Abstract
Background
Non-adherence to immunosuppressant therapy (IST) is a major risk factor for graft rejection. Limited reports are available regarding the prevalence of non-adherence to IST in kidney transplant recipients (KTRs) as well as the predictors and barriers of non-adherence.
Material/Methods
The study included ambulatory KTRs, ≥18 years of age, with a functional kidney, from January 2017 to November 2018. The primary outcome was the prevalence of non-adherence, assessed with: 1) A telephone interview to complete the Arabic-translated and validated Immunosuppressant Therapy Adherence Instrument Scale (ITAS) and 2) IST serum blood levels within therapeutic levels. The secondary outcomes were the barriers to adherence using the validated Immunosuppressant Therapy Barriers of Adherence Scale (ITBS).
Results
We enrolled 102 of 141 patients screened. The mean±SD for age, body mass index, and the baseline of the estimated glomerular filtration rate were 45.5±15.6 years, 29.1±6 kg/m2, and 72.7±21.9 ml/min/1.73 m2, respectively. The prevalence of non-adherence was 5.9%, 95% CI (2.19–12.36%) and 14.7%, 95% CI (8.47–23.09%) using the ITAS and the average blood serum drug levels, respectively. The concordance of the 2 methods demonstrated an agreement of 81.3%, kappa of 0.01, and 95% CI (−0.16 to 0.18). The median, interquartile range (IQR) for ITBS, and uncontrollable and controllable barriers for adherence were 21, (18–25), 15, (12–18), and 6, (5–8), respectively.
Conclusions
The current study demonstrated a low to moderate prevalence of non-adherence to IST in KTRs. The barriers for adherence with IST necessitate additional targeted interventions to manage and optimize therapeutic and clinical outcomes.
Keywords: Calcineurin, Immunosuppressive Agents, Kidney Transplantation, Medication Adherence, Patient Compliance
Background
Chronic kidney disease is a progressive illness [1]. The United States (US) data for 2019 reported that ~37 millions (15%) of the US population have chronic kidney disease and that per day, more than 340 patients with end-stage renal disease (ESRD) require the intiation of renal replacement therapy, either dialysis or transplantation [2]. In Saudi Arabia in 2017, 18,270 patients required hemodialysis, with 2848 (16%) patients on the waiting list for kidney transplantation. There were ~11,509 KTRs in Saudi Arabia from 1990 to 2017 [3].
A kidney transplantation is the ultimate renal replacement therapy for ESRD patients as it improves their quality of life and survival, compared to other renal replacement therapies such as hemodialysis or peritoneal dialysis [4–7]. Although kidney transplantation is the preferred renal replacement modality, it is associated with many short- and long-term medical complications [8–10]. KTRs usually receive induction IST before, during the perioperative period, post-kidney transplantation, followed by a maintenance immunosuppressive regimen to suppress their immune system and prevent acute and chronic episodes of rejection [11].
The main objectives of the IST are to prevent graft rejection and to reduce morbidities, hospital admissions, and complications associated with loss of graft function [12]. The most frequently used regimen, maintenance IST, consists of calcineurin inhibitors (CNIs) such as tacrolimus or cyclosporine, an antiproliferative agent such as mycophenolate mofetil, or mycophenolate sodium or azathioprine, and/or the mammalian target of rapamycin inhibitor with or without oral corticosteroids [11,13]. Patients have a complex medication regimen, including IST, prophylactic anti-infective agents, and other medications used for the treatment of chronic comorbid conditions, leading to polypharmacy and a high daily pill burden, which may influence the adherence to their medications [11,14].
Non-adherence to IST results in a higher a rate of acute or chronic rejection, loss of graft function, a decrease in the quality of life, and an increase of the economic burden on the healthcare system [15–19]. Various methods have been used to assess adherence and these are discussed as follow. (1) Subjective methods, such as cross-sectional surveys with KTRs, may overestimate the adherence or physician-reported adherence, which underestimates adherence [16,20]. (2) Objective methods, such as laboratory monitoring of the blood serum level of CNIs, which is one of the most accurate methods to assess adherence. However, it may be subject to bias if the KTRs adhere to their medication prior to the follow-up clinic visits [21]. (3) Indirect measures through pharmacy refill records, which may be inaccurate in case of multiple refills from different pharmacies, and it does not necessarily reflect the adherence behavior related to actually taking the IST [22]. (4) Electronic monitoring (EM) is expensive and opening the EM device is only a marker of pill consumption [23,24]. (5) A combination of these methods have been suggested by a recent expert of the FDA panel for optimum assessment of adherence, given the limitations of each method [25].
A recent systematic review, including 37 studies, reported that non-adherence in KTRs varies from 1.6% to 96% [26]. The review identified the following risk factors associated with non-adherence to IST: young age (≤50 years of age), male gender, low social support, unemployment, low level of education, ≥3 months post-transplant, living donor, presence of ≥6 comorbidities, administration of ≥5 medications per day, a negative attitude, and depression [26]. Another study found that only 34% of KTRs were adherent to their medication regimen and there was a strong association between the level of satisfaction and the adherence to treatment using subjective instruments [27].
To the best of our knowledge, there is a paucity of data related to the non-adherence patterns in KTRs in our center and the association with possible risk factors, which may explain non-adherence. Therefore, this study aimed to assess the non-adherence to IST in our cohort of KTRs and to identify potential barriers and predictors of non-adherence.
Material and Methods
Setting and Time Frame
We conducted a cross-sectional study and a retrospective chart review in the Ambulatory Care Center of King Khalid Hospital, Ministry of National Guard-Health Affairs, Jeddah, Saudi Arabia from January 2017 to November 2018.
Sampling and Study Population
We used a convenience sampling technique of all accessible KTRs who met the eligibility criteria. We included participants who were a KTR with a functional kidney (not being dialyzed), ≥18 years old, had the kidney transplant before June 2017, and were prescribed IST, which includes CNIs or sirolimus, which requires routine laboratory monitoring of serum blood levels during follow-up visits. We excluded KTRs who were admitted during the study period or who refused to participate in the study.
Study Outcomes
The primary outcome was the rate of non-adherence to IST in KTRs in our center. The secondary outcomes were to determine the barriers related to non-adherence of the IST and identify if there were any episodes of rejection due to non-adherence to IST.
Data Collection
We identifed the cohort of KTRs through a list provided by the kidney transplant coordinators. The study investigators screened patients in terms of the inclusion criteria and eligible patients were invited to participate in the survey through a telephone interview. The study investigators completed the structered questionnaire via a telephone call. We used the Electronic Health Records (EHRs) to identify the baseline demographic information, cormorbidties, and various clinical characteristics, including the transplant donor type, the serum creatinine at the last clinic visit, and any episodes of rejection from January 2017 to June 2018.
Outcome Assessment
The primary outcome of non-adherence was assessed with 2 methods:
Subjective: We used the validated ITAS, a 4-item instrument developed and validated to assess adherence to IST in transplant recipients; reliability testing demonstrated a Cronbach alpha of 0.81 [28].
Objective: We reviewed the EHRs to document serum levels of immunosuppressive medication, specifically tacrolimus, cyclosporine, or sirolimus, obtained during the routine follow-up prior to clinic visits for each patient during the study period.
The secondary outcomes of the barriers for non-adherence were assessed with the ITBS, a validated 13-item instrument, using a 5-point Likert scale. The instrument had 2 subscales of 8 uncontrollable and 5 controllable barriers to adherence in KTRs [29]. The Cronbach alpha of the uncontrollable barriers, controllable barriers, and the combined ITBS was 0.93, 0.86, and 0.91, respectively [29]. The uncontrollable barriers refer to those factors which are not controlled by the patient, such as being prescribed too many medications, depression, confusion or lack of understanding; these factors, to a large extent, are within the control of the healthcare system. The controllable barriers include factors that are within the control of the patient or willingness not to adhere to their IST, such as not remembering to take their medication, choosing to stop them due to adverse effects or when feeling better, or financial reasons.
We combined the questions of the ITAS and ITBS instruments into one questionnaire, with a total of 17 questions. The study investigators, who are native Arabic speakers, translated the survey into the Arabic language. Subsequently, we pre-tested for the appropriateness of wording and clarity with a sample of 8 transplant recipients. We revised the questionnaire based on the results of the pre-testing and changed 2 words in question 6 (confused) and question 7 (I don’t understand) of ITBS. Tables 1 and 2 present the questions for the ITAS and ITBS, administered to the study participants.
Table 1.
Immunosuppressant Transplant Adherence Instrument scale (ITAS) [28].
| Questions of ITAS | None | 1–20% | 21–50% | >50% yery frequent |
|---|---|---|---|---|
| 1. In the past 3 months, how often did you forget to take your immunosuppressant medications? | ||||
| 2. In the last 3 months, how often were you careless about taking your immunosuppressant medication(s)? | ||||
| 3. In the last 3 months, how often did you stop taking your immunosuppressant medication because you felt worse? | ||||
| 4. In the last 3 months, how often did you miss taking your immunosuppressant medication(s) for any reason? |
Table 2.
Immunosuppressant Therapy Barriers Scale (ITBS) [29].
| Questions* | Strongly disagree | Disagree | Neutral | Agree | Strongly agree |
|---|---|---|---|---|---|
| 1. I have to take the immunosuppressant medication(s) too many times per day. | |||||
| 2. I have to take too many capsules (or tablets) of my immunosuppressant medication(s) at one time? | |||||
| 3. I can’t tell if my immunosuppressant medication(s) is (are) helping me? | |||||
| 4. I skip doses of my immunosuppressant medication(s) when I go out of town? | |||||
| 5. I miss doses of my immunosuppressant medication(s) when I feel depressed? | |||||
| 6. I get confused about how to take my immunosuppressant medication. | |||||
| 7. I do not understand when to take my immunosuppressant medication(s). | |||||
| 8. I often run out (or do not have enough) of immunosuppressant medication(s). | |||||
| 9. It is hard for me to remember to take my immunosuppressant medication(s). | |||||
| 10. I miss a dose of my immunosuppressant medication(s) when I think there may be side effects | |||||
| 11. I sometimes skip doses of my immunosuppressant medication(s) when I feel good (or better). | |||||
| 12. I miss doses of my immunosuppressant medication(s) when I get out of my daily routine. | |||||
| 13. I skip doses of my immunosuppressant medication(s) when I am short of money. |
The eight uncontrollable barriers are questoions 1–8, while the contrrollable barriers are questions 9–13.
Ethics
The study recieved an IRB approval from King Abdullah International Medical Research Center (RSS18/043/J). Verbal consent was obtained from the eligible participants through a telephone interview, which was witnessed via a loudspeaker and signed by an independent non-study investigator.
Sample Size
All eligible KTRs (~140 patients) were invited to participate in the study. A sample of 90–98 transplant recipients was estimated to detect a prevalence of 20–30% [26] for non-adherence with a 95% confidence interval, 5% precision, and an alpha of 0.05 [30].
Statistical Analysis
Descriptive statistics were used to present the baseline characteristics, as deemed necessary. The responses of the ITAS questions were coded as 0, 1, 2, and 3 for the frequencies of >50% of the time, 21–50% of the time, 1–20% of the time, and zero% (never), respectively. A maximum score of 12 represented highest adherence to IST and the lowest score of 0 represented non-adherence [28]. We assumed a cut-off for the adherence rate of 80% or above (a score ≥10 on the ITAS Score) [28].
The responses of the ITBS questions were coded as 1, 2, 3, 4, and 5 for the frequencies of strongly disagree, disagree, neutral, agree, and strongly agree, respectively. The maximum score of 65 and a minimum score of 13 represented the highest and lowest barriers for adherence. The uncontrollable barriers subscale had a minimum score of 8 and a maximum of 40, and the controllable barriers subscale had a minimum score of 5 and a maximum of 25 [29].
The objective assessment of the adherence using the average serum levels of immunosuppressant agents was classified as a binary outcome of adherent: achieving the target therapeutic levels or non-adherent if the level was below the target therapeutic levels, using 80% as a cut-off point to define adherence [28]. The following target therapeutic levels were used: 4–15 ng/ml, 100–400 ng/ml, and 4–10 ng/ml for tacrolimus, cyclosporine, and sirolimus, respectively [28]. We conducted a sensitivity analysis to assess the primary outcome using a target cyclosporine level of 75 since this cut-off is mostly used in practice for KTRs who are using IST for more than 2–3 years.
A binomial exact test was used for the estimation of the 95% confidence intervals for the prevalence of non-adherence. The agreement between the subjective and objective measures for the assessment of non-adherence was assessed using Kappa-statistics with 95% confidence intervals (CI). Two-sided tests and a significance level of 0.05 were used for all analyses. Analyses was conducted using STATA 14 (StataCorp LP, College Station, TX, USA).
Results
We enrolled and consented 102 of 141 KTRs who were screened and invited to participate in the study. We excluded 39 KTRs for the following reasons: 24 KTRs did not have active follow-up visits during the study period, 7 refused to participate in the study, 5 were admitted at the time of the interview, and we could not contact 3 KTRs due to inaccurate contact telephone numbers.
In terms of the baseline characteristics, the mean age was 48.55 years ±SD 15.64, 64.71% were male, and the mean body mass index (BMI) was 29.14±SD 5.9. The cohort included 53.9% KTRs with a high school education or above and the mean baseline estimated glomerular filteration rate was 72.67±SD 21.89 ml/min/1.73 m2.
Tacrolimus was the CNI most frequently prescribed (75.49%) and most (55.88%) of the recipients had a living-related donor. The most frequent comorbidities were hypertension (68.63%) and diabetes mellitus (42.16%). Table 3 presents the details related to the baseline characteristics of the sample.
Table 3.
Baseline characteristics of the sample.
| Baseline characteristics | N=102* |
|---|---|
| Demographic information | |
| Age (years) | 48.55±15.64 |
| Sex (Male) | 66 (64.71%) |
| Body mass index (kg/m2) | 29.14±5.94 |
| Under weight | 1 (0.98%) |
| Normal weight | 26 (25.49%) |
| Overweight | 33 (32.35%) |
| Obese | 42 (41.18%) |
| Material status (married) | 76 (74.51%) |
| Educational level | |
| Not educated | 18 (17.65%) |
| Below high school | 29 (28.43%) |
| High school | 23 (22.55%) |
| Graduate and post graduate | 32 (31.37%) |
| Transplant and medications related | |
| Years post kidney transplant | 10 (6–14) |
| Type of donor** | |
| Living-related | 39 (38.24%) |
| Living non-related | 57 (55.88%) |
| Deceased | 6 (5.88%) |
| History of previous rejection | 3 (2.94%) |
| Estimated glomerular filtration rate (ml/min/1.73 m2)# | 72.67±21.89 |
| Immunosuppression therapy (IST) | |
| Tacrolimus-based | 77 (75.49%) |
| Cyclosporine-based | 25 (24.51%) |
| Number of prescribed medications | 9 (6–12) |
| Self-administer medications | 91 (89.22%) |
| Comorbidities | |
| Hypertension | 70 (68.63%) |
| Diabetes mellitus | 43 (42.16%) |
| Thyroid disorders | 19 (18.63%) |
| Dyslipidemia | 14 (13.73%) |
| Ischemic heart diseases | 6 (5.88%) |
| Liver diseases | 6 (5.88%) |
| Others## | 6 (5.88%) |
Data are presented as frequency (percentage): n (%), mean±Standard Deviation (SD), Median; Interquartile Range (IQR);
One participant is missing for the type of donor;
estimated glomerular filtration rate is based on Modified diet and renal disease equation (MDRD);
others include 2 patients who had gout, 2 patients with cancer and 2 patients had heart failure.
Primary Outcome
Prevalence of non-adherence: The subjective assessment indicated non-adherence at 5.9% (6/102) with a 95% CI (2.19–12.36%) using the ITAS survey. The majority (n=72) had a score of 12, 21 a score of 11, 3 a score of 9 and 10, 2 a score of 8, and 1 had a score of 6 (Figure 1). The objective assessment of non-adherence indicated a rate of 14.7% (15/102), 95% CI (8.47–23.09%). The concordance of assessing non-adherence with the 2 methods demonstrated a significant agreement of 81.3% with a kappa of 0.01, 95% CI (−0.16 to 0.18). The sensitivity analysis, using a cut-off for cyclosporine of 75, resulted in a non-adherence rate of 7.14% (7/102).
Figure 1.
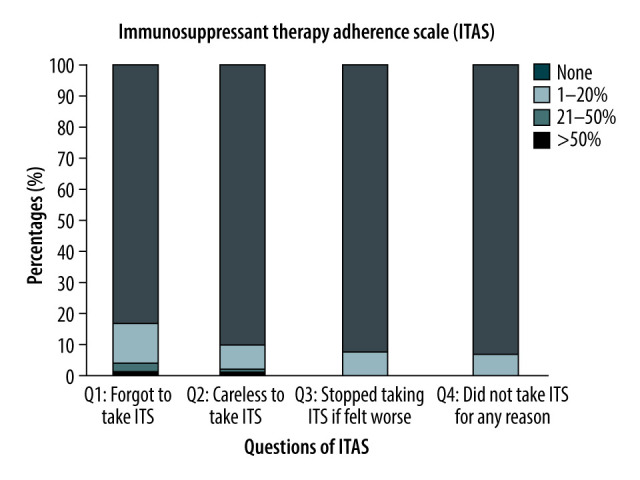
Distribution of the Responses for the Immunosuppressant Transplant Adherence Instrument scale (ITAS).
Secondary Outcomes
The median ITBS score to identify the combined barriers for non-adherence, uncontrollable and controllable subcales were 21 (IQR 18–25), 15 (IQR 12–18), and 6 (IQR 5–8), respectively. For the uncontrollable barriers, the majority (83.3%) strongly agreed that they take ITS too many times per day. However, the sample strongly disagreed with several questions. Almost half (44.1%) disagreed that they take too many ITS tablets and capsules at the same time, 65.7% that they cannot tell if the ITS medications help them or not, 91.2% disagreed that they skip doses if out of town, 92.6% that they miss doses when depresssed, 83.3% that they were confused in terms of how to take their medication, 80.2% that they do not understand when to take their medication, and 65.7% that they often run out of their medication (Figure 2).
Figure 2.
Proportion of the responses related to the uncontrollable barriers “ Healthcare system-related” of ITBS.
Similary for the controllable barriers, the sample strongly disagreed with most questions. The majority (63.7%) disagreed that they found it hard to remember the ITS, 79.4% that they miss their medication doses due to adverse effects, 86.3% that they skip doses if they feel good, 78.4% that they miss the dose if out of their routine, and 90.2% that they skip a dose if short of money (Figure 3).
Figure 3.

Proportion of the responses related to the controllable barriers “Patient-related” of ITBS.
Three patients had a history of rejection; all of them had a maximum ITAS score and average blood serum levels of CNIs within the therapeutic range.
Discussion
The current study’s estimate of a low non-adherence rate to ITS, using the subjective ITAS assessment and the objective direct assessment of serum therapeutic blood levels, are consistent with studies reporting non-adherence rates of 28.3% and 14.3%, using similar subjective methodology [15,31] and for the objective assessment using the therapeutic drug levels [32]. Contrary to the current study, some studies reported a higher non-adherence rate. For example, a study including 151 KTRs reported a non-adherence rate of 64% with participants with comparable baseline characteristics. However, different assessment tools were used and the authors acknowleged they may have overestimated the non-adherence rate [27]. Another descriptive correlational study with 230 KTRs reported a non-adherence rate of 57.8%, using the ITAS [33]. There were some differences in the responses of the current study and their study for the second ITAS question, as only 62.6% were never careless to take their ITS in the last 3 months, compared to 90% in our study [33]. It is important to realize that carelessness may be linked to differences in the level of medication understanding and the patient’s beliefs about the ITS, which are important parameters affecting non-adherence, and not clearly reported in their study [12,34].
In our center, the transplant team consists of transplant nephrologists, coordinators, and pharmacists who educate the KTRs at different encounters. The pharmacists provide counseling, educate the patients, and discuss the importance of using the ITS with all KTRs immediately after surgery, during inpatient admission, and before discharge. Subsequently, counseling is enforced at the outpatient setting by the transplant coordinators and the physicans at different follow-up visits, and in some occassions by the pharmacist, to improve any knowledge deficits or misunderstanding of ITS or any other medication regimen. Our current standard of practice may explain our low non-adherence rate and why some of the factors associated with non-adherence to ITS reported in literature, such as longitvity of the transplant and living donors, were not major concerns, although the KTRs in the current study had a median of 10 years after transplant, IQR (6–14) years, and 94% had a living donor [33]. Although non-adherence to ITS is associated with a high rejection rate in the literature [19,21,29,31], it was not evident in the current study with a low non-adherence rate. The 3 cases with rejection were adherent to their ITS.
The high rate of adherence reported in the current study detected with the 2 methods may be justified by the low ITBS scores in terms of the barriers encountered as there was a significant negative correlation between the measures of adherence and the ITBS, consistent with previous reports [29,31].
Although the overall ITBS score in the current study was consistently low, in support of another cross-sectional study with 252 adult KTRs, the barriers related to non-adherence differed in the 2 studies [31]. The differences may reflect variability in the practice setting, type of support offered to patients, and characteristics of the study population. The barriers provide opportunities for interventions and future patient-centered goals to be achieved by the multidisciplinary transplant team, with the pharmacist playing a vital role [31,35]. For the uncontrollable barriers, the majority of our KTRs agreed with “taking ITS many times per day” and more than one-third agreed that they “take many tablets and capsules at the same time”. These 2 healthcare system-related barriers were also reported in another study, although the non-adherence rate was lower in the current study (5.9% vs 55%), and it may reflect the complexity of the medication regimen, the multiple comorbities of KTRs, and the high pill burden [36].
However, the 2 barriers may provide an opportunity to the pharmacist to conduct a structural review of the medication using the Medication Therapy Management (MTM) services in the outpatient setting to simplify, de-prescribe unnecessary drug-use, schedule the administration of medications throughout the day, empower patients with the necessary tools to optimize their adherence, and achieve the target therapeutic and clinical outcomes [35,37].
The majority of the sample agreed that they think ITS benefits them, they do not skip doses if out of town or depressed, are not confused about their medication, and understand when to take the medication. These findings demonstrate that our patients understand their medication and have positive beliefs regarding the usefullness of ITS. In contrast, a study with 161 KTRs identified that the patients could not say if the ITS benefit them or not, which highlights the variability of staff resources, education, and counseling by the multidisciplinary healthcare transplant team in different settings [36]. In the current study, less than 20% of the sample agreed that they run out of their ITS, compared to 2.8% in another study [31]; this uncontrollable healthcare system-related barrier should be managed to improve access to IST.
Regarding the controllable barriers, the sample agreed or strongly agreed that they remember to take ITS, do not miss doses due to adverse effects, when they feel good, or if they are out of their daily routine, which emphasizes the importance of patients’ self-efficacy and its impact on adherence [29,38,39]. In contrast, another study, with 161 KTRs, reported a non-adherence rate of 55%. The non-adherent group had signficantly higher scores for 3 of the 5 controllable barriers (cannot remember to take medications, skip doses when out of their daily routine, or when they are short of money) compared to the adherent group, which indicate the need to design interventions to overcome these modificable factors to improve adherence [36]. Finally, skipping medications when patients are short of money was not considered a barrier in our setting, as the majority of the KTRs are insured with access granted to IST, contrary to other studies highlighting the importance of a consistent medication supply [33,36].
The current study has several limitions. Firstly, the study presents a single-center experience. Secondly, due to the cross-sectional design, we did not assess the history of drug–drug interactions or possible drug–food interactions that may have influenced drug levels at specific visits. However, we used the average blood serum levels for every patient to minimize factors affecting the drug levels and probable intraindividual variability. Thirdly, the study has limited generalizability to settings with similar standards of care, as the transplant team includes a pharmacist who provide education and support to KTRs. Fourthly, we did not assess other sociodemographic factors and depression, which may be associated with self-efficacy and possible non-adherence [31,32].
Our study has several strengths. Firstly, we assessed non-adherence in the KTRs with 2 methods to present a precise estimate and reduce the posssible limitations of each measure [21,25,26,40]. Secondly, there was a signficant agreement between the 2 methods, although some studies reported some variability between assessment methods for non-adherence [21,26]. Thirdly, we recorded the serum blood level extracted from the EHRs for ~18 months to reflect a consistent pattern of behavior. Fourthly, the ITBS scores supported limited barriers and the low non-adherence rate in our sample. Future studies should target specific multidimensional interventions to identify barriers and characterize patterns of non-adherence experienced by KTRs and evaluate effective strategies to manage these challenges to optimize therapeutic outcomes.
Conclusionss
Our study demonstrated a low to moderate prevalence of non-adherence to IST in kidney transplant recipients, based on subjective and objective measures. Although we identified limited barriers for non-adherence to IST, these require additional targeted interventions to overcome the factors and to optimize therapeutic and clinical outcomes for KTRs.
Acknowelgement
We acknoweledge Mrs. Nouf Saad, a transplant coordinator, for her support to identify the contact information of KTRs, faciltating the telephone interview, and enrollment of patients.
Abbreviations
- IST
immunosuppressant therapy
- KTRs
kidney transplant recipients
- ITAS
Immunosuppressant Therapy Adherence Instrument Scale
- ITBS
Immunosuppressant Therapy Barriers of Adherence Scale
- IQR
interquartile range
- ESRD
end-stage renal disease
- CNIs
calcineurin inhibitors
- EHRs
electronic health records
- CI
confidence intervals
- Q
question
Footnotes
A poster of the study was presented at the American Transplant Congress (ATC) in June 2019, in Boston, MA, USA. The abstract was published in the Am J Transplant in 2019. Furthermore, a poster of the study was presented at the 10th Research Summer School, King Abdullah International Medical Research Center, in August 2018, and at the Saudi International Pharmaceutical Sciences (SIPHA) Annual meeting in January 2019, in Jeddah, Saudi Arabia
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Luyckx VA, Tonellib M, Stanifer JW. The global burden of kidney disease and the sustainable development goals. Bull World Health Organ. 2018;96:414–22D. doi: 10.2471/BLT.17.206441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and prevention. Chronic Kidney Disease Initiative. Chronic Kidney Disease, Common-Serious-Costly. [Accessed on 12 August, 2020]. https://www.cdc.gov/kidneydisease/pdf/CKD-common-serious-costly-h.pdf.
- 3.Saudi Center for Organ Transplantation. Annual Report 2017 Organ Transplantation in Kingdom of Saudi Arabia. [Accessed on 12 August 2020]. http://www.scot.gov.sa/images/Report_En_F_annual%20report%202017.pdf.
- 4.Locatelli F, Pozzoni P, Del Vecchio L. Renal replacement therapy in patients with diabetes and end-stage renal disease. J Am Soc Nephrol. 2004;15(Suppl 1):S25–29. doi: 10.1097/01.asn.0000093239.32602.04. [DOI] [PubMed] [Google Scholar]
- 5.Maglakelidze N, Pantsulaia T, Tchokhonelidze I, et al. Assessment of health-related quality of life in renal transplant recipients and dialysis patients. Transplant Proc. 2011;43(1):376–79. doi: 10.1016/j.transproceed.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 6.Ogutmen B, Yildirim A, Sever MS, et al. Health-related quality of life after kidney transplantation in comparison intermittent hemodialysis, peritoneal dialysis, and normal controls. Transplant Proc. 2006;38(2):419–21. doi: 10.1016/j.transproceed.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Kumnig M, Rumpold G, Hofer S, et al. Patient-reported outcome reference values for patients after kidney transplantation. Wien Klin Wochenschr. 2014;126(1–2):15–22. doi: 10.1007/s00508-013-0448-6. [DOI] [PubMed] [Google Scholar]
- 8.Ojo AO, Wolfe RA, Held PJ, et al. Delayed graft function: Risk factors and implications for renal allograft survival. Transplantation. 1997;63(7):968–74. doi: 10.1097/00007890-199704150-00011. [DOI] [PubMed] [Google Scholar]
- 9.Coemans M, Süsal C, Döhler B, et al. Analyses of the short- and long-term graft survival after kidney transplantation in Europe between 1986 and 2015. Kidney Int. 2018;94(5):964–73. doi: 10.1016/j.kint.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Silkensen JR. Long-term complications in renal transplantation. J Am Soc Nephrol. 2000;11(3):582. doi: 10.1681/ASN.V113582. [DOI] [PubMed] [Google Scholar]
- 11.KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 12.Patzer RE, Serper M, Reese PP, et al. Medication understanding, non-adherence, and clinical outcomes among adult kidney transplant recipients. Clin Transplant. 2016;30(10):1294–305. doi: 10.1111/ctr.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neuwirt H, Rudnicki M, Schratzberger P, et al. Immunosuppression after renal transplantation. memo – Magazine of European Medical Oncology. 2019;12(3):216–21. [Google Scholar]
- 14.Rana G, Ahmed M, Mckane W. Polypharmacy in renal transplant recipients. Transplantation. 2018;102:S549. [Google Scholar]
- 15.Lalic J, Velickovic-Radovanovic R, Mitic B, et al. Immunosuppressive medication adherence in kidney transplant patients. Med Princ Pract. 2014;23(4):351–56. doi: 10.1159/000362792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinsky BW, Takemoto SK, Lentine KL, et al. Transplant outcomes and economic costs associated with patient noncompliance to immunosuppression. Am J Transplant. 2009;9(11):2597–606. doi: 10.1111/j.1600-6143.2009.02798.x. [DOI] [PubMed] [Google Scholar]
- 17.Prihodova L, Nagyova I, Rosenberger J, et al. Adherence in patients in the first year after kidney transplantation and its impact on graft loss and mortality: A cross-sectional and prospective study. J Adv Nurs. 2014;70(12):2871–83. doi: 10.1111/jan.12447. [DOI] [PubMed] [Google Scholar]
- 18.Tsapepas D, Langone A, Chan L, et al. A longitudinal assessment of adherence with immunosuppressive therapy following kidney transplantation from the Mycophenolic Acid Observational REnal Transplant (MORE) study. Ann Transplant. 2014;19:174–81. doi: 10.12659/AOT.890216. [DOI] [PubMed] [Google Scholar]
- 19.Williams AF, Manias E, Gaskin CJ, Crawford K. Medicine non-adherence in kidney transplantation. J Ren Care. 2014;40(2):107–16. doi: 10.1111/jorc.12063. [DOI] [PubMed] [Google Scholar]
- 20.Pabst S, Bertram A, Zimmermann T, et al. Physician reported adherence to immunosuppressants in renal transplant patients: Prevalence, agreement, and correlates. J Psychosom Res. 2015;79(5):364–71. doi: 10.1016/j.jpsychores.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Scheel J, Reber S, Stoessel L, et al. Patient-reported non-adherence and immunosuppressant trough levels are associated with rejection after renal transplantation. BMC Nephrol. 2017;18(1):107. doi: 10.1186/s12882-017-0517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Low JK, Williams A, Manias E, Crawford K. Interventions to improve medication adherence in adult kidney transplant recipients: A systematic review. Nephrol Dial Transplant. 2015;30(5):752–61. doi: 10.1093/ndt/gfu204. [DOI] [PubMed] [Google Scholar]
- 23.Nevins TE, Nickerson PW, Dew MA. Understanding medication nonadherence after kidney transplant. J Am Soc Nephrol. 2017;28(8):2290–301. doi: 10.1681/ASN.2017020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Low JK, Manias E, Crawford K, et al. Improving medication adherence in adult kidney transplantation (IMAKT): A pilot randomised controlled trial. Sci Rep. 2019;9(1):7734. doi: 10.1038/s41598-019-44002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ettenger R, Albrecht R, Alloway R, et al. Meeting report: FDA public meeting on patient-focused drug development and medication adherence in solid organ transplant patients. Am J Transplant. 2018;18(3):564–73. doi: 10.1111/ajt.14635. [DOI] [PubMed] [Google Scholar]
- 26.Belaiche S, Decaudin B, Dharancy S, et al. Factors relevant to medication non-adherence in kidney transplant: A systematic review. Int J Clin Pharm. 2017;39(3):582–93. doi: 10.1007/s11096-017-0436-4. [DOI] [PubMed] [Google Scholar]
- 27.Alkatheri AA, Albekairy AM, Jarab A, et al. Medication adherence and treatment satisfaction among renal transplant recipients. Ann Transplant. 2016;21:270–78. doi: 10.12659/aot.897101. [DOI] [PubMed] [Google Scholar]
- 28.Chisholm MA, Lance CE, Williamson GM, et al. Development and validation of the immunosuppressant therapy adherence instrument (ITAS) Patient Educ Couns. 2005;59(1):13–20. doi: 10.1016/j.pec.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Chisholm MA, Lance CE, Williamson GM, et al. Development and validation of an immunosuppressant therapy adherence barrier instrument. Nephrol Dial Transplant. 2005;20(1):181–88. doi: 10.1093/ndt/gfh576. [DOI] [PubMed] [Google Scholar]
- 30.Open Souce Statistics for Public Health. http://www.openepi.com/SampleSize/SSPropor.htm [Available on 22.7.2018]
- 31.Weng FL, Chandwani S, Kurtyka KM, et al. Prevalence and correlates of medication non-adherence among kidney transplant recipients more than 6 months post-transplant: A cross-sectional study. BMC Nephrol. 2013;14:261. doi: 10.1186/1471-2369-14-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paterson TSE, O’Rourke N, Shapiro RJ, et al. Medication adherence in renal transplant recipients: A latent variable model of psychosocial and neurocognitive predictors. PLoS One. 2018;13(9):e0204219. doi: 10.1371/journal.pone.0204219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shabany Hamedan M, Mohamad Aliha J. Relationship between immunosuppressive medications adherence and quality of life and some patient factors in renal transplant patients in Iran. Glob J Health Sci. 2014;6(4):205–12. doi: 10.5539/gjhs.v6n4p205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butler JA, Peveler RC, Roderick P, et al. Modifiable risk factors for non-adherence to immunosuppressants in renal transplant recipients: A cross-sectional study. Nephrol Dial Transplant. 2004;19(12):3144–49. doi: 10.1093/ndt/gfh505. [DOI] [PubMed] [Google Scholar]
- 35.Alloway RR, Dupuis R, Gabardi S, et al. Evolution of the role of the transplant pharmacist on the multidisciplinary transplant team. Am J Transplant. 2011;11(8):1576–83. doi: 10.1111/j.1600-6143.2011.03601.x. [DOI] [PubMed] [Google Scholar]
- 36.Cossart AR, Staatz CE, Campbell SB, et al. Investigating barriers to immunosuppressant medication adherence in renal transplant patients. Nephrology (Carlton) 2017;24(1):102–10. doi: 10.1111/nep.13214. [DOI] [PubMed] [Google Scholar]
- 37.Staino C, Pilch N, Patel S, et al. Optimizing finite resources: Pharmacist chart reviews in an outpatient kidney transplant clinic. J Am Pharm Assoc (2003) 2015;55(6):613–20. doi: 10.1331/JAPhA.2015.14241. [DOI] [PubMed] [Google Scholar]
- 38.Sanders-Pinheiro H, Colugnati FAB, Marsicano EO, et al. Prevalence and correlates of non-adherence to immunosuppressants and to health behaviours in patients after kidney transplantation in Brazil – the ADHERE BRAZIL multicentre study: A cross-sectional study protocol. BMC Nephrol. 2018;19(1):41. doi: 10.1186/s12882-018-0840-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chisholm-Burns MA, Spivey CA, Graff Zivin J, et al. Improving outcomes of renal transplant recipients with behavioral adherence contracts: Arandomized controlled trial. Am J Transplant. 2013;13(9):2364–73. doi: 10.1111/ajt.12341. [DOI] [PubMed] [Google Scholar]
- 40.Schäfer-Keller P, Steiger J, Bock A, et al. Diagnostic accuracy of measurement methods to assess non-adherence to immunosuppressive drugs in kidney transplant recipients. Am J Transplant. 2008;8(3):616–26. doi: 10.1111/j.1600-6143.2007.02127.x. [DOI] [PubMed] [Google Scholar]



