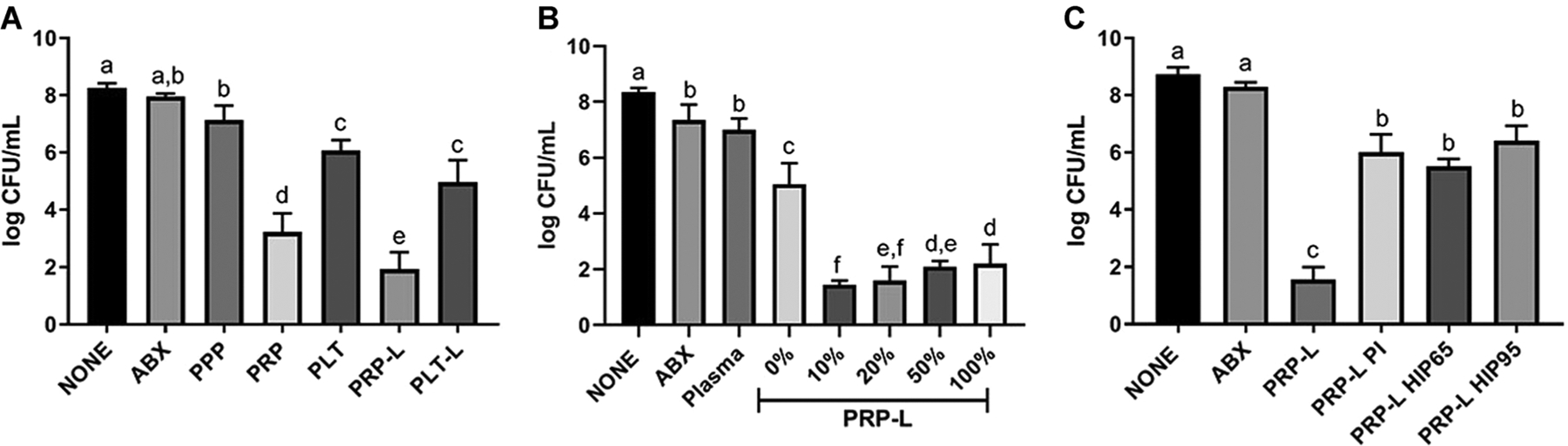
Antibiofilm effects of PRP-L are dependent upon proteolytic activity within the plasma. Synovial fluid infection with S. aureus ATCC 25923 and left untreated (NONE) or treated with 10× MIC amikacin (ABX) and platelet-poor plasma (PPP). A, Platelets from 50× PRP were washed, suspended in PBS at equivalent volume (PLT) and subsequently lysed by five consecutive freeze-thaw cycles (PLT-L). These formulations were used alongside PRP and PRP-L at the same 50× platelet concentration. B, PRP-L was generated in different percentages of plasma as indicated by the percent (%) following PRP-L. C, PRP-L was compared to PRP treated with proteinase inhibitors prior to lysis (PRP-L PI) and to PLT combined with plasma that was heat-inactivated at either 65°C or 95°C for 30 minutes prior to lysis (PRP-L HIP65 or PRP-L HIP95, respectively). Platelet formulations were generated from individual horses (n = 6). Bars are means and standard deviations. Differing letters indicate statistical significance of P < .05. PBS, phosphate-buffered saline; PLT, platelets in buffer; PLT-L, platelet lysed in buffer; PRP, platelet-rich plasma; PRP-L, PRP lysate
3.6 |. Proteolytic activity in plasma during lysis is important to the antibiofilm activity of PRP-L
Most AMPs are derived from proteolytic cleavage from larger precursors30; for example, LL-37 is processed by proteinase 3 from the larger cathelicidin (hCAP-18).31 In order to determine if the proteolytic activity was important to the activity of PRP-L, we added protease inhibitors to PRP prior to platelet lysis (Figure 4C). The PRP-L that was treated with protease inhibitors (PRP-L PI) lost significant antibiofilm activity compared to untreated PRP-L (P < .0001). In order to determine if the proteolytic activity was plasma-derived, we heat-inactivated plasma at 65°C or 95°C prior to platelet lysis within the plasma. We found that heat inactivation of plasma decreased the antibiofilm effects of PRP-L (Figure 4C), and concluded that the proteolytic activity in plasma is also a crucial component of the antibiofilm activity of PRP-L.
3.7 |. PRP-L pooled from multiple individuals decreases variability and increases the antimicrobial potency
The use of pooled PRP-L (pPRP-L) is a common practice in the development of a serum substitute for the culture of stem cells to reduce the variability observed in individual donors.32 Therefore, we set out to determine if pPRP-L would be more efficacious than PRP-L from individual horses. pPRP-L was generated from 8 horses pooled on four separate occasions and decreased bacterial load to a greater extent than PRP-L from individual horses (P < .03) (Figure 5). These findings led to the use of pPRP-L in all subsequent assays.
FIGURE 5.

Increased antibiofilm activity of pooled versus individual PRP-L. Synovial fluid was infected with S. aureus ATCC 25923 and treated with 50× PRP-L generated from individual horses (n = 8, INDIVIDUAL) or PRP-L pooled amongst the same eight individual horses on four separate collection occasions (n = 4, POOLED). Bars are means and standard deviations. Differing letters indicate statistical significance of P < .05. PRP, platelet-rich plasma; PRP-L, PRP lysate
3.8 |. The bioactive components of pPRP-L are cationic and low-molecular weight
We hypothesized that the bioactivity of PRP-L was due to AMPs which are small (<10 kDa) and cationic in nature.22 In order to test this hypothesis, we fractionated pPRP-L into its anionic and cationic components using ion exchange resin and tested each fraction for antibiofilm properties. We found that the cationic fraction of pPRP-L was significantly more active against synovial fluid biofilms than the anionic fraction (P < .0001); the cationic-rich fraction was also more active when compared to the parent pPRP-L (P < .008) (Figure 6). We then used centrifugation filters (50 and 10 kDa) in sequential order to divide the cationic pPRP-L components into high, medium and low-molecular-weight fractions. From there we determined that the <10 kDa or low-molecular-weight enriched fraction had similar antibiofilm activity than its parent cationic pPRP-L fraction (Figure 6).
FIGURE 6.

Crude protein fractionation of pooled PRP-L (pPRP-L) to determine the active antibiofilm components. Synovial fluid was infected with S. aureus ATCC 25923 and treated with 50× pPRP-L fractionated into its respective anionic and cationic components. The cationic fraction was subsequently passed consecutively through molecular weight filters (cutoffs 50 and 10 kDa). The retentate of each passage was collected and the flow-thru was placed on the next filter until 10 kDa from which the flow-thru was recovered. Bars are means and standard deviations. Differing letters indicate statistical significance of P < .05
3.9 |. The cationic, low-molecular-weight pPRP-L is antimicrobial and synergistic with aminoglycosides against both gram-negative and gram-positive species
Last, we wanted to explore whether the fractionated pPRP-L maintained its antibiofilm activity and synergism with the aminoglycoside amikacin against the clinical isolates that form biofilm aggregates in synovial fluid. We found that the cationic, low-molecular-weight pPRP-L has antibiofilm properties against all clinical isolates (P < .02) (Figure 7). However, when compared to PRP-L shown in Figure 2, pPRP-L was more active against gram-positive biofilm aggregates than gram-negative biofilm aggregates (P < .05). We also showed that the fractionated pPRP-L maintained synergism with amikacin (P < .03), albeit more so against gram-positive biofilm aggregates (P < .02) (Figure 7).
FIGURE 7.

Efficacy of fractionated cationic, low-molecular-weight pPRP-L and synergism with aminoglycosides against gram-positive and gram-negative synovial fluid biofilms. Synovial fluid was infected using the four clinical isolates from equine septic arthritis cases and left untreated (NONE) or treated with the cationic low-molecular-weight fractionated pooled 50× PRP-L (CAT, <10 kDa pPRP-L) alone or in combination with antimicrobial treatment with amikacin at 10× MIC (ABX). Bars are means and standard deviations. Differing letters indicate statistical significance of P < .05. PRP, platelet-rich plasma; pPRP-L, pooled PRP-L
4 |. DISCUSSION
S. aureus, as well as other gram-positive and gram-negative isolates, form free-floating biofilms in both human and equine synovial fluid that are tolerant to traditional antimicrobial therapy.12–15 In this study, we utilized an in vitro equine model to investigate the ability of different PRP formulations to combat biofilm aggregates that form in synovial fluid. We found that all PRP formulations tested herein displayed antimicrobial properties but to varying degrees. Formulations with higher concentrations of platelets without leukocytes displayed the highest antimicrobial activity. Activation or lysis of PRP to release soluble bioactive factors and remove the platelet cell structures increased the antibiofilm activity. Pooling PRP-L from multiple horses further increased the antibiofilm activity as compared to PRP-L from individual horses. We discovered that antibiofilm activity was lost when the plasma component was removed or proteolytic activity within the plasma was inhibited. Additionally, we found that the bioactive components within PRP-L were cationic and low-molecular weight (<10 kDa). Overall, our results suggest that PRP-L could be a promising therapeutic avenue to combat biofilms that form in synovial fluid.
To date, the utility of PRP has mostly been focused on its regenerative and antiinflammatory capacities.18 However, platelets can interact with the host immune response, recognize and respond to bacterial pathogens, and, when activated, release a large number of bioactive factors including those with antimicrobial properties.33 Recently, studies have investigated the potential antimicrobial properties of PRP in vitro.20,21 These studies have collectively observed a bacteriostatic to the bactericidal effect of PRP against a variety of bacterial pathogens including S. aureus. Within those studies, several formulations have been investigated that differ in the presence or absence of leukocytes and the concentrations of platelets. Our investigations provide evidence that PRP can successfully combat free-floating S. aureus biofilm aggregates that form in synovial fluid. We show that the antibiofilm effects of PRP are platelet-dependent and do not rely upon the presence of leukocytes. We also showed a dose dependency, wherein higher concentrations of platelets in PRP correlated with increased antimicrobial activity.
Reports describing the antimicrobial activity of PRP have used both PRP and PRP gels to combat bacteria.19,34–36 These gels, also called platelet-rich fibrin, are processed by activating PRP with thrombin or calcium chloride resulting in an increased release of platelet-derived bioactive factors.37 Lysis is a similar method to increase platelet factor release without the formation of a fibrin clot and is used commonly as an additive for stem cell culture.32 Both activated and lysed PRP have been reported to have increased antimicrobial activity.23,35 We observed similar findings during our investigations where activated or lysed PRP potentiate the antibiofilm effects of PRP against biofilm aggregates in synovial fluid. We speculate that this is due to the removal of the physical platelet structure which may deplete the ability of S. aureus to antagonize platelet antimicrobial functions while maintaining the platelet and plasma-derived antimicrobial factors. The interaction of S. aureus and platelets has been well described as a balance between pro-bacterial and antibacterial roles.38 For example, S. aureus can physically adhere to platelets during infective endocarditis to build vegetative lesions on cardiac valves.26 Therefore, removal of the cellular structure may prevent undesirable S. aureus interactions with platelets. Additionally, in our study, we found that lysed PRP had more activity than A-PRP. We speculate that this is due to the ability of lysis to release more bioactive factors from platelets compared to activation. In accordance with this speculation, researchers have shown that lysis results in higher growth factor release than activation.39 Additionally, another study showed that lysed PRP was more antimicrobial against planktonic bacteria than A-PRP.35 These findings are likely due to the fact that activation is often partial, leading to the release of some but not all of the platelet contents.
Several studies have described the antimicrobial nature of platelets in the absence of plasma.40,41 However, one report showed that plasma is a vital component to maintain antimicrobial activity of PRP.42 Therefore, we investigated the necessity of plasma for PRP and PRP-L to retain antibiofilm functions. We found that the combination of both plasma and platelets was necessary and that 10% plasma resulted in the best PRP formulation when compared to 0% or 100% plasma and platelets. These observations correlate with another report showing that 5% to 10% plasma was synergistic with platelets but 50% or greater plasma decreased platelet antimicrobial activity.43 We speculate that 10% maintains the concentration of proteases required to cleave platelet-derived proteins as described below while decreasing the amount of fibrinogen in the preparation. This is important, since fibrinogen can be used by S. aureus as an extracellular matrix protein to form biofilms.44
The use of pPRP-L is a common practice in the development of a serum substitute for the culture of stem cells to reduce the variability observed in individual donors.32 Our group recently reported on the ability of pPRP-L to protect chondrocytes and increase the production of hyaluronic acid by synoviocytes in vitro.45 Therefore, we investigated the difference in antimicrobial activity between PRP-L generated from individual horses vs PRP-L pooled from multiple individuals. Therein, we found that pPRP-L displayed greater antimicrobial potency than individual PRP-L. We also found that pooling PRP-L from multiple individuals decreased the variability in the antibiofilm effects seen from a single individual. Literature has shown that there is tremendous variability in the quality and biological potency of PRP based on the factors such as age or gender of the donor.27–29 For example, PRP from men has higher levels of interleukin-1 (IL-1) receptor antagonist protein, and PRP from older individuals possesses less insulin-like growth factor-1.28 It has even been shown that circadian rhythm can affect platelet function.46 These factors lead to uncontrollable differences in growth factor and cytokine concentrations within the PRP leading to large variations in regenerative capacity. Further supporting this, one study showed that pPRP-L was able to lower IL-1β release by inflamed monocytes while individual PRP-L had no effect.47 Undoubtedly, this biological variability impacts the antimicrobial activity of PRP-L as well; therefore, a pooled product is able to capitalize on variability, resulting in a product with more antibiofilm activity than an individual product.
Platelets release AMPs22 that are synergistic with traditional antimicrobial agents against resistant pathogens or bacteria within biofilms.48–50 We observed a synergistic effect of PRP and PRP-L with the aminoglycoside amikacin against synovial fluid biofilms. In addition, most AMPs are derived from proteolytic cleavage from larger precursors30; for example, LL-37 is processed by proteinase 3 from the larger cathelicidin (hCAP-18).31 In accordance with that literature, we found that the addition of protease inhibitors abrogated the antibiofilm function of PRP-L. Yeaman et al,41 was the first to describe small, cationic antimicrobial proteins derived from thrombin-stimulated rabbit platelets. Through the use of protein fractionation techniques, we determined that the bioactive components of pPRP-L were indeed within the fraction containing small (<10 kDa), cationic peptides. Future studies identifying the equine specific platelet-derived AMPs within this PRP-L fraction could lead to a novel therapeutic peptide for the treatment of biofilm infections.
In conclusion, the findings of this study demonstrate that PRP-L has potent antibiofilm properties and is synergistic with the aminoglycoside amikacin against synovial fluid biofilms. While we have previously reported on the chondroprotective effects of unfractionated 10× pPRP-L in vitro,45 further testing is needed to determine if these effects are maintained with the fractionated 50× pPRP-L. The next step is to utilize a large animal model of infectious arthritis in order to fully investigate the efficacy of fractionated 50× PRP-L in vivo with the presence of resident cells and immune cells that cannot be fully recapitulated in vitro. An animal model is also essential to study the antimicrobial activity of PRP-L against other infective reservoirs within the joint apart from free-floating biofilm aggregates such as synovial and cartilage surface biofilms and intracellular bacteria. Results derived from such a translational and clinically relevant animal model would support the continued development of these findings into novel therapeutic strategies for difficult to treat infectious arthritis and periprosthetic joint infection cases with an underlying biofilm infection. More importantly, our observations of restoring the activity of conventional antimicrobials such as amikacin, suggests that PRP-L could be a valuable therapeutic augmentation of existing antimicrobial regimes.
ACKNOWLEDGMENTS
This work was supported by the Raymond Firestone Trust (JMG and TPS), American Quarter Horse Foundation (JMG, TPS, and LVS), Grayson-Jockey Club Research Foundation, Inc (JMG, TPS, LVS), Morris Animal Foundation (JMG and LVS) and the Fund for Orthopedic Research in honor of Gus and Equine athletes (F.O.R.G.E; LVS). The contents of this manuscript are the subject of a provisional patent filed by North Carolina State University and the University of Pennsylvania by the authors (JMG, TPS, LVS). The authors would like to thank the North Carolina State University CVM Laboratory Animal Resources staff for their help with animal care and handling, Ms Anna Rogers in the Department of Population Health and Pathobiology at North Carolina State University CVM for her technical assistance with the microbiological assays and Dr Donna Kelly at the PADLS New Bolton Center Clinical Microbiology Laboratory, University of Pennsylvania School of Veterinary Medicine for her aid in collecting the clinical isolates.
Funding information
American Quarter Horse Foundation; Raymond Firestone Trust; Grayson-Jockey Club Research Foundation; Fund for Orthopedic Research in honor of Gus and Equine athletes (F.O.R.G.E); Morris Animal Foundation
REFERENCES
- 1.Morton AJ. Diagnosis and treatment of septic arthritis. Vet Clin North Am—quine Pract. 2005;21(3):627–649. [DOI] [PubMed] [Google Scholar]
- 2.Mathews CJ, Weston VC, Jones A, Field M, Coakley G. Bacterial septic arthritis in adults. Lancet. 2010;375(9717):846–855. [DOI] [PubMed] [Google Scholar]
- 3.Shirtliff ME, Mader JT. Acute septic arthritis. Clin Microbiol Rev. 2002; 15(4):527–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tong SYCC, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015; 28(3):603–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev. 2014; 27(2):302–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nair R, Schweizer ML, Singh N. Septic arthritis and prosthetic joint infections in older adults. Infect Dis Clin North Am. 2017;31(4):715–729. [DOI] [PubMed] [Google Scholar]
- 7.Cobo J, Miguel LGS, Euba G, et al. Early prosthetic joint infection: outcomes with debridement and implant retention followed by antibiotic therapy. Clin Microbiol Infect. 2011;17(11):1632–1637. [DOI] [PubMed] [Google Scholar]
- 8.Byren I, Bejon P, Atkins BL, et al. One hundred and twelve infected arthroplasties treated with “DAIR” (debridement, antibiotics and implant retention): antibiotic duration and outcome. J Antimicrob Chemother. 2009;63(6):1264–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otto M Staphylococcal biofilms. Curr Top Microbiol Immunol. 2008; 322:207–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall-Stoodley L, Stoodley P. Evolving concepts in biofilm infections. Cell Microbiol. 2009;11(7):1034–1043. [DOI] [PubMed] [Google Scholar]
- 11.Bjarnsholt T, Alhede M, Alhede M, et al. The in vivo biofilm. Trends Microbiol. 2013;21(9):466–474. [DOI] [PubMed] [Google Scholar]
- 12.Dastgheyb S, Parvizi J, Shapiro IM, Hickok NJ, Otto M. Effect of biofilms on recalcitrance of staphylococcal joint infection to antibiotic treatment. J Infect Dis. 2015;211(4):641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dastgheyb SS, Hammoud S, Ketonis C, et al. Staphylococcal persistence due to biofilm formation in synovial fluid containing prophylactic cefazolin. Antimicrob Agents Chemother. 2015;59(4):2122–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez K, Patel R. Biofilm-like aggregation of Staphylococcus epidermidis in synovial fluid. J Infect Dis. 2015;212(2):335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilbertie JM, Schnabel LV, Hickok NJ, et al. Equine or porcine synovial fluid as a novel ex vivo model for the study of bacterial free-floating biofilms that form in human joint infections. PLoS One. 2019; 14(8):e0221012. Al-Bakri A, ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoodley P, Ehrlich GD, Sedghizadeh PP, et al. Orthopaedic biofilm infections. Curr Orthop Pract. 2011;22(6):558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolcott RD, Ehrlich GD. Biofilms and chronic infections. JAMA. 2008; 299(22):2682. [DOI] [PubMed] [Google Scholar]
- 18.Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA. Platelet-rich plasma: a milieu of bioactive factors. Arthrosc—J Arthrosc Relat Surg. 2012;28(3):429–439. [DOI] [PubMed] [Google Scholar]
- 19.Moojen DJF, Everts PAM, Schure RM, et al. Antimicrobial activity of platelet-leukocyte gel against staphylococcus aureus. J Orthop Res. 2008;26(3):404–410. [DOI] [PubMed] [Google Scholar]
- 20.Lopez C, Carmona JU, Giraldo CE, Alvarez M. Bacteriostatic effect of equine pure platelet-rich plasma and other blood products against methicillin-sensitive Staphylococcus aureus. An in vitro study. Vet Comp Orthop Traumatol. 2014;27:372–378. [DOI] [PubMed] [Google Scholar]
- 21.López C, Álvarez ME, Carmona JU. Temporal bacteriostatic effect and growth factor loss in equine platelet components and plasma cultured with methicillin-sensitive and methicillin-resistant staphylococcus aureus: a comparative in vitro study. Vet Med Int. 2014;2014: 525826–525828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang Y-Q, Yeaman MR, Selsted ME. Antimicrobial peptides from human platelets. Infect Immun. 2002;70(12):6524–6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rózalski MI, Micota B, Sadowska B, Paszkiewicz M, Wiȩckowska-Szakiel M, Rózalska B. Antimicrobial/antibiofilm activity of expired blood platelets and their released products. Postepy Hig Med Dosw. 2013;67(545):321–325. [DOI] [PubMed] [Google Scholar]
- 24.Al-Ajlouni J, Awidi A, Samara O, et al. Safety and efficacy of autologous intra-articular platelet lysates in early and intermediate knee osteoarthrosis in humans. Clin J Sport Med. 2015;25(6):524–528. [DOI] [PubMed] [Google Scholar]
- 25.Tyrnenopoulou P, Diakakis N, Karayannopoulou M, Savvas I, Koliakos G. Evaluation of intra-articular injection of autologous platelet lysate (PL) in horses with osteoarthritis of the distal interphalangeal joint. Vet Q. 2016;36(2):56–62. [DOI] [PubMed] [Google Scholar]
- 26.Liesenborghs L, Verhamme P, Vanassche T. Staphylococcus aureus, master manipulator of the human hemostatic system. J Thromb Haemost. 2018;16(3):441–454. [DOI] [PubMed] [Google Scholar]
- 27.Giraldo CE, López C, Álvarez ME, Samudio IJ, Prades M, Carmona JU. Effects of the breed, sex and age on cellular content and growth factor release from equine pure-platelet rich plasma and pure-platelet rich gel. BMC Vet Res. 2013;9(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiong G, Lingampalli N, Koltsov JCB, et al. Men and women differ in the biochemical composition of platelet-rich plasma. Am J Sports Med. 2018;46(2):409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rinnovati R, Romagnoli N, Gentilini F, Lambertini C, Spadari A. The influence of environmental variables on platelet concentration in horse platelet-rich plasma. Acta Vet Scand. 2016;58(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang LJ, Gallo RL. Antimicrobial peptides. Curr Biol. 2016;26(1): R14–R19. [DOI] [PubMed] [Google Scholar]
- 31.Sørensen OE, Follin P, Johnsen AH, et al. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001;97(12): 3951–3959. [DOI] [PubMed] [Google Scholar]
- 32.Burnouf T, Strunk D, Koh MBC, Schallmoser K. Human platelet lysate: replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials. 2016;76:371–387. [DOI] [PubMed] [Google Scholar]
- 33.Yeaman MR. Platelets: at the nexus of antimicrobial defence. Nat Rev Microbiol. 2014;12(6):426–437. [DOI] [PubMed] [Google Scholar]
- 34.Li GY, Yin JM, Ding H, Jia WT, Zhang CQ. Efficacy of leukocyte- and platelet-rich plasma gel (L-PRP gel) in treating osteomyelitis in a rabbit model. J Orthop Res. 2013;31(6):949–956. [DOI] [PubMed] [Google Scholar]
- 35.Burnouf T, Chou ML, Wu YW, Su CY, Lee LW. Antimicrobial activity of platelet (PLT)-poor plasma, PLT-rich plasma, PLT gel, and solvent/detergent-treated PLT lysate biomaterials against wound bacteria. Transfusion. 2013;53(1):138–146. [DOI] [PubMed] [Google Scholar]
- 36.Bielecki TM, Gazdzik TS, Arendt J, Szczepanski T, Kròl W, Wielk-oszynski T. Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances: an in vitro study. J Bone Jt Surg Br. 2007;89-B(3):417–420. [DOI] [PubMed] [Google Scholar]
- 37.Textor JA, Tablin F. Activation of equine platelet-rich plasma: comparison of methods and characterization of equine autologous thrombin. Vet Surg. 2012;41(7):784–794. [DOI] [PubMed] [Google Scholar]
- 38.Yeaman MR. Bacterial-platelet interactions: virulence meets host defense. Future Microbiol. 2010;5(3):471–506. [DOI] [PubMed] [Google Scholar]
- 39.Etulain J, Mena HA, Meiss RP, et al. An optimised protocol for platelet-rich plasma preparation to improve its angiogenic and regenerative properties. Sci Rep. 2018;8(1):1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeaman MR, Norman DC, Bayer AS. Staphylococcus aureus susceptibility to thrombin-induced platelet microbicidal protein is independent of platelet adherence and aggregation in vitro. Infect Immun. 1992;60(6):2368–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeaman MR, Tang YIQ, Shen AJ, Bayer AS, Selsted ME. Purification and in vitro activities of rabbit platelet microbicidal proteins. Infect Immun. 1997;65(3):1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drago L, Bortolin M, Vassena C, Romanò CL, Taschieri S, Del Fabbro M. Plasma components and platelet activation are essential for the antimicrobial properties of autologous platelet-rich plasma: an in vitro study. PLoS One. 2014;9(9):e107813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trier DA, Gank KD, Kupferwasser D, et al. Platelet antistaphylococcal responses occur through P2X1 and P2Y12 receptor-induced activation and kinocidin release. Infect Immun. 2008;76(12):5706–5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zapotoczna M, O’Neill E, O’Gara JP. Untangling the diverse and redundant mechanisms of Staphylococcus aureus biofilm formation. PLoS Pathog. 2016;12(7):e1005671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gilbertie JM, Long JM, Schubert AG, Berglund AK, Schaer TP, Schnabel LV. Pooled platelet-rich plasma lysate therapy increases synoviocyte proliferation and hyaluronic acid production while protecting chondrocytes from synoviocyte-derived inflammatory mediators. Front Vet Sci. 2018;5:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scheer FAJL, Michelson AD, Frelinger AL, et al. The human endogenous circadian system causes greatest platelet activation during the biological morning independent of behaviors. PLoS One. 2011;6(9):e24549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naskou MC, Norton NA, Copland IB, Galipeau J, Peroni JF. Innate immune responses of equine monocytes cultured in equine platelet lysate. Vet Immunol Immunopathol. 2018;195:65–71. [DOI] [PubMed] [Google Scholar]
- 48.Dosler S, Mataraci E. In vitro pharmacokinetics of antimicrobial cationic peptides alone and in combination with antibiotics against methicillin resistant Staphylococcus aureus biofilms. Peptides. 2013; 49:53–58. [DOI] [PubMed] [Google Scholar]
- 49.Grassi L, Maisetta G, Esin S, Batoni G. Combination strategies to enhance the efficacy of antimicrobial peptides against bacterial biofilms. Front Microbiol. 2017;8(DEC):2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mercier R-C, Dietz RM, Mazzola JL, Bayer AS, Yeaman MR. Beneficial influence of platelets on antibiotic efficacy in an in vitro model of Staphylococcus aureus-induced endocarditis. Antimicrob Agents Chemother. 2004;48(7):2551–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]


