Abstract
Metastatic burden is a critical factor for therapy decision‐making in metastatic hormone‐sensitive prostate cancer. The present study aimed to identify prognostic factors in men with high‐ or low‐metastatic burden treated with primary androgen‐deprivation therapy. The study included 2450 men with de novo metastatic prostate cancer who were treated with primary androgen‐deprivation therapy at 30 institutions across Japan between 2008 and 2017. We investigated the prognostic value of various clinicopathological parameters for progression‐free survival (PFS) and overall survival (OS) in patients stratified by low‐ or high‐metastatic burden. Among the 2450 men, 841 (34.3%) and 1609 (65.7%) were classified as having low‐ and high‐metastatic burden, respectively. Median PFS of the low‐ and high‐burden groups were 44.5 and 16.1 months, respectively, and the median OS was 103.2 and 62.7 months, respectively. Percentage of biopsy‐positive core, biopsy Gleason grade group, T‐stage, and N‐stage were identified to be differentially prognostic. M1a was associated with worse PFS than was M1b in the low‐burden group, whereas lung metastasis was associated with better PFS and OS than was M1b in the high‐burden group. Differential prognostic factors were identified for patients with low‐ and high‐burden metastatic prostate cancer. These results may assist in decision‐making to select the optimal therapeutic strategies for patients with different metastatic burdens.
Keywords: androgen‐deprivation therapy, hormone‐sensitive prostate cancer, metastatic burden, metastatic prostate cancer, prognostic factor
This study investigated prognostic factors according to metastatic burden, and found that prognostic impact of some factors was different. This finding may be valuable in decision‐making to select the optimal therapeutic strategies for patients with different metastatic burdens.

1. INTRODUCTION
The CHAARTED and STAMPEDE (arm C) trials of docetaxel chemotherapy with androgen‐deprivation therapy (ADT) showed beneficial effects on both progression‐free survival (PFS) and overall survival (OS) in men with metastatic hormone‐sensitive prostate cancer (HSPC). 1 , 2 , 3 Since then, survival benefit has also been demonstrated with the cytochrome P450 17α‐hydroxylase/17,20‐lyase (CYP17) inhibitor abiraterone (LATITUDE and STAMPEDE [arm G] trials) and the novel antiandrogens enzalutamide (ENZAMET trial) and apalutamide (TITAN trial) with ADT. 4 , 5 , 6 , 7 Two randomized trials, HORRAD and STAMPEDE (arm H), have additionally shown a potential survival benefit with prostate‐targeted radiotherapy for patients with low metastatic burden HSPC. 8 , 9 Based on these findings, novel therapeutic modalities combined with ADT are now recommended therapeutic options for metastatic HSPC. 10
The survival benefits of these novel therapies differ according to tumor aggressiveness, burden, and spread. In the CHAARTED trial, upfront docetaxel chemotherapy with ADT significantly prolonged the survival of patients with high‐volume disease (defined as visceral metastasis or ≥4 bone metastases beyond the vertebrae and pelvis), but it is not yet known whether the same regimen increases the survival of patients with low‐volume disease. 11 In this regard, although the STAMPEDE (arm C) trial suggested comparable survival benefits for patients with low‐ and high‐volume disease, the effects did not achieve statistical significance. 12 Local radiotherapy has been shown to benefit patients with oligometastatic, but not high‐volume metastatic, HSPC. The STOPCAP meta‐analysis of the HORRAD and STAMPEDE (arm H) trials showed improved survival of men with ≤4 bone metastases in response to local radiotherapy. 13 Then, androgen receptor pathway inhibitors (ARPI) and/or radical treatment are currently recommended therapeutic options for oligometastatic HSPC, whereas the benefit of metastasis‐directed therapies remains under investigation. ARPI or docetaxel are recommended therapeutic options for high‐volume HSPC. 14
The results of these studies suggest that metastatic burden should be taken into account when selecting therapeutic strategies for patients with metastatic HSPC. However, it is not known whether the prognostic factors differ for patients with low‐ and high‐burden metastatic HSPC. Here, we report the results of a multi‐institutional study in Japan to investigate prognostic factors differentially associated with PFS and OS in patients with de novo metastatic HSPC with low‐ or high‐metastatic burden.
2. MATERIALS AND METHODS
2.1. Patients
This study retrospectively enrolled patients who were newly diagnosed with de novo metastatic prostate cancer between 2008 and 2017 at 30 institutions, mainly academic hospital and cancer centers, participating in the Japanese Urological Oncology Group (JUOG). 15 The study was approved by the institutional review board of each institute. 15 All patients were pathologically diagnosed with adenocarcinoma of the prostate, and distant metastasis was detected by computed tomography and bone scans performed at the time of diagnosis. Of the 2829 patients with metastatic prostate cancer described in our previous report, 15 we excluded 379 patients with (i) unknown number of bone metastases, (ii) unknown M1 sub‐stage, (iii) unknown/undetermined Gleason grade group, or pathological diagnosis other than adenocarcinoma, (iv) initial treatment (eg, upfront docetaxel and upfront ARPI) other than castration monotherapy or combined androgen blockade (CAB), or (vi) unknown prognosis. In total, data were analyzed for 2450 patients.
2.2. Methods
Demographic, clinicopathological, and survival data were obtained from medical records. Clinical staging was determined by the unified TNM criteria. 16 Gleason grade categories at initial diagnosis were evaluated as <3 + 4 = 7 (I); 3 + 4 (II); 4 + 3 (III); 4 + 4 (IV); or 9‐10 (V). Gleason scores 3 + 5 and 5 + 3 were included in category IV, according to a previous report. 17 Patients were dichotomized into low‐burden (lymph node metastasis and/or <4 bone metastases without visceral metastasis) and high‐burden (≥4 bone metastases and/or visceral metastasis) groups. Hormone therapy was given as castration monotherapy (surgical or medical castration) or CAB (surgical or medical castration plus a first‐generation nonsteroidal antiandrogen [bicalutamide and flutamide]). PFS was calculated from the date of diagnosis to the date of progression, defined as PSA levels increased by 25% from nadir and higher than 2.0 ng/mL, and OS was calculated as the date of diagnosis to the date of death due to any cause. Surviving patients without disease progression were censored at the last follow‐up visit.
2.3. Statistical analysis
All analyses were carried out using JMP13 software (SAS Institute, Cary, NC, USA). Continuous and categorical data were analyzed by Wilcoxon rank sum and Pearson’s chi squared tests, respectively. Survival analyses were conducted by the Kaplan‐Meier method and log‐rank test. A Cox proportional hazards model was used to estimate hazard ratios (HR). The differential prognostic value of clinicopathological parameters was investigated through interaction tests. All P values were two‐sided; P < .05 was considered significant.
3. RESULTS
Of the 2450 men retrospectively analyzed, 841 (34.3%) and 1609 (65.7%) were classified as having low‐ and high‐burden disease, respectively. When patient characteristics were compared, pain level, PSA value, percentage of biopsy positive cores, biopsy Gleason grade group, T‐stage, N‐stage, M‐stage, and extent of disease (EOD) score were all less favorable for patients in the high‐burden compared with the low‐burden group (Table 1). Most patients (86.7%) were treated with CAB, and a minority (n = 342; 14.0%) received local radiotherapy. Among the patients with lung (n = 268) or liver (n = 33) metastasis, 198 (73.9%) and 27 (81.8%), respectively, also had bone metastasis. None of the patients had concomitant lung and liver metastases.
TABLE 1.
Patient characteristics stratified by metastatic burden
| Variable | Metastasis burden | P‐value | |
|---|---|---|---|
| Low burden (n = 841) | High burden (n = 1609) | ||
| Age at diagnosis, years (IQR) | 73 (67‐79) | 73 (66‐78) | .084 |
| NA | 3 | 5 | |
| Pain | |||
| Absence | 758 (91.7%) | 1150 (72.5%) | |
| Presence | 69 (8.3%) | 436 (27.5%) | <.0001* |
| NA | 14 | 23 | |
| PSA value at diagnosis, ng/mL (IQR) | 80.4 (26.1‐249) | 365 (105‐1078) | <.0001* |
| NA | 5 | 8 | |
| Percentage of biopsy positive core, n (%) | |||
| ≤66% | 268 (33.1%) | 371 (23.9%) | |
| >66% | 542 (66.9%) | 1184 (76.1%) | <.0001* |
| NA | 31 | 54 | |
| Biopsy Gleason grade group, n (%) | |||
| Group ≤ IV | 348 (41.4%) | 561 (34.9%) | |
| Group V | 493 (58.6%) | 1048 (65.1%) | .0015* |
| Clinical T‐stage, n (%) | |||
| T1/2 | 169 (20.5%) | 218 (13.9%) | |
| T3 | 465 (56.3%) | 815 (52.0%) | |
| T4 | 192 (23.2%) | 535 (34.1%) | <.0001* |
| NA | 15 | 41 | |
| Clinical N‐stage, n (%) | |||
| N0 | 342 (41.0%) | 589 (36.9%) | |
| N1 | 492 (59.0%) | 1009 (63.1%) | .046* |
| NA | 7 | 11 | |
| Clinical M‐stage, n (%) | |||
| M1a | 188 (22.4%) | – | |
| M1b | 653 (77.6%) | 1308 (81.3%) | |
| M1c (lung) | – | 268 (16.7%) | |
| M1c (liver) | – | 33 (2.1%) | <.0001* |
| EOD score, n (%) | |||
| EOD1 | 653 (100%) | 318 (20.7%) | |
| EOD2 | – | 623 (40.6%) | |
| EOD3 | – | 433 (28.2%) | |
| EOD4 | – | 159 (10.4%) | <.0001* |
| Therapeutic modality, n (%) | |||
| Castration | 125 (14.9%) | 201 (12.5%) | |
| CAB | 716 (85.1%) | 1408 (87.5%) | .10 |
Abbreviations: CAB, combined androgen blockade; EOD, extent of disease; IQR, interquartile range; PSA, prostate‐specific antigen.
Statistically significant.
During follow up, 424 (50.4%) and 231 (27.5%) patients experienced disease progression and death due to any cause in the low‐burden group while 1109 (68.9%) and 661 (41.1%) patients experienced disease progression and death due to any cause in the high‐burden group, respectively. Median follow‐up time for men alive at the date of censor was 39.2 months (interquartile range [IQR], 22.1‐66.8 months). Median PFS and OS were 21.3 months (95% confidence interval [CI] 20.1‐23.1 months) and 76.3 months (95% CI 70.4‐82.0 months), respectively. In the low‐ and high‐burden groups, the median PFS was 44.5 months (95% CI 36.9‐51.9 months) and 16.1 months (95% CI 15.0‐17.5 months), respectively (P < .0001; Figure 1A), and the median OS was 103.2 months (95% CI 94.2 months–not reached) and 62.7 months (95% CI 57.0‐66.4 months), respectively (P < .0001; Figure 1B).
FIGURE 1.

Kaplan‐Meier survival analysis of men with metastatic prostate cancer stratified by metastatic burden. A and B, Progression‐free survival (A) and overall survival (B) of patients with high or low metastatic burden
Next, we analyzed the prognostic value of various parameters for PFS. PFS was significantly associated with age, PSA value, percentage of biopsy positive cores, biopsy Gleason grade group, T‐stage, and N‐stage in the low‐burden group, and with pain, PSA value, biopsy Gleason grade group, T‐stage, N‐stage, and therapeutic modality in the high‐burden group (Table 2). When interaction P values as prognostic value of clinicopathological parameters were calculated, percentage of biopsy‐positive core, biopsy Gleason grade group, T‐stage, and N‐stage were identified to be differentially prognostic for PFS. Among the patients with low metastatic burden, M1a was associated with worse PFS than was M1b (HR, 1.39; 95% CI, 1.11‐1.73; P = .0037), whereas the reverse was true for the all‐patient group (HR, 0.77; 95% CI, 0.63‐0.94; P = .0093) (Table 2). In the high‐burden group, M1c (lung metastasis) was associated with better PFS than was M1b (HR, 0.65; 95% CI, 0.55‐0.77; P < .0001), whereas PFS was comparable between patients with lung metastasis and M1b within the all‐patient group (HR, 0.86; 95% CI, 0.73‐1.02; P = .077; Table 2). High EOD score and liver metastasis were unfavorable prognostic factors for PFS in both the high‐burden and all‐patient groups (Table 2).
TABLE 2.
Associations between clinicopathological parameters and progression‐free survival
| Variable | Low burden | High burden | Interaction P‐value | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | ||
| Age at diagnosis, years | |||||||
| ≤70 | Ref | – | – | Ref | – | – | .071 |
| >70 | 0.75 | 0.62‐0.91 | .0037* | 0.93 | 0.82‐1.04 | .21 | |
| Pain | |||||||
| Absence | Ref | – | – | Ref | – | – | .42 |
| Presence | 1.10 | 0.79‐1.54 | .57 | 1.24 | 1.09‐1.41 | .0009* | |
| PSA value at diagnosis, ng/mL | |||||||
| ≤100 | Ref | – | – | Ref | – | – | .55 |
| >100, ≤500 | 1.43 | 1.15‐1.78 | .0012* | 1.21 | 1.03‐1.43 | .022* | |
| >500 | 1.69 | 1.30‐2.20 | <.0001* | 1.60 | 1.37‐1.87 | <.0001* | |
| Percentage of biopsy positive core | |||||||
| ≤66% | Ref | – | – | Ref | – | – | <.0001* |
| >66% | 2.32 | 1.84‐2.93 | <.0001* | 1.14 | 0.99‐1.32 | .064 | |
| Biopsy Gleason grade group | |||||||
| Group ≤ IV | Ref | – | – | Ref | – | – | .0071* |
| Group V | 2.33 | 1.89‐2.88 | <.0001* | 1.57 | 1.38‐1.79 | <.0001* | |
| Clinical T‐stage | |||||||
| T1/2 | Ref | – | – | Ref | – | – | .0005* |
| T3 | 1.62 | 1.21‐2.17 | .0012* | 1.06 | 0.88‐1.28 | .56 | |
| T4 | 3.31 | 2.42‐4.54 | <.0001* | 1.50 | 1.24‐1.83 | <.0001* | |
| Clinical N‐stage | |||||||
| N0 | Ref | – | – | Ref | – | – | .0028* |
| N1 | 2.03 | 1.65‐2.49 | <.0001* | 1.32 | 1.17‐1.50 | <.0001* | |
| Clinical M‐stage | |||||||
| M1a | 1.39 | 1.11‐1.73 | .0037* | – | – | – | – |
| M1b | Ref | – | – | Ref | – | – | |
| M1c (lung) | – | – | – | 0.65 | 0.55‐0.77 | <.0001* | |
| M1c (liver) | – | – | – | 1.41 | 0.94‐2.12 | .095 | |
| EOD score | |||||||
| EOD1 | – | – | – | Ref | – | – | – |
| EOD2 | – | – | – | 1.71 | 1.43‐2.04 | <.0001* | |
| EOD3 | – | – | – | 2.22 | 1.85‐2.68 | <.0001* | |
| EOD4 | – | – | – | 3.63 | 2.89‐4.55 | <.0001* | |
| Therapeutic modality | |||||||
| Castration | Ref | – | – | Ref | – | – | .97 |
| CAB | 0.78 | 0.60‐1.02 | .069 | 0.83 | 0.69‐0.997 | .046* | |
Abbreviations: CAB, combined androgen blockade; CI, confidence interval; EOD, extent of disease; HR, hazard ratio; PSA, prostate‐specific antigen.
Statistically significant.
We analyzed the prognostic factors for OS according to metastatic burden in the same manner. OS was significantly associated with pain, percentage of biopsy positive cores, biopsy Gleason grade group, T‐stage, and N‐stage in the low‐burden group, and with age, biopsy Gleason grade group, and T‐stage in the high‐burden group (Table 3). When interaction P values as prognostic value of clinicopathological parameters were calculated, only T‐stage was identified to be differentially prognostic for OS. OS was comparable between the M1a and M1b subgroups of patients with low metastatic burden (HR, 1.27; 95% CI, 0.93‐1.74; P = .13), whereas the M1a subgroup had better OS than M1b when all patients were assessed (HR, 0.73; 95% CI, 0.55‐0.97; P = .030) (Table 3). As noted for PFS, OS was better for patients with lung metastasis than with M1b in the high‐burden group (HR, 0.66; 95% CI, 0.52‐0.83; P = .0004), but it was comparable in the M1a and M1b subgroups of all patients (HR, 0.83; 95% CI, 0.66‐1.05; P = .13) (Table 3). High EOD score and liver metastasis were unfavorable prognostic factors for OS in both the high‐burden and all‐patient groups, as noted for PFS (Table 3).
TABLE 3.
Associations between clinicopathological parameters and overall survival
| Variable | Low burden | High burden | Interaction P‐value | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | ||
| Age at diagnosis, years | |||||||
| ≤70 | Ref | – | – | Ref | – | – | .85 |
| >70 | 1.16 | 0.89‐1.52 | .27 | 1.18 | 1.01‐1.37 | .040* | |
| Pain | |||||||
| Absence | Ref | – | – | Ref | – | – | .14 |
| Presence | 1.54 | 1.02‐2.31 | .039* | 1.10 | 0.93‐1.31 | .25 | |
| PSA value at diagnosis, ng/mL | |||||||
| ≤100 | Ref | – | – | Ref | – | – | .20 |
| >100, ≤500 | 1.20 | 0.90‐1.61 | .22 | 0.87 | 0.71‐1.07 | .20 | |
| >500 | 1.21 | 0.85‐1.74 | .29 | 1.03 | 0.85‐1.25 | .77 | |
| Percentage of biopsy positive core | |||||||
| ≤66% | Ref | – | – | Ref | – | – | .091 |
| >66% | 1.57 | 1.16‐2.11 | .0032* | 1.14 | 0.95‐1.37 | .17 | |
| Biopsy Gleason grade group | |||||||
| Group ≤ IV | Ref | – | – | Ref | – | – | .22 |
| Group V | 1.97 | 1.48‐2.62 | <.0001* | 1.57 | 1.33‐1.86 | <.0001* | |
| Clinical T‐stage | |||||||
| T1/2 | Ref | – | – | Ref | – | – | .021* |
| T3 | 1.25 | 0.84‐1.87 | .27 | 1.02 | 0.80‐1.30 | .88 | |
| T4 | 2.50 | 1.63‐3.82 | <.0001* | 1.33 | 1.03‐1.72 | .026* | |
| Clinical N‐stage | |||||||
| N0 | Ref | – | – | Ref | – | – | .17 |
| N1 | 1.36 | 1.04‐1.78 | .026* | 1.08 | 0.92‐1.26 | .36 | |
| Clinical M‐stage | |||||||
| M1a | 1.27 | 0.93‐1.74 | .13 | – | – | – | – |
| M1b | Ref | – | – | Ref | – | – | |
| M1c (lung) | – | – | – | 0.66 | 0.52‐0.83 | .0004* | |
| M1c (liver) | – | – | – | 2.08 | 1.33‐3.26 | .0013* | |
| EOD score | |||||||
| EOD1 | – | – | – | Ref | – | – | – |
| EOD2 | – | – | – | 1.52 | 1.19‐1.93 | .0007* | |
| EOD3 | – | – | – | 1.82 | 1.42‐2.34 | <.0001* | |
| EOD4 | – | – | – | 3.45 | 2.61‐4.58 | <.0001* | |
| Therapeutic modality | |||||||
| Castration | Ref | – | – | Ref | – | – | .31 |
| CAB | 1.13 | 0.74‐1.72 | .59 | 0.93 | 0.72‐1.19 | .56 | |
Abbreviations: CAB, combined androgen blockade; CI, confidence interval; EOD, extent of disease; HR, hazard ratio; PSA, prostate‐specific antigen.
Statistically significant.
Kaplan‐Meier survival curves confirmed the differential prognostic values of percentage of biopsy positive cores, biopsy Gleason grade group, T‐stage, N‐stage, and metastatic sites in patients with low and high metastatic burden (Figures 2 and 3).
FIGURE 2.

Kaplan‐Meier analysis of progression‐free survival in men with metastatic prostate cancer stratified by clinicopathological factors. A‐E, Patients with low and high metastatic burden stratified by percentage biopsy positive cores (A), biopsy Gleason grade group (B), clinical T‐stage (C), clinical N‐stage (D), and metastatic site (E)
FIGURE 3.
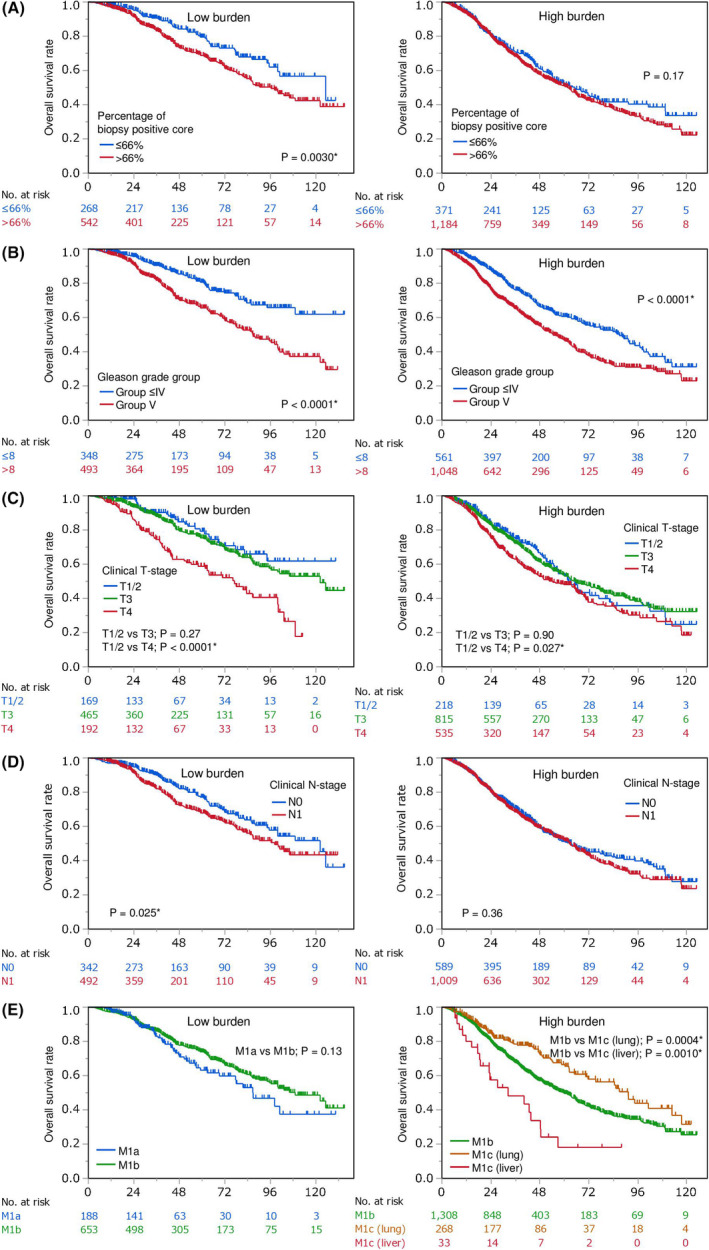
Kaplan‐Meier overall survival analysis of men with metastatic prostate cancer stratified by clinicopathological factors. A‐E, Patients with low and high burden stratified by percentage of biopsy‐positive cores (A), biopsy Gleason grade group (B), clinical T‐stage (C), clinical N‐stage (D), and metastatic site (E)
4. DISCUSSION
The criteria used in this study are similar to that in CHAARTED criteria although metastasis beyond vertebrae and pelvis was not included. Accordingly, the discrimination ability for PFS and OS between low‐ and high‐metastatic burden in this study was similar to those by CHAARTED criteria. 18 , 19
To our knowledge, this is the first investigation of how the prognostic factors for PFS and OS in Japanese patients with metastatic HSPC is affected by the metastatic burden. We identified how the percentage of biopsy‐positive cores, biopsy Gleason grade group, T‐stage, and N‐stage differentially impact on the prognosis between low and high burden. Those findings suggest that locoregional tumor burden and cancer malignant potential have relatively greater impact in patients with low burden, but less in patients with high burden. In addition, this study suggested that M1a was a possible unfavorable prognostic factor in low burden, suggesting that M1a diseases may be more aggressive than oligometastatic bone metastatic disease.
In the present study, high EOD score and liver metastasis, but not lung metastasis, were unfavorable prognostic factors in the all‐patient group, consistent with previous reports, 20 , 21 , 22 as well as in the high‐burden group, whereas lung metastasis was associated with a favorable prognosis in the high‐burden group. These findings are consistent with previous work suggesting that liver and lung metastasis have distinct prognostic value for patients with metastatic prostate cancer. 23 In the present study of mostly Japanese men, lung and liver metastases were observed in 13.2% and 1.3% of patients, respectively, which contrasts with the almost equivalent prevalence rates of 5.7% and 4.5%, respectively, among patients with stage IV prostate cancer in the USA (Surveillance, Epidemiology and End Results–Medicare database). 21 These findings suggest the possibility that metastatic prostate cancer may differ biologically and clinically among patients of different races. 23 Otherwise, the difference of the medical care system such as access to chest computed tomography scan may affect the prevalence rate of lung metastasis. The present study demonstrates for the first time the differential prognostic value of metastatic sites and other factors in Japanese patients with high‐ or low‐metastatic burden HSPC.
In recent years, risk stratification by metastatic burden has been used to determine the optimal therapeutic strategy for patients with metastatic prostate cancer. ARPI, including CYP17 inhibitors and novel antiandrogens, have been shown to improve the survival of patients with HSPC, regardless of metastatic burden, and are currently in clinical use. In contrast, upfront docetaxel chemotherapy and local radiotherapy are thought to be more suitable for patients with high‐ and low‐volume metastatic disease, respectively. 7 , 13 The results of the present study suggest that patients with low‐burden disease and locoregional risk factors such as high percentage of biopsy positive cores, high Gleason grade group, high T‐stage (especially T4), and, possibly, N1 may be ideal candidates for local therapy. A subgroup analysis of the STAMPEDE trial found that local radiotherapy had a favorable effect on OS for the T4, Gleason score ≥8, and N1 patient subgroups, although the impact of percentage of biopsy positive cores was not reported. 9 Patients with low‐burden disease and unfavorable prognostic factors may benefit more from therapies such as ARPI, whereas patients with high‐burden disease and unfavorable prognostic factors may require more intensive therapies, such as docetaxel in combination with ARPI; notably, the latter combination is under investigation in the PEACE‐I and ARASENS trials. The results of those studies will undoubtedly be valuable in developing novel therapeutic strategies for patients with metastatic HSPC who have differing metastatic burdens.
The present study has several limitations. First, the study design was retrospective. Second, the study cohort consisted mostly of Japanese patients, which may limit the applicability of the findings to other patient ethnicities. Third, the accrual period included the era before novel agents for hormone‐refractory prostate cancer were introduced, and several of our patients died before abiraterone acetate, enzalutamide, radium‐233, and cabazitaxel became available. However, all patients had access to docetaxel chemotherapies. Finally, most metastasis was diagnosed by imaging modalities only without biopsy, where accuracy of metastasis is dependent on the diagnostic ability of imaging.
5. CONCLUSION
In conclusion, the results of this study identified differential prognostic factors for patients with low‐ and high‐metastatic burden HSPC. These findings have several potential clinical implications; for example, they may assist in providing more accurate prognoses of patients with metastatic HSPC, in identifying novel therapeutic strategies for patients with differing metastatic burden, and in identifying patients who may benefit from more or less intensive therapies.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENT
We thank Anne M. O’Rourke, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Shiota M, Terada N, Saito T, et al; Japanese Urological Oncology Group (JUOG) . Differential prognostic factors in low‐ and high‐burden de novo metastatic hormone‐sensitive prostate cancer patients. Cancer Sci.2021;112:1524–1533. 10.1111/cas.14722
REFERENCES
- 1. Shiota M, Eto M. Current status of primary pharmacotherapy and future perspectives toward upfront therapy for metastatic hormone‐sensitive prostate cancer. Int J Urol. 2016;23:360‐369. [DOI] [PubMed] [Google Scholar]
- 2. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone‐sensitive prostate cancer. N Engl J Med. 2015;373:737‐746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first‐line long‐term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163‐1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration‐sensitive prostate cancer. N Engl J Med. 2017;377:352‐360. [DOI] [PubMed] [Google Scholar]
- 5. James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with standard first‐line therapy in metastatic prostate cancer. N Engl J Med. 2019;381:121‐131. [DOI] [PubMed] [Google Scholar]
- 7. Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration‐sensitive prostate cancer. N Engl J Med. 2019;381:13‐24. [DOI] [PubMed] [Google Scholar]
- 8. Boevé LMS, Hulshof MCCM, Vis AN, et al. Effect on survival of androgen deprivation therapy alone compared to androgen deprivation therapy combined with concurrent radiation therapy to the prostate in patients with primary bone metastatic prostate cancer in a prospective randomised clinical trial: Data from the HORRAD trial. Eur Urol. 2019;75:410‐418. [DOI] [PubMed] [Google Scholar]
- 9. Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392:2353‐2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nuhn P, De Bono JS, Fizazi K, et al. Update on systemic prostate cancer therapies: Management of metastatic castration‐resistant prostate cancer in the era of precision oncology. Eur Urol. 2019;75:88‐99. [DOI] [PubMed] [Google Scholar]
- 11. Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone‐sensitive prostate cancer: Long‐term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36:1080‐1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clarke NW, Ali A, Ingleby FC, et al. Addition of docetaxel to hormonal therapy in low‐ and high‐burden metastatic hormone sensitive prostate cancer: long‐term survival results from the STAMPEDE trial. Ann Oncol. 2019;30:1992‐2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burdett S, Boevé LM, Ingleby FC, et al. Prostate radiotherapy for metastatic hormone‐sensitive prostate cancer: A STOPCAP systematic review and meta‐analysis. Eur Urol. 2019;76:115‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gillessen S, Attard G, Beer TM, et al. Management of patients with advanced prostate cancer: Report of the advanced prostate cancer consensus conference 2019. Eur Urol. 2020;77:508‐547. [DOI] [PubMed] [Google Scholar]
- 15. Terada N, Mizowaki T, Saito T, et al. Potential effectiveness of local radiotherapy for extending survival and reducing urinary‐related events in patients with de novo metastatic prostate cancer. BJUI Compass. 2020;1:165‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. International Union Against Cancer . Urologic Tumors. Prostate. In: Sobin LH, Wittekind CH, eds. TNM classification of malignant tumors, 5th edn. New York, NY: John Wiley & Sons; 1997:170‐173. [Google Scholar]
- 17. Epstein JI, Zelefsky MJ, Sjoberg DD, et al. A contemporary prostate cancer grading system: a validated alternative to the Gleason score. Eur Urol. 2016;69:428‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shiota M, Namitome R, Kobayashi T, et al. Prognostic significance of risk stratification in CHAARTED and LATITUDE studies among Japanese men with de novo metastatic prostate cancer. Int J Urol. 2019;26:426‐428. [DOI] [PubMed] [Google Scholar]
- 19. Okamoto T, Hatakeyama S, Narita S, et al. Validation and development of the CHAARTED criteria in patients with hormone‐naïve metastatic prostate cancer: A multi‐institutional retrospective study in Japan. Int J Urol. 2020;27:90‐91. [DOI] [PubMed] [Google Scholar]
- 20. Soloway MS, Hardeman SW, Hickey D, et al. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer. 1988;61:195‐202. [DOI] [PubMed] [Google Scholar]
- 21. Gandaglia G, Karakiewicz PI, Briganti A, et al. Impact of the site of metastases on survival in patients with metastatic prostate cancer. Eur Urol. 2015;68:325‐334. [DOI] [PubMed] [Google Scholar]
- 22. Akamatsu S, Kubota M, Uozumi R, et al. Development and validation of a novel prognostic model for predicting overall survival in treatment‐naïve castration‐sensitive metastatic prostate cancer. Eur Urol Oncol. 2019;2:320‐328. [DOI] [PubMed] [Google Scholar]
- 23. Shiota M. Editorial Comment to Validation and development of the CHAARTED criteria in patients with hormone‐naïve metastatic prostate cancer: A multi‐institutional retrospective study in Japan. Int J Urol. 2020;27:92. [DOI] [PubMed] [Google Scholar]


