Abstract
Background and Purpose :
The search for the development of a suitable novel antimicrobial agent for fungal diseases continues to be a key problem in the current clinical field. The present investigation was aimed to determine the antifungal effect of the ethanolic crude extracts of Woodfordia fruticosa leaf (Wfl) and Punica granatum peel (Pgp) in uncontrolled diabetic patients wearing removable dentures.
Materials and Methods:
The ethanolic extracts of both plants were prepared using the soxhlet extraction method, and the obtained metabolites were confirmed by thin- layer chromatography. After the preparation of the mouthwash, a total of 100 subjects were randomly divided into two groups. Each subject was given physiological saline at the baseline. Group I was provided with P. granatum mouthwash, while Group II was given W. fruticosa mouthwash. Following the administration of the mouthwash, the patients were requested to rinse the mouthwash using the oral rinse technique twice daily 5 ml/rinse for 30 sec. Subsequently, colony-forming units (CFU) were evaluated in the participants. Post-therapeutic samples were collected 1 h and 1 week after the mouthwash use.
Results:
The mean reduction of CFU was calculated at the baseline, as well as 1 h and 1 week after using mouthwash. The results indicated a drastic reduction in CFU 1 h and 1 week after the application of Wfl mouthwash.
Conclusion:
The obtained data revealed that Wfl had potential anticandidal activity against Candida yeast cells, probably owing to its bioactive compounds like glycosides. Therefore, this agent can be used effectively as a natural remedy for the treatment of oral candidiasis. However, the exact mechanism of action of this plant needs to be elucidated.
Keywords: Antifungal agents, Glycosides, Oral candidiasis, Punica granatum, Woodfordia fruticosa
Introduction
Oral candidiasis is an opportunistic fungal infection that is more prevalent in patients with xerostomia or immunosuppressive conditions (e.g., HIV infection), denture wearers, and those on anticancer chemotherapy or high carbohydrate diet. Moreover, this infection is considered to be a hallmark of systemic diseases, such as diabetes mellitus [ 1 ]. Candida species, namely C. albicans, C. glabrata, and C. tropicalis, account for 80% of fungal infections in the oral cavity. Candida species have the isolation rates of 20-75% and 50-65% from the oral cavity in the general population and individuals wearing removable dentures, respectively [ 2 , 3 ].
The widespread use of antifungal agents, particularly azole drugs, has caused a considerable increase in clinical resistance and failure to respond in the current scenario of fungal diseases [ 4 ]. This has implicated the utmost need for replacing chemical drugs with herbal medicines, which date back centuries ago. There are drawbacks to the conventional antifungal arsenal due to the high toxicity of the compounds, cost factors, drug interactions, and more importantly antimicrobial resistance. Therefore, there is a substantial need to explore a novel antifungal agent that enhances the bioavailability in order to improve the antifungal spectrum and combat resistance [ 5 ]. The plants of Lythraceae, also called the loosestrife family, have either wild or cultivated species and have gained medicinal importance due to their numerous pharmacological activities [ 6 ]. With an aim to discover a novel antifungal agent, the present study was conducted to investigate the antifungal activity of two plants from the same family, namely Punica granatum (Pg) and Woodfordia fruticosa (Wf), for uncontrolled diabetic patients wearing removable dentures.
The Wf, also called fire flame bush or Dhawai, contains various natural compounds, such as tannins, phenols, terpenoids, steroids, and carbohydrates [ 7 ]. There is evidence on the in vitro antimicrobial effect of Wf leaves and flowers; however, these effects have not been examined in any in vivo studies till date. The results of multiple studies, such as those conducted by Parekh et al. (2007) [ 8 ], Kumaraswamy et al. (2008) [ 9 ], Chougale et al. (2009) [ 10 ], Kaur et al. (2010) [ 11 ], and Kharate et al. (2018) [ 12 ], have confirmed the inhibitory effect of Wf against various bacterial strains.
The Pg, also called pomegranate, is a fruit distributed worldwide. It has innumerable bioconstituents, such as flavonoids, polyphenols, terpenoids, alkaloids, tannins, ellagic acid, and gallic acid [ 13 ]. The findings of various studies, such as those performed by Vasconcelos et al. (2003, 2006) [ 14 ], Duman et al. (2009) [ 15 ], Anibal et al. (2010) [ 13 ], Ferrazzano et al. (2017) [ 16 ], and Bassiri-Jahromi et al. (2015, 2018) [ 17 ], are suggestive of the potent antifungal efficacy of Pg and its role in the inhibition of the growth of various Candida species. The overview of the literature on these two plants indicates the scarcity of the studies investigating the antifungal effect (especially with regard to Wf) against Candida species. Regarding this, the present study was conducted to specifically examine the antifungal activity of the ethanolic extracts of these two plants in uncontrolled diabetic patients wearing removable dentures. It was also aimed to identify and isolate the active substance in both plants.
Materials and Methods
The selected plants, namely Wf and Pg, were collected from the SM Heena Industries in India in June 2019. The chosen plants were taxonomically authenticated and identified at the Deccan branch, Botanical Survey of India Hyderabad, with the herbarium voucher No. D-95.
Extraction of secondary metabolites and biochemical test for Woodfordia fruticosa leaf and Punica granatum peel
The dried Wf leaves (Wfl) and Pg peel (Pgp; 1500 gm) were put in a mechanical grinder. The obtained coarse powder was then subjected to extraction with ethanol using a soxhlet extractor. Finally, the obtained extract was dried in a vacuum using a rotary evaporator. The yield percentage was calculated using the following formula [ 18 ]:
Extract yield%=R/S×100
where R is the weight of the extracted plant residues and S is the weight of the raw plant sample.
For phytochemical analysis, the extracts were dissolved in ethanol (1 mg/ml). Phytochemical screening was performed, and biochemical tests were conducted. Some of the secondary metabolites found in Wfl were glycosides and carbohydrates, and those in Pgp were flavonoids, steroids, and terpenoids.
To confirm the presence of these phytoconstituents, high-performance thin-layer chromatography (TLC) was carried out. In doing so, the mobile phase of ethanol: isopropyl alcohol: triethylamine was taken at the ratio of 6:5:3:5:1.0, and the stationary phase of silica gel was used. The spraying reagents, like glacial acetic acid and concentrated sulfuric acid, were used to visualize the number of spots. Based on color and retention factor (Rf) value, the plates were visualized at visible light with the UVs of 254 and 366 nm, respectively. The Rf value was calculated using the following formula (Kumar S et al., 2013) [ 19 ]:
Rf=Distance travelled by component Distance travelled by solvent
The identified metabolites were then separated by means of TLC.
Preparation of mouthwash
To prepare the mouthwash, 0.8 gm of each plant extract was superadded with 10 ml glycerol, 0.2 gm L- menthol, 0.05 gm peppermint oil, and 0.2 gm citric acid. Subsequently, sufficient water was added to make a total volume of 100 ml. The mouthwash was formulated according to the procedure adopted by Sritrairat et al. (2011) [ 20 ].
Study population
The present study was conducted at the Department of Oral Medicine and Radiology between 2019 and 2020. The study procedures were in accordance with the CONSORT and Declaration of Helsinki (revised in 2013) guidelines. The study protocol was approved by the Institutional Ethics Committee of the Central Drugs Standard Control Organisation (No. ECR/804/Inst/AP/2016). Informed consent was obtained from all the subjects willing to participate in the research. The present study was a continuation of our previous research published in the Journal of Clinical and Diagnostic Research in 2016 with the Clinical Trial Registry number of REF/2016/03/11053.
A total of 100 subjects who were diabetic and wore denture, either removable or fixed types, were randomly selected by a simple randomization method using a coin toss. The participants were evaluated for blood sugar levels by glycosylated hemoglobin assay, and the test results were standardized according to the American Diabetes Association. Those with a hemoglobin A1C level of > 7 were considered poor diabetic and included in the study. Some of the patients had such clinical symptoms as the burning and painful sensation of oral cavity or change of taste; however, most of them were asymptomatic. The inclusion criteria were: 1) pregnancy or lactation, 2) allergy to prepared mouthwash, 3) diagnosis of other systemic diseases, and 4) use of herbal products or form of mouthwash in the last 2 months.
Study groups and procedure for the estimation of antifungal activity
A total of 100 subjects were investigated in this double-blinded study in two groups based on the coin toss (n=100, age: 35-65 years, both women and men). Group I (n=50) were provided with Pgp mouthwash, while Group II (n=50) was given Wfl mouthwash. The participants, as well as the physician, were blinded to the allocated product. Following the administration of the mouthwash, the patients were requested to rinse the mouthwash using the oral rinse technique. The samples were collected at three time points. In this regard, at the baseline, each subject was asked to rinse his/her mouth using physiological saline; subsequently, the swish (i.e., amount of mouthwash mix collected from patients' mouth after using mouthwash) was collected in sterile containers, which was then subjected to CFU estimation.
Later, the subjects were randomly allotted to use either of the mouthwash, and the post-therapeutic samples were collected after 1 h and 1 week. The subjects were advised to use the given mouthwash twice a day (5 ml/rinse for 30 sec). They were also instructed to write about their compliance in a diary on a daily basis to obtain a home assessment of the treatment. The participants’ subjective satisfaction with the mouthwash taste and smell was assessed using a 9-point hedonic scale [ 21 ]. In this regard, positive numbers were interpreted as moderate to strong satisfaction, whereas negative numbers were representative of weak satisfaction.
The burning sensation was assessed using the Visual Analog Scale [ 22 ]. In addition, allergy to the prescribed mouthwash was evaluated at different time points. After the collection of samples in sterile containers, a serial dilution technique (3 dilutions for each sample) was performed where 0.2 ml of each sample was diluted in 9.8 ml of physiological saline. The third dilution was used for the determination of the total microbial count. The CFU was estimated using the following formula:
CFU/mL=1,000×number of colonies/3
The identification of Candida species was accomplished using HiChrome Candida differential agar (HiMedia Lab, Mumbai, India). In this medium, the observation of green, metallic blue, and cream or white colors were regarded to denote C. albicans, C. tropicalis, and C. glabrata, respectively (Figure 1).
Figure 1.
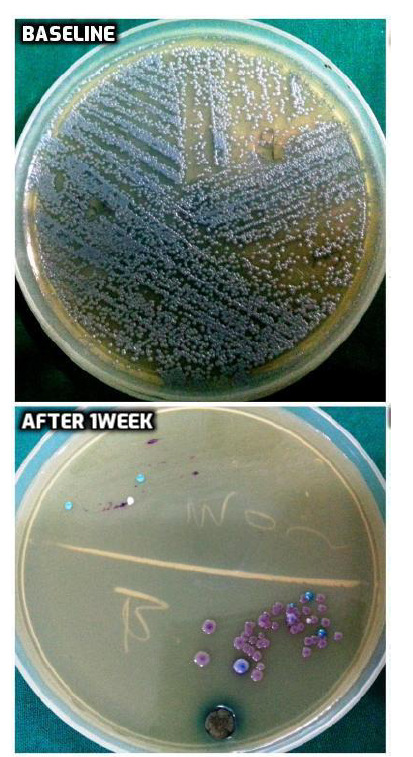
Colony forming units in HiChrome agar at the baseline and 1-week time after using Woodfordia leaf mouthwash
Statistical analysis
The data were analyzed in SPSS software (version 21.0) for windows. The data were expressed as mean±standard deviation. In addition, the confidence interval was calculated using inferential statistics to find the uncertainties associated with the sampling method. A p-value of ≤ 0.05 was considered statistically significant, and a p-value of ≤ 0.01 was regarded as highly significant.
Results
The results were indicative of a decrease in the mean CFU count in both groups at different time points. This mean value showed a drastic reduction in the Wfl group both 1 h and 1 week after the intervention. Accordingly, Wfl mouthwash was more effective in reducing CFU count at all-time points. This result can be attributed to the excellent antifungal activity of Wfl (Figure 2). Given the higher antifungal efficacy of Wfl mouthwash, a spectral analysis was performed on this product in order to explore the exact phytoconstituent responsible for its antifungal effect.
Figure 2.
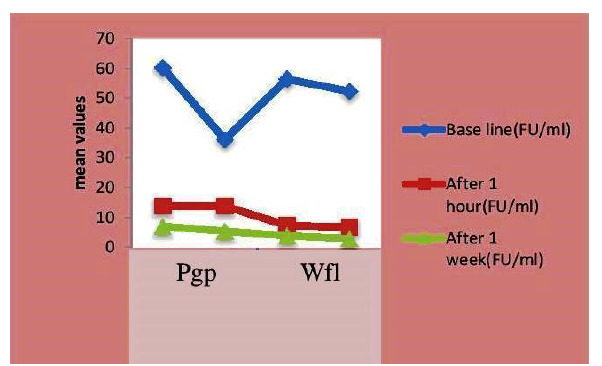
Line graph representing mean reduction of colony forming units per millimeter at different time points in both groups
Spectral analysis of Woodfordia fruticosa leaf
The interpretation of the spectral data was based on physicochemical properties and their comparison with the data presented in the literature. The functional groups, number of protons and carbons in the structure, and molecular weight of the compound were recorded by the infrared spectra, proton nuclear magnetic resonance spectroscopy, carbon-13 nuclear magnetic resonance, and mass spectrometry, respectively. The spectral analysis revealed glycosides and carbohydrates as the bioconstituents of Wfl. The details are described in The details are described in Table 1.
Table 1.
Quantitative analysis of Woodfordia fruticosa leaf showing chemical characterization
| 3rPosition | C atom | δ 13C (ppm) | δ 1H (ppm) |
|---|---|---|---|
| 1 | C | 145.11 | - |
| 2 | CH | 94.40 | 6.25 |
| 3 | C | 153.71 | - |
| 4 | CH | 95.51 | 6.24 |
| 5 | C | 157.16 | - |
| 6 | CH | 92.25 | 6.13 |
| 1 | OCH3 | 57.41 | 3.96-3.90 |
| 3 | OCH3 | 56.83 | 3.746-3.70 |
| 11 | CH | 100.83 | 4.97(Glu-H) |
| 21 | CH-OH | 78.23 | 3.29 (Glu-H) |
| 31 | CH-OH | 77.51 | 3.41(Glu-H) |
| 41 | CH-OH | 74.71 | 3.38(Glu-H) |
| 51 | CH-OH | 77.26 | 3.27(Glu-H) |
| 6a1 | CH-OH | 69.76 | 3.28(Glu-H) |
| 6b1 | CH-OH | 69.76 | 3.26(Glu-H) |
C: Carbon atom, δ: delta scale showing chemical shifts, 13C: carbon- 13, ppm: parts per million,1H: hydrogen-1, CH-methane, CH-OH - Hydroxy methyl group, OCH3- Oxygen ,methyl group- Methoxygroup, Glu-H: glucosyl ions
Discussion
In recent times, the rise in the incidence of fungal infections and clinical resistance to conventional antifungal therapy has implicated an imperative need for the introduction of novel, preventive, and therapeutic approaches for the treatment of oral candidiasis [ 23 ]. Appropriate target identification and rational drug design technologies using the small molecules of natural products can accelerate the development of antifungal agents [ 24 ]. There has been much emphasis on the in vitro antimicrobial activity of Wf. Moreover, the results of several studies have been confirmative of the antifungal activity of Punica. However, to the best of our knowledge, the antifungal activity of the ethanolic extracts of these two plants has not yet been discussed in patients with uncontrolled diabetes.
Endocrine disorders, such as diabetes mellitus, can be a predisposing factor for oral candidiasis. The review of the literature in such databases as the Embase, Medline, Science citation index, NIH public access, PubMed, and Cochrane database of systematic reviews, revealed no relevant information on this type of clinical trial. Hence, our research particularly targeted the antifungal activity of the ethanolic extract of these two plants in patients with uncontrolled diabetes. Before the implementation of the present in vivo trial, the toxic study parameters were considered based on a study performed by Palande et al. [ 25 ] addressing the acute oral toxicity of Wfl in Wistar rats and proving its safety up to a dose of 2,000 mg/kg.
Likewise, Patel et al. [ 26 ] examined the acute toxicity of pomegranate peel extract (PPE) and revealed that it could be safely administered up to a dose of 5,000 mg/kg. Hence, in the present research, 1,500 mg of each plant was used, which was within the determined limit. The ethanolic extracts were preferred in the current study due to their capacity to penetrate the cellular membrane and facilitate the extraction of the intracellular ingredients from the plant, resulting in enhanced efficacy against Candida isolates. Moreover, ethanol is considered to be the best solvent for the extraction of the majority of phytoconstituents [ 27 ].
In the present study, the ethanolic extract of Wfl mouthwash showed a superior antifungal activity with respectively 50% and 85% decrease in viable colonies 1 h and 1 week after the intervention, compared to Pgp. Similar to our study, Dubey et al. reported effective antimicrobial activity for the ethanolic extracts of Wf, and their findings clearly supported our study [ 28 ]. Kharate et al. also determined the antibacterial activity of the methanolic, hexane, chloroform, and acetone extracts of Wf, where methanolic extract showed superior antimicrobial activity [ 12 ]. Similarly, Dabur et al. evaluated the antibacterial activity of Wf against various bacterial strains and C. albicans using different solvents and observed efficient activity against the mentioned microorganisms, which is consistent with our research findings [ 29 ].
In another study performed by Sahu et al. [ 7 ], evaluating the antimicrobial activity of Wf, such metabolites as tannins, saponins, glycosides, and flavonoids were found to be responsible for the effect. However, in our study, the spectral data particularly revealed glycosides to be responsible for the antifungal effect. These findings are in accordance with those obtained by Dubey et al. [ 30 ], Grover et al. [ 31 ], and Kabesh et al. [ 32 ].
The antifungal activity of phytoglycosides has been mediated through multiple targets. The results of a study carried out by Zhang et al. [ 33 ] revealed that antifungal glycosides damage the plasma membrane, and that the leakage of cytoplasmic material causes cell death. Park et al. [ 34 ] indicated that this activity may be the result of the membrane disruption mechanisms. Likewise, Freiesleben et al. [ 35 ] suggested that after the complex formation of cholesterol, glycosides attach to the lyophilic moiety inside the membrane and hydrophilic moiety outside the cell, thereby suppressing the fungal growth.
A recently proposed mechanism by Lee et al. [ 36 ] is the inhibition of the calcineurin pathway. Calcineurin is a highly specific Ca2+-dependent serine- threonine phosphatase that has a key role in mediating stress cell response, which particularly helps in suppressing the growth of fungal species, primarily C. albicans. Accordingly, these possible mechanisms can account for the marked antifungal effect with the glycosides of Wfl as observed in our study. However, further research is required to determine the exact mechanisms involved in this particular effect.
The present study also involved the investigation of the antifungal effect of Pgp. Our results revealed a higher decrease in fungal colonies 1 week after the use of Pgp mouthwash. However, Wfl exhibited superior antifungal efficacy inducing a more significant decrease in viable colonies. There are no comparable studies addressing the antifungal activity of these two plants. Accordingly, the present study is the first of its kind, which effectively demonstrated the therapeutic protocol and determined the antifungal potential of these two plants.
The antifungal mechanism of Pgp has been confirmed by the presence of its active inhibitors, such as phenolics and flavonoids [ 37 ]. Different solvents have been used in various studies for the extraction of phytocompounds in different parts of Punica; however, it depends on the type of solvent used and the polarity. Lavaee et al. compared the efficacy of the methanolic and ethanolic extracts of the bark and roots of Punica in the isolation of Candida species and found that the methanolic extracts were more effective [ 38 ]. Accordingly, in the current study, ethanolic extracts were used.
Several studies have been performed on the antifungal effect of Candida. In this regard, Mansourian et al. reported that PPE had definite anti- Candidal activity based on the agar well diffusion method in vitro [ 39 ]. Likewise, Endo et al. described PPE as a potent inhibitor of C. albicans [ 40 ]. Moreover, Tayel et al. demonstrated that the application of PPE aerosol was an efficient method for complete sanitization and prevention against C. albicans growth in semi-closed places [ 41 ].
The presence of chemical components, such as flavonoids and terpenoids, in the current study revealed the antifungal effect of Pgp. Flavonoids inhibit fungal growth with several underlying mechanisms, such as the disruption of plasma membrane, induction of mitochondrial dysfunction, inhibition of the cell wall formation, cell division, RNA and protein synthesis, and efflux-mediated pumping systems [ 42 ]. Lolita et al. [ 43 ] evaluated the antifungal effect of the ethanolic extracts of red pomegranate peel against C. albicans and revealed flavonoids as the secondary metabolites in ethanolic extract responsible for the effect. The results of the mentioned study are evidently in accordance with our findings where the ethanolic extract of Pgp and metabolites, like flavonoids, were responsible for the antifungal effect.
Terpenoid was found to be another agent in Pgp accounting for the antifungal effect. The mechanism of action of this compound is the inhibition of morphogenesis, cell adhesion, and biofilm formation of Candida [ 44 ]. Based on the abovementioned findings, it is required to perform studies that can evaluate the in vivo use of phototherapeutic products, such as W. fruticosa and Punica, to obtain accurate results regarding their potential inhibitory effects against oral candidal infections and use them as a suitable target for discovering a novel anticandidal agent.
Conclusion
The data obtained in our research indicated that Wfl was a potential inhibitor of oral Candida infection in patients with uncontrolled diabetes wearing removable dentures. The bioactive compounds, like glycosides, in Wf would have exerted their antifungal effect through multiple mechanisms, as described by various researchers. Furthermore, the advances in the discovery of new antifungal targets may help develop new formulations by encouraging the researchers to work on the isolation and characterization of phytoconstituents. Such new drug leads could help identify candidate medications for the treatment of the fungal infections of the oral cavity.
Acknowledgement
We declare no source of funding
Author’s contribution
B. S. contributed substantially to the conception and design of the study, acquisition of data, drafting of the article, and approval of the final version. S. S. designed the experiment and co-wrote the paper. B. R. P. analyzed the data and wrote the manuscript. C. R. performed the critical revision of the article and statistical analysis. K. N. M. revised the article and performed the final approval.
Conflict of Interest: We declare no conflicts of interest.
Financial disclosure
The authors have no relevant financial disclosures in this article.
References
- 1.Manning DJ, Coughlin RP, Poskitt EM. Candida in mouth or on dummy? . Arch Dis Child. 1985;60(4):381–2. doi: 10.1136/adc.60.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berdicevsky I, Ben-Aryeh H, Szargel R, Gutman D. Oral Candida in children. Oral Surg Oral Med Oral Pathol. 1984; 57(1):37–40. doi: 10.1016/0030-4220(84)90257-3. [DOI] [PubMed] [Google Scholar]
- 3.Cumming CG, Wight C, Blackwell CL, Wray D. Denture stomatitis in the elderly. Oral Microbiol Immunol. 1990; 5(2):82–5. doi: 10.1111/j.1399-302x.1990.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 4.Ostrosky-Zeichner L, Casadevall A, Galgiani JN, Odds FC, Rex JH. An insight into antifungal pipeline: selected new molecules and beyond. Nat Rev Drug Discov. 2010; 9(9):719–27. doi: 10.1038/nrd3074. [DOI] [PubMed] [Google Scholar]
- 5.Scorzoni L, De Paula e Silva, Marcos CM, Assato PA. Antifungal therapy: new advances in understanding and treatment of mycosis. Front Microbiol. 2017; 8:36. doi: 10.3389/fmicb.2017.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maberley DJ. Mabberley's plant-book: a portable dictionary of plants, their classification and uses. Cambridge: Cambridge University Press; 2017. [Google Scholar]
- 7.Sahu VK, Ahmad S. Phytochemical screening of secondary metabolites present in Woodfordia fruticosa leaves and their antibacterial properties with different solvent extracts. Int J Quart Environ Science. 2015; 7(1):260–6. [Google Scholar]
- 8.Parekh J, Chanda S. In vitro antibacterial activity of the crude methanol extract of Woodfordia fruticosa Kurz. flower (Lythraceae) Braz J Microbiol. 2007; 38(2):204–7. [Google Scholar]
- 9.Kumaraswamy MV, Kavitha HU, Satish S. Antibacterial potential of extracts of Woodfordia fruticosa kurz on human pathogens. World J Med Sci. 2008; 3(2):93–6. [Google Scholar]
- 10.Chougale AD, Padul MV, Arfeen S, Kakad SL. Antibacterial activity directed fractionation of Woodfordia fruticosa kurz. Leaves. 2009; 8(31):75–81. [Google Scholar]
- 11.Kaur R, Kaur H. Antimicrobial activity of essential oil and plant extracts of Woodfordia fruticosa. Arch Appl Sci Res. 2010; 2(1):302–9. [Google Scholar]
- 12.Kharate MS, Pandhure NB. Phytochemical and antimicrobial properties of medicinal plant crude extract, Woodfordia fruticosa Linn from Marathwada, Maharashtra state. Int J Res Appl Sci Eng Technol. 2018; 6(1):38. [Google Scholar]
- 13.Anibal C. Antifungal activity of the ethanolic extracts of Punicagranatum L. and evaluation of the morphological and structural modifications of its compounds upon the cells of Candida spp. Braz J Microbiol. 2013; 44(3):839–48. doi: 10.1590/S1517-83822013005000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.218884545656500Vasconcelos LC, Sampaio FC, Sampaio MC, Pereira Mdo S, Higino JS, Peixoto MH. Minimum inhibitory concentration of adherence of Punicagranatum Linn (pomegranate) gel against S. mutans, S. mitis and C. albicans. Braz Dent J. 2006; 17(3):223–7. doi: 10.1590/s0103-64402006000300009. [DOI] [PubMed] [Google Scholar]
- 15.Duman AD, Ozgen M, Dayisoylu KS, Erbil N, Durgac C. Antimicrobial activity of six pomegranate (Punicagranatum L. ) varieties and their relation to some of their pomological and phytonutrient characteristics. Molecules. 2009; 14(5):1808–17. doi: 10.3390/molecules14051808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrazzano GF, Scioscia E, Sateriale D, Pastore G, Colicchio R, Pagliuca C. In vitro antibacterial activity of pomegranate juice and peel extracts on cariogenic bacteria. Biomed Res Int. 2017; 2017:2152749. doi: 10.1155/2017/2152749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bassiri-Jahromi S, Pourshafie MR, Ardakani EM, Ehsani AH, Doostkam A, Katirae F. In vivo comparative evaluation of the Pomegranate (Punicagranatum) peel extract as an alternative agent to Nystatin against Oral Candidiasis. Iran J Med Sci. 2018; 43(3):296–304. [PMC free article] [PubMed] [Google Scholar]
- 18.Mostafa AA, Al-Askar AA, Almaary KS, Dawoud TM, Sholkamy EN, Bakri MM. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J Biol Sci. 2018; 25(2):361–6. doi: 10.1016/j.sjbs.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar S, Jyotirmayee K, Sarangi M. Thin layer chromatography: a tool of biotechnology for isolation of bioactive compounds from medicinal plants. Int J Pharm Sci Rev Res. 2013; 18(1):126–32. [Google Scholar]
- 20.Sritrairat N, Nukul N, Inthasame P, Sansuk A, Prasirt J, Leewatthanakon T, et al. Antifungal activity of lawsone methyl ether in comparison with chlorohexidine. J Oral Pathol Med. 2011; 40(1):90–6. doi: 10.1111/j.1600-0714.2010.00921.x. [DOI] [PubMed] [Google Scholar]
- 21.Lim J. Hedonic scaling: a review of methods and theory. Food Qual Prefer. 2011; 22(8):733–47. [Google Scholar]
- 22.Kakoei S, Pardakhty A, Maryam-Al-Sadat Hashemipour HL, Kalantari B, Tahmasebi E. Comparison the pain relief of amitriptyline mouthwash with benzydamine in oral mucositis. J Dent. 2018; 19(1):34. [PMC free article] [PubMed] [Google Scholar]
- 23.Wise R, Hart T, Cars O, Streulens M, Helmuth R, Huovinen P, et al. Antimicrobial resistance. Is a major threat to public health. BMJ. 1998; 317(7159):609–10. doi: 10.1136/bmj.317.7159.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheng C, Zhang W. New lead structures in antifungal drug discovery. Curr Med Chem. 2011; 18(5):733–66. doi: 10.2174/092986711794480113. [DOI] [PubMed] [Google Scholar]
- 25.Palande N. Acute oral toxicity study of extract derived from Woodfordia fruticosa in Albino Wistar Rats. Glob J Res Scholar. 2016; 6(1):8–11. [Google Scholar]
- 26.Patel C, Dadhaniya P, Hingorani L, Soni MG. Safety assessment of pomegranate fruit extract: acute and sub-acute toxicity studies. Food Chem Toxicol. 2008; 46(8):2728–35. doi: 10.1016/j.fct.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 27.Chandel SS. Preliminary phytochemical and physicochemical investigation of Woodfordia fruticosa (Linn) Kurz root. Int J Green Pharmacy. 2018; 12(4):840–6. [Google Scholar]
- 28.Dubey D, Sahu MC, Rath S, Paty BP, Debata NK, Padhy RN. Antimicrobial activity of medicinal plants used by Aborigines of kalahandi, orissa, India against multidrug resistant bacteria. Asian Pac J Trop Biomed. 2012; 2(2): S846–54. [Google Scholar]
- 29.Rani S, Rahman K, Younis M, Basar SN. Dhawa (Woodfordia fruticosa (L. ) Kurz. ): a versatile medicinal plant . Int J Pharm Sci Drug Res. 2015; 7(4):315–20. [Google Scholar]
- 30.Dubey D, Patnaik R, Ghosh G, Padhy RN. In vitro antibacterial activity, gas chromatography–mass spectrometry analysis of Woodfordia fruticosa Kurz. leaf extract and host toxicity testing with in vitro cultured lymphocytes from human umbilical cord blood. Osong Publ Health Res Perspect. 2014; 5(5):298–312. doi: 10.1016/j.phrp.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grover N, Meena R, Patni V. Physiochemical evaluation, phytochemical screening and chromatographic fingerprint profile of Woodfordia fruticose (L. ) Kurz extracts. Int J Pharm Sci Res. 2014; 5(7):2772–82. [Google Scholar]
- 32.Kabesh K, Senthilkumar P, Ragunathan R, Kumar RR. Phytochemical analysis of Catharanthus roseus plant extract and its antimicrobial activity. Int J Pure App Biosci. 2015; 3(2):162–72. [Google Scholar]
- 33.Zhang JD, Xu Z, Cao YB, Chen HS, Yan L, An MM, et al. Antifungal activities and action mechanisms of compounds from Tribulus terrestris L. J Ethnopharmacol. 2006; 103(1):76–84. doi: 10.1016/j.jep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 34.Cana PA, Jaeyong CH, Hwang B, Hwang IS, Mi Ran Kim ER, Lee DG. Styraxjaponoside A and B, Antifungal Lignan Glycosides Isolated from Styrax japonica S. et Z. Biomol Ther. 2010; 18(4):420–5. [Google Scholar]
- 35.Freiesleben S, Jäger A. Correlation between plant secondary metabolites and their antifungal mechanisms–a review. Med Aromatic Plants. 2014; 3(2):1–6. [Google Scholar]
- 36.Lee WJ, Moon JS, Kim SI, Bahn YS, Lee H, Kang TH, et al. A phenylpropanoid glycoside as a calcineurin inhibitor isolated from Magnolia obovata Thunb. J Microbiol Biotechnol. 2015; 25(9):1429–32. doi: 10.4014/jmb.1506.06031. [DOI] [PubMed] [Google Scholar]
- 37.Al-Zoreky NS. Antimicrobial activity of pomegranate (Punicagranatum L. ) fruit peels. Int J Food Microbiol. 2009; 134(3):244–8. doi: 10.1016/j.ijfoodmicro.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Lavaee F, Motaghi D, Jassbi AR, Jafarian H, Ghasemi F. Antifungal effect of the bark and root extracts of Punica granatum on oral Candida isolates. Curr Med Mycol. 2018; 4(4):20. doi: 10.18502/cmm.4.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mansourian A, Boojarpour N, Ashnagar S, Beitollahi JM, Shamshiri AR. The comparative study of antifungal activity of Syzygium aromaticum, Punica granatum and nystatin on Candida albicans; an in vitro study. J Mycol Med. 2014; 24(4): e163–8. doi: 10.1016/j.mycmed.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Endo EH, Ueda-Nakamura T, Nakamura CV, Filho BP. Activity of spray-dried micro particles containing pomegranate peel extract against Candida albicans. Molecules. 2012; 17(9):10094–107. doi: 10.3390/molecules170910094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tayel AA, El-Tras WF. Anticandidal activity of pomegranate peel extract aerosol as an applicable sanitizing method. Mycoses. 2010; 53(2):117–22. doi: 10.1111/j.1439-0507.2008.01681.x. [DOI] [PubMed] [Google Scholar]
- 42.Saleh MS, Mickymaray S. Antifungal efficacy and mechanisms of flavonoids. Antibiotics. 2020; 9(2):45. doi: 10.3390/antibiotics9020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parera LA, Lawa Y, More E. The effect of ethanol extracts of red pomegranate peel (Punica granatum L. ) on activities Candida albicans. Environ Conservat. 2019; 25:81–6. [Google Scholar]
- 44.Zore GB, Thakre AD, Jadhav S, Karuppayil SM. Terpenoids inhibit Candida albicans growth by affecting membrane integrity and arrest of cell cycle. Phytomedicine. 2011; 18(13):1181–90. doi: 10.1016/j.phymed.2011.03.008. [DOI] [PubMed] [Google Scholar]


