Abstract
Tumor metastasis is the leading cause of death worldwide and involves an extremely complex process composed of multiple steps. Our previous study demonstrated that apoptosis signal‐regulating kinase 1 (ASK1) deficiency in mice attenuates tumor metastasis in an experimental lung metastasis model. However, the steps of tumor metastasis regulated by ASK1 remain unclear. Here, we showed that ASK1 deficiency in mice promotes natural killer (NK) cell‐mediated intravascular tumor cell clearance in the initial hours of metastasis. In response to tumor inoculation, ASK1 deficiency upregulated immune response‐related genes, including interferon‐gamma (IFNγ). We also revealed that NK cells are required for these anti‐metastatic phenotypes. ASK1 deficiency augmented cytokine production chemoattractive to NK cells possibly through induction of the ligand for NKG2D, a key activating receptor of NK cells, leading to further recruitment of NK cells into the lung. These results indicate that ASK1 negatively regulates NK cell‐dependent anti‐tumor immunity and that ASK1‐targeted therapy can provide a new tool for cancer immunotherapy to overcome tumor metastasis.
Keywords: ASK1, intravascular tumor cell clearance, lung metastasis, NK cell, NKG2D
ASK1 regulates the initial steps of tumor lung metastasis in an NK cell‐dependent fashion. ASK1 deficiency in mice enhances cytokine production of NK cells and NK cell recruitment in response to tumor inoculation. ASK1 deficiency leads to upregulation of NKG2D ligands H60, which might augment NK cell‐dependent intravascular tumor cell clearance.
1. INTRODUCTION
Tumor metastasis is the process of cancer cells spreading from primary lesions to distant organs and accounts for a large part of cancer mortality. 1 However, limited progress has been made in the treatment of tumor metastasis, mainly due to the complex metastatic process. Although some molecular mechanisms of metastasis have been elucidated to date, there remain many unanswered questions, especially how intravascular tumor cells are eliminated during dissemination. Hence, further elucidation of the molecular mechanisms is needed to develop novel therapies to prevent tumor metastasis at early stages.
Apoptosis signal‐regulating kinase 1 (ASK1) is a MAPK kinase‐kinase that activates downstream MAPKs, JNK, and p38 in response to various stimuli. 2 , 3 We previously demonstrated that metastasis was markedly attenuated in ASK1‐deficient (ASK1−/−) mice at 14 d after iv injection of tumor cells in an experimental lung metastasis model. Furthermore, time‐dependent analysis demonstrated the involvement of ASK1 in the metastatic process within 3 d after tumor cell inoculation. The experimental metastatic process in the first few days involves many steps. Injected tumor cells travel through the bloodstream and are arrested in the lung microvasculature within a few minutes. The trapped tumor cells then form emboli and adhere to endothelial cells. Extravasation and initial seeding into the stroma usually occur within 1‐3 d after initial arrest. 4 Among these multiple steps, those involving ASK1 are unclear.
Natural killer (NK) cells are cytotoxic lymphocytes of the innate immune system involved in early immune responses against tumor cells. Accumulating evidence suggests that NK cells can prevent tumor metastasis in an experimental lung metastasis model. 5 , 6 Moreover, NK cells can kill intravascular tumor cells in the initial steps of the metastatic process. 7 , 8
In this study, we revealed that ASK1 deficiency in mice promotes intravascular tumor cell clearance through enhanced cytokine production of NK cells as well as NK cell recruitment to the metastatic lung.
2. MATERIALS AND METHODS
2.1. Mice
ASK1−/− mice and mice harboring the ASK1 floxed allele (ASK1F/F) on a C57BL/6 background were generated and described previously. 3 , 9 , 10 Ncr1‐cre mice were kindly provided by Dr. Veronika Sexl. 11 Pf4‐cre mice were purchased from the Jackson Laboratory. Lysm‐cre mice were provided from RIKEN. Cdh5‐cre mice were obtained from National Institutes of Biomedical Innovation, Health and Nutrition. 12 The mice were maintained in a specific pathogen‐free facility and age‐matched female 8‐ to 16‐wk‐old mice were used. All the experiments were performed following the experimental protocol approved by the animal ethics committee of the University of Tokyo.
2.2. Cell culture
MCA205 methylcholanthrene‐induced fibrosarcoma was generously provided by S. A. Rosenberg (National Cancer Institute, Bethesda, MD). Lewis lung carcinoma 3LL cells and sarcoma MCA205 cells with luciferase expression (3LL‐Luc2 cells and MCA205‐Luc2 cells, respectively) were kindly provided by Dr. Yoshihiro Hayakawa. 3LL‐Luc2 cells were maintained in RPMI‐1640 medium (Sigma) with 100 units/mL penicillin G and 10% FBS. MCA205‐Luc2 cells were maintained in RPMI‐1640 medium with 100 units/mL penicillin G, 10% FBS and 200 μg/mL hygromycin B. Melanoma B16F10 cells with luciferase expression (B16F10‐luc‐G5 cells; Xenogen Corp.) were maintained in DMEM‐high glucose medium (Sigma) with 100 units/mL penicillin G, 10% FBS and 0.25 mg/mL zeocin. RAW264.7 cells were maintained in RPMI‐1640 medium with 10% FBS. HEK293T cells were maintained in DMEM‐high glucose medium with 10% FBS. Cells were cultured in 5% CO2 at 37°C.
2.3. Tumor cell inoculation
For experimental lung metastasis model, the following numbers of tumor cells or control phosphate‐buffered saline (PBS) were injected iv: 6 × 105 or 1 × 106 cells/mouse for 3LL‐Luc2 cells, 7 × 105 cells/mouse for B16F10‐luc‐G5 cells and 8 × 105 cells/mouse for MCA205‐Luc2 cells. At indicated time points after iv injection, lungs were dissected and analyzed by luciferase activity assay, flow cytometry, ELISA, DNA microarray, and/or quantitative PCR.
2.4. Luciferase activity assay
Lungs were homogenized in Luciferase Culture Lysis 5× Reagent (Promega) diluted with distilled water. The supernatants were collected after centrifugation and analyzed by Luciferase Assay System (Promega).
2.5. Flow cytometry and NK cell isolation
Lungs were minced and digested with 1 mg/mL Collagenase D (Roche) for 1 h in a shaker set to 37°C. Cell suspensions were filtered through 70‐μm cell strainers and lysed with erythrocyte lysis buffer (0.083% NH4Cl, 2.05% Tris), followed by separation with 30% Percoll (GE Healthcare) if necessary and resuspension in Staining Buffer (eBioscience). Cell suspensions were blocked with Fc Block (BD Biosciences) if necessary and stained with the antibodies. Flow cytometry analysis and NK cell isolation (gated by CD3e−NK1.1+) were performed with FACSAria (BD Biosciences). NK cell ratio was calculated out of mononucleated cells (MNCs). Isolated NK cells were collected into 750 μL ISOGEN‐LS (Wako), followed by RNA isolation.
2.6. ELISA
Lungs were homogenized in RIPA buffer (50 mM Tris‐HCl pH 8.0, 150 mM NaCl, 1% NP‐40, 0.5% DOC, 0.1% SDS, 1 mM PMSF, 5 μg/mL leupeptin, 8 mM NaF, 12 mM beta‐glycerophosphate, 1 mM Na3VO4, 1.2 mM Na2MoO4, 5 µM cantharidin and 2 mM imidazole) with protease inhibitor cOmplete, Mini Protease Inhibitor Cocktail (Roche). The lysates were cleared by centrifugation and analyzed using IFNγ Mouse ELISA Kit Quantikine M (R&D Systems).
2.7. DNA microarray
Microarray hybridization and scanning were performed as previously described. 9 Total RNA was pooled from the lungs of 3‐ to 8‐wk‐old female mice at 3 h after iv injection of 3LL‐Luc2 cells. Database for Annotation, Visualization and Integrated Discovery (DAVID) analysis was performed against the genes whose expression were more than 2‐fold higher in the lungs of ASK1−/− mice compared with those of WT mice.
2.8. RNA isolation
RNA was isolated from lungs or RAW264.7 cells using ISOGEN (Wako) as previously described. 9 RNA was extracted from sorted NK cells using ISOGEN‐LS, and glycogen (Wako) was used to increase RNA yields.
2.9. Quantitative PCR
Reverse transcription and designing of primers were performed as described previously. 9 LightCycler 96 (Roche) and SYBR Green PCR Master Mix were utilized and each gene expression was normalized to Rps18. Primer sequences are listed in Table S1.
2.10. Antibodies and reagents
For NK cell depletion, 150 μg anti‐asialo GM1 antibody (Wako) was intraperitoneally injected 2 d prior to tumor inoculation. Anti‐mouse CD3e APC and anti‐mouse NK1.1 PE‐Cyanine7 antibodies were purchased from eBioscience for flow cytometry analysis. Anti‐mouse PECAM‐1 (BD Biosciences), anti‐mouse FLT4 (R&D Systems) antibodies, and Alexa Fluor 594 and 488 secondary antibodies (Thermo Fisher Scientific) were used for immunofluorescence imaging. For western blot, anti‐ASK1 (EP553Y; Abcam) and anti‐α‐tubulin (Santa Cruz) antibodies were used.
2.11. Transfection
siRNA transfection for RAW264.7 cells was performed using Lipofectamine RNAiMAX (Invitrogen), in accordance with the manufacturer's instructions. siRNA sequences are listed in Table S1.
2.12. Construction of ASK1‐deficient RAW264.7 cells
Lentiviral plasmid vectors carrying Cas9 endonuclease, and sgRNA targeting Ask1 or carrying non‐targeting sgRNA, were prepared utilizing lentiCRISPRv2 plasmid (Addgene). The annealed oligos were cloned between the BsmBI restriction sites of the plasmid. The sequences of top and bottom oligos are listed in Table S1. To produce lentivirus, HEK293T cells were transfected with Ask1‐targeting or non‐targeting lentiviral vectors, psPAX2 and pCMV‐SVS‐G (Addgene) plasmids using Lipofectamine 3000 (Thermo Fisher) in accordance with the manufacturer's protocols. To reduce cytotoxicity, the medium was replaced at 5 h after incubation. At 2 d after transfection, the supernatants containing lentivirus were harvested and filtered with 0.45 µm PVDF filter (Millipore). RAW264.7 cells were transfected with lentivirus and supplemented with 8 µg/mL polybrene (Nacalai Tesque). The medium was replaced at 1 d after transfection. Next day, the transfected cells were selected with 4 µg/mL puromycin (GIBCO).
2.13. Preparation of lysates
RAW264.7 cells were lysed with immunoprecipitation (IP) lysis buffer + DOC (20 mM Tris‐HCl pH 7.5, 150 mM NaCl, 4 mM EDTA pH8.0, 1% sodium deoxycholate, 1% Triton X‐100, 1 mM phenylmethylsulfonyl fluoride, 5 µg/mL leupeptin). The lysates were cleared by centrifugation, mixed with an equal amount of 2× loading buffer (4% SDS, 100 mM Tris‐HCl pH 8.8, 10% bromophenol blue, 36% glycerol and 10 mM dithiothreitol) and subjected to western blot after boiling.
2.14. Western blot
Lysates were resolved on SDS‐PAGE and electroblotted onto PVDF membranes. After blocking with 5% skimmed milk in TBS‐T (50 mM Tris‐HCl, 150 mM NaCl and 0.05% Tween 20, pH 8.0), the membranes were probed with appropriate antibodies. The antibody‐antigen complexes were detected using the ECL system (GE Healthcare).
2.15. Immunofluorescence imaging
Whole embryos were dissected at embryonic day 14.5 and fixed in 4% paraformaldehyde (PFA)/PBS at 4°C for 15‐20 min. Back skin was peeled off and further fixed in 4% PFA/PBS at 4°C overnight. Tissues were washed with 0.2% Triton X‐100/PBS (PBT) at 4°C for 30 min, blocked in 1% bovine serum albumin/PBT for 1 h at room temperature (r.t.) and stained with primary antibodies in blocking solution at 4°C overnight. Tissues were washed with PBT, followed by staining with secondary antibodies in blocking solution at 4°C for overnight. Tissues were washed with PBT and were flat‐mounted on slide glasses. Images were obtained using a fluorescence microscope BZ‐X710 (Keyence).
2.16. Statistical analysis
All data were presented as mean ± SEM. Unpaired two‐tailed Student t test, two‐way ANOVA followed by Bonferroni multiple comparisons test or one‐way ANOVA followed by Tukey‐Kramer multiple comparisons were used. Statistical tests were carried out using the statistical programming language R and its available packages.
3. RESULTS
3.1. ASK1 deficiency in mice enhances tumor cell clearance and upregulates immune response‐related genes
To study the involvement of ASK1 in tumor metastasis in more detail, we used Lewis lung carcinoma cells stably expressing luciferase (3LL‐Luc2 cells), in an experimental lung metastasis model as described previously. 13 In our previous report, we did not investigate the potential involvement of ASK1 at the early stage of tumor metastasis, such as the initial hours after tumor inoculation. We therefore assessed the time‐dependent changes in the luciferase activity of the lung lysates from 10 min to 24 h after iv injection of 3LL‐Luc2 cells. In an experimental metastasis model, the accumulation of tumor cells in the lung peaked at 10 min after tumor cell inoculation. 14 We found no significant difference in the luciferase activity of the lung lysates at 10 min after injection between WT and ASK1−/− mice, suggesting that 3LL‐Luc2 cell circulation is unaffected by ASK1 deficiency. In contrast, ASK1−/− mice had less luciferase activity in the lung lysates at 3, 18 and 24 h after injection compared with WT mice (Figure 1A). Moreover, luciferase activity in the lung lysates of ASK1−/− mice was also decreased when injected with as many as 1 × 106 cells/mouse of 3LL‐Luc2 cells (Figure 1B). Of note, ASK1−/− mice showed essentially the same phenotype 3 h after injection with murine sarcoma MCA205 cells stably expressing luciferase (MCA205‐Luc2 cells) (Figure 1C). Because the extravasation of iv injected tumor cells starts approximately 3 h in an experimental lung metastasis model, 15 the rapid decrease in the luciferase activity of the lung lysates from 10 min to 3 h points to the lysis of the injected tumor cells in the lung microvasculature. Therefore, the accelerated decrease in luciferase activity in ASK1−/− mice indicates that intravascular tumor cell clearance is promoted in these mice.
FIGURE 1.
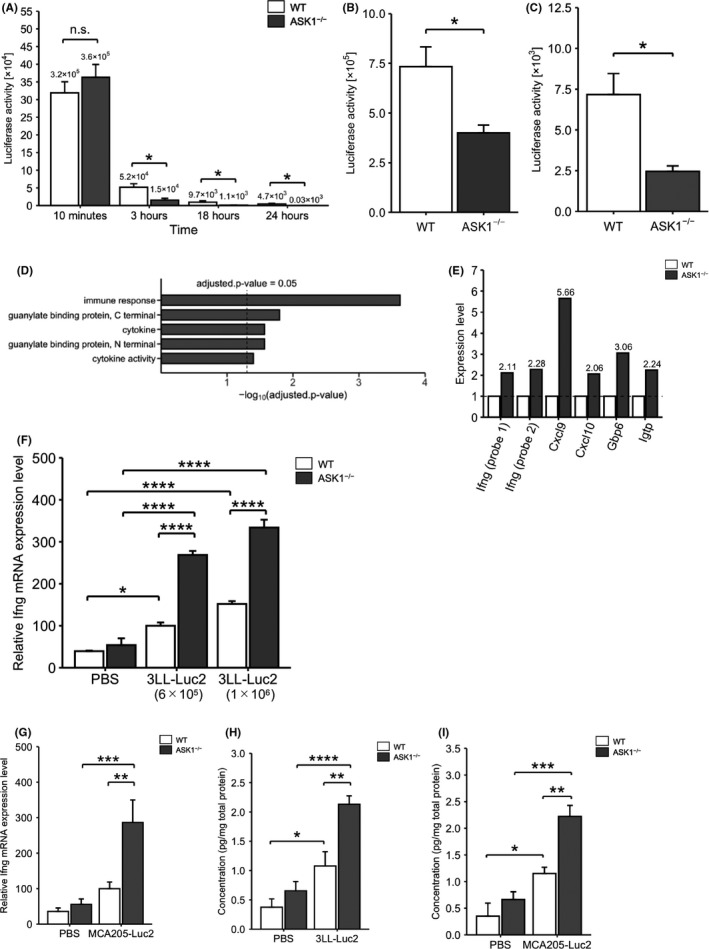
ASK1−/− mice show enhanced tumor cell clearance and upregulation of immune response‐related genes. A‐C, Luciferase activity of the lung lysates at the indicated time points after iv injection of 6 × 105 cells/mouse for 3LL‐Luc2 cells (A; 18 h: n = 6, other groups: n = 3) or at 3 h after iv injection of 1 × 106 cells/mouse for 3LL‐Luc2 cells (B; WT: n = 6, ASK1−/−: n = 5) or MCA205‐Luc2 cells (C; n = 3). D, DAVID pathway analysis of candidate genes whose expression was upregulated more than 2‐fold in the lungs of ASK1−/− mice in DNA microarray. E, Expression ratios of Ifng and the downstream genes in DNA microarray. F, G, Ifng mRNA expression in the lungs at 3 h after iv injection of 3LL‐Luc2 cells (F), MCA205‐Luc2 cells (G), or PBS. E, n = 3. F, WT‐PBS, ASK1−/−‐PBS: n = 3, ASK1−/−‐3LL‐Luc2 (1 × 106 cells/mouse): n = 5, other groups: n = 6. G, WT‐PBS, ASK1−/−‐PBS: n = 6, WT‐MCA205‐Luc2: n = 5, ASK1−/−‐MCA205‐Luc2: n = 4. H, I, IFNγ protein concentration in the lungs at 3 h after iv injection of 3LL‐Luc2 cells (H), MCA205‐Luc2 cells (I), or PBS. H, ASK1−/−‐3LL‐Luc2: n = 6, other groups: n = 4, I, n = 3. Unpaired Student t test (A‐C) or two‐way ANOVA followed by Bonferroni's multiple comparisons (F‐I). *P < .05, **P < .01, ***P < .001, ****P < .0001
To explore the mechanism by which ASK1−/− mice show resistance against early metastasis, we performed a DNA microarray using lungs from WT and ASK1−/− mice 3 h after iv injection of 3LL‐Luc2 cells. Gene ontology analysis revealed that immune response‐related genes, including interferon‐gamma (Ifng; encoding IFNγ) and its downstream genes, C‐X‐C motif chemokine ligand 9 (Cxcl9), Cxcl10, 16 guanylate binding protein family member 6 (Gbp6) 17 and interferon gamma‐induced GTPase (Igtp), 18 were upregulated in the lungs of ASK1−/− mice (Figure 1D,E). IFNγ is a well known anti‐tumor and anti‐metastatic cytokine. 5 Quantitative PCR analysis confirmed the upregulation of Ifng mRNA in the lungs of ASK1−/− mice at 3 h after iv injection of 6 × 105 or 1 × 106 cells/mouse 3LL‐Luc2 cells (Figure 1F) or MCA205‐Luc2 cells (Figure 1G). Furthermore, the protein level of IFNγ was also enhanced (Figure 1H,I). These data suggest that ASK1 regulates the expression of immune response‐related genes in response to tumor inoculation. We have previously revealed that lung metastasis is attenuated in ASK1−/− mice 14 d after iv injection of melanoma B16F10 cells stably expressing luciferase (B16F10‐luc‐G5 cells) as well as 3LL‐Luc2 cells. 13 However, at 3 h after injection of B16F10‐luc‐G5 cells, WT and ASK1−/− mice had comparable luciferase activity in the lung lysates (Figure S1A). Consistently, Ifng mRNA was not increased in the lungs of ASK1−/− mice (Figure S1B). Therefore, in contrast with the results with 3LL‐Luc2 cells and MCA205‐Luc2 cells, ASK1−/− mice did not show enhancement of anti‐metastatic immune response against B16F10‐luc‐G5 cells (see Section 4).
3.2. NK cells trigger enhanced tumor cell clearance in ASK1−/− mice
NK cells are innate immune cells that can rapidly recognize and kill tumor cells and produce high levels of IFNγ. 5 , 19 Considering that the lungs of ASK1−/− mice had decreased tumor cells and enhanced IFNγ expression as early as 3 h after iv injection of tumor cells, we investigated the involvement of NK cells. We depleted NK cells using anti‐asialo GM1 antibody before 3LL‐Luc2 cell inoculation (Figure 2A). As expected, NK cell depletion abolished the resistance against metastasis observed in ASK1−/− mice (Figure 2B). Additionally, Ifng mRNA upregulation in the lungs of ASK1−/− mice was completely eliminated by treatment with anti‐asialo GM1 antibody (Figure 2C). These data indicated that NK cell‐dependent intravascular tumor cell clearance is enhanced in ASK1−/− mice.
FIGURE 2.
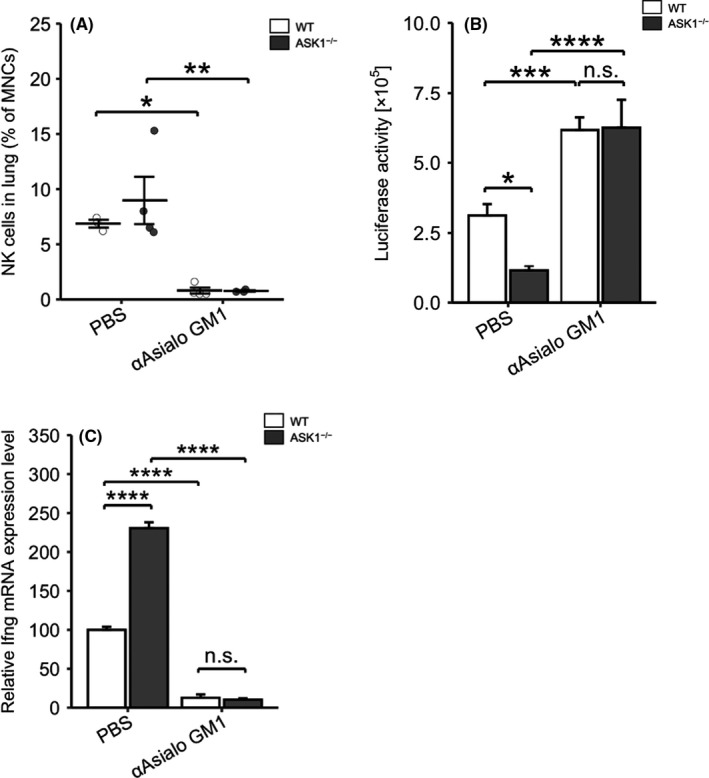
NK cells are required for enhanced tumor cell clearance in ASK1−/− mice. A, Ratio of NK cells in mononucleated cells (MNCs) in the lungs at 2 d after treatment with anti‐asialo GM1 antibody or PBS (WT‐PBS, ASK1−/−‐αAsialo GM1: n = 3, ASK1−/−‐PBS, WT‐αAsialo GM1: n = 4). B, Luciferase activity of the lung lysates of PBS‐ or anti‐asialo GM1 antibody‐treated mice at 3 h after iv injection of 3LL‐Luc2 cells (ASK1−/−‐αAsialo GM1: n = 7, other groups: n = 8). C, Ifng mRNA expression in the lungs of PBS‐ or anti‐asialo GM1 antibody‐treated mice at 3 h after iv injection of 3LL‐Luc2 cells (WT‐PBS, ASK1−/−‐PBS: n = 6, WT‐αAsialo GM1: n = 5, ASK1−/−‐αAsialo GM1: n = 4). Two‐way ANOVA followed by Bonferroni's multiple comparisons. n.s.: nonsignificant, *P < .05, ***P < .001, ****P < .0001
3.3. ASK1 in NK cells is dispensable for NK cell‐mediated tumor cell clearance
To examine whether ASK1 in NK cells contributes to intravascular tumor cell clearance, we generated NK cell‐specific ASK1‐deficient mice (Ncr1‐cre; ASK1F/F mice). Surprisingly, Ncr1‐cre; ASK1F/F mice and the corresponding control ASK1F/F mice had comparable luciferase activity in the lung lysates at 3 h after iv injection of 3LL‐Luc2 cells (Figure 3A) or MCA205‐Luc2 cells (Figure 3B). Similarly, Ifng mRNA in the lungs was not enhanced in Ncr1‐cre; ASK1F/F mice (Figure 3C,D). These results suggested that ASK1 in host cells other than NK cells regulated NK cell‐mediated tumor cell clearance.
FIGURE 3.
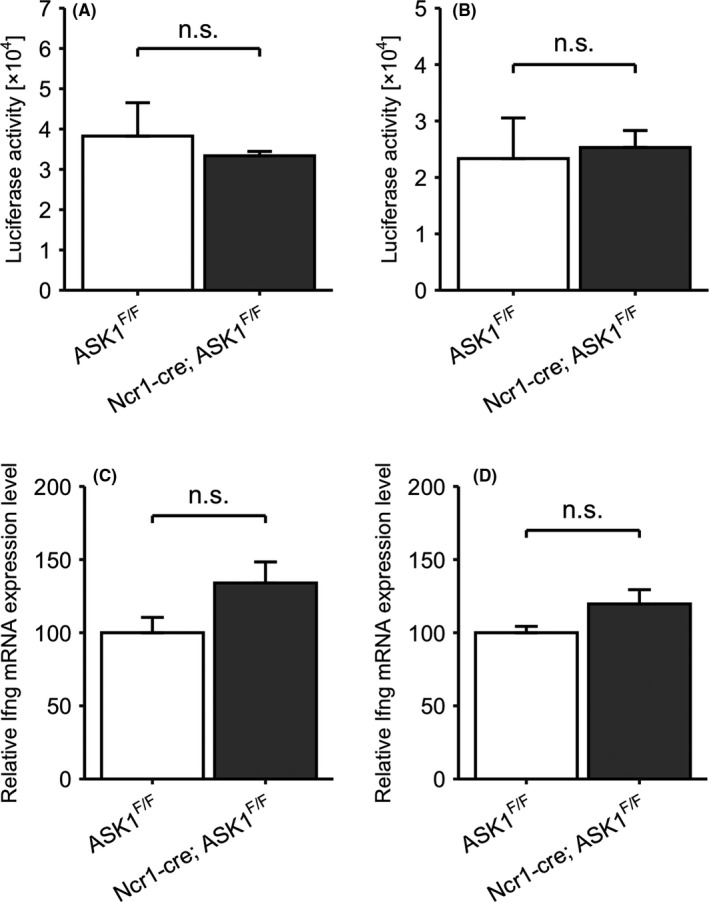
ASK1 in NK cells is dispensable for NK cell‐mediated tumor cell clearance. A, B, Luciferase activity of the lung lysates at 3 h after iv injection of 3LL‐Luc2 cells (A) or MCA205‐Luc2 cells (B) (n = 3). C, D, Ifng mRNA expression in the lungs at 3 h after iv injection of 3LL‐Luc2 cells (C) or MCA205‐Luc2 cells (D) (n = 3). Unpaired Student t test. n.s.: nonsignificant
We have previously revealed using bone marrow chimeric mice that ASK1 in both bone marrow‐derived cells and the other types of cells from recipient mice contributed to tumor metastasis. We focused on bone marrow‐derived cells and revealed that platelet‐intrinsic ASK1 facilitates tumor metastasis, while myeloblast‐intrinsic ASK1 does not. 13 Platelets promote intravascular tumor cell survival by forming aggregates with them. 20 Therefore, platelet‐intrinsic ASK1 was expected to enhance intravascular tumor cell survival. However, at 3 h after iv injection of 3LL‐Luc2 cells, platelet‐specific ASK1‐deficient mice (Pf4‐cre; ASK1F/F mice; Figure S2A) and myeloblast‐specific ASK1‐deficient mice (Lysm‐cre; ASK1F/F mice; Figure S2B) had comparable luciferase activity in the lung lysates to ASK1F/F mice. These data indicated that ASK1 in platelets and myeloblasts contributed only slightly to NK cell‐mediated tumor cell clearance.
More recently, endothelial overexpression of ASK1 in mice was reported to enhance ovarian cancer dissemination (local metastasis) through VE‐cadherin degradation and transmigration of tumor‐associated macrophages. 21 However, whether endogenous ASK1 contributes to tumor dissemination or whether endothelial ASK1 regulates distal metastasis, such as lung metastasis, remains unknown. We therefore generated endothelial‐specific ASK1‐deficient mice (Cdh5‐cre; ASK1F/F mice). Both blood and lymphatic vascular structures in Cdh5‐cre; ASK1F/F mice were equivalent to those in ASK1F/F mice, suggesting that endothelial ASK1 had little impact on physiological vascular formation (Figure S2C). Consistent with the study mentioned above, 21 Cdh5‐cre; ASK1F/F mice showed a dramatic attenuation of lung metastasis at 14 d after iv injection of 3LL‐Luc2 cells (Figure S2D). At 3 h after injection, however, the luciferase activity in the lung lysates of Cdh5‐cre; ASK1F/F and ASK1F/F mice was comparable (Figure S2E). These results suggested that endothelial ASK1 had little effect on intravascular tumor cell clearance, although it regulates tumor metastasis at relatively late stages.
3.4. ASK1 deficiency in mice enhances cytokine production of NK cells and NK cell recruitment into the lung
As Ncr1‐cre; ASK1F/F mice did not show enhanced NK cell‐mediated tumor cell clearance, we speculated that cell non‐autonomous ASK1 deficiency other than NK cells enhanced cytokine production and/or recruited NK cells in response to tumor inoculation. First to examine the capacity of cytokine production of NK cells, we isolated NK cells from the lungs of WT and ASK1−/− mice at 3 h after tumor inoculation and evaluated cytokine expression. Similar to observations in whole lung lysates (Figure 1), iv injection of 3LL‐Luc2 cells induced the transcription of Ifng and Csf2 (encoding granulocyte‐macrophage colony‐stimulating factor; GM‐CSF) (Figure 4A), which is often observed in activated NK cells. 22 Ifng and Csf2 mRNA expression in naïve NK cells (PBS‐injected control) from the lungs of WT and ASK1−/− mice was indistinguishable (Figure 4A). We next evaluated NK cell recruitment into the lungs in response to tumor inoculation. Flow cytometry analysis revealed that the ratio of NK cells in mononucleated cells (MNCs) in the lungs of WT and ASK1−/− mice was comparable at the basal state or at 3 h after iv injection of 3LL‐Luc2 cells (Figure 4B). Interestingly, however, the NK cell ratio significantly increased in ASK1−/− mice at 1 h after injection (Figure 4B), when the NK cell number in the lung reached its peak in an experimental lung metastasis model. 7 Collectively, the enhanced recruitment and cytokine production of NK cells may contribute to the anti‐metastatic phenotype observed in ASK1−/− mice.
FIGURE 4.
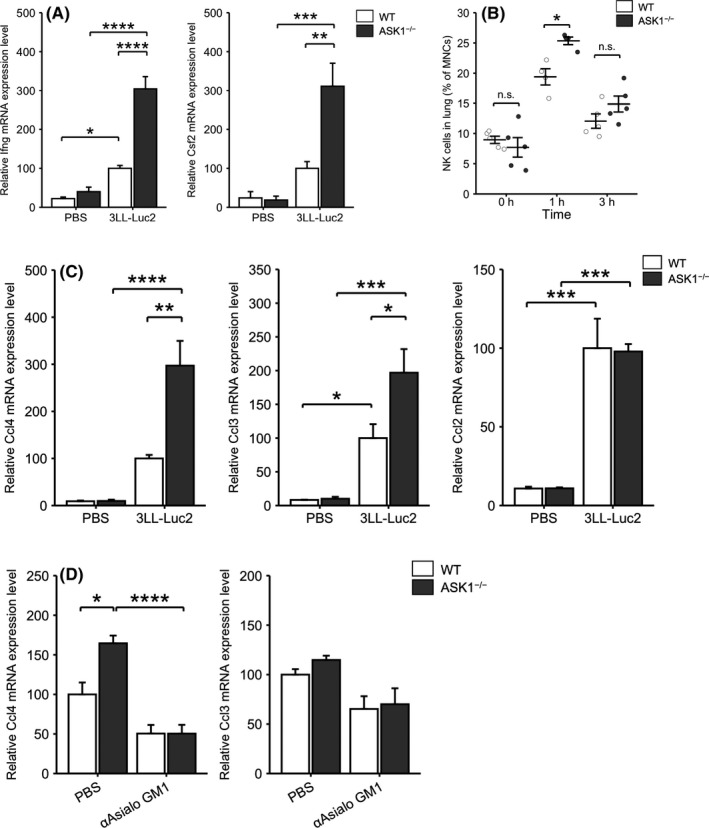
ASK1 deficiency in mice enhances cytokine production of NK cells and NK cell recruitment into the lung. A, Ifng (left) or Csf2 (right) mRNA expression in lung NK cells at 3 h after iv injection of 3LL‐Luc2 cells or PBS (WT‐PBS, ASK1−/−‐3LL‐Luc2: n = 4, ASK1−/−‐PBS: n = 3, WT‐3LL‐Luc2: n = 5). B, Ratio of NK cells in mononucleated cells (MNCs) in the lungs at the indicated time points (1 h: n = 4, other groups: n = 5). C, mRNA expression of various chemokines in the lungs at 1 h after iv injection of 3LL‐Luc2 cells or PBS (n = 4). D, Ccl4 (left) or Ccl3 (right) mRNA expression in the lungs of PBS‐ or anti‐asialo GM1 antibody‐treated mice at 1 h after iv injection of 3LL‐Luc2 cells (WT‐PBS: n = 3, other groups: n = 4). Two‐way ANOVA followed by Bonferroni's multiple comparisons. n.s.: nonsignificant, *P < .05, **P < .01, ***P < .001, ****P < .0001
Among various chemokines recruiting NK cells, 23 , 24 iv injection of 3LL‐Luc2 cells induced C‐C motif chemokine ligand 4 (Ccl4), Ccl3 and Ccl2 mRNA in the lungs of WT mice at 1 h after injection (Figure 4C). Although indistinguishable at the basal state, Ccl4 and Ccl3 were significantly upregulated in the lungs of ASK1−/− mice compared with WT mice at 1 h after injection of 3LL‐Luc2 cells (Figure 4C). We did not observe a significant difference in other major chemokines, including Ccl5, Ccl8, Cxcl10 and C‐X3‐C motif chemokine ligand 1 (Cx3cl1) (Figure S3), suggesting that CCL4 and CCL3 might be responsible for the ASK1‐mediated regulation of NK cell recruitment into the lung. Next, we depleted NK cells using anti‐asialo GM1 antibody followed by injection of 3LL‐Luc2 cells and analyzed Ccl4 and Ccl3 mRNA in the lungs at 1 h after tumor inoculation. Anti‐asialo GM1 antibody treatment abolished Ccl4 upregulation in the lungs of ASK1−/− mice (Figure 4D). Although we did not observe a significant difference in Ccl3 in the lungs of NK cell‐intact ASK1−/− mice, there was a trend of Ccl3 downregulation with anti‐asialo GM1 antibody treatment in WT and ASK1−/− mice (Figure 4D). Therefore, ASK1 deficiency in mice enhanced cytokine production of NK cells and NK cell recruitment into the lung in response to tumor cell inoculation.
3.5. ASK1 mediates the expression of the NKG2D ligands H60
Beside Ifng and other chemokines (Figure 1D), we found that NKG2D ligands H60a and H60b showed a more than 2‐fold increase in the lungs of ASK1−/− mice at 3 h after iv injection of 3LL‐Luc2 cells (Figure 5A). H60a and H60b belong to the H60 family and are stress‐induced ligands for NKG2D, which is a key activating receptor expressed on NK cells. 25 In C57BL/6 mice, H60a is known as a pseudogene. 26 Surprisingly, these 2 genes were sharply upregulated in the lungs of ASK1−/− mice in the basal state, as well as 1 h after injection of 3LL‐Luc2 cells (Figure 5B). To investigate the effect of ASK1 on H60 expression, we knocked down and knocked out Ask1 in mouse macrophage‐like cell line RAW264.7 using siRNA and CRISPR‐Cas9 system, respectively (Figure 5C,E). A previous report suggested that macrophages are one of the cell types that mediate tumor cell killing by NK cells through binding of NKG2D ligands to NKG2D expressed on NK cells. 27 In RAW264.7 cells, ASK1 knockdown and knockout markedly increased H60b expression but not H60a expression (Figure 5D,F). These results suggested that ASK1 negatively regulates H60b expression, which seems contradictory to the data showing that Lysm‐cre; ASK1F/F mice did not exhibit any anti‐metastatic response (Figure S2B). However, given that NKG2D ligands can be expressed on several cell types, H60b upregulation only in macrophages might be insufficient for NK cell activation (see Section 4).
FIGURE 5.
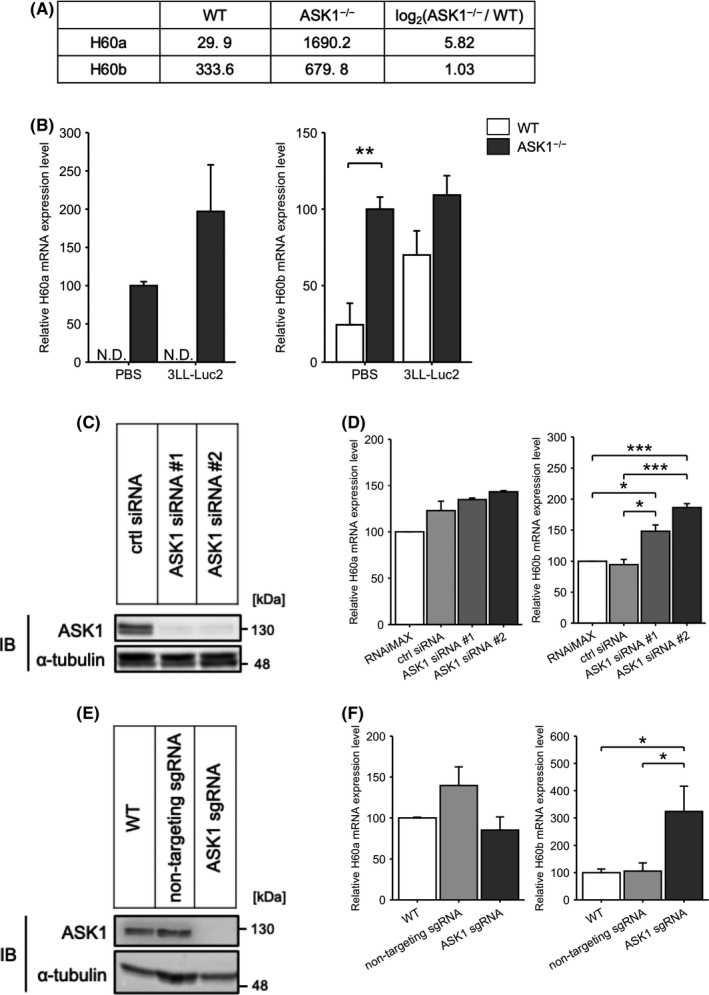
ASK1 mediates the expression of the NKG2D ligands H60. A, Signal intensity of H60a and H60b in the DNA microarray. B, H60a (left) or H60b (right) mRNA expression in the lungs at the basal state or 1 h after iv injection of 3LL‐Luc2 cells (n = 4). C, ASK1 protein expression in RAW246.7 cells transfected with the indicated siRNA. D, H60a (left) or H60b (right) mRNA expression in RAW246.7 cells transfected with the indicated siRNA (n = 3). E, ASK1 protein expression in RAW246.7 cells expressing Cas9 and the indicated sgRNA. F, H60a (left) or H60b (right) mRNA expression in RAW246.7 cells expressing Cas9 and the indicated sgRNA (n = 5). Unpaired Student t test (B) or one‐way ANOVA followed by Tukey‐Kramer multiple comparisons (D, F). *P < .05, **P < .01
4. DISCUSSION
In this study, we newly identified ASK1 as a negative regulator of NK cell‐mediated anti‐tumor immunity in experimental lung metastasis. We revealed that ASK1 regulates NK cell‐mediated tumor cell clearance in the initial hours of tumor metastasis. ASK1 deficiency enhanced cytokine production of NK cells and NK cell recruitment into the lung through upregulation of chemokines such as Ccl3 and Ccl4 in response to tumor inoculation, which might augment intravascular tumor cell clearance (Figure 6). Therefore, NK cells from the lungs of ASK1−/− mice can produce higher amount of cytokines and subsequently, more NK cells are recruited into the lung through CCL3 and CCL4, which forms a positive feedback loop to facilitate intravascular tumor cell clearance. In our phenotypic analysis with multiple types of tumor cells, ASK1−/− mice did not show an anti‐metastatic response against B16F10‐luc‐G5 cells (Figure S1A,B), in striking contrast to 3LL‐Luc2 cells (Figure 1A,B,F) and MCA205‐Luc2 cells (Figure 1C,G). In general, NKG2D is critical for NK cell activation. 28 Most of the tumor cells frequently used in experimental lung metastasis models, such as 3LL and MCA205 cells, express NKG2D ligands. 29 , 30 In addition, B16F10 cells do not express NKG2D ligands 29 and therefore might escape from NKG2D‐mediated NK cell surveillance. Hence our data supported the importance of NKG2D in enhancing intravascular tumor cell clearance observed in ASK1−/− mice.
FIGURE 6.
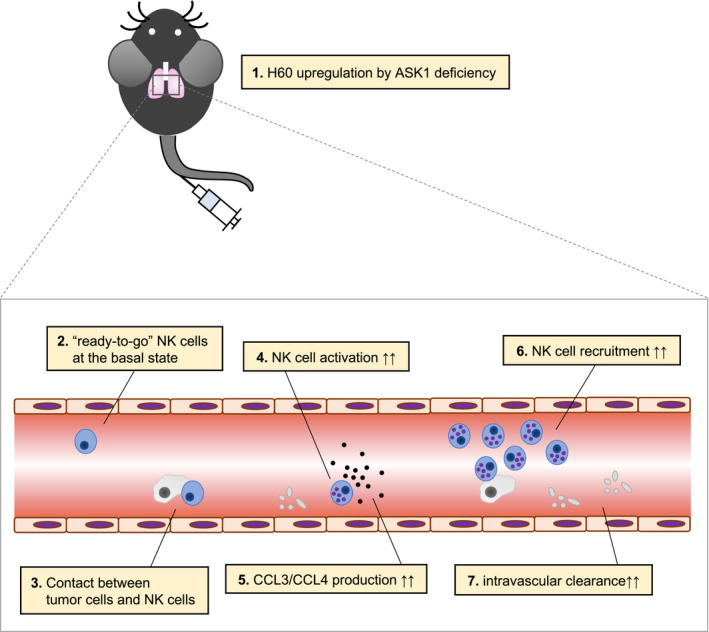
Schematic model for enhanced intravascular tumor cell clearance in ASK1−/− mice
ASK1 deficiency led to upregulation of the NKG2D ligands H60a and H60b in the lungs even without tumor cell inoculation (Figure 5B). However, there were minor differences in Ifng and Csf2 expression in the naïve NK cells of WT and ASK1−/− mice (Figure 4A,B). One of the possible reasons is that H60 upregulation did not exceed a threshold for cytokine production of NK cells but contributed to lowering the threshold. In other words, H60 upregulation by ASK1 deficiency without stimulation may lead to a “ready‐to‐go” state, but not a “hyperactivation” state, of NK cells in ASK1−/− mice. Only in response to additional NKG2D stimulation by tumor cells might NK cells in ASK1−/− mice enhance cytokine production. Meanwhile, enhancement in cytokine production of NK cells, NK cell recruitment into the lung and intravascular tumor cell clearance observed in ASK1−/− mice can be regulated by combination of H60 and other NKG2D ligands. NKG2D ligands, including H60, are transcriptionally induced by various stimuli. 28 Although macrophages expressed H60 (Figure 5D,F), Lysm‐cre; ASK1F/F mice did not show enhanced tumor cell clearance (Figure S2B), suggesting that H60 upregulation by ASK1 deletion in several cell types in addition to macrophages, and/or activation by other ligands might be required for full activation of NKG2D in NK cells and subsequent NK cell‐mediated tumor cell clearance. Identifying cell types that express H60b other than macrophages using single‐cell RNAseq would be the key to elucidate the whole mechanism of the anti‐metastatic phenotype observed in ASK1−/− mice.
In summary, our study indicated that ASK1 regulates the initial steps of tumor metastasis through enhanced cytokine production of NK cells and NK cell recruitment (Figure 6). Considering that ASK1 deficiency did not affect subcutaneous tumor growth 13 and that lung‐resident NK cells are superior to rapidly clear the metastatic invasion of cancer cells compared to other target organs, 31 the enhanced intravascular tumor cell clearance in ASK1−/− mice might be organ‐specific. To date, numerous small molecule drugs targeting ASK1 have been actively developed. 32 Among them, Selonsertib developed by Gilead was especially considered as a promising ASK1 inhibitor for treating nonalcoholic steatohepatitis (NASH). Although Selonsertib was ineffective in reducing NASH‐induced liver fibrosis in 2 clinical trials in phase 3, 33 our data demonstrated that ASK1 inhibition with this drug might be a potential strategy for cancer immunotherapy against tumor metastasis. Further development of ASK1 inhibitors for treating cancer is expected.
DISCLOSURE
The authors declare no potential conflicts of interest.
Supporting information
Fig S1‐S3
Table S1
ACKNOWLEDGMENTS
We thank Dr. Ayako Nishihara for technical assistance. We thank all the members of the Laboratory of Cell Signaling. This work was supported by the Japan Agency for Medical Research and Development (AMED) under the Project for Elucidating and Controlling Mechanisms of Aging and Longevity (JP20gm5010001 to HI), the Japan Society for the Promotion of Science (JSPS) under the Grant‐in‐Aid for Scientific Research on Innovative Areas (KAKENHI; JP26114009 to HI, JP17H06419 to IN), the Grants‐in‐Aid for Scientific Research (KAKENHI; JP18H03995 and JP18K19469 to HI, JP18H02569 and JP15K14445 to IN), the Grants‐in‐Aid for Challenging Research (KAKENHI; 18K19469 to HI), the Yasuda Medical Foundation (2017Y‐1 to HI) and the Japan Science and Technology Agency (JST) Moonshot R&D – MILLENNIA Program (JPMJMS2022‐18 to HI).
Fujimoto M, Kamiyama M, Fuse K, et al. ASK1 suppresses NK cell‐mediated intravascular tumor cell clearance in lung metastasis. Cancer Sci. 2021;112:1633–1643. 10.1111/cas.14842
Makoto Fujimoto and Miki Kamiyama contributed equally to this study.
Contributor Information
Miki Kamiyama, Email: kamiyama@mol.f.u-tokyo.ac.jp.
Hidenori Ichijo, Email: ichijo@mol.f.u-tokyo.ac.jp.
REFERENCES
- 1. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646‐674. [DOI] [PubMed] [Google Scholar]
- 2. Ichijo H, Nishida E, Irie K, et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275(5296):90‐94. [DOI] [PubMed] [Google Scholar]
- 3. Tobiume K, Matsuzawa A, Takahashi T, et al. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2(3):222‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Labelle M, Hynes RO. The initial hours of metastasis: the importance of cooperative host‐tumor cell interactions during hematogenous dissemination. Cancer Discov. 2012;2(12):1091‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Takeda K, Nakayama M, Sakaki M, et al. IFN‐γ production by lung NK cells is critical for the natural resistance to pulmonary metastasis of B16 melanoma in mice. J Leukoc Biol. 2011;90(4):777‐785. [DOI] [PubMed] [Google Scholar]
- 6. Teng MWL, Andrews DM, McLaughlin N, et al. IL‐23 suppresses innate immune response independently of IL‐17A during carcinogenesis and metastasis. Proc Natl Acad Sci USA. 2010;107(18):8328‐8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grundy MA, Zhang T, Sentman CL. NK cells rapidly remove B16F10 tumor cells in a perforin and interferon‐gamma independent manner in vivo. Cancer Immunol Immunother. 2007;56(8):1153‐1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spiegel A, Brooks MW, Houshyar S, et al. Neutrophils suppress intraluminal NK cell‐mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov. 2016;6(6):630‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hattori K, Naguro I, Okabe K, et al. ASK1 signalling regulates brown and beige adipocyte function. Nat Commun. 2016;7:11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kanki H, Suzuki H, Itohara S. High‐efficiency CAG‐FLPe deleter mice in C57BL/6J background. Exp Anim. 2006;55(2):137‐141. [DOI] [PubMed] [Google Scholar]
- 11. Eckelhart E, Warsch W, Zebedin E, et al. A novel Ncr1‐Cre mouse reveals the essential role of STAT5 for NK‐cell survival and development. Blood. 2011;117(5):1565‐1573. [DOI] [PubMed] [Google Scholar]
- 12. Kogata N, Arai Y, Pearson JT, et al. Cardiac ischemia activates vascular endothelial cadherin promoter in both preexisting vascular cells and bone marrow cells involved in neovascularization. Circ Res. 2006;98(7):897‐904. [DOI] [PubMed] [Google Scholar]
- 13. Kamiyama M, Shirai T, Tamura S, et al. ASK1 facilitates tumor metastasis through phosphorylation of an ADP receptor P2Y12 in platelets. Cell Death Differ. 2017;24(12):2066‐2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Purushotham AD, McCulloch P, George WD. Enhancement of pulmonary tumour seeding by human coagulation factors II, IX, X–an investigation into the possible mechanisms involved. Br J Cancer. 1991;64(3):513‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sindelar WF, Tralka TS, Ketcham AS. Electron microscopic observations on formation of pulmonary metastases. J Surg Res. 1975;18(2):137‐161. [DOI] [PubMed] [Google Scholar]
- 16. Rauch I, Müller M, Decker T. The regulation of inflammation by interferons and their STATs. JAKSTAT. 2013;2(1):e23820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martens S, Howard J. The interferon‐inducible GTPases. Annu Rev Cell Dev Biol. 2006;22:559‐589. [DOI] [PubMed] [Google Scholar]
- 18. Taylor GA, Jeffers M, Largaespada DA, Jenkins NA, Copeland NG, Vande Woude GF. Identification of a novel GTPase, the inducibly expressed GTPase, that accumulates in response to interferon gamma. J Biol Chem. 1996;271(34):20399‐20405. [DOI] [PubMed] [Google Scholar]
- 19. Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon‐gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75(2):163‐189. [DOI] [PubMed] [Google Scholar]
- 20. Gay LJ, Felding‐Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yin M, Zhou HJ, Zhang J, et al. ASK1‐dependent endothelial cell activation is critical in ovarian cancer growth and metastasis. JCI Insight. 2017;2(18):e91828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Costanzo MC, Kim D, Creegan M, et al. Transcriptomic signatures of NK cells suggest impaired responsiveness in HIV‐1 infection and increased activity post‐vaccination. Nat Commun. 2018;9(1):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robertson MJ. Role of chemokines in the biology of natural killer cells. J Leukoc Biol. 2002;71(2):173‐183. [PubMed] [Google Scholar]
- 24. Menten P, Wuyts A, Van Damme J. Macrophage inflammatory protein‐1. Cytokine Growth Factor Rev. 2002;13(6):455‐481. [DOI] [PubMed] [Google Scholar]
- 25. Takada A, Yoshida S, Kajikawa M, et al. Two novel NKG2D ligands of the mouse H60 family with differential expression patterns and binding affinities to NKG2D. J Immunol. 2008;180(3):1678‐1685. [DOI] [PubMed] [Google Scholar]
- 26. Zhang H, Hardamon C, Sagoe B, Ngolab J, Bui JD. Studies of the H60a locus in C57BL/6 and 129/Sv mouse strains identify the H60a 3’UTR as a regulator of H60a expression. Mol Immunol. 2011;48(4):539‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou Z, Zhang C, Zhang J, Tian Z. Macrophages help NK cells to attack tumor cells by stimulatory NKG2D ligand but protect themselves from NK killing by inhibitory ligand Qa‐1. PLoS One. 2012;7(5):e36928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3(10):781‐790. [DOI] [PubMed] [Google Scholar]
- 29. Smyth MJ, Swann J, Kelly JM, et al. NKG2D recognition and perforin effector function mediate effective cytokine immunotherapy of cancer. J Exp Med. 2004;200(10):1325‐1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hayakawa Y, Sato‐Matsushita M, Takeda K, Iwakura Y, Tahara H, Irimura T. Early activation and interferon‐γ production of tumor‐infiltrating mature CD27high natural killer cells. Cancer Sci. 2011;102(11):1967‐1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yamamoto Y, Miyazato K, Takahashi K, Yoshimura N, Tahara H, Hayakawa Y. Lung‐resident natural killer cells control pulmonary tumor growth in mice. Cancer Sci. 2018;109(9):2670‐2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fujisawa T. Therapeutic application of apoptosis signal‐regulating kinase 1 inhibitors. Adv Biol Regul. 2017;66:85‐90. [DOI] [PubMed] [Google Scholar]
- 33. Harrison SA, Wong VW‐S, Okanoue T, et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: results from randomized phase III STELLAR trials. J Hepatol. 2020;73(1):26‐39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S3
Table S1


