Abstract
Background/Aim:
A sufficiently open papilla is needed to remove common bile duct stones (CBDS) but endoscopic sphincterotomy (EST) requires a high level of skill and is difficult with endoscopic papillary balloon dilation (EPBD). The main adverse event of EST is bleeding and perforation and that of EPBD is post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis. To reduce these adverse events we employed minimal EST followed by papillary dilation (ESBD), and retrospectively evaluated its efficacy and safety compared with EST.
Patients and Methods:
CBDS patients who underwent EST (n = 114) or ESBD (n = 321) at Juntendo University Hospital from January 2009 to December 2018 were consecutively enrolled, retrospectively. The exclusion criteria were large-balloon dilation (≥ 12 mm), large CBDS (>12 mm), and previous EST/EPBD. We compared the overall stone removal rate, incidence of adverse event, procedure time, number of ERCP procedures, and rate of mechanical lithotripsy (ML) between the two groups.
Results:
Complete stone removal was successful in both ESBD and EST group. However, the rate of multiple ERCP sessions was significantly lower (35.1% vs. 12.8%, P < 0.001), procedure time was shorter (31.6 vs. 25.8 min, P = 0.01), and rate of ML was lower (16.7% vs. 7.8%, P = 0.01) in ESBD group. Bleeding was significantly more frequent in the EST group (9.6% vs. 1.2%, P < 0.001), particularly acute bleeding (7.9% vs. 0.9%, P < 0.001).
Conclusions:
ESBD is more efficient and safer in the management of CBD stones than EST. A prospective randomized study comparing ESBD with EST is needed to establish this combination technique.
Keywords: Bleeding, common bile duct stones, endoscopic papillary balloon dilation, endoscopic sphincterotomy, post-ERCP pancreatitis
INTRODUCTION
Common bile duct stone (CBDS) is one of the most common benign diseases requiring endoscopic retrograde cholangiopancreatography (ERCP)-related procedures. Endoscopic sphincterotomy (EST) is a standard procedure for managing CBDS, and endoscopic papillary balloon dilation (EPBD) is performed for patients with bleeding tendency. However, making an adequate incision for EST requires a high level of skill and increases the risk of bleeding and perforation. In addition, even if EST with a medium incision is performed, it is difficult to achieve adequate papillary dilatation for removing stones. EPBD does not require a high level of skill to achieve sufficient papillary dilation and can be safely performed by novices. However, EPBD has a risk of post-ERCP pancreatitis (PEP).[1,2] Although the incidence of PEP is low in specialized EPBD centers,[3] clinical trials in multiple centers have revealed that EPBD is associated with a significantly higher incidence of PEP compared with EST.[4]
To remove large stones, endoscopic papillary large balloon dilation (EPLBD), using a balloon of ≥12 mm diameter, is often performed. When performing EPLBD, an additional small incision is typically made, similar to minimal EST. EPLBD with EST decreases the incidence of hemorrhage without increasing the incidence of PEP compared to without EST.[5,6,7] Based on the result of EPLBD, even in normal EPBD (≤10 mm), minimal EST may reduce the risk of papillary hemorrhage without increasing the risk of PEP. The procedure on the papilla used to remove small stones (<12 mm diameter) from a small-diameter bile duct (<12 mm) needs to be improved. We investigated retrospectively whether minimal EST followed by EPBD (ESBD) decreases the incidence of hemorrhage and the risk of PEP in the removal of small CBDS (≤12 mm diameter).
PATIENTS AND METHODS
Patient
This study was a retrospective chart review at a single academic center. We enrolled endoscopically treated cases of CBDS who met the following inclusion criteria: 1) age 18 years or older, 2) ability to give informed consent, and 3) received endoscopic treatment for ESBD or EST alone. The exclusion criteria for this study were as follows: 1) EPLBD (papillary dilation of ≥12 mm), 2) CBDS of >12 mm, 3) previous EST or EPBD, and 4) performance of EPBD alone, 5) surgically altered anatomy. Among 778 patients with naïve papilla who received endoscopic treatment with a papillary procedure from January 2009 to March 2018, 435 patients who met the inclusion and exclusion criteria were consecutively enrolled in this retrospective study at Juntendo University Hospital. This study was approved by the Institutional Review Board and written informed consent was obtained from all of the patients before ERCP.
The patients were divided into two groups according to the procedure: 114 patients received EST alone (EST group), and 321 underwent ESBD (ESBD group). ESBD was performed beginning in 2012, and the procedure was performed at the discretion of the endoscopist; many of the endoscopists selected ESBD. Trainee endoscopists performed endoscopic procedures under the supervision of experts because our hospital is a teaching hospital.
Methods
ERCP was performed in a standard manner using side-viewing endoscopes (TJF-260V and JF-260V, Olympus, Tokyo, Japan and ED-580T, Fujifilm, Tokyo, Japan). The patients were sedated with a standard dose of midazolam and pethidine hydrochloride. A conventional contrastmedium injection cannulation technique using a cannula (ERCP catheter; MTW Endoskopie, Wesel, Germany) with a 0.035-inch standard guidewire (Jagwire; Boston Scientific Japan, Tokyo, Japan, and Revowave; Piolax Medical Devices Inc, Kanagawa, Japan) or a 0.035-inch hydrophilic guidewire (Radifocus; Terumo, Tokyo, Japan) was employed. In cases of difficult cannulation, the pancreatic guidewire/stent placement technique, endoscopic ultrasonography/percutaneous transhepatic cholangio drainage-guided rendezvous technique, or precut technique using a needle knife was used. A pull-type sphincterotome (Clever Cut 3V; Olympus, Tokyo, Japan) was employed and an electrosurgical unit (ERBE VIO300, ERBE, Tubingen, Germany) was used with effect 3 in Endocut I mode (output limit, 120 W).
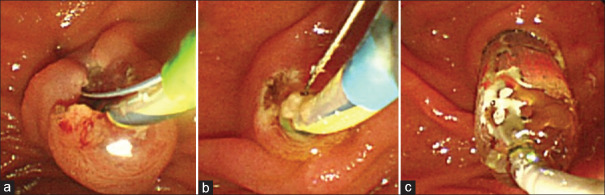
The extent of the incision was divided into full, medium, and minimum. A full EST was defined as an EST extending to almost the total length of the ampullary-protruding portion. EST that extended over the covering fold was defined as medium EST and EST that did not extend over the covering fold was defined as small EST. In small EST, a shorter incision of 4 mm or less was defined as minimal EST. Medium and minimal EST were performed in the EST and ESBD groups, respectively [Figure 1]. In the ESBD group, balloon size (8 or 10 mm Hurricane RX; Boston Scientific Corporation, Natik, MA) was selected based on the diameter of the lower bile duct. The balloon was gradually infiated under fluoroscopic guidance with half-diluted contrast material, with saline, until the waist of the balloon disappeared; the balloon was then immediately deflated. Stones were removed using a retrieval balloon catheter or a four-wire basket catheter. Endoscopic mechanical lithotripsy (ML) was performed for patients with large stones that were difficult to retrieve using a conventional balloon/basket catheter.
Figure 1.
Endoscopic image of (a) EST and (b and c) ESBD. a: Medium EST shows one to two thirds incision of the ampulla. Very small EST (b: within one third incision of the ampulla) followed by Balloon dilation (b: 6 mm, 8 mm or 10 mm)
Definitions
Complete stone removal was confirmed by a balloon-occluded cholangiogram at the end of the procedure or by the follow-up cholangiogram if a naso-biliary drainage tube had been placed. If any stones remained, an additional endoscopic procedure was performed to retrieve the remaining CBDS.
Severity of adverse event (AE) related to the procedures was defined according to the Lexicon criteria. Hyperamylasemia was defined as a serum amylase level exceeding three times the upper limit of normal without abdominal pain. Hyperamylasemia with persistent abdominal pain for more than 24 h was defined as PEP.
Post-ERCP bleeding was defined as endoscopically confirmed bleeding within 1 week of ERCP that required hemostatic treatment. Early bleeding was defined as bleeding that occurred during or within 24 h after ERCP. Delayed bleeding was defined as bleeding not evident at the end of the procedure, but which occurred 24 h after the procedure. The following constitute our step-up strategies of hemostasis when we encounter hemorrhage from the papilla: spraying with diluted epinephrine was the initial treatment, followed by compression with a retrieval balloon catheter. If these methods do not adequately control bleeding, then we performed injection of hypertonic saline epinephrine solution (HSE), hemostatic clipping, or electrical coagulation. A covered metallic stent was placed when various hemostatic procedures failed. Bleeding that was controlled during the procedure by spraying diluted epinephrine was not considered an AE, because such degree of bleeding seemed to stop by merely waiting for several minutes. Successful hemostasis was considered when hemorrhage had stopped for more than 5 min during endoscopic observation.
Cholangitis was defined as a fever of >38°C accompanied by leukocytosis, elevated liver enzyme levels after the procedure. Cholecystitis was defined as pain in the right upper quadrant accompanied by gallbladder swelling on some imaging modalities. All AEs were classified and graded according to the severity grading system of the Lexicon of the American Society for Gastrointestinal Endoscopy.[8]
Endpoints
The primary endpoint was overall stone removal rate. The secondary endpoints were the incidence of AE (PEP, acute cholangitis and cholecystitis, acute/delayed bleeding and perforation), procedure time, number of ERCP procedures, the rate of ML, and the recurrence of CBDS.
Statistical analysis
Data are presented as means ± standard deviation. Categorical parameters were compared by Chi-squared test or the Fisher exact test, and continuous variables by Student's t-test. Differences were considered significant when the P value was <0.05. Factors with P < 0.05 by univariate analysis were considered to be potential risk factors for bleeding and were further analyzed in a multivariate analysis. The data were analyzed using SPSS 19 software (Chicago, IL).
RESULTS
A total of 435 patients were analyzed in this retrospective study. Their mean age was 72.7 ± 12.8 years (range, 25 to 101 years) at the time of the initial procedure, and there were 275 males and 160 females. The clinical features of the patients in the EST (n = 114) and ESBD (n = 321) groups are presented in Table 1. There were no significant differences in terms of sex, laboratory data pre-ERCP, mean diameter of stones, number of stones, and presence of periampullary diverticulum between the two groups. In contrast, age, BMI, accompanying cardiovascular disease, and number of patients taking antithrombotic drugs differed significantly between the two groups. The frequency of accompanying cardiac diseases (EST group 11.4% vs. ESBD group 22.7%, P = 0.01) and antithrombotic drugs (EST group 14.1% vs. ESBD: 29.6%, P < 0.001) was higher in the ESBD group than in the EST group.
Table 1.
Patients’ characteristics
| EST (n=114) | ESBD (n=321) | P | |
|---|---|---|---|
| Age (mean±SD) | 75.1±13.0 | 71.7±13.2 | 0.02 |
| Sex (male:female) | 66:48 | 209:112 | 0.17 |
| BMI | 22.2±3.5 | 23.2±3.8 | 0.02 |
| Accompanying disease, n (%) | |||
| Cardiovascular disease | 13 (11.4%) | 73 (22.7%) | 0.01 |
| Chronic renal failure | 14 (12.3%) | 60 (18.7%) | 0.15 |
| Liver cirrhosis | 3 (2.6%) | 12 (3.7%) | 0.58 |
| Chronic pancreatitis | 2 (1.8%) | 2 (0.6%) | 0.28 |
| Diabetes mellitus | 26 (22.8%) | 66 (20.6%) | 0.60 |
| Antithrombotic drugs, n (%) | 16 (14.0%) | 95 (29.6%) | <0.001 |
| Number of Antithrombotic drugs, 1/2- | 13/3 | 70/25 | 0.76 |
| Withdrawn/continuous/heparin replacement | 8/3/5 | 42/8/45 | 0.31 |
| Laboratory data before ERCP | |||
| Platelet count | 22.5±10.6 | 22.6±8.1 | 0.94 |
| PT% | 86.2±13.1 | 87.9±13.0 | 0.26 |
| Total bilirubin | 1.8±2.0 | 2.1±3.1 | 0.37 |
| Characteristics of stones | |||
| Mean diameter of stones (mm) | 5.1±2.9 | 5.6±3.0 | 0.17 |
| Number of stones | 1.6±1.3 | 1.9±2.3 | 0.21 |
| Number of stones (1/2/≥3) | 84/11/19 | 215/42/64 | 0.07 |
| Periampullary diverticula | 36 (31.6%) | 91 (28.3%) | 0.52 |
| Dilation balloon diameter, 6/8/10 mm | - | 3/263/55 | - |
EST: Endoscopic sphincterotomy, ESBD: Endoscopic minimal-sphincterotomy followed by papillary balloon dilation, BMI: Body mass index
The details of procedures are shown in Table 2. There were no significant differences in the overall stone removal rate or the frequency of difficult cannulation between the two groups. The rate of multiple ERCP sessions was significantly lower in the ESBD group than in the EST group (P < 0.01). The procedure time of 16 cases was unknown, and the analysis of procedure time was performed excluding these missing data. The mean procedure time was significantly shorter in the ESBD group (31.6 ± 16.8 vs. 25.8 ± 18.8 min, 95%CI 1.78-9.78, P = 0.01). In addition, the rate of ML was significantly lower in the ESBD group (EST group 16.7% vs. ESBD group 7.8%, P = 0.01).
Table 2.
Details of the procedures
| EST (n=114) | ESBD (n=321) | P | |
|---|---|---|---|
| Overall stone removal | 100% | 100% | 1.00 |
| Required multiple sessions | 40 (35.1%) | 41 (12.8%) | <0.001 |
| Mean procedure time (min) | 31.6±16.8 | 25.8±18.8 | 0.01 |
| Mechanical lithotripsy | 19 (16.7%) | 25 (7.8%) | 0.01 |
| Mean duration of follow-up (months) | 75.7±29.0 | 40.1±23.8 | <0.001 |
| Difficult cannulation & employed procedure | 6 (5.3%) | 22 (6.9%) | 0.66 |
| Pancreatic guidewire | 1 (0.9%) | 8 (2.5%) | 0.30 |
| Pancreatic stent | 3 (2.6%) | 6 (1.9%) | 0.62 |
| Pre-cut | 0 | 5 (1.6%) | 0.18 |
| Rendezvous | 2 (1.8%) | 3 (0.9%) | 0.48 |
| Prophylactic pancreatic stent | 5 (4.4%) | 9 (2.8%) | 0.41 |
| Prophylactic biliary stent | 9 (7.9%) | 26 (8.1%) | 0.95 |
EST: Endoscopic sphincterotomy, ESBD: Endoscopic minimal-sphincterotomy followed by papillary balloon dilation
The details of AEs are shown in Table 3. The frequency of AEs was significantly higher in the EST group than in the ESBD group (EST group 15.8% vs. ESBD group 4.4%, P < 0.001). There were no significant differences in the frequency of PEP, perforation, acute cholangitis, or acute cholecystitis. Bleeding was significantly more frequent in the EST group than in the ESBD group (EST group 9.6% vs.ESBD group 1.2%, P < 0.001). Also, early bleeding was significantly more frequent in the EST group (EST group 7.9% vs.ESBD group 0.9%, P < 0.001). Recurrence of CBDS was more frequent in the EST group than in the ESBD group (EST group 11.4% vs.ESBD group 5.9%, P = 0.05), albeit not significantly so.
Table 3.
Adverse events related to the procedures
| EST (n=114) | ESBD (n=321) | P | |
|---|---|---|---|
| Total number of adverse events | 18 (15.8%) | 14 (4.4%) | <0.001 |
| Post-ERCP pancreatitis | 3 (2.6%) | 6 (1.9%) | 0.62 |
| Mild/moderate/severe | 3/0/0 | 6/0/0 | - |
| Bleeding | 11 (9.6%) | 4 (1.2%) | <0.001 |
| Early bleeding (within 1 week) | 9 (7.9%) | 3 (0.9%) | <0.001 |
| Delayed bleeding (after 1 week) | 2 (1.8%) | 1 (0.3%) | 0.11 |
| Perforation | 2 (1.8%) | 1 (0.3%) | 0.11 |
| Acute cholangitis | 1 (0.9%) | 3 (0.9%) | 0.96 |
| Acute cholecystitis | 1 (0.9%) | 0 | 0.09 |
| Mortality | 0 | 0 | - |
| Recurrence of CBD stones | 13 (11.4%) | 19 (5.9%) | 0.05 |
| Recurrence of CBD stones (<1 year) | 6 (5.3%) | 8 (2.5%) | 0.15 |
EST: Endoscopic sphincterotomy, ESBD: Endoscopic minimal-sphincterotomy followed by papillary balloon dilation, CBD: Common bile duct stones
The management of early and delayed bleeding is shown in Table 4. All patients with post-ERCP bleeding were successfully treated by endoscopic hemostasis. Early bleeding was observed in nine patients in the EST group, and three patients in the ESBD group. Among the nine patients in the EST group, eight (88.9%) required balloon tamponade, and one (11.1%) required HSE injection. Among the three patients in the ESBD group, two (66.7%) required balloon tamponade, and one (33.3%) required hemostatic clipping. These patients with early bleeding required unplanned hospital admission or prolongation of hospital stay for ≤3 nights and graded as mild in the Lexicon criteria.
Table 4.
Management of bleeding
| Early bleeding⊛ (n=12) |
Delayed bleeding† (n=3) |
|||
|---|---|---|---|---|
| EST | ESBD | EST | ESBD | |
| Patients, n | 9 | 3 | 2 | 1 |
| Mild/moderate/severe | 9/0/0 | 3/0/0 | 0/2/0 | 0/1/0 |
| Endoscopic treatment, n (%) | ||||
| Balloon tamponade | 9 (100%) | 3 (100%) | 1 (50.0%) | 1 (100%) |
| Injection with HSE | 1 (11.1%) | 0 | 0 | 0 |
| Hemostatic clipping | 0 | 1 (33.3%) | 0 | 0 |
| Blood transfusion | 0 | 0 | 1 (50.0%):4unit | 0 |
EST: Endoscopic sphincterotomy, ESBD: Endoscopic minimal-sphincterotomy followed by papillary balloon dilation, HSE: Hypertonic saline epinephrine. ⊛within 1 week, †after 1 week
Delayed bleeding was observed in two patients in the EST group and in one patient in the ESBD group. Of the two patients in the EST group, one (50.0%) required balloon tamponade and one (50.0%) required blood transfusion. The patient transfused with four units of blood had a melena and a drop in the hemoglobin level of 5 g/dL from baseline. In the ESBD group, one patient with delayed bleeding required balloon tamponade. These patients with delayed bleeding were required repeat endoscopy and graded as moderate in the Lexicon criteria.
ESBD had a low risk of bleeding, and did not increase the risk of PEP, perforation, acute cholangitis, or acute cholecystitis. Medium EST, chronic pancreatitis and stone diameter were risk factors for bleeding in the univariate analyses. Only medium EST remained as an independent risk factor for bleeding (odds ratio 0.118, 95% confidence interval 0.037-0.379; <0.001) in a multivariate analysis [Table 5].
Table 5.
Univariate and multivariate analysis of risk factors for bleeding
| Univariate analysis | Multivariate analysis |
||
|---|---|---|---|
| P | Odds ratio (95% CI) | P | |
| Age | 0.61 | - | - |
| Sex | 0.79 | - | - |
| Cardiovascular disease | 0.32 | - | - |
| Chronic renal failure | 1.00 | - | - |
| Liver cirrhosis | 1.00 | - | - |
| Chronic pancreatitis | 0.02 | 7.16 (0.57-89.84) | 0.13 |
| Diabetes mellitus | 0.63 | - | - |
| Platelet count | 0.71 | - | - |
| PT% | 0.10 | - | - |
| Antithrombotic drugs | 0.13 | - | - |
| diameter of stones | 0.01 | 0.99 (0.83-1.19) | 0.93 |
| Periampullary diverticula | 0.13 | - | - |
| ESBD | <0.001 | 0.12 (0.04-0.38) | <0.001 |
| Mechanical lithotripsy | 0.67 | - | - |
| Procedure time | 0.56 | - | - |
ESBD, endoscopic minimal-sphincterotomy followed by papillary balloon dilation
DISCUSSION
EST is the standard technique for the removal of CBDS. However, EST carries an 8% to 13% risk of acute AEs, such as PEP, cholangitis, bleeding, and perforation.[9,10,11,12,13] EPBD using balloons of diameter 6 to 10 mm is an alternative to EST as a treatment for CBDS. However, EPBD is associated with a high risk of PEP. DiSario et al. reported a high frequency of PEP (15.4%), including two deaths from acute severe pancreatitis, among patients who underwent EPBD in a prospective randomized multicenter trial, and EPBD was the only reason for PEP.[2] Incomplete dilation of the papilla, intramucosal bleeding, and local edema was thought to be the main causes of PEP with EPBD.[14]
ESBD was developed to decrease the risk of bleeding without increasing the risk of PEP because the pancreatic orifice is separated from the biliary orifice after minimal EST. We evaluated the safety and effectiveness of ESBD compared with EST for the removal of CBDS of ≤12 mm diameter. Ding et al. reported that ESBD as a combination technique might be better for achieving a wide opening based on the two-ring theory.[14] In theory, the stone-extraction tunnel consists of two segments: the intraduodenal portion of the papilla forms the distal segment, and the distal bile duct the proximal segment. EST, EPBD, and ESBD have different effects on the stone-extraction tunnel. EST opens the distal segment and shortens the stone-extraction tunnel but does not affect the proximal segment. EPBD dilates both segments but maintains the whole structure intact. ESBD cuts the distal segment to shorten the stone extraction tunnel and dilate the proximal ring. As a result, the combination procedure may result in a wider opening, facilitating stone removal. In the present study, the final stone removal rate was 100% in both groups. However, the first stone removal rate was significantly higher in the ESBD group than the EST group (87.2% vs. 64.9%, P < 0.001). In addition, the rate of ML was significantly lower and the procedure time was significantly shorter in the ESBD group, despite the additional time required for papillary balloon dilation. These results suggest that the addition of papillary dilation after minimal incision may result in a more open papilla than medium EST. Therefore, ESBD was considered a more efficient procedure for CBDS removal.
Bleeding is one of the most frequent AEs of EST and the incidence is reported to be 1-10%.[12,15,16,17] Despite technical improvements and enhanced knowledge of the AEs associated with EST, bleeding remains one of its most problematic AEs.[18] The number of patients taking antithrombotic drugs is increasing as the elderly population increases and most patients cannot stop these drugs safely due to the high risk of cardiovascular or thromboembolic events.[19,20] Patients with any underlying coagulopathy have an increased risk of procedural or postprocedural bleeding.[15,21,22,23] EPBD has been reported to reduce the risk of bleeding compared to EST.[10,24,25] ESBD is likely to reduce the risk of bleeding as well as EPBD because of the minimal incision required. In the present study, the frequency of acute bleeding was significantly lower in the ESBD group than in the EST group, although the number of patients taking antithrombotic drugs was significantly higher in the ESBD group (EST group 14.0% vs.ESBD group 29.6%, P < 0.001).
Obstruction of the outflow of the pancreatic juice by intramucosal bleeding and/or local edema after EPBD is assumed to be a main cause of PEP.[26] Additionally, an insufficiently enlarged orifice of the bile duct hampers insertion of endoscopic devices and subsequent stone removal, and this stress on the pancreatic orifice results in PEP. EST drains pancreatic juice by incising the common channel and separating the pancreatic duct orifice and the bile duct orifice. Notably, EPBD has an estimated 5-7% risk of PEP, which is higher than that of EST.[4,10,27] In the present study, PEP occurred in three patients (2.6%) in the EST group and in six patients (1.9%) in the ESBD group. The incidence of PEP was similarly low in both groups. Our results showed that the additional papillary dilation did not increase the risk of PEP. Separation of the pancreatic and biliary ducts was achieved using even a minimal incision of the papilla, which reduced the incidence of PEP.
There were several limitations to this study. The major limitation was the retrospective collection and analysis of data. The other limitations were the relatively small number of patients in the EST group, and the different follow-up periods between the groups. The latter was because EST alone was performed mainly before 2012 and ESBD mainly after 2012. Moreover, accurate assessment of stone recurrence is difficult because patients without biliary symptoms do not come to hospital if the stone has recurred. We also acknowledge that some patients with complications would not return to our hospital and might have been missed. A further prospective study is needed to assess these limitations. We did not calculate or compare the cost of the procedures. Both additional balloon dilation and treatment for AEs may increase.
In conclusion, ESBD was more efficient and safer in the management of CBD stones than EST. ESBD showed lesser ERCP sessions, shorter procedure time and lower rate of using ML than EST group. Concern with adverse event, bleeding, and PEP were less in ESBD group. A prospective randomized controlled study comparing ESBD with EST is needed to establish this combination technique.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The present study did not receive any financial supports.
REFERENCES
- 1.Fujisawa T, Kagawa K, Hisatomi K, Kubota K, Nakajima A, Matsuhashi N. Is endoscopic papillary balloon dilatation really a risk factor for post-ERCP pancreatitis? World J Gastroenterol. 2016;22:5909–16. doi: 10.3748/wjg.v22.i26.5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Disario JA, Freeman ML, Bjorkman DJ, Macmathuna P, Petersen BT, Jaffe PE, et al. Endoscopic balloon dilation compared with sphincterotomy for extraction of bile duct stones. Gastroenterology. 2004;127:1291–9. doi: 10.1053/j.gastro.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Tsujino T, Kawabe T, Komatsu Y, Yoshida H, Isayama H, Sasaki T, et al. Endoscopic papillary balloon dilation for bile duct stone: Immediate and long-term outcomes in 1000 patients. Clin Gastroenterol Hepatol. 2007;5:130–7. doi: 10.1016/j.cgh.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Fujita N, Maguchi H, Komatsu Y, Yasuda I, Hasebe O, Igarashi Y, et al. Endoscopic sphincterotomy and endoscopic papillary balloon dilatation for bile duct stones: A prospective randomized controlled multicenter trial. Gastrointest Endosc. 2003;57:151–5. doi: 10.1067/mge.2003.56. [DOI] [PubMed] [Google Scholar]
- 5.Lin CK, Lai KH, Chan HH, Tsai WL, Wang EM, Wei MC, et al. Endoscopic balloon dilatation is a safe method in the management of common bile duct stones. Dig Liver Dis. 2004;36:68–72. doi: 10.1016/j.dld.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Attasaranya S, Cheon YK, Vittal H, Howell DA, Wakelin DE, Cunningham JT, et al. Large-diameter biliary orifice balloon dilation to aid in endoscopic bile duct stone removal: A multicenter series. Gastrointest Endosc. 2008;67:1046–52. doi: 10.1016/j.gie.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 7.Heo JH, Kang DH, Jung HJ, Kwon DS, An JK, Kim BS, et al. Endoscopic sphincterotomy plus large-balloon dilation versus endoscopic sphincterotomy for removal of bile-duct stones. Gastrointest Endosc. 2007;66:720–6. doi: 10.1016/j.gie.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 8.Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–54. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 9.Boender J, Nix GA, de Ridder MA, van Blankenstein M, Schutte HE, Dees J, et al. Endoscopic papillotomy for common bile duct stones: Factors influencing the complication rate. Endoscopy. 1994;26:209–16. doi: 10.1055/s-2007-1008945. [DOI] [PubMed] [Google Scholar]
- 10.Bergman JJ, Rauws EA, Fockens P, van Berkel AM, Bossuyt PM, Tijssen JG, et al. Randomised trial of endoscopic balloon dilation versus endoscopic sphincterotomy for removal of bileduct stones. Lancet. 1997;349:1124–9. doi: 10.1016/S0140-6736(96)11026-6. [DOI] [PubMed] [Google Scholar]
- 11.Huibregtse K. Complications of endoscopic sphincterotomy and their prevention. N Engl J Med. 1996;335:961–3. doi: 10.1056/NEJM199609263351309. [DOI] [PubMed] [Google Scholar]
- 12.Loperfido S, Angelini G, Benedetti G, Chilovi F, Costan F, De Berardinis F, et al. Major early complications from diagnostic and therapeutic ERCP: A prospective multicenter study. Gastrointest Endosc. 1998;48:1–10. doi: 10.1016/s0016-5107(98)70121-x. [DOI] [PubMed] [Google Scholar]
- 13.Freeman ML. Complications of endoscopic biliary sphincterotomy: A review. Endoscopy. 1997;29:288–97. doi: 10.1055/s-2007-1004193. [DOI] [PubMed] [Google Scholar]
- 14.Ding J, Li F, Zhu HY, Zhang XW. Endoscopic treatment of difficult extrahepatic bile duct stones, EPBD or EST: An anatomic view. World J Gastrointest Endosc. 2015;7:274–7. doi: 10.4253/wjge.v7.i3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balmadrid B, Kozarek R. Prevention and management of adverse events of endoscopic retrograde cholangiopancreatography. Gastrointest Endosc Clin N Am. 2013;23:385–403. doi: 10.1016/j.giec.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Saeed M, Kadir S, Kaufman SL, Murray RR, Milligan F, Cotton PB. Bleeding following endoscopic sphincterotomy: Angiographic management by transcatheter embolization. Gastrointest Endosc. 1989;35:300–3. doi: 10.1016/s0016-5107(89)72796-6. [DOI] [PubMed] [Google Scholar]
- 17.Sherman S, Hawes RH, Nisi R, Lehman GA. Endoscopic sphincterotomy-induced hemorrhage: Treatment with multipolar electrocoagulation. Gastrointest Endosc. 1992;38:123–6. doi: 10.1016/s0016-5107(92)70375-7. [DOI] [PubMed] [Google Scholar]
- 18.Kim KO, Kim TN, Kim SB, Lee JY. Characteristics of delayed hemorrhage after endoscopic sphincterotomy. J Gastroenterol Hepatol. 2010;25:532–8. doi: 10.1111/j.1440-1746.2009.06123.x. [DOI] [PubMed] [Google Scholar]
- 19.Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126–30. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 20.Mauri L, Hsieh WH, Massaro JM, Ho KK, D'Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356:1020–9. doi: 10.1056/NEJMoa067731. [DOI] [PubMed] [Google Scholar]
- 21.Hamada T, Yasunaga H, Nakai Y, Isayama H, Matsui H, Horiguchi H, et al. Bleeding after endoscopic sphincterotomy or papillary balloon dilation among users of antithrombotic agents. Endoscopy. 2015;47:997–1004. doi: 10.1055/s-0034-1392408. [DOI] [PubMed] [Google Scholar]
- 22.Mok SR, Arif M, Diehl DL, Khara HS, Ho HC, Elfant AB. Safety and efficacy of minimal biliary sphincterotomy with papillary balloon dilation (m-EBS+EPBD) in patients using clopidogrel or anticoagulation. Endosc Int Open. 2017;5:E157–e64. doi: 10.1055/s-0042-120225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909–18. doi: 10.1056/NEJM199609263351301. [DOI] [PubMed] [Google Scholar]
- 24.Ochi Y, Mukawa K, Kiyosawa K, Akamatsu T. Comparing the treatment outcomes of endoscopic papillary dilation and endoscopic sphincterotomy for removal of bile duct stones. J Gastroenterol Hepatol. 1999;14:90–6. doi: 10.1046/j.1440-1746.1999.01798.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhao HC, He L, Zhou DC, Geng XP, Pan FM. Meta-analysis comparison of endoscopic papillary balloon dilatation and endoscopic sphincteropapillotomy. World J Gastroenterol. 2013;19:3883–91. doi: 10.3748/wjg.v19.i24.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hakuta R, Hamada T, Nakai Y, Isayama H, Kogure H, Mizuno S, et al. Multicenter retrospective and comparative study of 5-minute versus 15-second endoscopic papillary balloon dilation for removal of bile duct stones. Endosc Int Open. 2017;5:E1027–34. doi: 10.1055/s-0043-118479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vlavianos P, Chopra K, Mandalia S, Anderson M, Thompson J, Westaby D. Endoscopic balloon dilatation versus endoscopic sphincterotomy for the removal of bile duct stones: A prospective randomised trial. Gut. 2003;52:1165–9. doi: 10.1136/gut.52.8.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]