Abstract
Background:
The role of Epstein–Barr virus (EBV) in inflammatory bowel disease (IBD) remains to be elucidated. The aim of this study was to investigate the presence of EBV in the blood and intestinal mucosa of patients with IBD and evaluate the association between EBV positivity and IBD.
Methods:
Patients with IBD, hospitalized between January 2015 and April 2018, were enrolled. The EBV-DNA load in blood samples from each subject was analyzed by quantitative real-time polymerase chain reaction. EBV-encoded small-RNA 1 (EBER-1) was detected by in-situ hybridization in intestinal mucosa tissue sections of patients with IBD.
Result:
EBV-DNA was detected in 48 out of 568 patients with IBD (8.4%), and EBER-1 positivity was detected in 27 of these patients (56.3%). Refractory IBD and severe mucosal inflammation were more common in patients with detectable levels of EBER-1 than in those without; the number of EBER-1-positive cells positively correlated with mucosal inflammation (P value < 0.05). Age (≥60 years old) and use of azathioprine were risk factors for EBV infection. There was no significant difference in clinical remission rate and surgical rate between the EBER-1 positive group and EBER-1 negative group, antiviral group and the non-antiviral group, among IBD patients who tested positive for EBV-DNA.
Conclusion:
Elderly patients with IBD, treated with azathioprine, are more susceptible to EBV positivity. Further, EBV mucosal detection correlated with the severity of mucosal damage and refractoriness, but not prognosis.
Keywords: Colonic mucosa, EBER-1, EBV-DNA, Epstein–Barr virus, inflammatory bowel disease
INTRODUCTION
Inflammatory bowel disease (IBD) is characterized by chronic inflammation in the intestinal mucosa and manifests with alternating periods of exacerbation and remission.[1] The disease has two main subsets: ulcerative colitis (UC) and Crohn's disease (CD). IBD is commonly treated with immunosuppressive and biological drugs; however, this treatment also increases the risk of tumors and opportunistic infections,[2,3] including infection with Epstein–Barr virus (EBV). EBV is a ubiquitous herpes virus, which infects more than 90% of individuals worldwide.[4,5] EBV mostly infects B lymphocytes and is capable of persisting throughout the lifetime of the host.[6] Furthermore, the virus can reactivate when the host is immunosuppressed, and EBV is associated with clinical conditions that range from asymptomatic infections to infectious mononucleosis, and even lymphoma.[6] Recent studies[5,7,8] indicate that patients with IBD on thiopurine therapy have a three- to five-fold increase in the risk of lymphoma, specifically EBV-positive lymphoma. However, to date, information regarding the role and risk factors of EBV in IBD exacerbation and therapeutic approaches has been inconclusive.[2,6,9,10] Most studies have focused on the prevalence of EBV seropositivity among patients with IBD[11,12] or EBV infection in the colonic mucosa.[4,6,9] Only a few studies have referred to both EBV-DNA in peripheral blood and EBV-positive cells in the colonic mucosa of patients with IBD. It is well known that EBV IgG screening is suggested prior to the initiation of immunomodulating treatment; anti-TNF can be considered in preference to thiopurines in EBV seronegative patients.[3] The choice of treatment program in patients with IBD and EBV-DNA in peripheral blood remains to be explored. It is not known if immunomodulator therapy is safe for these patients and if they need prophylactic antiviral treatment. All of these questions are worth exploring.
In addition, data on EBV prevalence in patients from China with IBD are limited. Therefore, the objectives of this study were to (1) investigate the prevalence of EBV in the blood and intestinal mucosa of Chinese patients with IBD, (2) explore the risk factors for EBV infection and the prognosis, and (3) investigate the relationship between EBV positivity and refractory IBD.
METHODS
Patients and methods
Consecutive in-patients with IBD in the West China Hospital of Sichuan University were enrolled from January 2015 to April 2018 in this prospective study. Patients were diagnosed with IBD according to established clinical, endoscopic, and histological features. Patients with IBD who also developed acquired immune deficiency syndrome, congenital immunodeficiency, or required organ transplantation, were excluded from this study. The local ethical committee of Sichuan University West China Hospital approved the protocol.
Blood sampling for EBV DNA detection and quantification was performed for each participant. Second and third samples were taken consecutively in EBV-DNA-positive patients 2 and 4 weeks after the initial sampling. All participants underwent a lower endoscopy procedure and mucosal damage was assessed by endoscopists. A representative tissue block with severe lesions was chosen for each patient, and the edge of the ulcer was used for the biopsy from the inflamed areas for highly sensitive EBV-encoded small-RNA 1 (EBER-1) in-situ hybridization in patients positive for EBV DNA in the blood. Patients with detectable blood levels of EBV-DNA were further selected from the total patients with IBD. Clinical and analytical data obtained from EBV-DNA-positive patients included sex, current age, the type of IBD (UC or CD), clinical features, and therapeutic regimens (prior to and postadmission, including 5-aminosalicylate (5-ASA), glucocorticoids, azathioprine (AZA)/6-mercaptopurine (6-MP), infliximab (IFX), or combined treatment with IFX and AZA). Patients with EBV-DNA-positive received antiviral therapy and recorded observations were made, such as the change in viral status. These patients were followed up annually for three years, and the prognosis data were collected.
In all cases, disease activity was assessed using the Mayo Clinic score for UC[13] and CD activity index (CDAI) for CD.[14] Disease activity was classified as follows: remission (Mayo <3, CDAI <150), and mild (Mayo 3–5, CDAI 150–220), moderate (Mayo 6–10, CDAI 221–450), and severe (Mayo 11–12, CDAI >450). The criteria for the degree of intestinal mucositis were as follows[15,16]: mild activity: cryptitis does not exceed 25% of the total crypt, rare crypt abscesses (every piece of biopsy tissue of no more than one); moderate activity: >25% of cryptitis, or multiple crypt abscess, or a few small mucosal erosion; severe activity: ulceration or multiple erosion.
The following criteria were applied to establish the refractoriness: (1) steroid dependence was identified in patients that could not be tapered off prednisone below 10 mg/day, despite maintaining remission; or after three months of steroid therapy, relapse occurred within 3 months after steroid cessation. (2) Steroid resistance was identified in patients that had active disease after 4 weeks of treatment with the equivalent of 0.75 mg/kg/day prednisone.[17](3) Resistance to AZA/6-MP was identified as lack of clinical improvement after patient dosage adjustment.[9,18](4) Nonresponse to IFX was defined by a lack of clinical improvement with induction therapy or recurrence of symptoms or disease activity during maintenance therapy despite an appropriate interval adjustment.[9,18](5) Refractoriness to combination therapies was defined as the persistence of active disease despite a treatment duration of at least 4 weeks.[9,18]
Based on the presence or absence of EBV DNA in the blood, patients were divided into the EBV-DNA-positive or EBV-DNA-negative groups. EBV-DNA-positive cases were scored quantitatively by the DNA peak values in blood and further divided into two categories: low EBV-DNA load (<102.5copies/mL) and high EBV-DNA load (opi2.5copies/mL). Patients were classified by the EBER-1 level detected in the intestinal mucosa: the EBER-1-positive or EBER-1-negative group. EBER-positive cases were scored quantitatively in the area of the highest EBV concentration per high-power field (HPF, 0.2 mm2, 400 × magnification) and divided into two categories: low EBV concentration (<10 EBER-positive cells per HPF) and high EBV concentration (≥10 EBER-positive cells per HPF).
EBV Viral Load in Blood by qRT-PCR
Three milliliter of venous blood was extracted from patients, and the citrate anticoagulant blood was centrifuged at 4000 r/min for 10 min to separate the plasma. EBV nucleic acid was extracted by automatic nucleic acid extractor (Natch S, China), and virus DNA was detected and quantified by a commercial quantitative real-time PCR (well Real-Time PCR System, Roche L LightCycler 480, America), artus EBV RG PCR kit (Qiagen). Primers and probes for gene amplification were provided by Hunan Shengxiang Biotechnology Co., LTD. (product standard YZB/country 1636-2015). Ctvalue ≤39 was considered positive.
EBER in situ hybridization
The gold standard for identifying EBV in biopsies is in-situ hybridization for EBV-encoded RNA (EBER-ISH). All mucosal biopsies, from the most significant active inflamed areas with erosions/ulcers in colon or terminal ileum, were fixed by formalin immediately and embedded in paraffin blocks. Only 1 biopsy per patient was selected for molecular examination. ISH was done by EBER ISH Kit (ZSGB-BIO, Ltd., Beijing, China). Prepared slices and decarboxylation of xylene was done for 10 min; rehydrated with anhydrous alcohol for 5 min; and gastric enzyme digestion (1 mg/L) for 30 min at room temperature. Samples were incubated at 37°C overnight with hybridization solution containing the EBER-probe (oligonucleotide probe with digoxin marker) and washed with PBS; Signals were amplified with antibiotin antibodies. A tissue was considered EBER-positive if the nuclei of cells appeared brown.
Statistical analyses
The quantitative variables with normal distribution are presented as the mean ± standard deviation, those with skewed distribution as median, and categorical variables were described as absolute frequencies (n) and relative frequencies (%). For between-group comparisons, Pearson's Chi-square test/Fisher's exact test were used for categorical variables, according to the sample number. Correlation between discontinuous variables was assessed using Wilcoxon, as appropriate. Univariate regression and multivariate logistic regression analyses were performed to evaluate the risk factors for EBV infection in blood and in intestinal mucosa of IBD patients. The results were considered significant when P was <0.05. All analyses were conducted using the software SPSS, version 20.0 (SPSS, Inc., Chicago, Illinois, USA).
RESULTS
A total of 572 in-patients with IBD (315 males and 257 females) with an average age of 46.1 ± 4.24 years were recruited in this study. There were 258 patients with UC and 314 patients with CD. However, four patients were misdiagnosed with IBD and eventually rediagnosed with lymphoma during follow-up visits. The characteristics of the four misdiagnosed patients were recurrent high fever, positive EBV-DNA in the blood, and positive EBER in the colonic mucosa. Three of them had liver and spleen enlargement or lymphadenectasis. One case was confirmed by performing repeated mucosal biopsy by colonoscopy. The other three cases, including two cases of colonic perforation and one case of uncontrollable massive gastrointestinal hemorrhage, were confirmed by surgery and colonic mucosal pathology.
In all, 568 patients with IBD were enrolled in the study. EBV-DNA was detected in the blood of 8.4% (48 out of 568) of IBD patients; EBER-1 positivity was present in 56.3% (27 out of 48) of EBV-DNA-positive IBD patients. Characteristics of the enrolled patients are shown in Table 1. Compared with patients with IBD and negative for EBV-DNA, male sex, fever, liver and spleen enlargement or lymphadenectasis, and influenza-like symptoms were more common in IBD patients with positive EBV-DNA (P < 0.05). Clinical disease activities revealed that severe activity was more common in IBD patients with EBV-DNA positivity (68.3%), compared with EBV-DNA-negative IBD patients (53.8%, P = 0.047). However, no significant difference was found in the degree of intestinal mucositis between the two groups (P > 0.05). Severe inflammation of the intestinal mucosa was more common in the EBER-1-positive IBD patient group than in the EBER-1-negative group (P = 0.030). However, there was no statistically significant difference in age, gender, clinical feature, and clinical disease activities between the groups of IBD patients with or without detectable EBER-1.
Table 1.
Clinical characteristics of IBD patients: EBV-DNA positive and EBV-DNA negative in blood, EBER-1 positive and EBER-1 negative in intestinal mucosa
| EBV-DNA in blood |
EBER-1 intestinal mucosa |
|||||
|---|---|---|---|---|---|---|
| + (n=48) | − (n=520) | P | + (n=27) | − (n=21) | P | |
| Mean age (mean±SD) (years) | 35.11±15.83 | 47.56±17.91 | <0.001 | 48.89±17.67 | 44.90±17.81 | 0.500 |
| Sex (male/female), n | 37/11 | 275/245 | 0.001 | 19/8 | 18/3 | 0.185 |
| UC/CD, n | 28/20 | 230/290 | 0.060 | 19/8 | 9/12 | 0.055 |
| Fever, n (%) | 25 (52.1%) | 180 (24.6%) | 0.012 | 15 (55.6%) | 10 (47.6%) | 0.586 |
| Liver and spleen enlargement or lymphadenectasis, n (%) | 4 (8.3%) | 2 (0.4%) | <0.001 | 2 (7.4%) | 3 (14.3%) | 0.439 |
| Influenza-like symptoms, n (%) | 7 (14.6%) | 10 (1.9%) | <0.001 | 5 (18.5%) | 3 (14.3%) | 0.696 |
| Clinical disease activities*, n (%) | ||||||
| Mild | 0 (0.0%) | 0 (0.0%) | - | 0 (0.0%) | 0 (0.0%) | - |
| Moderate | 15 (31.2%) | 240 (46.2%) | 0.047 | 9 (33.3%) | 6 (28.6%) | 0.724 |
| Severe | 33 (68.6%) | 280 (53.8%) | 0.047 | 18 (66.7%) | 15 (71.4%) | 0.724 |
| The degree of intestinal mucositis, n (%) | ||||||
| Mild | 1 (2.1%) | 10 (1.9%) | 0.939 | 0 (0.0%) | 1 (4.8%) | 0.263 |
| Moderate | 18 (37.5%) | 235 (45.2%) | 0.305 | 7 (25.9%) | 11 (52.4%) | 0.060 |
| Severe | 29 (60.4%) | 275 (52.9%) | 0.317 | 20 (74.1%) | 9 (42.6%) | 0.030 |
IBD, Inflammatory bowel disease; UC, ulcerative colitis; CD, Crohn’s disease; EBV, Epstein-Barr virus; EBER-1, Epstein-Barr virus-encoded small-RNA 1. *Clinical disease activities: the Mayo Clinic score for ulcerative colitis (UC), Crohn’s disease activity index for Crohn’s disease (CD)
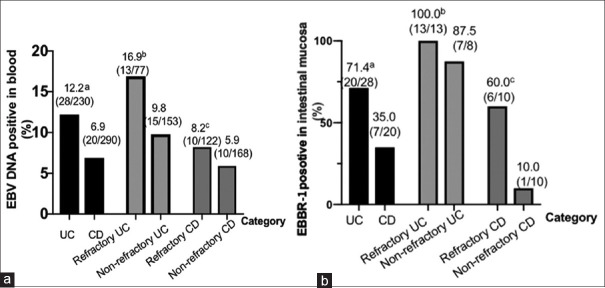
In this study, refractoriness was observed in 47.9% (23 out of 48) of patients with detectable EBV-DNA in their blood, compared to 33.8% (176 out of 520) of patients with no detectable EBV-DNA (P = 0.051). Further, refractoriness was more common in 70.4% (19 out of 27) of the IBD patients with EBER-1 detected in their intestinal mucosa compared to 33.3% (7 out of 21) of the IBD patients with no EBER-1 detected in the mucosa (P = 0.011). As shown in Figure 1a, EBV-DNA was detected more frequently in the blood of patients with UC (12.2%) than CD (6.2%), but no significant difference was found between refractory UC (or CD) and non-refractory UC (or CD). As shown in Figure 1b, EBER-1 was detected more frequently in the intestinal mucosa of UC patients compared with that of CD patients (P = 0.012). Similarly, EBER-1 was detected more frequently in refractory CD patients than in non-refractory CD patients (P = 0.019), but no statistical difference was found between patients with refractory UC and non-refractory UC (P = 0.083).
Figure 1.
(a) Percentage of patients with EBV-DNA positivity in blood in different groups aP, 0.039, UC versus CD; bP,0.121, refractory UC versus nonrefractory UC; cP, 0.458, refractory CD versus nonrefractory CD Percentage of patients with EBER-1 positive in mucosal in different groups. aP, 0.012, UC versus CD;bP, 0.191, refractory UC versus nonrefractory UC; cP, 0.019, refractory CD versus nonrefractory CD
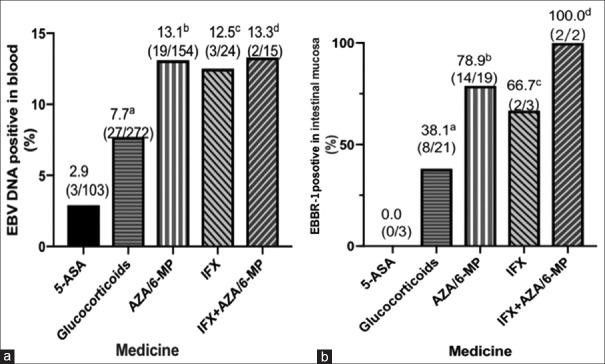
The pre-admission treatment of patients with IBD are shown in Figure 2. The prevalence of EBV-DNA in blood was higher in patients under therapy with AZA/6-MP (13.3%), IFX (12.5%), or AZA/6-MP + IFX (13.3%) than in patients under 5-ASA (2.9%) [Figure 2a]. The prevalence of EBER-1 in mucosa was also higher in patients treated with AZA/6-MP (78.9%) or AZA/6-MP + IFX (100.0%) than in those treated with 5-ASA [Figure 2b]. Patients under therapy with 5-ASA did not have any detectable EBER-1 in their intestinal mucosal tissues.
Figure 2.
(a) Percentage of patients with EBV DNA in blood in different groups. aP, 0.09, glucocorticoids versus ASA; bP, 0.008, AZA/6-MP versus ASA; cP, 0.045, IFX versus ASA; dP, 0.048, AZA/6-MP + IFX versus ASA (b) Percentage of patients with EBER-1 positivity in mucosal in different groups. aP, 0.151, glucocorticoids versus ASA; bP, 0.014, AZA/6-MP versus AZA; cP, 0.083, IFX versus ASA. dP, 0.025 AZA/6-MP + IFX versus ASA
Multivariate logistic regression analysis identified that age (≥60 years) (OR = 4.535, 95% CI 2.356–8.729) and the use of AZA/6-MP (OR = 2.020, 95% CI 1.030–3.962) were statistically significant risk factors for EBV-DNA positivity in the blood. In addition, ages ≥ 60 years (OR=4.603, 95% CI 1.005-21.093), use of AZA/6-MP (OR=7.668, 95% CI 1.515-38.826) and patients with UC (OR=6.616, 95% CI 410-31.0240) were independent risk factors for EBER positivity in mucosal tissues [Table 2].
Table 2.
Univariate analysis and multivariate analysis of risk factors for EBV-DNA positive, EBER-1 positive in IBD patients
| EBV-DNA positive in blood |
EBER-1 positive in mucosa |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis |
Multivariate analysis |
Univariate analysis |
Multivariate analysis |
|||||||||
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Age ≥60 years | 4.424 | 2.354-8.312 | <0.01 | 4.535 | 2.356-8.729 | <0.01 | 4.000 | 1.066-15.012 | 0.40 | 4.603 | 1.005-21.093 | 0.049 |
| Male gender | 1.445 | 0.783-2.669 | 0.239 | - | - | - | 0.441 | 0.101-1.930 | 0.277 | - | - | |
| UC | 1.689 | 0.934-3.052 | 0.083 | - | - | - | 3.750 | 1.114-12.640 | 0.033 | 6.616 | 1.410-31.040 | 0.017 |
| Clinical disease activities*:severe | 1.935 | 1.020-3.638 | 0.041 | 1.468 | 0.732-2.940 | 0.280 | 0.857 | 0.246-9.963 | 0.875 | - | - | - |
| The degree of intestinal mucositis:severe | 1.201 | 0.665-2.171 | 0.370 | - | - | - | 3.167 | 0.958-10.467 | 0.059 | - | - | - |
| 5-ASA | 0.918 | 0.460-2.092 | 0.904 | - | - | - | 0.000 | 0.000 | 0.999 | - | - | - |
| GS | 0.840 | 0.463-1.524 | 0.625 | - | - | - | 0.455 | 0.141-1.467 | 0.187 | - | - | - |
| AZA | 2.052 | 1.119-3.764 | 0.020 | 2.020 | 1.030-3.962 | 0.041 | 4.615 | 1.228-17.341 | 0.024 | 7.668 | 1.515-38.826 | 0.014 |
| IFX | 1.584 | 0.445-5.516 | 0.439 | - | - | - | 1.462 | 0.123-17.318 | 0.764 | - | - | - |
| IFX + AZA | 1.696 | 0.371-7.745 | 0.515 | - | - | - | 1.243E+9 | 0.000 | 0.999 | - | - | - |
EBV, Epstein-Barr virus; EBER-1, Epstein-Barr virus-encoded small-RNA 1; UC, ulcerative colitis; 5-ASA, 5-aminosalicylates; CS, corticosteroids; AZA, azathioprine; IFX, infliximab; IFX+AZA, infliximab in combination with azathioprine, all patients. *Clinical disease activities: the Mayo Clinic score for ulcerative colitis (UC), Crohn’s disease activity index for Crohn’s disease (CD)
Patients in both the EBV-DNA-positive and -negative groups had a clinical remission rate of more than 65% within 3 months [Table 3]. There was no significant difference in clinical remission rate and surgery rate between the two groups within 3 months (P > 0.05). During the follow-up period (38.07 ± 0.38 months), MALT lymphoma was observed in one patient (2.1%) with EBER-1 positivity in the mucosa in the EBV-DNA-positive group, while MALT lymphoma was also observed in one patient (0.2%) in the EBV-DNA-negative group (P = 0.034). Although we found a positive correlation between EBER positivity and severe mucosal inflammation in patients with IBD [Table 1], there was no significant difference in clinical remission rate and surgery rate between patients that were EBER-1 positive and EBER-1 negative within 3 months (P > 0.05) [Table 3].
Table 3.
Prognosis of IBD patients: EBV-DNA positive and EBV-DNA negative in blood, EBER-1 positive and EBER-1 negative in mucosa
| EBV-DNA |
EBER |
|||||
|---|---|---|---|---|---|---|
| + (n=48) | − (n=520) | P | + (n=27) | − (n=21) | P | |
| Clinical remission within 3 months | ||||||
| UC n (n) (%) | 20 (28) (71.4%) | 175 (230) (76.1%) | 0.558 | 13 (19) (68.4%) | 7 (9) (77.8%) | 0.609 |
| CD n (n) (%) | 13 (20) (65.0%) | 220 (290) (75.9%) | 0.277 | 4 (8) (50.0%) | 7 (12) (58.3%) | 0.714 |
| Surgery within 3 months, n (%) | 9 (18.8%) | 93 (17.8%) | 0.881 | 6 (22.2%) | 3 (14.2%) | 0.485 |
| Lymphoma was observed during follow-up, n (%) | 1 (2.1%) | 1 (0.2%) | 0.034 | 1 (3.7%) | 0 (0.0%) | 0.373 |
| CAEBV was observed during follow-up, n (%) | 0 (%) | 0 (0.0%) | - | 0 (0%) | 0 (0.0%) | - |
IBD, Inflammatory bowel disease; UC, ulcerative colitis; CD, Crohn’s disease; EBV, Epstein-Barr virus; EBER-1, Epstein-Barr virus-encoded small-RNA 1; CAEBV: chronic activity Epstein-Barr virus
As shown in supplementary file Table 1., 35.5% (17 out of 48) of IBD patients positive for EBV-DNA in their blood received ganciclovir therapy, while 64.5% (31 out of 48) did not. Among the IBD patients who did not receive ganciclovir therapy, some were treated with AZA/6-MP (38.7%), IFX (6.5%), or IFX + AZA (6.5%), and their EBV-DNA level became negative after 4 weeks. No statistical differences were found in the conversion, operation, and clinical remission rates between the patients receiving antiviral therapy and those without antiviral therapy.
Supplementary Table 1.
Prognosis of IBD patients with EB-DNA positive in blood: patients with antiviral therapy and patients without antiviral therapy
| Antiviral therapy (n=17) | Non-antiviral therapy (n=31) | P | |
|---|---|---|---|
| Post - admission treatment, n (%) | |||
| GS | 10 (58.8%) | 16 (51.6%) | 0.632 |
| AZA/6-MP | 5 (29.4%) | 11 (35.4%) | 0.670 |
| IFX | 2 (11.8%) | 2 (6.5%) | 0.524 |
| IFX+AZA | 0 | 2 (6.5%) | 0.285 |
| EBV-DNA was negative in duplicates after 2 weeks | 14 (82.4%) | 26 (83.9%) | 0.893 |
| EBV-DNA was negative in duplicates after 4 weeks | 17 (100.0%) | 31 (100.0%) | - |
| Clinical remission within 3 months | |||
| UC n (n) (%) | 8 (12) (66.7%) | 12 (16) (75.0%) | 0.629 |
| CD n (n) (%) | 3 (5) (60.0%) | 10 (15) (66.7%) | 0.787 |
| Surgery within 3 months, n (%) | 4 (23.6%) | 5 (16.1%) | 0.530 |
| Lymphoma was observed during the follow-up, n (%) | 1 (5.9%) | 0 (0.0%) | 0.172 |
| CAEBV was observed during the follow-up, n (%) | 0 (0.0%) | 0 (0.0%) | - |
IBD, Inflammatory bowel disease; UC, ulcerative colitis; CD, Crohn’s disease; CAEBV: chronic activity Epstein-Barr virus; EBV, Epstein-Barr virus; EBER-1, Epstein-Barr virus-encoded small-RNA 1; 5-ASA, 5-aminosalicylates; CS, corticosteroids; AZA, azathioprine; IFX, infliximab; IFX + AZA, infliximab in combination with azathioprine, all patients
There were no statistical differences in age, sex, refractoriness, disease activity, and outcome between the low EBV-DNA load and high EBV-DNA load groups [Table 4]. However, refractory IBD and severe intestinal mucositic were detected more frequently in patients with high EBER-1 concentration compared to those with low EBER-1 concentration in the mucosa.
Table 4.
Characteristics and prognosis of IBD patients: EBV-DNA positive and EBV-DNA negative in blood, EBER-1 positive and EBER-1 negative in mucosa
| EB-DNA loading in blood |
EBER-1 concentration in mucosa |
|||||
|---|---|---|---|---|---|---|
| Low EB-DNA loading (n=31) | High EB-DNA loading (n=17) | P | Low EBER-1 concentration (n=16) | High EBER-1 concentration (n=11) | P | |
| Age, (mean±SD) (years) | 45.19±19.2 | 51.29±12.9 | 0.316 | 49.33±18.6 | 52.72±15.5 | 0.721 |
| Sex, male/female, n | 26/5 | 11/6 | 0.131 | 10/6 | 9/2 | 0.280 |
| UC/CD, n | 18/13 | 10/7 | 0.959 | 10/6 | 9/2 | 0.280 |
| Refractory, n (%) | 16 (19.4%) | 7 (41.2%) | 0.489 | 8 (50.0%) | 11 (100.0%) | 0.005 |
| Clinical disease activities*, n (%) | ||||||
| Mild | 0 (0.0%) | 0 (0.0%) | - | 0 | 0 | - |
| Moderate | 10 (32.3%) | 5 (29.4%) | 0.839 | 5 (31.3%) | 4 (36.4%) | 0.782 |
| Severe | 21 (67.7%) | 12 (70.6%) | 0.839 | 11 (68.8%) | 7 (63.7%) | 0.782 |
| The degree of intestinal mucositis, n (%) | ||||||
| Mild | 1 (3.2%) | 0 (0.0%) | 0.454 | 0 (0.0%) | 0 (0.0%) | - |
| Moderate | 12 (38.7%) | 6 (35.3%) | 0.815 | 9 (56.3%) | 1 (9.1%) | 0.013 |
| Severe | 18 (58.1%) | 11 (64.7%) | 0.653 | 7 (43.8%) | 10 (90.9%) | 0.013 |
| Clinical remission within 3 months, n (%) | 22 (80.0%) | 12 (70.6%) | 0.978 | 12 (75.0%) | 5 (45.5%) | 0.118 |
| Surgery within 3 months, n (%) | 5 (16.1%) | 3 (17.6%) | 0.893 | 3 (18.8%) | 3 (27.3%) | 0.601 |
| Lymphoma was observed during follow-up, n (%) | 0 (0.0%) | 1 (5.8%) | 0.172 | 0 (0.0%) | 1 (9.1%) | 0.219 |
IBD, inflammatory bowel disease; UC, ulcerative colitis; CD, Crohn’s disease; EBV, Epstein-Barr virus; EBER-1, Epstein-Barr virus-encoded small-RNA 1. *Clinical disease activities: the Mayo Clinic score for ulcerative colitis (UC) and Crohn’s disease activity index for Crohn’s disease (CD). OR, Odds ratio; CI, confidence interval.
In addition, we used the Wilcoxon test to explore the relationship between the number of EBER positive cells in the mucosa and EBV-DNA load in the blood. No statistical difference was found in EBV-DNA load between the low and high EBER concentration groups, as shown in Figure 3.
Figure 3.
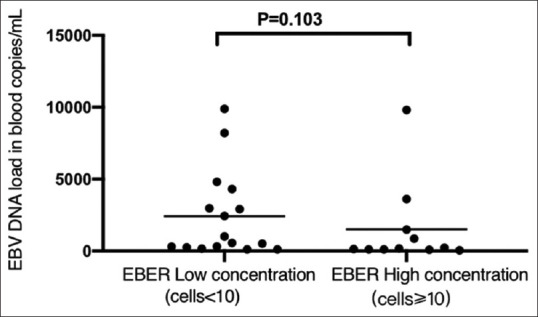
The comparison of EB-DNA load in blood between low EBER concentration group and high EBER concentration group in intestinal mucosa. Wilcoxon test reveals that the EBV load in blood of high EBER concentration group is not any higher than that of low EBER concentration group (P = 0.103)
DISCUSSION
In spite of the growing interest in EBV infections in patients with IBD, several criticalities are yet to be fully understood, such as the prevalence of refractoriness and surgical intervention as well as the risk factors involved. Few studies have explored EBV infection in patients with IBD in the Chinese population. Research concerning EBV infection in the blood of patients with IBD is inconclusive.[1,5,7,13,17,19] The results of this research show that 8.4% of patients with IBD have detectable levels of EBV-DNA in their blood, which is lower than the rate reported by other researchers (20–35%).[6,9,20] This difference may be due to the variations in the enrolled patient populations. Previously, the prevalence of EBV in the intestinal mucosa of patients with IBD was reported to range from 30% to 67%.[1,4,9,10,16,21] However, in our study, the focus was on IBD patients with EBV-DNA positivity in blood samples; the results showed that EBER-1 was present in 53.5% of EBV-DNA-positive patients, which is not higher than levels reported in previous studies.
IBD patients' refractoriness to conventional treatment may be related to the EBV load in the diseased mucosa.[4,9] In the study by Ciccocioppo et al.,[9] higher EBV-DNA loads were found in refractory IBD compared with that of responder IBD. Pezhouh et al.[4] found that EBER-1 positivity was seen in 60% of patients with refractory IBD, and EBER-1 positivity was associated with mucosal ulceration in patients with IBD. A similar finding was observed in our study; refractoriness was present in 70% of patients with EBER-1 positivity, which was higher than that of patients with EBER-1 negativity. EBER-1 positivity was more common in refractory CD patients than in non-refractory CD patients. Increased prevalence of this virus was observed in the colonic mucosa of patients with refractory IBD, indicating either a potential role of EBV in IBD refractoriness and severely inflamed areas of ulceration.[20] There was a positive correlation between EBER positivity and severe inflammation of the mucosa in patients with IBD in our study. This high percentage may be caused by both the increased number of infiltrating B lymphocytes due to inflammation and the increased EBV replication rate as a result of immunosuppression.[22] In this regard, viral agents can escape host immune surveillance directly at the mucosal level through their suggested viral tropism to sites of inflammation, and their effect on cytokine production.[23] Therefore, active EBV replication may cause and aggravate the inflammation of colon mucosa, which may be related to refractoriness.[4,19]
Our study found that severely inflamed areas of ulceration caused by EBV replication may be associated with the number of EBER-1 positive cells. Severe inflammation of the mucosa was more common in IBD patients with high EBER compared to those with low EBER concentration. Similarly, focal EBER-1 positivity (EBER ≥5/HPF) accounted for the highest proportion of refractory IBD patients in the study by Pezhouh et al.[4] In addition, a disconnection between the viral loads was found in the peripheral blood and the number of EBER-1-positive cells was revealed in our investigation. Thus, it is possible that viral colitis may exist independently from systemic involvement.[24]
Furthermore, our investigation revealed that advanced age and AZA/6-MP therapy were associated with EBV prevalence in the colonic mucosa of IBD patients. It is possible that the action of T cells in controlling viral replication is impaired by concurrent immunosuppressive agents, thus resulting in the uncontrolled activation of the lytic phase of the viral life cycle.[5,25] On the other hand, elderly patients often have suppressed immune responses related to aging; elderly patients have thinner intestinal mucosa with increased permeability,[6,26] which causes barrier dysfunction. Thus, EBV in the intestinal mucosa of elderly patients with IBD receiving AZA/6-MP treatment should be taken into consideration. In addition, our study suggested that UC was also found to be a risk factor for EBER positivity in IBD patients. Similarly, EBER-1 positivity in the intestinal mucosa was found to be more frequent among patients with UC than in patients with CD in a previously reported study.[21] Previous research suggested that UC with a predominance of Th2 lymphocytes may disturb the localized immunological niches and be conducive to the expansion of EBV-infected cells.[21,27]
Although EBER-1 positive cells in the diseased mucosa positively correlated with the severity of mucosal damage and refractoriness, no statistical difference was found in surgery rate between EBER-1-positive and -negative IBD patients. This is different from previous reports, which inferred that IBD patients with high EBV load in the mucosa were more likely to receive surgery.[5] It may be suggested that although EBV infection may aggravate local mucosal lesion, it does not indicate a worse prognosis. In this study, there were some IBD patients with EBV-DNA positivity, who were treated with AZA or IFX without ganciclovir, who showed EBV-DNA negativity during re-examinations. Replication of EBV is essentially a marker of more aggressive disease, but it does not seem to modify the outcome and antiviral therapy is thus less useful. Therefore, we suggest that EBV-DNA positivity in the blood may be a self-limiting infection in most patients.
Conversely, during the follow-up period, only one case of MALT lymphoma was observed in the group of IBD patients with EBV-DNA positive, while four cases of lymphoma were reported in the patients with EBV-DNA positivity and EBER-1 positive, which was concluded as misdiagnosed with IBD. These four patients had recurrent high fever and EBV-DNA positivity in the blood. Therefore, misdiagnosis of lymphoma should be taken into account with IBD, especially in patients with EBV-DNA positivity.
There were some limitations to this study. First, the study population was comprised wholly of in-patients, and hence there may be a selection bias. Second, since the EBER status in IBD patients who first tested positive for EBER in the mucosa could not regularly be reviewed, it is not clear when EBER became negative.
In conclusion, EBV replication may be linked to the aggravation of mucosal inflammation and refractoriness in patients with IBD, but it does not necessarily indicate poor prognosis. EBV-DNA positivity in the blood may be a self-limiting infection in most patients, and immunosuppression did not appear to aggravate the disease or induce activation of severe EBV infections in IBD patients with EBV-DNA positivity in the blood. In addition, the differentiation and diagnosis of IBD and lymphoma should be noted, especially in patients with recurrent fever, positive EBV-DNA, and positive EBER-1.
Financial support and sponsorship
The present work was supported by (1) The Science Foundation from the Science Technology Department of Sichuan Province, PR China (No. 2019YFS0262). (2) 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University. (3) Research Project from Health Commission of Sichuan Province for Cadres Health Care (No. 2018-102). (4) National Natural Science Foundation of China (No. 81270447).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Malik TA. Inflammatory bowel disease: Historical perspective, epidemiology, and risk factors. Surg Clin North Am. 2015;95:1105–22. doi: 10.1016/j.suc.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Li X, Chen N, You P, Peng T, Chen G, Wang J, et al. The status of Epstein-Barr virus infection in intestinal mucosa of Chinese patients with inflammatory bowel disease. Digestion. 2019;99:126–32. doi: 10.1159/000489996. [DOI] [PubMed] [Google Scholar]
- 3.Rahier JF, Magro F, Abreu C, C Conlon, P De Munter, G D'Haens, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8(6):443–468. doi: 10.1016/j.crohns.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Pezhouh MK, Miller JA, Sharma R, Borzik D, Eze O, Waters K, et al. Refractory inflammatory bowel disease: Is there a role for Epstein–Barr virus? A case controlled study using highly sensitive EBV encoded small RNA1 in situ hybridization. Hum Pathol. 2018;82:187–92. doi: 10.1016/j.humpath.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Nissen LHD, Nagtegaal ID, De Jong DJ, Kievit W, Derikx LA, Groenen PJ, et al. Epstein-Barr virus in inflammatory bowel disease: The spectrum of intestinal lymphoproliferative disorders. J Crohns Colitis. 2015;9:398–403. doi: 10.1093/ecco-jcc/jjv040. [DOI] [PubMed] [Google Scholar]
- 6.Magro F, Santos-Antunes J, Albuquerque A, Vilas-Boas F, Macedo GN, Nazareth N, et al. Epstein–Barr virus in inflammatory bowel disease — correlation with different therapeutic regimens. Inflamm Bowel Dis. 2013;19:1710–6. doi: 10.1097/MIB.0b013e318281f31c. [DOI] [PubMed] [Google Scholar]
- 7.Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005;54:1121–5. doi: 10.1136/gut.2004.049460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sokol H, Beaugerie L, Maynadie M, Laharie D, Dupas JL, Flourié B, et al. Excess primary intestinal lymphoproli- ferative disorders in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2063–71. doi: 10.1002/ibd.22889. [DOI] [PubMed] [Google Scholar]
- 9.Ciccocioppo R, Racca F, Paolucci S, Campanini G, Pozzi L, Betti E, et al. Human cytomegalovirus and Epstein-Barr virus infection in inflammatory bowel disease: Need for mucosal viral load measurement. World J Gastroenterol. 2015;21:1915–26. doi: 10.3748/wjg.v21.i6.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopes S, Andrade P, Conde S, Liberal R, Dias CC, Fernandes S, et al. Looking into enteric virome in patients with IBD: Defining guilty or innocence? Inflamm Bowel Dis. 2017;23:1278–84. doi: 10.1097/MIB.0000000000001167. [DOI] [PubMed] [Google Scholar]
- 11.de Francisco R, Castaño-García A, Martínez-González S, Pérez-Martínez I, González-Huerta AJ, Morais LR, et al. Impact of Epstein-Barr virus serological status on clinical outcomes in adult patients with inflammatory bowel disease. Aliment Pharm Ther. 2018;48:723–30. doi: 10.1111/apt.14933. [DOI] [PubMed] [Google Scholar]
- 12.Hradsky O, Copova I, Zarubova K, Durilova M, Nevoral J, Maminak M, et al. Seroprevalence of Epstein-Barr virus, cytomegalovirus, and polyomaviruses in children with inflammatory bowel disease. Digest Dis Sci. 2015;60:3399–407. doi: 10.1007/s10620-015-3764-z. [DOI] [PubMed] [Google Scholar]
- 13.D'Haens G, Sandborn WJ, Feagan BG, Geboes K, Hanauer SB, Irvine EJ, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763–86. doi: 10.1053/j.gastro.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 14.Best WR, Becktel JM, Singleton JW, Kern F., Jr Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70:439–44. [PubMed] [Google Scholar]
- 15.Marchal-Bressenot A, Salleron J, Boulagnon-Rombi C, Bastien C, Cahn V, Cadiot G, et al. Development and validation of the Nancy histological index for UC. Gut. 2017;66:43–9. doi: 10.1136/gutjnl-2015-310187. [DOI] [PubMed] [Google Scholar]
- 16.Feakins RM. British Society of Gastroenterology. Inflammatory bowel disease biopsies: Updated British Society of Gastroenterology reporting guidelines. J Clin Pathol. 2013;66:1005–26. doi: 10.1136/jclinpath-2013-201885. [DOI] [PubMed] [Google Scholar]
- 17.Dignass A, Eliakim R, Magro F, Maaser C, Chowers Y, Geboes K, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: Definition and diagnosis. J Crohn Colitis. 2012;6:965–90. doi: 10.1016/j.crohns.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Bradford K, Shih DQ. Optimizing 6-mercaptopurine and azathioprine therapy in the management of inflammatory bowel disease. World J Gastroenterol. 2011;17:4166–73. doi: 10.3748/wjg.v17.i37.4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dimitroulia E, Pitiriga VC, Piperaki ET, Spanakis NE, Tsakris A. Inflammatory bowel disease exacerbation associated with Epstein-Barr virus infection. Dis Colon Rectum. 2013;56:322–7. doi: 10.1097/DCR.0b013e31827cd02c. [DOI] [PubMed] [Google Scholar]
- 20.Juan A, Lobaton T, Tapia G, Mañosa M, Cabré E, Domènech E. Epstein-Barr virus-positive mucocutaneous ulcer in Crohn's disease. A condition to consider in immunosuppressed IBD patients. Dig Liver Dis. 2017;49:934–7. doi: 10.1016/j.dld.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Hosomi S, Watanabe K, Nishida Y, Yamagami H, Yukawa T, Otani K, et al. Combined infection of human herpes viruses: A risk factor for subsequent colectomy in ulcerative colitis. Inflamm Bowel Dis. 2018;24:1307–15. doi: 10.1093/ibd/izy005. [DOI] [PubMed] [Google Scholar]
- 22.Ryan JL, Shen YJ, Morgan DR, Thorne LB, Kenney SC, Dominguez RL, et al. Epstein-Barr virus infection is common in inflamed gastrointestinal mucosa. Dig Dis Sci. 2012;57:1887–98. doi: 10.1007/s10620-012-2116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loewendorf A, Benedict CA. Modulation of host innate and adaptive immunedefenses by cytomegalovirus: Timing is everything. J Intern Med. 2010;267:483–501. doi: 10.1111/j.1365-2796.2010.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciccocioppo R, Racca F, Scudeller L, Piralla A, Formagnana P, Pozzi L, et al. Differential cellular localization of Epstein–Barr virus and human cytomegalovirus in the colonic mucosa of patients with active or quiescent inflammatory bowel disease. Immunol Res. 2016;64:191–203. doi: 10.1007/s12026-015-8737-y. [DOI] [PubMed] [Google Scholar]
- 25.Vogl BA, Fagin U, Nerbas L, Schlenke P, Lamprecht P, Jabs WJ. Longitudinal analysis of frequency and reactivity of Epstein-Barr virus-specific T lymphocytes and their association with intermittent viral reactivation. J Med Virol. 2012;84:119–31. doi: 10.1002/jmv.22258. [DOI] [PubMed] [Google Scholar]
- 26.Tran L, Greenwood-Van Meerveld B. Age-associated remodeling of the intestinal epithelial barrier. J Cerontol Biol Sci Med Sci. 2013;68:1045–56. doi: 10.1093/gerona/glt106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spieker T, Herbst H. Distribution and phenotype of Epstein-Barr virus-infected cells in inflammatory bowel disease. Am J Pathol. 2000;157:51–7. doi: 10.1016/S0002-9440(10)64516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]