Abstract
Nivolumab can cause interstitial lung disease (ILD), which may be fatal; however, mortality risk factors have not been identified. This postmarketing study evaluated the poor prognostic factors of ILD in nivolumab‐treated patients with non–small cell lung cancer (NSCLC) in Japan. Clinical and chest imaging findings for each ILD case were assessed by an expert central review committee, and prognosis was evaluated by radiographic findings, including the presence/absence of peritumoral ground‐glass opacity (peritumoral‐GGO). Poor prognostic factors were identified by univariate and multivariate Cox regression analysis. Of the 238 patients with nivolumab‐induced ILD, 37 died. The main radiographic patterns of ILD were cryptogenic organizing pneumonia/chronic eosinophilic pneumonia–like (53.4%), faint infiltration pattern/acute hypersensitivity pneumonia–like (20.2%), diffuse alveolar damage (DAD)‐like (10.9%), and nonspecific interstitial pneumonia–like (6.3%). The main poor prognostic factors identified were DAD‐like pattern (highest hazard ratio: 10.72), ≤60 days from the start of nivolumab treatment to the onset of ILD, pleural effusion before treatment, lesion distribution contralateral or bilateral to the tumor, and abnormal change in C‐reactive protein (CRP) levels. Of the 37 deaths due to ILD, 17 had DAD‐like radiographic pattern, three had peritumoral‐GGO, and five had a change in radiographic pattern from non‐DAD at the onset to DAD‐like. Patients with NSCLC who develop ILD during nivolumab treatment should be managed carefully if they have poor prognostic factors such as DAD‐like radiographic pattern, onset of ILD ≤60 days from nivolumab initiation, pleural effusion before nivolumab treatment, lesion distribution contralateral or bilateral to the tumor, and abnormal changes in CRP levels.
Keywords: computed X‐ray tomography, interstitial lung diseases, nivolumab, non‐small‐cell lung carcinoma, prognostic factors
This postmarketing study showed that diffuse alveolar damage (DAD)‐like pattern, ≤60 days from the start of nivolumab treatment to the onset of interstitial lung disease (ILD), pleural effusion before treatment, lesion distribution contralateral or bilateral to the tumor, and abnormal change in C‐reactive protein levels were common poor prognostic factors of ILD in nivolumab‐treated patients with non–small cell lung cancer (NSCLC). Of the 37 deaths due to ILD, 17 had DAD‐like radiographic pattern, and five had a change in radiographic pattern from non‐DAD at the onset to DAD‐like. Patients with NSCLC who develop ILD during nivolumab treatment should be managed carefully if they have poor prognostic factors.
Abbreviations
- CEP
chronic eosinophilic pneumonia
- CIP
checkpoint inhibitor pneumonitis
- COP
cryptogenic organizing pneumonia
- CRP
C‐reactive protein
- DAD
diffuse alveolar damage
- ECRC
expert central review committee
- EGFR
epidermal growth factor receptor
- GGO
ground‐glass opacity
- HP
hypersensitivity pneumonia
- HR
hazard ratio
- ILD
interstitial lung disease
- KL‐6
Krebs von den Lungen‐6
- NSCLC
non–small cell lung cancer
- NSIP
nonspecific interstitial pneumonia
- PD‐1
programmed death‐1
- PD‐L1
programmed death‐ligand 1
- PMS
postmarketing surveillance
- PR
partial response
- TKI
tyrosine kinase inhibitor
1. INTRODUCTION
The immune checkpoint inhibitor nivolumab is a human monoclonal antibody against programmed death‐1 (PD‐1). 1 Nivolumab inhibits binding of PD‐1 to programmed death‐ligand 1 (PD‐L1) or 2 (PD‐L2), thereby enhancing the immune response to tumors. 1 Nivolumab is the first approved human anti‐human PD‐1 monoclonal antibody; it was initially approved in Japan in 2014 for the treatment of unresectable or metastatic melanoma and has subsequently been approved for the treatment of other types of cancer including non–small cell lung cancer (NSCLC). 2 The efficacy of nivolumab in previously treated patients with advanced NSCLC was demonstrated in the randomized phase 3 CheckMate‐017 and CheckMate‐057 studies. 3 , 4 Pooled analysis of the CheckMate‐017 and CheckMate‐057 studies showed that nivolumab was associated with a 5‐year survival rate of 13.4% compared with 2.6% for docetaxel. 5 Overall, nivolumab has a favorable safety profile. 1 , 3 , 4 However, owing to its mechanism of action, nivolumab is associated with immune‐related adverse events, including interstitial lung disease (ILD)/pneumonitis. 1 , 3 , 4 ILD may lead to a fatal outcome and therefore requires careful attention.
In the global CheckMate‐017 and CheckMate‐057 trials, the incidence of nivolumab‐associated ILD or pneumonitis was 4.6% (6/131 patients) and 3.5% (10/287 patients), respectively. 3 , 4 In Japanese patients with NSCLC, rates of nivolumab‐associated ILD of 7.2% and 9.6% have been reported in a pooled analysis of two phase 2 studies 6 (n = 111) and a postmarketing surveillance (PMS) study (n = 3606), respectively. 7 Nivolumab‐associated ILD may result in death; however, whether a particular type of ILD is related to death is not yet clear. Baba et al 8 published a study on imaging characteristics of different types of ILD and their outcomes in patients treated with nivolumab in NSCLC. However, this study did not identify the mortality risk factors related to nivolumab‐associated ILD.
Understanding the characteristics of nivolumab‐associated ILD and identifying mortality risk factors would allow more careful observation of the clinical course of ILD. The aim of the current study was to evaluate radiographic characteristics and poor prognostic factors of ILD in nivolumab‐treated patients with NSCLC in cases of ILD reported by treating physicians as part of a PMS for nivolumab in Japan.
2. PATIENTS AND METHODS
2.1. Study design
This was a retrospective observational postmarketing study of patients with NSCLC receiving nivolumab treatment in Japan.
2.2. Study population
Previously treated patients with NSCLC who experienced ILD while receiving nivolumab and who had chest radiographic and clinical findings available were eligible for this study. An ILD expert central review committee (ECRC) assessed radiographic images (computed tomography, X‐ray) and clinical data for each nivolumab‐treated patient. The ECRC consisted of eight radiologists and eight pulmonologists. For each case, two radiologists independently evaluated radiographs and two pulmonologists independently evaluated clinical data. Patients determined by the ECRC to fall into one of the following categories were considered not to have nivolumab‐associated ILD and were excluded from the analyses: primary disease exacerbation; infectious disease; ILD not associated with nivolumab treatment; not evaluable; and another drug could not be ruled out as the cause of ILD.
2.3. Data collection
Evaluations included the antitumor effect of nivolumab, ILD radiographic pattern, presence/absence of peritumoral ground‐glass opacity (peritumoral‐GGO; composed mainly of GGOs confined to the area around the tumor, previously called peritumoral infiltration 8 ), and clinical outcomes. Data were collected on the following: ILD type classification (typical or atypical); time from the initial dose of nivolumab to the onset of ILD; severity of ILD before treatment; pleural effusion before treatment; emphysema before treatment; percentage of normal lung tissue (≤50% or >50%) before treatment; presence/absence of honeycomb lung before treatment; presence/absence of GGO, pulmonary consolidation, reticular pattern, and traction bronchiectasis at ILD onset; change in pleural effusion at ILD onset; lesion distribution; ILD radiographic pattern (as defined below); presence/absence of peritumoral‐GGO; radiotherapy history; effect of steroid treatment; and abnormal changes in Krebs von den Lungen‐6 (KL‐6), lactate dehydrogenase (LDH), and C‐reactive protein (CRP). Changes in KL‐6, LDH, and CRP were based on laboratory values for each parameter and were confirmed at the discretion of the ECRC. Abnormal changes were determined as relatively large elevations in the levels of these parameters after ILD onset compared with their levels before nivolumab treatment. If the laboratory value was within the normal range at the time of ILD onset, or there was no apparent increase compared with before nivolumab treatment, a classification of no abnormal change was determined. Patients determined by the ECRC to have nivolumab‐associated ILD were classified by the ECRC into one of the following radiographic patterns: acute interstitial pneumonia/diffuse alveolar damage (DAD)‐like pattern characterized by GGOs or consolidations predominantly in dependent lung regions, which may be accompanied by linear opacities corresponding to lung volume loss and traction bronchiectasis; faint infiltration/acute hypersensitivity pneumonia (HP)‐like pattern characterized by diffuse GGOs and centrilobular nodularities, sometimes accompanied by air trapping; cryptogenic organizing pneumonia (COP)‐like pattern characterized by multifocal bilateral parenchymal consolidations with peripheral and lower‐lung distribution, sometimes accompanied by GGOs and reticular opacities; chronic eosinophilic pneumonia (CEP)‐like pattern characterized by GGOs, infiltrative shadows, nodule‐like shadows, mediastinal lymphadenopathy, pleural effusion, interlobular septum, and thickening of bronchovascular bundles; nonspecific interstitial pneumonia (NSIP)‐like pattern characterized by GGOs and reticular opacities predominantly in peripheral and lower‐lung distribution, sometimes accompanied by traction bronchiectasis and lower‐lobe volume loss; and “other.” 9 , 10 For patients who displayed multiple radiographic patterns, classification was assigned according to the most prominent feature exhibited. In addition, patients with atypical radiographic features were classified according to the presence/absence of peritumoral‐GGO, exacerbation of radiation fibrosis (GGO or consolidation around radiation fibrosis consistent with the radiation field), intensified infections (diffuse pulmonary infiltration radiologically resembling interstitial pneumonia with exacerbation of subclinical infection), or abnormal opacities largely confined to the lung ipsilateral to the tumor. 8
2.4. Statistical analysis
Univariate analysis was performed for all prognostic factors for nivolumab‐associated ILD (Fine and Gray model 11 ) on which data were collected. Poor prognosis was defined as ILD‐associated death, as determined by the ECRC. Eleven poor prognostic factors of high clinical significance were identified. Of these, DAD‐like pattern had the highest hazard ratio (HR) in the univariate analysis and hence was identified as a poor prognostic factor, and the other 10 factors were considered candidate poor prognostic factors. To examine these poor prognostic factors, multivariate analyses were performed using the Fine and Gray model 11 as in univariate analyses. For the multivariate analysis, DAD‐like pattern was paired one on one with each of the remaining 10 prognostic factors, and poor prognostic factors were identified using HR of <0.5 or >2 as a guide.
3. RESULTS
3.1. Patient disposition
Of the 325 nivolumab‐treated patients with NSCLC who reported symptoms of ILD between December 17, 2015 and March 31, 2016, 273 patients were determined by the ECRC to have ILD (Figure 1). The cause of ILD was determined to be nivolumab for 238 of the 273 patients with ILD; the effects of other agents (eg, epidermal growth factor receptor tyrosine kinase inhibitors [EGFR TKIs]) could not be excluded for the remaining 35 patients (Figure 1). In total, 37 of the 238 patients died of ILD.
FIGURE 1.
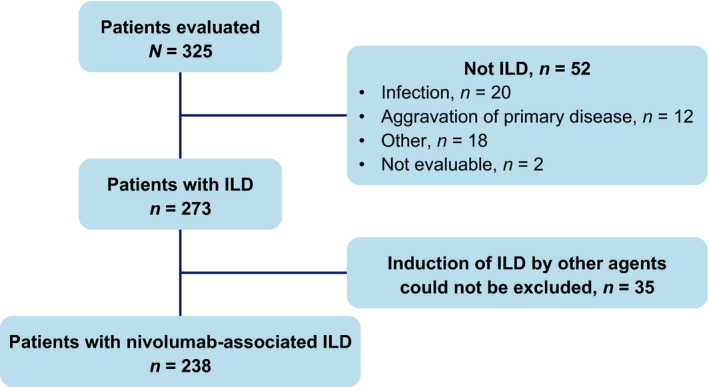
Patient disposition. ILD, interstitial lung disease
3.2. Prognosis by ILD radiographic pattern and peritumoral‐GGO
In this population of 238 patients with NSCLC and nivolumab‐associated ILD, the most common radiographic pattern of ILD was COP/CEP‐like (53.4%), followed by faint infiltration/acute HP‐like (20.2%) and DAD‐like (10.9%) (Table 1, Figure 2). Overall, 11.3% of patients had peritumoral‐GGO, and 87.8% of patients did not have peritumoral‐GGO (Table 1, Figure 2). Of the 37 patients who died of ILD, 17 patients had DAD‐like radiographic pattern (Table 1).
TABLE 1.
Prognosis by ILD radiographic pattern and peritumoral‐GGO status
| Factor | All patients (N = 238) | Patients survived (n = 201) a | Patients died of ILD (n = 37) a |
|---|---|---|---|
| ILD radiographic pattern, n (%) | |||
| DAD‐like | 26 (10.9) | 9 (34.6) | 17 (65.4) |
| Faint infiltration/acute HP‐like | 48 (20.2) | 46 (95.8) | 2 (4.2) |
| COP/CEP‐like | 127 (53.4) | 113 (89.0) | 14 (11.0) |
| NSIP‐like | 15 (6.3) | 14 (93.3) | 1 (6.7) |
| Other | 22 (9.2) | 19 (86.4) | 3 (13.6) |
| Peritumoral‐GGO status, n (%) a | |||
| Peritumoral‐GGO | 27 (11.3) | 24 (88.9) | 3 (11.1) |
| Non–peritumoral‐GGO | 209 (87.8) | 175 (83.7) | 34 (16.3) |
| Undeterminable | 2 (0.8) | 2 (100) | 0 |
Abbreviations: CEP, chronic eosinophilic pneumonia; COP, cryptogenic organizing pneumonia; DAD, diffuse alveolar damage; GGO, ground‐glass opacity; HP, hypersensitivity pneumonia; ILD, interstitial lung disease; NSIP, nonspecific interstitial pneumonia.
For each ILD radiographic pattern and peritumoral‐GGO status category, the percentage of patients who survived and the percentage of patients who died of ILD were calculated as a percentage of the subset of patients with the indicated radiographic pattern or peritumoral‐GGO status.
FIGURE 2.
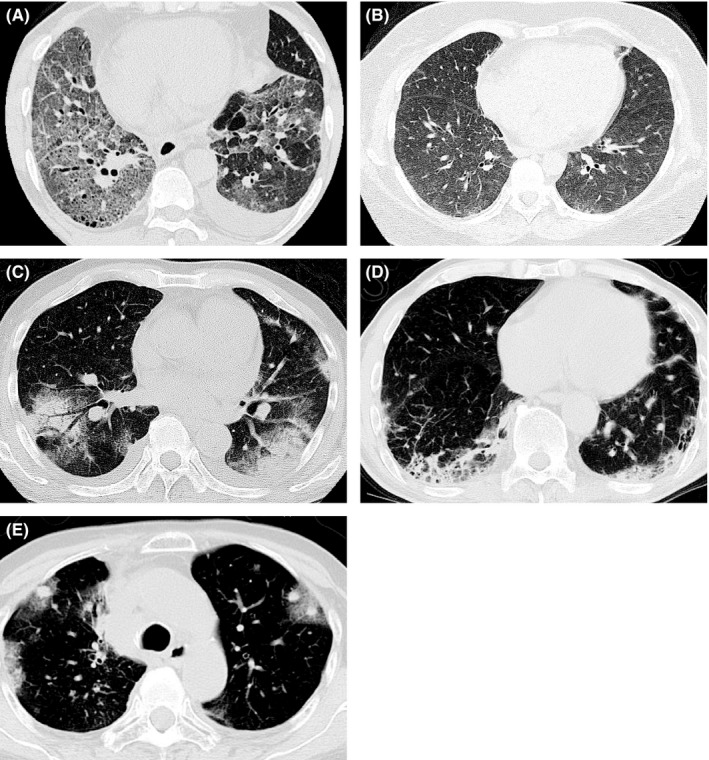
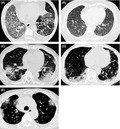
Representative radiological patterns of nivolumab‐related interstitial lung disease (ILD). A, Typical diffuse alveolar damage (DAD)‐like radiographic pattern in a 57‐year‐old male with squamous non–small cell lung cancer (NSCLC). Ground‐glass opacities (GGOs) were apparent in almost the whole lung field. Distorted interlobular septum, pleura, and vessels indicated a DAD‐like pattern. B, Typical faint infiltration/hypersensitivity pneumonia (HP)‐like ILD radiographic pattern in a 44‐year‐old female with lung adenocarcinoma. Faint infiltration was apparent in both lung fields. C, Typical cryptogenic organizing pneumonia (COP)/chronic eosinophilic pneumonia (CEP)‐like ILD radiographic pattern in a 74‐year‐old male with squamous NSCLC. Patchy GGO with partial consolidation was distributed in both peripheral lung fields. D, Typical nonspecific interstitial pneumonia (NSIP)‐like ILD radiographic pattern in a 68‐year‐old male with lung adenocarcinoma. Peribronchovascular consolidation was observed. E, Typical peritumoral‐GGO in a 70‐year‐old female with lung adenocarcinoma. GGO surrounding tumors was apparent after nivolumab treatment
3.3. Prognostic risk factor analysis
The risk factor most strongly linked with ILD‐associated mortality was DAD‐like radiographic pattern (HR: 10.72; 95% confidence interval [CI]: 5.65‐20.34) (Table 2). Ten poor prognostic factors of high clinical relevance (based on omission of predicted confounding factors [eg, typical vs atypical pattern and lesion distribution/peritumoral‐GGO or traction bronchiectasis and DAD‐like pattern]) were identified for ILD‐associated mortality among univariate risk factor analyses. These 10 poor prognostic factors were ≤60 days from the start of treatment to the onset of ILD, mild to moderate to severe ILD before treatment, ≤50% of normal lung tissue, no emphysema before treatment, pleural effusion before treatment, no peritumoral‐GGO, no history of radiotherapy, lesion distribution contralateral to tumor and bilateral, abnormal fluctuation in KL‐6, and abnormal change in CRP (Table 2).
TABLE 2.
Univariate analysis of poor prognostic factors for ILD‐associated death
| Risk factor | Category |
Patients n |
Patients died of ILD, n (%) | Category comparison | Hazard ratio (95% CI) | |
|---|---|---|---|---|---|---|
| Patient clinical characteristics | Gender | Male | 200 | 30 (15.0) | Male vs female | 0.81 (0.35‐1.85) |
| Female | 38 | 7 (18.4) | ||||
| Age, years | <65 | 84 | 10 (11.9) | ≥65 vs <65 | 1.50 (0.72‐3.13) | |
| ≥65 | 154 | 27 (17.5) | ||||
| <75 | 188 | 30 (16.0) | ≥75 vs <75 | 0.93 (0.41‐2.13) | ||
| ≥75 | 50 | 7 (14.0) | ||||
| ECOG PS | 0‐1 | 188 | 22 (11.7) | 2‐4 vs 0‐1 | 3.32 (1.73‐6.38) | |
| 2‐4 | 50 | 15 (30.0) | ||||
| Smoking history | Non‐smoker | 29 | 9 (31.0) | Smoker vs non‐smoker | 0.40 (0.19‐0.84) | |
| Smoker | 204 | 27 (13.2) | ||||
| Unknown | 5 | 1 (20.0) | ||||
| Previous or comorbid disease | No | 52 | 9 (17.3) | Yes vs no | 0.77 (0.36‐1.68) | |
| Yes | 186 | 28 (15.1) | ||||
| Liver disease | No | 223 | 34 (15.2) | Yes vs no | 1.27 (0.42‐3.82) | |
| Yes | 15 | 3 (20.0) | ||||
| Kidney disease | No | 225 | 35 (15.6) | Yes vs no | 0.99 (0.23‐4.18) | |
| Yes | 13 | 2 (15.4) | ||||
| Heart disease | No | 216 | 32 (14.8) | Yes vs no | 1.54 (0.62‐3.80) | |
| Yes | 22 | 5 (22.7) | ||||
| Autoimmune disease | No | 226 | 36 (15.9) | Yes vs no | 0.50 (0.07‐3.60) | |
| Yes | 12 | 1 (8.3) | ||||
| Lung infection | No | 225 | 33 (14.7) | Yes vs no | 2.25 (0.80‐6.39) | |
| Yes | 13 | 4 (30.8) | ||||
| Number of prior chemotherapy regimens | 0 | 4 | 1 (25.0) | ≥3 vs 0‐2 | 1.35 (0.71‐2.57) | |
| 1 | 64 | 10 (15.6) | ||||
| 2 | 68 | 8 (11.8) | ||||
| ≥3 | 102 | 18 (17.6) | ||||
| History of radiotherapy (including chest) | Yes | 100 | 11 (11.0) | No vs yes | 1.86 (0.93‐3.75) | |
| No | 135 | 26 (19.3) | ||||
| Not evaluable | 3 | 0 (0.0) | ||||
| ILD type classification | Typical | 152 | 30 (19.7) | Typical vs atypical | 3.06 (1.27‐7.33) | |
| Atypical | 85 | 6 (7.1) | ||||
| Not evaluable | 1 | 1 (100.0) | ||||
| Days from start of treatment to onset of ILD | ≤60 days | 151 | 34 (22.5) | ≤60 days vs >60 days | 7.57 (2.36‐24.33) | |
| >60 days | 87 | 3 (3.4) | ||||
| Image findings of existing lung before treatment | Severity of ILD | None | 156 | 21 (13.5) | Mild to moderate to severe vs none | 1.45 (0.76‐2.76) |
| Mild | 61 | 12 (19.7) | ||||
| Moderate | 16 | 3 (18.8) | ||||
| Severe | 3 | 1 (33.3) | ||||
| Undeterminable | 1 | 0 (0.0) | ||||
| Unknown | 1 | 0 (0.0) | ||||
| Presence of honeycomb lung | No | 222 | 32 (14.4) | Yes vs no | 2.09 (0.80‐5.45) | |
| Yes | 14 | 4 (28.6) | ||||
| Undeterminable | 1 | 1 (100.0) | ||||
| Percentage of normal lung tissue | >50% | 220 | 33 (15.0) | ≤50% vs >50% | 1.72 (0.60‐4.98) | |
| ≤50% | 17 | 4 (23.5) | ||||
| Unknown | 1 | 0 (0.0) | ||||
| Severity of emphysema | None | 115 | 18 (15.7) | None vs mild to moderate to severe | 1.03 (0.54‐1.96) | |
| Mild | 74 | 13 (17.6) | ||||
| Moderate | 37 | 5 (13.5) | ||||
| Severe | 11 | 1 (9.1) | ||||
| Unknown | 1 | 0 (0.0) | ||||
| Presence of pleural effusion | No | 136 | 13 (9.6) | Yes vs no | 2.72 (1.39‐5.32) | |
| Yes | 102 | 24 (23.5) | ||||
| Image findings at ILD onset | GGO | No | 12 | 3 (25.0) | No vs yes | 1.75 (0.51‐5.98) |
| Yes | 226 | 34 (15.0) | ||||
| Pulmonary consolidation | No | 104 | 20 (19.2) | No vs yes | 1.62 (0.85‐3.07) | |
| Yes | 134 | 17 (12.7) | ||||
| Reticular pattern | No | 193 | 32 (16.6) | No vs yes | 1.53 (0.60‐3.88) | |
| Yes | 45 | 5 (11.1) | ||||
| Traction bronchiectasis | No | 211 | 28 (13.3) | Yes vs no | 2.79 (1.34‐5.79) | |
| Yes | 27 | 9 (33.3) | ||||
| Change in pleural effusion | None | 30 | 2 (6.7) | No change vs none | 2.94 (0.70‐12.41) | |
| No change | 110 | 19 (17.3) | ||||
| Decrease | 87 | 16 (18.4) | Decrease vs none | 3.29 (0.77‐14.06) | ||
| Increase | 11 | 0 (0.0) | Increase vs none | – | ||
| Lesion distribution | Bilateral | 137 | 26 (19.0) | Contralateral to tumor and bilateral vs ipsilateral to tumor | 5.62 (1.38‐22.84) | |
| Contralateral | 43 | 8 (18.6) | ||||
| Ipsilateral | 51 | 2 (3.9) | ||||
| Not applicable | 7 | 1 (14.3) | ||||
| Image findings | ILD radiographic pattern | DAD‐like | 26 | 17 (65.4) | DAD vs non‐DAD | 10.72 (5.65‐20.34) |
| Faint infiltration/acute HP‐like | 48 | 2 (4.2) | ||||
| COP/CEP‐like | 127 | 14 (11.0) | ||||
| NSIP‐like | 15 | 1 (6.7) | ||||
| Other | 22 | 3 (13.6) | ||||
| Peritumoral‐GGO | No | 209 | 34 (16.3) | No vs yes | 1.52 (0.47‐4.86) | |
| Yes | 27 | 3 (11.1) | ||||
| Undeterminable | 2 | 0 (0.0) | ||||
| Clinical findings | KL‐6 | Abnormal change | 117 | 23 (19.7) | With abnormal change vs no abnormal change | 2.37 (1.01‐5.57) |
| No abnormal change | 82 | 7 (8.5) | ||||
| Not evaluable | 39 | 7 (17.9) | ||||
| CRP | Abnormal change | 154 | 32 (20.8) | With abnormal change vs no abnormal change | 8.93 (2.19‐36.49) | |
| No abnormal change | 72 | 2 (2.8) | ||||
| Not evaluable | 12 | 3 (25.0) | ||||
| LDH | Abnormal change | 103 | 26 (25.2) | With abnormal change vs no abnormal change | 3.77 (1.81‐7.83) | |
| No abnormal change | 130 | 10 (7.7) | ||||
| Not evaluable | 5 | 1 (20.0) | ||||
| Effect of steroid treatment | Yes | 180 | 11 (6.1) | No vs yes | 57.00 (23.11‐140.62) | |
| No | 22 | 21 (95.5) | ||||
| Not evaluable | 36 | 5 (13.9) | ||||
Note: The risk factors in bold were considered clinically significant and were selected for multivariate analyses.
Abbreviations: CEP, chronic eosinophilic pneumonia; CI, confidence interval; COP, cryptogenic organizing pneumonia; CRP, C‐reactive protein; DAD, diffuse alveolar damage; ECOG PS, Eastern Cooperative Oncology Group performance status; GGO, ground‐glass opacity; HP, hypersensitivity pneumonia; ILD, interstitial lung disease; KL‐6, Krebs von den Lungen‐6; LDH, lactate dehydrogenase; NSIP, nonspecific interstitial pneumonia.
Multivariate analysis of ILD radiographic pattern (the preselected prognostic factor with the highest HR in the univariate analysis [DAD‐like pattern]) paired one on one with the remaining 10 prognostic factors confirmed the poor prognostic factors for survival (Table 3 and Table S1). In the multivariate model, the following were identified as poor prognostic factors when paired with DAD‐like pattern on the basis of HR >2: days from the start of treatment to the onset of ILD: ≤60 days; pleural effusion before treatment: yes; lesion distribution: contralateral to tumor and bilateral; and change in CRP: abnormal change.
TABLE 3.
Multivariate analysis of poor prognostic factors for ILD‐associated death
| Risk factor | Category comparison |
|---|---|
| ILD radiographic pattern | DAD vs non‐DAD |
| Days from start of treatment to onset of ILD | ≤60 days vs >60 days |
| Pleural effusion before treatment | Yes vs no |
| Lesion distribution | Contralateral to tumor and bilateral vs ipsilateral to tumor |
| Change in CRP | Abnormal change vs no abnormal change |
Abbreviations: CRP, C‐reactive protein; DAD, diffuse alveolar damage; ILD, interstitial lung disease.
3.4. Change in ILD radiographic pattern
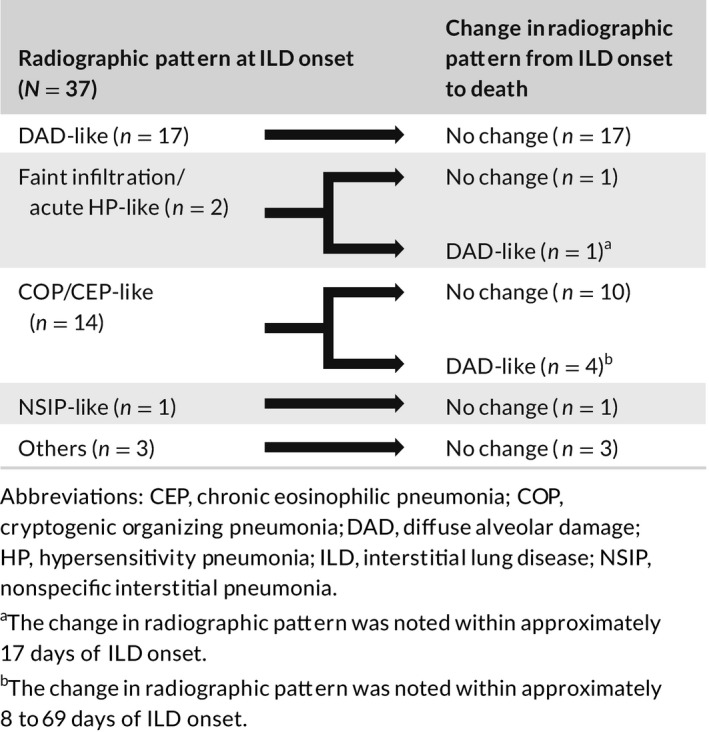
Among the 37 patients who died of ILD, 17 patients had DAD‐like pattern, and 20 patients had non–DAD‐like pattern. Of the 20 patients with a non–DAD‐like pattern, a change in radiographic pattern was observed for five patients during the course of their ILD. The radiographic pattern changed to DAD‐like (in all five patients) from COP/CEP‐like in four patients and from faint infiltration pattern/acute HP‐like pattern in one patient (Table 4). The change in radiographic pattern from faint infiltration pattern/acute HP‐like and from COP/CEP‐like to DAD‐like was observed within approximately 17 days and 8 to 69 days from ILD onset, respectively. All five patients received steroid treatment; however, only one patient responded, three of them did not respond, and one patient received treatment a day before death and hence was not evaluated.
TABLE 4.
Change in radiographic pattern from ILD onset to death in 37 patients with ILD‐related death
3.5. Tumor response by ILD radiographic pattern and peritumoral‐GGO status
No patients had a complete response; the partial response (PR) rate for the study population was 34.0% (81/238 patients, Table 5). The PR rate was 7.7% in patients with the DAD‐like radiographic pattern compared with 34.7% to 46.7% in patients with other radiographic patterns of ILD (Table 5). The PR rate was 40.7% in patients with peritumoral‐GGO and 33.5% in patients without peritumoral‐GGO (Table 5). In the peritumoral‐GGO group, 0/27 patients (0%) had the DAD‐like pattern compared with 26/209 patients (12.4%) in the non–peritumoral‐GGO group.
TABLE 5.
Tumor response by ILD radiographic pattern and peritumoral‐GGO status
| Factor | All patients | Tumor response, n (%) a | ||||
|---|---|---|---|---|---|---|
| n | CR | PR | SD | PD | Undeterminable | |
| ILD radiographic pattern, n (%) a | ||||||
| DAD‐like | 26 | 0 | 2 (7.7) | 12 (46.2) | 8 (30.8) | 4 (15.4) |
| Faint infiltration/acute HP‐like | 48 | 0 | 18 (37.5) | 15 (31.3) | 13 (27.1) | 2 (4.2) |
| COP/CEP‐like | 127 | 0 | 44 (34.7) | 44 (34.7) | 27 (21.3) | 12 (9.4) |
| NSIP‐like | 15 | 0 | 7 (46.7) | 5 (33.3) | 2 (13.3) | 1 (6.7) |
| Other | 22 | 0 | 10 (45.5) | 4 (18.2) | 1 (4.6) | 7 (31.8) |
| Peritumoral‐GGO status, n (%) a | ||||||
| Peritumoral‐GGO | 27 | 0 | 11 (40.7) | 12 (44.4) | 4 (14.8) | 0 |
| Non–peritumoral‐GGO | 209 | 0 | 70 (33.5) | 68 (32.5) | 46 (22.0) | 25 (12.0) |
| Undeterminable | 2 | 0 | 0 | 0 | 1 (50.0) | 1 (50.0) |
| All patients | 238 | 0 | 81 (34.0) | 80 (33.6) | 51 (21.4) | 26 (10.9) |
Abbreviations: CEP, chronic eosinophilic pneumonia; COP, cryptogenic organizing pneumonia; CR, complete response; DAD, diffuse alveolar damage; GGO, ground‐glass opacity; HP, hypersensitivity pneumonia; ILD, interstitial lung disease; NSIP, nonspecific interstitial pneumonia; PD, progressive disease; PR, partial response; SD, stable disease.
For each ILD radiographic pattern and peritumoral‐GGO status category, the percentage of patients with each type of tumor response was calculated as a percentage of the subset of patients with the indicated radiographic pattern or peritumoral‐GGO status.
3.6. History of radiotherapy to lungs
In total, 93 of 238 patients had a history of radiotherapy to the chest area before receiving nivolumab treatment, of whom 10 patients died of ILD. The ILD mortality rate was 10.8% in patients with a history of radiotherapy (10/93 patients) and 18.2% in patients without a history of radiotherapy (26/143 patients) (Table 6). The period between the end of radiotherapy and the start of nivolumab treatment was <6 months for seven of the 10 patients with a history of radiotherapy who died of ILD (Table 6).
TABLE 6.
Summary of history of radiation therapy to lungs
| Total, n (%) | Outcome, n (%) | |||
|---|---|---|---|---|
| Survived | Death | Unknown | ||
| History of radiation to lung | ||||
| No | 143 (60.1) | 115 (80.4) | 26 (18.2) | 2 (1.4) |
| Yes | 93 (39.1) | 82 (88.2) | 10 (10.8) | 1 (1.1) |
| Unknown | 2 (0.8) | 1 (50.0) | 1 (50.0) | 0 |
| Total | 238 (100.0) | 198 (83.2) | 37 (15.5) | 3 (1.3) |
| Period between end of chest irradiation and start of nivolumab treatment | ||||
| <1 month | 4 (4.3) | 3 (75.0) | 1 (25.0) | 0 |
| ≥1 month and <6 months | 21 (22.6) | 15 (71.4) | 6 (28.6) | 0 |
| ≥6 months and <1 year | 22 (23.7) | 20 (90.9) | 2 (9.1) | 0 |
| ≥1 year and <3 years | 27 (29.0) | 26 (96.3) | 0 | 1 (3.7) |
| ≥3 years | 10 (10.8) | 9 (90.0) | 1 (10.0) | 0 |
| Unknown | 9 (9.7) | 9 (100.0) | 0 | 0 |
| Total | 93 (100.0) | 82 (88.2) | 10 (10.8) | 1 (1.1) |
4. DISCUSSION
In this postmarketing study, the radiographic characteristics and mortality risk factors of nivolumab‐associated ILD were evaluated in 238 patients with NSCLC. A spectrum of radiographic patterns was observed, of which the COP/CEP‐like pattern was the most common. The ILD mortality rate varied according to ILD radiographic pattern and was substantially higher in patients with the DAD‐like radiographic pattern than in patients with the other patterns. A DAD‐like radiographic pattern was one of five poor prognostic factors of nivolumab‐associated ILD identified by univariate/multivariate analyses, along with ≤60 days from the initial dose of nivolumab to the onset of ILD, pleural effusion present before nivolumab treatment, abnormal opacities distributed contralateral to the tumor or bilaterally, and abnormal change in CRP levels. Given the potentially fatal outcome of ILD, patients with NSCLC and nivolumab‐associated ILD who have these poor prognostic factors should be managed carefully during nivolumab treatment.
In this postmarketing study, of the 238 patients with NSCLC with nivolumab‐associated ILD, 37 patients died, which is in the order of that reported in other studies of nivolumab‐treated patients with cancer. Ohe et al 7 reported an overall ILD mortality rate of 9.9% in a PMS study of 345 patients with NSCLC and nivolumab‐associated ILD. Delaunay et al 12 reported an ILD mortality rate of 9.4% in a retrospective study of 64 patients with cancer (mostly NSCLC or melanoma) who experienced ILD during immune checkpoint inhibitor treatment (cytotoxic T‐lymphocyte–associated protein 4, PD‐1, or PD‐L1 inhibitors).
As reported previously, 8 , 13 multiple radiographic patterns were observed in the current analysis of patients with nivolumab‐associated ILD, including COP/CEP‐like, infiltration/acute HP‐like, DAD‐like, and NSIP‐like patterns. DAD in the lung in ILD patients is generally associated with a poor prognosis and high mortality. 14 , 15 The ILD mortality rate was highest in patients with DAD‐like radiographic pattern, with a rate of 65.4%. This finding is consistent with the postmarketing study reported by Baba et al, 8 in which the ILD mortality rate was 68.4% (13/19 patients with DAD‐like pattern). Of note, in five patients who died in the current analysis, mortality was preceded by a change in radiographic pattern to the DAD‐like pattern. However, this may be complicated by the fact that imaging findings of an early‐stage DAD‐like pattern may be difficult to diagnose, 9 so the initial diagnosis of ILD may have been perceived as a non–DAD‐like pattern. The radiographic change may indicate worsening ILD. 15 Furthermore, four of these five patients had the COP/CEP‐like pattern at the early stage of diagnosis, which is usually responsive to steroid treatment. 16 , 17 However, although all five patients received steroids, most did not respond; this could be because they had DAD‐like pattern from the start, which rarely responds to treatment and has a poor prognosis. 9 This highlights the importance of continued observation of patients with ILD—including patients with the more favorable ILD radiographic patterns—with the aim of preventing the worsening of the existing ILD.
The identification of ILD onset ≤60 days from the initial dose of nivolumab as a poor prognostic factor indicates that careful observation is required for patients with early onset of ILD during nivolumab treatment; this is because their risk of death is higher compared with patients who develop ILD >60 days after starting nivolumab treatment. Similar results were observed in NSCLC patients treated with immune checkpoint inhibitors, where the median time for the onset of checkpoint inhibitor pneumonitis (CIP) was 82 days, with most cases occurring <6 months after the initiation of immune checkpoint inhibitor treatment, irrespective of grade, and grade 5 CIP occurring at 12 days from treatment initiation. 18 The presence of pleural effusion was identified as a poor prognostic factor; however, the mechanism by which pleural effusion contributes to ILD‐associated death is not known. Recently, malignant pleural effusion has been associated with shorter progression‐free survival and overall survival in NSCLC patients treated with anti‐PD‐1 antibodies. 19 , 20 , 21 Taken together, the presence of pleural effusion before treatment may be one of the noteworthy factors when considering nivolumab as a therapeutic candidate for NSCLC. The identification of abnormal change in CRP levels as a poor prognostic factor may be related to its role as a marker of inflammation. Aggravated inflammation may be related to the degree of lung injury. Of note, history of radiotherapy to the chest area was not identified as a poor prognostic factor in the current analysis.
Peritumoral‐GGO may indicate an antitumor immune response in patients with ILD. In the previously reported postmarketing study of nivolumab‐associated ILD in 144 patients with NSCLC or melanoma, the PR rate tended to be higher (52.2% vs 24.0%) and the ILD mortality rate tended to be lower (4.3% vs 19.8%) in the peritumoral‐GGO group (previously called peritumoral infiltration; n = 23) than in the non–peritumoral‐GGO group (n = 121). 8 In the current analysis also, the PR rate tended to be higher (40.7% vs 33.5%) and the ILD mortality rate tended to be lower (11.1% vs 16.3%) in patients with peritumoral‐GGO than in patients without peritumoral‐GGO; however, the differences between the two groups were less marked. In this study, the proportion of patients with the DAD‐like pattern was lower in the peritumoral‐GGO group (0%) than in the non–peritumoral‐GGO group (12.4%), and the lower response rate observed in the non–peritumoral‐GGO group may be a result of the higher proportion of patients with the DAD‐like pattern. The cause of the uneven distribution of DAD‐like pattern between the peritumoral‐GGO groups is unknown.
In order to focus on nivolumab‐associated ILD, patients who received other drugs associated with ILD, such as EGFR TKIs, 22 were excluded from the current analysis. In total, 29 patients were excluded because they had also received an EGFR TKI, including 22 patients who had received the third‐generation EGFR TKI osimertinib after the end of nivolumab treatment. ILD has been reported in patients with NSCLC who received osimertinib after nivolumab treatment, 23 , 24 and a PMS study of osimertinib treatment in Japan identified prior nivolumab treatment as a potential factor associated with ILD onset during osimertinib treatment in patients with EGFR T790M–positive NSCLC. 25
The main strength of this study was the evaluation of nivolumab‐associated ILD in a real‐world setting, as opposed to a clinical trial setting where patients with poor performance status or underlying ILD are likely to be excluded. Other strengths included sufficiently large sample size (>200 patients) to allow identification of risk factors contributing to a poor prognosis, and central evaluation of ILD radiographic images by a committee with a high degree of expertise. The main limitation was the postmarketing study design, which is based on spontaneous reporting of chest images and clinical course by the treating physician, and therefore may not include all cases of nivolumab‐associated ILD occurring in patients with NSCLC during the surveillance period. Additionally, tumor response was assessed via chest imaging and clinical course and was not based on RECIST guidelines.
In conclusion, the following were identified as risk factors for a poor prognosis of nivolumab‐associated ILD in patients with NSCLC: a DAD‐like radiographic pattern; onset of ILD ≤60 days from the initial dose of nivolumab; pleural effusion present before nivolumab treatment; abnormal opacities distributed contralateral to the tumor or bilaterally; and abnormal change in CRP levels. Given the potentially fatal outcome of ILD, all nivolumab‐treated patients should be carefully managed to prevent the development or worsening of ILD.
DISCLOSURE
Y. Saito reports personal fees from AstraZeneca, Boehringer Ingelheim, Chugai, Novartis, and Ono. S. Sasaki reports personal fees and/or grants from Boehringer Ingelheim, Chugai, Kyorin, MSD, Ono, Pfizer, Shionogi, Taiho, and Torii. K. Oikado, J. Tominaga, M. Sata, and M. Endo report personal fees from Ono. F. Sakai reports personal fees and/or grants and/or nonfinancial support from AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Daiichi Sankyo, Eisai, Fuji, Japanese Ministry of Health, Labour and Welfare, Japanese Ministry of the Environment, Merck Serono, Ono, Shionogi, and Canon Medical Systems. T. Kato reports personal fees and/or grants from AbbVie, Astellas, AstraZeneca, BMS, Boehringer Ingelheim, Chugai, Eli Lilly, F. Hoffmann‐La Roche, Kyorin, Kyowa Kirin, MSD, Merck Serono, Nitto Denko, Novartis, Ono, Pfizer, Regeneron, Sumitomo Dainippon, Taiho, and Takeda. T. Iwasawa reports personal fees and/or grants from AstraZeneca, Bayer, Boehringer Ingelheim, Canon Medical Systems, FujiFilm Medical Systems, GE Healthcare, KAKENHI, Nihon Medi‐Physics, Ono, Shionogi, and Tsuchiya Foundation. H. Kenmotsu reports personal fees and/or grants from AstraZeneca, Boehringer Ingelheim, BMS, Chugai, Eli Lilly, Kyowa Hakko Kirin, MSD, Novartis, Ono, and Taiho. M. Kusumoto reports personal fees from AstraZeneca, Canon Medical Systems, MSD, and Ono. T. Baba reports personal fees and/or nonfinancial support from AMCO, Asahi Kasei, Astellas, AstraZeneca, BMS, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Kyorin, MSD, Ono, Shionogi, Taiho, and Toray Industries. Y. Fujiwara reports grants and/or personal fees from AbbVie, AstraZeneca, BMS, Chugai, Daiichi Sankyo, Eisai, Eli Lilly, Incyte, Merck Serono, MSD, Novartis, Ono, Sysmex, and Taiho. H. Sugiura reports personal fees from Ono and MSD. N. Yanagawa reports personal fees and/or grants and/or nonfinancial support from Ono. Y. Ito and T. Sakamoto are employed by Ono. Y. Ohe reports personal fees and/or grants from Amgen, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Celltrion, Chugai, Daiichi Sankyo, Dainippon‐Sumitomo, Eli Lilly, Ignyta, Janssen, Kissei, Kyorin, MSD, Novartis, Ono, Pfizer, Taiho, and Takeda. K. Kuwano reports personal fees and/or grants from Astellas, AstraZeneca, Boehringer Ingelheim, Chugai, Daiichi Sankyo, Eisai, GSK, Kyorin, MSD, Ono, Shionogi, Taiho, and Tsumura.
ROLE OF THE SPONSOR
The study sponsor was involved in the study design, writing of the report, and in the decision to submit the article for publication.
OTHER CONTRIBUTORS/ACKNOWLEDGMENTS
The authors would like to thank all study participants.
Supporting information
Table S1
ACKNOWLEDGMENTS
This work was supported by Ono Pharmaceutical Co., Ltd. and Bristol‐Myers Squibb K.K. Medical writing assistance was provided by Sandra Kurian, MPharm, and Tania Dickson, PhD, CMPP, of ProScribe – Envision Pharma Group and was funded by Ono Pharmaceutical Co., Ltd. and Bristol‐Myers Squibb K.K. ProScribe's services complied with international guidelines for Good Publication Practice (GPP3).
Saito Y, Sasaki S, Oikado K, et al. Radiographic features and poor prognostic factors of interstitial lung disease with nivolumab for non–small cell lung cancer. Cancer Sci.2021;112:1495–1505. 10.1111/cas.14710
Funding information
Bristol‐Myers Squibb K.K. Ono Pharmaceutical Co., Ltd.
See related article https://onlinelibrary.wiley.com/doi/10.1111/cas.14715
REFERENCES
- 1. Guo L, Zhang H, Chen B. Nivolumab as programmed death‐1 (PD‐1) inhibitor for targeted immunotherapy in tumor. J Cancer. 2017;8:410‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pharmaceuticals and Medical Devices Agency . Review Report: Opdivo (nivolumab) intravenous infusion. 2017. http://www.pmda.go.jp/files/000223201.pdf. Accessed January 22, 2020.
- 3. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med. 2015;373:123‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borghaei H, Paz‐Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med. 2015;373:1627‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gettinger S, Borghaei H, Brahmer J, et al. Five‐year outcomes from the randomized, phase 3 trials CheckMate 017/057: nivolumab vs docetaxel in previously treated NSCLC. J Thorac Oncol. 2019;14:OA14.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kato T, Masuda N, Nakanishi Y, et al. Nivolumab‐induced interstitial lung disease analysis of two phase II studies patients with recurrent or advanced non‐small‐cell lung cancer. Lung Cancer. 2017;104:111‐118. [DOI] [PubMed] [Google Scholar]
- 7. Ohe Y, Gemma A, Nakagawa K, et al. Real‐world safety of nivolumab in patients with non‐small cell lung cancer (NSCLC) in Japan: interim summary of post‐marketing all‐case surveillance. Ann Oncol. 2018;29:viii400‐viii441. [Google Scholar]
- 8. Baba T, Sakai F, Kato T, et al. Radiologic features of pneumonitis associated with nivolumab in non‐small‐cell lung cancer and malignant melanoma. Future Oncol. 2019;15:1911‐1920. [DOI] [PubMed] [Google Scholar]
- 9. Kubo K, Azuma A, Kanazawa M, et al. Consensus statement for the diagnosis and treatment of drug‐induced lung injuries. Respir Investig. 2013;51:260‐277. [DOI] [PubMed] [Google Scholar]
- 10. Nishino M, Chambers ES, Chong CR, et al. Anti‐PD‐1 inhibitor‐related pneumonitis in non‐small cell lung cancer. Cancer Immunol Res. 2016;4:289‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496‐509. [Google Scholar]
- 12. Delaunay M, Cadranel J, Lusque A, et al. Immune‐checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J. 2017;50. [DOI] [PubMed] [Google Scholar]
- 13. Nishino M, Ramaiya NH, Awad MM, et al. PD‐1 inhibitor‐related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res. 2016;22:6051‐6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Azuma A, Kudoh S. High prevalence of drug‐induced pneumonia in Japan. JMAJ. 2007;50:405‐411. [Google Scholar]
- 15. Kaarteenaho R, Kinnula VL. Diffuse alveolar damage: a common phenomenon in progressive interstitial lung disorders. Pulm Med. 2011;2011:531302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Disayabutr S, Calfee CS, Collard HR, Wolters PJ. Interstitial lung diseases in the hospitalized patient. BMC Med. 2015;13:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suzuki Y, Suda T. Long‐term management and persistent impairment of pulmonary function in chronic eosinophilic pneumonia: a review of the previous literature. Allergol Int. 2018;67:334‐340. [DOI] [PubMed] [Google Scholar]
- 18. Suresh K, Voong KR, Shankar B, et al. Pneumonitis in non‐small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol. 2018;13:1930‐1939. [DOI] [PubMed] [Google Scholar]
- 19. Kawachi H, Tamiya M, Tamiya A, et al. Association between metastatic sites and first‐line pembrolizumab treatment outcome for advanced non–small cell lung cancer with high PD‐L1 expression: a retrospective multicenter cohort study. Invest New Drugs. 2020;38:211‐218. [DOI] [PubMed] [Google Scholar]
- 20. Shibaki R, Murakami S, Shinno Y, et al. Malignant pleural effusion as a predictor of the efficacy of anti‐PD‐1 antibody in patients with non‐small cell lung cancer. Thorac Cancer. 2019;10:815‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adachi Y, Tamiya A, Taniguchi Y, et al. Predictive factors for progression‐free survival in non‐small cell lung cancer patients receiving nivolumab based on performance status. Cancer Med. 2020;9:1383‐1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen YM, Luo YH. Interstitial lung disease induced by targeted therapy for non‐small cell lung cancer: a review of diagnosis, workup, and management. J Palliat Care Med. 2015;5:204. [Google Scholar]
- 23. Kotake M, Murakami H, Kenmotsu H, Naito T, Takahashi T. High incidence of interstitial lung disease following practical use of osimertinib in patients who had undergone immediate prior nivolumab therapy. Ann Oncol. 2017;28:669‐670. [DOI] [PubMed] [Google Scholar]
- 24. Uchida T, Kaira K, Yamaguchi O, et al. Different incidence of interstitial lung disease according to different kinds of EGFR‐tyrosine kinase inhibitors administered immediately before and/or after anti‐PD‐1 antibodies in lung cancer. Thorac Cancer. 2019;10:975‐979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gemma A, Kusumoto M, Sakai F, et al. Real‐world evaluation of factors for interstitial lung disease incidence and radiologic characteristics in patients with epidermal growth factor receptor T790M‐positive non‐small cell lung cancer treated with osimertinib in Japan. J Thorac Oncol. 2020;S1556‐0864(20)30717‐6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


