Abstract
Background
Coronavirus disease 2019 (COVID-19) causes thromboembolic complications during or post-infection period despite a lack of conventional risk factors. The study aims to learn fundamental changes in COVID-19 patients who underwent embolectomy in terms of clinical characteristics and clot composition.
Methods
In a retrospective cohort study design, we evaluated 21 patients who underwent embolectomy in our clinic between March 12, 2020, and December 31, 2020. Demographics, characteristics, and laboratory values were abstracted and analyzed. Histopathological assessment was held in the pathology department.
Results
Of these 21 patients, 11 (52.3%) were SARS-CoV-2 positive and 10 (47.6%) were SARS-CoV-2 negative. There is no statistical difference in terms of anatomic distribution, diagnostic method, length of hospital stay, amputation or mortality levels. Thromboembolic material of COVID-19 patients include significantly less red blood cell (RBC) (21.2–32.6%; P= 0.01), more lymphocyte (14.1–2.6%; P< 0.001), and more leukocyte (27.1–22.1%; P= 0.05). There was no statistical difference between the fibrin ratio.
Conclusions
Inflammatory cells are prominent in arterial thromboembolic material of COVID-19 patients. A combination of hyperinflammation and prothrombotic status may be responsible for this phenomenon.
Keywords: COVID-19, Thromboembolism, Embolectomy
Introduction
SARS-CoV-2 was recognized as a respiratory pathogen whose effects were primarily pulmonary in nature. As the pandemic evolved, other systemic influences have been widely experienced and described. Acute thromboembolic phenomena were pointed in the context of excessive inflammation, hypoxia, endothelial injury, immobilization, and platelet dysfunction at cardiovascular, cerebrovascular, and peripheral venous/arterial levels, affecting as high as 49% of patients and overshadowing the prognosis of the disease.1., 2., 3., 4., 5., 6., 7. Arterial thromboembolic complications may lead to devastating consequences as amputation, premature intubation, multiorgan dysfunction, stroke, and death.
The exact relationship between acute arterial thrombosis and COVID-19 remains unclear. The aim of this study is first, to describe demographic data, laboratory tests, and clinical characteristics of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) positive and negative patients with peripheral arterial thromboembolism. Second, we aimed to learn whether there is any difference in the histological composition of thromboembolic material between these groups. Our last aim is to seek any correlation between clot composition and severity of COVID-19.
Methods
Patients above 18 years of age who underwent embolectomy at Izmir Katip Celebi University Ataturk Research and Training Hospital from March 12, 2020, to December 31, 2020, were included. SARS-CoV-2 status was obtained from medical records. Polymerase chain reaction (PCR) from a nasopharyngeal swab was used for the diagnose of COVID-19. One person receives one test, the second test is performed if the clinical features and blood tests are highly suggestive of COVID-19. SARS-CoV-2 positive patients either admitted to the emergency ward with symptoms of arterial thromboembolism and tested for SARS-CoV-2 as a part of the preprocedural evaluation, or were already tested positive and had home quarantine as they had mild symptoms, and a third group was consulted to vascular surgery department due to suspicion of arterial thromboembolism during hospital follow-up. Patients with cardiac thrombus, myxoma, history of former or active neoplasia, and deep venous thrombosis were planned to exclude from the study but there were no patients with such characteristics according to the medical records.
Embolectomy was performed under general or local anesthesia as required. Arterial clamping took place 3 minutes after 80U/Kg systemic unfractionated heparin. An inguinal incision was preferred for lower extremity occlusion, femoral bifurcation was sought for optimal thrombectomy. Brachial exploration was performed for upper extremity occlusion. Thromboembolectomy was executed by using an embolectomy catheter of optimal size. Embolectomy material was sent to histopathologic evaluation as a part of the procedural routine. Of 21 patients, 20 had a successful surgery, which can be defined as complete removal of the blood clot and subsequent presence of adequate run-off and backflow. One patient in the COVID-19 group had tibiopedal thrombosis and very poor backflow was seen after clot removal.
Patients were divided into two groups according to SARS-Cov-2 test results. Patients who were tested positive had a range of COVID-19 symptomatology (mild, moderate, and severe) according to the previous description of the World Health Organization.8 The primary sources of patient data were the hospital database and archive of the pathology department. Retrospective chart review abstracted demographic variables including age, gender, body mass index (BMI), history of comorbidities, history of former or active smoking, and current use of antiaggregant or anticoagulant therapy. Additional variables assessed included laboratory values on the day of identification of acute thromboembolism such as full blood count, CRP, ferritin, LDH, procalcitonin, and d-dimer levels. Anatomic distribution, diagnostic method, length of hospital stay, amputation, and mortality numbers were also noted.
Histopathologic Analysis
The retrieved thrombus material was fixed in 10% neutralized buffered formalin immediately after retrieval. The formalin-fixed, paraffin-embedded thrombus was cut at 2-μm thickness. All thrombi were stained with hematoxylin-eosin (H&E). H&E is the most widely used histology stain and is considered the gold-standard for the diagnosis of many diseases and to date most studies analyzing clot composition.9., 10., 11. The slides with the stained specimens were scanned at x10 and × 40 with a BX51 device (Olympus, Tokyo, Japan) and digitally stored. The histopathological analysis was performed by an experienced pathologist and two investigators blinded to the clinical and interventional data. Quantification of fibrin, RBC, WBC, and lymphocyte was performed manually, relying on visual inspection. The histopathological assessment included organization, degree of the main composition, presence of calcifications, cholesterol crystals, and endothelial cells.
The Shapiro-Wilk test was used to analyze the homogeneity of the variables. The independent sample t-test was used to analyze group differences among demographic variables. The Mann-Whitney U test was used for nonhomogenous variables. Chi-square or Fisher exact analysis was used to analyze associations between categorical variables. Spearman correlation test was used for evaluating the relationship between clot composition and severity of COVID-19. Data analysis was performed using the SPSS 22.0 software package and Pvalues <0.05 were considered statistically significant.
This study was approved by the platform of scientific research of the Ministry of Health (Turkey) and the Institutional Review Board of Izmir Katip Celebi University with a waiver of informed consent.
Results
During the study period, our hospital, which is the largest research and training hospital in the Aegean region, cared for 3765 patients with COVID-19. Of the 21 patients who underwent embolectomy, 11 (52.3%) were SARS-CoV-2 positive and 10 (47.6%) were SARS-CoV-2 negative. If we consider the same calendar period (March–December) in 2019, the incidence rate of patients presenting with arterial thromboembolism in 2020 was significantly higher (12 vs. 21). In COVID-19 group, 4 (36.4%) had mild presentation, 4 (36.4%) had moderate presentation and 3 (27.3%) had severe presentation. SARS-CoV-2 positive patients who underwent embolectomy tended to be older (71.2 vs. 61.8 years; P= 0.15), and more frequently female (54.5% vs. 40%, P= 0.67), although these findings did not reach significance. SARS-CoV-2 positive patients were less frequently smokers (18.2% vs. 70%; P= 0.03). Two groups were relatively equal concerning other demographic variables such as BMI, comorbidities, and antiaggregant/anticoagulant therapy. No difference was detected regarding Rutherford classification (P= 0.36). In the COVID-19 group, 3 patients who were on anticoagulant therapy were receiving therapeutic-dose enoxaparin sodium as a part of the COVID-19 therapy algorithm of our hospital. The rest of the patients in this group were in home-quarantine before admission for limb ischemia. It is important to note that none of COVID-19 patients with arterial thromboembolism had atrial fibrillation, mechanical cardiac valve, or cardiac thrombus. In the SARS-CoV-2 negative group, among 4 patients receiving subtherapeutic dosages of warfarin, 2 of them had atrial fibrillation and 2 of them had a history of mechanical mitral valve replacement (Table I ).
Table I.
Demographic factors and clinical characteristics for patients with and without COVID-19
| Characteristics | No Covid-19 (N = 10) Mean ± SD /n(%)/min-max |
Covid-19 positive (N = 11) Mean ± SD /n(%)/min-max |
P value | |
|---|---|---|---|---|
| Age | 61.8 ± 15.51 (40–79) | 71.27 ± 13.99 (41–88) | 0.15 | |
| Sex | Female | 4 (40) | 6 (54.5) | 0.67 |
| Male | 6 (60) | 5 (44.5) | ||
| BMI | 24.9 ± 4.86 (18–32) | 26.54 ± 3.83 (22–32) | 0.39 | |
| History of HT | 6 (60) | 7 (63.6) | 0.86 | |
| History of DM | 2 (20) | 5 (45.5) | 0.21 | |
| History of HL | 2 (20) | 1 (9.1) | 0.58 | |
| History of AF | 2 (20) | 0 (0) | 0.21 | |
| History of CAD | 3 (30) | 3 (27.3) | 0.89 | |
| History of CHF | 3 (30) | 1 (9.1) | 0.31 | |
| History of PAD | 5 (50) | 3 (27.3) | 0.38 | |
| History of smoking | 7 (70) | 2 (18.2) | 0.03* | |
| History of using antiaggregant | 7 (70) | 4 (36.4) | 0.19 | |
| History of using anticoagulant | 4 (40) | 3 (27.3) | 0.65 | |
| Rutherford staging | I | 1 (10) | 0 | 0.36 |
| IIA | 9 (90) | 10 (90.9) | ||
| IIB | 0 | 1 (9.1) | ||
| III | 0 | 0 | ||
BMI, body mass index; HT, hypertension; DM, diabetes mellitus; HL, hyperlipidemia; AF, Atrial Fibrilation; CAD, coronary artery disease; CHF, congestive heart failure; PAD, pulmonar artery disease; SD, Standart Deviation.
p<0.05.
SARS-COV-2 positive patients had higher CRP, Ferritin, and LDH levels (P= 0.004, P= 0.003, P< 0.001*, respectively) There were no significant differences in WBC count, hemoglobin, lymphocyte count, neutrophil count, platelet count, and D-dimer. Although the COVID-19 positive patients trended toward higher procalcitonin levels, these differences did not reach statistical significance (Table II ).
Table II.
Laboratory values for patients with and without coronavirus disease COVID-19
| Variables | No Covid-19 (N = 10) Mean ± SD/Median (IQR)/min-max |
Covid-19 positive (N = 11) Mean ± SD/Median (IQR)/min-max |
P value |
|---|---|---|---|
| Wbc, k/ml | 10.25 ± 2.29 (6.40–14.7) | 11.4 ± 4.09 (5.40–17) | 0.44 |
| Hemoglobin, g/dL | 11.87 ± 2.22 (8.75–16) | 12.96 ± 1.63 (11–16) | 0.21 |
| Hematocrit, % | 35.7 ± 6.63 (26–46) | 38.63 ± 4.92 (32–47) | 0.26 |
| Lymphocyte count, k/mL | 1747.2 ± 812.9 (660–2800) | 1233.63 ± 404.25 (600–1900) | 0.09 |
| Neutrophil count, k/mL | 7768 ± 2813.3 (4300–13,500) | 9527.27 ± 3901.5 (4400–15,000) | 0.25 |
| Platelet Count, k/mL | 290 ± 91.87 (173–447) | 281.09 ± 83.7 (135–412) | 0.81 |
| CRP | 3.85 (3.55) (0–30) |
27 (93) (3.5–138) |
0.004* |
| Ferritin | 678.81 ± 488.95 (125–1450) | 91.3 ± 46.88 (8–155) | 0.003* |
| LDH | 61 (27.5) 40-130 |
275 (143) 128-420 |
<0.001* |
| Procalcitonin | 0.01 (0.31) (0–1.29) |
0.16 (0.73) (0.01–2.4) |
0.06 |
| D-Dimer | 565 (555) (200–1450) |
345 (415) (185–2900) |
0.48 |
Wbc, White blood cell; SD, standard deviation; IQR, Interquartile range.
p<0.05.
There was no significant difference between groups in terms of anatomic distribution of thrombi and length of hospital stay. Diagnosis of thromboembolism by physical examination was executed more frequently SARS-CoV-2 negative group (P= 0.02). Below-knee amputation was required in one patient who had a history of peripheral arterial disease and had his third embolectomy in the SARS-CoV-2 negative group. Femoral and popliteal pulses were palpable but anterior and tibial pulses were absent right after embolectomy despite medical therapy. The patient who underwent below-knee amputation in the COVID-19 group had tibiopedal thrombosis. No further intervention could be planned because of hemodynamic instability, unfortunately, he died 2 days after amputation. No significant difference was detected between the mortality rate of the 2 groups (P= 0.58; Table III ).
Table III.
Anatomic distribution, diagnostic details and outcomes for patients with acute arterial thrombosis
| Characteristics | No Covid-19 (N = 10) Mean ± SD /n(%)/min-max |
Covid-19 positive (N = 11) Mean ± SD /n(%)/min-max |
P value |
|---|---|---|---|
|
Anatomic distribution Aortoiliac |
2 (20) | 3 (27.3) | 0.69 |
| Femoropopliteal | 1 (10) | 2 (18.2) | 0.69 |
| Tibiopedal | 1 (10) | 1 (9.1) | 0.89 |
| Upper extremity | 2 (20) | 2 (18.2) | 0.85 |
| Aortoiliac+ Femoropopliteal | 0 (0) | 1 (9.1) | 0.92 |
| Femoropopliteal + Tibiopedal | 4 (40) | 2 (18.2) | 0.36 |
|
Diagnostic Method CT angiogram |
3 (30) | 5 (45.5) | 0.65 |
| Doppler USG | 1 (10) | 5 (45.5) | 0.14 |
| Physical Examination | 6 (60) | 1 (9.1) | 0.02* |
| Length of hospital stay | 14.5±9 (3-30) | 12.81±9.29 (3-30) | 0.67 |
| Amputation | 1 (10) | 1 (9.1) | 0.94 |
| Mortality | 2 (20) | 1 (9.1) | 0.58 |
p<0.05.
Histopathological Findings
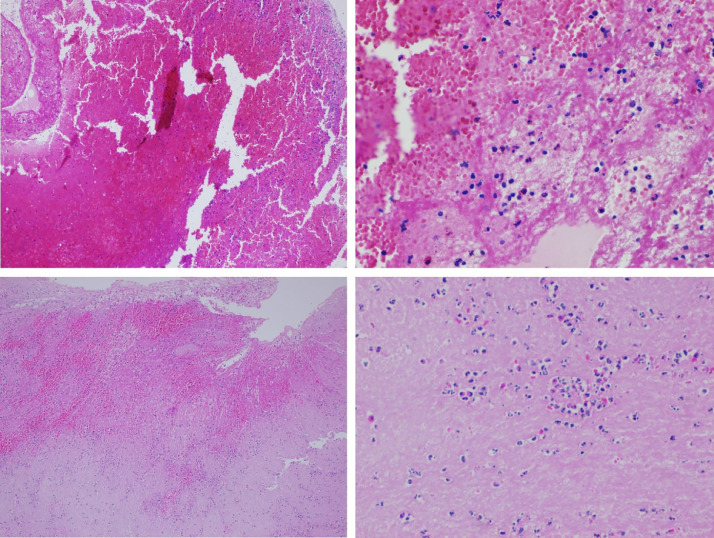
All clots displayed a heterogeneous pattern of four different components: fibrin, RBC, and leukocytes, lymphocytes. There were no cholesterol crystals, calcified materials, smooth-muscle cells, or other components of the intima or vessel wall structures. Thromboembolic material of COVID-19 patients include less red blood cell (RBC; 21.2–32.6%; P= 0.01), more lymphocyte (14.1–2.6%; P< 0.001), and more leukocyte (27.1–22.1%; P= 0.05; Fig. 1). There was no statistical difference between fibrin levels (Table IV ).
Fig. 1.
Photomicrographs of thrombi representing SARS-CoV positive and negative groups, H&E staining. A and B, Predominantly lymphocyte infiltration within the thrombi of a COVID-19 patient, X10 magnification (A) and X40 magnification (B). C and D, Clot composition of a patient from SARS-Cov-2 negative group, x10 magnification (C) and x40 magnification (D).
Table IV.
Histopathological structure of thrombus relation with COVID-19
| Component | No Covid-19 (N = 10) Mean/min-max |
Covid-19 positive (N = 11) Mean±SD/n(%)/min-max |
P value |
|---|---|---|---|
| RBC, % | 32.68% (18.18–40) | 21.24% (18.18–30) | 0.01* |
| Lymphocyte, % | 2.65% (0–9.09) | 14.17% (8.33–20) | <0.001* |
| Fibrin, % | 27.78% (18.18–33.33) | 23.8%(18.18–30) | 0.06 |
| Leukocyte, % | 22.13% (18.18–27.27) | 27.13% (16.67–36.3) | 0.05* |
| Connective tissue, % | 14.74% (8.33–18.18) | 13.64% (7.69–25) | 0.46 |
RBC, red blood cell.
p<0.05.
There is no correlation between the severity of COVID-19 and the ratio of RBC, WBC, lymphocyte, fibrin, and connective tissue in the clot (P= 0.679, P= 0.287, P= 0.836, P= 0.722, and P= 0.297, respectively).
Discussion
This is a single-center, retrospective, cohort study in one of the largest tertiary academic medical centers in Turkey. The first documented case of COVID-19 in Turkey occurred on March 11, 2020, and the first documented case in Izmir at our hospital on March 13, 2020. Our hospital cared for 3765 patients until the end of the calendar year, this number has been increasing in the writing process of this paper. Compared to the same period of 2019, we were asked to consult on a nearly twofold higher number of patients with the suspect of arterial thromboembolism. This increase evoked curiosity to study the relationship between COVID-19 and arterial thromboembolic events.
Causes of thrombosis in SARS-CoV-2 positive patients have been widely studied all over the world. It was reported that the untempered inflammation, along with direct viral-mediated effects and hypoxia, probably contributes to the high rates of thrombotic complications in COVID-19.1 , 2 Ackermann et al. revealed that the increased expression of angiotensin converting enzyme-2 (ACE-2) in endothelial cells after infection with SARS-CoV-2 may perpetuate a vicious cycle of endothelialitis and promotes thromboinflammation.3 Endothelial cell damage under ACE2-mediated entry of SARS-CoV-2 causes subsequent inflammation and the generation of a prothrombotic status.3 , 12 , 13 Infection-mediated endothelial injury (characterized by elevated levels of von Willebrand factor) and endothelialitis (marked by the presence of activated neutrophils and macrophages), found in multiple vascular beds (including the lungs, kidney, heart, small intestine, and liver) in patients with COVID-19, can trigger excessive thrombin production, inhibit fibrinolysis, and activate complement pathways, initiating thromboinflammation and ultimately leading to microthrombi deposition and microvascular dysfunction.13., 14., 15., 16., 17. Hypoxia-mediated hyperviscosity and upregulation of the HIF-1 (hypoxia-inducible factor 1) signaling pathway after acute lung injury may also contribute to the prothrombotic state.18
Small case reports and case series have demonstrated the presence of fibrinous exudates and microthrombi in histopathological examinations in patients with COVID-19.19., 20., 21., 22. To our knowledge, however, there are no papers dealing with clot composition retrieved from COVID-19 patients. Thrombi are complex, heterogeneous structures containing fibrin, platelets, erythrocytes, and leukocytes. These formed elements are placed to modify thrombus persistence or lysis, and leukocytes clearly represent another local variable capable of modifying thrombus stability in vivo.23 Neutrophil extracellular traps (NETs), which are released upon neutrophil activation may also play a prothrombotic role since they form a fibrin-like base for platelet adhesion, activation, and aggregation. Furthermore, NETs promote the accumulation of prothrombotic molecules, like von Willebrand factor and fibrinogen, thus significantly contributing to thrombus formation. Notably, there is vast data linking NETs to arterial and venous thrombosis in animal models, as well as in humans.24 In our study, WBC and lymphocyte ratio of clot is significantly high in SARS-CoV-2 positive patients when compared to the negative group, whereas there is no statistically significant difference in their levels in blood. There is no statistically significant difference regarding demographic data between groups, and COVID-19 patients are less frequently smokers, with a lower risk of thromboembolic events on this aspect.
In our cohort, patients with COVID-19 have a mean age of 71.2, thus it is impossible to rule out atherosclerotic lesion formation owing to aging, which is the dominant risk factor. Several lines of evidence functionally link lymphocytes and platelets in the development and clinical manifestations of atherosclerosis. In the study of Gori et al., lymphocyte master cytokines such as IFN-γ and IL-4 were shown to associate significantly with residual platelet reactivity in acute coronary syndrome patients on dual antiplatelet therapy, pointing to a role for T cell effector function in the development of thrombosis.25 Experimental studies have shown that IFN-γ can enhance platelet-dense granule secretion and conjugation with lymphocytes,26 and IL-2 was shown to reduce platelet adhesion and increase α-granule secretion.27 Furthermore, lymphocyte ecto-ATPase may convert ATP released extracellularly by platelets themselves or other cell types into ADP, which subsequently enhances platelet aggregation.28 Notably, however, the interaction of platelets and lymphocytes appears to be bidirectional, and platelets appear to modulate the various aspect of lymphocyte function and to influence their engagement in atherosclerosis. Platelets can adhere to lymphocytes to form platelet–lymphocyte conjugates,29 which may facilitate the adhesion of lymphocytes under sheer stress conditions30 and recruit them in sites of arterial thrombi.31 COVID-19 may be responsible for the accumulation of lymphocytes according to this algorithm.
In our cohort, arterial thromboses were nearly equally seen in SARS-CoV-2 positive patients that ranged from mild to severe. This finding is compatible with the study of Indes et al., which suggested that there was no observed correlation between the severity of COVID-19 and the frequency of arterial thrombosis.32
Our study group was homogenous regarding age, gender, and other demographic characteristics except smoking. SARS-CoV-2 positive patients were less frequently smokers in our cohort. SARS-COV-2 positive patients with arterial thromboembolism had significantly higher CRP, Ferritin, and LDH levels. In addition, we noticed that SARS-CoV-2 positive patients exhibited a trend toward higher WBC and neutrophil count and lower lymphocyte and platelet count, which may indicate an augmented inflammatory response. Although the Covid-19 positive patients trended toward higher procalcitonin levels, these differences did not reach statistical significance. In contrary to other reports, we did not have significantly elevated levels of D-dimer.7 , 32., 33., 34., 35. It was clearly stated that elevated D-dimer levels indicate the most severe forms of COVID-19.4 Elevated levels of D-dimer at admission (reported in up to 46% of hospitalized patients) and a longitudinal increase during hospitalization have been linked with worse mortality in COVID-19.4 , 5 Of 11 SARS-CoV-2 positive patients, 3 had severe symptomatology and 1 of them died in our cohort. Therefore, by having 8 mild and moderate COVID-19 patients, who were successfully discharged at the end of their therapy, our finding supports those recent papers. We found no statistically significant difference between groups in terms of anatomic distribution of thrombi. Diagnosis of thromboembolism by physical examination was executed more frequently SARS-CoV-2 negative group. Clinical characteristics were clear to identify thromboemboli like palpable pulses in the opposite limb and acute history. On the other hand, one patient from the COVID-19 group was too unstable for transport to imaging, and the diagnosis was based on clinical signs of acute ischemia. For the other patients in this group, it was hard to palpate pulses with personal protective equipment, so we asked for doppler USG or CT angiograms for accurate diagnosis. No statistical difference occurred between SARS-CoV-2 negative and positive groups regarding amputation and mortality rates. There are several limitations of our study one of which is the small sample size, therefore, it is difficult to draw any meaningful conclusions based on our data. The significance of Pvalues should be viewed with caution. The results of SARS-CoV-2 positive patients with arterial thromboembolism were not compared with SARS-CoV-2 patients without clinically detected arterial thromboemboli. Therefore, it is not possible to conclude that arterial thromboemboli is more frequently seen in older patients with atherosclerosis. There were 3 patients, in the SARS-CoV-2 negative group in whom there was strong clinical suspicion of COVID-19 may have had false-negative tests, even on repeat testing. Two of them had cough and fatigue while the other one had fever and dyspnea on exertion. Thorax CT scan revealed diffuse bilateral pulmonary ground-glass opacities. The retrieved thrombus material might not always reflect the whole thrombus, and a bias toward more stable clot components can not be ruled out. The manual segmentation of the thrombus components may be associated with an operator bias.
Conclusion
Our study is the first to investigate the histopathologic aspect of COVID-19 patients with arterial thromboembolism, one of the common features associated with COVID-19, Our aim to compare the clot composition of SARS-CoV positive and negative patients was for investigating the effects of COVID-19 related thromboembolic process in the first place. We found that this augmented inflammatory state provides changes in clot composition on a cellular basis, with elevated levels of WBC and lymphocyte in thromboembolic material. No correlation between the composition of the clot and the severity of COVID-19 was identified. There is an urgent need for further clinical and histopathological investigation to get certain knowledge of the cause and effect relationship for COVID-19 patients with arterial thromboembolism.
Footnotes
The study was held in Izmir Katip Celebi University Ataturk Training and Research Hospital.
References
- 1.Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 2.The Lancet Haematology COVID-19 coagulopathy: an evolving story. Lancet Haematol. 2020;7:e425. doi: 10.1016/S2352-3026(20)30151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klok FA, Hak MJ, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thrombos Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization (WHO) 2020. Clinical management of COVID-19: interim guidance.WHO/2019-nCoV/clinical/2020 [Google Scholar]
- 9.Boeckh-Behrens T, Schubert M, Förschler A, et al. The impact of histological clot composition in embolic stroke. Clin Neuroradiol. 2016;26:189–197. doi: 10.1007/s00062-014-0347-x. [DOI] [PubMed] [Google Scholar]
- 10.Moftakhar P, English JD, Cooke DL, et al. Density of thrombus on admission CT predicts revascularization efficacy in large vessel occlusion acute ischemic stroke. Stroke. 2013;44:243–245. doi: 10.1161/STROKEAHA.112.674127. [DOI] [PubMed] [Google Scholar]
- 11.Mokin M, Morr S, Natarajan SK, et al. Thrombus density predicts successful recanalization with Solitaire stent retriever thrombectomy in acute ischemic stroke. J Neurointerv Surg. 2015;7:104–107. doi: 10.1136/neurintsurg-2013-011017. [DOI] [PubMed] [Google Scholar]
- 12.Teuwen LA, Geldhof V, Pasut A, et al. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020 doi: 10.1038/s41577-020-0343-0. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varga A, Flammer AJ, Steiger P, et al. Endothelial cell infection and endothelilitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 15.Levi M, van der Poll T. Coagulation and sepsis. Thromb Res. 2017;149:38–44. doi: 10.1016/j.thromres.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Jackson SP, Darbousset R, Schoenwaelder SM. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. 2019;133:906–918. doi: 10.1182/blood-2018-11-882993. [DOI] [PubMed] [Google Scholar]
- 17.Bikdeli B, Madhavan MV, Gupta A, et al. Pharmacological agents targeting thromboinflammation in COVID-19: review and implications for future research. Thromb Haemost. 2020;120:1004–1024. doi: 10.1055/s-0040-1713152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta N, Zhao YY, Evans CE. The stimulation of thrombosis by hypoxia. Thromb Res. 2019;181:77–83. doi: 10.1016/j.thromres.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Deshpande C. Thromboembolic findings in COVID-19 autopsies: pulmonary thrombosis or embolism? Ann Intern Med. 2020;173:394–395. doi: 10.7326/M20-3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H., Zhou P., Wei Y., et al. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann. Intern. Med. 2020;172:629–632. doi: 10.7326/M20-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolhnikoff M, Duarte-Neto AN, de Almeida Montiro RA, et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J. Thromb Haemost. 2020;18:1517–1519. doi: 10.1111/jth.14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Copin MC, Parmentier E, Duburcq T, et al. Time to consider histologic pattern of lung injury to treat critically ill patients with COVID-19 infection. Intensive Care Med. 2020;46:1124–1126. doi: 10.1007/s00134-020-06057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robbie L, Libby P. Inflammation and atherothrombosis. Ann NY Acad Sci. 2001;947:167–180. doi: 10.1111/j.1749-6632.2001.tb03939.x. [DOI] [PubMed] [Google Scholar]
- 24.Moschonas IC, Tselepis AD. The pathway of neutrophil extracellular traps towards atherosclerosis and thrombosis. Atherosclerosis. 2019;288:9–16. doi: 10.1016/j.atherosclerosis.2019.06.919. [DOI] [PubMed] [Google Scholar]
- 25.Gori AM, Cesari F, Marcucci R, et al. The balance between proand anti-inflammatory cytokines is associated with platelet aggregability in acute coronary syndrome patients. Atherosclerosis. 2009;202:255–262. doi: 10.1016/j.atherosclerosis.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Todoroki N, Watanabe Y, Akaike T, et al. Enhancement by IL-1 beta and IFN-gamma of platelet activation: adhesion to leukocytes via GMP-140/PADGEM protein (CD62) Biochem Biophys Res Commun. 1991;179:756–761. doi: 10.1016/0006-291x(91)91881-c. [DOI] [PubMed] [Google Scholar]
- 27.Oleksowicz L, Paciucci PA, Zuckerman D, et al. Alterations of platelet function induced by interleukin-2. J Immunother. 1991;10:363–370. doi: 10.1097/00002371-199110000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Stafford NP, Pink AE, White AE, et al. Mechanisms involved in adenosine triphosphate-induced platelet aggregation in whole blood. Arterioscler Thromb Vasc Biol. 2003;23:1928–1933. doi: 10.1161/01.ATV.0000089330.88461.D6. [DOI] [PubMed] [Google Scholar]
- 29.Li N, Ji Q, Hjemdahl P. Platelet–lymphocyte conjugation differs between lymphocyte subpopulations. J Thromb Haemost. 2006;4:874–881. doi: 10.1111/j.1538-7836.2006.01817.x. [DOI] [PubMed] [Google Scholar]
- 30.Diacovo TG, Roth SJ, Morita CT, et al. Interactions of human alpha/beta and gamma/delta T lymphocyte subsets in shear flow with E-selectin and P-selectin. J Exp Med. 1996;183:1193–1203. doi: 10.1084/jem.183.3.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu H, Zhu L, Huang Z, et al. Platelets enhance lymphocyte adhesion and infiltration into arterial thrombus. Thromb Haemost. 2010;104:1184–1192. doi: 10.1160/TH10-05-0308. [DOI] [PubMed] [Google Scholar]
- 32.Indes JE, Koleilat I, Hatch AN, et al. Early experience with arterial thromboembolic complications in patents with COVID-19. J Vasc Surg. 2020;73:381–389. doi: 10.1016/j.jvs.2020.07.089. .e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sánchez JB, Cuipal Alcalde JD, Ramos-Isidro R, et al. Acute limb ischemia in a peruvian cohort infected by Covid-19. Ann Vasc Surg. 2021 doi: 10.1016/j.avsg.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veyre F, Poulain-Veyre C, Esparcieux A, et al. Femoral arterial thrombosis in a young adult after nonsevere COVID-19. Ann Vasc Surg. 2020 doi: 10.1016/j.avsg.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Etkin Y, Conway AM, Silpe J, et al. Acute arterial thromboembolism in patients with COVID-19 in the New York City area. Ann Vasc Surg. 2021;70:290–294. doi: 10.1016/j.avsg.2020.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]