Abstract
Objectives:
Tobacco smoke exposure reduces CFTR functional expression in vitro and contributes to acquired CFTR dysfunction. We investigated whether it also inhibits the clinical benefit of CFTR modulators, focusing on tezacaftor/ivacaftor, approved in February 2018 for individuals with CF age ≥12 years.
Methods:
A retrospective longitudinal analysis of encounter-based data from the CF Foundation Patient Registry (2016-2018) compared the slope of change in lung function (GLI FEV1% predicted) before and after tezacaftor/ivacaftor initiation in smoke-exposed vs unexposed age-eligible pediatric patients. Tobacco smoke exposure (Ever/Never) was determined from caregiver self-report. Statistical analyses used hierarchical linear mixed modeling and fixed effects regression modeling.
Results:
The sample included 6,653 individuals with a total of 105,539 person-period observations. Tezacaftor/ivacaftor was prescribed to 19% (1,251) of individuals, mean age 17 years, mean baseline ppFEV1 83%, 28% smoke-exposed. Tezacaftor/ivacaftor users who were smoke-exposed had a lower baseline ppFEV1 and experienced a greater lung function decline. Over two years, the difference in ppFEV1 by smoke exposure among tezacaftor/ivacaftor users increased by 1.2% (7.6% to 8.8%, p<0.001). In both mixed effects and fixed effects regression models, tezacaftor/ivacaftor use was associated with improved ppFEV1 among unexposed individuals (1.2% and 1.7%, respectively; p<0.001 for both) but provided no benefit among smoke-exposed counterparts (0.3%, p=0.5 and 0.6%, p=0.07, respectively).
Conclusion:
Tobacco smoke exposure nullifies the therapeutic benefit of tezacaftor/ivacaftor among individuals with CF aged 12-20 years old. To maximize the therapeutic opportunity of CFTR modulators, every effort must be taken to eliminate smoke exposure in CF.
INTRODUCTION
Tobacco smoke exposure includes exposure to smoke from burning tobacco products or exhaled by a smoker, as well as exposure to the residue from tobacco smoke that accumulates in dust, objects, and on surfaces and is reemitted into the air, ingested, or absorbed via skin contact.1 Despite evidence for the adverse effects of tobacco smoke exposure on CF lung health,2–6 approximately one-third of U.S. children and adolescents with CF are regularly exposed, per caregiver self-report.4,5 Among those exposed, 80% never change exposure status between 6 and 18 years of age (CF Foundation Patient Registry data from 2006 to 2016).5
Animal models and human studies show that smoke exposure reduces CFTR functional expression7–10 and contributes to acquired CFTR dysfunction in people without CF.11,12 Published data strongly indicate that exposure to cigarette smoke inhibits anion transport by the CFTR,11,13–16 leading to delayed mucociliary transport and mucus stasis.11,17 In vivo studies in mice,10,18 ferrets,17 and humans10–12,19,20 also demonstrate that acquired CFTR dysfunction persists even after smoking cessation and affects organs beyond the respiratory tract.
Despite the deleterious effects of cigarette smoke on CFTR-dependent anion transport in vitro,12,21 the effect of tobacco smoke exposure on the clinical efficacy of CFTR modulator drugs has not been investigated. We hypothesize that clinical response to CFTR modulator drugs is blunted in CF patients exposed to tobacco smoke. In light of the considerable investments in the development of CFTR modulators, it is imperative to quantify the consequence of smoke exposure on their therapeutic benefit.
The current retrospective longitudinal study used population-level data from the U.S. CF Foundation Patient Registry22 (CFFPR) to investigate whether tobacco smoke exposure inhibits the clinical benefit of tezacaftor/ivacaftor (Symdeko), which was FDA-approved on February 12, 2018 for people with CF age 12 years and older who are F508del homozygous or have at least one CFTR mutation responsive to tezacaftor/ivacaftor. We focus on tezacaftor/ivacaftor because it is the most recent CFTR modulator for which data from the CFFPR is available. Specifically, we compared change in lung function (GLI ppFEV1) as a function of tezacaftor/ivacaftor initiation in smoke-exposed vs unexposed pediatric patients with CF, age 12-20 years old.
METHODS
The study cohort comprised all individuals in the CFFPR who were born between 1/1/1998 and 12/31/2006. This pediatric cohort was between 12 years old (FDA-approved minimum age to receive tezacaftor/ivacaftor) and 20 years old at the end of 2018. Lung function measures were obtained from encounters in 2016–2018 so that we could examine the trajectory of lung function before and after the introduction of this CFTR modulator. To be included in the analytic sample, individuals had to be age-eligible and have at least one lung function measure in 2018. The study was approved by the Institutional Review Board of the University of Alabama at Birmingham (protocol 300002076).
Measures
Outcome variable.
Using participants’ lung function measures from each recorded clinical encounter between 1/1/2016 and 12/31/2018, we estimated the slope of change in lung function before and after tezacaftor/ivacaftor initiation. Lung function was measured as forced expiratory volume in 1 second, percent predicted (ppFEV1), calculated with the Global Fung Function Initiative [GLI] reference equations.20
Exposure variables.
Tezacaftor/ivacaftor use (Yes/No) based on physician-reported prescription was obtained from encounter data. The CFFPR reports whether tezacaftor/ivacaftor has been prescribed (=1). For encounters that do not record tezacaftor/ivacaftor (=1), we assume no tezacaftor/ivacaftor prescription at that encounter. In the analytical sample, we included those with uninterrupted tezacaftor/ivacaftor use, those with only one interruption in tezacaftor/ivacaftor use, and those who used tezacaftor/ivacaftor but then discontinued all subsequent use. Individuals with inconsistent tezacaftor/ivacaftor use (two or more interruptions of tezacaftor/ivacaftor) were excluded from our analyses.
Self-reported tobacco smoke exposure (Yes/No), measured at each age, was determined based on response to the questions, “Does anyone in the patient’s household smoke cigarettes?” (Yes/No) and “During the reporting year, how often was this patient exposed to second-hand smoke?” (Daily/Several times per week/Several times per month or less/Never). Tobacco smoke exposure was coded as ‘Yes’ if either the response to the first question was affirmative or the response to the second question was “Daily” or “Several times per week.” Smoke exposure was treated as a time-invariant measure: individuals were coded as smoke-exposed if the above was true at any age since age 6 years old. Variations in the coding of smoke exposure, including ‘currently exposed’ or ‘exposed since age 12 years’, produced substantively similar results.
Covariates.
Sex (Male/Female), race/ethnicity (Non-Hispanic white/Non-Hispanic other race/Hispanic any race), health insurance (Private/Public/Both private and public/Other or none), paternal education (Less than high school/High school/Some college/College degree), genotype (delF508 homozygous/delF508 heterozygous/Other), and P. aeruginosa in past 12 months (Y/N), selected a priori, were included in the hierarchical linear mixed models.
Statistical analysis
We use both hierarchical linear mixed modeling and fixed effects regression modeling to gain an understanding of the effect of tezacaftor/ivacaftor on lung function and the extent to which smoke exposure impacts this association. Hierarchical linear mixed models use information from both within-individual exposure-outcome association and between-individual exposure-outcome association. However, variation between individuals may introduce confounding bias due to unmeasured time-invariant factors that are associated with both the exposure and the outcome. Therefore, we also use fixed effects models, as their estimators rely only on variation within individuals and are unaffected by confounding from unmeasured time-invariant factors. The fixed effect coefficients represent how much of a change in the predictor variable is associated with the slope of lung function, or change in lung function net of any time-invariant predictors. The impact of time-invariant predictors (such as race, sex, or parent education) is already accounted for, as individuals are used as their own controls.23
In the mixed effects models, the slope, or change over time, is measured in days, centered at the first observation. This allows for interpretable intercepts that represent expected lung function in 2016, accounting for covariates. For ease of interpretation, in the results we present time as annual change rather than daily change. The non-linear change in slope is measured as the quadratic of time. Age is assessed at the end of each year, and encounter date is used as a measure of time. To account for the likelihood that younger individuals have better lung function and slower rates of decline then older individuals, we interact age in 2016 (centered) with time and time squared. We estimate two models: Model 1 includes the predictor variables (tezacaftor/ivacaftor and smoke exposure) and all covariates, while Model 2 additionally includes the interaction between tezacaftor/ivacaftor and smoke exposure. A significant interaction suggests that the association between tezacaftor/ivacaftor and ppFEV1 is moderated by smoke exposure.
In the fixed effects modeling, the impact of predictors that do not have much or any within-person variation (e.g., smoke exposure) cannot be estimated directly, but we can examine whether the association between predictor and outcome variables varies by such time-invariant measures. Thus, we are able to evaluate whether smoke exposure blunts the potentially beneficial effect of tezacaftor/ivacaftor on ppFEV1 by interacting ‘ever exposed to smoke’ with tezacaftor/ivacaftor use. Therefore, we estimate two fixed effects models: Model 1 includes age, time presented as annual change, and the interaction between age in 2016 and time (to account for the variation in ppFEV1 change by age); Model 2 additionally includes the interaction between tezacaftor/ivacaftor and smoke exposure. As in the mixed effect models, time is measured in days, but for simplicity we present the regression coefficients of annual change. In a sensitivity analysis, an interaction between tezacaftor/ivacaftor and father’s education (1=college degree or higher; 0=all else) was included to assess for confounding by socioeconomic status.
Change from lumacaftor/ivacaftor to tezacaftor/ivacaftor.
To assess for potential bias introduced by use of lumacaftor/ivacaftor prior to tezacaftor/ivacaftor initiation, we performed sensitivity analyses in which we interacted lumacaftor/ivacaftor (Orkambi) and tezacaftor/ivacaftor.
Discontinuation of prescribed tezacaftor/ivacaftor.
Some individuals who begin taking tezacaftor/ivacaftor have subsequent encounters that do not record tezacaftor/ivacaftor use. To address the possibility that tezacaftor/ivacaftor use may not be consistently recorded although a patient continues to take the drug, we performed a sensitivity analysis where we included ZIP code as a level of variation in the mixed models, thereby accounting for the possibility that some care centers may be entering prescription data in the CFFPR less consistently.
Approximately 8% of observations had missing values on covariates. Missing data were addressed with multiple imputations (n=10 data sets) using Markov Chain Monte Carlo.24 Multiple imputations were conducted on the wide version (one observation per individual) of the data. In our analyses, we use imputed time-varying variables from 2016, 2017, and 2018 and non-time varying variables. Analyses were performed with Stata 16 (College Station, TX: StataCorp LLC).
RESULTS
The analytic sample included 6,653 individuals, who contributed a total of 105,539 person-period observations, or an average of 5 lung function measures per person per year. A STROBE diagram of the study population is presented in Supplementary Figure 1.
Descriptive statistics of the sample in 2018, overall and by tezacaftor/ivacaftor use, are shown in Table 1. Individuals in the sample were primarily non-Hispanic White (82%), with private insurance (49%), F508del homozygous (47%), and P. aeruginosa negative (65%). One-third (33%) had tobacco smoke exposure, and one-fifth (19%) were prescribed tezacaftor/ivacaftor.
Table 1.
Characteristics of the sample, overall and by tezacaftor/ivacaftor use, 2018 (N=6,653)
| Total Mean (SD) or % | Teza/iva N=1,251 (18.8%) | No teza/iva N=5,402 (81.2%) | p-value | |
|---|---|---|---|---|
|
|
||||
| SOCIODEMOGRAPHIC | ||||
| Age | 16.5 (2.45) | 16.7 (2.48) | 16.4 (2.20) | <0.001 |
| Female, % | 48.8 | 53.9 | 47.6 | <0.001 |
| Race/ethnicity, % | ||||
| White | 81.9 | 89.3 | 80.2 | <0.001 |
| Hispanic any race | 10.5 | 6.4 | 11.5 | |
| Other | 7.5 | 4.3 | 8.3 | |
| Father’s education, % | ||||
| Less than high school | 3.5 | 2.4 | 3.8 | <0.001 |
| High school | 21.2 | 18.7 | 21.8 | |
| Some college | 17.6 | 16.0 | 17.9 | |
| College degree or more | 57.7 | 62.9 | 56.5 | |
| Health insurance, % | ||||
| Private only | 49.4 | 57.5 | 47.5 | <0.001 |
| Public only | 39.8 | 32.4 | 41.6 | |
| Both | 9.7 | 9.5 | 9.8 | |
| Other/none | 1.1 | 0.5 | 1.2 | |
| Tobacco smoke exposure, % | 33.2 | 27.8 | 34.5 | <0.001 |
| CLINICAL | ||||
| Lung function, ppFEV1 | 84.2 (20.1) | 82.5 (19.8) | 84.6 (20.1) | <0.001 |
| BMI percentile | 50.2 (28.0) | 48.8 (26.7) | 50.5 (28.2) | <0.041 |
| F508del homozygous, % | 47.1 | 90.2 | 37.1 | <0.001 |
| P. aeruginosa, % | 35.3 | 49.6 | 34.3 | <0.001 |
| Hospitalizations in 12 months, % | ||||
| None | 59.1 | 54.9 | 60.1 | <0.001 |
| One | 20.8 | 24.3 | 20.0 | |
| Two or more | 20.1 | 20.8 | 16.8 | |
| Exacerbations in 12 months, % | ||||
| None | 63.5 | 59.4 | 64.5 | <0.001 |
| One | 19.7 | 23.8 | 18.7 | |
| Two or more | 16.8 | 16.8 | 16.8 | |
Teza/iva=tezacaftor/ivacaftor
Compared to those who did not receive tezacaftor/ivacaftor, those who were prescribed the modulator had a slightly lower ppFEV1 (83% vs 85%), higher prevalence of P. aeruginosa (50% vs 34%), and more hospitalizations (21% vs 17% with ≥2 per year). However, tezacaftor/ivacaftor users had more advantageous sociodemographic characteristics than non-users. For example, they were more likely to be non-Hispanic White (89% vs 80%), to have private health insurance (58% vs 48%), a college-educated father (63% vs 57%), and not to be smoke exposed (28% vs 35%) (Table 1).
Figure 2 is a cross-sectional illustration of the annualized mean ppFEV1 of tezacaftor/ivacaftor users from 2016 to 2018, by smoke exposure status. Tezacaftor/ivacaftor users who were exposed to tobacco smoke had a nearly 8% lower baseline ppFEV1 than unexposed counterparts. In addition to this diminished baseline lung function, they also experienced a greater decline in lung function: over the course of 2 years, the difference in ppFEV1 by smoke exposure status increased by 1.2% (7.6% to 8.8%, p<0.001) among tezacaftor/ivacaftor users.
Figure 2. Mean ppFEV1 of tezacaftor-ivacaftor1 users, by tobacco smoke exposure (TSE).
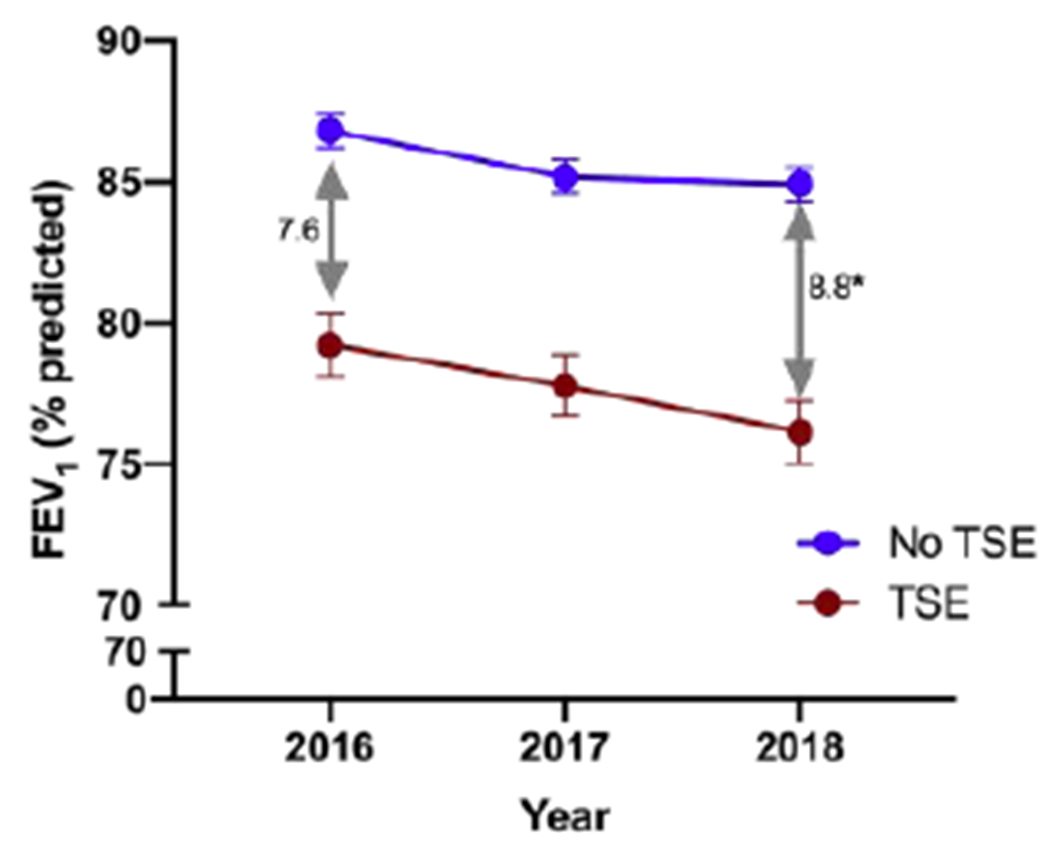
1 FDA-approved in February, 2018.
*Statistical significance of the increased difference in ppFEV1 by TSE from 2016 to 2018.
Table 2 presents results from the mixed effects models. Model 1 includes the main predictor variables (tezacaftor/ivacaftor and smoke exposure), time (measured in exact annual change and annual change squared), and the covariates (age, sex, race, health insurance, father’s education, genotype, and P. aeruginosa). In Model 1, tezacaftor/ivacaftor use is associated with increased ppFEV1 (b=1.01, 95% CI 0.63 to 1.39, p<0.001) whereas smoke exposure is associated with decreased ppFEV1 (b= −3.27, 95% CI −4.23 to −2.30, p<0.001). Model 2 additionally includes the interaction of tezacaftor/ivacaftor with smoke exposure. The interaction is significant and negative, indicating that the beneficial impact of tezacaftor/ivacaftor is reduced in smoke-exposed individuals compared to unexposed (b= −0.84, 95% CI −1.65 to −0.03, p=0.041). These results are displayed graphically in Figure 3. Among smoke-exposed individuals, the predicted ppFEV1 of tezacaftor/ivacaftor users is not statistically different from the predicted ppFEV1 of tezacaftor/ivacaftor non-users (82.9, 95% CI 81.8 to 83.9 vs 82.5, 95% CI 81.7 to 83.2). In contrast, among those unexposed to smoke, tezacaftor/ivacaftor use is associated with a 1.2% improvement in ppFEV1 (87.0, 95% CI 86.3 to 87.7 vs 85.8, 95% CI 85.2 to 86.3, p<0.001) (Figure 3).
Table 2.
Multivariable regression: mixed models of ppFEV1 (N=6,653)
| ppFEV1 |
||||
|---|---|---|---|---|
| Model 1 | Model 2 | |||
| β (95% CI) | p-value | β (95% CI) | p-value | |
|
|
||||
| Intercept (ppFEV1 in 2016) | 90.35 (89.47, 91.24) | <0.001 | 90.36 (89.47, 91.24) | <0.001 |
| Smoke exposure | −3.27 (−4.23, −2.30) | <0.001 | −3.27 (−4.24, −2.31) | <0.001 |
| Teza/iva | 1.01 (0.63, 1.39) | <0.001 | 1.24 (0.80, 1.69) | <0.001 |
| Teza/iva × smoke exposure | - | - | −0.84 (−1.65, −0.03) | 0.041 |
| Paternal education | ||||
| Less than high school | −4.58 (−7.19, −1.98) | 0.001 | −4.58 (−7.19, −1.98) | 0.001 |
| High school | −4.02 (−5.21, −2.82) | <0.001 | −4.02 (−5.21, −2.81) | <0.001 |
| Some college | −1.66 (−2.93, −0.40) | 0.010 | −1.66 (−2.92, −0.40) | 0.010 |
| College degree | 1.00 (reference) | 1.00 (reference) | ||
| Health insurance | ||||
| Private only | 1.00 (reference) | 1.00 (reference) | ||
| Public only | −0.46 (−0.86, −0.07) | 0.021 | −0.46 (−0.86, −0.07) | 0.021 |
| Both private and public | −0.08 (−0.48, 0.32) | 0.700 | −0.08 (−0.48, 0.32) | 0.706 |
| None/other | −0.07 (−1.82, 0.40) | 0.209 | −0.07 (−1.82, 0.40) | 0.210 |
| Female sex | −1.18 (−2.03, −0.33) | 0.006 | −1.18 (−2.03, −0.33) | 0.006 |
| Race/ethnicity | ||||
| White | 1.00 (reference) | 1.00 (reference) | ||
| Other | −1.08 (−2.73, 0.57) | 0.198 | −1.08 (−2.73, 0.57) | 0.198 |
| Hispanic | −3.70 (−5.19, −2.21) | <0.001 | −3.70 (−5.19, −2.21) | <0.001 |
| Age in 2016 (centered) | −1.63 (−1.79, −1.46) | <0.001 | −1.63 (−1.79, −1.46) | <0.001 |
| P. aeruginosa | −0.59 (−0.81, −0.37) | <0.001 | −0.59 (−0.81, −0.37) | <0.001 |
| delF608 homozygous | −0.84 (−1.71, 0.03) | 0.059 | −0.84 (−1.71, 0.03) | 0.059 |
|
| ||||
| Annual rate of change × age in 2016 | −1.85 (−2.03, −1.67) | <0.001 | −1.85 (−2.03, −1.67) | <0.001 |
| 0.03 (−0.04, 0.10) | 0.418 | 0.03 (−0.04, 0.10) | 0.420 | |
| Annual rate of change, squared × age in 2016 | 0.29 (0.22, 0.37) | <0.001 | 0.29 (0.22, 0.37) | <0.001 |
| −0.07 (−0.10, −0.04) | <0.001 | −0.07 (−0.10, −0.04) | <0.001 | |
Figure 3.
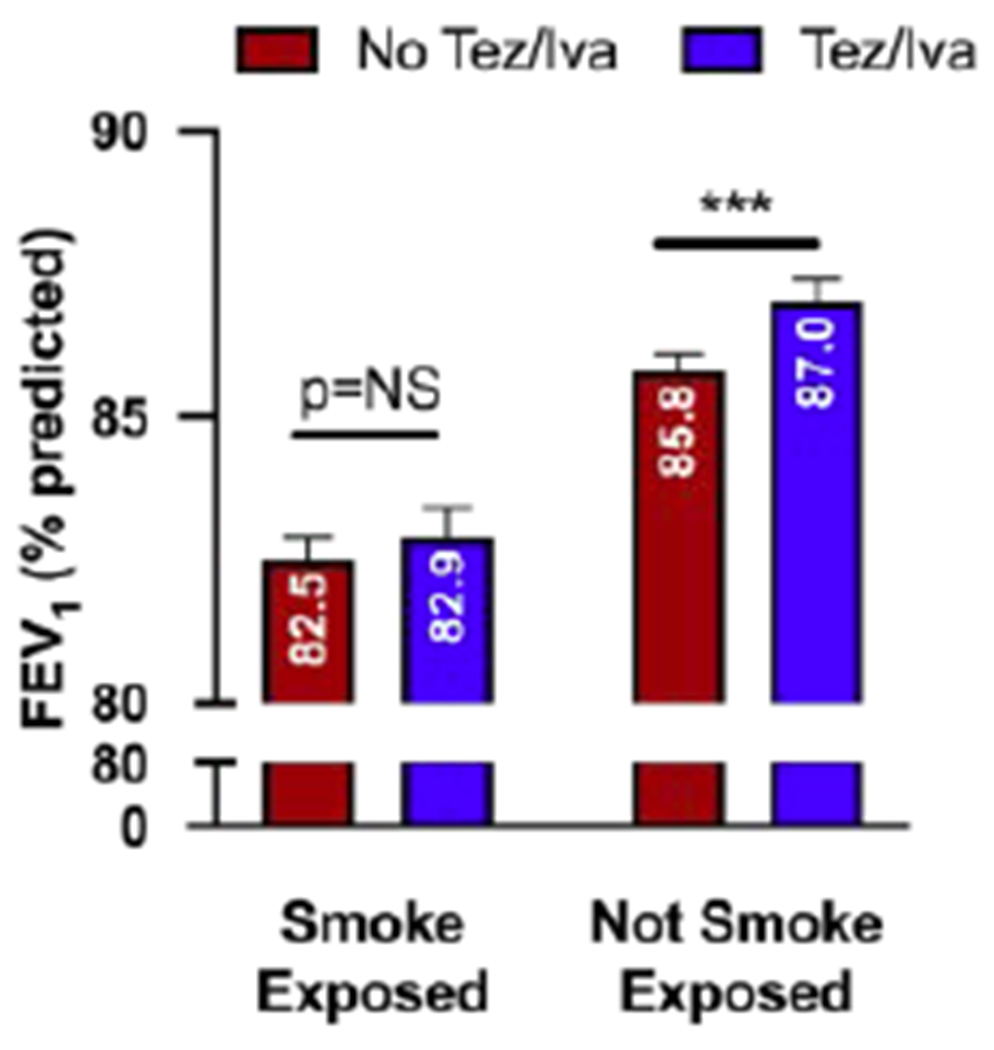
ppFEV1 estimates from mixed effects regression
In fixed effects modeling (Table 3), Model 1 includes time (presented as annual change), the interaction of time with age in 2016, and tezacaftor/ivacaftor. We find that tezacaftor/ivacaftor use is associated with improved ppFEV1 (b=1.41, 95% CI 1.07 to 1.75, p<0.001). Model 2 adds the interaction of tezacaftor/ivacaftor with smoke exposure. The interaction is significant and negative, indicating that the beneficial impact of tezacaftor/ivacaftor is reduced in smoke-exposed individuals compared to unexposed (b= −1.10, 95% CI 1.84 to 0.36, p=0.004). The association between tezacaftor/ivacaftor and ppFEV1 of the unexposed represents the main effect of tezacaftor/ivacaftor (b=1.71, 95% CI 1.31 to 2.11, p<0.001). The main effect of tezacaftor/ivacaftor plus the interaction between tezacaftor/ivacaftor and smoke exposure represents the effect of tezacaftor/ivacaftor among smoke-exposed (b=1.71 + (−1.10) = 0.61). Additional analyses indicate that the effect of tezacaftor/ivacaftor among smoke-exposed is non-significant (p=0.06). In both models, ppFEV1 declines by 1.57% each year (p<0.001) and by another 0.14% for each year of age (p<0.001).
Table 3.
Multivariable regression: fixed effects models of ppFEV1 (N=6,653)
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| b | 95% CI | b | 95% CI | |||
|
|
||||||
| Intercept | 82.0 *** | 81.92 | 82.08 | 82.0 *** | 81.92 | 82.08 |
| Time (year) | −1.57 *** | −1.63 | −1.50 | −1.57 *** | −1.63 | −1.50 |
| Time × age (2016 centered) | −0.14 *** | −0.17 | −0.12 | −0.14 *** | −0.17 | −0.12 |
| Teza/iva | 1.41 *** | 1.07 | 1.75 | 1.71 *** | 1.31 | 2.11 |
| Teza/iva × smoke exposure | - | - | - | −1.10 ** | −1.84 | −0.36 |
Boldface indicates statistical significance:
p<0.01,
p<0.001; two-tailed tests.
Teza/iva=tezacaftor/ivacaftor
In a sensitivity analysis, ‘ever prescribed lumacaftor/ivacaftor’ was interacted with tezacaftor/ivacaftor use, and the interaction term was included in all models above. It did not change the interaction between smoke exposure and tezacaftor/ivacaftor, indicating that smoke exposure negates the beneficial effect of tezacaftor/ivacaftor even after accounting for lumacaftor/ivacaftor use.
Inclusion of ZIP code as a level of variation in the mixed models did not change the interpretation of our main variables and produced a model with worse model fit statistics, indicating that any potential variation in data entry between care centers does not substantively impact our findings.
In fixed effects modeling, the interaction between tezacaftor/ivacaftor and father’s education was significant, but when included in all models, it did not change the significance or the magnitude of interaction between smoke exposure and tezacaftor/ivacaftor. This indicates that smoke exposure negates the beneficial effect of tezacaftor/ivacaftor regardless of whether socioeconomic status influences the impact of tezacaftor/ivacaftor on lung function.
DISCUSSION
We conducted a retrospective longitudinal analysis of data from the CF Foundation Patient Registry to investigate whether tobacco smoke exposure inhibits the clinical benefit of tezacaftor/ivacaftor (Symdeko), approved in February 2018 for people with CF age 12 years and older. The results show beneficial impact of tezacaftor/ivacaftor among children and adolescents who were never exposed to smoke. Among smoke-exposed individuals, however, tezacaftor/ivacaftor did not improve lung function. These epidemiologic findings from a national pediatric CF cohort corroborate evidence from animal models and human studies that tobacco smoke exposure reduces CFTR functional expression and function.7–11,13–17 As CFTR modulators become a transformative therapeutic approach for people with CF, these results suggest that exposure to smoke may contribute to the heterogeneity of benefit.25,26 The consequence of smoke exposure on CFTR-directed therapeutics now needs to be examined in additional genotypes and in the recently approved triple combination agent (elexacaftor/tezacaftor/ivacaftor).
We and others have outlined the impact of smoke exposure on CF lung disease in children.4–6 Despite these observations, approximately 30% of U.S. children with CF remain smoke-exposed.4,5 The current study expands the rationale for smoking cessation beyond disease prevention to therapeutic optimization. Tremendous resources have been deployed to develop CFTR modulators and make them available to all people with CF. To increase the health return on this investment, effective smoking cessation strategies should be implemented at CF care centers. As the risk of smoke exposure is doubled in low-income or low-education households,5 the differential effect of novel CFTR drugs in smoke-exposed children and adolescents with CF may exacerbate existing socioeconomic inequities in CF outcomes and contribute to health disparities. Therefore, strategies and interventions to eliminate smoke exposure in CF households are critically needed.
The 1.2%–1.7% improvement in ppFEV1 attributable to tezacaftor/ivacaftor in this national pediatric cohort, age 12-20 years old, appears smaller than the effect size reported in randomized clinical trials of the drug: 4% improvement in individuals homozygous for F508del27 and 6.8% improvement in individuals heterozygous for F508del and a residual function mutation.28 This is not unexpected, as our study reflects a “real-world” experience that often is distinct from clinical trials. Our findings are based on a longer observational period (at least 12 months) compared to the clinical trials above (6 months and 2 months, respectively), and ppFEV1 improvement with F508del corrector-potentiator therapy is known to diminish over time as disease continues to progress.29,30 Sample size difference (N=6,653 for our study, compared to N=510 and N=248 for the clinical trials, respectively) and selection bias may also have influenced the effect sizes. Our sample was younger (mean age 17 years vs 26 years and 35 years, respectively, for the two clinical trials), and age-based differences in tezacaftor/ivacaftor effects have been reported.31 A ceiling effect for ppFEV1 is also a major and impactful consideration, as 61% of our pediatric cohort had a baseline ppFEV1 over 90%. The annual decline in ppFEV1 observed in this study (1.9% plus additional 0.03% for each year of age) is comparable to the annual decline reported previously: 1.9% among individuals with CF aged 18-24 year32 and 2.3% among those aged 13-17 years.33
As tezacaftor/ivacaftor provides only 4% improvement in ppFEV1,27 corresponding to approximately a 25% improvement in F508del CFTR function in vitro,34 it is not surprising that the deleterious effect of smoke exposure decreases CFTR benefit below the therapeutic threshold. The key question going forward is how smoke exposure impacts the benefit of more effective CFTR combinations such as elexacaftor/tezacaftor/ivacaftor (Trikafta) for F508del correction, read-through agents for nonsense mutations such as G542X, or mutation-agnostic gene-editing agents under development. Our findings serve as a warning sign that smoke exposure has a clinically significant impact on CFTR therapeutics that nullifies marginally effective strategies and likely reduces the benefit of more effective ones, such as the triple-combination therapy. This could also impact clinical trials with experimental CFTR modulators, impairing the potential for benefit observed in participants with smoke exposure.
The major limitation of this study is the self-reported nature of the smoke exposure data. For example, in our sample, approximately 15% of caregivers who responded affirmatively to the question, “Does anyone in the patient’s household smoke cigarettes?”, also responded that their child is “Never” exposed to smoke. The difficulty in measuring the exposure led to treating it as a non-time varying variable (‘ever exposed’ rather than ‘currently exposed’), as this approach produced a better model fit. Clearly, biochemical verification of exposure would be preferred to validate our conclusions. It should also be noted that we only assessed the effect of smoke exposure rather than active smoking. However, the proportion of smokers in the sample was less than 0.5% among individuals age ≥15. We also acknowledge the large variation in duration of tezacaftor/ivacaftor use (range 0-672 days) and pulmonary function tests (range 1-44) before and after drug initiation. Finally, in a recent study of individuals who did not tolerate lumacaftor/ivacaftor but were able to tolerate tezacaftor-ivacaftor, there was no diminished effect from tezacaftor/ivacaftor after lumacaftor/ivacaftor use.35 It is therefore unlikely that prior lumacaftor/ivacaftor use biased our results, and an interaction analysis confirmed that.
The major finding of this study is that exposure to tobacco smoke cancels the benefit of the CFTR modulator tezacaftor/ivacaftor. This finding demands two actions: (1) development and implementation of enhanced smoking cessation strategies for caregivers of smoke-exposed children on CFTR modulators, and (2) determining the effect of smoke exposure in next-generation CFTR modulators.
CONCLUSION
Tobacco smoke exposure nullifies the therapeutic benefit of tezacaftor/ivacaftor in individuals with CF age 12-20 years old. Smoking cessation and exposure prevention should be prioritized further to improve CFTR modulator efficacy in pediatric CF care.
Supplementary Material
Highlights.
Among individuals with CF aged 12-20 years who were not exposed to tobacco smoke, use of tezacaftor/ivacaftor was associated with 1.2%–1.7% improvement in ppFEV1.
Among smoke-exposed counterparts, tezacaftor/ivacaftor use was not associated with ppFEV1 improvement.
Tobacco smoke exposure nullifies the therapeutic benefit of tezacaftor/ivacaftor among individuals with CF aged 12-20 years old.
To maximize the therapeutic opportunity of CFTR modulators, every effort should be taken to eliminate tobacco smoke exposure in CF.
Acknowledgments:
The authors would like to thank the Cystic Fibrosis Foundation for the use of CF Foundation Patient Registry data to conduct this study. Additionally, we thank the patients, care providers, and clinic coordinators at CF centers throughout the United States for their contributions to the CF Foundation Patient Registry.
Funding: This study was supported by grants from the National Institutes of Health (P30DK72482, K08HL140190, HARRIS19A0-KB).
Conflicts of interest: WTH and SMR have received support through their institution (UAB) from Vertex Pharmaceuticals to conduct and oversee CFTR modulator clinical trials.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.U.S. Department of Health and Human Services. The Health Consequences of Smoking – 50 Years of Progress: A Report of the Surgeon General. Rockville, MD, 2014. [Google Scholar]
- 2.Sanders DB, Emerson J, Ren CL, et al. Early Childhood Risk Factors for Decreased FEV1 at Age Six to Seven Years in Young Children with Cystic Fibrosis. Ann Am Thorac Soc. 2015;12(8):1170–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collaco JM, Vanscoy L, Bremer L, et al. Interactions between Secondhand Smoke and Genes That Affect Cystic Fibrosis Lung Disease. JAMA. 2008;299(4):417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ong T, Schechter M, Yang J, et al. Socioeconomic Status, Smoke Exposure, and Health Outcomes in Young Children with Cystic Fibrosis. Pediatrics. 2017;139(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oates GR, Baker E, Rowe SM, et al. Tobacco Smoke Exposure and Socioeconomic Factors Are Independent Predictors of Pulmonary Decline in Pediatric Cystic Fibrosis. J Cyst Fibros. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopp BT, Ortega-Garcia JA, Sadreameli SC, et al. The Impact of Secondhand Smoke Exposure on Children with Cystic Fibrosis: A Review. Int J Environ Res Public Health. 2016;13(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raju SV, Rasmussen L, Sloane PA, Tang LP, Libby EF, Rowe SM. Roflumilast Reverses Cftr-Mediated Ion Transport Dysfunction in Cigarette Smoke-Exposed Mice. Respir Res. 2017;18(1):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rab A, Rowe SM, Raju SV, Bebok Z, Matalon S, Collawn JF. Cigarette Smoke and Cftr: Implications in the Pathogenesis of Copd. Am J Physiol-Lung Cell Mol Physiol. 2013;305(8):L530–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasmussen JE, Sheridan JT, Polk W, Davies CM, Tarran R. Cigarette Smoke-Induced Ca2+ Release Leads to Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Dysfunction. J Biol Chem. 2014;289(11):7671–7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raju SV, Jackson PL, Courville CA, et al. Cigarette Smoke Induces Systemic Defects in Cystic Fibrosis Transmembrane Conductance Regulator Function. Am J Respir Crit Care Med. 2013;188(11):1321–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sloane PA, Shastry S, Wilhelm A, et al. A Pharmacologic Approach to Acquired Cystic Fibrosis Transmembrane Conductance Regulator Dysfunction in Smoking Related Lung Disease. PLoS One. 2012;7(6):e39809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dransfield MT, Wilhelm AM, Flanagan B, et al. Acquired CFTR Dysfunction in the Lower Airways in COPD. Chest. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cantin AM, Hanrahan JW, Bilodeau G, et al. Cystic Fibrosis Transmembrane Conductance Regulator Function Is Suppressed in Cigarette Smokers. Am J Respir Crit Care Med. 2006;173(10):1139–1144. [DOI] [PubMed] [Google Scholar]
- 14.Kreindler JL, Jackson AD, Kemp PA, Bridges RJ, Danahay H. Inhibition of Chloride Secretion in Human Bronchial Epithelial Cells by Cigarette Smoke Extract. Am J Physiol-Lung Cell Mol Physiol. 2005;288(5):L894–902. [DOI] [PubMed] [Google Scholar]
- 15.Clunes LA, Davies CM, Coakley RD, et al. Cigarette Smoke Exposure Induces CFTR Internalization and Insolubility, Leading to Airway Surface Liquid Dehydration. FASEB J. 2012;26(2):533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savitski AN, Mesaros C, Blair IA, Cohen NA, Kreindler JL. Secondhand Smoke Inhibits Both Cl− and K+ Conductances in Normal Human Bronchial Epithelial Cells. Respir Res. 2009;10:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin VY, Fain MD, Jackson PL, et al. Vaporized E-Cigarette Liquids Induce Ion Transport Dysfunction in Airway Epithelia. Am J Respir Cell Mol Biol. 2019;61(2):162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raju SV, Tate JH, Peacock SK, et al. Impact of Heterozygote CFTR Mutations in COPD Patients with Chronic Bronchitis. Respir Res. 2014;15(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Courville CA, Tidwell S, Liu B, Accurso FJ, Dransfield MT, Rowe SM. Acquired Defects in CFTR-Dependent Beta-Adrenergic Sweat Secretion in Chronic Obstructive Pulmonary Disease. Respir Res. 2014;15(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Courville CA, Raju SV, Liu B, Accurso FJ, Dransfield MT, Rowe SM. Recovery of Acquired Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Dysfunction Following Smoking Cessation. Submitted. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raju SV, Lin VY, Liu L, et al. The Cystic Fibrosis Transmembrane Conductance Regulator Potentiator Ivacaftor Augments Mucociliary Clearance Abrogating Cystic Fibrosis Transmembrane Conductance Regulator Inhibition by Cigarette Smoke. Am J Respir Cell Mol Biol. 2017;56(1):99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knapp EA, Fink AK, Goss CH, et al. The Cystic Fibrosis Foundation Patient Registry. Design and Methods of a National Observational Disease Registry. Ann Am Thorac Soc. 2016;13(7):1173–1179. [DOI] [PubMed] [Google Scholar]
- 23.Gunasekara FI, Richardson K, Carter K, Blakely T. Fixed Effects Analysis of Repeated Measures Data. Int J Epidemiol. 2014;43(1):264–269. [DOI] [PubMed] [Google Scholar]
- 24.Allison P. Missing Data: Quantitative Applications in the Social Sciences. Vol 136. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- 25.Taylor-Cousar J, Niknian M, Gilmartin G, Pilewski JM, investigators VX. Effect of Ivacaftor in Patients with Advanced Cystic Fibrosis and a G551d-CFTR Mutation: Safety and Efficacy in an Expanded Access Program in the United States. J Cyst Fibros. 2016;15(1):116–122. [DOI] [PubMed] [Google Scholar]
- 26.Hebestreit H, Sauer-Heilborn A, Fischer R, Kading M, Mainz JG. Effects of Ivacaftoron Severely Ill Patients with Cystic Fibrosis Carrying a G551d Mutation. J Cyst Fibros. 2013;12(6):599–603. [DOI] [PubMed] [Google Scholar]
- 27.Taylor-Cousar JL, Munck A, McKone EF, et al. Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del. N Engl J Med. 2017;377(21):2013–2023. [DOI] [PubMed] [Google Scholar]
- 28.Rowe SM, Daines C, Ringshausen FC, et al. Tezacaftor-Ivacaftor in Residual-Function Heterozygotes with Cystic Fibrosis. N Engl J Med. 2017;377(21):2024–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sagel SD, Khan U, Heltshe SL, et al. Clinical Effectiveness of Lumacaftor/Ivacaftor in Cystic Fibrosis Patients Homozygous for F508del-CFTR. Annals ATS. 2020. [Google Scholar]
- 30.Konstan MW, McKone EF, Moss RB, et al. Assessment of Safety and Efficacy of Long-Term Treatment with Combination Lumacaftor and Ivacaftor Therapy in Patients with Cystic Fibrosis Homozygous for the F508del-CFTR Mutation (Progress): A Phase 3, Extension Study. Lancet Respir Med. 2017;5(2):107–118. [DOI] [PubMed] [Google Scholar]
- 31.Balk E, Trikalinos T, Kuntz K, al e. Modulator Treatments for Cystic Fibrosis: Effectiveness and Value; Final Evidence Report and Meeting Summary. Boston, MA: Institute for Clinical and Economic Review; June7, 2018. [Google Scholar]
- 32.Konstan MW, Wagener JS, Vandevanter DR, et al. Risk Factors for Rate of Decline in FEV1 in Adults with Cystic Fibrosis. J Cyst Fibros. 2012;11(5):405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konstan MW, Morgan WJ, Butler SM, et al. Risk Factors for Rate of Decline in Forced Expiratory Volume in One Second in Children and Adolescents with Cystic Fibrosis. J Pediatr. 2007;151(2):134–139, 139 e131. [DOI] [PubMed] [Google Scholar]
- 34.Van Goor F, Hadida S, Grootenhuis PD, et al. Correction of the F508del-CFTR Protein Processing Defect in Vitro by the Investigational Drug Vx-809. Proc Natl Acad Sci USA. 2011;108(46):18843–18848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwarz C, Sutharsan S, Epaud R, et al. Tezacaftor/Ivacaftor in People with Cystic Fibrosis Who Stopped Lumacaftor/Ivacaftor Due to Respiratory Adverse Events. J Cyst Fibros. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


