Abstract
Nonalcoholic fatty liver disease (NAFLD) is an increasingly common condition, affecting up to 25% of the population worldwide. NAFLD has been linked to several conditions, including hepatic inflammation, fibrosis, and hepatocellular carcinoma (HCC), however the role of NAFLD in cholangitis and the development of cholangiocellular carcinoma (CCC) remains poorly understood. This study investigated whether a high‐fat diet (HFD) promotes cholangitis and the development of CCC in mice. We used liver‐specific E‐cadherin gene (CDH1) knockout mice, CDH1∆Liv, which develop spontaneous inflammation in the portal areas along with periductal onion skin‐like fibrosis, similar to that of primary sclerosing cholangitis (PSC). An HFD or normal diet (ND) was fed to CDH1∆Liv mice for 7 mo. In addition, CDH1∆Liv mice were crossed with LSL‐KrasG12D mice, fed an HFD, and assessed in terms of liver tumor development. The extent of cholangitis and number of bile ductules significantly increased in mice fed an HFD compared with ND‐administered CDH1∆Liv mice. The numbers of Sox9 and CD44‐positive stem cell‐like cells were significantly increased in HFD mice. LSL‐KrasG12D /CDH1∆Liv HFD mice exhibited increased aggressiveness along with the development of numerous HCC and CCC, whereas LSL‐KrasG12D/CDH1∆Liv ND mice showed several macroscopic tumors with both HCC and CCC components. In conclusion, NAFLD exacerbates cholangitis and promotes the development of both HCC and CCC in mice.
Keywords: carcinoma, cholangitis, steatohepatitis
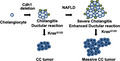
Cdh1 deletion in cholangiocytes develops spontaneous cholangitis and ductular reaction. The extent of cholangitis and ductular reaction is increased in mice with NAFLD. Additional Kras mutation develops cholangiocellular tumors in mice with Cdh1 deleted cholangiocytes and NAFLD promotes development of cholangiocellular tumors.
1. INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is becoming increasingly common, due in part to the wide spectrum of disease pathologies, ranging from simple liver fat deposition to more severe presentations such as nonalcoholic steatohepatitis (NASH), liver cirrhosis (LC), and hepatocellular carcinoma (HCC). It is estimated that 25% of the world's population has NAFLD. 1 The etiology of the disease is related to diet, body mass index, gut microbiota, and genetic factors. 2
Liver cancer is one of the major types of cancer‐related death worldwide. 3 Common forms include HCC and cholangiocellular carcinoma (CCC), which can sometimes present as mixed type carcinomas. The prognosis of patients with liver cancer has improved recently due to advances in diagnosis and treatment; however, the long‐term prognosis remains unsatisfactory. 4 A greater understanding of the molecular mechanisms underlying liver carcinogenesis is therefore needed to address these shortcomings.
Recent evidence has suggested that NAFLD with fibrosis or cirrhosis increases the risk of developing HCC. 5 Such an association is consistent with recent studies suggesting that between 4% to 22% of HCC cases may be related to NAFLD. 5 Another type of liver cancer, CCC, arises from various cell types within the biliary tree. There are several major risk factors for the development of CCC, including primary sclerosing cholangitis (PSC), viral infection (hepatitis B virus [HBV] and hepatitis C virus [HCV]), LC, and biliary stone disease. 6 Metabolic conditions including diabetes and obesity have also been identified as risk factors for CCC. 7
Recent studies have reported several mechanisms, such as the epithelial‐mesenchymal transition (EMT), which may promote carcinogenesis. 8 Mutation or decreased expression of E‐cadherin is associated with malignant progression of various cancers, including HCC and CCC. 9 , 10 As deletion of E‐cadherin is known to promote invasiveness and EMT, liver‐specific knockout of E‐cadherin has been used as a model for examining tumor progression in the liver. 11 Furthermore, liver‐specific deletion of E‐cadherin was shown to induce spontaneous periportal inflammation resembling PSC, indicating that these mice can also be used as a model of cholangitis.
Nonalcoholic fatty liver disease is associated with several important comorbidities, including hepatic inflammation, fibrosis, and the development of HCC, however the role of NAFLD in cholangitis and the development of CCC remains poorly understood. This study investigated whether HFD promotes cholangitis and the development of CCC in mice.
2. MATERIALS AND METHODS
2.1. Animals
CDH1F/F, Alb‐Cre, Alb‐CreERT, CK19‐CreERT, KrasLSLG12D, and Rosa26tdTomato mice were purchased from the Jackson Laboratory (Bar Harbor, MN, USA). All knockout strains were developed on the C57BL/6 genetic background. Mice were maintained in filter‐topped cages on autoclaved food and water at Yokohama City University. All animal experiments were approved by the Ethics Committee for Animal Experimentation of Yokohama City University and conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals.
2.2. Reagents
The following antibodies were used in the experiments: anti‐CD44 (AbD Serotec); anti‐Sox9 and anti‐K19 (Santa Cruz Biotechnology), anti‐F4/80 (Caltag), anti‐Ki67 (Gene Tex), anti‐HNF4α (Abcam), and anti‐RFP (Rockland).
2.3. High‐fat diet
Mice were fed with a normal or high‐fat diet (HFD) (40% kcal fat [22% trans‐fat and 26% saturated fatty acids by weight], 22% fructose, 10% sucrose, 2% cholesterol; D09100301, Research Diets).
2.4. Immunohistochemical analyses
Liver tissue was fixed in 10% formaldehyde, dehydrated, embedded in paraffin, and sectioned (5 μm thickness). Sections were deparaffinized, rehydrated, treated with 3% H2O2 in PBS, and incubated overnight at 4°C with the appropriate antibodies. Binding of the primary antibody was detected using biotin‐labeled anti‐rabbit IgG or anti‐rat IgG antibodies (1:500 dilution; Vector Laboratories), followed by the streptavidin‐horseradish peroxidase (HRP) reaction and visualization with 3,3‐diaminobenzidine (DAB; Sigma) and counterstaining with hematoxylin. For the double staining, sections were incubated with 1st primary antibody (1:500) overnight at 4°C, and the immunoreactivity was visualized with DAB (brown staining) with a peroxidase‐based Histofine Simple Stain Kit (MAX PO R, Nichirei). For the immunoreactivity to another target, 2nd primary anti (1:500) was incubated overnight at 4°C and visualized with Fast Red II Substrate (Nichirei) using an alkaline phosphatase‐based Histofine Simple Stain Kit (AP R, Nichirei).
2.5. Statistical analysis
Data are expressed as the mean ± standard error of the mean (SEM). Significant differences were determined using Student t test. P‐values ≤ .05 were considered significant.
3. RESULTS
3.1. NAFLD exacerbates cholangitis caused by E‐cadherin deletion
Previously, we have shown that liver‐specific E‐cadherin knockout mice (CDH1ΔL) developed spontaneous periportal inflammation as well as periductal fibrosis, which resembles PSC. To determine the role of NAFLD in cholangitis, CDH1ΔL mice were fed an HFD for 20 wk to induce NAFLD CDH1∆L mice fed the HFD exhibited significant increases in body weight and fat deposition, similar to HFD‐administered CDH1F/F control mice (Figure 1A,B, Figure S1). HFD‐administered CDH1∆L mice exhibited steatosis, ballooning, and inflammation similar to that of CDH1F/F mice, and the cholangitis in the periportal area was more severe compared with that seen in mice fed a ND (Figure 1B and Figure S1). To better assess the inflammatory response, we performed immunostaining of F4/80 myeloid cells in CDH1ΔL mice, and observed a significant increase in the number of F4/80‐positive cells in the periportal area of mice fed an HFD relative to ND controls (Figure 1C). CD45‐ and CD3‐positive lymphoid cells were also increased in HFD mice, although the overall number of CD45‐positive cells was relatively small compared with F4/80 myeloid cells (data not shown). We observed increased ALT expression in both CDH1ΔL and CDH1F/F control mice fed the HFD, with no significant difference between strains. Serum levels of total alkaline phosphatase (ALP) and bile acid were increased in CDH1ΔL mice compared with CDH1F/F control mice, however the ALP and total bile acid levels were not different between ND and HFD mice (Figure 1D).
FIGURE 1.
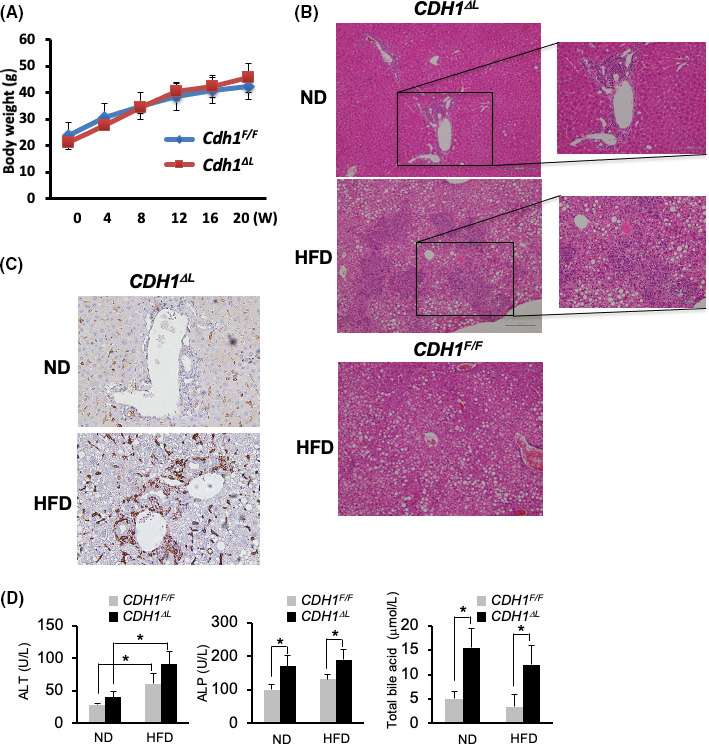
NAFLD exacerbates cholangitis caused by E‐cadherin deletion. A, Body weight curve of CDH1ΔL mice (n = 5) and CDH1F/F (n = 5) after HFD. B, H&E staining of the liver in ND and HFD CDH1ΔL mice and CDH1F/F HFD mice. ×100 (left panel) and ×200 (right panel) magnification. C, Immunostaining of F4/80 in CDH1ΔL ND and HFD mice. ×200 magnification. D, Serum ALT, ALP, and total bile acid levels of ND and HFD mice of each genotype (both n = 5). Values represent the mean ± standard error of the mean (SEM). *P < .05 as determined by Student t test
CDH1ΔL showed numerous primitive duct cells expressing progenitor markers, such as Sox9 and CD44, in the periportal area. 11 CDH1ΔL HFD mice showed increased numbers of Sox9‐ and CD44‐positive cells compared with ND mice (Figure 2A,B). CK19‐positive ductular cells were also increased by HFD (Figure 2A,B). Interestingly, Sox9‐positive cells were observed not only in zone 1, but also in in zone 2 of CDH1ΔL HFD mice compared with only zone 1 in the CDH1ΔL ND mice (Figure 2A,B). CDH1F/F HFD mice showed slightly but not significantly increased numbers of Sox9, CD44 and CK19‐positive cells compared with ND mice (Figure S2). We performed double staining of Sox9/CK19 and Sox9/CD44 (Figure 2B), and found that Sox9 and CK19 were stained in almost the same cells. Most of the Sox9‐positive cells were positive for CD44, however CD44 single positive cells were frequently found, possibly because of the positivity of CD44 in mesenchymal cells in addition to immature cholangiocytes. In contrast, CDH1F/F HFD mice showed slightly, but not significantly, increased numbers of Sox9‐, CD44‐, and CK19‐positive cells (Figure 2C). These results suggested a significant increase in the extent of cholangitis and the number of bile ductules in CDH1ΔL HFD mice relative to CDH1ΔL ND mice. Fibrosis, as determined using α‐smooth muscle actin (α‐SMA) immunostaining, was slightly increased in CDH1ΔL HFD mice relative to ND controls (data not shown).
FIGURE 2.
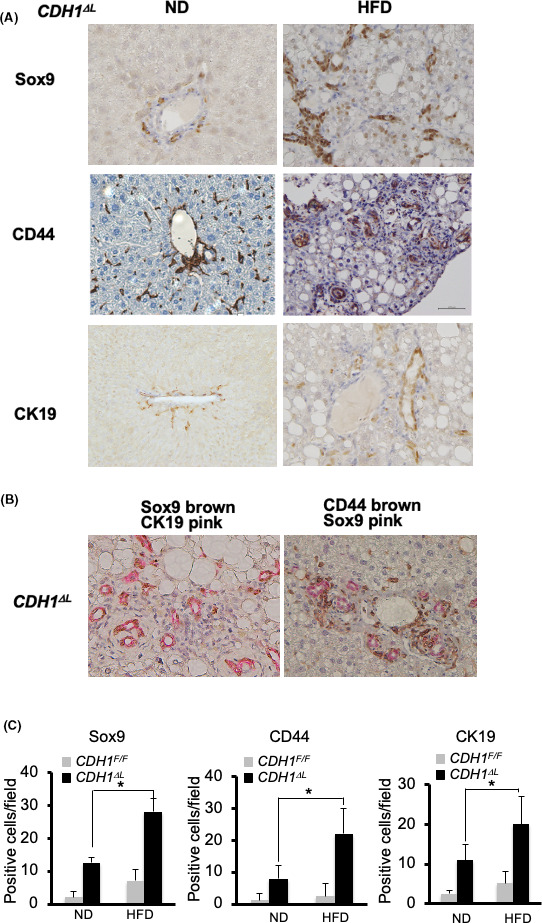
NAFLD increased Sox9‐, CD44‐, and CK19‐positive cells in E‐cadherin‐deleted liver. A, Immunostaining of Sox9, CD44, and CK19 in CDH1ΔL ND and HFD mice (×200 magnification). B, Double immunostaining of Sox9 (brown)/CK19 (pink) (left panel) and CD44 8brown)/Sox9 (pink) (right panel) in CDH1ΔL HFD mice (×200 magnification). C, Quantification of Sox9, CD44, and CK19 cells in CDH1ΔL (n = 5) and CDH1F/F (n = 5) mice. Positive cell numbers per field. *P < .05
3.2. The NAFLD‐induced exacerbated ductular reaction might be irreversible
To determine whether the ductular reaction seen in these mice is reversible, mice fed the HFD diet for 5 mo were switched to the ND for 3 wk. We found that severe cholangitis and fat deposition in CDH1∆L were significantly reversed after 3 wk; however, the increased ductular reaction, as well as the increased numbers of Sox9, CD44 and CK19‐positive cells were not reversed (Figure 3A,B). In contrast, the number of F4/80 myeloid cells was reduced in these mice (Figure 3C), suggesting that the NAFLD‐induced bile duct reaction may be irreversible over short time periods.
FIGURE 3.
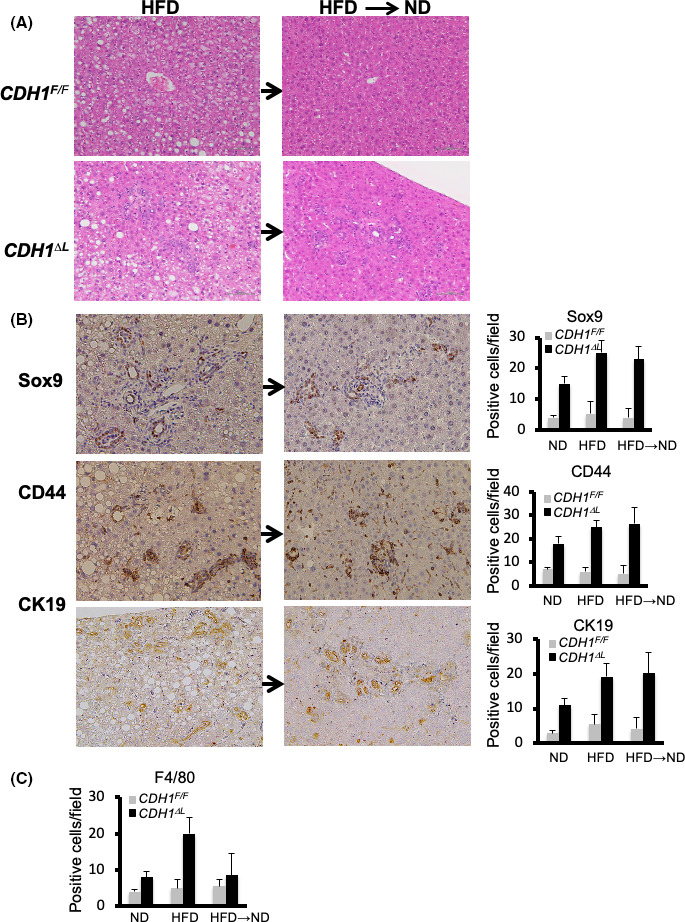
NAFLD‐induced exacerbation of the ductular reaction may be irreversible. A, H&E staining of CDH1ΔL (n = 4) and CDH1F/F (n = 4) mice fed an HFD for 5 mo before being switched to ND for 3 wk. B, Sox9, CD44, and CK19 immunostaining and quantification. ×200 magnification. C, Quantification of F4/80‐positive cells
3.3. Increased ductular reaction induced by HFD may be due to bile duct epithelial cells
We have previously reported that loss of E‐cadherin in bile duct epithelial cells (BECs) rather than hepatocytes induces cholangitis and ductular reaction in CDH1∆L. 11 We also showed that E‐cadherin was deleted both in hepatocytes and BECs of CDH1∆L mice. 11 Therefore, we wanted to determine whether the increased ductular reaction associated with HFD was also associated with BECs. To delete E‐cadherin only in BECs, we crossed CDH1F/F mice with K19CreERT mice expressing a tamoxifen (TAM)‐inducible Cre ERT in the endogenous K19 locus (CDH1F/F/K19CreERT; CDH1∆ich) (Figure 4A). At 12 wk after TAM injection, increased cholangitis (F4/80‐positive cells), ductular reaction (CK19‐positive), and Sox9‐positive staining were observed in ND mice, as described previously 11 (Figure 4B). In CDH1Δich mice, HFD increased cholangitis (F4/80‐positive cells), ductular reactivity (CK19‐positive cells), and Sox9‐positive staining compared with ND mice (Figure 4B,C). These observations suggested that the increased cholangitis and ductular reactivity induced by HFD were dependent on BECs (Figure 4A–C). However, compared with CDH1∆L HFD mice, CK19‐ and Sox9‐positive cell expression in CDH1∆ich HFD mice was relatively limited around zone 1. To confirm that the increased ductular reaction was derived from E‐cadherin‐deleted BECs, we crossed Rosa26tdTomato mice (tdTomato mice) with CDH1∆ich mice and found that most of the ductular cells were tdTomato‐positive, suggesting that the increase in ductular cells was derived from CK19‐positive BECs in HFD‐administered CDH1∆ich mice (Figure 4D).
FIGURE 4.
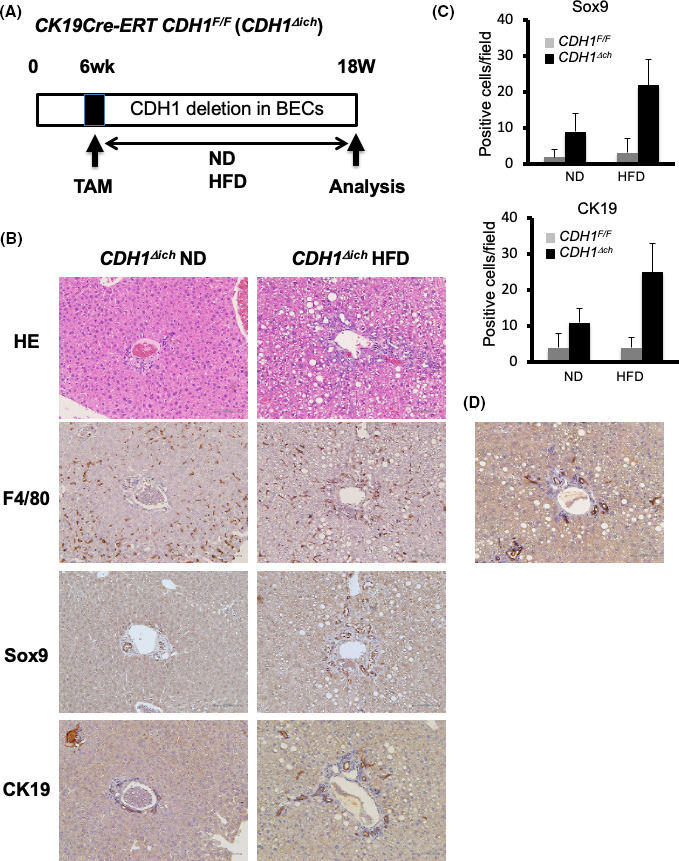
Increased ductular reaction induced by the HFD may be due to bile duct epithelial cells. A, CDH1F/F mice crossed with K19CreERT mice, in which a TAM‐inducible CreERT was inserted into the endogenous K19 locus (CDH1F/F/K19CreERT;CDH1∆ich). B, At 12 wk after TAM injection, mice were fed with a ND or HFD. H&E and immunostaining results of F4/80, Sox9, and CK19 are shown (×100 magnification). C, Quantification of Sox9‐ and CK19‐positive cells of CDH1F/F and CDH1∆ich mice fed the ND or HFD are shown (n = 5 for all groups). D, Rosa26tdTomato mice were crossed with CDH1∆ich mice; most of the ductular cells were tdTomato‐positive, suggesting that increased ductular cell expression in HFD‐administered mice was due to BECs (×100 magnification)
3.4. Increased primitive cell expression induced by HFD was also due to hepatocytes
Biphenotypic hepatocytes, also known as ductular hepatocytes, express ductal markers and give rise to ductal cells, and have been reported in cholestatic liver injury models. 12 , 13 , 14 Furthermore, pre‐existing populations of Sox9‐expressing periportal hepatocytes and other bile‐duct‐enriched genes have shown extensive proliferation after chronic injuries. 15 In CDH1∆L mice, an HFD increased Sox9‐positive cells, not only in zone 1, but also in zone 2 (Figure 2A). Therefore, we wanted to determine whether hepatocytes other than BECs were the source of Sox9‐positive cells. To generate hepatocyte‐specific E‐cadherin knockout mice, we crossed CDH1F/F mice with Alb‐CreERT mice expressing a TAM‐inducible Cre ERT in the endogenous albumin locus (CDH1F/F/Alb‐CreERT; CDH1∆ihep). CDH1∆ihep and control mice were injected with TAM at 6 wk of age and fed with the HFD or ND for 12 wk (Figure 5A). No obvious phenotype was found in CDH1∆ihep ND mice, consistent with our previous report of no apparent periportal inflammation associated with the injection of adenovirus‐expressing Cre recombinase in CDH1F/F mice. 11 Interestingly, CDH1∆ihep HFD mice showed increased numbers of small Sox9‐positive hepatocyte‐like cells, with no such phenotype seen in control HFD mice (Figure 5B–D). As shown in Figure 5C, Sox‐9 cells were located in both zone 1 and zone 2. CK19‐positive cells were slightly increased in CDH1∆ihep HFD mice relative to both CDH1∆ihep ND and CDH1F/F HFD mice.
FIGURE 5.
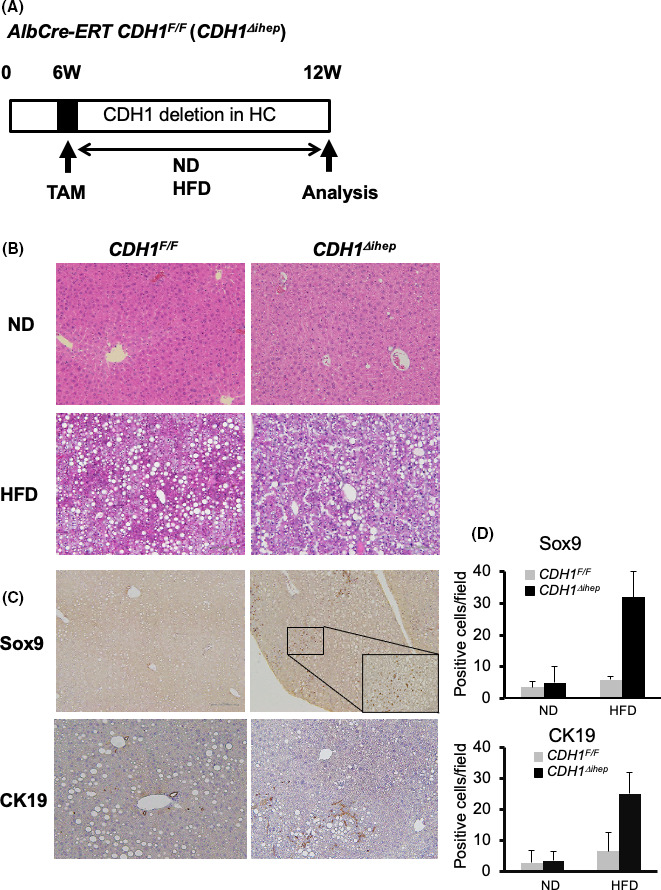
HFD‐induced Sox9‐positive cells were derived from hepatocytes. A, CDH1F/F mice were crossed with Alb‐CreERT mice, in which a TAM‐inducible CreERT was inserted into the endogenous albumin locus (CDH1F/F/Alb‐CreERT;CDH1∆ihep). CDH1∆ihep or control mice were injected with TAM at 6 wk of age and fed a ND or HFD for 12 wk. B, H&E staining of the liver of CDH1F/F and CDH1∆ihep mice fed the ND or HFD (×100 magnification). C, Sox9 and CK19 immunostaining in the liver of CDH1F/F and CDH1∆ihep mice fed the HFD. D, Quantification of Sox‐9‐ and CK19‐positive cells in CDH1F/F and CDH1∆ihep mice fed the HFD are shown (n = 5 for all groups)
To determine the source of the Sox9‐positive cells in CDH1∆ihep HFD mice, these mice were crossed with tdTomato mice, as described above. We found that the Sox‐9‐positive cells in zone 2 were derived from Alb‐positive hepatocytes. These observations indicated that E‐cadherin deletion in hepatocytes led to the production of hepatoblast‐like cells by steatosis. These results suggested that increased Sox9‐positive cell expression in CDH1∆hep was due to both hepatocytes and BECs.
3.5. High‐fat diet promotes tumorigenesis in CDH1ΔL
Previously, we showed that a small percentage of male CDH1ΔL mice (2/12, 16.7%) spontaneously developed liver tumors by 11 mo of age. 11 In this study, we analyzed tumorigenesis in 8‐mo‐old CDH1ΔL HFD mice over 6 mo. The overall tumor incidence was 2/11 (22.2%), representing a small, but noticeable, difference compared with CDH1ΔL ND mice (0/8; 0%). The majority of the tumors in the CDH1ΔL HFD mice contained both hepatocellular and ductular (cholangiocellular) components (Figure 6A, left panel); the others contained only ductular or hepatocellular component (Figure 6A, right panel). As only 2 CDH1ΔL HFD mice developed spontaneous tumors, it was difficult to determine whether mixed type, hepatocellular, or cholangiocellular tumors were more common in this group.
FIGURE 6.
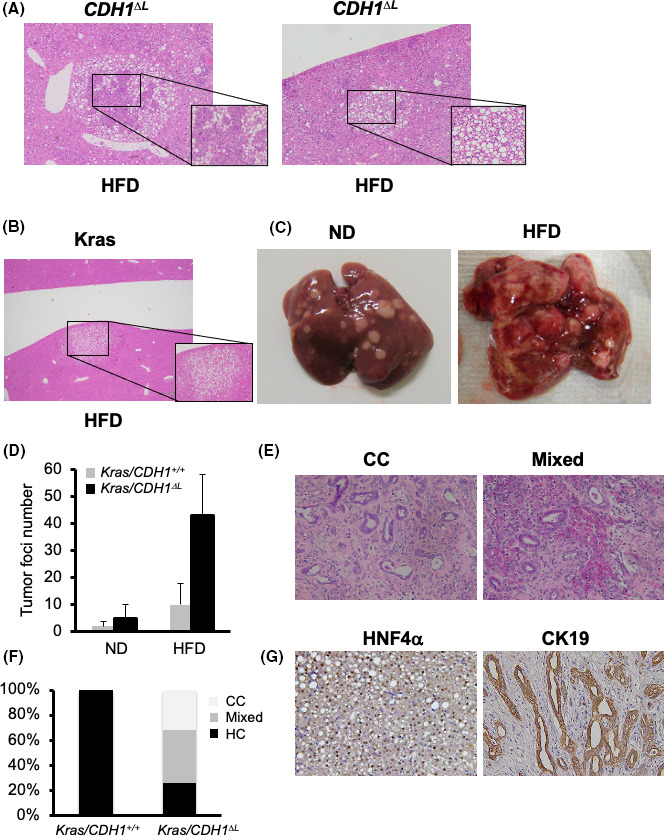
High‐fat diet promotes tumorigenesis in CDH1ΔL. A, Tumors in 8‐mo‐old CDH1ΔL mice fed an HFD for 6 mo (×100 magnification). B, Tumors of Alb‐cre/LSL‐KrasG12D (Krashep) HFD mice. Krashep mice were started on an HFD at 2 mo of age and sacrificed at 5 mo (3‐mo HFD). C, Macroscopic features of Krashep/CDHF/F and Krashep/CDH1ΔL mice fed the HFD. D, The number of tumor foci in Krashep/CDHF/F (n = 5) and Krashep/CDH1ΔL (n = 7) mice fed the HFD for 3 mo, starting at 2 mo of age. E, H&E staining of CC and Mixed tumors of Krashep/CDH1ΔL HFD mice (×100 magnification). F, Tumor histology in the tumors of Krashep/CDHF/F and Krashep/CDH1ΔL HFD mice. G, HNF4α and CK19 immunostaining in the HC (left panel) and CC (right panel) tumors of Kras hep/CDH1 ΔL HFD mice (×100 magnification)
As the incidence of tumors in CDH1ΔL HFD mice was too low to analyze further, we expanded our analysis to tumor incidence in liver‐specific Kras‐expressing mice (Alb‐cre/KrasLSLG12D:Kras). At 5 mo of age, there were no visible tumors in any of the Kras ND mice (0/8; 0%). Kras mice fed the HFD (starting at 2 mo of age) sacrificed at 5 mo old (3‐mo HFD) showed visible white lesions containing histologically confirmed hepatocellular tumors (8/8:10%; Figure 6B,C, right panel). The number of tumor foci was increased in Kras HFD mice compared with ND controls (Figure 6D). These results suggested that Kras HFD mice can be used as a model for hepatocarcinogenesis.
To analyze the role of cholangitis in HFD‐induced hepatocarcinogenesis, Kras/CDH1ΔL mice were transitioned to an HFD at 2 mo and analyzed after 3 mo (5 mo of age). Kras/CDH1ΔL ND mice developed a small number of visible liver tumors at 5 mo of age, however the Kras/CDH1ΔL HFD group exhibited a significant increase in tumor burden compared with Kras/CDH1+/+ mice (Figure 6C,D). Histological analyses revealed a variety of tumor types, including cholangiocellular (CC) (34%), cholangiocellular/hepatocellular (Mixed) (42%) and hepatocellular (24%) (HC) tumors (Figure 6F) determined using immunostaining of CK19 for CC and HNF4α for HC tumors (Figure 6G). These results suggested that loss of E‐cadherin exacerbated HFD‐related increases in Ras signaling, resulting in enhanced development of both hepatocellular and cholangiocellular tumors.
4. DISCUSSION
In this study, we observed a significant increase in the extent of cholangitis and fibrosis, and in the number of bile ductules in CDH1∆Liv mice fed an HFD for 7 mo compared with ND‐administered mice (Figure 7). The numbers of CD44‐ and Sox9‐positive stem cell‐like cells were also increased in HFD mice.
FIGURE 7.
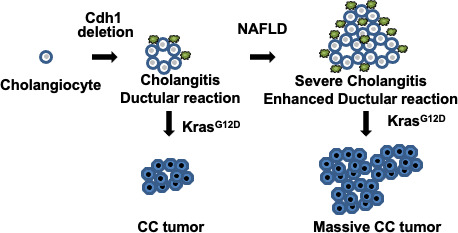
Graphic schema of this study. Cdh1 deletion in cholangiocytes develops spontaneous inflammation (cholangitis) and ductular reaction. The extent of cholangitis and ductular reaction is increased in mice with NAFLD. Additional Kras mutation develops cholangiocellular (CC) tumors in mice with Cdh1 deleted cholangiocytes and NAFLD promotes development of CC tumors
Overweight and obese PSC patients have been found to exhibit more advanced fibrosis, as well as more rapid progression of fibrosis, compared with normal weight patients. 16 Several other liver diseases are known to elicit ductular reactions such as chronic viral hepatitis. 17 Therefore, an increased ductular reaction in response to HFD may be more common than previously thought and may be associated with cancer development.
The mechanism by which obesity or NAFLD leads to more aggressive inflammation and an increased ductular reaction is unknown. We have shown that the ductular reaction in response to E‐cadherin deletion is dependent on impairment of the intrahepatic biliary network. In this study, we did not observe any increase in the number of tubules in HFD‐fed CDHF/F mice, suggesting that HFD itself is not sufficient to induce a ductular reaction. In contrast, CDH1∆Liv showed an increased ductular reaction in response to HFD. Recent reports have suggested that pathogen‐associated molecular pattern (PAMP)‐sensing receptors, such as Toll‐like receptors, and the subsequent expression of cytokines triggered by their activation, are important for increased ductular reaction. 18 , 19 PAMPs are known to activate inflammatory cells, such as Kupffer cells and macrophages, in the liver and also induce the production of inflammatory cytokines and growth factors, such as tumor necrosis factor (TNF)‐α and interleukin (IL)‐6. Although inflammation is an important factor seen in association with ductule formation, the factors driving their proliferation remain poorly understood. We aimed to induce the ductular reaction in TNF‐α knockout mice using antibiotics; however, no significant effect was seen (data not shown).
Based on these observations, we aimed to determine whether this enhanced ductular response promoted CCC. We showed that liver‐specific LSL‐KrasG12D/CDH1∆Liv with ND reveled 2‐10 macroscopically visible tumors containing both hepatocellular and cholangiocellular components, and HFD administration induced the development of more numerous and aggressive CCCs. To investigate the source of cholangiocellular tumors, we crossed the CDH1∆ich and LSL‐KrasG12D mice, and maintained them on an HFD for 3 mo. Small precancerous lesions were found in all mice, suggesting that the cholangiocellular tumors in Kras/CDH1∆Liv mice were at least partially due to the increased expression of CK‐19‐positive ductular cells (Figure S3). The model CK19‐CreERT showed relatively low efficiency of knockdown and CDH1 was expressed in around 70% of the cholangiocytes (Figure S4). We assume that this potentially explains the low number of tumors in the mouse line when crossed with Kras. In contrast, a previous mouse model of cholangiocarcinoma, in which hepatocytes and cholangiocytes were labeled with heritable, cell type–specific reporters, found that cholangiocarcinomas were generated by biliary lineage cells derived from hepatocytes, rather than from cholangiocytes. 20
In this study, Alb‐CreERT/Kras (Krasihep) mice showed no visible tumors, but histologically small foci were observed in all mice. However, there were more than 10 visible tumors in the Krasihep/CDH1∆ihep HFD mice (Figure 6D). Furthermore, the number of Sox9‐positive cells was increased in the background and tumors of Krasihep/CDH1∆ihep mice. To trace these cells, tdTomato mice were crossed with Krasihep/CDH1∆ihep. Histological analyses showed that the majority of these cells were Tomato‐positive, suggesting that Sox9 positive cells of Krasihep/CDH∆ihep mice originated from hepatocytes. Most of the tumors were AFP‐positive HCC, with only a small proportion of CK19‐positive CCC cells. These observations suggested that E‐cadherin deleted hepatocytes, which exhibit a Sox9‐positive premature NAFLD phenotype, may represent an important source of tumors.
The bile canalicular reaction is caused by the proliferation of liver stem or progenitor cells, such as oval cells. A typical bile duct reaction is characterized by an increase in relatively well‐shaped bile ducts with a clear lumen, usually associated with rapid biliary obstruction. Conversely, an atypical bile duct reaction is an irregular form of bile duct hyperplasia often observed in various pathological conditions, such as cirrhosis, alcoholic liver injury, and fulminant hepatitis with inflammation and fibrosis. The atypical bile duct reaction is caused by the proliferation of existing BECs, although the underlying cause of this reaction remains unclear. However, many researchers consider the cause to be the proliferation of stem cells activated by injury, as well as the proliferation of oval cells.
Loss of E‐cadherin expression is associated with mutations of the E‐cadherin gene CDH1, loss of heterozygosity in 16q22.1, or a transcriptional down‐regulation of CDH1 by promoter methylation. 21 , 22 Clinically, a decrease or deletion of E‐cadherin has been reported in some liver diseases. For example, both HCV and HBV proteins were shown to induce promoter hypermethylation of E‐cadherin, resulting in decreased expression and induction of EMT. 23 , 24 The presence of hypermethylated E‐cadherin in serum has been linked to HCC in patients with HCV‐related cirrhosis. 25
In summary, NAFLD exacerbates cholangitis and becomes a strong promoter not only of HCC, but also of CCC in mice (Figure 7). As the number of cancer patients with fatty liver continues to rise, a better understanding of the precise molecular mechanisms underlying tumor growth in both normal and NAFLD livers is needed to improve the diagnosis and treatment of these patients.
DISCLOSURE
All authors have no competing interests.
Supporting information
Fig S1‐S4
ACKNOWLEDGMENTS
SM was supported by Grants‐in‐Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (#19K08373 and #19K34567).
Maeda S, Hikiba Y, Fujiwara H, et al. NAFLD exacerbates cholangitis and promotes cholangiocellular carcinoma in mice. Cancer Sci. 2021;112:1471–1480. 10.1111/cas.14828
REFERENCES
- 1. Araújo AR, Rosso N, Bedogni G, Tiribelli C, Bellentani S Global epidemiology of non‐alcoholic fatty liver disease/non‐alcoholic steatohepatitis: What we need in the future. Liver Int. 2018;38(Suppl. 1):47‐51. [DOI] [PubMed] [Google Scholar]
- 2. Buzzetti E, Pinzani M, Tsochatzis EA The multiple‐hit pathogenesis of non‐alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038‐1048. [DOI] [PubMed] [Google Scholar]
- 3. Bertuccio P, Alicandro G, Malvezzi M, et al. Cancer mortality in Europe in 2015 and an overview of trends since 1990. Ann Oncol. 2019;30:1356‐1369. [DOI] [PubMed] [Google Scholar]
- 4. Chew SA, Moscato S, George S, Azimi B, Danti S Liver cancer: Current and future trends using biomaterials. Cancers (Basel). 2019;11(12):2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M Global epidemiology of nonalcoholic fatty liver disease—Meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73‐84. [DOI] [PubMed] [Google Scholar]
- 6. Labib PL, Goodchild G, Pereira SP Molecular pathogenesis of cholangiocarcinoma. BMC Cancer. 2019;19:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Petrick JL, Yang B, Altekruse SF, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: A population‐based study in SEER‐Medicare. PLoS One. 2017;12:e0186643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gao D, Vahdat LT, Wong S, Chang JC, Mittal V Microenvironmental regulation of epithelial‐mesenchymal transitionsin cancer. Cancer Res. 2012;72:4883‐4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Terashita K, Chuma M, Hatanaka Y, et al. ZEB1 expression is associated with prognosis of intrahepatic cholangiocarcinoma. J Clin Pathol. 2016;69:593‐599. [DOI] [PubMed] [Google Scholar]
- 10. Giannelli G, Koudelkova P, Dituri F, Mikulits W Role of epithelial to mesenchymal transition in hepatocellular carcinoma. J Hepatol. 2016;65:798‐808. [DOI] [PubMed] [Google Scholar]
- 11. Nakagawa H, Hikiba Y, Hirata Y, et al. Loss of liver E‐cadherin induces sclerosing cholangitis and promotes carcinogenesis. Proc Natl Acad Sci. 2014;111:1090‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanimizu N, Nishikawa Y, Ichinohe N, Akiyama H, Mitaka T Sry HMG box protein 9‐positive (Sox9+) epithelial cell adhesion molecule‐negative (EpCAM‐) biphenotypic cells derived from hepatocytes are involved in mouse liver regeneration. J Biol Chem. 2014;289:7589‐7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tarlow B, Pelz C, Naugler W, et al. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell. 2014;15:605‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yanger K, Zong Y, Maggs LR, et al. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev. 2013;27:719‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Font‐Burgada J, Shalapour S, Ramaswamy S, et al. Hybrid periportal hepatocytes regenerate the injured liver without giving rise to cancer. Cell. 2015;162:766‐779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reyes JL, Vannan DT, Vo T, et al. Neutralization of IL‐15 abrogates experimental immune‐mediated cholangitis in diet‐induced obese mice. Sci Rep. 2018;8:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sato K, Marzioni M, Meng F, Francis H, Glaser S, Alpini G Ductular reaction in liver diseases: pathological mechanisms and translational significances. Hepatology. 2019;69:420‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Odena G, Chen J, Lozano JJ, et al. LPS‐TLR4 pathway mediates ductular cell expansion in alcoholic hepatitis. Sci Rep. 2016;6:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Joshi‐Barve S, Kirpich I, Cave MC, Marsano LS, McClain CJ Alcoholic, nonalcoholic, and toxicant‐associated steatohepatitis: Mechanistic similarities and differences. Cell Mol Gastroentreol Hepatol. 2015;1:356‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sekiya S, Suzuki A Intrahepatic cholangiocarcinoma can arise from Notch‐mediated conversion of hepatocytes. J Clin Invest. 2012;122:3914‐3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Y, Shang Y Epigenetic control of epithelial‐to‐mesenchymal transition and cancer metastasis. Exp Cell Res. 2013;319:160‐169. [DOI] [PubMed] [Google Scholar]
- 22. Fan X, Jin S, Li Y, et al. Genetic and epigenetic regulation of e‐cadherin signaling in human hepatocellular carcinoma. Cancer Manag Res. 2019;11:8947‐8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park J, Jang KL Hepatitis C virus represses E‐cadherin expression via DNA methylation to induce epithelial to mesenchymal transition in human hepatocytes. Biochem Biophys Res Commun. 2014;446:561‐567. [DOI] [PubMed] [Google Scholar]
- 24. Arzumanyan A, Friedman T, Kotei E, Ng IOL, Lian Z, Feitelson MA Epigenetic repression of E‐cadherin expression by hepatitis B virus x antigen in liver cancer. Oncogene. 2012;31:563‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. El‐Bendary M, Nour D, Arafa M, Neamatallah M Methylation of tumour suppressor genes RUNX3, RASSF1A and E‐Cadherin in HCV‐related liver cirrhosis and hepatocellular carcinoma. Br J Biomed Sci. 2020;77:35‐40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S4


