Abstract
Objective
Renal autotransplant (RA) is an underutilized procedure to treat major ureteric loss. Studies on long-term outcomes and follow-up after RA are scarce. This study aimed to report the long-term outcomes and follow-up after RA.
Material and methods
We identified 9 patients, from 2007 to 2019, who underwent RA after major ureteric loss (where direct restoration of continuity was not possible). We collected data regarding the etiology of ureteric loss, preoperative differential renal function, method of nephrectomy (laparoscopic or open), method of anastomosing the residual ureter/pelvis to the bladder, postoperative complications, duration of hospital stay, and renal function and drainage postoperatively and until the last follow-up. Changes in renal function and/or any obstruction to urinary drainage of the ipsilateral kidney postoperatively or during follow-up were measured. The Wilcoxon matched-pairs signed-rank test was used to compare the mean creatinine values preoperatively, postoperatively, and at last follow-up (p<0.05 was considered statistically significant).
Results
All the patients had uneventful intraoperative and postoperative periods. The mean hospital stay was 6.4 (5–8) days. The median follow-up was 132 (46–156) months. The mean preoperative serum creatinine level was 1.0 (0.7–1.7) mg/dL. The mean creatinine value postoperatively and at last follow-up had no significant difference with preoperative value (p=0.96 and 0.75, respectively). The postoperative diethylene triamine pentaacetic acid scan demonstrated good perfusion and drainage. There was no deterioration of renal function or drainage during the follow-up.
Conclusion
RA is an excellent modality to treat major ureteric loss. It preserves renal function and avoids the problems related to bowel interposition and the need for long-term follow-up.
Keywords: Autografts, kidney, stricture, ureter, ureteral diseases
Introduction
Long-segment ureteral loss/injury is a disastrous complication for any patient and a nightmare for the treating surgeon. It is usually iatrogenic and could be caused by ureteroscopy, which is the most common cause of iatrogenic ureteric injury.[1] Progressive miniaturization of scopes has made the procedure more favorable for the ureter. However, complications do happen, of which ureter avulsion is a catastrophe. Among numerous options to restore continuity of the kidney with the bladder, renal autotransplant (RA) is often underutilized. We lack the literature regarding the long-term results after this procedure. Here we present 9 patients with long-segment ureteric injuries, managed with autotransplantation and the follow-up of over a decade.
Material and methods
This is a retrospective study of 9 patients of autotransplant, operated and followed up between 2007 and 2019. It may be noted that patient information was used for this study after taking consent from the patients. Ethical clearance was not needed per the institutional protocol.
A total of 8 patients had ureteric avulsion during the semi-rigid ureteroscopy for stone removal (7–10 mm stones in the upper and mid ureter) (Figure 1a), and 1 patient had a gunshot injury to the abdomen. The preoperative serum creatinine levels were 0.7–1.7 (mean: 1.04) mg/dL. All the patients had a normal functioning opposite kidney. At our center, 2 patients had a ureteric avulsion, after which an immediate nephrostomy was placed; 5 patients had percutaneous nephrostomy (PCN) placed elsewhere after the uretroscopic injury and were referred to our clinic (Figure 2); and 1 patient had a malpositioned nephrostomy with septic collection in the flank, which required ultrasound-guided percutaneous drainage. In the patient with the gunshot injury, ureteral injury was missed during the initial exploration elsewhere and was only diagnosed after an obvious urinary leak. A PCN was placed after 4 days. All the patients had less than 2–4 cm of viable upper ureter. In 7 ureteric avulsions, almost all of the ureter was lying in the bladder. A detailed preoperative evaluation included review of the preoperative images, operative notes (if available), and fresh diethylene triamine pentaacetic acid (DTPA) scans/dye studies. Direct restoration of continuity of the kidney with the urinary bladder was not possible in any of the patients. All the patients underwent retrieval of the kidney, followed by autotransplantation, after detailed discussion and consent. All the patients had sterile preoperative urine cultures and received 3 days of perioperative antibiotics per our departmental protocol. Nephrectomy was performed by primarily via open method in 1, laparoscopy to open conversion in 1, and primarily laparoscopically in 7 patients. Details of individual patients regarding the timing of the nephrostomy placement after the ureteral injury, duration of the PCN staying in situ, and timing of surgery after the ureteral injury have been given in the Supplementary table 1 and the details accompanying the table.
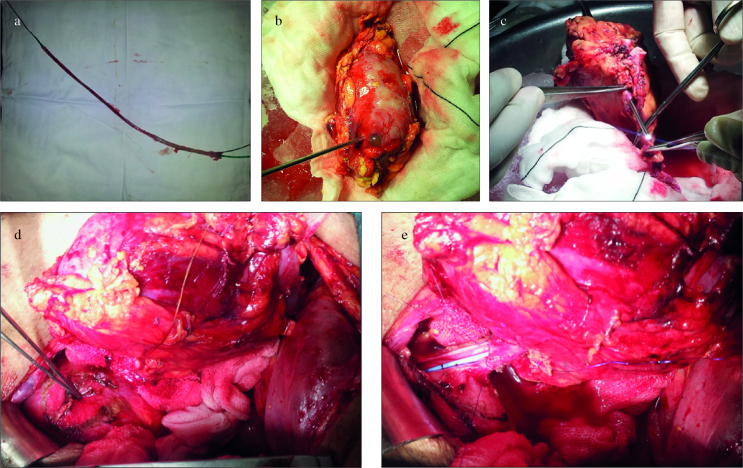
Figure 1. a–e.
(a) Complete avulsion of the ureter after ureteroscopy. (b) Inserting ureteroscopy via nephrostomy site to remove the stone on the bench. (c) Ureteroscope coming out of the ureter. Kindly note the almost absent ureter length after ureteropelvic junction. (d) Small Boari flap being fashioned for ureteroneocystostomy. (e) Ureteroneocystostomy being fashioned with a double J stent in situ
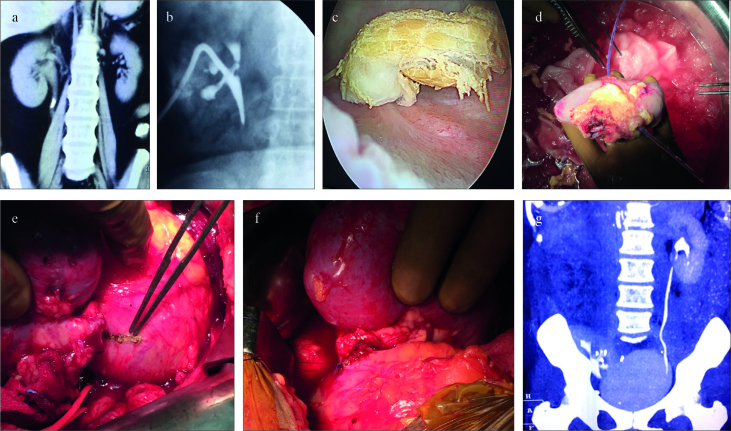
Figure 2. a–g.
(a) Preoperative computed tomography (CT). (b) Postavulsion nephrostogram. (c) Avulsed ureter segment seen inside the urinary bladder. (d) Retrieved kidney with feeding tube from nephrostomy site coming out from the ureter. (e) Bladder marked for ureteroneocystostomy. (f) Completed ureteroneocystostomy. (g) Postoperative CT scan with autotransplanted kidney in the right iliac fossa region
Open nephrectomy
In 1 patient (with a history of septic collection in the flank), the left kidney was retrieved by a standard lumbar incision. A lower iliac fossa incision was made on the right side for autotransplantation. The stone that had up-migrated into the lower calyx during the previous ureteroscopy was retrieved on bench (Figure 1b). Retroperitoneoscopic kidney retrieval was attempted in the patient with gunshot injury in the view of previous laparotomy and colostomy, but it required conversion to open approach. The remaining 7 kidneys were removed laparoscopically.
Laparoscopic nephrectomy
After complete mobilization of the kidney, the pre-marked Gibson incision was made on the ipsilateral side to the level of the peritoneum. The peritoneum was opened, and the kidney was manually retrieved and perfused with cold Eurocollins solution. The peritoneum was closed, and the patient was repositioned in the supine position.
Engraftment and follow-up
The kidney was transplanted in the pelvis with end-to-side/side-to-side vascular anastomoses to the external/internal iliac artery and external iliac vein. The collecting system was anastomosed to the bladder by modified Lich-Gregoir technique or Boari flap (Figures 1 and 2) and stented. Oral feeds were started on the evening of surgery. Urethral catheter was removed on the 5th day, and the ureteral stent was removed at 2 weeks. The patients were then followed with a color Doppler ultrasound of the graft at 3 months. DTPA scan was performed at 6 months to assess graft function and urinary drainage. The patients were then asked to follow-up annually with serum creatinine and ultrasonography. The last follow-up was conducted in December 2019.
Statistical analysis
The mean preoperative creatinine value was compared with the postoperative value and value at the last follow up using the Wilcoxon matched-pairs signed-rank test. A p value of <0.05 was considered statistically significant.
Results
The mean age of the patients was 36.44 (24–78) years (Table 1). The mean preoperative creatinine level was 1.04 (0.7–1.7) mg/dL. The mean warm ischemia time was 3.69 (3.0–4.2) min. The mean postoperative creatinine level was 1.04 (0.7–1.5) mg/dL. The mean operative time was 364.9 (300–400) min out of which a mean of 174 (160–200) min were spent on performing nephrectomy. The mean hospital stay was 6.4 (5–8) days. There were no intraoperative complications; 2 patients had a low-grade fever on postoperative day 1, which responded to antipyretics (no change in the antibiotics) (Table 2). We could successfully restart oral feeds from the evening of the surgery, and our patients stayed an average of 6.4 (5–8) days in the hospital. All the patients were doing well till their last follow-up (median: 132; range: 46–156 months) (Table 2). The mean serum creatinine level till the last follow-up was 1.07 (0.9–1.6) mg/dL. There was no significant change in the mean serum creatinine level postoperatively (p=0.96) and until the last follow-up (p=0.75). Unobstructed urinary drainage was noted in all the patients during postoperative DTPA scan. None of the patients had any hydronephrosis in the ultrasonographic evaluation during the follow-up.
Table 1.
Preoperative patient profile
| No. | Age (years) | Sex | Side | History | Intervention | Creatinine (mg/dL) | Percentage function of affected kidney (%) | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 34 | M | Left | Ureteral stone | PCN | 0.9 | 49 | Ureteral avulsion |
| 2 | 28 | M | Left | Ureteral stone | PCN | 0.7 | 45 | Ureteral avulsion |
| 3 | 38 | F | Right | Ureteral stone | Replacement of malpositioned PCN | 0.8 | 50 | Ureteral avulsion with retroperitoneal collection |
| 4 | 32 | M | Left | Ureteral stone | PCN | 0.8 | 47 | Ureteral avulsion |
| 5 | 24 | M | Left | Gun shot | PCN after detecting urinary leak | 1.4 | 43 | Retroperitoneal collection with ureteral loss |
| 6 | 30 | F | Left | Ureteral stone | PCN | 0.9 | 50 | Ureteral avulsion |
| 7 | 33 | M | Right | Ureteral stone | PCN | 1.2 | 46 | Ureteropelvic junction disruption |
| 8 | 31 | M | Left | Ureteral stone | PCN | 1.0 | 48 | Ureteral avulsion |
| 9 | 78 | M | Left | Ureteral stone | PCN | 1.7 | 45 | Ureteral avulsion |
M: male; F: female; PCN: percutaneous nephrosotomy tube placement. This table gives the basic patient profile with individual etiologies, preoperative creatinine levels, interventions performed before definitive surgery, split function (based on diethylene triamine pentaacetic acid scan), and the outcome of ureteric injuries.
Table 2.
Operative findings and outcomes
| Time (min) | Total time | Warm ischemia time | Stay | Follow-up | Postoperative creatinine | Creatinine at last follow-up | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Intraoperative findings | Reconstruction | Nephrectomy | (min) | (min) | Complications | (days) | (months) | (mg/dL) | (mg/dL) |
| 1 | No fibrosis, 3 cm proximal ureter | Ureteroneocystostomy | 170 | 340 | 3.5 | - | 7 | 156 | 1.1 | 1.0 |
| 2 | Fibrosis, 4 cm proximal ureter | Ureteroneocystostomy | 160 | 350 | 4.0 | Fever | 8 | 145 | 0.8 | 0.9 |
| 3 | Severe fibrosis, short extrarenal pelvis | Pyelovesicostomy | 190 | 380 | 3.8 | - | 6 | 132 | 1.2 | 1.2 |
| 4 | No fibrosis, no proximal ureter | Pyelovesicostomy | 180 | 390 | 4.1 | - | 5 | 107 | 0.9 | 1.0 |
| 5 | Severe fibrosis, no proximal ureter | Pyelovesicostomy | 200 | 400 | 4.2 | Fever | 8 | 82 | 1.3 | 1.2 |
| 6 | No fibrosis, 4 cm proximal ureter | Ureteroneocystostomy | 120 | 300 | 3.0 | - | 5 | 142 | 1.0 | 0.9 |
| 7 | Fibrosis, 3 cm proximal ureter | Ureteroneocystostomy | 180 | 380 | 3.9 | - | 7 | 102 | 0.7 | 0.9 |
| 8 | No fibrosis, 4 cm proximal ureter | Ureteroneocystostomy | 196 | 384 | 3.7 | - | 5 | 143 | 0.9 | 1.0 |
| 9 | No fibrosis, no proximal ureter | Pyelovesicostomy | 170 | 360 | 3.0 | - | 6 | 46 | 1.5 | 1.6 |
This table describes the intraoperative findings and technique of anastomosing the residual renal pelvis/ureter to the urinary bladder. The time taken to perform the nephrectomy, total duration of the surgery, and warm ischemia time are discussed. This is followed by the description of the in-hospital stay of each patient with complications, and side by side comparison of postoperative serum creatinine values with serum creatinine at the last follow-up.
Discussion
Ureteric injuries are the cause of significant morbidity and unplanned hospitalization. Iatrogenic causes include more than 75% of the ureteric injuries.[2] Traditionally, gynecological surgeries used to be the most common cause of iatrogenic ureteric injuries followed by general surgical/pelvic procedures. However, lately, ureteroscopy has been reported to be the most common cause,[1] although the incidence has reduced with refinement of techniques and miniaturization of instruments.[3] During endourological procedures, ureteric injuries are usually diagnosed intraoperatively. In other settings, the presentation may be delayed. This is especially true in penetrating/blunt trauma injuries and requires a high index of suspicion.[4] If immediate repair is not possible, ipsilateral renal moiety should be urgently diverted with a PCN.[2] In ureteric avulsion, the ureter gets avulsed near the ureteropelvic junction (UPJ) and often is pulled out of the urethra or lies in the bladder. Various options to treat long-segment ureteric loss are Boari flap, ureteral substitution with bowel segments, and RA. Reconstruction of the urinary tract should be preferably performed through urothelium-lined tissue because it is not absorptive and resists the effects of urine.[5]
The Boari flaps have been described to bridge the gaps from up to mid ureter (10–15 cm defects) comfortably. Very few authors have described addressing the defects up to the renal pelvis in elective settings.[6] However, Boari flap may not always be applicable where the anastomosis is required at or just below the UPJ. For successful bridging of longer defects, the urinary bladder should have a very good capacity. To find that the Boari flap is not reaching up to the renal pelvis in the midst of the procedure is a disaster.
The ileum is most commonly used bowel segment for substitution. Goodwin et al.[7] popularized the ileum as a safe and useful tissue source for reconstruction of long length of ureter. However, Tanagho[8] had strong reservations against using bowel segments in a closed urinary system. He felt that the bowel segment is subjected to the variable and high intraluminal pressure of the urinary system, and the bowel segment assumes a partial reservoir function permitting selective absorption, which results in fluid and electrolyte imbalance. Chung et al.[9] questioned Tanagho on their selection of the patients for poor results. They proposed careful selection of patients and close attention to the surgical technique to get good results with acceptable complications for the ileal ureter. The same conclusions were reiterated by many other authors.[10–13]
In an ideal scenario, there should not be any bowel-related complications during genitourinary reconstruction. The complication rates secondary to bowel substitution have varied in different series. The commonly described complications include electrolyte imbalance, reabsorption, mucous secretion, and so on.[9–14] The reported rate of metabolic acidosis is nearly 12%. In the same study, 2 of the 17 patients developed bladder stones during the follow-up.[15] Chronic UTI has been found in up to 66% of cases, with Pseudomonas spp. as causative agent in 25%.[14] Chronic bacteriuria is estimated to cause renal failure in 25%–34% of all the patients. Modification of the ileal ureter replacement using modifications, such as Yang-Monti principle, could mitigate the metabolic complications of using the ileal ureter as such.[16] However, it should be noted that in this report of 16 patients, the authors admitted that 1 of the reasons for the absence of metabolic complications after this procedure could be owing to the inclusion of patients with creatinine ≤1.8 gm/dL only. In patients with compromised renal functions, there is a higher chance of metabolic abnormalities and progressive renal failure. The contraindications to an ileal ureteral substitution are baseline renal insufficiency, with a serum creatinine level of greater than 2 mg/dL, bladder dysfunction or outlet obstruction, inflammatory bowel disease, and radiation enteritis.[17] RA can be used safely and reliably in all the above situations. Wolff et al.[15] found progressive deterioration of renal function after ileal ureter substitution in patients with compromised preoperative renal function. The mean creatinine levels before surgery, 1 month after surgery, and at the last follow-up visit were 1.3±0.3 (0.6–3.4), 1.4±0.4 (0.6–3.6), and 1.8±0.6 (0.7–4.7) mg/dL, respectively. At the end of the follow-up period, 15 patients still had ileal ureters. Of these, 3 required dialysis. It is quite remarkable that none of our patients showed any significant deterioration in renal function till their last follow-up (Table 2) neither did they face any metabolic complications.
The most significant complication after bowel substitution is malignancy. Although having a very low incidence rate (0.8%), it has a varied latent period ranging from 4 to 32 years, and authors have recommended annual surveillance.[18] We believe that even a low possibility of development of malignancy should deter the urologists from using bowel segments in the urinary system if any other method is available.
Hardy[19] was the first to describe RA in 1963 for management of extensive ureteric loss. Since then, many series have described RA for varied indications. The biggest advantage of RA is maintaining continuity of the urothelium, thereby avoiding adverse effects arising from the contact of urine with the intestinal mucosa. Various authors have published their experience with RA with good long-term renal preservation.[20–23] In all our patients, renal moiety was preserved.
Meng et al.[21] encountered dense perihilar fibrosis in all their patients. All of their patients had urinary extravasation and a history of prior manipulation. They kept the patients on nephrostomy and performed nephrectomy after 4 to 6 months of initial injury to allow the perinephric inflammation to subside. Novick et al.[20] have also described dense perihilar fibrosis because of prior surgeries, which lead to vascular spasm, inadequate perfusion, and ultimately loss of the autograft. We encountered dense fibrosis in 2 patients, 1 with a history of a gunshot wound and the other with a history of septic collection. This led to conversion from retroperitoneoscopic to open approach in the 1st patient, whereas the 2nd patient did not consent for laparoscopic nephrectomy.
The patients in whom PCN was secured immediately after the injury with proper diversion of urine, inflammation was significantly controlled, and autotransplantation was performed within a few days with gratifying results.
In renal allograft transplantation, vascular complications are rare, occurring in 1.4%–6.6% of cases, with pseudoaneurysms reported in 0.14%–0.2% of all transplants.[24,25] Reviews on autotransplantation have described almost double the rate of vascular complications in autotransplantations, especially in cases of renovascular diseases and ureteral injuries, citing higher infection rates in these cases. However, if we analyze the data of various series and case reports, we could find 8 cases of graft loss secondary to vascular complications in 199 cases (4%). Complications, such as hilar bleeding,[23] arterial dissection, and graft thrombosis,[22] could be ascribed to technical errors and not autograft transplantation. Graft nephrectomy had to be performed after RA following arterial dissection in 1 study.[23] However, in our limited number of patients, we did not encounter such complications. Renal vascular thromboses are another catastrophic complication observed after RA. As a protocol, we flushed the kidney with Eurocollins solution during perfusion after nephrectomy. Heparinization was limited to the intraoperative period only. The external iliac vessels were flushed with heparinized saline after performing venotomy and arteriotomy, before starting vascular anastomosis. They were flushed again before the completion of vascular anastomosis. None of our patients developed vascular thrombosis.
Novick et al.[20] also described a graft nephrectomy with hemorrhage by vascular erosion by the nephrostomy tube. A primary non-function of the graft had been explained secondary to non-perfusion post-retrieval. Ruiz et al.[26], while concluding their experience of over 26 years with RA, opined that RA is quite similar to living-donor kidney transplant, and when it is performed by experienced transplant surgeons, it has low morbidity and mortality rates, as well as acceptable long-term results. One of the authors (AK) of this study has a vast experience in renal allograft transplantation. In all of our patients, we used RA as the primary approach after ensuring proper urinary diversion after primary insult, strict attention to details, and proper asepsis. We did not encounter any vascular complications.
In the literature, both ileal replacement and RA have been described as elective reconstructive procedures of significant ureteric loss when all other options are not feasible. Many authors consider ileal replacement as a better option for the replacement of long-segment ureteral loss, citing increased exposure of urologists in handling bowels and potential vascular complications, which may lead to autograft loss. However, bowel interposition is associated with its own share of complications, some of which may not be innocuous. Bowel interposition may not always be a viable option as discussed earlier. The latest available literature on RA reports excellent long-term outcomes. The 1st multi-institutional study on RA was reported by Cowan et al.[23], with a total of 54 procedures with a shorter follow-up period and 93% rate of functioning grafts. One of the largest series of laparoscopic nephrectomies for auto transplantation was reported by Tran et al.[27] in 2015. It included 52 patients with more than 90%success rate over a 6-year follow-up period. They opined that in patients with recurrent nephrolithiasis, more efficient urinary drainage provided after RA, owing to shorter or absent ureteral length, may prevent urinary stasis and stone formation. Excellent long-term results of RA, after an average follow-up of 55 months, were reported by Bourgi et al.[28] in 2018. Currently, completely intracorporeal robot-assisted nephrectomy with RA is a feasible approach for renal preservation after major ureteral injury.[29] Once stable, the patient can be released from specialty care with excellent long-term outcomes. Tran et al.[27] reported outcomes in 41 patients with ureteral stricture (among other indications for RA), with a median follow-up of 73.5 months. We report the outcomes of RA in 9 patients with the longest known median follow-up (132 months) according to the best of our knowledge. One of the limitations of our study is the small number of patients.
In conclusion, the prevention of ureteric injury is vital; however, no patient should suffer bowel-related complications after undergoing surgical correction for total ureteric loss, especially when options, such as RA, are available. In the hands of an experienced transplant surgeon, primary RA remains an invaluable tool to reconstruct the defect with excellent long-term outcomes, irrespective of the current renal function. RA spares the patient from recurring pyelonephritis, metabolic complications, deterioration of renal function, and risk of malignancy. Oral feeds may be resumed early, and recovery is faster. The patient may not require any long-term follow-up. We would advocate RA as the first surgical option in future, with long ureteral defects, based on almost 13 years of our experience with this procedure and follow-up of our operated patients. We feel that an adequately powered randomized study comparing RA to another approach like ileal interposition may help to settle the debate. Promising results of this study emphasize the need to approach RA with less apprehension.
Supplementary material
Supplementary 1.
This table gives the details of individual patients regarding the etiology of ureteral injury, timing of nephrostomy placement, duration on PCN, and timing after surgery with intraoperative findings
| Intraoperative findings | Etiology | Time of nephrostomy placement after ureteral injury | Duration on PCN | Time of surgery after injury | |
|---|---|---|---|---|---|
| 1 | No fibrosis, 3 cm proximal ureter | Ureteroscopy for ureteral stone | Same day as injury | 3 days | 3 days |
| 2 | Fibrosis, 4 cm proximal ureter | Ureteroscopy for ureteral stone | No details available | No details available | 21 days |
| 3 | Severe fibrosis, short extrarenal pelvis | Ureteroscopy for ureteral stone | First PCN placed intraoperatively Second PCN placed later to replaced malpositioned right PCN | Duration on First PCN was 4 days, Duration on second PCN was 41 days. | 45 days |
| 4 | No fibrosis, no proximal ureter | Ureteroscopy for ureteral stone | Same day as surgery | 3 days | 3 days |
| 5 | Severe fibrosis, no proximal ureter | Gunshot | 4 days after surgery | 61 days | 65 days |
| 6 | No fibrosis, 4 cm proximal ureter | Ureteroscopy for ureteral stone | Same day as injury | 7 days | 7 days |
| 7 | Fibrosis, 3 cm proximal ureter | Ureteroscopy for ureteral stone | No details available | No details available | 30 days |
| 8 | No fibrosis, 4 cm proximal ureter | Ureteroscopy for ureteral stone | Same day as injury | 4 days | 4 days |
| 9 | No fibrosis, no proximal ureter | Ureteroscopy for ureteral stone | Same day as injury | 7 days | 7 days |
PCN: percutaneous nephrostomy
Patient 1 and patient 4 had ureteral injury in our surgical unit, at our institute, after ureteroscopy, and PCN was placed intraoperatively. These patients underwent renal autotransplantation within 3 days after the injury, with a successful laparoscopic nephrectomy.
Patient 5 had a gunshot injury to his abdomen for which he underwent exploratory laparotomy and formation of end colostomy in the emergency surgical unit. Ureteral injury was missed intraoperatively. It was diagnosed later when he had a high drain output, which proved to be urine after confirming high drain fluid creatinine levels. Percutaneous nephrostomy (PCN) was placed after 4 days. The patient was later discharged and was transferred to our unit after 2 months. He underwent renal autotransplantation once he was deemed fit for surgery by the anesthesia team. Retroperitoneoscopic approach was used initially for nephrectomy, but in view of adhesions, it was converted to open approach.
Patient 6 was diagnosed as having ureteric avulsion intraoperatively by a different surgical unit. Intraoperative consultation was attended by our team, and a PCN was placed. This patient underwent laparoscopic nephrectomy followed by renal autotransplant after a week. The rest of the patients were referred to us from other facilities.
Patient 2 and 7. No details could be retrieved about the timing of the placement of PCN after the ureteral injury. These patients underwent renal autotransplantation within 1 week of being referred to us. Some adhesions were encountered intraoperatively, but laparoscopic nephrectomy could be completed successfully.
Patient 3. The first PCN was placed intraoperatively, which was later found to be malpositioned, and a second PCN was placed to replace it after 4 days. This patient was discharged afterwards. The patient underwent renal autotransplantation 4 days after being referred to us. The patient did not consent for laparoscopic nephrectomy; hence, he underwent open nephrectomy to retrieve the kidney for autotransplantation. Dense adhesions were encountered intraoperatively, but the kidney was retrieved safely.
Patients 8 and 9 had early PCN placement and were operated within a week of injury, with successful laparoscopic nephrectomy.
Main Points.
Bowel interposition is a popular choice among surgeons to reconstruct major ureteric loss, with apprehensions toward the results of renal autotransplantation.
The patients suffer serious metabolic complications after bowel interposition and are required to have lifelong follow-up.
Renal autotransplant offers excellent results in the long term without the risk of any metabolic complication and with no need for long-term follow-up.
Renal autotransplant should be adopted as the preferred modality to restore continuity of kidney with the bladder where direct restoration of continuity is not possible otherwise.
Footnotes
Ethics Committee Approval: Since only data was collected, ethical approval was not needed.
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – A.K., R.M., S.C.; Design – P.D., R.M.; Supervision – A.K., S.C.; Resources – A.B., P.D., V.D.; Data Collection and/or Processing – A.B., S.C.; Analysis and/or Interpretation – A.B., P.D.; Literature Search – A.B., R.M., P.D., S.C.; Writing Manuscript – A.B., R.M.; Critical Review – A.K., S.C.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Johnson DB, Pearle MS. Complications of ureteroscopy. Urol Clin North Am. 2004;31:157–71. doi: 10.1016/S0094-0143(03)00089-2. [DOI] [PubMed] [Google Scholar]
- 2.Bryk DJ, Zhao LC. Guideline of guidelines: a review of urological trauma guidelines. BJU Int. 2016;117:226–34. doi: 10.1111/bju.13040. [DOI] [PubMed] [Google Scholar]
- 3.Al-Awadi K, Kehinde EO, Al-Hunayan A, Al-Khayat A. Iatrogenic ureteric injuries: incidence, aetiological factors and the effect of early management on subsequent outcome. Int Urol Nephrol. 2005;37:235–41. doi: 10.1007/s11255-004-7970-4. [DOI] [PubMed] [Google Scholar]
- 4.Taqi KM, Nassr MM, Al Jufaili JS, Abu-Qasida AI, Mathew J, Al-Qadhi H. Delayed Diagnosis of Ureteral Injury Following Penetrating Abdominal Trauma: A Case Report and Review of the Literature. Am J Case Rep. 2017;18:1377–81. doi: 10.12659/AJCR.905702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harzmann R, Kopper B, Carl P. Cancer induction by urinary drainage or diversion through intestinal segments? Urologe A. 1986;25:198–203. [PubMed] [Google Scholar]
- 6.Mauck RJ, Hudak SJ, Terlecki RP, Morey AF. Central role of Boari bladder flap and downward nephropexy in upper ureteral reconstruction. J Urol. 2011;186:1345–9. doi: 10.1016/j.juro.2011.05.086. [DOI] [PubMed] [Google Scholar]
- 7.Goodwin WE, Winter CC, Turner RD. Replacement of the ureter by small intestine: clinical application and results of the ileal ureter. J Urol. 1959;81:406–18. doi: 10.1016/S0022-5347(17)66035-X. [DOI] [PubMed] [Google Scholar]
- 8.Tanagho EA. A case against incorporation of bowel segments into the closed urinary system. J Urol. 1975;113:796–802. doi: 10.1016/S0022-5347(17)59582-8. [DOI] [PubMed] [Google Scholar]
- 9.Chung BI, Hamawy KJ, Zinman LN, Libertino JA. The use of bowel for ureteral replacement for complex ureteral reconstruction: long-term results. J Urol. 2006;175:179–84. doi: 10.1016/S0022-5347(05)00061-3. [DOI] [PubMed] [Google Scholar]
- 10.Bejany DE, Lockhart JL, Politano VA. Ileal segment for ureteral substitution or for improvement of ureteral function. J Urol. 1991;146:302–5. doi: 10.1016/S0022-5347(17)37776-5. [DOI] [PubMed] [Google Scholar]
- 11.Verduyckt FJH, Heesakkers JPFA, Debruyne FMJ. Long-term results of ileum interposition for ureteral obstruction. Eur Urol. 2002;42:181–7. doi: 10.1016/S0302-2838(02)00266-X. [DOI] [PubMed] [Google Scholar]
- 12.Bonfig R, Gerharz EW, Riedmiller H. Ileal ureteric replacement in complex reconstruction of the urinary tract. BJU Int. 2004;93:575–80. doi: 10.1111/j.1464-410X.2003.04672.x. [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Gomez E, Malde S, Spilotros M, Shah PJ, Greenwell JT, Ockrim JL. A tertiary experience of ileal-ureter substitution: Contemporary indications and outcomes. Scand J Urol. 2016;50:192–9. doi: 10.3109/21681805.2015.1106579. [DOI] [PubMed] [Google Scholar]
- 14.Shokeir AA, Gaballah MA, Ashamallah AA, Ghoneim MA. Optimization of replacement of the ureter by ileum. J Urol. 1991;146:306–10. doi: 10.1016/S0022-5347(17)37777-7. [DOI] [PubMed] [Google Scholar]
- 15.Wolff B, Chartier-Kastler E, Mozer P, Haertig A, Bitker MO, Rouprêt M. Long-term functional outcomes after ileal ureter substitution: a single-center experience. Urology. 2011;78:692–5. doi: 10.1016/j.urology.2011.04.054. [DOI] [PubMed] [Google Scholar]
- 16.Esmat M, Abdelaal A, Mostafa D. Application of Yang-Monti principle in ileal ureter substitution: is it a beneficial modification? Int Braz J Urol. 2012;38:779–87. doi: 10.1590/1677-553820133806779. [DOI] [PubMed] [Google Scholar]
- 17.Nakada S, Hsu T. Management of Upper Urinary Tract Obstruction. In: Wein AJ editor-in-chief, Kavoussi LR, Partin AW, Novick AC, Peters CA, editors. Campbell-Walsh Urology. Published online 2012. [Google Scholar]
- 18.Ali-El-Dein B, El-Tabey N, Abdel-Latif M, Abdel-Rahim M, El-Bahnasawy MS. Late uro-ileal cancer after incorporation of ileum into the urinary tract. J Urol. 2002;167:84–8. doi: 10.1016/S0022-5347(05)65388-8. [DOI] [PubMed] [Google Scholar]
- 19.Hardy JD. High ureteral injuries. Management by autotransplantation of the kidney. JAMA. 1963;184:97–101. doi: 10.1001/jama.1963.03700150051008. [DOI] [PubMed] [Google Scholar]
- 20.Novick AC, Jackson CL, Straffon RA. The role of renal autotransplantation in complex urological reconstruction. J Urol. 1990;143:452–7. doi: 10.1016/S0022-5347(17)39988-3. [DOI] [PubMed] [Google Scholar]
- 21.Meng MV, Freise CE, Stoller ML. Expanded experience with laparoscopic nephrectomy and autotransplantation for severe ureteral injury. J Urol. 2003;169:1363–7. doi: 10.1097/01.ju.0000054927.18678.5e. [DOI] [PubMed] [Google Scholar]
- 22.Hau HM, Bartels M, Tautenhahn HM, Morgul MH, Fellmer P, Ho-Thi P, et al. Renal autotransplantation--a possibility in the treatment of complex renal vascular diseases and ureteric injuries. Ann Transplant. 2012;17:21–7. doi: 10.12659/AOT.883690. [DOI] [PubMed] [Google Scholar]
- 23.Cowan NG, Banerji JS, Johnston RB, Duty BD, Bakken B, Hedges JC, et al. Renal Autotransplantation: 27-Year Experience at 2 Institutions. J Urol. 2015;194:1357–61. doi: 10.1016/j.juro.2015.05.088. [DOI] [PubMed] [Google Scholar]
- 24.Parada B, Figueiredo A, Mota A, Furtado A. Surgical complications in 1000 renal transplants. Transplant Proc. 2003;35:1085–6. doi: 10.1016/S0041-1345(03)00318-X. [DOI] [PubMed] [Google Scholar]
- 25.Orlic P, Vukas D, Drescik I, Ivancic A, Blecic G, Budiselic B, et al. Vascular complications after 725 kidney transplantations during 3 decades. Transplant Proc. 2003;35:1381–4. doi: 10.1016/S0041-1345(03)00506-2. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz M, Hevia V, Fabuel JJ, Fernández AA, Gómez V, Burgos FJ. Kidney autotransplantation: long-term outcomes and complications. Experience in a tertiary hospital and literature review. Int Urol Nephrol. 2017;49:1929–35. doi: 10.1007/s11255-017-1680-1. [DOI] [PubMed] [Google Scholar]
- 27.Tran G, Ramaswamy K, Chi T, Meng M, Freise C, Stoller ML. Laparoscopic Nephrectomy with Autotransplantation: Safety, Efficacy and Long-Term Durability. J Urol. 2015;194:738–43. doi: 10.1016/j.juro.2015.03.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bourgi A, Aoun R, Ayoub E, Moukarzel M. Experience with Renal Autotransplantation: Typical and Atypical Indications. Adv Urol. 2018;2018 doi: 10.1155/2018/3404587. 3404587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordon ZN, Angell J, Abaza R. Completely intracorporeal robotic renal autotransplantation. J Urol. 2014;192:1516–22. doi: 10.1016/j.juro.2014.02.2589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary 1.
This table gives the details of individual patients regarding the etiology of ureteral injury, timing of nephrostomy placement, duration on PCN, and timing after surgery with intraoperative findings
| Intraoperative findings | Etiology | Time of nephrostomy placement after ureteral injury | Duration on PCN | Time of surgery after injury | |
|---|---|---|---|---|---|
| 1 | No fibrosis, 3 cm proximal ureter | Ureteroscopy for ureteral stone | Same day as injury | 3 days | 3 days |
| 2 | Fibrosis, 4 cm proximal ureter | Ureteroscopy for ureteral stone | No details available | No details available | 21 days |
| 3 | Severe fibrosis, short extrarenal pelvis | Ureteroscopy for ureteral stone | First PCN placed intraoperatively Second PCN placed later to replaced malpositioned right PCN | Duration on First PCN was 4 days, Duration on second PCN was 41 days. | 45 days |
| 4 | No fibrosis, no proximal ureter | Ureteroscopy for ureteral stone | Same day as surgery | 3 days | 3 days |
| 5 | Severe fibrosis, no proximal ureter | Gunshot | 4 days after surgery | 61 days | 65 days |
| 6 | No fibrosis, 4 cm proximal ureter | Ureteroscopy for ureteral stone | Same day as injury | 7 days | 7 days |
| 7 | Fibrosis, 3 cm proximal ureter | Ureteroscopy for ureteral stone | No details available | No details available | 30 days |
| 8 | No fibrosis, 4 cm proximal ureter | Ureteroscopy for ureteral stone | Same day as injury | 4 days | 4 days |
| 9 | No fibrosis, no proximal ureter | Ureteroscopy for ureteral stone | Same day as injury | 7 days | 7 days |
PCN: percutaneous nephrostomy