Abstract
The 5-HT1A receptor is a G protein coupled receptor (GPCR) that activates G proteins of the Gαi/o family. 5-HT1A receptors expressed in the raphe, hippocampus and prefrontal cortex are implicated in the control of mood and are targets for anti-depressant drugs. Regulators of G protein signaling (RGS) proteins are members of a large family that play important roles in signal transduction downstream of G protein coupled receptors (GPCRs). The main role of RGS proteins is to act as GTPase accelerating proteins (GAPs) to dampen or negatively regulate GPCR-mediated signaling. We have shown that a mouse expressing Gαi2 that is insensitive to all RGS protein GAP activity has an anti-depressant-like phenotype due to increased signaling of postsynaptic 5-HT1A receptors, thus implicating the 5-HT1A receptor–Gαi2 complex as an important target. Here we confirm that RGS proteins act as GAPs to regulate signaling to adenylate cyclase and the mitogen-activated protein kinase (MAPK) pathway downstream of the 5-HT1A receptor, using RGS-insensitive Gαi2 protein expressed in C6 cells. We go on to use short hairpin RNA (shRNA) to show that RGS19 is responsible for the GAP activity in C6 cells and also that RGS19 acts as a GAP for 5-HT1A receptor signaling in human neuroblastoma SH-SY5Y cells and primary hippocampal neurons. In addition, in both cell types the synergy between 5-HT1A receptor and the fibroblast growth factor receptor 1 in stimulating the MAPK pathway is enhanced following shRNA reduction of RGS19 expression. Thus RGS19 may be a viable new target for anti-depressant medications.
Keywords: Regulator of G protein signaling, Serotonin 1A receptor (5-HT1A), FGF2, MAP kinase, SH-SY5Y cells, Hippocampal neurons
1. Introduction
Regulators of G protein signaling (RGS) proteins make up a large family of proteins comprising over 20 members that are defined by the presence of a RGS homology (RH) domain. This region of the protein binds to the active form of Gαi/o and Gαq proteins to accelerate GTP hydrolysis and form inactive Gα-GDP. Thus, RGS proteins act as GTPase accelerating proteins (GAPs) and afford a negative modulation of G-protein coupled receptor (GPCR) signaling [1-3]. Mutation of a glycine to serine in the switch I region of Gαi/o and Gαq proteins prevents the binding of Gα subunits to RH domain of all RGS proteins and so inhibits this negative regulation, leading to increased downstream signaling [4-8].
We have shown that a mouse with knock-in of a mutant Gαi2 that does not bind to RH domains (Gαi2GSGS) exhibits an anti-depressant- and anti-anxiety-like phenotype [9]. The phenotypic behavior is reversed by the 5-HT1A receptor antagonist WAY-100635. Moreover, heterozygotes show enhanced responses to the 5-HT1A receptor agonist 8-OH-DPAT and the selective serotonin reuptake inhibitor (SSRI) fluvoxamine in tests for anti-depressant-like activity. This behavior is hypothesized to be due to an enhanced activity of postsynaptic 5-HT1ARs in the hippocampus and/or prefrontal cortex, rather than autoreceptors in the raphe nucleus, and is specific for Gαi2 [9]. However, the particular RGS protein or proteins that are responsible for endogenous GAP activity at these postsynaptic 5-HT1A receptors and whose action is inhibited by the genetic manipulation of Gαi2 have not been identified. Very recently, Stewart et al. [10] have shown that a RGS6 knock-out mouse exhibits very similar anti-depressant- and anti-anxiety-like phenotypic behaviors to the Gαi2GSGS mouse. On the other hand the underlying biochemistry appears to be different between the genotypes, with the Gαi2GSGS mouse showing alteration compared to wild-type littermates in the phosphorylation status of GSK3β [9], while the RGS6 mouse does not show these changes but rather variation in the level of phospho-CREB [10]. Therefore, we have sought alternative candidate RGS proteins that could be responsible and/or contribute to the phenotypic 5-HT1A receptor-mediated anti-depressant-like behavior in the Gαi2GSGS mouse.
There is evidence that in addition to selectivity for Gα subtypes RGS proteins show specificity for particular receptors [11-14]. Studies of the effect of RGS proteins on 5-HT1A receptor signaling are limited. In addition to the studies with the RGS6 knockout mouse, RGS4, RGS10 and RGS20 have been reported to significantly attenuate 5-HT1A receptor signaling using heterologous cell lines and/or overexpression or inhibition of RGS proteins in raphe or cortical neurons [11,15,16]. We have previously demonstrated that RGS19, also known as Gα interacting protein or GAIP [17], acts as an effective GAP for mu-opioid receptor signaling in SH-SY5Y cells [14]. These cells also endogenously express 5-HT1A receptors [18]. 5-HT1A receptors and mu-opioid receptors show crosstalk [18] and also form functional heterodimers [19]. Therefore we hypothesized that RGS19 would act as an effective GAP for 5-HT1A receptor signaling.
Here we use C6 cells expressing RGS-insensitive Gαi2 to confirm endogenous GAP activity at 5-HT1A receptors linked to Gαi2. We then employ lentiviral delivery of short hairpin RNA (shRNA) to demonstrate that endogenous RGS19 protein in C6 cells, SH-SY5Y cells as well as mouse primary hippocampal neurons acts as a GAP for 5-HT1A receptor signaling in a brain region that is involved in the actions of serotonergic drugs in mood disorders. Finally, since fibroblast growth factor-2 (FGF2) acting at the FGF receptor 1 is reported to synergize with 5-HT1A receptor activation in hippocampal neurons [20], we ask if this synergy is modulated by RGS19.
2. Materials and methods
2.1. Cell culture
C6 and SH-SY5Y cells from ATCC are maintained in Dulbecco's modified Eagles medium (DMEM) with high glucose, l-glutamine, and pyridoxine HCl, without sodium pyruvate containing 10% fetal bovine serum (FBS, Invitrogen) under 5% CO2 at 37 °C. The C6 cell line stably expressing either pertussis toxin (PTX) insensitive Gαi2 (Gαi2CI) or both PTX insensitive and RGS insensitive Gαi2 (Gαi2CIGS) has been previously described [8,21].
Preparation of primary culture of hippocampal neurons is as follows: dissociated hippocampal cell culture was prepared as described previously [22]. Briefly, hippocampi were removed from postnatal day 0 (P0) mice (ICR) and dissociated with 0.5% trypsin. Dissociated hippocampal cells were plated at 60,000 cells per coverslip on 12 mm glass coverslips coated with poly-D-lysine (Millipore) in 24 well-plates, and maintained in neurobasal medium with B27 supplement (Invitrogen). All animal care and use were in accordance with the institutional guidelines and approved by the University Committee on Use and Care of Animals.
2.2. Western blot analysis
Whole-cell lysates were prepared from SH-SY5Y cells, C6 cells, and primary culture of hippocampal neurons as previously described [13]. Briefly, cells were suspended in ice-cold radio-immunoprecipitation assay (RIPA) lysis buffer containing protease inhibitor cocktail [13], then homogenized, and centrifuged at 20,000 ×g for 10 min. The supernatant (~20 μg) was subjected to SDS-PAGE on a 12% mini-gel and transferred to an Immobilon-P transfer membrane. The membrane was blocked with 1% bovine serum albumin in TBST (10 mM Tris–HCl, pH 8.0, 150 mM NaCl, 0.05% Tween 20) for 1 h and incubated with RGS19-specific or RGS4-specific anti-serum at a 1:8000 dilution overnight on a rocking shaker in the cold room. After three consecutive washes, the membrane was incubated with 1:10,000 dilution of secondary antibody (goat anti-rabbit IgG-horseradish peroxidase) for 1 h at room temperature. Prestained SDS-PAGE protein standards (Bio-Rad, Precision Plus Protein standards, Kaleidoscope) were used to determine the size of detected proteins. The membranes were cut at the 37-kDa marker, and the upper membrane was blotted with anti-α-tubulin antibody at 1:10,000 dilution as an internal control for protein loading. Proteins were visualized by chemiluminescence with SuperSignal West Pico (Pierce) and exposed to X-ray film or using the Odyssey FC imaging system (LI-COR, Inc., Lincoln, NE), then quantified.
2.3. cAMP assay
One day before transfection, stable PTX insensitive (PTX-i) and RGS/PTX insensitive (RGS/PTX-i) Gαi2 expressing C6 cells were plated into a 24-well plate so as to reach 60–80% confluency at transfection. HA tagged 5-HT1A plasmid DNA was transiently transfected with Lipofectamine 2000 for 48 h. Cells were treated with PTX (100 ng/ml) overnight before assay. On the day of assay, cells were washed once with fresh serum-free medium, and the medium was replaced with 1 mM 3-isobutyl-1-methylxanthine (IBMX) in serum-free medium for 15 min at 37 °C and then replaced with medium containing 1 mM IBMX, 30 μM forskolin, and 10 μM 8-OH-DPAT for 5 min at 37 °C. Reactions were stopped by replacing the medium with ice-cold 3% perchloric acid, and samples were kept at 4 °C for at least 30 min. An aliquot (0.4 ml) from each sample was removed, neutralized with 0.08 ml of 2.5 M KHCO3, vortexed, and centrifuged at 15,000 ×g for 1 min to pellet the precipitates. Accumulated cAMP was measured by radioimmunoassay in a 15 μl aliquot of the supernatant from each sample following the manufacturer's instructions (Diagnostic Products, Los Angeles, CA) and calculated as pmol/μg protein accumulation of cAMP.
2.4. MAPK assay
C6 Cells were plated in 24-well plates and treated with PTX (100 ng/ml) for 7–8 h in serum-free medium before assay. SH-SY5Y cells, were maintained in serum-free medium for 48 h before assay. For primary culture of hippocampal neurons, they were in serum-free medium for 3 h only. Then cells were washed once with fresh serum-free medium and stimulated with varying concentrations of 8-OH-DPAT (Sigma-Aldrich) as indicated with or without FGF2 (0.5 ng/ml, Sigma-Aldrich) or distilled H2O, respectively, for 5 or 10 min at 37 °C. The reaction was stopped by adding 0.1 ml of ice-cold SDS sample buffer (62.5 mM Tris–HCl, pH 6.8, 2% SDS, 10% glycerol, 50 mM dithiothreitol, and 0.01% bromophenol blue), and samples were removed from the wells, boiled for 5 min, and then subjected (10–15 μl each) to electrophoresis using a 12% SDS-PAGE mini-gel, followed by transfer to an Immobilon™-FL membrane (Fisher) for Western blotting. After blocking with an Odyssey blocking buffer for 1 h, the blot was probed with a 1:1000 dilution of mouse anti-phospho-p44/42MAPK (Thr202/Tyr204) antibody and rabbit anti-p44/42 MAPK antibody (Cell Signaling Technology) overnight on a rocking shaker in a cold room. After three consecutive washes, the blot was incubated with secondary antibody (1:10,000 dilution) of anti-mouse IRdye 680 RD and anti-rabbit IRdye 800 CW for 1 h at room temperature. Then images were acquired using an Odyssey FC imaging system (Li-COR Biosciences, Lincoln, NE) and quantified using building in program. MAPK activity was calculated as the ratio of normalized arbitrary units (a.u.) of phosphorylated ERK1/2 over total ERK1/2 or presented as percent of vehicle-treated control.
2.5. Lentiviral infection of primary neurons and establishment of SH-SY5Y and C6 cell lines stably expressing RGS4 shRNA, RGS19 shRNA, or control GFP shRNA
Lentiviral RGS4 shRNA and RGS19 shRNA were generated as described previously [13,14,23]. SH-SY5Y, C6 cells or primary cultures of hippocampal neurons were plated (at ~50% confluence) in 35-mm dishes or 24 well plate (with coverslips) and infected with a mixture of the four lentiviral stocks encoding shRNA against RGS4 or RGS19 in complete medium. A control cell line was obtained using lentivirus stock encoding shRNA against GFP (from Dr. Didier Trono's laboratory) [24]. The stable cell lines were generated by passaging cells into larger dishes. The virus also encodes for GFP and expression of this protein was used as an indicator for the presence of shRNA throughout cell culture maintenance. The knockdown of RGS4 and RGS19 protein was determined by Western blot with specific anti-RGS19 and anti-RGS4 antibodies compared to the control cell line with GFP shRNA expression.
2.6. Data and statistical analysis
Data from at least three separate experiments are presented as means ± S.E. Data were compared by two-way analysis of variance with Bonferroni posttest analysis of variance in GraphPad Prism, version 5.0 (GraphPad Software, La Jolla, CA), and differences were considered significant if p < 0.05.
3. Results
3.1. RGS-insensitive Gαi2 enhances 5-HT1A receptor-mediated signaling in C6 cells
To confirm the overall effect of RGS protein GAP activity on 5-HT1A receptor signaling and so support the proposed mechanism underlying the phenotypic behavior in the Gαi2GSGS mouse, we used a C6 cell line stably expressing either pertussis toxin (PTX) insensitive Gαi2 (Gαi2CI) or both PTX insensitive and RGS insensitive Gαi2 (Gαi2CIGS) as a model system. These cells have been previously described and express comparable levels of Gαi2 protein [5,21]. To determine the effect of endogenous RGS GAP activity, Gαi2CI and Gαi2CIGS cells were treated with PTX (100 ng/ml) for 8 h to prevent coupling between endogenous Gα proteins and 5-HT1A receptors. Treatment of cells for 5 min with or without varying concentrations of the 5-HT1A receptor agonist 8-OH-DPAT did not show a significant increase in ERK1/2 phosphorylation in Gαi2CI control cells up to the highest concentration (10 μM) of 8-OH-DPAT tested. However, there was a significantly increased effect in the Gαi2CIGS cells even at lower doses of 8-OH-DPAT compared with their Gαi2CI controls (Fig. 1A). To confirm this finding, we compared 5-HT1A receptor mediated inhibition of forskolin-stimulated cAMP accumulation. In native C6 cells, inhibition of cAMP by 8-OH-DPAT was not detectable, presumably due to the low endogenous expression of 5-HT1A receptors [18]. Therefore, Gαi2CI and Gαi2CIGS cells were transiently transfected with HA-tagged 5-HT1A receptors to confirm a similar expression level in the two cell lines using anti-HA antibody (Fig. 1B). After PTX treatment as above, the ability of the 5-HT1A receptor agonist 8-OH-DPAT (10 μM) to inhibit forskolin (30 μM)-stimulated cAMP was determined. In Gαi2CI cells, 8-OH-DPAT reduced forskolin-stimulated cAMP levels to 68 ± 5% of the control levels but gave a significantly larger inhibition to 43 ± 7% of control levels in the Gαi2CIGS cells.
Fig. 1.
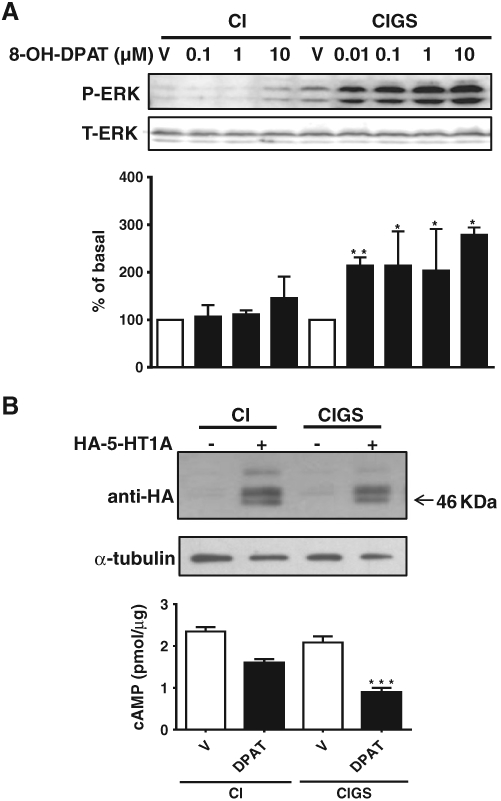
Expression of RGS-insensitive Gαi2 (CIGS) in C6 cells enhances 5-HT1A receptor-mediated increase in ERK1/2 phosphorylation and inhibition of adenylate cyclase. A, Effect of global RGS inhibition on 5-HT1A receptor signaling to MAPK. C6 cell lines stably expressing either PTX insensitive (PTXi) (CI) or both PTX insensitive (PTXi) and RGS insensitive (RGSi) Gαi2 (CIGS) subunits were plated into 24 well plates and serum starved together with PTX (100 ng/ml) treatment for 8 h; the MAPK assay was performed for 5′ with or without (V) varying concentrations of 5-HT1A receptor agonist 8-OH-DPAT from 10−8 to 10−5 M as described under Materials and methods. Treatment with 8-OH-DPAT did not significantly increase ERK1/2 phosphorylation in Gαi2CI control cells. There was a significantly increased effect in the Gαi2CIGS cells from the lowest concentration of 0.01 μM of 8-OH-DPAT compared with their Gαi2CI controls. *, p ≤ 0.05; and **, p < 0.01 (n = 3–4). B, Effect of RGS modulation on 5-HT1A receptor signaling to adenylate cyclase. HA-tagged 5-HT1A receptors were transiently transfected into C6 cell lines stably expressing either PTX insensitive (CI) or both PTX insensitive and RGS insensitive Gαi2 subunits (CIGS) using Lipofectamine 2000. Western blot analysis with anti-HA antibody confirmed equal expression of HA-5-HT1A receptor in the two cell lines (B, upper). No bands were detected in the mock transfected cells. Inhibition of forskolin (30 μM)-stimulated cAMP by the 5-HT1A receptor agonist 8-OH-DPAT (10 μM) measured at 5′ was enhanced in C6 cells overexpressing 5-HT1AR and RGS insensitive Gαi2 (CIGS) (B, lower). ***, p < 0.001 (n = 6).
3.2. RGS19 but not RGS4 modulates 5-HT1A receptor signaling in C6 cells and SH-SY5Y cells
RGS19 is highly expressed in C6 and SH-SY5Y cells at both the mRNA and protein levels as determined by RT-PCR with RGS19-specific primers and specific anti-RGS19 antibody [14]. We have shown that this protein, but not RGS4, negatively regulates opioid mu-receptor signaling [14].
To determine if RGS19 plays a role in 5-HT1A receptor signaling in C6 cells, we knocked down RGS19 by stably expressing RGS19 shRNA in Gαi2CI cells using lentiviral delivery. Western blot analysis showed that RGS19 protein expression was reduced to ~33% of the level in control cells that expressed shRNA against GFP (Fig. 2A). Cells were treated with PTX overnight to prevent coupling of the 5-HT1A receptor to Gα proteins other than the PTX-insensitive Gαi2. 8-OH-DPAT did not significantly increase ERK1/2 phosphorylation in control GFP shRNA cells, but the ability of 8-OH-DPAT to stimulate phospho-ERK1/2 formation was markedly enhanced in the cells expressing RGS19 shRNA compared with the control cells expressing shRNA against GFP (Fig. 2B). C6 cells do not express RGS4 [13].
Fig. 2.
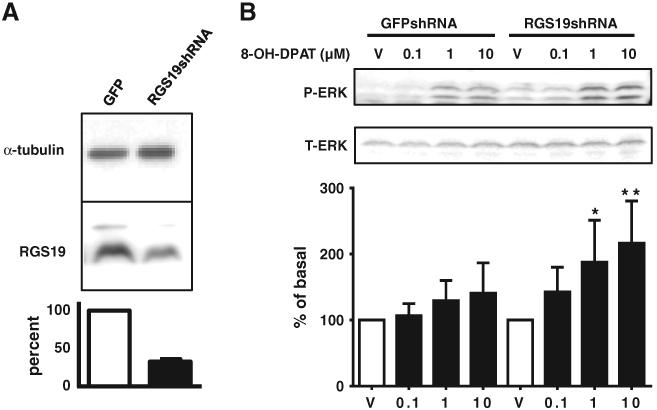
Specific role of RGS19 in modulating 5-HT1A receptor signaling in C6 cells. A, Generation of C6 cell line stably expressing RGS19 shRNA. C6 cells expressing PTX-insensitive Gαi2 (CI) were infected with a mixture of the four lentiviral stocks encoding shRNA against RGS19. A control cell line was obtained using lentivirus stocks encoding shRNA against GFP. Whole cell lysates were prepared and knockdown of endogenously expressed RGS19 was determined by Western blot analysis with anti-RGS19 antibody. B, MAPK assay in RGS19-deficient C6 cells. Cells were treated with PTX (100 ng/ml) in serum-free medium for 7 h before the assay. 8-OH-DPAT did not significantly increase ERK1/2 phosphorylation in control GFPshRNA cells, but concentration (10−7 to 10−5 M)-dependently increased ERK1/2 phosphorylation in RGS19-deficient C6 cells (RGS19shRNA) compared with control cells expressing GFP shRNA cells. *, p ≤ 0.05; and **, p < 0.01 (n= 7).
In SH-SY5Y cells, both RGS4 and RGS19 are endogenously highly expressed [13,14]. SH-SY5Y cell lines stably expressing shRNA against RGS4 and RGS19 were established as described previously [13,14]. Knockdown of these proteins was confirmed by Western blot analysis with specific antibodies for either RGS4 or RGS19 [13,14]. SH-SY5Y cells expressing shRNA against GFP were used as controls. In control SH-SY5Y cells, 8-OH-DPAT was unable to stimulate ERK1/2 phosphorylation, presumably due to the low level of expression of endogenous 5-HT1A receptor [18]. However, knockdown of RGS19 allowed 8-OH-DPAT to concentration-dependently-increase ERK1/2 phosphorylation especially at higher concentrations compared with the control cells expressing shRNA against GFP (Fig. 3). No effect was seen in RGS4-deficient cells.
Fig. 3.
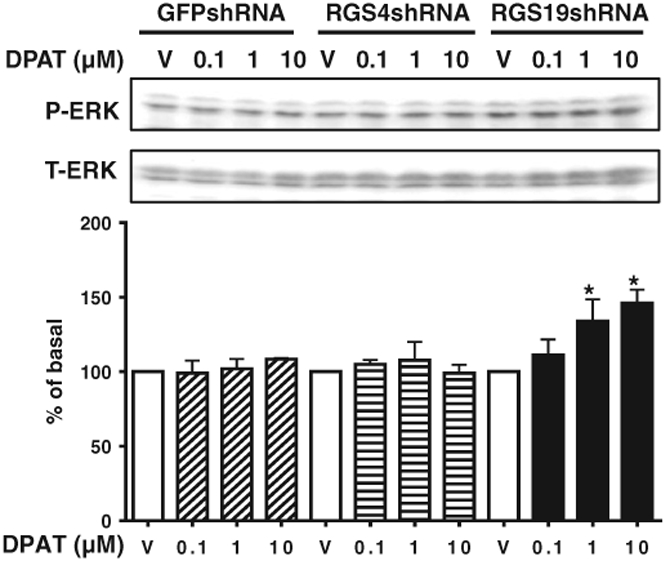
RGS19 but not RGS4 modulates 5-HT1A receptor signaling in SH-SY5Y cells. SH-SY5Y cells stably expressing shRNA specifically targeting either RGS4 or RGS19 or control GFP shRNA were used. After serum-starvation for 48 h, cells were treated with vehicle (V) and varying concentrations of 8-OH-DPAT (10−7 to 10−5 M) for 5 min and ERK1/2 phosphorylation was determined. *, p < 0.05 (n = 3–4) for the RGS19-deficient RGS19 shRNA cell line, compared with control cells expressing shRNA against GFP. There is no difference in phosphorylation of ERK1/2 in RGS4 shRNA cells compared with the control cells with GFP shRNA following 8-OH-DPAT stimulation. Data are presented as percent of vehicle-treated controls.
It has been shown that fibroblast growth factor 2 (FGF2) enhances the action of 5-HT1A receptor agonists to stimulate ERK1/2 phosphorylation in hippocampal neurons [20]. In control SH-SY5Y cells, FGF2 did not increase ERK1/2 phosphorylation alone nor did it significantly alter the inability of 8-OH-DPAT to cause ERK1/2 phosphorylation. However, in SH-SY5Y cells deficient in RGS19, 8-OH-DPAT did stimulate ERK1/2 phosphorylation (Fig. 4) and there was also a marked synergistic increase in ERK1/2 phosphorylation when 8-OH-DPAT and FGF2 were co-administered (Fig. 4). Knockdown of RGS4 had no effect on 8-OH-DPAT's ability to stimulate ERK1/2 phosphorylation alone or combined with FGF2 treatment.
Fig. 4.
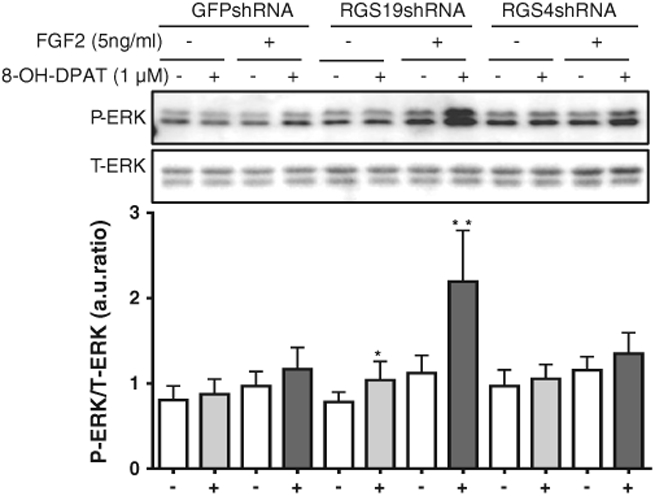
Synergistic activation of ERK1/2 phosphorylation by 8-OH-DPAT and FGF2 is enhanced by RGS19 but not RGS4 knockdown in SH-SY5Y cells. SH-SY5Y cells stably expressing control GFP shRNA, RGS19 shRNA and RGS4 shRNA, respectively, were serum-starved for 48 h and a MAPK assay was performed for 10′ with (+)or without (−) the 5-HT1A receptor agonist (8-OH-DPAT, 1.0 μM) and with (+) or without (−) FGF2 (5 ng/ml). (+) and (−) signs at bottom of figure indicate absence or presence of 8-OH-DPAT. *, p < 0.05; and **, p < 0.01 (n = 5) for the RGS19-deficient cell line, compared with control cells expressing shRNA against GFP with (+) or without (−) FGF2 treatment. There was no difference in cells expressing shRNA against RGS4 compared with the control cells expressing shRNA against GFP. Data are presented as a ratio of normalized arbitrary units (a.u.) of phosphorylated ERK1/2 (P-ERK) over total ERK1/2 (T-ERK).
3.3. Synergistic activation of ERK1/2 phosphorylation by 5-HT1A and FGFR1 receptors is enhanced by RGS19 knockdown in primary cultures of hippocampal neurons
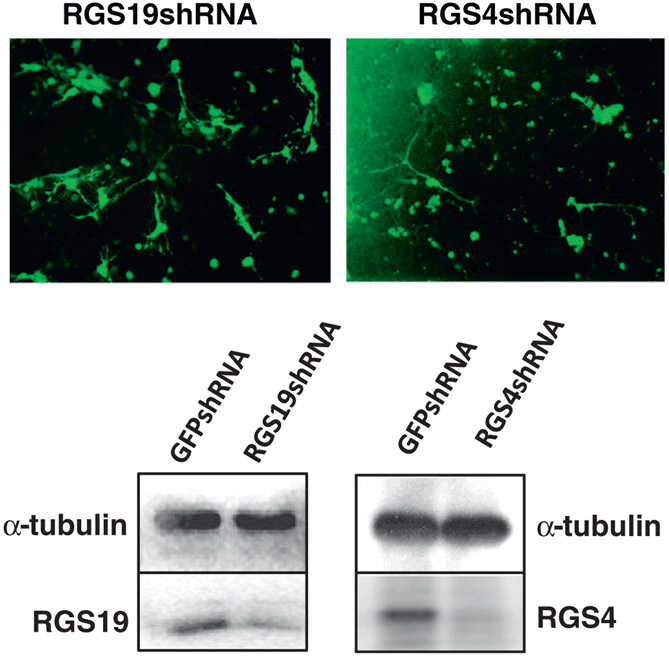
Both RGS4 and RGS19 proteins are highly expressed in hippocampal neurons. Primary cultures of hippocampal neurons were prepared from postnatal day 0 (P0) mice, and infected with lentiviruses encoding shRNA targeting either GFP (control), RGS4 or RGS19. Infection occurred in ~80% of the cells as measured by GFP expression (Fig. 5A), and resulted in a reduction of RGS19 and RGS4 to ~35% and ~15% of control levels, respectively (Fig. 5B) by Western blot analysis.
Fig. 5.
Knockdown of RGS4 and RGS19 by lentiviral infection of shRNA in primary cultures of mouse hippocampal neurons. Primary cultures of mouse hippocampal neurons were prepared from postnatal day 0 (P0) mice, and infected with lentiviruses containing shRNA targeting either GFP (control), RGS 4 or RGS19, respectively, at day 3 in vitro. The expressed RGS shRNAs were monitored by GFP gene expression and showed about an 80% infection rate (top). After 7 days in vitro (4 days post-infection), hippocampal neurons were lysed and subjected to Western blot analysis; RGS4 and RGS19 proteins were knocked down to ~15% and ~35% of GFP shRNA control levels, respectively (bottom).
In control neurons expressing shRNA against GFP, 8-OH-DPAT (0.5 μM) alone did not induce ERK1/2 activation. However, a small but not significant degree of ERK1/2 activation was seen with FGF2 in the presence or absence of 8-OH-DPAT. In contrast, in cells with reduced expression of RGS19, ERK1/2 activation was increased by 2-fold. In addition, there was a marked synergistic effect of 8-OH-DPAT and FGF2 on ERK1/2 phosphorylation in neurons with reduced RGS19 levels (Fig. 6). Cells expressing shRNA against RGS4 behaved the same as the control cells (Fig. 6).
Fig. 6.
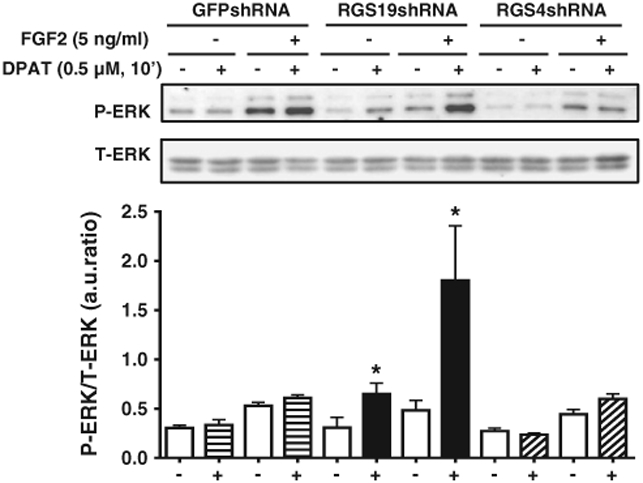
Synergistic activation of ERK1/2 phosphorylation by 8-OH-DPAT and FGF2 is enhanced in RGS19-deficient hippocampal neurons, but not in RGS4-deficient hippocampal neurons. Primary cultures of mouse hippocampal neurons were infected with lentiviral delivery of shRNAs against GFP (control), RGS4 or RGS19 on day 3. On day 8 neurons were serum-starved for 3 h and ERK1/2 phosphorylation was determined after 10′ with (+) or without (−) 8-OH-DPAT (0.5 μM) and with (+) or without (−) FGF2 (5 ng/ml). (+) and (−) signs at bottom of figure indicate absence or presence of 8-OH-DPAT. *, p < 0.05 (n = 3) for the hippocampal neurons with reduced RGS19 expression, compared with control neurons expressing shRNA against GFP. No difference was observed in hippocampal neurons with reduced RGS4 expression. Data are presented as a ratio of normalized arbitrary units (a.u.) of phosphorylated ERK1/2 (P-ERK) over total ERK1/2 (T-ERK).
4. Discussion
The present findings confirm that the Gαi2-coupled 5-HT1A receptor is susceptible to GAP activity and that RGS19 is at least partly responsible for this action. Moreover, RGS19 acts as a GAP for 5-HT1A receptor signaling in human neuroblastoma SH-SY5Y cells, as well as primary cultures of mouse hippocampal cells. This is important since 5-HT1A receptors in the hippocampus are implicated in the control of mood and consequently RGS19 could play an important role in controlling these events. The fact that we see a significant effect with selective knockdown of RGS19 indicates that this RGS protein may be a key modulator of 5-HT1A receptor signaling in vivo and that loss of this activity in the mouse expressing RGS-insensitive Gαi2 could contribute, along with RGS6 [10] to be the observed anti-depressant- and anti-anxiety-like phenotype.
In addition to RGS19, SH-SY5Y and hippocampal cells (but not C6 cells) also express a high level of RGS4 [13,14] that has been previously shown to impact 5-HT1A receptor signaling in overexpression studies [11,16]. Moreover, RGS4 has been implicated in the action of a range of anti-depressant drugs in mouse models, although this includes both serotonergic and non-serotonergic drugs [25-27] and so is unlikely to be responsible for the specific 5-HT1A action seen in the Gαi2GSGS mouse. However, the present results provide no evidence for RGS4 regulation of 5-HT1A receptor signaling in SH-SY5Y cells or in primary hippocampal neurons. This lack of GAP action of RGS4 compared to previous studies could be due to tissue specific differences or more simply a reflection of use of overexpression methods and so highlights the benefit of using knockdown rather than overexpression methods. The reasons for the receptor selective effect of RGS19 versus RGS4 are not clear. Both are small RGS proteins with approximately 45% conserved amino-acids in the RH domains and the RGS4 inhibitor CCG50014 that prevents binding of the RH domain of RGS4 to Gαi/o proteins also has similar potency for RGS19 [28,29]. The N-terminus of RGS4 is thought to be responsible for its recognition of certain receptors, including the delta opioid receptor [13] and presumably is not optimal for binding to the 5-HT1A receptor. In contrast, although RGS19 is itself similar in structure to RGS4, with the addition of a cysteine string in the N-terminus [3] that may be palmitoylated, its active form is more complex since it has two partners, GAIP-interacting protein C-terminus (GIPC) and GAIP-interacting protein N-terminus (GIPN) that add additional sites for protein–protein binding and so could contribute to the specificity.
The 5-HT1A receptor activates the MAPK pathway via a Gαi/o-mediated mechanism [19,30,31]. However, there is considerable evidence that GPCRs can activate this pathway via transactivation of tyrosine kinase receptors [32-34]. Previously, 5-HT1A receptors and fibroblast growth factor (FGF) receptors in the hippocampus have been shown to synergize to activate ERK1/2 [20]. This possibly occurs by the formation of 5-HT1A receptor: FGF-receptor heteromers that lead to a 5-HT1A receptor-mediated phosphorylation of FGF receptors [20]. There is considerable evidence of a protective role for FGF2 in depression [35]. Thus, FGF2 given i.c.v. has anti-depressant-like actions in the rat [36]. Selective serotonin re-uptake inhibitors or serotonin-specific reuptake inhibitor (SSRIs) increase FGF2 expression in the hippocampus and cortex [37] and FGF2 mRNA expression in the human hippocampus is dysregulated in depression [38]. In the present study, FGF2 alone did not stimulate ERK1/2 phosphorylation in SH-SY5Y cells or primary hippocampal neurons, but 8-OH-DPAT did. However, both compounds added together showed a synergistic ERK1/2 phosphorylation. This synergism was greatly enhanced by the knockdown of RGS19. The mechanism of this synergy is unknown. One distinct possibility, based on previous literature, is a 5-HT1A receptor-dependent autophosphorylation of the FGF receptor 1 through an intracellular G protein-dependent mechanism [20]. This process would be enhanced by the increased signaling downstream of the 5-HT1A receptor due to the loss of negative regulatory actions of RGS19. On the other hand, additional components downstream of 5-HT1A receptor will also be enhanced by the loss of RGS19 and so could lead to synergy at points upstream of ERK1/2. The RGS19 C-terminus binding protein GIPC contains a PDZ domain that binds RGS19 and has been shown to link RGS19 to tyrosine kinase Trk A and B receptors [39], thus joining G protein signaling and tyrosine kinase pathways [39]. RGS19 could play a similar role in connecting 5-HT1A receptor and FGF receptor 1 signaling, although, in that case we might expect a loss of signaling in the RGS19 knockdown conditions.
Previous studies have implicated RGS6, a member of the R7 family of RGS proteins that has DEP and GGL-like binding domains [3] for additional protein binding, as a GAP for 5-HT1A receptor-mediated signaling. This suggestion is based on the finding that in cortical neurons from RGS6 knockout (RGS6−/−) mice, 8-OH-DPAT-mediated inhibition of adenylate cyclase is enhanced [10]. Moreover, the RGS6−/− mouse shows a very similar anti-depressant- and anti-anxiety-like behavioral phenotype to the Gαi2GSGS mouse suggesting that this is the critical RGS protein. On the other hand, increases in phosphorylation of the GSK3β, a process that occurs in response to a variety of anti-depressant drugs [40-42] that is seen in the hippocampus and cortex of the Gαi2GSGS mouse [9] were not reproduced in the RGS6−/− mouse, but rather CREB phosphorylation was decreased, suggesting differences in the biochemistry underlying the phenotype and that the specific Gα protein, not just the lack of RGS responsiveness, is critical for the phenotype behavior. In addition, a robust effect of RGS19 knockdown was seen on 5-HT1A receptor stimulation of ERK1/2 phosphorylation and the synergy with FGF2, suggesting that other RGS proteins are not able to fully substitute for RGS19 and therefore suggesting a lack of redundancy of RGS action in these systems. However we cannot rule out a contribution from RGS6 and so the respective roles for RGS6 and RGS19 as GAPs for 5-HT1A receptor signaling merit further investigation.
5. Conclusion
RGS19 acts as a highly effective GAP to modulate G protein signaling downstream of 5-HT1A receptors and may contribute to the phenotypic anti-depressant- and anti-anxiety-like behavior seen in Gαi2GSGS mice. Moreover, RGS19 may play an important role in controlling the degree of synergism between FGF-1 receptors and 5-HT1A receptors, that may work together to mediate an anti-depressant effect. Thus, inhibition of RGS19 may be a viable novel target for anti-depressant medications.
Acknowledgments
The authors thank Dr. Didier Trono (Department of Microbiology & Molecular Medicine, University of Geneva, Switzerland) for the LentiVector system and control GFP shRNA constructs and Dr. Marilyn Gist Farquhar (University of California San Diego, La Jolla) for providing the anti-RGS19 antibody. The authors thank Ms. Hellen Zhang for her assistance with fluorescence microscopy.
This work was supported by the National Institutes of Health [Grants: MH083754 and MH097021].
Abbreviations:
- GPCRs
G protein coupled receptors
- RGS
regulator of G protein signaling
- GAP
GTPase accelerating protein
- RH
RGS homology domain
- shRNA
short hairpin RNA
- GFP
green fluorescent protein
- IBMX
3-isobutyl-1-methylxanthine
- MAPK
mitogen-activated protein kinase
- AC
adenylate cyclase
- cAMP
cyclic adenosine monophosphate
- 5-HT1AR
serotonin receptor 5-HT1A
- FGFR1
fibroblast growth factor receptor 1
- FGF2
fibroblast growth factor 2
- GIPN
GAIP-interacting protein N-terminus
- GIPC
GAIP-interacting protein C-terminus
- PTX
pertussis toxin
- 8-OH-DPAT
(±)-8-hydroxy-2-dipropylaminotetralin hydrobromide
- GDP
guanosine diphosphate
- GTP
guanosine triphosphate
- RIPA
radio-immunoprecipitation assay
- ANOVA
analysis of variance
- i.c.v.
intracerebroventricular injection
- WAY-100635
N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridyl)cyclohexanecarboxamide
- SSRIs
selective serotonin reuptake inhibitors
- GSK3β
glycogen synthase kinase 3 beta
- Trk
tyrosine kinase
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- [1].Traynor JR, Neubig RR, Mol. Interv 5 (2005) 30–41. [DOI] [PubMed] [Google Scholar]
- [2].Neubig RR, Siderovski DP, Nat. Rev. Drug Discov 1 (2002) 187–197. [DOI] [PubMed] [Google Scholar]
- [3].Hollinger S, Hepler JR, Pharmacol. Rev 54 (2002) 527–559. [DOI] [PubMed] [Google Scholar]
- [4].Lan KL, Sarvazyan NA, Taussig R, Mackenzie RG, DiBello PR, Dohlman HG, Neubig RR, J. Biol. Chem 273 (1998) 12794–12797. [DOI] [PubMed] [Google Scholar]
- [5].Lan KL, Zhong H, Nanamori M, Neubig RR, J. Biol. Chem 275 (2000) 33497–33503. [DOI] [PubMed] [Google Scholar]
- [6].Zhong H, Neubig RR, J. Pharmacol. Exp. Ther 297 (2001) 837–845. [PubMed] [Google Scholar]
- [7].Clark MJ, Harrison C, Zhong H, Neubig RR, Traynor JR, J. Biol. Chem 278 (2003) 9418–9425. [DOI] [PubMed] [Google Scholar]
- [8].Fu Y, Zhong H, Nanamori M, Mortensen RM, Huang X, Lan K, Neubig RR, Methods Enzymol. 389 (2004) 229–243. [DOI] [PubMed] [Google Scholar]
- [9].Talbot JN, Jutkiewicz EM, Graves SM, Clemans CF, Nicol MR, Mortensen RM, Huang X, Neubig RR, Traynor JR, Proc. Natl. Acad. Sci. U. S. A 107 (2010) 11086–11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stewart A, Maity B, Wunsch AM, Meng F, Wu Q, Wemmie JA, Fisher RA, FASEB J. 28 (2014) 1738–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ghavami A, Hunt RA, Olsen MA, Zhang J, Smith DL, Kalgaonkar S, Rahman Z, Young KH, Cell. Signal 16 (2004) 711–721. [DOI] [PubMed] [Google Scholar]
- [12].Wang Q, Liu M, Mullah B, Siderovski DP, Neubig RR, J. Biol. Chem 277 (2002) 24949–24958. [DOI] [PubMed] [Google Scholar]
- [13].Wang Q, Liu-Chen LY, Traynor JR, J. Biol. Chem 284 (2009) 18357–18367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang Q, Traynor JR, Mol. Pharmacol 83 (2013) 512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Beyer CE, Ghavami A, Lin Q, Sung A, Rhodes KJ, Dawson LA, Schechter LE, Young KH, Brain Res. 1022 (2004) 214–220. [DOI] [PubMed] [Google Scholar]
- [16].Gu Z, Jiang Q, Yan Z, Mol. Pharmacol 71 (2007) 1030–1039. [DOI] [PubMed] [Google Scholar]
- [17].De Vries L, Lou X, Zhao G, Zheng B, Farquhar MG, Proc. Natl. Acad. Sci. U. S. A 95 (1998) 12340–12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Levitt ES, Purington LC, Traynor JR, Mol. Pharmacol 79 (2011) 461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cussac D, Rauly-Lestienne I, Heusler P, Finana F, Cathala C, Bernois S, De Vries L, Cell. Signal 24 (2012) 1648–1657. [DOI] [PubMed] [Google Scholar]
- [20].Borroto-Escuela DO, Romero-Fernandez W, Mudó G, Pérez-Alea M, Ciruela F, Tarakanov AO, Narvaez M, Di Liberto V, Agnati LF, Belluardo N, Fuxe K, Biol. Psychiatry 71 (2012) 84–91. [DOI] [PubMed] [Google Scholar]
- [21].Clark MJ, Traynor J, Methods Enzymol. 389 (2004) 155–169. [DOI] [PubMed] [Google Scholar]
- [22].Teranchi A, Johnson-Venkatesh EM, Toth AB, Javed D, Sutton MA, Umemori H, Nature 465 (2010) 783–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang Q, Traynor JR, J. Biol. Chem 286 (2011) 7854–7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wiznerowicz M, Trono D, J. Virol 77 (2003) 8957–8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Stratinaki M, Varidaki A, Mitsi V, Ghose S, Magida J, Dias C, Russo SJ, Vialou V, Caldarone BJ, Tamminga CA, Nestler EJ, Zachariou V, Proc. Natl. Acad. Sci. U. S. A 110 (2013) 8254–8259. [DOI] [PMC free article] [PubMed] [Google Scholar]; Erratum in: Proc. Natl. Acad. Sci. U S A 110, 2013:11660 [Google Scholar]
- [26].Rivero G, Gabilondo AM, García-Sevilla JA, Callado LF, La Harpe R, Morentin B, Meana JJ, Psychopharmacology (Berl) 226 (2013) 177–188. [DOI] [PubMed] [Google Scholar]
- [27].Huuhka K, Kampman O, Anttila S, Huuhka M, Rontu R, Mattila KM, Hurme M, Lehtimäki T, Leinonen E, Neurosci. Lett 437 (2008) 25–28. [DOI] [PubMed] [Google Scholar]
- [28].Storaska AJ, Neubig RR, Methods Enzymol. 522 (2013) 133–152. [DOI] [PubMed] [Google Scholar]
- [29].Storaska AJ, Mei JP, Wu M, Li M, Wade SM, Blazer LL, Sjögren B, Hopkins CR, Lindsley CW, Lin Z, Babcock JJ, McManus OB, Neubig RR, Cell. Signal 25 (2013) 2848–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Raymond JR, Olsen CL, Gettys TW, Biochemistry 32 (1993) 11064–11073. [DOI] [PubMed] [Google Scholar]
- [31].Raymond JR, Mukhin YV, Gelasco A, Turner J, Collinsworth G, Gettys TW, Grewal JS, Garnovskaya MN, Pharmacol. Ther 92 (2001) 179–212. [DOI] [PubMed] [Google Scholar]
- [32].Shah BH, Catt KJ, Trends Neurosci. 27 (2004) 48–53. [DOI] [PubMed] [Google Scholar]
- [33].Pyne NJ, Pyne S, Trends Pharmacol. Sci 32 (2011) 443–450. [DOI] [PubMed] [Google Scholar]
- [34].Delcourt N, Bockaert J, Marin P, Trends Pharmacol. Sci 28 (2007) 602–607. [DOI] [PubMed] [Google Scholar]
- [35].Jarosik J, Legutko B, Werner S, Unsicker K, von Bohlen Und Halbach O, Restor. Neurol. Neurosci 29 (2011) 153–165. [DOI] [PubMed] [Google Scholar]
- [36].Turner CA, Gula EL, Taylor LP, Watson SJ, Akil H, Brain Res. 1224 (2008) 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kato M, Okugawa G, Wakeno M, Takekita Y, Nonen S, Tetsuo S, Nishida K, Azuma J, Kinoshita T, Serretti A, Eur. Neuropsychopharmacol 19 (2009) 718–725. [DOI] [PubMed] [Google Scholar]
- [38].Gaughran F, Payne J, Sedgwick PM, Cotter D, Berry M, Brain Res. Bull 70 (2006) 221–227. [DOI] [PubMed] [Google Scholar]
- [39].Lou X, Yano H, Lee F, Chao MV, Farquhar MG, Mol. Biol. Cell 12 (2001) 615–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Beaulieu JM, Int. J. Neuropsychopharmacol 10 (2007) 3–6. [DOI] [PubMed] [Google Scholar]
- [41].Beaulieu JM, Zhang X, Rodriguiz RM, Sotnikova TD, Cools MJ, Wetsel WC, Gainetdinov RR, Caron MG, Proc. Natl. Acad. Sci. U. S. A 105 (2008) 1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Li X, Zhu W, Roh MS, Friedman AB, Rosborough K, Jope RS, Neuropsychopharmacology 29 (2004) 1426–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]