Abstract
TAS0313, a novel cancer vaccine cocktail, was developed to overcome the disadvantages of previously developed short and long peptide vaccines; it comprises several long peptides targeting multiple cancer antigens. We evaluated TAS0313 monotherapy in Japanese patients with advanced solid tumors for which no other therapies were available. In the dose‐finding cohort, patients received TAS0313 (9 or 27 mg) on days 1, 8, and 15 of cycles 1 and 2, and then on day 1 of each subsequent 21‐day cycle. The primary objective was the evaluation of safety and tolerability. Secondary objectives were evaluation of efficacy, tumor responses, and immune activation (CTL, IgG, and tumor‐infiltrating lymphocyte [TIL] levels). The full analysis set contained 10 patients in the 9‐mg group and seven in the 27‐mg group. No dose‐limiting toxicities were reported in cycle 1. All adverse drug reactions (ADRs) were grade 1 or 2; the most common ADRs were injection site‐related events. The best response was stable disease in four of 17 patients. The median progression‐free survival (PFS) duration was 2.2 (95% confidence interval, 1.0‐2.3) months overall; patients with baseline low lymphocyte counts (≤750/μL) had shorter PFS. Compared with baseline, TILs were increased in five patients. Although CTLs, IgG, and TILs were induced, no correlative pattern with clinical outcomes was observed. The safety, tolerability, and induction of immune responses in patients with advanced solid tumors receiving TAS0313 were confirmed. Further evaluation of TAS0313’s efficacy as monotherapy or in combination with pembrolizumab is underway. The study is registered at www.clinicaltrials.jp (JapicCTI‐183824).
Keywords: advanced solid tumor, cancer peptide vaccine, efficacy, phase I study, safety
This phase I, first‐in‐human clinical study confirmed the safety, tolerability, and induction of immune responses in patients with advanced solid tumors receiving TAS0313. We evaluated tumor‐infiltrating CD8+ T lymphocytes, CTLs, and IgG compared with pretreatment and confirmed the immune activation effects of TAS0313.
1. INTRODUCTION
Cancer vaccines are a new type of cancer immunotherapy that directs the host’s immune system against specific tumor factors. 1 Peptide‐ and protein‐based vaccines have the potential to target specific tumor types, through the recognition of complexes comprising tumor antigen‐derived peptide epitopes and human leukocyte antigen (HLA) by host CTLs. They are thought to hold great promise for the treatment of many malignancies. 2 Previous clinical studies have reported the partial efficacy of short peptide vaccines containing 8‐10 amino acid residues for urothelial carcinoma and glioblastoma. 3 , 4 However, in an analysis of multiple clinical trials, the response rate of these short peptide vaccines was only 2.9%, 5 indicating that short peptide vaccines represent a limited treatment option.
Limitations associated with short peptide vaccines include 6 : low immunogenicity of short peptides, CTL accumulation at the antigen‐rich vaccination site rather than in tumors, which induces CTL dysfunction, and accessibility of short peptides to HLA molecules, which induces unfavorable antigen presentation from nonprofessional antigen‐presenting cells that leads to immunological tolerance of CTLs. Another important limitation of peptide vaccines is the HLA restriction of the peptide as HLA gene frequency varies according to race. 7
Previous studies have attempted to overcome the disadvantage of low immunogenicity induced by short peptide vaccines by developing novel long peptide vaccines; results indicated that these products were generally well‐tolerated and induced cellular immune responses. 8 , 9 , 10 However, long peptide vaccines containing CTL epitopes derived from a single cancer antigen lack efficacy because cancer cells can escape tumor surveillance by losing expression of the specifically targeted cancer antigen. Furthermore, the limitation of HLA restriction remains unsolved. More recently, it has been suggested that multiple long peptides might improve the immunogenicity of cancer peptide vaccines 6 , 11 ; this could enable activation of an increased number of CTLs.
TAS0313 is a novel cancer vaccine cocktail that was developed to overcome the disadvantages of previously developed short and long peptide vaccines by containing multiple long peptides that target multiple cancer antigens and increasing patient eligibility for vaccination by targeting multiple HLA types. TAS0313 contains three long peptides (TAS0314, TAS0315, and TAS0316) (Table S1), that harbor 12 CTL epitope peptides restricted by HLA‐A24, A2, and A3‐supertype. These peptides are derived from eight cancer‐associated antigens that are highly expressed in various tumor types: squamous cell carcinoma antigen recognized by T‐cells 2 (SART2), 12 SART3, 13 , 14 , 15 multidrug resistance‐associated protein 3 (MRP3), 16 epidermal growth factor receptor (EGFR), 17 Wolf‐Hirschhorn syndrome candidate 2 protein (WHSC2), 18 transmembrane protein 189 (TMEM189) (also referred to as KUA), 18 tyrosine‐protein kinase LCK (LCK), 19 , 20 , 21 and parathyroid hormone‐related protein (PTHRP) 22 (Table S2). In regard to TMEM189, previous reports described the “RLQEWCSVI” epitope peptide as “UBE2V p43‐51”. Because the original protein of the peptide has proven to be TMEM189 (NCBI protein database accession number: NP_001155977.2), we described “RLQEWCSVI” as TMEM189 p43‐51.
This first‐in‐human, phase I/II clinical study aimed to evaluate the tolerability and safety of TAS0313 monotherapy in patients with a solid tumor. The study also investigated the efficacy and immune responses of TAS0313 in this patient population. This report focuses on the results of the phase I portion of the study, which was intended to examine the safety and efficacy of two doses of TAS0313, 9 and 27 mg.
2. MATERIALS AND METHODS
2.1. Study design and drug treatment
This was a phase I/II, open‐label, nonrandomized, multicenter study designed to examine the tolerability, safety, and efficacy of TAS0313 in patients with solid tumors. The study design was as follows: phase I, dose‐finding (cohort A); phase II, efficacy‐finding (cohort B); and assessment in urothelial carcinoma (cohort C). Only data from phase I (cohort A) will be presented in this report, and phase II data will be reported later.
Two doses of TAS0313, 9 mg (3 mg of each of the three peptides) and 27 mg (9 mg of each peptide), were given to patients with a solid tumor for which no further effective therapies were available. The study drug was prepared by dissolving 9 mg lyophilized TAS0313 in water, which was then mixed with an adjuvant, Montanide ISA 51 VG (Seppic), at a ratio of 1:1 for emulsification. The dose selection method involved evaluating TAS0313 at a starting dose of 9 mg, followed by 27 mg. Each dose group included six tolerability‐evaluable patients, and each cycle lasted for 21 days. TAS0313 was injected s.c. near the lymph node on days 1, 8, and 15 of cycles 1 and 2 and on day 1 of cycle 3 or later in 21‐day cycles until disease progression or an unacceptable adverse event (AE) was observed.
The criterion for the discontinuation of the dose increase to 27 mg was exceeding 33% of adverse drug reactions (ADRs) that met the tolerability evaluation criteria during cycle 1 in the 9‐mg dose group. The tolerability evaluation criteria included hematologic toxicity (grade 4 hematologic toxicity, febrile neutropenia, anemia, or thrombocytopenia requiring blood transfusion), nonhematologic toxicity (grade 3 or higher; except injection site reaction), and others (inability to start study drug within 14 days from day 1 of cycle 2 because of an ADR of grade 2 or higher). The decision of tolerability in each dose group was discussed and confirmed between the sponsor and investigator and in consultation with the data monitoring committee.
The study protocol and all amendments were reviewed by the institutional review board of each center. The study was carried out according to the protocol, good clinical practice guidelines, applicable local regulations, and the Declaration of Helsinki. Written informed consent was obtained from all patients at the time of enrollment. The study is registered at www.clinicaltrials.jp (JapicCTI‐183824).
2.2. Patients
Enrolled patients had at least one of the following HLA types: HLA‐A*02:01, ‐A*02:06, ‐A*02:07, ‐A*11:01, ‐A*24:02, ‐A*31:01, or ‐A*33:03. Key inclusion criteria were: (i) aged 20 years or older at the time of enrollment; (ii) histologically or cytologically confirmed advanced or metastatic solid tumor(s) for which no therapies are available; (iii) measurable or nonmeasurable lesion based on RECIST version 1.1; (iv) ECOG performance status (PS) of 0 or 1, and normal laboratory values; (v) an absolute neutrophil count of 1500/mm3 or higher; (vi) platelet count of 75 000/mm3 or higher; (vii) hemoglobin 8.0 g/dL or more; (viii) serum creatinine at least 1.5× upper limit of normal (ULN) or creatinine clearance 50 mL/min or higher; (ix) total bilirubin 1.5× ULN or less; and (x) aspartate aminotransferase and alanine aminotransferase 3.0× ULN or less.
2.3. Study assessments
2.3.1. Safety
The primary objective was to evaluate the tolerability and safety of TAS0313 according to the frequency of AEs and ADRs. These were summarized by system organ class and preferred term using the Medical Dictionary for Regulatory Activities, and their severity was graded according to the Common Terminology Criteria for Adverse Events version 4.03. If an ADR met the tolerability evaluation criteria within cycle 1, it was judged to be a dose‐limiting toxicity (DLT). The tolerability evaluation criteria for hematologic toxicity (defined as anemia, bone marrow hypocellular, lymphocyte count decreased, neutrophil count decreased, white blood cell count decreased, platelet count decreased, CD4 lymphocytes decreased, pancytopenia, and febrile neutropenia) were as follows: (i) a grade 4 event; (ii) febrile neutropenia (absolute neutrophil count less than 1000/mm3 with a single body temperature of more than 38.3°C or sustained fever of 38°C or higher for more than 1 hour); (iii) anemia requiring blood transfusion; and (iv) thrombocytopenia requiring blood transfusion. Additional tolerability evaluation criteria were nonhematologic toxicity of grade 3 or higher (except injection site reaction) and inability to start treatment within 14 days from the scheduled day 1 of cycle 2 due to a single ADR of grade 2 or higher.
2.3.2. Efficacy
The secondary objectives were assessment of clinical efficacy and tumor responses, evaluated according to RECIST version 1.1 and iRECIST. 23 Clinical efficacy was evaluated using the overall response rate (ORR), disease control rate (DCR), duration of response (DOR), progression‐free survival (PFS), and 6‐ and 12‐ month progression‐free rates (PFR). The best overall response in target lesions was evaluated by stable disease (SD; absence of tumor shrinkage corresponding to complete response [CR; complete disappearance of nonnodal target lesions] or partial response [decrease of 30% or more in diameters of target lesions compared with baseline] and absence of tumor growth corresponding to progressive disease [increase of 20% or more and 5 mm or more in diameters of target lesions compared with baseline]) and %DCR.
2.4. Immunological assays
The immune activation effect of TAS0313 was confirmed by measuring CTL epitope peptide‐specific counts and peptide‐specific IgG concentrations normalized by rabbit IgG standard samples in blood samples of patients; amino acid sequence of peptides and full experimental details are provided in Tables S2 and S3, respectively. At baseline, day 22 of cycle 2, and day 22 of cycle 3, 15 and 2 mL of blood were collected into two separate blood collection tubes containing an anticoagulant. One tube (15 mL) was for CTL measurement and the other (2 mL) for IgG measurement.
At baseline and day 22 of cycle 2, tumor‐infiltrating CD8+ T lymphocytes (TILs) were counted in tumor tissues by immunohistochemical staining, which was undertaken by SRL Laboratories. Tumor‐infiltrating CD8+ T lymphocytes in formalin‐fixed, paraffin‐embedded tissues were evaluated by immunohistochemical staining of slides with anti‐CD8 (SP57) rabbit monoclonal primary Ab (Roche Diagnostics) and Ventana iVIEW DAB Universal Kit (Roche Diagnostics) according to the manufacturer’s instructions. Two fields in hotspots with CD8 T cell infiltration at the tumor site were selected and CD8+ cells were counted.
The mRNA expression levels of immunological factors and target cancer‐associated antigens were measured at baseline from formalin‐fixed, paraffin‐embedded tissues by RikenGenesis using a modified nCounter PanCancer IO 360 Gene Expression Panel (nanoString) according to the manufacturer’s instructions. Measurement of TAS0313 target cancer antigens could be carried out in this modified panel by adding sets of probes.
2.5. Statistical analysis
A sample size of up to 30 tolerability‐evaluable patients was considered sufficient to examine the tolerability and safety of TAS0313.
The statistical analyses of the data reported herein were undertaken at the end of the phase I portion of the study; that is, data were analyzed when all patients enrolled in the phase I portion completed the study. The statistical software used for all the statistical analyses was SAS version 9.4 (SAS Institute).
The safety analysis set included all enrolled patients who received TAS0313 at least once. Tolerability‐evaluable patients were those in the safety analysis set in cycle 1, excluding patients who met any of the following conditions: used a prohibited concomitant medication or therapy before the onset of an ADR meeting the tolerability confirmation criteria, or who had no ADR meeting the tolerability confirmation criteria and failed to receive TAS0313 as specified in the protocol (days 1, 8, and 15 in cycle 1), or who did not undergo examination or observation. Adverse events and ADRs were described according to incidence, number of patients, and highest grade.
For efficacy analyses, the full analysis set (FAS) included all treated patients who had confirmed HLA‐A*02:01, ‐A*02:06, ‐A*02:07, ‐A*11:01, ‐A*24:02, ‐A*31:01, or ‐A*33:03, and histologically or cytologically confirmed advanced or metastatic solid tumor(s) for which no therapies were available at the time of study enrollment. The ORR and DCR were estimated with 95% confidence intervals (CI) by summarizing the best overall response. Time‐to‐event end points were described using the Kaplan‐Meier method to calculate the median and 95% CI.
The CTL cut‐off value of more than 250 spots/100 000 cells was set based on the difference in mean + three SDs between the negative control samples of the overall patient population. For the analysis of CTL induction, we calculated the change between the negative control samples taken pretreatment and the antigen stimulation samples taken at cycle 2 day 22 and cycle 3 day 22. We also normalized the changes to the pretreatment sample.
3. RESULTS
3.1. Patients and characteristics
For the phase I portion of the study, the period of patient recruitment was from 30 January 2018 to 5 September 2018, during which time 17 patients (10 in the 9‐mg group and seven in the 27‐mg group) were registered. Of the first six patients enrolled in the 9‐ and 27‐mg groups, one patient did not complete the treatment with TAS0313 in cycle 1, because of progression of the primary disease. Therefore, one more patient was added to each group. In six of seven patients in the 9‐mg group, the immune response using tumor tissue before and after treatment could not be evaluated because of exacerbation of the condition in three patients, consent for the biopsy could not be obtained in two patients, and lack of tumor in the tissue sample in one patient. Therefore, three more patients were added to the 9‐mg group; one of the three patients did not complete the TAS0313 treatment in cycle 1. Of the tolerability‐evaluable patients, there were eight patients in the 9‐mg TAS0313 group and six patients in the 27‐mg TAS0313 group. The FAS contained 10 and seven patients, respectively.
Patient characteristics of the safety analysis set are shown in Table 1. Age and PS were similar between the 9‐ and 27‐mg groups in phase I; lymphocyte counts were also similar between the dose groups.
TABLE 1.
Demographics and disease characteristics at baseline (safety analysis set) in patients with advanced solid tumors treated with cancer peptide vaccine TAS0313
|
TAS0313 9 mg dose (n = 10) |
TAS0313 27 mg dose (n = 7) |
|
|---|---|---|
| Sex | ||
| Male | 3 (30.0) | 5 (71.4) |
| Female | 7 (70.0) | 2 (28.6) |
| Age (y) | ||
| Median (range) | 64.5 (47, 70) | 65.0 (49, 67) |
| Height (cm) | ||
| Median (range) | 160.10 (138.6, 171.8) | 170.20 (150.0, 173.4) |
| Weight (kg) | ||
| Median (range) | 57.05 (45.1, 82.0) | 52.40 (44.3, 86.8) |
| ECOG PS | ||
| 0 | 5 (50.0) | 3 (42.9) |
| 1 | 5 (50.0) | 4 (57.1) |
| Cancer type | ||
| Biliary tract | 1 (10.0) | 1 (14.3) |
| Breast | 1 (10.0) | 0 (0.0) |
| Lung | 1 (10.0) | 1 (14.3) |
| Ovary | 1 (10.0) | 0 (0.0) |
| Pancreas | 3 (30.0) | 1 (14.3) |
| Carcinoma of unknown primary | 1 (10.0) | 0 (0.0) |
| Gall bladder | 1 (10.0) | 1 (14.3) |
| Retiform hemangioendothelioma | 1 (10.0) | 0 (0.0) |
| Palatal gingiva cancer | 0 (0.0) | 1 (14.3) |
| Duodenal papilla neuroendocrine carcinoma | 0 (0.0) | 1 (14.3) |
| Peritoneum mesothelioma | 0 (0.0) | 1 (14.3) |
| Histologic type | ||
| Adenocarcinoma | 4 (40.0) | 4 (57.1) |
| Small cell | 1 (10.0) | 0 (0.0) |
| Serous adenocarcinoma | 1 (10.0) | 0 (0.0) |
| Mucinous adenocarcinoma | 1 (10.0) | 0 (0.0) |
| Other | 3 (30.0) | 3 (42.9) |
| HLA genotyping test | ||
| A*02:01 | 3 (30.0) | 0 (0.0) |
| A*02:06 | 0 (0.0) | 2 (28.6) |
| A*02:07 | 1 (10.0) | 0 (0.0) |
| A*11:01 | 2 (20.0) | 2 (28.6) |
| A*24:02 | 7 (70.0) | 4 (57.1) |
| A*31:01 | 2 (20.0) | 2 (28.6) |
| A*33:03 | 0 (0.0) | 2 (28.6) |
| Other | 4 (40.0) | 1 (14.3) |
| Lymphocytes (per μL) | ||
| Median (range) | 939.60 (476.0, 1969.8) | 1144.00 (795.6, 1752.3) |
| History of surgery | ||
| No | 5 (50.0) | 3 (42.9) |
| Yes | 5 (50.0) | 4 (57.1) |
| Prior radiation therapy | ||
| No | 5 (50.0) | 5 (71.4) |
| Yes | 5 (50.0) | 2 (28.6) |
| Prior systemic drug therapies | ||
| Neoadjuvant | ||
| No | 9 (90.0) | 6 (85.7) |
| Yes | 1 (10.0) | 1 (14.3) |
| Adjuvant | ||
| No | 6 (60.0) | 7 (100.0) |
| Yes | 4 (40.0) | 0 (0.0) |
| Advanced/metastatic | ||
| Yes | 10 (100.0) | 7 (100.0) |
Abbreviations: HLA, human leukocyte antigen; PS, performance status.
3.2. Safety and tolerability
No DLTs met the tolerability criteria in cycle 1 in the tolerability evaluable population. Adverse drug reactions are summarized by dose in Table S4. The most common ADRs were injection site‐related events (which included injection site dermatitis, injection site pruritus, injection site rash, injection site reaction, and injection site ulcer) occurring in 70.0% of patients in the 9‐mg group and 85.7% in the 27‐mg group, and pyrexia, which occurred in 10.0% and 28.6%, respectively. All ADRs were grade 1 or 2. No serious ADRs (grade 3 or more) were observed in any patient, and no deaths or ADRs leading to treatment discontinuation occurred during the study period.
3.3. Clinical responses
No patients required assessment using iRECIST. Regarding the clinical responses by dose, in the 9‐mg group, no complete or partial responses were observed, two of 10 patients had a best overall response of SD, and the DCR was 20.0%. In the 27‐mg group, no complete or partial responses were observed, two of seven patients had a best overall response of SD, and the DCR was 28.6%. A total of two patients (one in the 9‐mg group and one in the 27‐mg group) had SD for more than 6 months (data not shown). The patient in the 9‐mg group had carcinoma of unknown primary and the patient in the 27‐mg group had palatal gingiva cancer. Furthermore, there was one case that showed tumor shrinkage of 15.5% in a patient with pancreatic cancer (Figure S1). As there were no complete or partial responses, DOR was not calculated.
The median PFS was 1.8 (95% CI, 0.4‐2.3) months in the 9‐mg group, 2.2 (95% CI, 0.6‐3.2) months in the 27‐mg group, and 2.2 (95% CI, 1.0‐2.3) months in all patients. Of note, patients with low lymphocyte counts (750/μL or lower) tended to have a shorter PFS (Figure 1). The 6‐month PFR was 10.0% (95% CI, 0.6‐35.8) for the 9‐mg group, 14.3% (95% CI, 0.7‐46.5) for the 27‐mg group, and 11.8% (95% CI, 2.0‐31.2) in all patients. The 12‐month PFR was 0% (95% CI not calculated) for the 9‐mg group, the 27‐mg group, and for all patients.
FIGURE 1.
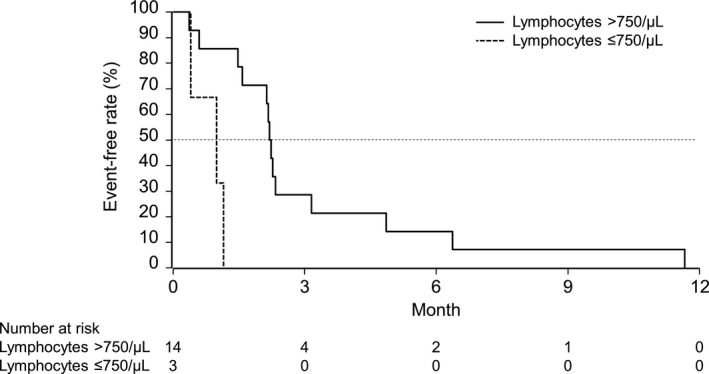
Progression‐free survival in patients with advanced solid tumors treated with cancer peptide vaccine TAS0313 in the phase I portion of the study, according to lymphocyte count (full analysis set)
3.4. Immune responses
There were no clear correlative patterns between immune response and ORR (Table 2).
TABLE 2.
Correlation between immunological response and overall response rate (full analysis set) in patients with advanced solid tumors treated with cancer peptide vaccine TAS0313
| Dose (mg) | Patient | Cancer | HLA‐A | IgG a | CTL b | TIL c | Antigen d | ORR |
|---|---|---|---|---|---|---|---|---|
| 9 | 1 | Biliary tract | 24 | + | − | ND | 8 | PD |
| 2 | Breast | 11 | + | − | ND | ND | PD | |
| 3 | Lung | 02 | + | − | ND | 7 | PD | |
| 4 | Carcinoma of unknown primary | 24 | + | + | ND | ND | SD | |
| 5 | Ovary | 11 | − | − | ND | ND | PD | |
| 6 | Pancreas | 24, 31 | + | − | ND | ND | PD | |
| 7 | Gall bladder | 02, 24 | + | + | − | 8 | PD | |
| 8 | Pancreas | 02, 24 | + | − | + | 8 | PD | |
| 9 | Pancreas | 24, 31 | + | − | ND | 8 | PD | |
| 10 | Retiform hemangioendothelioma | 02, 24 | + | − | + | 8 | SD | |
| 27 | 1 | Palatal gingiva cancer | 31, 33 | + | − | − | 8 | SD |
| 2 | Biliary tract | 24 | + | + | ND | 8 | PD | |
| 3 | Pancreas | 02 | + | − | ND | 7 | SD | |
| 4 | Lung | 11, 24 | + | + | + | 8 | PD | |
| 5 | Gall bladder | 02, 24 | + | + | ND | 8 | PD | |
| 6 | Duodenal papilla neuroendocrine carcinoma | 11, 31 | + | − | + | 8 | PD | |
| 7 | Peritoneum mesothelioma | 24, 33 | + | − | + | 8 | PD |
Abbreviations: HLA, human leukocyte antigen; ND, not determined (samples unavailable); ORR, overall response rate; PD, progressive disease; SD, stable disease; TIL, tumor‐infiltrating lymphocyte.
At least one IgG level was ≥30%, compared with baseline.
≥250 spots/100 000 cells.
Increased by 1 or more compared with baseline.
Target cancer‐associated antigen expression‐positive number out of eight antigens (Table S5).
3.4.1. Cytotoxic T lymphocytes
Human leukocyte antigen‐matched peptide cocktail‐specific CTL induction was assessed, because the required blood volume to undertake full analysis of each epitope peptide‐specific CTL measurement was limited. Cytotoxic T lymphocyte induction was defined as patients who had more than 250 spots/100 000 cells in a study period; induction was confirmed in two subjects in the 9‐mg group and three subjects in the 27‐mg group (Figure 2; Table 2).
FIGURE 2.
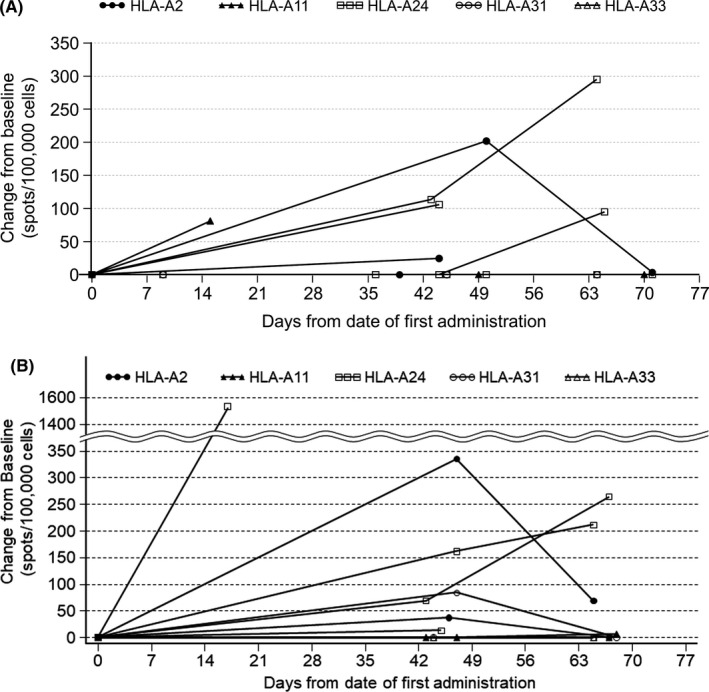
Induction of CTLs by TAS0313 showing the change from pretreatment in CTL spot number for each patient with advanced solid tumor. A, 9‐mg dose group. B, 27‐mg dose group (patients evaluable for pharmacodynamics analysis). Days from date of first treatment = (measurement date) − (date of first administration) + 1. When change from baseline was less than zero, it was treated as zero. Patients evaluable for pharmacodynamics analysis were all treated patients who had available data on CTL, IgG, or tumor‐infiltrating CD8+ T lymphocytes. HLA, human leukocyte antigen
3.4.2. Immunoglobulin G
Immunoglobulin G induction was defined as patients whose IgG to at least one epitope was elevated by more than 30% compared with baseline data; IgG induction was confirmed in nine subjects in the 9‐mg group and seven subjects in the 27‐mg group (Table 2).
3.4.3. Tumor‐infiltrating lymphocytes
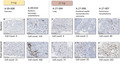
Tumor‐infiltrating lymphocyte induction was defined as patients who had TIL counts of 1 or more. Tumor‐infiltrating lymphocyte induction compared with baseline values was confirmed in two of three (66.7%) subjects in the 9‐mg group and three of four (75%) subjects in the 27‐mg group (Figure 3; Table 2).
FIGURE 3.
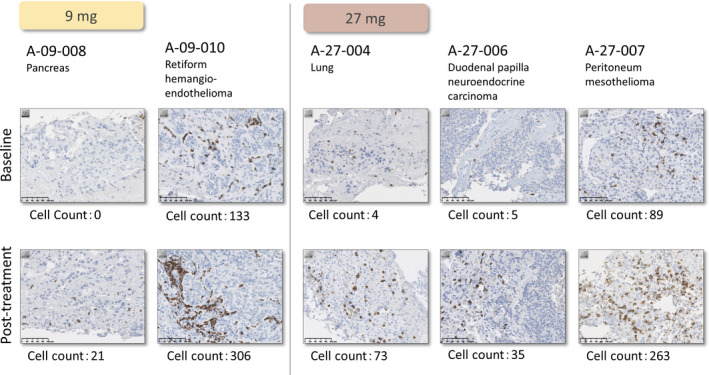
Induction of tumor‐infiltrating lymphocytes by TAS0313 dose in patients with advanced solid tumors, from baseline to posttreatment
3.4.4. Other measurements
The expression of the cancer‐related antigens targeted by TAS0313 was investigated. Six subjects in the 9‐mg group and seven subjects in the 27‐mg group showed target antigen and HLA‐A expression (Table 2). Presence of cancer‐related antigens and HLA‐A expression were defined as positive when the normalized values were three times higher than the SD of negative control values. Negative control values were measured using randomly designed eight primer sets that were included in the nCounter PanCancer IO 360 Gene Expression Panel.
4. DISCUSSION
Previously, most cancer peptide vaccines developed in Japan have used HLA‐A24‐restricted peptides because Japanese cancer patients are more likely to be HLA‐A24‐positive. 24 However, HLA allele frequency is known to vary according to race. 7 Importantly, due to the peptides it contains, TAS0313 could be an effective treatment for multiple HLA phenotypes, including patients with HLA‐A02, ‐A03, ‐A11, ‐A24, ‐A31, and ‐A33. The expression of these target cancer‐related antigens was confirmed in all patients. Based on these data, TAS0313 is expected to be effective for a wide range of patients with solid tumors.
We confirmed the tolerability of TAS0313 at 9 and 27 mg in patients in the phase I portion of the study, and, importantly, there were no differences in the safety profile of TAS0313 given at 9 or 27 mg. All ADRs in this study were grade 2 or lower, and the most common ADRs in both the 9‐ and 27‐mg groups were injection site‐related events, followed by pyrexia. There were no remarkable differences between the results of our study and those reported from previous studies using short cancer vaccines 4 ; however, as the number of cases evaluated to date is small, further investigation remains necessary.
Our data indicated that TAS0313 (9 and 27 mg) induced CTLs, IgGs, and elevated TILs compared with pretreatment, confirming the immune activation effects of TAS0313. In a phase I study on vaccination with a cancer vaccine that contained 10 or 20 mixed peptides that were compatible with various HLA genotypes, 25 , 26 peptide‐specific CTLs and IgG responses were induced. The CTL‐positive frequency in the current study seems likely to be low compared to previous reports of short peptide vaccine, although it is difficult to directly compare the results because many differences exist in methodology and the study protocols. However, considerable upregulation of peptide‐specific IgG titer and TIL infiltration were observed simultaneously. To draw a conclusion regarding the immunogenicity of TAS0313 in humans, further investigation with some improvements are required in future clinical trials.
Programmed death‐1 (PD‐1) Ab treatment is rapidly becoming the focal point of cancer treatment. However, PD‐1 Ab treatment is limited for so‐called “cold” tumors, which are poorly infiltrated by CD8+ T cells. Findings from the current study established that TIL induction was generated by treatment with TAS0313 and confirmed similar results observed in previous preclinical studies. 27 Thus, we can postulate that TAS0313 might be effective at converting “cold” tumors to T cell‐infiltrating “hot” tumors, and might have combinatorial efficacy with PD‐1 Abs. However, it is not clear whether this increase was a tumor antigen‐specific CTL. To confirm whether there is a benefit of TIL increase, we are now evaluating combination therapy with the anti‐PD‐1 Ab pembrolizumab during phase II (cohort C).
In this study, the DCR and median PFS were 20.0% and 1.8 months, respectively, in the 9‐mg group and 28.6% and 2.2 months, respectively, in the 27‐mg group. Efficacy results with cancer vaccines have been variable to date; in an open‐label, randomized phase II trial of personalized peptide vaccination in patients with bladder cancer, no improvement of PFS was reported. 3 In contrast, a phase I trial of 14 vaccine candidates (ITK‐1) in patients with recurrent or progressive glioblastoma multiforme reported a median PFS of 2.3 months and a 6‐month PFS rate of 16.7%. 4 Of note, in our study, patients with low lymphocyte counts (750/μL or fewer) tended to have a shorter PFS. Patients in this study had already exhausted other treatment options; most had received prior radiation therapy, and all had received prior chemotherapy, both of which cause lymphodepletion. Several studies have reported that a total low pretreatment lymphocyte count is associated with poor survival outcomes among patients with solid tumors receiving surgery and/or chemotherapy, radiotherapy, palliative therapy, or immune‐based therapies, including peptide tumor vaccines. 28 , 29 , 30 High neutrophil‐to‐lymphocyte ratio (NLR) tended to be associated with poor survival in several studies. 31 , 32 The current study also supported this observation in the high NLR (2.5 or higher) group (Figure S2); however, the PFS difference was not clear compared with the pretreatment lymphocyte count criterion (Figure 1). No significant differences in PFS were observed when patients were stratified by the induction of IgG or CTLs.
This study had some limitations, in addition to those already mentioned, which must be considered. These include the sample size, which was small, and a potential lack of generalizability to other patients based on differences in HLA type between Japan and other countries. In addition, due to the extensive prior treatment status and potential lymphodepletion of study patients, the necessary immune effector cells required for full vaccine effectiveness could have been reduced or eliminated, limiting the potential for optimal tumor response. 5
In summary, this phase I, first‐in‐human clinical study confirmed the safety, tolerability, and induction of immune responses in patients with advanced solid tumors receiving TAS0313 9 and 27 mg. Based on the results of the phase I portion of this study, including increased TILs, the phase II portion has been initiated. No DLTs met the tolerability criteria, and no clear dose dependence was observed in the efficacy. Therefore, based on the differences in immune activation effect between 9 and 27 mg, the highest dose of 27 mg was selected for monotherapy for glioblastoma, and 9 mg was selected for urothelial carcinoma for which pembrolizumab combination therapy was given.
CONFLICT OF INTEREST
T. Shimizu reports other fees from Novartis, Eli Lilly, Bristol‐Myers Squibb, Daiichi‐Sankyo, Millennium‐Takeda, AstraZeneca, Eisai, AbbVie, Incyte, Astellas Pharma, Symbio Pharmaceuticals, 3D‐Medicine, Five Prime, PharmaMar, and Chordia Therapeutics outside the submitted work. T. Koyama reports other fees from Chugai and Sysmex outside the submitted work. S. Iwasa reports personal fees from Taiho and Ono, and research funding from Bristol‐Myers Squibb, Merck Biopharma, and Astellas outside the submitted work. Y. Fujiwara reports research funding from AbbVie, Eli Lilly, and Incyte, and research funding and personal fees from AstraZeneca, Bristol‐Myers Squibb, Chugai, Daiichi‐Sankyo, MSD, and Novartis outside the submitted work. A. Shimomura reports research funding and personal fees from Chugai, AstraZeneca, and Daiichi Sankyo, and personal fees from Pfizer, Eli‐Lilly, Eisai, and Novartis outside the submitted work. S. Kitano reports research funding from Astellas, Gilead Sciences, AMED (Japanese Agency for Medical Research and Development), and JSPS (Japanese Society for the Promotion of Science), research funding and personal fees from Boehringer Ingelheim, Eisai, Ono, and REGENERON, and personal fees from AstraZeneca, Chugai, Pfizer, Sanofi, Nippon Kayaku, Meiji Seika Pharma, Taiho, Novartis, Daiichi‐Sankyo, MSD, Kyowa Hakko Kirin, Celgene, Sumitomo Dainippon Pharma, Bristol‐Myers Squibb, AYUMI Pharmaceutical Corporation, Rakuten Medical, PMDA (Japanese Pharmaceuticals and Medical Devices Agency), and GlaxoSmithKline outside the submitted work. N. Yamamoto reports research funding from Taiho, Quintiles, Astellas, Novartis, Daiichi‐Sankyo, Kyowa‐Hakko Kirin, Bayer, Janssen Pharma, MSD, Merck, and GlaxoSmithKline, research funding and personal fees from Ono, Chugai, Pfizer, Eli Lilly, Bristol‐Myers Squibb, Eisai, Takeda, and Boehringer Ingelheim, and personal fees from AstraZeneca, Otsuka, Cimic, and Sysmex outside the submitted work. No potential conflicts of interest were disclosed by the other authors. This study was funded by Taiho Pharmaceutical Co., Ltd., Tokyo, Japan. The study sponsor, Taiho Pharmaceutical Co., Ltd., contributed to the study design, the collection, analysis, and interpretation of data, the writing of the report, and in the decision to submit the paper for publication.
Supporting information
Fig S1‐S2
Table S1
Table S2
Table S3
Table S4
Table S5
ACKNOWLEDGMENTS
The study was sponsored by Taiho Pharmaceutical Co., Ltd. The authors thank Sally‐Anne Mitchell, PhD, of Edanz Medical Writing for providing medical writing services.
Kondo S, Shimizu T, Koyama T, et al. First‐in‐human study of the cancer peptide vaccine TAS0313 in patients with advanced solid tumors. Cancer Sci.2021;112:1514–1523. 10.1111/cas.14765
Study registration: JapicCTI‐183824
Protocol Number: 10 068 010
REFERENCES
- 1. Thomas S, Prendergast GC. Cancer vaccines: a brief overview. Methods Mol Biol. 2016;1403:755‐761. [DOI] [PubMed] [Google Scholar]
- 2. Zhang L, Huang Y, Lindstrom AR, Lin TY, Lam KS, Li Y. Peptide‐based materials for cancer immunotherapy. Theranostics. 2019;9:7807‐7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Noguchi M, Matsumoto K, Uemura H, et al. An open‐label, randomized phase II trial of personalized peptide vaccination in patients with bladder cancer that progressed after platinum‐based chemotherapy. Clin Cancer Res. 2016;22:54‐60. [DOI] [PubMed] [Google Scholar]
- 4. Terasaki M, Shibui S, Narita Y, et al. Phase I trial of a personalized peptide vaccine for patients positive for human leukocyte antigen‐A24 with recurrent or progressive glioblastoma multiforme. J Clin Oncol. 2011;29:337‐344. [DOI] [PubMed] [Google Scholar]
- 5. Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909‐915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Slingluff CL Jr. The present and future of peptide vaccines for cancer: single or multiple, long or short, alone or in combination? Cancer J. 2011;17:343‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Middleton D, Williams F, Meenagh A, et al. Analysis of the distribution of HLA‐A alleles in populations from five continents. Hum Immunol. 2000;61:1048‐1052. [DOI] [PubMed] [Google Scholar]
- 8. Sabbatini P, Tsuji T, Ferran L, et al. Phase I trial of overlapping long peptides from a tumor self‐antigen and poly‐ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clin Cancer Res. 2012;18:6497‐6508. [DOI] [PubMed] [Google Scholar]
- 9. Takahashi N, Ohkuri T, Homma S, et al. First clinical trial of cancer vaccine therapy with artificially synthesized helper/killer‐hybrid epitope long peptide of MAGE‐A4 cancer antigen. Cancer Sci. 2012;103:150‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leffers N, Lambeck AJA, Gooden MJM, et al. Immunization with a P53 synthetic long peptide vaccine induces P53‐specific immune responses in ovarian cancer patients, a phase II trial. Int J Cancer. 2009;125:2104‐2113. [DOI] [PubMed] [Google Scholar]
- 11. Rahat MA. Targeting angiogenesis with peptide vaccines. Front Immunol. 2019;10:1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakao M, Shichijo S, Imaizumi T, et al. Identification of a gene coding for a new squamous cell carcinoma antigen recognized by the CTL. J Immunol. 2000;164(5):2565‐2574. [DOI] [PubMed] [Google Scholar]
- 13. Minami T, Matsueda S, Takedatsu H, et al. Identification of SART3‐derived peptides having the potential to induce cancer‐reactive cytotoxic T lymphocytes from prostate cancer patients with HLA‐A3 supertype alleles. Cancer Immunol Immunother. 2007;56(5):689‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang D, Nakao M, Shichijo S, et al. Identification of a gene coding for a protein possessing shared tumor epitopes capable of inducing HLA‐A24‐restricted cytotoxic T lymphocytes in cancer patients. Cancer Res. 1999;59(16):4056‐4063. [PubMed] [Google Scholar]
- 15. Ito M, Shichijo S, Miyagi Y, et al. Identification of SART3‐derived peptides capable of inducing HLA‐A2‐restricted and tumor‐specific CTLs in cancer patients with different HLA‐A2 subtypes. Int J Cancer. 2000;88(4):633‐639. [DOI] [PubMed] [Google Scholar]
- 16. Yamada A, Kawano K, Koga M, Matsumoto T, Itoh K. Multidrug resistance‐associated protein 3 is a tumor rejection antigen recognized by HLA‐A2402‐restricted cytotoxic T lymphocytes. Cancer Res. 2001;61(17):6459‐6466. [PubMed] [Google Scholar]
- 17. Shomura H, Shichijo S, Komatsu N, et al. Identification of epidermal growth factor receptor‐derived peptides recognised by both cellular and humoral immune responses in HLA‐A24+ non‐small cell lung cancer patients. Eur J Cancer. 2004;40(11):1776‐1786. [DOI] [PubMed] [Google Scholar]
- 18. Ito M, Shichijo S, Tsuda N, et al. Molecular basis of T cell‐mediated recognition of pancreatic cancer cells. Cancer Res. 2001;61(5):2038‐2046. [PubMed] [Google Scholar]
- 19. Imai N, Harashima N, Ito M, et al. Identification of Lck‐derived peptides capable of inducing HLA‐A2‐restricted and tumor‐specific CTLs in cancer patients with distant metastases. Int J Cancer. 2001;94(2):237‐242. [DOI] [PubMed] [Google Scholar]
- 20. Naito M, Komohara Y, Ishihara Y, et al. Identification of Lck‐derived peptides applicable to anti‐cancer vaccine for patients with human leukocyte antigen‐A3 supertype alleles. Br J Cancer. 2007;97(12):1648‐1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harashima N, Tanaka K, Sasatomi T, et al. Recognition of the Lck tyrosine kinase as a tumor antigen by cytotoxic T lymphocytes of cancer patients with distant metastases. Eur J Immunol. 2001;31(2):323‐332. [DOI] [PubMed] [Google Scholar]
- 22. Yao A, Harada M, Matsueda S, et al. Identification of parathyroid hormone‐related protein‐derived peptides immunogenic in human histocompatibility leukocyte antigen‐A24+ prostate cancer patients. Br J Cancer. 2004;91(2):287‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143‐e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nukaya I, Yasumoto M, Iwasaki T, et al. Identification of HLA‐A24 epitope peptides of carcinoembryonic antigen which induce tumor‐reactive cytotoxic T lymphocyte. Int J Cancer. 1999;80:92‐97. [DOI] [PubMed] [Google Scholar]
- 25. Iwasa S, Yamada Y, Heike Y, et al. Phase I study of a new cancer vaccine of ten mixed peptides for advanced cancer patients. Cancer Sci. 2016;107:590‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Noguchi M, Arai G, Matsumoto K, et al. Phase I trial of a cancer vaccine consisting of 20 mixed peptides in patients with castration‐resistant prostate cancer: dose‐related immune boosting and suppression. Cancer Immunol Immunother. 2015;64:493‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tanaka Y, Wada H, Goto R, et al. TAS0314, a novel multi‐epitope long peptide vaccine, showed synergistic antitumor immunity with PD‐1/PD‐L1 blockade in HLA‐A*2402 mice. Sci Rep. 2020;10(1):17284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schueneman AJ, Sugar EA, Uram J, et al. Low total lymphocyte count is associated with poor survival in patients with resected pancreatic adenocarcinoma receiving a GM‐CSF secreting pancreatic tumor vaccine. Ann Surg Oncol. 2013;20(Suppl 3):S725‐S730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao J, Huang W, Wu Y, et al. Prognostic role of pretreatment blood lymphocyte count in patients with solid tumors: a systematic review and meta‐analysis. Cancer Cell Int. 2020;20:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Suekane S, Noguchi M, Terasaki M, et al. Biomarkers predictive of overall survival in advanced cancer patients treated with a peptide‐based cancer vaccine. Ann Oncology. 2019;30(Suppl 5):43. Abstract 137P. [Google Scholar]
- 31. Hazama S, Takenouchi H, Tsunedomi R, et al. Predictive biomarkers for the outcome of vaccination of five therapeutic epitope peptides for colorectal cancer. Anticancer Res. 2014;34(8):4201‐4205. [PubMed] [Google Scholar]
- 32. Hazama S, Nakamura Y, Tanaka H, et al. A phase ΙI study of five peptides combination with oxaliplatin‐based chemotherapy as a first‐line therapy for advanced colorectal cancer (FXV study). J Transl Med. 2014;12:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S2
Table S1
Table S2
Table S3
Table S4
Table S5


