Abstract
The penicillin allergy label has been consistently linked with deleterious effects that span the health care spectrum, including suboptimal clinical outcomes, the emergence of bacterial resistance, and increased health care expenditures. These risks have recently motivated professional organizations and public health institutes to advocate for the implementation of penicillin allergy delabeling initiatives; however, the burden of delabeling millions of patients is too expansive for any one discipline to bear alone. This review presents the unique perspectives and roles of various stakeholder groups involved in penicillin allergy diagnosis, assessment, and delabeling; we emphasize opportunities, barriers, and promising areas of innovation. We summarize penicillin allergy methods and tools that have proven successful in delabeling efforts. A multidisciplinary approach to delabeling patients with reported penicillin allergy, bolstered by evidence-based clinical practices, is recommended to reduce the risks that associate with the penicillin allergy label.
Keywords: Hypersensitivity, Delabeling, Delabel, Beta-lactam, Cephalosporin, Antibiotic, Utilization
Penicillin allergy is confirmed in less than 10% of patients with a reported penicillin allergy in the United States.1 There are well-defined harms associated with an unverified penicillin allergy: ineffective treatment, overtreatment, and more broad-spectrum treatment that results in antibiotic resistance and adverse effects that include Clostridioides difficile infection (CDI, Figure 1).2–7 As penicillin allergy evaluation becomes more widely recommended,1,7–9 a new challenge has emerged related to meeting the demand for penicillin allergy assessments and delabeling.
FIGURE 1.
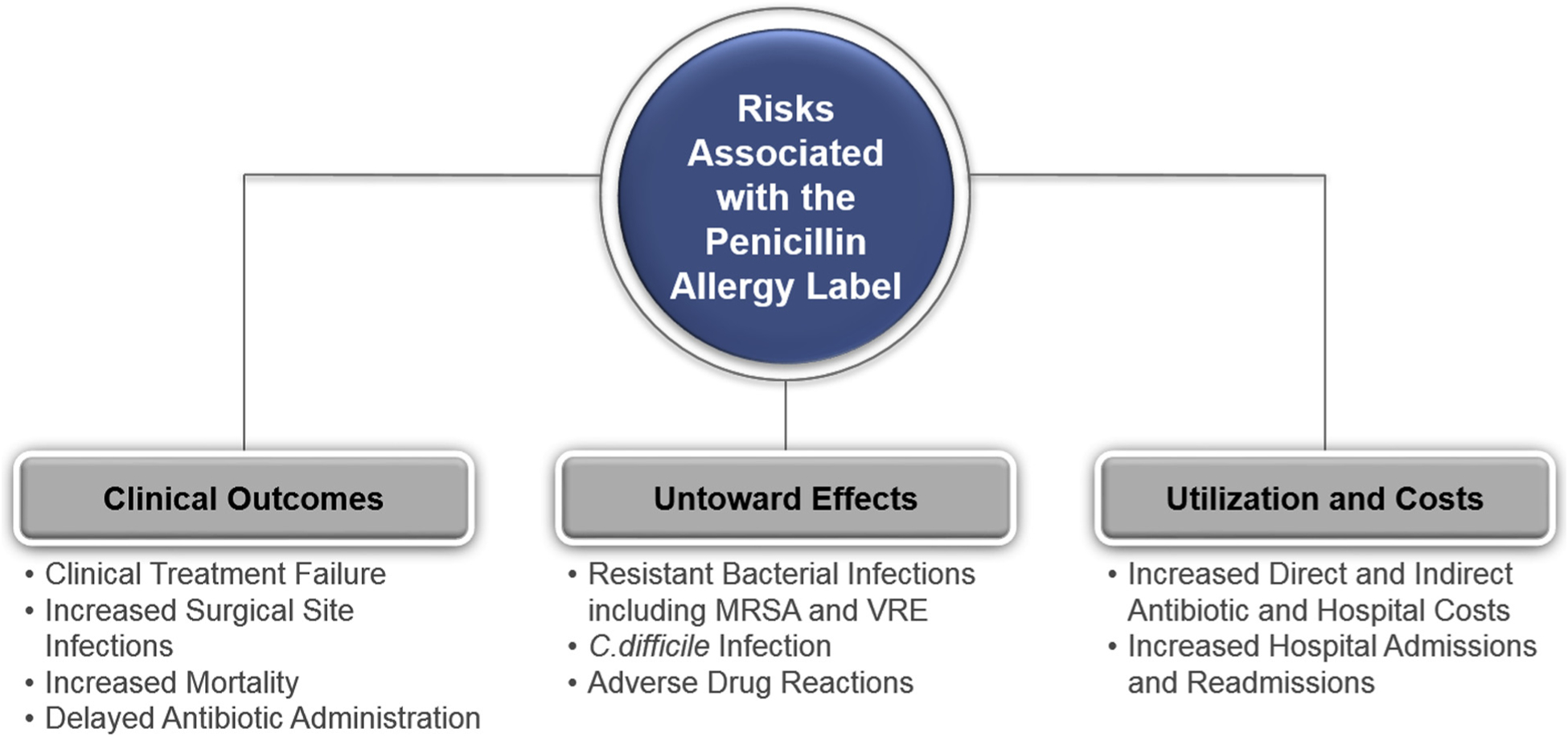
Summary of risks of the penicillin allergy label. The figure summarizes why penicillin allergy delabeling is important to the quality and safety of health care delivered. Recent studies have identified associations that showed that patients with a penicillin allergy label have inferior outcomes, untoward effects, and increased costs. C. difficile, Clostridioides difficile; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococcus.
Although penicillin allergy assessments are routine for allergy specialists to perform, it is not possible for allergists to evaluate all patients who carry a penicillin allergy label. Indeed, there are more than 30 million Americans with a penicillin allergy label and less than 5000 practicing allergists in the United States.10 The majority of acute care hospitals lack access to allergy specialists and penicillin allergy diagnostic testing.11 Extending penicillin allergy assessments to clinicians beyond allergy specialists requires those providers to gain additional knowledge. Systems must also design new workflows and infrastructure to accommodate penicillin allergy testing and delabeling. Despite these requirements, there are emerging examples of diverse clinicians addressing penicillin allergy and facilitating penicillin allergy delabeling across hospital locations and populations.12
Penicillin allergy assessment, to some degree, can occur in any location by any health care professional trained in taking an allergy history. The history includes core features that can assist in making a risk judgment about the reaction (Figure 2).1,13 It is possible for patients to be delabeled based on the history alone.14 For example, the penicillin “allergic” patient who recently took amoxicillin without incident can be delabeled and counseled about why there is no true penicillin allergy. Patients whose reaction to penicillin was clearly nonimmunologic, such as “headache,” “fatigue,” or “nausea,” can be delabeled without any further work up, provided that the patient understands and is agreeable. Rarely, a patient may describe a severe reaction to penicillin that does not qualify for a drug challenge and subsequent delabeling. Such reactions include hemolytic anemia, liver injury, renal failure, and severe cutaneous adverse reactions such as Stevens-Johnson syndrome and drug reaction with eosinophilia and systemic symptoms. However, the most commonly reported reactions to penicillins are itching, rash, and hives, as well as reactions that are unknown from early childhood.15,16 These reactions may be considered potentially IgE-mediated, and as such, performing skin testing and/or a drug challenge is needed to complete the penicillin allergy assessment (Table I).
FIGURE 2.
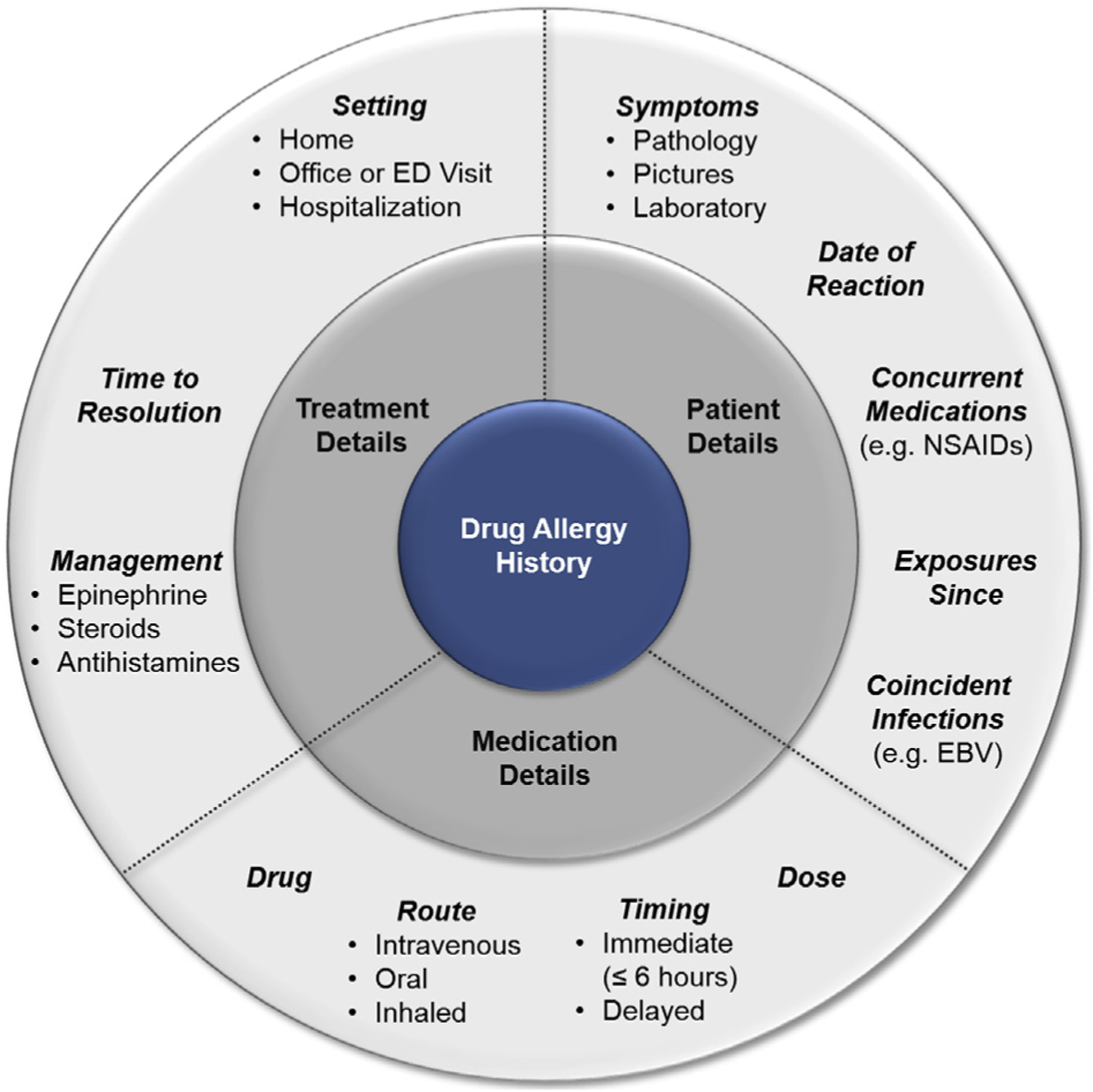
Core elements of the drug allergy history. The figure includes elements important to the drug allergy history, considering details related to the patient, medication, and treatment. In addition to considering the reaction history, it is also important to consider the general and current health of the patient. Specific patient factors such as pregnancy or having an oxygen requirement because of poor lung function may make a patient “high risk” for penicillin allergy delabeling even when the reaction history is “low risk.” EBV, Epstein-Barr virus; ED, emergency department; NSAID, nonsteroidal anti-inflammatory drug.
TABLE I.
Penicillin allergy procedures for patients with suspected immediate (IgE-mediated) allergies: penicillin skin testing, drug challenges, and penicillin induction of tolerance (desensitization)
| Penicillin skin testing | Drug challenge | Penicillin induction of tolerance (desensitization) | |
|---|---|---|---|
| Definition | Diagnostic reagent(s) used to detect the presence of allergen-specific IgE in the skin | Controlled introduction of a penicillin drug (usually amoxicillin) in patients with a low likelihood of an allergic reaction | Incrementally increasing doses of a drug to temporarily induce immune tolerance |
| Clinical indication |
|
|
|
| Process | Skin prick testing followed by intradermal testing, if negative. Confirm negative skin test results with an oral amoxicillin challenge | Administration of 1 full dose in 1–2 divided doses given 30–60 min apart | Administration of approximately 10 incrementally increasing doses given 15–30 min apart |
| Effect on immune system | Not intended to induce drug tolerance or alter the immune response | Not intended to induce drug tolerance or alter the immune response | Renders temporary state of tolerance that requires uninterrupted dosing after completion |
| High-acuity bed requirement | No | No | Yes |
| Interpretation of results | Positive predictive value: 40%–100% Negative predictive value: 97%–100% | Tolerance indicates that the patient is not allergic | Tolerance is temporary and maintained only as long as the patient continues to take the drug for a specific dose or course |
| Penicillin allergy label | Delabel patients only with negative skin test results who tolerate the drug challenge | Delabel patients who tolerate 1 full dose of a penicillin antibiotic | Patients are still considered allergic despite tolerability. Allergy label remains. If relevant, consider penicillin skin testing in the future when clinically appropriate |
MULTIDISCIPLINARY ENGAGEMENT
Allergy and immunology
Perspective.
After reports of penicillin anaphylaxis followed the rapid expansion of penicillin’s use during the World War II, allergy specialists responded by developing skin-testing techniques to evaluate for penicillin allergy.17,18 Since that time, allergists have been intimately involved in drug allergy research and patient care, especially because allergy is not routinely taught to nonallergist medical professionals.
The development of penicilloyl-polylysine (PPL, Pre-Pen) antigenic determinant in the 1970s and evolution of skin testing to include native penicillin G and minor determinant mixture (MDM) provided allergists with important diagnostic tools for penicillin allergy evaluation. However, penicillin skin-testing results must be interpreted in the context of pretest probability, given that the test is more often used for its high negative predictive value (>95%) rather than its positive predictive value, which varies from 40% to 100%.1,17,19 The field continued to develop expanded skin-testing reagents and techniques as well as explore pathways for lower risk patient assessments that use a direct challenge (ie, a drug challenge without preceding penicillin skin testing), particularly for pediatric patients.17,20–24
Practice and logistics.
Allergists are well suited to evaluate drug-induced and other hypersensitivity reactions as they have the greatest familiarity with allergy diagnostic testing (eg, skin testing and drug challenge performance) and interpretation. The skin-testing techniques and challenge procedures used for penicillin allergy assessment and delabeling are used to assess other hypersensitivities (eg, to food) evaluated by allergy specialists. Furthermore, Allergy and Immunology is the only field where skin testing is a core competency with defined practice standards.1 Allergists also have the largest experience with anaphylaxis management and in recognizing unusual manifestations of true drug allergy, both of which are Allergy and Immunology core competencies.1,25 Allergy training, therefore, lends a unique perspective to the practice of penicillin allergy evaluation. Although allergy practices are predominantly ambulatory, in the last decade, allergists have assumed vital leadership roles in some antibiotic stewardship programs (ASPs) that include inpatient penicillin allergy evaluations.12,23,26,27
Allergists can easily perform penicillin allergy assessments as part of their practice. First, allergists are experienced in mixing, diluting, and testing with different drug reagents. Allergists stock PPL and other drugs for skin testing and drug challenges; some allergist have experiencing mixing MDM. Allergists routinely order the tools needed for skin testing and drug challenges. Allergists also stock all emergency medications needed to treat anaphylaxis, and some allergy offices treat anaphylaxis on a weekly basis, given the robust practice of subcutaneous allergen immunotherapy for allergic rhinitis and asthma (“allergy shots”).
Barriers.
The largest barrier restricting more delabeling by allergy specialists relates to the population and practice of Allergy and Immunology in the United States. First, there are less than 5000 practicing US allergists.10 Second, Allergy and Immunology is a predominantly community-based practice (eg, just 1126 members of the American Academy of Allergy, Asthma, and Immunology reported being “medical school faculty” [Brandt R. Personal communication, 2019]). Given the diversity of conditions managed by Allergy and Immunology and limited drug allergy exposure in some training programs, not all graduating allergists have comfort assessing drug allergies.28 Graduating trainees may also not be interested in drug allergy due to the low pretest probability for finding true immunologic disease. Allergists also realize that inpatient skin testing is time consuming and challenging to complete due to competing scheduled patient tests, a patient’s clinical status (eg, new oxygen requirement), or even health care system pressure for short lengths of stay and swift discharge planning.29–32 Finally, the lack of commercially available comprehensive penicillin skin-testing reagents in the United States poses a barrier to delabeling. Even outside the United States, there are notable variations in penicillin testing reagent access.
Opportunities and innovation.
Penicillin allergy delabeling is an emerging multidisciplinary objective without clearly defined ownership, roles, or responsibilities.27 With their unique skills and training, allergists can substantially enhance penicillin allergy delabeling efforts through synergistic relationships with other clinicians, pharmacists, and ASPs.33,34 Allergists can help provide the evidence base for risk-stratified drug allergy management programs across the patient care continuum.18,24,32,35–37 Allergists can also assume a leadership role by designing or managing inpatient implementation of penicillin allergy delabeling programs as part of antimicrobial stewardship.18,23,32,35,36
Allergists are ideally positioned to innovate, using best practices,7 implementing telehealth,38,39 and assessing antibiotic allergies beyond penicillins.40 Ultimately, we envision the potential for hospitals to hire inpatient allergists who focus on drug allergy documentation, assessment, and management, with ASP membership.
Allergists can also lead in training other professionals. A wide variety of providers, including medical doctors from different disciplines, pharmacists, and advanced practice practitioners, could learn to perform penicillin skin testing and drug challenges. Allergists might also lead anaphylaxis trainings or simulations, helping colleagues and trainees become comfortable with evidence-based anaphylaxis diagnosis and management.41–43
Penicillin allergy delabeling presents an opportunity for sustainable revenue and future growth for allergy specialists in clinical practice. Through relationship building with ASPs, allergists would gain a consistent referral source for the patients with higher risk penicillin allergies and other drug allergies beyond penicillin (eg, sulfonamide antibiotics).27,40 Rising familiarity with direct challenges in low-risk patients by nonallergists18,32,35,36,44 may result in another referral source, patients with positive or indeterminate findings. Using penicillin allergy delabeling infrastructure as a gateway into multidisciplinary collaborative drug allergy practice and innovation provides a meaningful way in which allergists can impact the practice of medicine, patient care, and public health while bolstering their networks and patient referrals.
Infectious diseases, antibiotic stewardship programs, and infection prevention and control
Perspective.
Infectious diseases (ID) specialists have a unique role to play in penicillin allergy assessment and delabeling. ID specialists are experts in the selection of the most effective antibiotics for both prophylaxis and treatment of infections, and because of this are both consulted for specialty care and influence antibiotic prescribing policies and practices within their institutions.
Practice and logistics.
Given the wide range of infections for which penicillins and other beta-lactam antibiotics are considered first-line choices,7 ID specialists are often in a position of recommending these agents, as well as allergy evaluations to optimize antimicrobial selection. Consultation by ID physicians has been shown to improve adherence to guidelines and patient outcomes with serious infections.45 ID specialists can also obtain accurate and complete allergy histories and, with adequate resources, perform penicillin allergy evaluation with penicillin skin testing12,46 and/or direct challenges.35,36
However, the role of ID specialists extends beyond individual patients to practices, hospitals, and health systems. ID specialists are leaders in infection prevention and control (IPC) and ASPs, and as such they can proactively advocate for routine penicillin allergy assessments as an integral part of patient care and collaborate to implement programs.47 ID specialists have been integral collaborators in penicillin allergy evaluation programs in both the inpatient and outpatient settings.46
Barriers.
Despite the high value placed on penicillin allergy evaluation to improve antibiotic selection, appropriateness, and safety among ID specialists, less than half of ID specialists report having access to drug challenges and penicillin skin testing.33 ID fellowship programs have limited pharmacology and allergy training. Penicillin allergy assessments are notably not a part of routine ID training. However, few ASP/IPC fellowships may include a rotation with allergy specialists to learn drug allergy assessment procedures. The funding for hospital and health care systems to perform ASP/IPC is not large and must be justified annually; carving out designated funding for penicillin allergy programs may be infeasible.
Opportunities and innovation.
ID specialists must continue to engage, lead, and support multidisciplinary efforts to increase awareness of the importance of penicillin allergy evaluation for improving antibiotic choice by reducing unnecessary antibiotic use and establishing penicillin allergy delabeling as a routine component of ASP and infection prevention.
ID specialists are key stakeholders and leaders in the design of institutional formularies, including the use of restrictive formularies; the focus of restrictions is often on broad-spectrum therapies, many of which are employed in the setting of perceived beta-lactam allergy. Multiple studies have shown that the tools of ASPs, including preauthorization review and postprescription audit and feedback, both of which can incorporate consideration of antibiotic allergy, improve antimicrobial use.48 The importance of penicillin allergy evaluation is highlighted in the Centers for Disease Control and Prevention’s Core Elements of Hospital Stewardship Programs,49 and evaluation of antibiotic allergy is included in recommendations for training in ASP for leaders.50 Formal inclusion of both educational interventions and protocols for penicillin skin testing and/or drug challenges as part of ASPs has demonstrated reductions in broad-spectrum antimicrobial use.51 To date, ID specialists have designed and implemented large inpatient penicillin allergy evaluation programs12,34,52 within ASPs and demonstrated that ID trainees can implement inpatient penicillin skin testing.46
ASPs and IPC have shared a common interest in reducing the risk of antimicrobial resistance; however, IPC focuses on prevention of acquisition and transmission of resistant organisms as well as CDI, and reduction in surgical site infection (SSI), all of which are impacted by antimicrobial choice. Use of beta-lactam alternative agents has been associated with increased risk of SSI, whereas beta-lactam allergies have been associated with increased risk of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and CDI.2,5 In addition, an increase in risk of SSI has been associated with beta-lactam allergy, attributable to the use of beta-lactam alternatives.53–55 As such, there are key penicillin allergy delabeling opportunities for IPC, in addition to those led by ASPs.
Emergency medicine and the emergency department Perspective.
Antibiotics are the most commonly prescribed medications in the emergency department (ED)56; of all antibiotics prescribed in the ED from 2009 to 2014, 44% were broad-spectrum antibiotics.57 An estimated 14.5 million visits could have their antibiotic treatment affected by a reported penicillin allergy.56 In a recent study of pediatric ED providers, it was reported that in the presence of a penicillin allergy label, cefdinir (a broad-spectrum third-generation cephalosporin) would be the most likely alternative prescription provided.58 Delay in antimicrobial administration may also occur for patients with a reported penicillin allergy.59,60
Practice and logistics.
The ED is a complex environment in which a large volume of patients are treated for illnesses and injuries ranging from nonurgent to life threatening. There are approximately 145.6 million ED visits per year in the United States.56 The ED may provide a unique opportunity to identify and delabel patients with reported penicillin allergy given the high volume of patients with infections in a high-resource environment. The mechanisms for delabeling could be in real time while patients are waiting in the ED (eg, before discharge or transition to another unit).61,62 Given that the ED is equipped to diagnose and manage potential allergic reactions that could result from widespread penicillin allergy testing, its resources are ideal for penicillin allergy delabeling efforts. If delabeling were not feasible or supported in the ED setting, the ED could serve as the identification point for specialist referral for penicillin allergy diagnostic testing and alternative strategies, such as observed drug challenges to the indicated beta-lactam,63 that can be implemented to ensure optimal immediate treatment.
Barriers.
Although the ED may be an innovative location to delabel patients, several barriers to implementation exist. First, patients in need of critical medical intervention may not be ideal candidates to undergo real-time delabeling. Drug allergy evaluation is ideally suited for patients who are afebrile, hemodynamically stable, have stable respiratory status, and have no active rashes or swelling that could be confused with an allergic reaction. ED overcrowding in the United States has been associated with increased time to treatment and longer hospital stays.64 As such, ED overcrowding is a system-level focus with ED length of stay measured and tracked. ED overcrowding and the system desire for swift patient throughput poses a large barrier to encouraging ED clinicians and hospital leadership to embrace ED-based penicillin allergy assessments. Third, logistical considerations ranging from testing requirements, medication ordering and dispensing, patient billing/charges, and documentation must be considered. Considering documentation, ED-based delabeling efforts will be ineffective if allergy information is not communicated to the patients’ usual providers, who may not be in the same health care system or use the same electronic health record (EHR).
Opportunities and innovation.
Different testing strategies for addressing penicillin allergy in the ED have been explored to date. A 2009 study completed penicillin skin testing in adult ED patients and found that 91% patients tested negative.62 More recently, adult ED patients were penicillin skin tested and given a graded oral amoxicillin challenge (if skin test negative) and 81% were not allergic.65 Two-step beta-lactam challenges (“test doses”) in the ED based on an inpatient algorithm were implemented in a Boston-based academic medical center,35,66 which found that ED drug challenges were feasible and that hypersensitivity reactions were infrequent.63 However, although those challenges were feasible in the ED setting and optimized the patient’s immediate antibiotic regimen, they uncommonly resulted in penicillin allergy delabeling.67
Testing in pediatric ED patients, who comprise an estimated 1 in 5 ED visits,68 includes the delabeling of 100 of 100 low-risk children from a Wisconsin-based Pediatric ED who completed penicillin skin testing and amoxicillin challenges.59 After that initial study, direct drug challenges have been used to delabel low-risk children with penicillin allergy histories presenting to their pediatric ED.44 Over the course of the first year, 37 children participated and 36 tolerated amoxicillin.44
Innovations in penicillin allergy delabeling in the ED might include methods to identify patients with penicillin allergy labels for direct ED-based penicillin allergy delabeling or an automatic referral for penicillin allergy testing on discharge. ED observation units and urgent care units are ideal locations for penicillin allergy testing given their available staffing and access to requisite medications and equipment. Additional innovations should optimize urgent beta-lactam antibiotic administration; for example, order sets for sepsis and febrile neutropenia might provide specific guidance for patients reporting penicillin allergies to improve first-line treatment.
Internal medicine: hospitalists and adult primary care
Perspective.
Although inpatient antibiotic prescribing may be facilitated by ID specialists or monitored by ASPs, outpatient primary care prescriptions and prescribing practices are less commonly monitored systematically.69 Data from 2007 through 2009 showed that antibiotics were prescribed during 101 million (95% confidence interval: 91–111 million) ambulatory visits annually, representing 10% of all visits.70 Broad-spectrum agents were prescribed during 61% of those visits, with the most commonly prescribed antibiotics including fluoroquinolones (25% of antibiotics), macrolides (20% of antibiotics), and aminopenicillins (12% of antibiotics).70
Practice and logistics.
The majority of antibiotics in the United States are prescribed by internists providing care in inpatient or outpatient practice settings. Adult patients are often many years removed from their penicillin allergy history and are thus less likely to be truly penicillin allergic. It is therefore important for clinicians in these environments to identify patients with reported allergy for penicillin allergy delabeling. EHRs can identify eligible inpatients and facilitate antibiotic appropriateness.66,71 To date, primary care practices have identified patients for penicillin allergy evaluation performed by specialists,72,73 and hospitalists and internal medicine trainees have been instrumental to many acute care (inpatient) beta-lactam allergy pathways.12
Barriers.
Several barriers exist in implementing effective stewardship practices in both inpatient and outpatient general medicine settings. Limited provider knowledge and comfort with drug and penicillin allergy is a notable barrier.36,58,74,75 Another barrier is competing interests, given the large number of demands on our primary care doctors and hospitalists. When an outpatient presents for a short follow-up visit, it is likely that some active problems will not be addressed, let alone the historical penicillin allergy. For inpatients with active infections, the medicine focus is finding the right antibiotic considering a host of factors, with the patient’s allergy list one of many considerations. It is uncommon to prescribe drugs to patients that are on the patient’s allergy list because of patient safety, and potential associated medico-legal implications.76 Although allergy reconciliation is a theoretical requirement of EHR meaningful use, the EHR allergy list in practice is often incomplete and/or erroneous.77 To use the allergy list in the EHR to identify patients for internal medicine or primary care-based delabeling initiatives would first require improved EHR documentation.
Opportunities and innovation.
Inpatient penicillin allergy assessments are a safe and effective component of ASPs.12,29 The practice typically utilizes penicillin skin testing, and if a patient is found to be skin test negative, a therapeutic course of penicillin is prescribed. This method has been used in both medical “floor” and intensive care units.14,78 In a meta-analysis of inpatient penicillin skin tested patients, the proportion of negative tests ranged between 79% and 100%, with a population weighted mean of 95.1%.29 Overall, studies demonstrate that patients with negative skin tests have greater penicillin and cephalosporin use, lower broad spectrum antibiotic use, and in some studies, an associated cost savings.14,32 Alternative penicillin allergy assessment strategies include direct challenges in hospitalized patients.66
Providers in an outpatient setting will choose to use broad-spectrum antibiotics to treat common bacterial infections when patients report a penicillin allergy.58,79 However, these same providers would likely be amenable to altering their prescription patterns after the penicillin allergy was investigated.80 Although implementing delabeling initiatives may be too unfamiliar and/or too challenging for some internists, any penicillin allergy assessment efforts would likely benefit patients; given that outpatient testing is a reimbursable procedure, primary care practices might be incentivized to learn how to assess and delabel penicillin allergy.81 Implementation of improved EHR allergy documentation may improve prescribing.82
Pediatrics
Perspective.
At least 1 in 5 pediatric ambulatory visits result in the prescription of an antibiotic.83 Many of the prescribed antibiotics are beta-lactams prescribed as first-line treatment for sinusitis, acute otitis media, and Streptococcus pharyngitis. Based on recent census data, there are 74.2 million children in the United States84 with 10% reportedly allergic to penicillin.85 Penicillin allergy labels, which are applied to 75% of children before age 3,15 result in broad-spectrum treatment. At least half of the time an antibiotic is prescribed in ambulatory pediatric practice; it is a broad-spectrum antibiotic (commonly a macrolide).83
Practice and logistics.
Pediatricians provide outpatient and inpatient medical care to children from birth until the age of 18 years. As all pediatricians know, rashes occur for many reasons in children. Although a drug allergy is a potential reason for a child’s rash when on antibiotics, alternative explanations include a reaction from the underlying infection (eg, Epstein-Barr virus) or even an unrelated cause (eg, contact dermatitis). The initial diagnosis of a penicillin allergy is made most often by pediatricians, generally before age 3 years and commonly without a physical examination.15 Many reaction symptoms are consistent with an adverse and not allergic reaction.15,86,87 Adult patients frequently reporting a “rash” or “unknown” allergy when they were children are often relaying allergy information told to them by their pediatrician or parents. Ambulatory and inpatient pediatric practices are also in the unique position to address penicillin allergy overdiagnosis in addition to implementing penicillin allergy delabeling strategies.88 The majority of children with reported penicillin allergy could tolerate a penicillin without having an allergic reaction.89
Barriers.
Barriers to implementing delabeling strategies within pediatrics are similar to those faced by internal and emergency medicine, including limitations in knowledge and comfort,58 competing patient needs, and time constraints. In addition, parent resistance and/or fear may pose additional delabeling obstacles in pediatrics. One prior study identified that 18% of parents refused penicillin class antibiotics after negative skin testing because they still feared an adverse reaction.90
Opportunities and innovation.
It is important to address the initial point of penicillin allergy entry into the EHR. Almost 90% of families reported that their pediatrician diagnosed their initial allergic reaction.15 Correctly categorizing the type of reaction, with key details and photos, at the time of entry into the EHR is critical to give future providers more insight into the nature of the reaction for improved decision making. It is also important to have reaction types documented so that nonallergic symptoms (eg, diarrhea, single episode of emesis with medication administration, isolated rhinorrhea) are distinguished from allergy. If pediatrics practices cannot practically embrace penicillin allergy testing and drug challenges themselves, routine specialist referrals for penicillin allergy testing could be implemented.
Pharmacy
Perspective.
Clinical pharmacists review allergy documentation and prescriptions/orders, including those for beta-lactam alternative antibiotics as part of ASP practices. Clinical pharmacists have demonstrated a higher understanding of the natural history of penicillin allergy and beta-lactam cross-reactivity compared with none—allergy-trained health care professionals.75
Practice and logistics.
Clinical pharmacists routinely evaluate the essential components of pharmacotherapy and are considered the gatekeepers of medication management and safety across various health care settings. Clinical pharmacists can be integrated into the team-based health care to educate clinicians regarding the evaluation and management of patients with a penicillin allergy label. Pharmacists can prepare and distribute reagents with expertise and precision. ID pharmacists are integral members of ASPs. Multidisciplinary collaboration with pharmacists on delabeling initiatives and direct patient care has been shown to optimize management, including antibiotic therapy.14,91 The clinical and financial benefits of delabeling patients with a penicillin allergy history should incentivize pharmacy departments to prioritize penicillin allergy efforts by allocating sufficient resources.
The first pharmacist-driven penicillin allergy skin-testing program was reported by Wall et al in 2004.92 Since then, additional delabeling models and approaches have been successfully championed by pharmacists (Table II) with regulatory support present in more than half of the US states.14,93–97
TABLE II.
Pharmacist-associated penicillin allergy delabeling efforts
| Pharmacist activity performed | Chen et al14 (n = 252) |
Park et al93 (n = 503) |
Gugkaeva et al94 (n = 53) |
Phan et al95 (n = 140) |
Ramsey et al91 (n = 50) |
|---|---|---|---|---|---|
| Proactive patient identification | ✓ | ✓ | ✓ | ✓ | |
| Allergy history reconciliation | ✓ | ✓ | ✓ | ✓ | |
| Screening for penicillin skin testing | ✓ | ✓ | ✓ | ||
| Preparation of penicillin skin test reagents | ✓ | ✓ | |||
| Performing penicillin skin testing | ✓ | ✓ | |||
| Patient/provider education | ✓ | ✓ | ✓ | ||
| Antibiotic recommendations | ✓ | ✓ | ✓ | ✓ | |
| Allergy delabeling | ✓ | ✓ | |||
| Allergy relabeling monitoring | ✓ | ✓ | |||
| Inpatient setting | ✓ | ✓ | ✓ | ✓ | |
| Ambulatory setting | ✓ |
Barriers.
As the role of the clinical pharmacist in delabeling penicillin-allergic patients continues to expand, various obstacles have been identified. Current drug allergy education in pharmacy undergraduate and postgraduate curricula is limited.98 Although pharmacists may independently seek out additional drug allergy training, there is no established credentialing expectation and practice requirement. Another barrier is the pharmacist’s lack of “full provider” status that allows pharmacists to provide and gain reimbursement from rendered clinical services.99 Federal policy changes would be required to expand the pharmacist’s scope of practice and reimbursement for penicillin skin testing.81
Opportunities and innovation.
Pharmacists are ideal champions of inpatient penicillin allergy delabeling efforts based on their natural role in optimizing medication therapy and their greater routine inpatient availability. Some hospitals have specialized ID or ASP pharmacists who are involved with regular tracking of antibiotic utilization, including utilization of restricted antibiotics. Pharmacist-driven penicillin allergy assessment models provide a “boots on the ground” approach, which can augment and maintain efforts in all facilities, but particularly when allergists are not accessible.
Pharmacists are also well positioned to proactively identify patients who could benefit from a penicillin allergy evaluation early on in their clinical course. Medication allergy reconciliation, review of microbiology data, and oversight of second-line, beta-lactam alternative antibiotics are all opportunities where pharmacists can optimize clinical care in patients with a reported penicillin allergy.14,100,101
Efforts to integrate fundamental drug allergy education into pharmacy practice experiences are imperative to ensure that pharmacists are prepared to meet a growing need for penicillin allergy delabeling.98 The pharmacy profession should set a competency standard for pharmacists performing penicillin skin testing to ensure the delivery of quality clinical services and patient care.
Although the outpatient setting is rife with opportunity for penicillin allergy evaluation and delabeling with a variety of potential contributions from pharmacists, pharmacist-led delabeling programs to date have been reported predominantly in acute care inpatient settings.14,51,92 However, expansion of pharmacist services beyond the hospital may result in similar benefits on a wider scale.
SUMMARY AND COORDINATION OF EFFORTS
The penicillin allergy label is pervasive and infiltrates many aspects of health care, influencing outcomes in diverse patients from all health care settings where antibiotics are prescribed. Recognition of the harms associated with the penicillin allergy label has mobilized professional and public health organizations to encourage penicillin allergy evaluations and delabeling. Widespread collaborative efforts with innovative solutions are needed to implement systematic delabeling (Figure 3 and Figures E1–E7, available in this article’s Online Repository at www.jaci-inpractice.org). In this review, we described the contributions and perspectives of multiple disciplines to delabeling; although approaches have varied, many disciplines have already made substantial strides toward combatting erroneous penicillin allergy labels and improving antibiotic choices.
FIGURE 3.
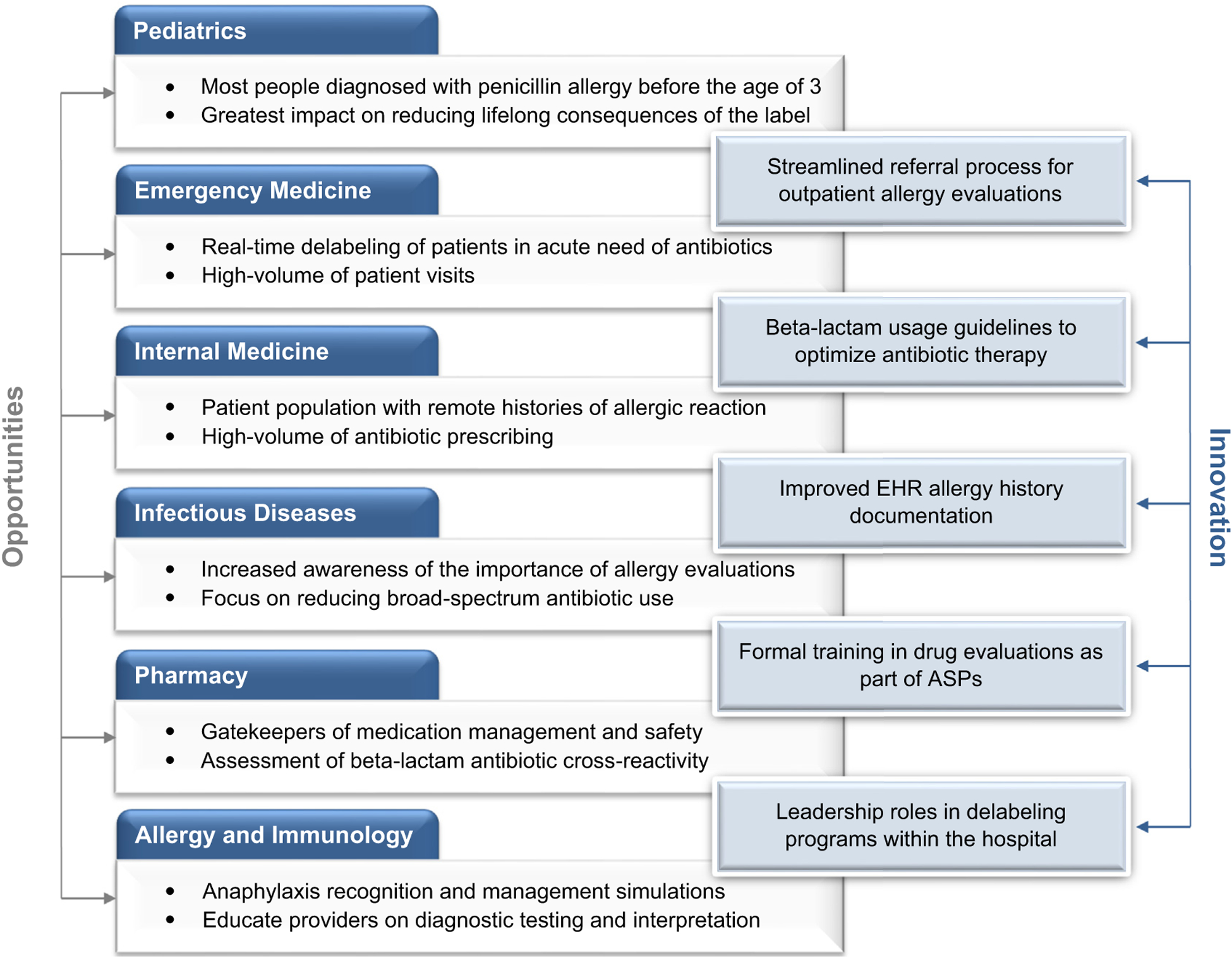
Multidisciplinary engagement opportunities for penicillin allergy delabeling. The figure summarizes the multidisciplinary opportunities and innovations that are critical to penicillin allergy delabeling efforts. ASP, Antibiotic Stewardship Program; EHR, electronic health record.
Supplementary Material
Acknowledgment
The authors thank Christian M. Mancini for his research assistance.
No funding was received for this work.
Abbreviations used
- ASP
Antibiotic stewardship program
- CDI
Clostridioides difficile infection
- ED
Emergency department
- EHR
Electronic health record
- ID
Infectious diseases
- IPC
Infection prevention and control
- MDM
Minor determinant mixture
- PPL
Penicilloyl-polylysine
- SI
Surgical site infection
Footnotes
Conflicts of interest: E. S. Shenoy and K. G. Blumenthal have a beta-lactam clinical decision support tool licensed to Persistent Systems, Inc. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Joint Allergy Task Force on Practice Parameters. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol 2010;105:259–73. [DOI] [PubMed] [Google Scholar]
- 2.Blumenthal KG, Lu N, Zhang Y, Li Y, Walensky RP, Choi HK. Risk of meticillin resistant Staphylococcus aureus and Clostridium difficile in patients with a documented penicillin allergy: population based matched cohort study. BMJ 2018;361:k2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeffres MN, Narayanan PP, Shuster JE, Schramm GE. Consequences of avoiding beta-lactams in patients with beta-lactam allergies. J Allergy Clin Immunol 2016;137:1148–53. [DOI] [PubMed] [Google Scholar]
- 4.Blumenthal KG, Lu N, Zhang Y, Walensky RP, Choi HK. Recorded penicillin allergy and risk of mortality: a population-based matched cohort study. J Gen Intern Med 2019;34:1685–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol 2014;133:790–6. [DOI] [PubMed] [Google Scholar]
- 6.MacFadden DR, LaDelfa A, Leen J, Gold WL, Daneman N, Weber E, et al. Impact of reported beta-lactam allergy on inpatient outcomes: a multicenter prospective cohort study. Clin Infect Dis 2016;63:904–10. [DOI] [PubMed] [Google Scholar]
- 7.Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and management of penicillin allergy: a review. JAMA 2019;321:188–99. [DOI] [PubMed] [Google Scholar]
- 8.Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016;62: e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Quality Forum. National Quality Partners playbook: antibiotic stewardship in acute care; 2016. Available from: http://www.qualityforum.org/NQP/Antibiotic_Stewardship_Playbook.aspx. Accessed February 4, 2020.
- 10.The Center for Health Workforce Studies. American Academy of Allergy Asthma and Immunology Report on the Allergy and Immunology Physician Workforce, 1999–2009/10. Rensselaer, NY: The Center for Health Workforce Studies; 2012. Available from: https://www.aaaai.org/Aaaai/media/MediaLibrary/PDF%20Documents/Practice%20and%20Parameters/2012-AI-Physician-Workforce-Report.pdf. Accessed February 4, 2020. [Google Scholar]
- 11.Mancini CM, Fu X, Zhang Y, Kuper K, Schulz LT, Bhowmick T, et al. Penicillin allergy evaluation access: a national survey [published online ahead of print May 18, 2020]. Clin Infect Dis. 10.1093/cid/ciaa567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfson AR, Huebner EM, Blumenthal KG. Acute care beta-lactam allergy pathways: approaches and outcomes. Ann Allergy Asthma Immunol 2019;123: 16–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blumenthal KG, Peter JG, Trubiano JA, Phillips EJ. Antibiotic allergy. Lancet 2019;393:183–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen JR, Tarver SA, Alvarez KS, Tran T, Khan DA. A Proactive Approach to penicillin allergy testing in hospitalized patients. J Allergy Clin Immunol Pract 2017;5:686–93. [DOI] [PubMed] [Google Scholar]
- 15.Vyles D, Chiu A, Simpson P, Nimmer M, Adams J, Brousseau DC. Parent-reported penicillin allergy symptoms in the pediatric emergency department. Acad Pediatr 2017;17:251–5. [DOI] [PubMed] [Google Scholar]
- 16.Wong A, Seger DL, Lai KH, Goss FR, Blumenthal KG, Zhou L. Drug hypersensitivity reactions documented in electronic health records within a large health system. J Allergy Clin Immunol Pract 2019;7:1253–1260.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banks TA, Tucker M, Macy E. Evaluating penicillin allergies without skin testing. Curr Allergy Asthma Rep 2019;19:27. [DOI] [PubMed] [Google Scholar]
- 18.Stone CA Jr, Trubiano J, Coleman DT, Rukasin CRF, Phillips EJ. The challenge of de-labeling penicillin allergy. Allergy 2020;75:273–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macy E, Richter PK, Falkoff R, Zeiger R. Skin testing with penicilloate and penilloate prepared by an improved method: amoxicillin oral challenge in patients with negative skin test responses to penicillin reagents. J Allergy Clin Immunol 1997;100:586–91. [DOI] [PubMed] [Google Scholar]
- 20.Solensky R, Jacobs J, Lester M, Lieberman P, McCafferty F, Nilsson T, et al. Penicillin allergy evaluation: a prospective, multicenter, open-label evaluation of a comprehensive penicillin skin test kit. J Allergy Clin Immunol Pract 2019; 7:1876–1885.e3. [DOI] [PubMed] [Google Scholar]
- 21.Iammatteo M, Alvarez Arango S, Ferastraoaru D, Akbar N, Lee AY, Cohen HW, et al. Safety and outcomes of oral graded challenges to amoxicillin without prior skin testing. J Allergy Clin Immunol Pract 2019;7: 236–43. [DOI] [PubMed] [Google Scholar]
- 22.Stevenson B, Trevenen M, Klinken E, Smith W, Yuson C, Katelaris C, et al. Multicenter Australian study to determine criteria for low-and high-risk penicillin testing in outpatients. J Allergy Clin Immunol Pract 2020;8: 681–689.e3. [DOI] [PubMed] [Google Scholar]
- 23.Stone CA Jr, Stollings JL, Lindsell CJ, Dear ML, Buie RB, Rice TW, et al. Risk-stratified management to remove low-risk penicillin allergy labels in the intensive care unit. Am J Respir Crit Care Med 2020;201: 1572–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trubiano JA, Vogrin S, Chua KYL, Bourke J, Yun J, Douglas A, et al. Development and validation of a penicillin allergy clinical decision rule. JAMA Intern Med 2020;180:745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieberman P, Nicklas RA, Randolph C, Oppenheimer J, Bernstein D, Bernstein J, et al. Anaphylaxis—a practice parameter update 2015. Ann Allergy Asthma Immunol 2015;115:341–84. [DOI] [PubMed] [Google Scholar]
- 26.Banks TA, Ressner RA, Gada SM. Antibiotic reclamation: penicillin allergy, antibiotic stewardship, and the allergist. Ann Allergy Asthma Immunol 2015; 115:451–2. [DOI] [PubMed] [Google Scholar]
- 27.Trubiano JA, Stone CA, Grayson ML, Urbancic K, Slavin MA, Thursky KA, et al. The 3 Cs of antibiotic allergy-classification, cross-reactivity, and collaboration. J Allergy Clin Immunol Pract 2017;5:1532–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerace KS, Karlin E, McKinnon E, Phillips E. Varying penicillin allergy testing practices in the United States: a time for consensus. J Allergy Clin Immunol Pract 2015;3:791–3. [DOI] [PubMed] [Google Scholar]
- 29.Sacco KA, Bates A, Brigham TJ, Imam JS, Burton MC. Clinical outcomes following inpatient penicillin allergy testing: a systematic review and meta-analysis. Allergy 2017;72:1288–96. [DOI] [PubMed] [Google Scholar]
- 30.Warrington RJ, Lee KR, McPhillips S. The value of skin testing for penicillin allergy in an inpatient population: analysis of the subsequent patient management. Allergy Asthma Proc 2000;21:297–9. [DOI] [PubMed] [Google Scholar]
- 31.Geng B, Thakor A, Clayton E, Finkas L, Riedl MA. Factors associated with negative histamine control for penicillin allergy skin testing in the inpatient setting. Ann Allergy Asthma Immunol 2015;115:33–8. [DOI] [PubMed] [Google Scholar]
- 32.Blumenthal KG, Wickner PG, Hurwitz S, Pricco N, Nee AE, Laskowski K, et al. Tackling inpatient penicillin allergies: assessing tools for antimicrobial stewardship. J Allergy Clin Immunol 2017;140:154–161.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trubiano JA, Beekmann SE, Worth LJ, Polgreen PM, Thursky KA, Slavin MA, et al. Improving antimicrobial stewardship by antibiotic allergy delabeling: evaluation of knowledge, attitude, and practices throughout the emerging infections network. Open Forum Infect Dis 2016;3:ofw153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trubiano J, Phillips E. Antimicrobial stewardship’s new weapon? A review of antibiotic allergy and pathways to ‘de-labeling’. Curr Opin Infect Dis 2013;26: 526–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blumenthal KG, Shenoy ES, Varughese CA, Hurwitz S, Hooper DC, Banerji A. Impact of a clinical guideline for prescribing antibiotics to inpatients reporting penicillin or cephalosporin allergy. Ann Allergy Asthma Immunol 2015;115:294–300.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blumenthal KG, Shenoy ES, Hurwitz S, Varughese CA, Hooper DC, Banerji A. Effect of a drug allergy educational program and antibiotic prescribing guideline on inpatient clinical providers’ antibiotic prescribing knowledge. J Allergy Clin Immunol Pract 2014;2:407–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blumenthal KG, Huebner EM, Fu X, Li Y, Bhattacharya G, Levin AS, et al. Risk-based pathway for outpatient penicillin allergy evaluations. J Allergy Clin Immunol Pract 2019; (7):2411–2414.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Staicu ML, Holly AM, Conn KM, Ramsey A. The use of telemedicine for penicillin allergy skin testing. J Allergy Clin Immunol Pract 2018;6:2033–40. [DOI] [PubMed] [Google Scholar]
- 39.Phadke NA, Wolfson AR, Mancini C, Fu X, Goldstein SA, Ngo J, et al. Electronic consultations in allergy/immunology. J Allergy Clin Immunol Pract 2019;7:2594–602. [DOI] [PubMed] [Google Scholar]
- 40.Krantz MS, Stone CA Jr, Abreo A, Phillips EJ. Oral challenge with trimethoprim-sulfamethoxazole in patients with “sulfa” antibiotic allergy. J Allergy Clin Immunol Pract 2020;8:757–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kolawole H, Guttormsen AB, Hepner DL, Kroigaard M, Marshall S. Use of simulation to improve management of perioperative anaphylaxis: a narrative review. Br J Anaesth 2019;123:e104–9. [DOI] [PubMed] [Google Scholar]
- 42.Mawhirt SL, Fonacier L, Aquino M. Utilization of high-fidelity simulation for medical student and resident education of allergic-immunologic emergencies. Ann Allergy Asthma Immunol 2019;122:513–21. [DOI] [PubMed] [Google Scholar]
- 43.Barni S, Mori F, Giovannini M, de Luca M, Novembre E. In situ simulation in the management of anaphylaxis in a pediatric emergency department. Intern Emerg Med 2019;14:127–32. [DOI] [PubMed] [Google Scholar]
- 44.Vyles D, Chiu A, Routes J, Castells M, Phillips EJ, Visotcky A, et al. Oral amoxicillin challenges in low-risk children during a pediatric emergency department visit. J Allergy Clin Immunol Pract 2020;8:1126–1128.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bai AD, Showler A, Burry L, Steinberg M, Ricciuto DR, Fernandes T, et al. Impact of infectious disease consultation on quality of care, mortality, and length of stay in Staphylococcus aureus bacteremia: results from a large multicenter cohort study. Clin Infect Dis 2015;60:1451–61. [DOI] [PubMed] [Google Scholar]
- 46.Heil EL, Bork JT, Schmalzle SA, Kleinberg M, Kewalramani A, Gilliam BL, et al. Implementation of an infectious disease fellow-managed penicillin allergy skin testing service. Open Forum Infect Dis 2016;3:ofw155.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ostrowsky B, Banerjee R, Bonomo RA, Cosgrove SE, Davidson L, Doron S, et al. Infectious diseases physicians: leading the way in antimicrobial stewardship. Clin Infect Dis 2018;66:995–1003. [DOI] [PubMed] [Google Scholar]
- 48.Reed EE, Stevenson KB, West JE, Bauer KA, Goff DA. Impact of formulary restriction with prior authorization by an antimicrobial stewardship program. Virulence 2013;4:158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention. Core elements of hospital antibiotic stewardship. 2019. Available from: https://www.cdc.gov/antibiotic-use/core-elements/index.html. Accessed February 4, 2020.
- 50.Cosgrove SE, Hermsen ED, Rybak MJ, File TM Jr, Parker SK, Barlam TF, et al. Guidance for the knowledge and skills required for antimicrobial stewardship leaders. Infect Control Hosp Epidemiol 2014;35:1444–51. [DOI] [PubMed] [Google Scholar]
- 51.Jones BM, Avramovski N, Concepcion AM, Crosby J, Bland CM. Clinical and economic outcomes of penicillin skin testing as an antimicrobial stewardship initiative in a community health system. Open Forum Infect Dis 2019;6: ofz109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leis JA, Palmay L, Ho G, Raybardhan S, Gill S, Kan T, et al. Point-of-care beta-lactam allergy skin testing by antimicrobial stewardship programs: a pragmatic multicenter prospective evaluation. Clin Infect Dis 2017;65: 1059–65. [DOI] [PubMed] [Google Scholar]
- 53.Pool C, Kass J, Spivack J, Nahumi N, Khan M, Babus L, et al. Increased surgical site infection rates following clindamycin use in head and neck free tissue transfer. Otolaryngol Head Neck Surg 2016;154:272–8. [DOI] [PubMed] [Google Scholar]
- 54.Kawakita T, Huang CC, Landy HJ. Choice of prophylactic antibiotics and surgical site infections after cesarean delivery. Obstet Gynecol 2018;132:948–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blumenthal KG, Ryan EE, Li Y, Lee H, Kuhlen JL, Shenoy ES. The impact of a reported penicillin allergy on surgical site infection risk. Clin Infect Dis 2018;66:329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Centers for Disease Control and Prevention. Antibiotic use in the United States, 2017: Progress and opportunities. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2017. Available from: https://www.cdc.gov/antibiotic-use/stewardship-report/pdf/stewardship-report.pdf. Accessed February 4, 2020. [Google Scholar]
- 57.Poole NM, Shapiro DJ, Fleming-Dutra KE, Hicks LA, Hersh AL, Kronman MP. Antibiotic prescribing for children in United States emergency departments: 2009–2014. Pediatrics 2019;143:e20181056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vyles D, Mistry RD, Heffner V, Drayna P, Chiu A, Visotcky A, et al. Reported knowledge and management of potential penicillin allergy in children. Acad Pediatr 2019;19:684–90. [DOI] [PubMed] [Google Scholar]
- 59.Kim RY, Ng AM, Persaud AK, Furmanek SP, Kothari YN, Price JD, et al. Antibiotic timing and outcomes in sepsis. Am J Med Sci 2018;355:524–9. [DOI] [PubMed] [Google Scholar]
- 60.Conway EL, Lin K, Sellick JA, Kurtzhalts K, Carbo J, Ott MC, et al. Impact of penicillin allergy on time to first dose of antimicrobial therapy and clinical outcomes. Clin Ther 2017;39:2276–83. [DOI] [PubMed] [Google Scholar]
- 61.Vyles D, Adams J, Chiu A, Simpson P, Nimmer M, Brousseau DC. Allergy testing in children with low-risk penicillin allergy symptoms. Pediatrics 2017; 140:e20170471. [DOI] [PubMed] [Google Scholar]
- 62.Raja AS, Lindsell CJ, Bernstein JA, Codispoti CD, Moellman JJ. The use of penicillin skin testing to assess the prevalence of penicillin allergy in an emergency department setting. Ann Emerg Med 2009;54:72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maguire M, Hayes BD, Fuh L, Elshaboury R, Gandhi RG, Bor S, et al. Beta-lactam antibiotic test doses in the emergency department. World Allergy Organ J 2020;13:100093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verelst S, Wouters P, Gillet JB, Van den Berghe G. Emergency department crowding in relation to in-hospital adverse medical events: a large prospective observational cohort study. J Emerg Med 2015;49:949–61. [DOI] [PubMed] [Google Scholar]
- 65.Marwood J, Aguirrebarrena G, Kerr S, Welch SA, Rimmer J. De-labelling self-reported penicillin allergy within the emergency department through the use of skin tests and oral drug provocation testing. Emerg Med Australas 2017;29: 509–15. [DOI] [PubMed] [Google Scholar]
- 66.Blumenthal KG, Li Y, Hsu JT, Wolfson AR, Berkowitz DN, Carballo VA, et al. Outcomes from an inpatient beta-lactam allergy guideline across a large US health system. Infect Control Hosp Epidemiol 2019;40:528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wright A, Rubins D, Shenoy ES, Wickner PG, McEvoy D, Wolfson AR, et al. Clinical decision support improved allergy documentation of antibiotic test dose results. J Allergy Clin Immunol Pract 2019;7:2919–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McDermott KW, Stocks C, Freeman WJ. Overview of pediatric emergency department visits, 2015. Statistical Brief #242. Rockville, MD: Healthcare Cost and Utilization Project; 2006. [PubMed] [Google Scholar]
- 69.Klepser ME, Dobson EL, Pogue JM, Labreche MJ, Adams AJ, Gauthier TP, et al. A call to action for outpatient antibiotic stewardship. J Am Pharm Assoc (2003) 2017;57:457–63. [DOI] [PubMed] [Google Scholar]
- 70.Shapiro DJ, Hicks LA, Pavia AT, Hersh AL. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–09. J Antimicrob Chemother 2014;69: 234–40. [DOI] [PubMed] [Google Scholar]
- 71.Devchand M, Stewardson AJ, Urbancic KF, Khumra S, Mahony AA, Walker S, et al. Outcomes of an electronic medical record (EMR)-driven intensive care unit (ICU)-antimicrobial stewardship (AMS) ward round: assessing the “Five Moments of Antimicrobial Prescribing. Infect Control Hosp Epidemiol 2019;40:1170–5. [DOI] [PubMed] [Google Scholar]
- 72.Jones EP, Kim AS. Penicillin allergy testing: a strategic approach to increasing referrals from primary care physicians. Ann Allergy Asthma Immunol 2019; 123:96–7. [DOI] [PubMed] [Google Scholar]
- 73.Sundquist BK, Bowen BJ, Otabor U, Celestin J, Sorum PC. Proactive penicillin allergy testing in primary care patients labeled as allergic: outcomes and barriers. Postgrad Med 2017;129:915–20. [DOI] [PubMed] [Google Scholar]
- 74.Stukus DR, Green T, Montandon SV, Wada KJ. Deficits in allergy knowledge among physicians at academic medical centers. Ann Allergy Asthma Immunol 2015;115:51–55.e1. [DOI] [PubMed] [Google Scholar]
- 75.Staicu ML, Soni D, Conn KM, Ramsey A. A survey of inpatient practitioner knowledge of penicillin allergy at 2 community teaching hospitals. Ann Allergy Asthma Immunol 2017;119:42–7. [DOI] [PubMed] [Google Scholar]
- 76.Jeffres MN, Hall-Lipsy EA, King ST, Cleary JD. Systematic review of professional liability when prescribing beta-lactams for patients with a known penicillin allergy. Ann Allergy Asthma Immunol 2018;121:530–6. [DOI] [PubMed] [Google Scholar]
- 77.Blumenthal KG, Acker WW, Li Y, Holtzman NS, Zhou L. Allergy entry and deletion in the electronic health record. Ann Allergy Asthma Immunol 2017; 118:380–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rimawi RH, Mazer MA. Expanding the pool of healthcare providers to perform penicillin skin testing in the ICU. Intensive Care Med 2014;40: 462–3. [DOI] [PubMed] [Google Scholar]
- 79.McGrath LJ, Becker-Dreps S, Pate V, Brookhart MA. Trends in antibiotic treatment of acute otitis media and treatment failure in children, 2000–2011. PLoS One 2013;8:e81210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vyles D, Chiu A, Routes J, Castells M, Phillips EJ, Kibicho J, et al. Antibiotic use after removal of penicillin allergy label. Pediatrics 2018;141: e20173466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kufel WD, Justo JA, Bookstaver PB, Avery LM. Penicillin allergy assessment and skin testing in the outpatient setting. Pharmacy (Basel) 2019;7:E136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shah NS, Ridgway JP, Pettit N, Fahrenbach J, Robicsek A. Documenting penicillin allergy: the impact of inconsistency. PLoS One 2016;11: e0150514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hersh AL, Shapiro DJ, Pavia AT, Shah SS. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics 2011;128:1053–61. [DOI] [PubMed] [Google Scholar]
- 84.The Annie E. Casey Foundation. The Changing Child Population of the United States: An Analysis of the U.S. Population Under 18 Using Data from the 2010 Census Baltimore, MD: The Annie E. Casey Foundation; 2011. Available from: https://files.eric.ed.gov/fulltext/ED527048.pdf. Accessed February 4, 2020. [Google Scholar]
- 85.Blumenthal KG, Shenoy ES. Is my child allergic to penicillin? JAMA Pediatr 2019;173:708. [DOI] [PubMed] [Google Scholar]
- 86.Bass JW, Crowley DM, Steele RW, Young FS, Harden LB. Adverse effects of orally administered ampicillin. J Pediatr 1973;83:106–8. [DOI] [PubMed] [Google Scholar]
- 87.Caubet JC, Kaiser L, Lemaitre B, Fellay B, Gervaix A, Eigenmann PA. The role of penicillin in benign skin rashes in childhood: a prospective study based on drug rechallenge. J Allergy Clin Immunol 2011;127:218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Allen HI, Vazquez-Ortiz M, Murphy AW, Moylett EM. De-labeling penicillin-allergic children in outpatients using telemedicine: potential to replicate in primary care. J Allergy Clin Immunol Pract 2020;8:1750–2. [DOI] [PubMed] [Google Scholar]
- 89.Mill C, Primeau MN, Medoff E, Lejtenyi C, O’Keefe A, Netchiporouk E, et al. Assessing the diagnostic properties of a graded oral provocation challenge for the diagnosis of immediate and nonimmediate reactions to amoxicillin in children. JAMA Pediatr 2016;170:e160033. [DOI] [PubMed] [Google Scholar]
- 90.Picard M, Paradis L, Nguyen M, Begin P, Paradis J, Des Roches A. Outpatient penicillin use after negative skin testing and drug challenge in a pediatric population. Allergy Asthma Proc 2012;33:160–4. [DOI] [PubMed] [Google Scholar]
- 91.Ramsey A, Staicu ML. Use of a penicillin allergy screening algorithm and penicillin skin testing for transitioning hospitalized patients to first-line antibiotic therapy. J Allergy Clin Immunol Pract 2018;6:1349–55. [DOI] [PubMed] [Google Scholar]
- 92.Wall GC, Peters L, Leaders CB, Wille JA. Pharmacist-managed service providing penicillin allergy skin tests. Am J Health Syst Pharm 2004;61: 1271–5. [DOI] [PubMed] [Google Scholar]
- 93.Park MA, McClimon BJ, Ferguson B, Markus PJ, Odell L, Swanson A, et al. Collaboration between allergists and pharmacists increases beta-lactam antibiotic prescriptions in patients with a history of penicillin allergy. Int Arch Allergy Immunol 2011;154:57–62. [DOI] [PubMed] [Google Scholar]
- 94.Gugkaeva Z, Crago JS, Yasnogorodsky M. Next step in antibiotic stewardship: pharmacist-provided penicillin allergy testing. J Clin Pharm Ther 2017;42: 509–12. [DOI] [PubMed] [Google Scholar]
- 95.Phan A, Allen B, Epps K, Alikhil M, Kamataris K, Tucker C. Initiative to reduce aztreonam use in patients with self-reported penicillin allergy: effects on clinical outcomes and antibiotic prescribing patterns. Am J Health Syst Pharm 2018;75(Suppl 3):S58–62. [DOI] [PubMed] [Google Scholar]
- 96.King EA, Challa S, Curtin P, Bielory L. Penicillin skin testing in hospitalized patients with beta-lactam allergies: effect on antibiotic selection and cost. Ann Allergy Asthma Immunol 2016;117:67–71. [DOI] [PubMed] [Google Scholar]
- 97.Bland CM, Bookstaver PB, Griffith NC, Heil EL, Jones BM, Ann Justo J, et al. A practical guide for pharmacists to successfully implement penicillin allergy skin testing. Am J Health Syst Pharm 2019;76:136–47. [DOI] [PubMed] [Google Scholar]
- 98.University of South Carolina College of Pharmacy. Penicillin allergy assessment & skin testing (PAAST) certificate program. Available from: https://www.sc.edu/study/colleges_schools/pharmacy/centers/penicillin_allergy_skin_testing_certificate_program/index.php. Accessed February 4, 2020.
- 99.Gebhart F On the road to provider status. DrugTopics. June 13, 2019. Available from: https://www.drugtopics.com/latest/road-provider-status. Accessed February 4, 2020. [Google Scholar]
- 100.Mann KL, Wu JY, Shah SS. Implementation of a pharmacist-driven detailed penicillin allergy interview. Ann Pharmacother 2020;54:364–70. [DOI] [PubMed] [Google Scholar]
- 101.Chen JR, Tarver SA, Alvarez KS, Wei W, Khan DA. Improving aztreonam stewardship and cost through a penicillin allergy testing clinical guideline. Open Forum Infect Dis 2018;5:ofy106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


