INTRODUCTION
Venous thromboembolism (VTE) is responsible for 3% of all maternal deaths worldwide according to data from the World Health Organization.1 Data from the developed world suggest that death rates from VTE are significantly higher, as VTE remains one of the leading causes of maternal deaths.1,2 Recent analysis of maternal mortality in the United States showed that VTE accounted for 15% of all maternal deaths between 2003 and 2011.3 Sub-standard care occurred in more than half of all deaths4 from pulmonary embolism (PE) in the confidential enquiry into maternal deaths in the United Kingdom, highlighting the importance of understanding and overcoming challenges in the care of this condition in pregnancy. A clinician who does not treat pregnant women on a regular basis may not routinely consider many of the pregnancy-specific risk factors for VTE. Pretest probability is also quite complicated in pregnancy, as pretest probability rules have not been validated in this population, compelling the clinician to rely more heavily on imaging tests. Diagnostic procedures are also fraught with concerns about diagnostic accuracy in this population, as well as concerns for fetal safety, teratogenicity, and oncogenicity. Treatment strategies are complex, and providers need to balance efficacy while considering fetal safety, teratogenicity, pharmacodynamics, the unexpected nature of labor and delivery, and the subsequent need to weigh the risk of anticoagulation with the risk of clot recurrence perinatally. In this review, we examine the pregnancy-specific nuances in the risk assessment pretest probability, diagnostic evaluation, and therapeutic considerations.
Epidemiology
The risk of peripartum VTE is increased, with the postpartum period conferring a higher day-to-day risk than the antepartum period. The risk of VTE is estimated at 5 to 12 per 100,000 pregnancies antepartum compared with age-matched nonpregnant women, translating into a 0.1% absolute risk.5,6 Although the absolute risk of VTE is lower in the postpartum period compared with the antepartum period, estimated at 0.05%,7,8 the day-to-day risk is significantly higher when considering the significantly shorter postpartum period. The risk for VTE is highest in the first 6 weeks postpartum. Although previous data had suggested that the epidemiologic risk9,10 and the biochemical hematological changes11,12 that occur in pregnancy return to baseline at 6 weeks postpartum, recent claims data have shown a twofold increase in the risk of venous thrombosis (odds ratio [OR] 2.2, 95% confidence interval [CI] 1.4–3.3) from 7 to 12 weeks postpartum compared with the same time period a year later.13
Pathophysiology of Thromboembolic Disease in Pregnancy
Venous stasis, vascular injury, and hypercoagulable state (Virchow triad) are all responsible for the increased risk of VTE in pregnancy. Inherited thrombophilias and other risk factors also contribute to the development of VTE in pregnancy.
Venous stasis
In pregnancy, through progesterone-induced veno-dilation,14 renal vasodilation occurs simultaneously with systemic vasodilation and leads to 30% to 50% increase in renal blood flow and glomerular filtration rate (GFR). This rise in GFR increases distal sodium delivery, allowing for escape from the sodium-retaining effect of aldosterone. The volume expansion secondary to aldosterone increases the atrial natriuretic peptide, which in turn inhibits sodium reabsorption in the distal tubules leading to an increase in systemic volume and Na retention.15 The increase in total body blood volume leads to an increase in blood volume in the lower extremities, from 94.7 ± 27.3 mL in nonpregnant individuals to 110.1 ± 30.2 mL in pregnancy. There is also an increase in the diameter of the common femoral vein from 10.14 ± 1.24 mm to 12.72 ± 2.27 mm, as well as proportional increases in the saphenous and popliteal vein diameters.16 This rise in venous blood volume and pressure, along with resulting distension of the vessels, leads to stasis and increased lower extremity edema. However, it has been proposed that, unlike VTE in the general population, VTE in pregnancy may start in the pelvis17 rather than the lower extremities, as the percentage of isolated pelvic deep venous thrombosis is significantly higher in pregnancy.18 As the right common iliac artery crosses over the left common iliac vein, a pulsatile compression of the left-sided venous system ensues. This compression is implicated in the increase in left-sided DVTs in pregnant women, with an occurrence of 90% on the left side compared with 55% of the time in nonpregnant individuals.19,20
Vascular dysfunction and injury
In normal pregnancy there are circulating cytokines and growth factors that may contribute to the breakdown of the endothelial monolayer. This can lead to vascular dysfunction and injury by degrading or removing cell junctional proteins.21 Endothelial injury also can occur during normal labor, as well as during surgical delivery.22 In addition, the increase in blood volume and diameter of vessels causes sheer stress on the vessels, potentially leading to vascular damage.21
Hypercoagulable state
During pregnancy, the blood becomes hypercoagulable with increases in procoagulation factors V, VII, VIII, IX, X, and XII and von Willebrand factor. Factor VII increases up to 10-fold, whereas fibrinogen rises 2-fold.23 Von Willebrand and factor VIII are elevated in late gestation and factor XI tends to decrease during pregnancy. There is also a decrease in anticoagulant activity with a decrease in protein S with gestational age, whereas protein C activity remains unchanged.23 Fibrinolysis is reduced in pregnancy as a result of an enhanced activity of plasminogen activator inhibitor type I and II and a decreased activity of tissue plasminogen activator.24
Inherited thrombophilias
Up to 40% of women who develop VTE while pregnant are found to have an inherited thrombophilia.19 In addition, a reported OR of 51.8 (95% CI 38.7–69.2) for thrombophilia was described in women with VTE in a study of the National Inpatient Sample evaluating risk factors for VTE.25 Inherited thrombophilias associated with increased risk of VTE in pregnancy include factor V Leiden, Prothrombin G20210 A mutation, antithrombin deficiency, protein C deficiency, and protein S deficiency.26 Estimates of reported thrombophilias vary in the literature and are based on populations studied.27–29 Heterozygous factor V Leiden is associated with an absolute risk of up to 3% and has been observed in up to 40% of VTE cases occurring during pregnancy. Homozygous factor V Leiden is not as common, but carries a significantly higher absolute risk, reported to be up to 14%, whereas the absolute risk of VTE among women with protein C or protein S deficiency has been reported to be up to 6%. Prothrombin G20210 A mutation has a prevalence of 3% and has been reported in up to 17% of VTE cases in pregnancy. Antithrombin deficiency has a prevalence of up to 0.6% with a 25-fold increased risk for VTE. Protein C deficiency has a prevalence of 0.2% to 0.3% with a risk for VTE of 2% to 7%, whereas protein S deficiency has a prevalence of less than 0.1% with a risk for VTE of 6% to 7%.30
Other risk factors
Additional risk factors for VTE in pregnancy include the same risk factors for VTE in the general nonpregnant population, as well as risk factors that are specific to pregnancy itself. Nonpregnancy-related risk factors29 include age older than 35, obesity, varicose veins, paraplegia, sickle cell disease, and heart disease, along with other medical comorbidities such as nephrotic syndrome, systemic lupus erythematosus, and inflammatory bowel disease. Prior VTE carries an OR for subsequent VTE of 24.8 (95% CI 17.1–36).25 Some risk factors are specific for the antepartum period, whereas others are specific for the postpartum period.
Risk factors associated with antepartum VTE include immobility, assisted reproductive technique, smoking, obesity, and antepartum hemorrhage.29 Risk factors for postpartum VTE include immobility, placenta abruption, preeclampsia, growth restriction, Cesarean delivery,31 postpartum infection, postpartum hemorrhage, and obesity.29 More recently, a UK-based registry has developed a postpartum VTE risk prediction model. This model was then validated in a Sweden-based registry and showed that the most predictive risk factors included varicose veins, stillbirth, preeclampsia, postpartum infection, emergency Cesarean delivery, and medical comorbidities. Of note, this model did not assess thrombophilia or past VTE.8 In addition, some risk factors have a synergistic effect in pregnancy. For instance, strict antepartum immobilization for at least a week in women with an elevated body mass index at the first prenatal visit is associated with a 62-fold risk for antepartum VTE and a 40-fold risk for postpartum VTE.32
DIAGNOSIS
Signs and symptoms of VTE in pregnancy are similar to those in nonpregnant individuals. These include shortness of breath, tachycardia, leg pain or swelling, pelvic discomfort, and chest pain. Shortness of breath is the most common presenting symptom (34.7%), followed by tachycardia (30.4%), leg pain or weakness (9.6%), and chest pain (13%).33 In addition to history and physical examination findings, multiple laboratory and imaging tests are used in the general population to help guide and diagnose VTE in pregnancy. These tests include D-dimer, brain natriuretic peptide (BNP), troponin, chest radiographs (CXR), multidetector computed tomography with pulmonary angiogram (CTPa), compression Doppler ultrasonography (CUS), and ventilation perfusion scan (V/Q scan).
Clinical Pretest Probability Rules
In the general population, pretest probability tools are used to determine likelihood of PE. These tests can then further guide the diagnostic approach toward imaging or D-dimer testing. Pretest probability rules have not been validated in pregnancy, and although some studies have suggested good correlation between rules, such as the modified Wells score and the diagnosis of PE, these rules have not been validated to date. The existing rules do not consider pregnancy and pregnancy-specific risk factors, and include some factors that may not be relevant or applicable to the pregnant population. Although sensitivity and negative predictive value appear to be excellent in retrospective cohorts using the Wells’ criteria in peripartum women, these studies are limited by their retrospective design, the lack of combining the tool with D-dimer testing in their diagnostic approach, and the small number of patients.34,35
Diagnostic and Prognostic Laboratory Testing
D-dimer is a fibrin degradation product that is detected in the blood following blood clot degradation. D-dimer levels increase in pregnancy with the highest levels in the third trimester in one study.36 Levels return to baseline approximately 6 weeks postpartum.37 In general, normal D-dimer levels in pregnancy are less than 0.95 μg/mL in the first trimester, less than 1.29 μg/mL in the second trimester, and less than 1.7 μg/mL during the third trimester.36 Sensitivity of D-dimers has varied in different retrospective studies from 73% to 100%.38 However, D-dimers remain nonvalidated in prospective diagnostic cohort studies in pregnancy and cannot safely exclude the diagnosis of PE.39
BNP is commonly used to identify patients with PE that are at high risk of clinical deterioration. In a longitudinal sample, BNP levels were similar across 3 trimesters and postpartum; however, perinatal levels were approximately twice as high as those of nonpregnant controls.40 These findings were demonstrated with other studies.41 In patients with preexisting heart disease, BNP levels increase throughout pregnancy and the postpartum period.42 A BNP level of less than 100 pg/mL was shown in one study to have a negative predictive value of 100% for identifying decompensation of heart disease42; however, the study did not examine right heart strain as it relates to BNP. The utility of using BNP to risk stratify women with PE needs to be evaluated further.
Troponin I (TnI) is another marker commonly used in the risk stratification of patients with PEs. The use of TnI in risk stratifying pregnant women with PE has not been studied and requires validation.43
Imaging
Although the use of ionizing radiation is best minimized in pregnancy due to the potential risk of teratogenicity and oncogenicity, this small risk needs to be weighed against the risk of missing a PE, which could have deleterious effects on both the mother and the fetus. A false-positive diagnosis is associated with unnecessary risk of therapeutic anticoagulation during the index pregnancy, which complicates labor and delivery, unneeded prophylactic anticoagulation in future pregnancies, and slimmer contraceptive options. Imaging used to diagnose a PE in pregnancy includes CUS, CXR, V/Q scans, and CTPa.
The biggest concerns regarding exposure of the fetus to radiation are teratogenicity and oncogenicity. The minimum dose of radiation associated with an increased risk of teratogenicity in humans is yet to be firmly established. Animal studies in mice and rats have shown that a minimum exposure to radiation at levels of 0.05 to 0.25 Gy is needed to cause teratogenicity. Current guidelines suggest an exposure greater than 0.1 Gy at any time during gestation as the threshold beyond which congenital abnormalities are possible.24 To place this information into context, performing one CXR, a V/Q scan, and a CT of the chest exposes the fetus to significantly less than 0.01 Gy.44 Despite the relative safety of these studies in terms of teratogenicity, the clinician should work with the radiology team and their institution’s physicists to take necessary steps to minimize the dose of radiation with every imaging study that exposes to ionizing radiation. These steps may include modifying imaging protocols,45 performing ventilation scans only if perfusion scans are abnormal, frequent voiding to avoid pooling of radioactive material in the bladder, and the use of abdominal and breast shielding.
Imaging Diagnostic Approach
In 2011, the American Thoracic Society (ATS) published guidelines regarding diagnostic testing for PE in pregnancy that were based on low-quality, and very low quality, evidence,46 and, hence, not universally adopted.47 The recommendations from this document are quite different from the current standard of care in the nonpregnant population for many reasons that extend beyond the risk of teratogenicity. Technical limitations with enhanced studies in pregnancy constitute a major challenge48 due to the significant increase in plasma volume, heart rate, and cardiac output potentially diluting contrast and reducing vascular enhancement. These limitations depend on injection protocol as well as dose of contrast medium used, and may be minimized with protocol modifications.45,49 Breast radiation dose is significantly higher in CT studies compared with V/Q scans50 and could potentially translate into a higher risk of developing breast cancer during a woman’s lifetime, especially in younger women.51,52 However, breast shields, which are now being used for imaging of any body part in women, can reduce the amount of breast radiation without significantly affecting resolution of chest studies.53 Lastly, the use of iodine-containing contrast media, which are known to cross the placenta, is another concern with CT imaging, given the potential to interfere with thyroid function at birth. However, a study of nearly 350 newborns born to women exposed to iodinated contrast during pregnancy showed no significant effect on neonatal thyroid function.54
The ATS/Society of Thoracic Radiology (STR) guidelines recommend CUS as the first imaging for pregnant women with lower extremity symptoms. CUS is noninvasive and has no radiation exposure. In the nonpregnant population, the number needed to test to avoid further diagnostic workup is 11.55 In a randomized diagnostic noninferiority trial comparing CT/D-dimer approach with a CUS/CT/D-dimer approach, DVT was diagnosed by CUS in 9% of patients suspected of PE who were randomized to this approach.55 These statistics may be even lower in pregnancy, with the number needed to test being significantly higher, as isolated pelvic DVTs are more common17 and the ability of this test to detect pelvic DVTs suboptimal.43 In addition, this diagnostic modality may not be an adequate first-line test in critically ill patients with hemodynamic instability, and chest imaging may be a better approach (Fig. 1). However, in symptomatic patients, if the CUS reveals evidence of a lower extremity VTE, no further imaging is required and treatment for VTE can be initiated.
Fig. 1.
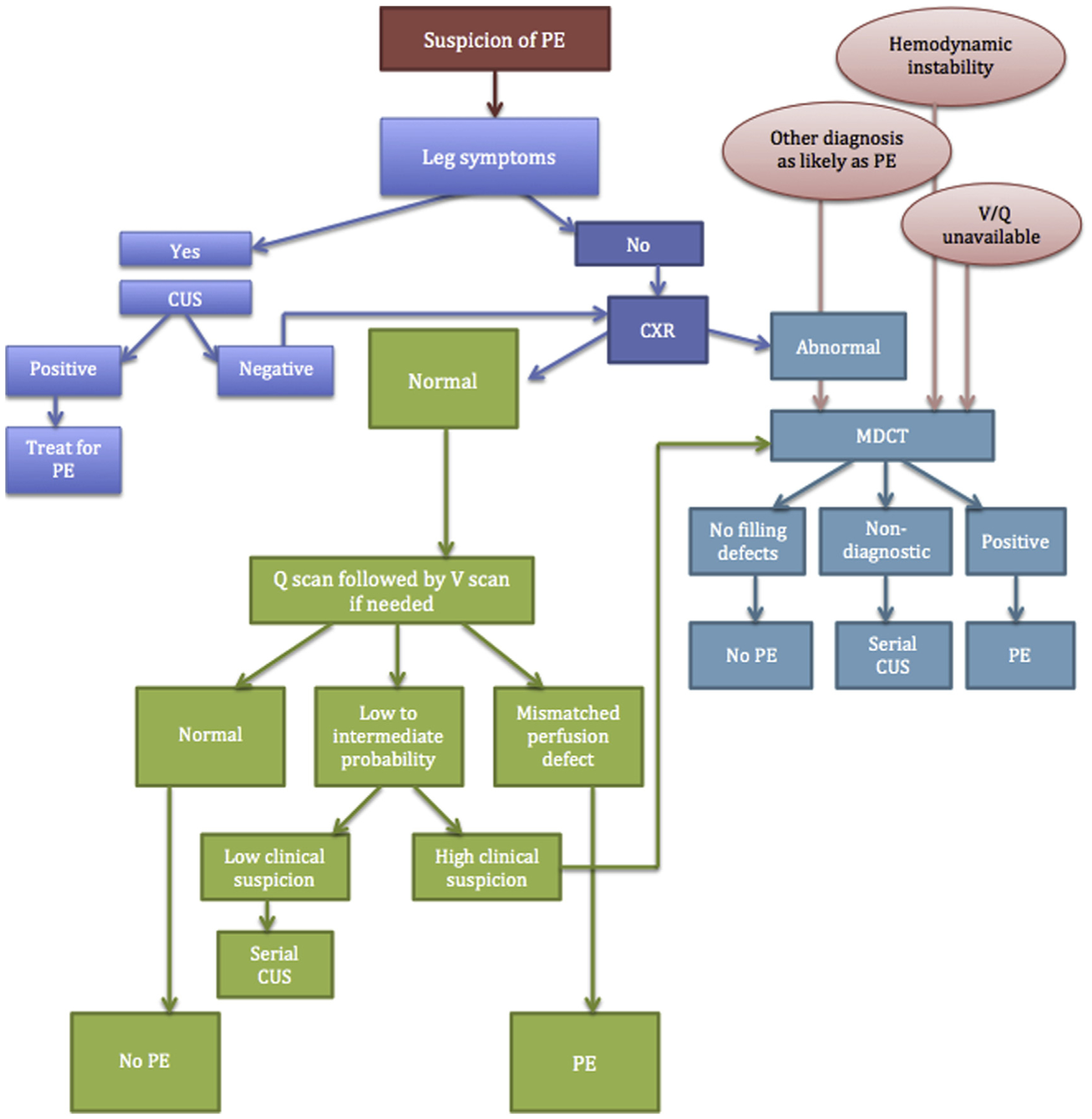
Diagnostic approach to suspected PE in pregnancy. MDCT, multidetector computed tomography.
If the patient does not have any lower extremity symptoms or the CUS is negative for any signs of VTE, then a CXR should be the first imaging test.46 The approximate dose of radiation the fetus is exposed to with a maternal CXR is 0.000001 Gy. If the CXR is abnormal and explains the clinical picture and the clinician is no longer concerned for VTE, it is reasonable to withhold additional imaging and treat the underlying condition diagnosed by CXR. However, if a clinical suspicion for VTE remains despite an abnormal CXR, a CTPa should be obtained. On the other hand, if the CXR is normal, V/Q scan is an appropriate next imaging study.
The approximate amount of radiation exposure for the fetus in a V/Q scan is 0.00028 to 0.00051. This is slightly higher but comparable to the amount of radiation exposure of a CTPa. As noted in the 2011 ATS/STR guidelines,46 most evidence in the literature is based on retrospective studies, hence limiting the ability to ascertain the diagnostic capabilities of various approaches. Both V/Q scans and CTPa appear to safely exclude PE based on these studies; however, as the incidence of PE in pregnant patients suspected of PE is quite low (close to 3%),56–58 larger studies are needed to confirm diagnostic accuracy of these imaging approaches. Based on retrospective data, women with a normal CXR are more likely to achieve a diagnosis when evaluated using a V/Q scan as opposed to a CTPa.59 The rate of nondiagnostic test for PE in a pregnant patient with a normal CXR is 30% for CTPa versus 5.6% when using a V/Q scan.59 However, the rate of nondiagnostic CTPa studies decreases from 30.0% to 16.4% when initial CXR is abnormal, and the rate of nondiagnostic test in V/Q scan increases from 5.6% to 40.0%.59
Though the previously suggested approach (see Fig. 1) is feasible and reasonable when the patient is being evaluated in a hospital setting, deciding on the next available diagnostic study following a CXR may prove to be a logistical challenge in patients being evaluated in an outpatient setting. In most cases, we opt for referring a patient for CTPa. In addition, given the better availability of CT imaging compared with nuclear studies, especially after hours, and that CT may offer an alternative diagnosis when PE is not present in a population with low incidence of PE among suspected patients, many, including the authors, prefer starting with a multidetector CT. Imaging with CT may also be advantageous in patients with severe hypoxemia or hemodynamic instability.
TREATMENT
In hemodynamically stable patients, acute treatment consists of anticoagulants as the first-line treatment. Low molecular weight heparin (LMWH) and unfractionated heparin (UFH) can both be used safely in pregnancy, as they do not cross the placental barrier (Table 1). However, LMWH is the anticoagulant of choice for antenatal VTE due to its better bioavailability,60 low risk of bleeding,61–63 and low rates of heparin-induced thrombocytopenia61 and osteopenia.64,65 The standard dose of enoxaparin is 1 mg/kg subcutaneous injection every 12 hours given insufficient data on once-daily dosing in pregnancy. Dosing and monitoring of LMWH remains open to debate.29,66 Plasma volume changes significantly in pregnancy and weight is a dynamic entity in pregnancy, and thus volume of distribution of drugs, including heparins, differs significantly. Although recent guidelines do not recommend monitoring,29 we perform anti-Xa monitoring weekly for 2 to 3 weeks at initiation, and then once per trimester. Anti-Xa levels 4 hours after injection with goal anti-Xa level of 0.5 to 1.1 units/mL are generally used,66 although those levels have not been linked to better clinical outcomes or lower risk of recurrence.
Table 1.
Safety of anticoagulant use in pregnancy
| Anticoagulant | Safety and Use in Pregnancy |
|---|---|
| LMWH | Enoxaparin, Daltaparin; dose 1 mg/kg BID; does not cross the placenta, blood thinner of choice in pregnancy; may need to adjust dose with increasing weight/may need to monitor anti-Xa levels (goal 0.5–1.1 U/mL). |
| UFH | May be used in place of LMWH in setting of renal dysfunction, high-risk PE, or during delivery, as it has a short half-life; IV drip or injections; need to monitor anti-Xa levels as volume of distribution and weight changes during pregnancy. |
| NOAC/DOAC | Clinical trials for DOACs/NOACs excluded pregnant women. Association with congenital anomalies, bleeding, implantation failure in animal studies. Slight increase in risk in human reports. Registry available: http://www.surveygizmo.com/s3/2394649/international-registry-of-pregnancy-during-NOAC-use-inclusion. |
| VKA | Warfarin crosses the placenta and has potential of teratogenicity; 3.7%–6.4% risk of congenital abnormalities. Also risk of fetal hemorrhage after organogenesis. Should not be used for treatment of VTE in pregnancy. |
| Synthetic heparins | Fondaparinux is not thought to increase risk of congenital malformations. Small amounts cross the placenta and 10% detected in neonates’ blood with measurable effect on neonatal anti-Xa levels. This effect was not enough to lead to a full antithrombotic effect; hence clinical significance of placental transfer is unknown. |
| Thrombin inhibitors | Lepirudin is not expected to increase the risk of congenital anomalies. Human case reports and animal studies do not show evidence of malformation. Lepirudin does cross the rat placenta. Argatroban use in animal studies was performed at doses lower than human doses. Rare case reports do not show evidence of congenital anomalies; safety cannot be ascertained. Argatroban has been used in cases of HIT and heparin allergy. |
Abbreviations: BID, twice daily; HIT, heparin-induced thrombocytopenia; IV, intravenous; LMWH, low molecular weight heparins; NOAC/DOAC, novel/direct anticoagulants; PE, pulmonary embolism; UFH, unfractionated heparins; VKA, vitamin K antagonists; VTE, venous thromboembolism.
UFH is still used in pregnancy in areas of the world where LMWH is not available. It is also preferred in cases of renal dysfunction and is used intravenously66 in patients with high burden of thrombosis possibly requiring thrombolysis, or around labor and delivery or an anticipated procedure, as it has a short half-life and is readily reversible. Because of changes in volume of distribution and bioavailability of this drug during pregnancy and the poor reliability of activated partial thromboplastin time levels, dosing should be monitored with heparin levels.61,67
The duration of treatment for VTE in pregnancy is not well studied, therefore recommendations for nonpregnant patients are followed.68 Given that the postpartum patients are high risk for recurrent VTE, it is recommended that therapy be extended for at least 6 weeks postpartum.69
Warfarin, an oral vitamin K agonist (VKA), is not used in pregnancy, as it crosses the placenta and can be teratogenic. The risk of congenital abnormalities with VKAs is 3.7% to 6.4%.66 Given that LMWH and UFH are similarly efficacious but safer in pregnancy, the use of VKAs in pregnancy cannot be justified.70 In the postpartum period, coumadin has been detected in breast milk in tiny amounts (Table 2) but is generally considered safe to use while breast feeding.71 Postpartum patients on therapeutic anticoagulation may need to be bridged from LMWH or UFH to warfarin so as not to significantly increase their risk of recurrent VTE.72 The risk of recurrence needs to be weighed against the risk of bleeding following an intervention and timing of the bridging carefully considered, especially in the case of a surgical delivery.
Table 2.
Anticoagulant use and lactation
| Anticoagulant | Safety and Use in Lactation |
|---|---|
| LMWH | Enoxaparin, Dalteparin; small amount excreted in breast milk but combination of low concentration and poor bioavailability when taken orally makes any clinically meaningful effect on infant unlikely. |
| UFH | High molecular weight, unlikely to transfer. Safe in lactation. |
| NOAC/DOAC | Clinical trials for DOACs/NOACs excluded pregnant women; animal studies show excretion into breast milk; contraindicated in breastfeeding women. |
| VKA | Detected in breast milk in small amounts but considered safe in lactation. May bridge from UFH or LMWH to VKA in postpartum patient. |
| Synthetic heparins | Fondaparinux transfers to breast; however, such transfer is inconsistent. |
| Thrombin inhibitors | One case report showed no transfer of Hirudin into breast milk; data are missing otherwise. Lepirudin is unlikely to transfer to human milk given its structure; oral bioavailability is low. Data on Argatroban use during lactation are lacking. |
The direct oral anticoagulants (DOACs) are newer non–vitamin K oral anticoagulants (NOACS) that directly inhibit clotting factors. Their use has become widespread in the nonpregnant population. The largest clinical trials for DOACs excluded pregnant women. DOACs cross the placenta,73,74 and animal studies have shown an elevated risk of anomalies, hemorrhage, and embryopathies (reprotox.org). Case reports and cohorts of pregnant women exposed to DOACs in pregnancy show an elevated risk of congenital anomalies and increased maternal bleeding.75 Hence, DOACs should be avoided throughout pregnancy or in women trying to conceive until there are better data and better understanding of their risks.76,77 Nonpregnant women receiving DOACs need to have proper contraceptive counseling, because use of contraceptives is complicated by history of VTE.
Pregnant women who are hemodynamically unstable or severely hypoxemic should be treated with thrombolysis if there are no contraindications. Data on managing pregnant patients in shock from VTE with other interventions besides systemic anticoagulation are limited to case reports. There are case reports of the administration of thrombolytics to pregnant patients with no resulting complications to the mother or the newborn,78 and tissue plasminogen activator (t-PA) appears to be the best thrombolytic agent. In one case series, however, the fetal fatality rate in the setting of thrombolytics was 8%.79 The overall risk of thrombolytic administration in pregnancy for decompensating patients is similar to nonpregnant patients80,81 and is best described in the management of acute stroke.82 The main risk of thrombolysis is maternal bleeding, which has been reported to occur in 8% of patients treated, with no reported cases of intracranial hemorrhage reported.81 Although reported cases of fetal death may be related to thrombolytics, it is as likely that they may be related to a hemodynamically unstable PE. Although catheter-based thrombolytic administration may have lower complication risk, as lower medication dosages are used than systemic lysis, there are currently no data to support its use in pregnancy.83
Other salvage therapies have been described in pregnant patients with hemodynamically unstable PE, which theoretically may have less bleeding risk than systemic thrombolytics and include extracorporeal membrane oxygenation (ECMO) and surgical thrombectomy. One recent case series described 2 cases of veno-arterial extracorporeal life support as an alternative to thrombolysis to restore hemodynamic stability in pregnant patients with massive PE.84 As ECMO is used in severe life-threatening conditions, it is hard to withhold this therapy from pregnant patients because of safety concerns; however, a decision to use such therapy should be weighed against efficacy, safety, and availability of other options. Surgical thrombectomy is another alternative to systemic thrombolysis and also may be considered when thrombolytic therapy has failed. Limited data suggested that although maternal survival may be high after surgical thrombectomy, fetal death rates can be elevated. In one case series, of eight cases reviewed there were no maternal deaths; however, 3 fetal deaths (37.5%) and 4 preterm deliveries (50.0%) were described.80 Temporary intravenous filters have also been successfully used in pregnancy in cases in which anticoagulation is contraindicated or to manage the risk of clot recurrence around labor and delivery when clot occurs at term. Given the impact of pregnancy on inferior vena cava (IVC) anatomy, filters are usually placed suprarenally. A recent systematic review, which included 124 pregnancies with IVC filters placed, found no fatal PEs in women with IVC filters placed along with no recorded fetal morbidity or mortality.85 There is, however, concern for more complicated retrieval of the filter in pregnancy, possibly due to angulation and distortion of the filter by the gravid uterus.86
Peripartum Anticoagulation
Management of VTE during the peripartum period can be challenging. Several clinical questions need to be addressed, including the duration of an anticoagulation window and whether an IVC filter is an option in high-risk patients. The biggest challenge for anticoagulation during the peripartum period is balancing the risk of postpartum hemorrhage with the risk of clot recurrence and the need for neuraxial analgesia.
Depending on the gestational age at which VTE has occurred (Table 3), several management options (eg, intravenous heparin, with-holding anticoagulation, placement of IVC filter) are available,87 but may vary based on institutional preferences.
Table 3.
Management of anticoagulation around labor and delivery
| Gestational Age at Time of VTE | Management Plan |
|---|---|
| <2 wk before labor |
|
| 2–4 wk before labor |
|
| More than 1 mo before labor |
|
| Recommendations for all women on anticoagulation during pregnancy |
|
PREVENTION
Pharmacologic thromboprophylaxis is usually initiated when the absolute risk of VTE exceeds a certain threshold and the benefit of thromboprophylaxis exceeds the risk associated with such therapy. However, risk assessment methods that estimate the risk of VTE are lacking in the perinatal population, and although it is clear that each additional risk factor increases the relative risk of VTE, the absolute risk estimate remains a challenge. This knowledge gap partly explains the discrepancies in recommendations for thromboprophylaxis by various societies.28,88,89
Risk stratification for VTE should be undertaken before consideration of preventive strategy; however, recommendations are mainly based on expert opinion rather than high-quality trials.90 Possible risk factors include age older than 35, obesity, parity greater than 3, previous VTE, gross varicose veins, paraplegia, medical comorbidities, and inheritable thrombophilias.91 One recent meta-analysis concluded that the thrombophilias associated with the highest risk in pregnancy were antithrombin deficiency, protein C deficiency, protein S deficiency, and homozygous factor V Leiden deficiency.92 Prothrombin gene mutation has also been considered a high-risk thrombophilia.28 Hence, guidelines have differed in their recommendations for pharmacotherapy, especially in the antepartum period (Table 4). Although most recommend thromboprophylaxis with LMWH for homozygous factor V Leiden or prothrombin gene mutation, recommendations for protein C, S, or antithrombin deficiency are debated.28,88,92 Postpartum pharmacoprophylaxis is recommended for most patients with inherited thrombophilia, including those with weak thrombophilias, especially those with a family history of VTE or additional risk factors, and obviously in all women with thrombophilias associated with a high risk for VTE.28,88 The Royal College of Obstetricians and Gynecologists also recommends thromboprophylaxis in patients with class 3 obesity and any woman with at least 2 persisting risk factors postpartum for at least 10 days.89
Table 4.
Summary of recommendations for prevention recurrent VTE
| Source of Recommendation/Level of Evidence | Antepartum | Postpartum | |
|---|---|---|---|
|
ACCP (Grade 1C)28 | Surveillance | AC prophylaxis |
| ACOG96 | Surveillance | AC prophylaxis | |
| RCOG (C)89 | Individual basis | AC prophylaxis | |
| Bates et al,29 2016 | Individual basis | AC prophylaxis | |
|
ACCP (Grade 2C)28 | Surveillance or AC prophylaxis | AC prophylaxis |
| ACOG96 | AC prophylaxis | AC prophylaxis | |
| RCOG (C)89 | AC prophylaxis | AC prophylaxis | |
| Bates et al,29 2016 | Individual basis | AC prophylaxis | |
|
ACCP (Grade 1C)28 | AC prophylaxis or surveillance | AC prophylaxis |
| ACOG96 | AC prophylaxis | AC prophylaxis | |
| RCOG (C)89 | Individual basis | AC prophylaxis | |
| Bates et al,29 2016 | |||
|
ACCP (Grade 1C)28 | AC prophylaxis or surveillance | AC prophylaxis |
| ACOG96 | AC prophylaxis or surveillance | Surveillance or AC | |
| RCOG (B)89 | AC prophylaxis | AC prophylaxis | |
| Bates et al,29 2016 | AC prophylaxis | AC prophylaxis | |
|
ACCP (Grade 2C)28 | AC prophylaxis | AC prophylaxis |
| ACOG96 | AC prophylaxis | AC | |
| RCOG (C)89 | AC prophylaxis | ||
| Bates et al,29 2016 | |||
|
ACCP (Grade 2C)28 | AC prophylaxis | AC prophylaxis |
| ACOG96 | AC prophylaxis | AC prophylaxis | |
| RCOG89 | AC prophylaxis | AC prophylaxis | |
| Bates et al,29 2016 | |||
|
ACCP (2C)28 | Surveillance if – FH | Surveillance AC prophylaxis |
| ACOG96 | Surveillance or AC prophylaxis | ||
| RCOG (D)89 | Consider AC prophylaxis | AC prophylaxis | |
| Bates et al,29 2016 | Consider AC prophylaxis if + FH | ||
|
ACCP (2B)28 | AC prophylaxis if + FH | AC if + FH |
| ACOG96 | AC prophylaxis if + FH Consider | Consider if + FH | |
| RCOG (C/D)89 | AC prophylaxis if + FH | ||
| Bates et al,29 2016 | |||
Abbreviations: −, negative; +, positive; AC, anticoagulation; ACCP, American College of Chest Physicians; ACOG, American College of Obstetricians and Gynecologists; FH, family history; H, hormonal; NH, non-hormonal; RCOG, Royal College of Obstetricians and Gynecologists; VTE, venous thromboembolism.
In low-risk patients, early mobilization and graduated elastic compression stockings are advised. Patients with high risk factors, including previous VTE or thrombophilic disorder, should be offered chemical prophylaxis.92 The pharmacologic prophylaxis of choice for antenatal and postnatal thromboprophylaxis is LMWH, as it does not cross the placenta.90 There is no evidence for the routine use of aspirin. For patients who are intolerant to heparin, fondaparinux may be used (see Table 2), especially in cases of heparin-induced thrombocytopenia.22
SUMMARY
The diagnosis and treatment of PE in pregnancy is complicated by pregnancy physiology. Much research is needed to develop pregnancy-specific pretest clinical pretest probability rules and to examine the negative predictive power of a given cutoff of D-dimers in diagnostic cohort studies. In addition, prospective research is needed to examine the positive and negative predictive value of imaging studies in confirming or excluding PE, and to determine the imaging study of choice in pregnancy from a cost-effectiveness and radiation-exposure standpoint, while examining pregnancy-specific imaging protocols. There are also numerous gaps in preventive and treatment strategies: mainly duration of therapy, appropriate dosing, adequacy of therapeutic levels in reducing recurrence, and in determination of risk assessment tools to use in prevention.
KEY POINTS.
Venous thromboembolism (VTE) is responsible for 3% of all maternal deaths worldwide and 15% in the United States.
The increased risk of VTE in pregnancy peaks immediately postpartum and may continue up to 12 weeks postpartum.
The increased risk is attributed to the Virchow triad, inherited thrombophilias, as well as other common risk factors.
The algorithm for diagnosing VTE differs during and immediately after pregnancy due to physiologic factors and fear of teratogenicity.
Low molecular weight heparin and unfractionated heparin are medications of choice, as warfarin is teratogenic and novel oral anticoagulants have increased rates of bleeding and congenital anomalies.
Funding:
G. Bourjeily is funded by NICHD R01HL-130702 and R01HD-078515.
Footnotes
Conflicts of Interest: All authors have no conflicts of interest to report.
REFERENCES
- 1.Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2014;2:e323–33. [DOI] [PubMed] [Google Scholar]
- 2.Knight M, Tuffnell D, Kenyon S, et al. , editors. Saving lives, improving mothers’ care—surveillance of maternal deaths in the UK 2011–13 and lessons learned to inform maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2009–13. Oxford (England): National Perinatal Epidemiology Unit, University of Oxford; 2015. [Google Scholar]
- 3.Kuriya A, Piedimonte S, Spence AR, et al. Incidence and causes of maternal mortality in the USA. J Obstet Gynaecol Res 2016;42:661–8. [DOI] [PubMed] [Google Scholar]
- 4.Cantwell R, Clutton-Brock T, Cooper G, et al. Saving mothers’ lives: reviewing maternal deaths to make motherhood safer: 2006–2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG 2011;118(Suppl 1): 1–203. [DOI] [PubMed] [Google Scholar]
- 5.Heit JA, Kobbervig CE, James AH, et al. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med 2005;143:697–706. [DOI] [PubMed] [Google Scholar]
- 6.Liu S, Rouleau J, Joseph KS, et al. Epidemiology of pregnancy-associated venous thromboembolism: a population-based study in Canada. J Obstet Gynaecol Can 2009;31:611–20. [DOI] [PubMed] [Google Scholar]
- 7.Gherman RB, Goodwin TM, Leung B, et al. Incidence, clinical characteristics, and timing of objectively diagnosed venous thromboembolism during pregnancy. Obstet Gynecol 1999;94:730–4. [DOI] [PubMed] [Google Scholar]
- 8.Sultan AA, West J, Grainge MJ, et al. Development and validation of risk prediction model for venous thromboembolism in postpartum women: multinational cohort study. BMJ 2016;355:i6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobsen AF, Skjeldestad FE, Sandset PM. Incidence and risk patterns of venous thromboembolism in pregnancy and puerperium–a register-based case-control study. Am J Obstet Gynecol 2008;198:233.e1–7. [DOI] [PubMed] [Google Scholar]
- 10.Pomp ER, Lenselink AM, Rosendaal FR, et al. Pregnancy, the postpartum period and prothrombotic defects: risk of venous thrombosis in the MEGA study. J Thromb Haemost 2008;6:632–7. [DOI] [PubMed] [Google Scholar]
- 11.Epiney M, Boehlen F, Boulvain M, et al. D-dimer levels during delivery and the postpartum. J Thromb Haemost 2005;3:268–71. [DOI] [PubMed] [Google Scholar]
- 12.Kjellberg U, Andersson NE, Rosen S, et al. APC resistance and other haemostatic variables during pregnancy and puerperium. Thromb Haemost 1999;81:527–31. [PubMed] [Google Scholar]
- 13.Kamel H, Navi BB, Sriram N, et al. Risk of a thrombotic event after the 6-week postpartum period. N Engl J Med 2014;370:1307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbagallo M, Dominguez LJ, Licata G, et al. Vascular effects of progesterone: role of cellular calcium regulation. Hypertension 2001;37:142–7. [DOI] [PubMed] [Google Scholar]
- 15.Tkachenko O, Shchekochikhin D, Schrier RW. Hormones and hemodynamics in pregnancy. Int J Endocrinol Metab 2014;12:e14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goulart VB, Cabral AC, Reis ZS, et al. Anatomical and physiological changes in the venous system of lower limbs in pregnant women and findings associated with the symptomatology. Arch Gynecol Obstet 2013;288:73–8. [DOI] [PubMed] [Google Scholar]
- 17.Chan WS, Spencer FA, Ginsberg JS. Anatomic distribution of deep vein thrombosis in pregnancy. CMAJ 2010;182:657–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldhaber SZ, Tapson VF. A prospective registry of 5,451 patients with ultrasound-confirmed deep vein thrombosis. Am J Cardiol 2004;93:259–62. [DOI] [PubMed] [Google Scholar]
- 19.Greer IA. Thrombosis in pregnancy: maternal and fetal issues. Lancet 1999;353:1258–65. [DOI] [PubMed] [Google Scholar]
- 20.James AH, Tapson VF, Goldhaber SZ. Thrombosis during pregnancy and the postpartum period. Am J Obstet Gynecol 2005;193:216–9. [DOI] [PubMed] [Google Scholar]
- 21.Boeldt DS, Bird IM. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J Endocrinol 2017;232:R27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim A, Samarage A, Lim BH. Venous thromboembolism in pregnancy. Obstet Gynaecol Reprod Med 2016;26:133–9. [Google Scholar]
- 23.Brenner B Haemostatic changes in pregnancy. Thromb Res 2004;114:409–14. [DOI] [PubMed] [Google Scholar]
- 24.Rosenkranz A, Hiden M, Leschnik B, et al. Calibrated automated thrombin generation in normal uncomplicated pregnancy. Thromb Haemost 2008;99: 331–7. [DOI] [PubMed] [Google Scholar]
- 25.James AH, Jamison MG, Brancazio LR, et al. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol 2006;194:1311–5. [DOI] [PubMed] [Google Scholar]
- 26.Dobbenga-Rhodes Y Shedding light on inherited thrombophilias: the impact on pregnancy. J Perinat Neonatal Nurs 2016;30:36–44. [DOI] [PubMed] [Google Scholar]
- 27.Gerhardt A, Scharf RE, Greer IA, et al. Hereditary risk factors for thrombophilia and probability of venous thromboembolism during pregnancy and the puerperium. Blood 2016;128:2343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012;141:e691S–736S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bates SM, Middeldorp S, Rodger M, et al. Guidance for the treatment and prevention of obstetric-associated venous thromboembolism. J Thromb Thrombolysis 2016;41:92–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American College of Obstetricians and Gynecologists Women’s Health Care Physicians. ACOG Practice Bulletin No. 138: inherited thrombophilias in pregnancy. Obstet Gynecol 2013;122:706–17. [DOI] [PubMed] [Google Scholar]
- 31.Blondon M, Casini A, Hoppe KK, et al. Risks of venous thromboembolism after cesarean sections: a meta-analysis. Chest 2016;150:572–96. [DOI] [PubMed] [Google Scholar]
- 32.Jacobsen AF, Skjeldestad FE, Sandset PM. Ante- and postnatal risk factors of venous thrombosis: a hospital-based case-control study. J Thromb Haemost 2008;6:905–12. [DOI] [PubMed] [Google Scholar]
- 33.Heyl PS, Sappenfield WM, Burch D, et al. Pregnancy-related deaths due to pulmonary embolism: findings from two state-based mortality reviews. Matern Child Health J 2013;17:1230–5. [DOI] [PubMed] [Google Scholar]
- 34.O’Connor C, Moriarty J, Walsh J, et al. The application of a clinical risk stratification score may reduce unnecessary investigations for pulmonary embolism in pregnancy. J Matern Fetal Neonatal Med 2011;24: 1461–4. [DOI] [PubMed] [Google Scholar]
- 35.Cutts BA, Tran HA, Merriman E, et al. The utility of the Wells clinical prediction model and ventilation-perfusion scanning for pulmonary embolism diagnosis in pregnancy. Blood Coagul Fibrinolysis 2014;25:375–8. [DOI] [PubMed] [Google Scholar]
- 36.Abbassi-Ghanavati M, Greer LG, Cunningham FG. Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gynecol 2009;114:1326–31. [DOI] [PubMed] [Google Scholar]
- 37.Boehlen F, Epiney M, Boulvain M, et al. Changes in D-dimer levels during pregnancy and the postpartum period: results of two studies. Rev Med Suisse 2005;1:296–8 [in French]. [PubMed] [Google Scholar]
- 38.Parilla BV, Fournogerakis R, Archer A, et al. Diagnosing pulmonary embolism in pregnancy: are bio-markers and clinical predictive models useful? AJP Rep 2016;06:e160–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bourjeily G D-dimer use in venous thromboembolic disease in pregnancy. BJOG 2015;122:401. [DOI] [PubMed] [Google Scholar]
- 40.Hameed AB, Chan K, Ghamsary M, et al. Longitudinal changes in the B-type natriuretic peptide levels in normal pregnancy and postpartum. Clin Cardiol 2009;32:E60–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Resnik JL, Hong C, Resnik R, et al. Evaluation of B-type natriuretic peptide (BNP) levels in normal and preeclamptic women. Am J Obstet Gynecol 2005;193:450–4. [DOI] [PubMed] [Google Scholar]
- 42.Tanous D, Siu SC, Mason J, et al. B-type natriuretic peptide in pregnant women with heart disease. J Am Coll Cardiol 2010;56:1247–53. [DOI] [PubMed] [Google Scholar]
- 43.Cutts BA, Dasgupta D, Hunt BJ. New directions in the diagnosis and treatment of pulmonary embolism in pregnancy. Am J Obstet Gynecol 2013;208: 102–8. [DOI] [PubMed] [Google Scholar]
- 44.Bourjeily G, Paidas M, Khalil H, et al. Pulmonary embolism in pregnancy. Lancet 2010;375:500–12. [DOI] [PubMed] [Google Scholar]
- 45.Boiselle PM, Goodman LR, Litmanovich D, et al. Expert opinion: CT pulmonary angiography in pregnant patients with suspected pulmonary embolism. J Thorac Imaging 2012;27:5. [DOI] [PubMed] [Google Scholar]
- 46.Leung AN, Bull TM, Jaeschke R, et al. An official American Thoracic Society/Society of Thoracic Radiology clinical practice guideline: evaluation of suspected pulmonary embolism in pregnancy. Am J Respir Crit Care Med 2011;184:1200–8. [DOI] [PubMed] [Google Scholar]
- 47.Skeith L, Rodger MA. Pulmonary complications of pregnancy: venous thromboembolism. Semin Respir Crit Care Med 2017;38:135–47. [DOI] [PubMed] [Google Scholar]
- 48.Gruning T, Mingo RE, Gosling MG, et al. Diagnosing venous thromboembolism in pregnancy. Br J Radiol 2016;89:20160021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schaefer-Prokop C, Prokop M. CTPA for the diagnosis of acute pulmonary embolism during pregnancy. Eur Radiol 2008;18:2705–8. [DOI] [PubMed] [Google Scholar]
- 50.Astani SA, Davis LC, Harkness BA, et al. Detection of pulmonary embolism during pregnancy: comparing radiation doses of CTPA and pulmonary scintigraphy. Nucl Med Commun 2014;35:704–11. [DOI] [PubMed] [Google Scholar]
- 51.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA 2007;298:317–23. [DOI] [PubMed] [Google Scholar]
- 52.Kim YK, Sung YM, Choi JH, et al. Reduced radiation exposure of the female breast during low-dose chest CT using organ-based tube current modulation and a bismuth shield: comparison of image quality and radiation dose. AJR Am J Roentgenol 2013;200: 537–44. [DOI] [PubMed] [Google Scholar]
- 53.Hopper KD, King SH, Lobell ME, et al. The breast: in-plane x-ray protection during diagnostic thoracic CT–shielding with bismuth radioprotective garments. Radiology 1997;205:853–8. [DOI] [PubMed] [Google Scholar]
- 54.Bourjeily G, Chalhoub M, Phornphutkul C, et al. Neonatal thyroid function: effect of a single exposure to iodinated contrast medium in utero. Radiology 2010;256:744–50. [DOI] [PubMed] [Google Scholar]
- 55.Righini M, Le Gal G, Aujesky D, et al. Diagnosis of pulmonary embolism by multidetector CT alone or combined with venous ultrasonography of the leg: a randomised non-inferiority trial. Lancet 2008;371: 1343–52. [DOI] [PubMed] [Google Scholar]
- 56.Bourjeily G, Khalil H, Raker C, et al. Outcomes of negative multidetector computed tomography with pulmonary angiography in pregnant women suspected of pulmonary embolism. Lung 2012;190: 105–11. [DOI] [PubMed] [Google Scholar]
- 57.Chan WS, Ray JG, Murray S, et al. Suspected pulmonary embolism in pregnancy: clinical presentation, results of lung scanning, and subsequent maternal and pediatric outcomes. Arch Intern Med 2002;162:1170–5. [DOI] [PubMed] [Google Scholar]
- 58.Shahir K, Goodman LR, Tali A, et al. Pulmonary embolism in pregnancy: CT pulmonary angiography versus perfusion scanning. AJR Am J Roentgenol 2010;195:W214–20. [DOI] [PubMed] [Google Scholar]
- 59.Cahill AG, Stout MJ, Macones GA, et al. Diagnosing pulmonary embolism in pregnancy using computed-tomographic angiography or ventilation-perfusion. Obstet Gynecol 2009;114:124–9. [DOI] [PubMed] [Google Scholar]
- 60.Couturaud F, Julian JA, Kearon C. Low molecular weight heparin administered once versus twice daily in patients with venous thromboembolism: a meta-analysis. Thromb Haemost 2001;86:980–4. [PubMed] [Google Scholar]
- 61.Greer IA, Nelson-Piercy C. Low-molecular-weight heparins for thromboprophylaxis and treatment of venous thromboembolism in pregnancy: a systematic review of safety and efficacy. Blood 2005;106:401–7. [DOI] [PubMed] [Google Scholar]
- 62.Lepercq J, Conard J, Borel-Derlon A, et al. Venous thromboembolism during pregnancy: a retrospective study of enoxaparin safety in 624 pregnancies. BJOG 2001;108:1134–40. [DOI] [PubMed] [Google Scholar]
- 63.Nelson-Piercy C, Powrie R, Borg JY, et al. Tinzaparin use in pregnancy: an international, retrospective study of the safety and efficacy profile. Eur J Obstet Gynecol Reprod Biol 2011;159:293–9. [DOI] [PubMed] [Google Scholar]
- 64.Pettila V, Leinonen P, Markkola A, et al. Postpartum bone mineral density in women treated for thromboprophylaxis with unfractionated heparin or LMW heparin. Thromb Haemost 2002;87:182–6. [PubMed] [Google Scholar]
- 65.Rodger MA, Kahn SR, Cranney A, et al. Long-term dalteparin in pregnancy not associated with a decrease in bone mineral density: substudy of a randomized controlled trial. J Thromb Haemost 2007;5: 1600–6. [DOI] [PubMed] [Google Scholar]
- 66.Bates SM. Pregnancy-associated venous thromboembolism: prevention and treatment. Semin Hematol 2011;48:271–84. [DOI] [PubMed] [Google Scholar]
- 67.Chunilal SD, Young E, Johnston MA, et al. The APTT response of pregnant plasma to unfractionated heparin. Thromb Haemost 2002;87:92–7. [PubMed] [Google Scholar]
- 68.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016;149:315–52. [DOI] [PubMed] [Google Scholar]
- 69.Schulman S, Rhedin AS, Lindmarker P, et al. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. Duration of Anticoagulation Trial Study Group. N Engl J Med 1995;332: 1661–5. [DOI] [PubMed] [Google Scholar]
- 70.Chan WS, Anand S, Ginsberg JS. Anticoagulation of pregnant women with mechanical heart valves: a systematic review of the literature. Arch Intern Med 2000;160:191–6. [DOI] [PubMed] [Google Scholar]
- 71.De Swiet M, Lewis PJ. Excretion of anticoagulants in human milk. N Engl J Med 1977;297:1471. [DOI] [PubMed] [Google Scholar]
- 72.Brandjes DP, Heijboer H, Buller HR, et al. Acenocoumarol and heparin compared with acenocoumarol alone in the initial treatment of proximal-vein thrombosis. N Engl J Med 1992;327:1485–9. [DOI] [PubMed] [Google Scholar]
- 73.Bapat P, Kedar R, Lubetsky A, et al. Transfer of dabigatran and dabigatran etexilate mesylate across the dually perfused human placenta. Obstet Gynecol 2014;123:1256–61. [DOI] [PubMed] [Google Scholar]
- 74.Bapat P, Pinto LS, Lubetsky A, et al. Examining the transplacental passage of apixaban using the dually perfused human placenta. J Thromb Haemost 2016; 14:1436–41. [DOI] [PubMed] [Google Scholar]
- 75.Beyer-Westendorf J, Michalski F, Tittl L, et al. Pregnancy outcome in patients exposed to direct oral anticoagulants—and the challenge of event reporting. Thromb Haemost 2016;116:651–8. [DOI] [PubMed] [Google Scholar]
- 76.Myers B, Neal R, Myers O, et al. Unplanned pregnancy on a direct oral anticoagulant (Rivaroxaban): a warning. Obstet Med 2016;9:40–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cohen H, Arachchillage DR, Middeldorp S, et al. Management of direct oral anticoagulants in women of childbearing potential: guidance from the SSC of the ISTH. J Thromb Haemost 2016;14:1673–6. [DOI] [PubMed] [Google Scholar]
- 78.Lonjaret L, Lairez O, Galinier M, et al. Thrombolysis by recombinant tissue plasminogen activator during pregnancy: a case of massive pulmonary embolism. Am J Emerg Med 2011;29:694.e1–2. [DOI] [PubMed] [Google Scholar]
- 79.Leonhardt G, Gaul C, Nietsch HH, et al. Thrombolytic therapy in pregnancy. J Thromb Thrombolysis 2006;21:271–6. [DOI] [PubMed] [Google Scholar]
- 80.te Raa GD, Ribbert LS, Snijder RJ, et al. Treatment options in massive pulmonary embolism during pregnancy; a case-report and review of literature. Thromb Res 2009;124:1–5. [DOI] [PubMed] [Google Scholar]
- 81.Turrentine MA, Braems G, Ramirez MM. Use of thrombolytics for the treatment of thromboembolic disease during pregnancy. Obstet Gynecol Surv 1995;50:534–41. [DOI] [PubMed] [Google Scholar]
- 82.Leffert LR, Clancy CR, Bateman BT, et al. Treatment patterns and short-term outcomes in ischemic stroke in pregnancy or postpartum period. Am J Obstet Gynecol 2016;214:723.e1–11. [DOI] [PubMed] [Google Scholar]
- 83.Weitz JI. Prevention and treatment of venous thromboembolism during pregnancy. Catheter Cardiovasc Interv 2009;74(Suppl 1):S22–6. [DOI] [PubMed] [Google Scholar]
- 84.Bataillard A, Hebrard A, Gaide-Chevronnay L, et al. Extracorporeal life support for massive pulmonary embolism during pregnancy. Perfusion 2016;31: 169–71. [DOI] [PubMed] [Google Scholar]
- 85.Harris SA, Velineni R, Davies AH. Inferior vena cava filters in pregnancy: a systematic review. J Vasc Interv Radiol 2016;27:354–60.e8. [DOI] [PubMed] [Google Scholar]
- 86.Gupta S, Ettles DF, Robinson GJ, et al. Inferior vena cava filter use in pregnancy: preliminary experience. BJOG 2008;115:785–8. [DOI] [PubMed] [Google Scholar]
- 87.Middeldorp S How I treat pregnancy-related venous thromboembolism. Blood 2011;118:5394–400. [DOI] [PubMed] [Google Scholar]
- 88.Chan WS, Rey E, Kent NE, et al. Venous thromboembolism and antithrombotic therapy in pregnancy. J Obstet Gynaecol Can 2014;36:527–53. [DOI] [PubMed] [Google Scholar]
- 89.Royal College of Obstetricians and Gynaecologists. Reducing the risk of venous thromboembolism during pregnancy and the puerperium. Green-top Guideline No. 37a. London: RCOG, 2015. Available at: https://www.rcog.org.uk/globalassets/documents/guidelines/gtg-37a.pdf. Accessed November 26, 2017. [Google Scholar]
- 90.Villani M, Ageno W, Grandone E, et al. The prevention and treatment of venous thromboembolism in pregnancy. Expert Rev Cardiovasc Ther 2017;15: 397–402. [DOI] [PubMed] [Google Scholar]
- 91.Skeith L, Carrier M, Robinson SE, et al. Risk of venous thromboembolism in pregnant women with essential thrombocythemia: a systematic review and meta-analysis. Blood 2017;129:934–9. [DOI] [PubMed] [Google Scholar]
- 92.Croles FN, Nasserinejad K, Duvekot JJ, et al. Pregnancy, thrombophilia, and the risk of a first venous thrombosis: systematic review and Bayesian meta-analysis. BMJ 2017;359:j4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.The reproductive toxicology center. Available at: http://www.reprotox.org/. Accessed September 20, 2017.
- 94.Mazer J, Zouein E, Bourjeily G. Treatment of pulmonary embolism in pregnancy. US Respir Dis 2012;8: 30–5. [Google Scholar]
- 95.Hale TW. Medications and mothers’ milk. Amarillo (TX): Pharmasoft Medical Pub; 2000. [Google Scholar]
- 96.James A Practice bulletin no. 123: thromboembolism in pregnancy. Obstet Gynecol 2011;118:718–29. [DOI] [PubMed] [Google Scholar]


