Dear Editor,
Iwasaki et al. previously demonstrated the usefulness of saliva polymerase chain reaction (PCR) in diagnosing coronavirus disease (COVID-19).1 Thereafter, several studies reported the simplicity and usefulness of saliva PCR and antigen tests.2 , 3 However, the most appropriate method for storing saliva specimens remains elusive.4
Any samples for COVID-19-related investigations should ideally be processed immediately after procurement. However, limited availability of measuring equipment and manpower may warrant the need to preserve samples in certain conditions until they are tested. Additionally, preserved samples may contribute to retrospective research. However, the differences in the results of several COVID-19-related tests under variable storage conditions (temperature, freezing/thawing, and solute medium) are not evaluated. We aimed to examine how variations in sample storage conditions affected results of antigen testing, PCR, and virus infectious titers for SARS-CoV-2. Antigen testing was performed by LUMIPULSE® (Fujirebio, Inc.,Tokyo, Japan).5 , 6 We analyzed specimens from eight patients who were examined at Hokkaido University Hospital from May 2020 to January 2021. The correspondence between patients and specimen numbers is as follows: Patient1 (swab1, saliva1), Patient2 (swab2), Ptatient3 (saliva2), Patient4 (swab3), Patient5 (saliva3), Patient6 (saliva4), Patient7 (saliva5), Patient8 (saliva6).
Nasopharyngeal samples were obtained using FLOQSwabs® (COPAN, Murrieta, CA, USA). The swab was inserted into the nostril up till the posterior nasopharynx and then gently removed while rotating. Thereafter, the swabs were placed in saline prior to evaluation. Saliva samples were self-collected by the patients. We instructed them to spit saliva o sterile PP Screw cup 50® (ASIAKIZAI Co., Tokyo, Japan) that naturally accumulates in the mouth into a cup twice and not to eat, drink, gargle, or brush their teeth for 10 min before specimen collection. Subsequently, 200 μL saliva was added to 600 μL phosphate-buffered saline, mixed vigorously, and centrifuged (20,000 x g; 5 min; 4 °C); the supernatant (140 μL) was used as a sample.
To investigate the PCR cycle threshold (Ct) values and antigen titers, swabs (swab1, 2) placed in saline were diluted using saline or CELLBANKER® (ZENOAQ RESOURCE Co., Fukushima, Japan), then preserved either at ambient temperature (AT), 4 °C, or −30 °C. Saliva samples (saliva1, 2) were preserved either at, 4 °C, or −30 °C, and diluted with PBS immediately before testing.
To investigate whether freezing and thawing affected virus cultures, we measured the virus infectious titer of one swab (swab 3) and four undiluted saliva (saliva 3,4,5,6) samples before freezing and after freezing and thawing. The detailed methodologies are reported previously7 and in online supplementary material.
Comparing the stability of saline and CELLBANKER® as preservation media for nasopharyngeal swabs
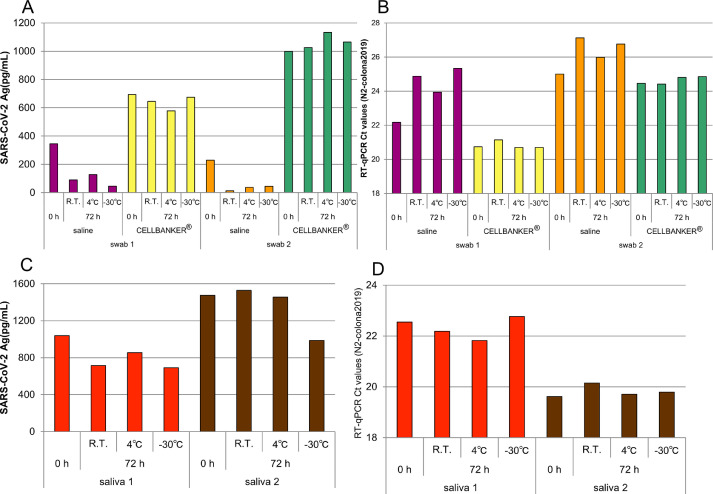
Fig. 1A shows the changes in antigen titers of the samples preserved in saline and CELLBANKER®. The antigen titers for samples preserved in saline decreased until 72 h (AT and 4 °C). Contrastingly, no such reduction was seen for samples preserved in CELLBANKER® (AT and 4 °C). When samples were frozen at −30 °C and then thawed, the antigen titers decreased in samples preserved with saline but not in those preserved with CELLBANKER®. However, Fig. 1B shows that the PCR Ct values remained unchanged under all preservation conditions.
Fig. 1.
Changes in virus antigen titer and PCR Ct values in Nasopharyngeal swabs and saliva samples. (A) virus antigen titer of nasopharyngeal swab, (B) RT-qPCR Ct value of nasopharyngeal swab, (C) virus antigen titer of saliva, (D) RT-qPCR Ct value of saliva.
Our study revealed that antigen titers were significantly lower in nasopharyngeal swab samples that were preserved in saline than in CELLBANKER®. This may be due to the trapping of antigen on the wall of the tube for non-specific binding with the former method. The decrease over time in antigen titers of samples preserved in saline relative to those preserved in CELLBANKER® persisted after 72 h (supplemental data). Therefore, preserving nasopharyngeal swab specimens in saline may incur false negative results.
CELLBANKER® was developed to prevent the degradation of nucleic acids and protect the cell membrane, particularly during experiments. Although the exact mechanism influencing the virus’ stability remains unclear, our findings indicate that CELLBANKER® is more effective than saline as a preservation medium for nasopharyngeal swab samples collected for antigen testing.
Stability of saliva samples in varying preservation conditions
For saliva samples preserved at AT and 4 °C, antigen titers and Ct values remained unchanged even after 72 h (Fig. 1C). Interestingly, for samples frozen at −30 °C and thawed, antigen titers decreased (Fig. 1C), whereas Ct values remained unchanged (Fig. 1D).
Effects of specimen storage methods and freezing and thawing on culture tests
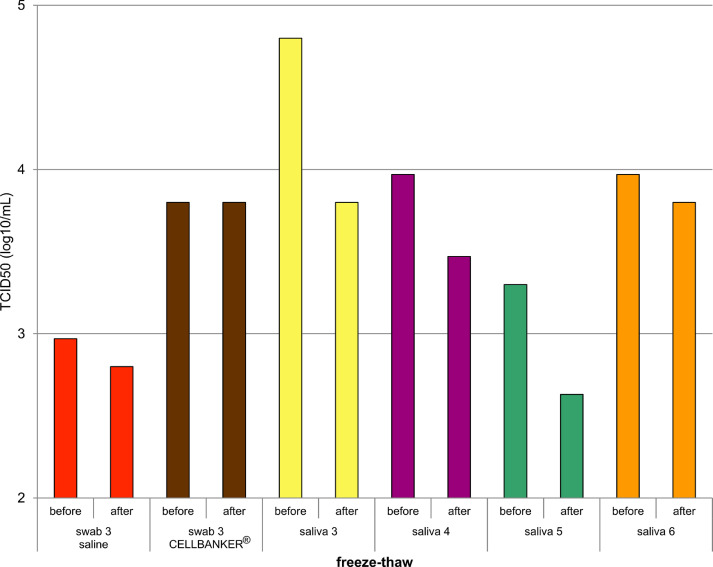
Fig. 2 shows the comparison of virus titers before freezing and after freezing and thawing in various storage solutions. When nasopharyngeal swab specimens were stored in CELLBANKER®, the infectious titers were unaffected; however, when stored in saline, the infectious titers decreased after freezing and thawing. Freezing and thawing of saliva samples also reduced infectious titers in culture tests.
Fig. 2.
Changes in culture virus titer before and after freezing and thawing.
Our results highlight the vulnerability of saliva samples to freezing and thawing. Although saliva was inferior to CELLBANKER® as preservation media, it maintained higher antigen titers than saline did. However, when the specimens were frozen and thawed, the titers decreased. Additionally, virus titers in the cultures may also decrease compared to that before freezing. Notably, freezing and thawing of saliva specimens may result in false negative results in antigen and culture tests.
The epitope recognized by LUMIPULSE® is speculated to be on the virus surface envelope. Therefore, freezing and thawing saliva degrades the epitope. We also found that saliva can be preserved for at least 72 h in AT or 4 °C. Contrastingly, the Ct value detected using PCR remained the same across all preservation conditions.
Our study has several limitations. First, the sample size is small due to the limited volume of cases at early phase of the disease, wherein the viral load and infectiousness quotient is high. This is because government policy demands suspected patients to either stay home or move into accommodation facilities after disease onset. It was difficult to collect nasopharyngeal swab specimens with positive viral cultures, because our institution recommended testing saliva specimens, and we were not able to match the number of both specimens. Second, there is the issue of heterogeneity of saliva as a medium. Salivary adulterants vary greatly among individuals, and the effect of freezing and thawing may also vary greatly among individuals. In fact, we found that the antigen titer decreased in saliva1 even before freezing.
Despite the limitations, the findings may greatly benefit clinical setting scenarios where antigen testing for COVID-19 cannot be performed immediately. Future studies must further investigate better storage solutions for saliva specimens.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Ethics
This study was approved by the Institutional Ethics Board (Hokkaido University Hospital Division of Clinical Research Administration Number: 020–0116 and 020–0111), and informed consent was obtained from all patients. All patients enrolled in this study were from the Hokkaido University Hospital.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2021.03.026.
Appendix. Supplementary materials
References
- 1.Iwasaki S., Fujiwara S., Nakakubo S., Kamada K., Yamashita Y., Fukumoto T., et al. Comparison of SARS-CoV-2 detection in nasopharyngeal swab and saliva. J Infect. 2020;81:e145–e147. doi: 10.1016/j.jinf.2020.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yokota I., Shane Peter Y., Okada K., Unoki Y., Yang Y., Inao T., et al. Mass screening of asymptomatic persons for SARS-CoV-2 using saliva. Clin Infect Dis. 2020:ciaa1388. doi: 10.1093/cid/ciaa1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medeiros da Silva R.C., Nogueira Marinho L.C., de Araújo Silva D.N., Costa de Lima K., Pirih F.Q., Luz de Aquino Martins A.R. Saliva as a possible tool for the SARS-CoV-2 detection: a review. Travel Med Infect Dis. 2020;38 doi: 10.1016/j.tmaid.2020.101920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.B Grisemer S., Van Slyke G., Ehrbar D., Strle K., Yildirim T., A Centurioni D., et al. Evaluation of specimen types and saliva stabilization solutions for SARS-CoV-2 testing. J Clin Microbiol. 2021 doi: 10.1128/jcm.01418-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirotsu Y., Maejima M., Shibusawa M., Amemiya K., Nagakubo Y., Hosaka K., et al. Prospective study of 1308 nasopharyngeal swabs from 1033 patients using the LUMIPULSE SARS-CoV-2 antigen test: comparison with RT-qPCR. Int J Infect Dis. 2021;105:7–14. doi: 10.1016/j.ijid.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishii T., Sasaki M., Yamada K., Kato D., Osuka H., Aoki K., et al. Immunochromatography and chemiluminescent enzyme immunoassay for COVID-19 diagnosis. J Infect Chemother. 2021 doi: 10.1016/j.jiac.2021.02.025. S1341-321X(21)00065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hattori T., Amishima M., Morinaga D., Kamada K., Nakakubo S., Yamashita Y., et al. Older age is associated with sustained detection of SARS-CoV-2 in nasopharyngeal swab samples. J Infect. 2021;82:159–198. doi: 10.1016/j.jinf.2020.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.