Abstract
Background
Abnormalities in the autonomic nervous system may occur in ischemic heart disease, but the mechanisms by which they are linked are not fully defined. The risk of cardiac events is increased during morning hours. Studying the contributions of autonomic mechanisms may yield insights into risk stratification and treatment. We hypothesize that autonomic dysfunction, measured by decreased heart rate variability (HRV), associates with abnormal stress myocardial perfusion imaging (MPI).
Methods
We performed a cross-sectional study of the association between abnormal myocardial stress perfusion with HRV using 276 middle-aged veteran twins without known ischemic heart disease. The primary independent variable was cardiac autonomic regulation measured with 24-hour electrocardiogram (ECG) monitoring, using linear and non-linear (multipole density, or Dyx) HRV metrics. The primary outcome was abnormal perfusion (>5% affected myocardium) during adenosine stress on [13N]-ammonia myocardial perfusion imaging with positron emission tomography.
Results
The mean (SD) age was 53 (3) years and 55 (20%) had abnormal perfusion. HRV (by Dyx) was reduced during morning hours in subjects with abnormal perfusion. At 7 AM, each standard deviation (SD) decrease in Dyx was associated a 4.8 (95% CI, 1.5 — 15.8) odds ratio (OR) for abnormal MPI. With Dyx < 2.0, the 7 AM OR for abnormal MPI was 11.8 (95% CI, 1.2 — 111.4).
Conclusions
Autonomic dysfunction, measured by non-linear HRV in the morning hours, was associated with an increased OR of abnormal MPI. These results suggest a potentially important role of ECG-based biomarkers in risk stratification for individuals with suspected ischemic heart disease.
Keywords: autonomic nervous system, myocardial perfusion, coronary artery disease, positron emission tomography, heart rate variability
1. INTRODUCTION
Almost half of sudden cardiac deaths occur in men and women without known ischemic heart disease (IHD).[1–3] Identification of those at higher risk may require evaluation of additional, non-traditional risk factors, such as autonomic dysfunction.[4–7] The autonomic nervous system (ANS) is implicated, for example, in the regulation of cardiac electrophysiology, contractility, and coronary vasomotor tone, among other effects.[8] Early identification of abnormalities in cardiac autonomic regulation may provide more insight into why certain individuals are at increased risk of sudden cardiac death.
Recent advances in electrocardiography (ECG) have led to the discovery of new risk markers for the identification of cardiovascular disease, which carry the advantages of being low cost and non-invasive performance.[9,10] These include markers of ANS function as reflected by heart rate variability (HRV),[11] a complex measure of the sinoatrial node responses to changes in autonomic outflow. HRV is dependent on multiple peripheral and central mechanisms that vary with physical position, psychological context, and the circadian rhythm.[11,12] Autonomic dysfunction, as indicated by low HRV, is associated with IHD and major adverse cardiovascular events,[7,13,14] and predicts worse outcomes in patients with myocardial infarction and ischemic cardiomyopathy,[15,16] as well as in the general population.[14,17] Low HRV is thought to be a measure of sympathovagal imbalance.[18,19] Increased sympathetic tone and vagal withdrawal is implicated in the pathogenesis of cardiac diseases, and dysregulation of the autonomic feedback mechanisms between the heart and brain may play a key role in the pathogenesis of cardiac diseases.[20,21] Prior studies have found a relationship between myocardial perfusion and HRV, but were limited by size and, more importantly, did not account for the context of HRV measurement, such as time of day, and are important to address before HRV can be considered a valid clinical tool.[9,10]
Our study evaluated the relationships between alterations in HRV and abnormalities in myocardial perfusion imaging (MPI) and coronary flow reserve (CFR) during pharmacological stress testing. The twin design reduces potential genetic and environmental confounding effects by evaluating differences within pairs.[22] We tested the hypothesis that low HRV is associated with abnormal coronary blood flow regulation during pharmacologic stress tests, measured by >5% perfusion deficits on MPI and low CFR.
2. METHOD
2.1. Study Population
This cross-sectional study was designed to evaluate the relationship of abnormal stress MPI and CFR with autonomic function, measured hourly over the course of 24 hours, in individuals without known IHD. Subjects were drawn from the Emory Twin Study,[23,24] which recruited middle-aged male twin pairs from the Vietnam Era Twin Registry.[25] Pairs of twins were examined at the Emory University General Clinical Research Center, and all data collection occurred during a 24-hour admission under controlled conditions. The twins in each pair maintained a nearly identical schedule, with all data collection beginning and ending at the same time. The twins arrived at 11 AM, with ECG recording started at approximately 1 PM, questionnaires and exam performed between 2 – 4 PM, dinner at 5 PM, bedtime at 10 PM, wake-up time at 6:30 AM, and PET scans performed between 8 – 10 AM the following morning. Subjects were excluded from analysis if they had known IHD, defined as previous diagnosis of myocardial infarction or previous coronary revascularization procedure, were unable to complete pharmacological stress testing, or used beta-blockers because of known effects on HRV and stress test results. The study was approved by the Emory Institutional Review Board and all subjects gave informed consent.
2.2. Study Measures
Baseline medical history was obtained by clinical personnel. Hypertension was defined by a measured systolic blood pressure > 140 mm Hg or by treatment with antihypertensive medications. Diabetes mellitus was defined by a fasting glucose level > 126 mg/dL or current treatment with antidiabetic medications. Plasma glucose was measured on the Beckman CX7 chemistry autoanalyzer. Cigarette smoking was classified into current, never, or former smoker. The Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) was used to define the diagnosis of post-traumatic stress disorder (PTSD).[26] Depressive symptoms were assessed with the Beck’s Depression Inventory.[27] Physical activity was assessed using the Baecke physical activity questionnaire.[28]
Twins wore an ambulatory electrocardiogram (Holter) monitor (GE Marquette SEER digital system; GE Medical Systems, Waukesha, Wisconsin) for 24 hours with matching recording times, schedules, and activity levels. The monitors were placed between 2 PM and 4 PM and removed the following afternoon. The sampling frequency was 125 Hz. The data were edited for noise and ectopic beats. Poor-quality ECG (> 20% interpolation) or limited duration of recordings (< 18 hours) were excluded. HRV was analyzed hourly through the commercial HeartTrends algorithm (Lev-El Diagnostics Ltd., Israel) which generated the Dyx measure. Dyx is derived from heart rate time series analysis and measures the variability and randomness of the heart rhythm. Dyx is generated through the multipole method analysis of Poincaré plot, in which beat-to-beat (RR) interval lengths are plotted as a function of prior RR intervals to form an ellipse, as seen in Figure 1. Dyx is calculated as the ratio of the kurtosis along the y-axis (long-term variability) and the x-axis (beat-to-beat random variation) of the ellipse, and higher values indicate more beat-to-beat randomness and/or decreased variability.[29,30] HRV was also measured by power spectral density through fast Fourier transform as previously described,[23] which was divided into three discrete frequency bands: very low frequency (VLF) 0.0033 to < 0.04 Hz; low frequency (LF) 0.04 to < 0.15 Hz; and high frequency (HF) 0.15 to < 0.40 Hz.[11] These frequency bands integrate heart rate fluctuations in response to many physiological stimuli. These include, among others, influences of the renin-angiotensin-aldosterone system (VLF), baroreceptor activity (LF), and respiration (HF).[31]
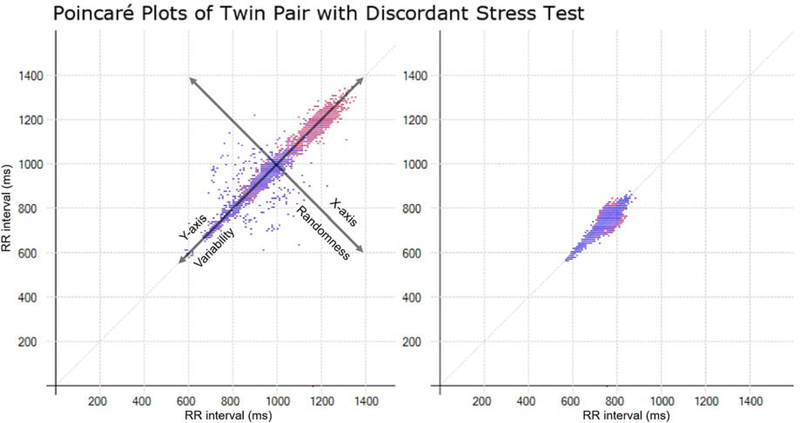
Fig. 1.
The two Poincaré plots represent ECG data at 7 AM for a twin pair that are discordant for stress test results. Both axes are RR interval lengths in milliseconds. The x-axis coordinate represents the RR interval for an initial beat, while the y-axis coordinate represents the RR interval for the following beat, such that the (x, y) coordinate represents (RRn,RRn+1). Each subsequent coordinate is plotted in this way. The red points are beats that were slower than the previous beat, while the blue points were faster than the previous beat. The shape of the resulting plot is abstracted into a single descriptive index called Dyx.The first twin (left) had no myocardial perfusion deficits on stress testing with a Dyx = 3.7. The second twin (right) had an abnormal stress test with a Dyx =1.7.
All subjects, for research purposes, underwent MPI using nitrogen-13-ammonia positron emission tomography with adenosine as the pharmacologic stressor. Adenosine doses were calculated to induce maximal coronary vasodilation.[32] Areas of diminished uptake indicate reduced capacity to maximally vasodilate, thereby causing relative coronary hypoperfusion. Images were visually interpreted by experienced cardiologists and radiologists with training in nuclear cardiology. Quantitative analysis was performed with the Emory Cardiac Toolbox to generate: a) coronary flow reserve (CFR) for absolute myocardial blood flow during stress and rest, and b) the stress total severity score (STSS), which measures the sum of the number of standard deviations below the expected value for each pixel compared to a database of normal controls.[33] CFR was defined as the ratio of mean stress to rest myocardial blood flow (mL/min/g), and low CFR was defined as a ratio < 1.5.[34] Abnormal MPI findings were defined as > 5% MPI deficit. For generalizability, semi-quantitative assessments were used as the primary outcome. Gallium (Ge-68) was used for attenuation correction, and thus coronary artery calcium scores could not be obtained.
2.3. Statistical Analysis
Statistical analysis was performed using R 3.4.0 (R Core Team 2017, Vienna, Austria). HRV measures of Dyx were computed hourly through methods previously published using the HeartTrends analysis, blinded to MPI outcomes.[9] Power spectral density measures of HF, LF, VLF were calculated in rolling 5-minute windows with one-minute increments. The power spectral density measures, as absolute units, were averaged hourly and then log-transformed. The HRV measures were z-transformed to allow for unit-less comparisons amongst each other. Ultra-low frequency power (<0.0033 Hz) was excluded as it is measured over 24 hours and could not be compared reliably hour-to-hour. To account for testing HRV over multiple hours, Bonferroni correction and repeat measure adjustments were made for each hypothesis tested, with p values multiplied by 24 before calculating statistical significance (p<0.05).[35] The cumulative distribution function of HRV over 24-hours between those with and without an abnormal MPI was compared using the two-sample Kolmogorov-Smirnov test. The entire 24-hour circadian profile of HRV was compared by abnormal MPI using cosinor analysis, which characterized the 24 hour distribution based on midline statistic of rhythm (MESOR), acrophase, and amplitude.[36] Each hourly HRV measure was compared by abnormal MPI using a Welch’s t-test.
Generalized linear mixed-effects models with Laplace approximations were used to account for clustering within twin pairs in regression analyses.[22] The models used HRV measures as continuous exposure variables, and Dyx was evaluated as the primary exposure of interested. PET stress perfusion was the primary outcome of interest and analyzed as a binary variable (cutoff 5%). Secondary exposures included frequency domain HRV and heart rate reserve. Secondary outcomes included quantitative global and regional measures of CFR and the STSS. Covariates including age, body mass index (BMI), hypertension, diabetes mellitus, smoking, PTSD, and depressive symptoms were analyzed for collinearity, and added to the adjusted model. Dyx < 2.0 has been suggested as a marker of poor prognosis,[9–11] and was analyzed as a dichotomous variable in additional regression models. Analyses were repeated within subgroups separated by comorbid conditions and Framingham Risk Score. Within-pair analysis for twins discordant for abnormal MPI was performed to control for demographic, familial, and early environmental influences as previously described.[22] Stratification by zygosity was not performed due to small number of twin pairs discordant for stress perfusion abnormalities.
3. RESULTS
3.1. Baseline Characteristics
From the original 526 individuals, 250 were excluded due to a combination of unusable Holter data (n = 197), known IHD (n = 94), beta blocker usage (n = 43), or incomplete PET data (n = 69) (Supplemental Table 2). The final sample consisted of 276 individuals. The mean age ± SD was 55 ± 3 years. The mean BMI was 29 ± 5 kg/m2. Comorbid conditions and cardiovascular risk factors were frequent in this sample of middle-aged veterans, including current/previous smoking (62%), hypertension (29%), diabetes (8%), PTSD (13%), and depressive symptoms (5.1 ± 6.6). Of the population, 3.6% reported having angina symptoms in the 4 weeks prior. There were 16 individuals with resting perfusion deficits. Fifty-five subjects had abnormal MPI (20%), as described in Table 1.
Table 1.
Description of subjects and co-morbid conditions.
| Normal Stress MPI (n = 221) | Abnormal Stress MPI (n = 55) | p-value | |
|---|---|---|---|
| Age (SD) | 55.3 (3.3) | 54.9 (3.0) | 0.34 |
| BMI (SD) | 29.3 (4.8) | 29.7 (5.1) | 0.53 |
| Smoking (%) | 139 (62.9) | 33 (60.0) | 0.81 |
| Hypertension (%) | 62 (28.1) | 19 (34.5) | 0.44 |
| Diabetes (%) | 18 (8.14) | 5 (9.1) | 0.79 |
| PTSD (%) | 28 (12.7) | 8 (14.5) | 0.88 |
| Depression (SD) | 5.15 (6.7) | 5.05 (6.2) | 0.92 |
| Antidepressants (%) | 38 (17.2) | 6 (10.9) | 0.35 |
| Lipid Lowering Drugs (%) | 3 (1.4) | 0 (0.0) | 1.00 |
| Antidiabetic Drugs (%) | 12 (5.4) | 4 (7.3) | 0.53 |
| Baecke score (SD) | 7.43 (1.7) | 7.29 (1.8) | 0.61 |
| FRS (SD) | 6.14 (2.2) | 5.91 (2.6) | 0.555 |
Smoking status indicates current and former smokers. Physical activity was assessed by the Baecke physical activity questionnaire. BMI = Body Mass Index. FRS = Framingham Risk Score. PTSD = Post Traumatic Stress Disorder.
3.2. Heart Rate Variability and Abnormal Myocardial Stress Perfusion
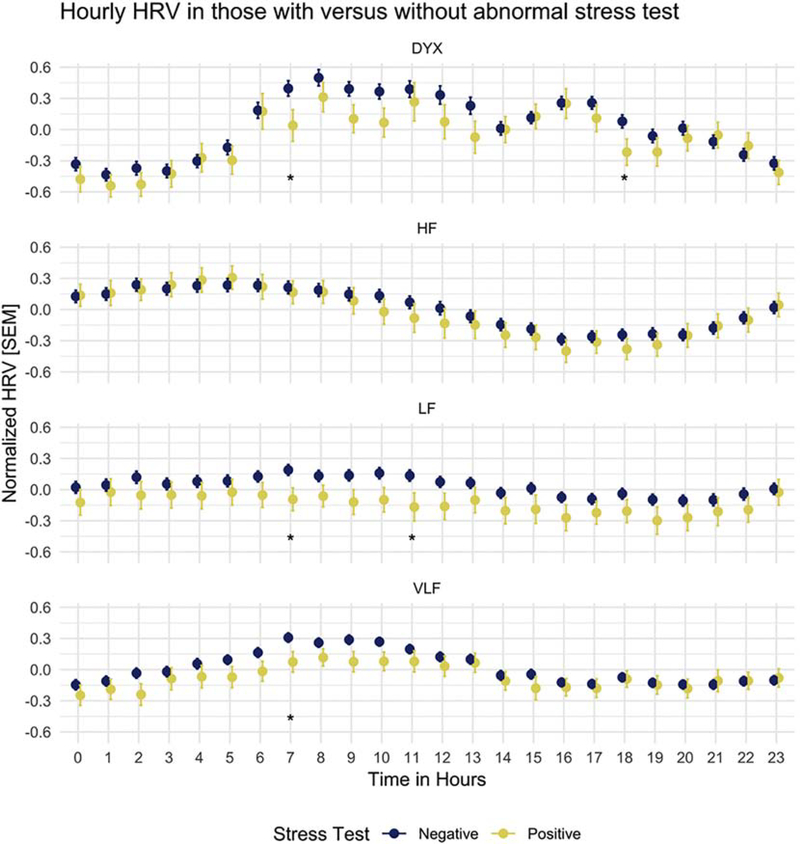
Dyx was calculated from Poincaré plots during each hour of ECG data, as shown in Figure 1 for sample individuals in different MPI categories. HRV measures had normal distributions. The 24-hour distribution of HRV in those with abnormal compared to those with normal MPI studies was different by two-sample Kolmogorov-Smirnov test (p < 0.01) for Dyx and LF only. In cosinor analysis, MESOR differed between groups for Dyx and LF HRV, while the amplitude and acrophase did not differ significantly. For all measures of HRV except HF, significantly lower values were found at 7 AM in those with abnormal compared to those with normal MPI studies (Figure 2). At 6 PM, in addition, Dyx was significantly lower in those with abnormal (vs. normal) MPI.
Fig. 2.
DYX, HF, LF, and VLF were grouped by hourly values and stress test status. *p b 0.05.
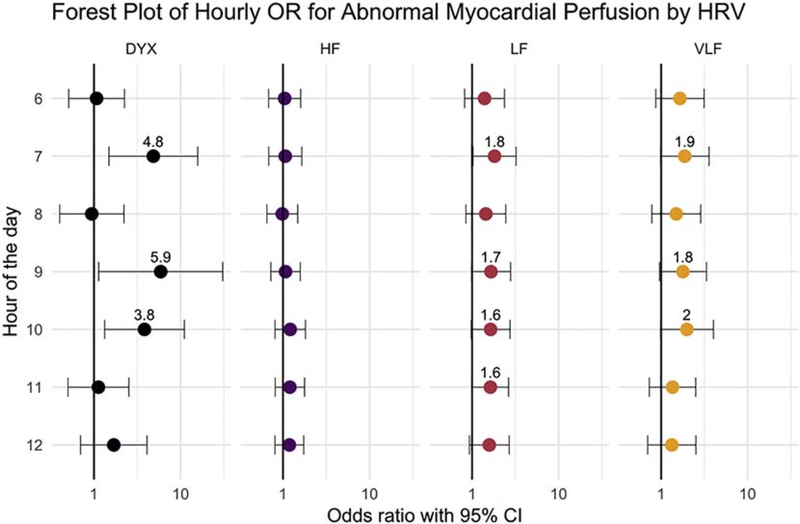
Multivariate regression modeling was performed for all hours and for all HRV measures (Figure 3). Over a continuous 24 hours, Dyx was associated with abnormal MPI, where a decrease of 1 SD in Dyx was associated with an odds ratio (OR) of 1.4 (95% confidence interval, 1.0 — 2.1). The strongest association with Dyx was at 7 AM, where the OR was 4.9 (1.5 — 15.8) odds ratio (OR) of abnormal MPI. For LF HRV, the OR was 1.8 (1.0 — 3.2) at 7 AM. No other hours or HRV measures had significant association with abnormal MPI. When Dyx was dichotomized with cutoff of 2.0 (n = 46 with low values),[9–11] low Dyx at 7 AM associated with OR of 11.8 (1.2 — 111.4) for abnormal MPI, consistent with an effect size of Cohen’s d = 0.42. In a separate analysis with abnormal tests defined as >10% myocardial perfusion deficits (n = 26), the OR of abnormal Dyx was 4.3 (1.2 – 34.0).
Fig. 3.
Visualization of hourly odds ratio for having abnormal myocardial perfusion imaging with lower HRV, from 6 AM to 12 PM. Other hours of the day were not found to be significant. When the confidence intervals had p b 0.1, the odds ratios were labeled.
Multivariable adjustments for demographic factors and comorbid conditions were performed, including age, BMI, smoking, hypertension, PTSD, and depressive symptoms. The effect of Dyx remained significant after adjustment, with an OR of abnormal MPI of 2.1 (1.3 — 3.0). When Dyx was adjusted for Framingham Risk Score, the OR of abnormal MPI remained significant at 1.6 (1.3 — 2.0). Heart rate reserve was not found to have a significant association with abnormal cardiac imaging in unadjusted and multivariate models.
Reduced CFR, through quantitative assessment, was also associated with Dyx. Every 1 SD decrease in Dyx was associated with a 0.13 (0.02 —0.23) decrease in global CFR. In logistic models with low CFR (< 1.5) as the outcome, Dyx was associated with an increased odd of low CFR, with OR=1.4 (1.1 — 2.0). Dyx also was associated with regional CFR, such that each SD decrease in Dyx associated with a decrease of 0.15 (0.05 – 0.26) in the left anterior descending artery territory, 0.11 (−0.01 – 0.22) in the left circumflex artery territory, and 0.15 (0.04, 0.23) in the right coronary artery territory. Both adjusted and multivariate models were tested (Supplemental Table 3). No associations of CFR with the other HRV metrics were found. STSS had no association with HRV in either adjusted or multivariate models.
3.3. Within-Pair and Subgroup Analyses
There were 38 pairs of twins that were discordant on the results of their MPI, and underwent subsequent hourly analysis in a similar fashion as above (Supplemental Figure 4). The results for Dyx were similar to the overall sample. Dyx was associated with abnormal MPI with OR of 2.5 (1.2 — 5.4) at 7 AM. There were no differences in LF HRV within pairs discordant for abnormal perfusion. The primary analysis was also performed in subgroups, divided by key demographic characteristics and risk factors. No significant differences were found based on the presence or absence of hypertension, diabetes, PTSD, depressive symptoms, or smoking (Supplemental Figure 5).
4. DISCUSSION
The major finding of our study is that alterations in the morning variation of heart rate variability are associated with alterations of coronary blood flow regulation. The strongest association with abnormal MPI imaging was a low Dyx at 7 AM, with an additional association at 6 PM. The effects remained significant after rigorous adjustment with traditional risk factors and familial effects in the within-pair analysis. Our data supports the growing evidence of Dyx as a marker of cardiovascular risk,[9,10,16,37] contextualizes the known relationship between the ANS and coronary blood flow regulation, and emphasizes the importance of circadian factors as triggers of cardiovascular events.[38,39]
Overall, Dyx has a moderate effect in predicting abnormal coronary flow regulation, with d = 0.42; this contrasts from traditional risk factors, which are not as predictive. Specifically, our findings show that 1 SD decrease in morning Dyx has the effect of a 5-fold increase in the odds of abnormal MPI, and a 12-fold increase in odds for Dyx < 2. Other studies have shown a similar effect size and independent association using exercise MPI. Goldkorn et al found an 8-fold increase in the odds of abnormal MPI in those with abnormal Dyx.[10] In a larger study, Dyx < 2.6 was found to have a 2-fold increase in the odds of positive exercise MPI.[40] However, our findings are focused on the relationship between abnormal HRV and coronary blood flow regulation, as we used adenosine for stress testing, and placed these findings into the context of time-of-day.
4.1. Heart Rate Variability
The reasons that Dyx is predictive of cardiac perfusion abnormalities are not clear. Dyx captures microscopic, random, non-linear patterns by evaluating individual beat-to-beat aberrations while frequency domain captures macroscopic patterns at the scale of multiple of hundreds to thousands of beats. The kurtosis, instead of the traditional standard deviations along the elliptical axes of a Poincaré plot, more heavily weighs the effect of a sudden, unpredictable beat and beat-to-beat interactions, which in turn reflects ANS dysfunction.[29,30] Dyx serves as a predictor of ventricular tachyarrhythmias in patients with defibrillators and as a prognostic marker of long-term mortality after myocardial infarctions.[16,41] Myocardial ischemia increases sympathetic burst activity and causes sudden aberrations in heart rhythm as well,[42] thereby reducing Dyx. Dyx contrasts from other traditional HRV metrics, such as frequency domain, that have been used to estimate the individual and mixed contributions of ANS outflow to the sinoatrial node.[31] Although HF largely reflects vagal activity, LF and VLF appear to be a combination of alterations in vagal and sympathetic outflow in relationship with other physiologic processes such as baroreflex activity and renin-angiotensin function.[31,43] These spectral metrics appear to focus on peripheral cardiovascular reflexes rather than intrinsic irregularities measured by Dyx.[44,45].
While Dyx has been shown to predict abnormal nuclear stress tests,[9,10] our study systematically evaluated the circadian rhythm in autonomic function as it relates to myocardial perfusion. We note that unlike the prior studies, time-of-day is an important moderator of this relationship. Only the MESOR cosinor metric was associated with abnormal MPI, but this was driven by the 7 AM and 6 PM time periods in which the difference between groups was largest. This pattern mirrors the temporal pattern in cardiac events, including sudden cardiac death, where events peak between 6 AM and 10 AM,[39,46] and secondarily between 6 PM and 8 PM.[47] There is a circadian pattern to autonomic outflow, melatonin, cortisol, and circulating catecholamines which could increase vulnerability to ischemia and cardiac death during morning hours.[46,48]
4.2. Coronary Blood Flow Regulation
Although MPI is normally used in a clinical setting to risk-stratify for obstructive CAD, the consistency of our findings among the primary outcome, stress perfusion, and secondary outcome, coronary flow reserve, support the hypothesis and prevailing evidence that myocardial blood flow is regulated by the cardiac autonomic nervous system. Our study has implications on the use of HRV in clinical context, pending further testing. Although resting perfusion deficits were not associated with HRV, the relationship of HRV and obstructive CAD remains to be seen. The dose of adenosine we used as the pharmacologic agent in our stress test induces maximal coronary vasodilation. When considering the low pre-test probability of our community-based sample for CAD with mean age of 55 years, many of them may have coronary endothelial dysfunction.[49] Regardless, participants with abnormal findings on perfusion may have an increased rate of cardiovascular events due to their underlying vasomotor and endothelial pathophysiology.[50,51] Whether or not HRV and myocardial blood flow regulation are causally related is unknown, and is an important direction for future research.
Many potential mechanistic pathways exist that may explain our findings.[52] It is well known that myocardial ischemia can increase the firing of afferent sensory fibers which transmit this increased input to the CNS via sympathetic visceral afferent pathways.[18,19] This results in reflex increases in efferent cardiac sympathetic efferent activity which can lead to regional coronary vasomotor changes as well as alteration in the control of heart rate.[53] One aspect of our study may help to tie together our findings. Adenosine, when administered either intravenously or intracoronary, often results in complaints of chest discomfort. Adenosine stimulates cardiac sympathetic afferents that travel to the spinal cord via sympathetic nerves prior to ascending to central nervous centers, as demonstrated in canine models by Thames et al.[54] These are likely the same nerve endings that are activated by myocardial ischemia resulting in angina.[55] In canine models, activation of cardiac sympathetic afferent neurons by myocardial ischemia induces reflex efferent sympathetic excitation, which is reduced by blockade of adenosine receptors and augmented by inhibition of the breakdown of adenosine.[8,54] Adenosine stress testing may lead to reflex sympathetic excitation which in turn alters coronary blood flow regulation, either at the macrovascular, microvascular level, or both.[56] Carefully designed studies are needed to test these hypotheses further.
4.3. Limitations and Future Direction
A weakness of our study is the absence of angiographic findings from cardiac catherization. As such, we do not know if the findings are due to significant epicardial obstructive atherosclerosis, although we feel it is unlikely in our patient population as patients with known IHD were excluded and coronary flow reserve was abnormal. Due to our use of gallium for attenuation correction, coronary artery calcifications could not be assessed limiting our ability to evaluate mechanisms specific to atherosclerosis and calcification. Although the twins maintained an identical schedule, actigraphy data were not available and sleep schedules could not be enforced, which may affect the individual circadian rhythms. This is a cross-sectional study and the directionality between abnormalities in autonomic control of HRV and altered coronary blood flow regulation was not tested directly. The generalizability of our study is limited by our population, which consisted of middle-aged, predominantly white, male veterans without prior IHD. Due to standards for HRV analysis, Holter data were considered unusable in a number of patients. Despite these limitations, our twin design allowed for a tightly controlled analysis when analyzing within twin pairs and allows for adjustment of unmeasured confounding. Future studies and prospective follow-up will be needed to elucidate the role of epicardial disease using coronary angiography and/or coronary microvascular disease in these autonomically-linked abnormalities in coronary blood flow regulation.
5. CONCLUSION
There is a circadian variation in autonomic control of HRV that is highly predictive of abnormal pharmacological stress MPI. The non-linear measure Dyx showed the strongest association, suggesting that decreased complexity is a representative feature of dysfunction of cardiac vasomotor regulation, which is likely involves autonomic mechanisms. These hypothesis-generating results should lead to further evaluation of the mechanisms underlying the relationship between autonomic control of HRV and coronary blood flow regulation.
Supplementary Material
HIGHLIGHTS.
Low heart rate variability is associated with abnormal stress myocardial perfusion and low coronary flow reserve
The autonomic differences between those with and without abnormal myocardial stress perfusion occur in the early morning hours, which is also when cardiac events are more likely to occur in general
The findings emphasize the importance of autonomic regulation of coronary blood flow and suggest heart rate variability may be helpful in risk stratification of those in whom ischemic heart disease is suspected
ACKNOWLEDGEMENTS
The United States Department of Veterans Affairs has provided financial support for the development and maintenance of the VET (Vietnam Era Twin) Registry. Numerous organizations have provided invaluable assistance, including: VA Cooperative Study Program; Department of Defense; National Personnel Records Center, National Archives and Records Administration; the Internal Revenue Service; National Institutes of Health (NIH); National Opinion Research Center; National Research Council, National Academy of Sciences; and the Institute for Survey Research, Temple University. NIH (K24HL077506, R01 HL68630, R01 AG026255, NIH K24 MH076955, R21HL093665- 01A1S1), the American Heart Association (0245115N), the National Center for Advancing Translational Sciences of the NIH under Award Number UL1TR000454, and by the Emory University General Clinical Research Center MO1-RR00039. We acknowledge the continued cooperation and participation of the members of the VET Registry and their families. Additionally, we would like to thank the tireless staff at Emory. The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Conflicts of Interest
None, no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Niemeijer MN, Van Den Berg ME, Leening MJ, Hofman A, Franco OH, Deckers JW, Heeringa J, Rijnbeek PR, Stricker BH, Eijgelsheim M, Declining incidence of sudden cardiac death from 1990–2010 in a general middle-aged and elderly population: The Rotterdam Study, Hear. Rhythm 12 (2015) 123–129. 10.1016/j.hrthm.2014.09.054. [DOI] [PubMed] [Google Scholar]
- [2].Madsen JK, Ischaemic heart disease and prodromes of sudden cardiac death. Is it possible to identify high risk groups for sudden cardiac death?, Br Hear. J 54 (1985) 27–32. 10.1136/hrt.54.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hayashi M, Shimizu W, Albert CM, The Spectrum of Epidemiology Underlying Sudden Cardiac Death, Circ. Res 116 (2015) 1887–1906. 10.1161/CIRCRESAHA.116.304521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Deyell MW, Krahn AD, Goldberger JJ, Sudden Cardiac Death Risk Stratification, Circ. Res 116 (2015) 1907–1918. 10.1161/CIRCRESAHA.116.304493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bauer A, Identifying high-risk post-infarction patients by autonomic testing — Below the tip of the iceberg, Int. J. Cardiol 237 (2017) 19–21. 10.1016/j.ijcard.2017.03.087. [DOI] [PubMed] [Google Scholar]
- [6].Liao D, Cai J, Rosamond WD, Barnes RW, Hutchinson RG, Whitsel EA, Rautaharju P, Heiss G, Cardiac autonomic function and incident coronary heart disease: A population-based case-cohort study: The ARIC study, Am. J. Epidemiol 145 (1997) 696–706. 10.1093/aje/145.8.696. [DOI] [PubMed] [Google Scholar]
- [7].La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ, Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction, Lancet. 351 (1998) 478–484. 10.1016/S0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- [8].Abboud FM, Thames MD, Interaction of Cardiovascular Reflexes in Circulatory Control, Compr. Physiol (2011) 675–753. 10.1002/cphy.cp020319. [DOI] [Google Scholar]
- [9].Oieru D, Moalem I, Rozen E, Naimushin A, Klempfner R, Goldenberg I, Goldkorn R, A novel heart rate variability algorithm for the detection of myocardial ischemia: pilot data from a prospective clinical trial., Isr. Med. Assoc. J 17 (2015) 161–5. http://www.ncbi.nlm.nih.gov/pubmed/25946767. [PubMed] [Google Scholar]
- [10].Goldkorn R, Naimushin A, Shlomo N, Dan A, Oieru D, Moalem I, Rozen E, Gur I, Levitan J, Rosenmann D, Mogilewsky Y, Klempfner R, Goldenberg I, Comparison of the usefulness of heart rate variability versus exercise stress testing for the detection of myocardial ischemia in patients without known coronary artery disease, Am. J. Cardiol 115 (2015) 1518–1522. 10.1016/j.amjcard.2015.02.054. [DOI] [PubMed] [Google Scholar]
- [11].Task Force of the ESC and NAS, Heart Rate Variability, Eur. Heart J 17 (1996) 354–381. 10.1161/01.CIR.93.5.1043. [DOI] [PubMed] [Google Scholar]
- [12].Goldstein DS, Bentho O, Park MY, Sharabi Y, Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes, Exp. Physiol 96 (2011) 1255–1261. 10.1113/expphysiol.2010.056259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mølgaard H, Sørensen KE, Bjerregaard P, Attenuated 24-h heart rate variability in apparently healthy subjects, subsequently suffering sudden cardiac death, Clin. Auton. Res 1 (1991) 233–237. 10.1007/BF01824992. [DOI] [PubMed] [Google Scholar]
- [14].Stein PK, Domitrovich PP, Huikuri HV, Kleiger RE, Traditional and nonlinear heart rate variability are each independently associated with mortality after myocardial infarction, J. Cardiovasc. Electrophysiol 16 (2005) 13–20. 10.1046/j.1540-8167.2005.04358.x. [DOI] [PubMed] [Google Scholar]
- [15].Harris PRE, Stein PK, Fung GL, Drew BJ, Prognostic value of heart rate turbulence for risk assessment in patients with unstable angina and non-ST elevation myocardial infarction, Vasc. Health Risk Manag 9 (2013) 465–473. 10.2147/VHRM.S43654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jørgensen RM, Abildstrøm SZ, Levitan J, Kobo R, Puzanov N, Lewkowicz M, Huikuri H, Peltola M, Haarbo J, Thomsen PEB, pilot study Group NICD, Heart Rate Variability Density Analysis (Dyx) and Prediction of Long-Term Mortality after Acute Myocardial Infarction., Ann. Noninvasive Electrocardiol 21 (2016) 60–68. 10.1111/anec.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Valencia JF, Vallverdú M, Porta A, Voss A, Schroeder R, Vázquez R, Bayés De Luna A, Caminal P, Ischemic risk stratification by means of multivariate analysis of the heart rate variability, Physiol. Meas 34 (2013) 325–338. 10.1088/0967-3334/34/3/325. [DOI] [PubMed] [Google Scholar]
- [18].Lombardi F, Sandrone G, Pernpruner S, Sala R, Garimoldi M, Cerutti S, Baselli G, Pagani M, Malliani A, Heart rate variability as an index of sympathovagal interaction after acute myocardial infarction, Am. J. Cardiol 60 (1987) 1239–1245. 10.1016/0002-9149(87)90601-1. [DOI] [PubMed] [Google Scholar]
- [19].Kleiger RE, Miller JP, Bigger JT, Moss AJ, Decreased heart rate variability and its association with increased mortality after acute myocardial infarction, Am. J. Cardiol 59 (1987) 256–262. 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- [20].Thayer JF, Yamamoto SS, Brosschot JF, The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors, Int. J. Cardiol 141 (2010) 122–131. 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- [21].Maheshwari A, Norby FL, Soliman EZ, Adabag S, Whitse EA, Alonso A, Chen LY, Low heart rate variability in a 2-minute electrocardiogram recording is associated with an increased Risk of sudden cardiac death in the general population: The Atherosclerosis Risk in communities study, PLoS One. 11 (2016). 10.1371/journal.pone.0161648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Carlin JB, Gurrin LC, Sterne JAC, Morley R, Dwyer T, Regression models for twin studies: A critical review, Int. J. Epidemiol 34 (2005) 1089–1099. 10.1093/ije/dyi153. [DOI] [PubMed] [Google Scholar]
- [23].Shah AJ, Lampert R, Goldberg J, Veledar E, Bremner JD, Vaccarino V, Posttraumatic stress disorder and impaired autonomic modulation in male twins, Biol. Psychiatry 73 (2013) 1103–1110. 10.1016/j.biopsych.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vaccarino V, Lampert R, Bremner JD, Lee F, Su S, Maisano C, V Murrah N, Jones L, Jawed F, Afzal N, Ashraf A, Goldberg J, Depressive symptoms and heart rate variability: Evidence for a shared genetic substrate in a study of twins, Psychosom. Med 70 (2008) 628–636. 10.1097/PSY.0b013e31817bcc9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Goldberg J, Curran B, Vitek ME, Henderson WG, Boyko EJ, The Vietnam era twin registry, Twin Res. 5 (2002) 476–481. 10.1375/twin.5.5.476. [DOI] [PubMed] [Google Scholar]
- [26].First MB, Spitzer RL, Miriam G, Williams JBW, Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV). Washington, D.C., Am. Psychiatr. Press. (1996). [Google Scholar]
- [27].Beck AT, Steer RA, Carbin MG, Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation, Clin. Psychol. Rev 8 (1988) 77–100. 10.1016/0272-7358(88)90050-5. [DOI] [Google Scholar]
- [28].Baecke JA, Burema J, Frijters JE, A short questionnaire for the measurement of habitual physical activity in epidemiological studies, Am. J. Clin. Nutr 36 (1982) 936–942. 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- [29].Lewkowicz M, Levitan J, Puzanov N, Shnerb N, Saermark K, Description of complex time series by multipoles, Phys. A Stat. Mech. Its Appl 311 (2002) 260–274. 10.1016/S0378-4371(02)00831-2. [DOI] [Google Scholar]
- [30].Olesen RM, Bloch Thomsen PE, Saermark K, Glikson M, Feldman S, Lewkowicz M, Levitan J, Statistical analysis of the DIAMOND MI study by the multipole method, Physiol. Meas 26 (2005) 591–598. 10.1088/0967-3334/26/5/002. [DOI] [PubMed] [Google Scholar]
- [31].Akselrod S, Gordon D, Ubel A, Shannon D, Barger C, Cohen R, Power Spectrum Analysis of Heart Rate Fluctuation: A Quantitative Probe of Beat-To-Beat Cardiovascular Control, 1981. file:///C:/Documents and Settings/avartak/Desktop/PhD Research/aniket research documents/HRV/AKSELROD_psd_SCIENCE_1981.pdf. [DOI] [PubMed]
- [32].Vaccarino V, Khan D, Votaw J, Faber T, Veledar E, Jones DP, Goldberg J, Raggi P, Quyyumi AA, Bremner JD, Inflammation is related to coronary flow reserve detected by positron emission tomography in asymptomatic male twins, J. Am. Coll. Cardiol 57 (2011) 1272–1279. 10.1016/j.jacc.2010.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hutchins GD, Schwaiger M, Rosenspire KC, Krivokapich J, Schelbert H, Kuhl DE, Noninvasive quantification of regional blood flow in the human heart using N-13 ammonia and dynamic positron emission tomographic imaging, J. Am. Coll. Cardiol 15 (1990) 1032–1042. 10.1016/0735-1097(90)90237-J. [DOI] [PubMed] [Google Scholar]
- [34].Gould KL, Johnson NP, Coronary Physiology Beyond Coronary Flow Reserve in Microvascular Angina: JACC State-of-the-Art Review, J. Am. Coll. Cardiol (2018). 10.1016/j.jacc.2018.07.106. [DOI] [PubMed] [Google Scholar]
- [35].Wright SP, Adjusted P-Values for Simultaneous Inference, Biometrics. 48 (1992) 1005. 10.2307/2532694. [DOI] [Google Scholar]
- [36].Guo YF, Stein PK, Circadian rhythm in the cardiovascular system: Chronocardiology, Am. Heart J 145 (2003) 779–786. 10.1016/S0002-8703(02)94797-6. [DOI] [PubMed] [Google Scholar]
- [37].Jørgensen RM, Levitan J, Halevi Z, Puzanov N, Abildstrøm SZ, Messier MD, Huikuri HV, Haarbo J, Thomsen PEB, Jons C, Heart rate variability density analysis (Dyx) for identification of appropriate implantable cardioverter defibrillator recipients among elderly patients with acute myocardial infarction and left ventricular systolic dysfunction, Europace. 17 (2015) 1848–1854. 10.1093/europace/euu394. [DOI] [PubMed] [Google Scholar]
- [38].Dolezel S, Gerová M, Gero J, Sládek T, Vasku J, Adrenergic innervation of the coronary arteries and the myocardium., Acta Anat. (Basel) 100 (1978) 306–16. http://www.ncbi.nlm.nih.gov/pubmed/619505 (accessed May 28, 2019). [PubMed] [Google Scholar]
- [39].Muller JE, Circadian variation in cardiovascular events., Am. J. Hypertens 12 (1999) 35S–42S. 10.1016/S0895-7061(98)00278-7. [DOI] [PubMed] [Google Scholar]
- [40].Goldenberg I, Goldkorn R, Shlomo N, Einhorn M, Levitan J, Kuperstein R, Klempfner R, Johnson B, Heart Rate Variability for Risk Assessment of Myocardial Ischemia in Patients Without Known Coronary Artery Disease: The HRV-DETECT (Heart Rate Variability for the Detection of Myocardial Ischemia) Study, J. Am. Heart Assoc 8 (2019). 10.1161/JAHA.119.014540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rozen G, Kobo R, Beinart R, Feldman S, Sapunar M, Luria D, Eldar M, Levitan J, Glikson M, Multipole analysis of heart rate variability as a predictor of imminent ventricular arrhythmias in ICD patients, PACE - Pacing Clin. Electrophysiol 36 (2013) 1342–1347. 10.1111/pace.12180. [DOI] [PubMed] [Google Scholar]
- [42].Hall TM, Gordon C, Roy R, Schwenke DO, Delayed coronary reperfusion is ineffective at impeding the dynamic increase in cardiac efferent sympathetic nerve activity following myocardial ischemia, Basic Res. Cardiol 111 (2016). 10.1007/s00395-016-0556-3. [DOI] [PubMed] [Google Scholar]
- [43].Lehrer PM, Vaschillo E, Vaschillo B, Lu SE, Eckberg DL, Edelberg R, Shih WJ, Lin Y, Kuusela TA, Tahvanainen KUO, Hamer RM, Heart rate variability biofeedback increases baroreflex gain and peak expiratory flow, Psychosom. Med 65 (2003) 796–805. 10.1097/01.PSY.0000089200.81962.19. [DOI] [PubMed] [Google Scholar]
- [44].Nozdrachev AD, John Newport Langley and his construction of the autonomic (Vegetative) nervous system, J. Evol. Biochem. Physiol 38 (2002) 537–546. 10.1023/A:1022056815191. [DOI] [PubMed] [Google Scholar]
- [45].Armour JA, Myocardial ischaemia and the cardiac nervous system., Eur. Heart J 16 (1999) 1751–2. https://academic.oup.com/cardiovascres/article-abstract/41/1/41/317013 (accessed September 27, 2018). [DOI] [PubMed] [Google Scholar]
- [46].Boudreau P, Dumont G, Kin NMKNY, Walker C-DD, Boivin DB, Correlation of heart rate variability and circadian markers in humans, in: 2011 Annu. Int. Conf. IEEE Eng. Med. Biol. Soc., IEEE, 2011: pp. 681–682. 10.1109/IEMBS.2011.6090153. [DOI] [PubMed] [Google Scholar]
- [47].Mulcahy D, Keegan J, Crean P, Quyyumi A, Shapiro L, Wright C, Fox K, Silent myocardial ischaemia in chronic stable angina: a study of its frequency and characteristics in 150 patients., Br. Heart J 60 (1988) 417–23. 10.1136/hrt.60.5.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Scheer FAJL, Hu K, Evoniuk H, Kelly EE, Malhotra A, Hilton MF, Shea SA, Impact of the human circadian system, exercise, and their interaction on cardiovascular function, Proc. Natl. Acad. Sci (2010). 10.1073/pnas.1006749107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chou R, Qaseem A, Biebelhausen J, Desai S, Feinberg L, Horwitch CA, Humphrey LL, McLean RM, Mir TP, Moyer DV, Skeff KM, Tape TG, Wiese J, Cardiac screening with electrocardiography, stress echocardiography, or myocardial perfusion imaging: Advice for high-value care from the american college of physicians, Ann. Intern. Med 162 (2015) 438–447. 10.7326/M14-1225. [DOI] [PubMed] [Google Scholar]
- [50].Gimelli A, Marzullo P, LAbbate A, Rovai D, ― False-positive‖ myocardial perfusion imaging: Correlation with cardiovascular risk factors and effect on event-free survival, J. Cardiovasc. Med 9 (2008) 707–713. 10.2459/JCM.0b013e3282f5ffc1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.