Abstract
Background
The Medicare population is increasing while the prevalence of obesity remains high. Bariatric surgery is the most efficacious treatment of obesity and its comorbidities. The objective of this investigation was to assess trends in utilization, readmission, mortality, and cost of bariatric surgery in the Medicare population.
Methods
Utilizing the Medicare Provider Analysis and Review database, patients with clinically severe obesity undergoing laparoscopic Roux‐en‐Y gastric bypass (RYGB), laparoscopic sleeve gastrectomy (SG), and laparoscopic adjustable gastric banding (LAGB) from 2011–2015 were identified. Trends in procedure selection, readmissions, mortality, and cost were examined. A multivariable logistic regression analysis to evaluate factors associated with readmission and mortality was performed.
Results
Of the 73,718 patients identified, 53,949 (73%) of patients were enrolled in Medicare due to disability, 19,191 (26%) due to age, and 578 (<1%) due to end stage renal disease (ESRD). Utilization of SG increased (1% in 2011 to 61% in 2015), while utilization of RYGB (68% to 32%) and LAGB (31% to 1%) decreased. Length of stay (LOS) was highest after RYGB (2.54 days), and lowest after LAGB (1.32 days). LOS decreased from 2.23 days in 2011 to 2.12 days in 2015. Thirty‐day readmissions were 8.24% for the disabled, 5.5% for the elderly, 12.8% with ESRD. Odds of readmission increased with black race, higher body mass index (BMI), and RYGB. Readmission decreased from 8% in 2011 to 7% in 2015. Thirty‐day mortality was 0.22% in the disabled, and 0.28% in the elderly. Odds of 30‐day mortality increased among men, those with higher BMI, some comorbidities, and those who underwent RYGB. Cost of SG decreased while cost of RYGB increased.
Conclusions
Among the Medicare population, an increase in SG while a decrease in RYGB and LAGB utilization was noted from 2011–2015. Readmissions and cost have decreased, while mortality has remained low.
Keywords: bariatric surgery, sleeve gastrectomy, Roux‐en‐Y gastric bypass, Medicare
1. INTRODUCTION
The elderly population of the United States is expected to double to 88 million by the year 2050. 1 Medicare, which provides health insurance for people over the age of 65, also provides coverage for working‐age people with disabilities and patients with end stage renal disease (ESRD) on dialysis. According to the Center for Medicare and Medicaid services (CMS), in 2017 over 58 million people are enrolled in traditional Medicare or Medicare advantage health plans. 2 As the elderly population of the United States increases and the number of Medicare enrollees continues to rise, health care utilization will also increase substantially. This increase in health service use is potentiated by the increase in health care utilization seen in people with obesity. 3 According to the Centers for Disease Control and Prevention, 42.8% of the elderly population (age ≥ 60) in the United States had obesity in 2017–2018. 4 Obesity also disproportionally affects the disabled population enrolled in Medicare services. 5
Obesity related metabolic dysfunction, such as decreased insulin sensitivity and increased intrahepatic triglyceride content, can be improved with even modest weight loss. 6 In the elderly population, a number of randomized trials have demonstrated that intentional weight loss in those with obesity leads to reduced mortality.7, 8 The lifestyle alterations required for sustained weight loss are difficult to implement in the clinical setting, and unfortunately weight regain is common.9, 10, 11 Bariatric surgery is the most efficacious treatment of obesity and its related comorbidities. Numerous trials have found that bariatric surgery outperforms medical management alone for sustained weight loss and improvement in obesity related comorbidities.12, 13, 14, 15 Although long‐term data are lacking, bariatric surgery appears to be safe and efficacious in the elderly population.16, 17, 18, 19 In 2006, CMS expanded coverage for beneficiaries seeking bariatric surgery. 20 Since then the number of Medicare patients undergoing bariatric surgery increased dramatically. 19
It appears that practice trends in bariatric surgery have dramatically changed over the last several years. Sleeve gastrectomy (SG) has now overtaken Roux‐en‐Y gastric bypass (RYGB) as the most common bariatric procedure performed in the United States.21, 22 This change in practice has further reduced the number of readmissions after bariatric surgery in that time. 21 Data evaluating practice trends in bariatric surgery, particularly among Medicare beneficiaries, remain limited. In a previous evaluation of the Medicare Provider Analysis and Review (MedPAR) database from 2006–2009, safety, mortality, and readmissions in the Medicare population were examined, but SG was not routinely performed and therefore not evaluated. 19 Given the current emphasis on health care utilization, cost, and readmissions, especially in the Medicare population, determining current practice trends in bariatric surgery is critical to forecast health care policy.
The objective of this study is to evaluate procedure selection, safety, readmissions, and cost of bariatric surgery in Medicare beneficiaries utilizing a population based approach. The hypothesis of this investigation was that procedure selection would shift toward increased utilization of the SG and safety, readmission, and cost of surgery would decrease over time.
2. MATERIALS AND METHODS
2.1. Data and patients
A retrospective cohort study including patients aged 18 and older undergoing bariatric surgery between 1 January 2011 and 31 September 2015 was performed using the MedPAR database. Based on original reason for Medicare entitlement, patients were classified into three groups, disabled, elderly, and ESRD. Types of bariatric surgery were classified by using combination of International Classification of Disease‐9th Revision (ICD‐9) and Current Procedural Terminology (CPT) codes. RYGB was classified using a combination of CPT codes 43644 and 43645, and ICD‐9 code 44.38. SG was classified using a combination of CPT code 43775 and ICD‐9 code 43.82. Laparoscopic adjustable gastric banding (LAGB) was classified using CPT code 43770 and ICD‐9 code 44.95. Revision of gastric band procedures were coded using CPT codes 43771, 43772, 34773, and 43774 and ICD‐9 codes 44.96, 44.97, and 44.98. Based on ICD‐9 and CPT codes, we are unable to reliably discern if revision was for complication or weight loss. Patient's body mass index (BMI) and comorbidities were identified by using primary or secondary ICD‐9 diagnosis codes from the inpatient or outpatient claims of the bariatric surgery admission. Mortality was identified by using the date of death from the Medicare member file in conjunction with hospital discharge status codes from inpatient claims.23, 24
2.2. Statistical analysis
Chi‐square tests were used to univariate analyses comparing patient demographic characteristics, BMI, comorbidities, hospital readmission, and mortality rate among study groups. Kruskal–Wallis Tests were performed to compare length of hospital stay (LOS) between the groups.
Payments were defined as the total dollar amount paid by Medicare for the patient's claim, represented as dollars of the corresponding year. “Adjusted procedure cost” was estimated using a generalized estimating equation multivariable model with gamma distribution, controlling for factors associated with medical expenditures for the surgery, including patient's year of surgery, demographics such as age, gender and race/ethnicity, BMI, comorbidities, and hospital regions. These adjusted costs are presented as a ratio compared with the reference adjusted cost (i.e., an adjusted cost ratio of 1.05 represents a 5% higher cost as compared to the reference group.) Kruskal–Wallis Tests were performed to compare these ratios among the groups listed.
Multivariable logistic regression models were used to examine factors associated with 90 days mortality and 30 days hospital readmission. The factors included were study group, type of procedure, year of surgery, age at surgery, age, gender, race, BMI, and comorbidities (diabetes, gastroesophageal reflux disease, hyperlipidemia, hypertension, coronary artery disease, congestive heart failure, peripheral arterial disease, rheumatoid arthritis, chronic obstructive pulmonary disease, chronic renal failure, cancer, depression, sleep apnea, and smoking). Odds ratio (OR) with associated 95% confidence intervals (CI) were reported for the multivariable models. 25 Reference groups for comorbidity OR are the groups without said disease, such as patients with diabetes compared to patients without diabetes. Statistical significance was evaluated at p < 0.05. All statistical analyses were performed using SAS Version 9.4 (SAS Institute Inc.).
3. RESULTS
3.1. Patient characteristics
From 2011 through the third quarter of 2015, 73,718 overall bariatric procedures in the Medicare population were captured. Demographic data are presented in Table 1. Of patients enrolled in Medicare, 53,949 (73%) were enrolled due to disability, 578 (<1%) due to ESRD, and 19,191 (26%) due to age. The majority (84%) of disabled patients were aged 35–64, the majority (81%) of ESRD patients were aged 35–64, and the majority (90%) of patients classified as elderly were >65. Explanations for this group including patients <65 include early enrollment based on birth date, and survivors benefits at age >60. 26 More women than men underwent bariatric surgery across all patients. White patients were more represented in this population.
TABLE 1.
Patient characteristics
| Patient Characteristics | ||||
|---|---|---|---|---|
| Disabled (n = 53,949) | ESRD (n = 578) | Elderly (n = 19,191) | p‐value a | |
| Age | ||||
| 18–34 | 4659 (8.6) | 93 (16.1) | <11 (0) | <0.0001 |
| 35–64 | 45,514 (84.4) | 469 (81.1) | 1866 (9.7) | |
| ≥65 | 3776 (7.0) | 16 (2.8) | 17,325 (90.3) | |
| Sex | ||||
| Men | 13,120 (24.3) | 237 (41.0) | 6066 (31.6) | <0.0001 |
| Women | 40,829 (75.7) | 341 (59.0) | 13,125 (68.4) | |
| BMI | ||||
| 35–39 | 8233 (15.3) | 87 (15.05) | 4908 (25.6) | <0.0001 |
| 40–44 | 13,729 (24.5) | 177 (30.6) | 6506 (33.9) | |
| 45–49 | 11,135 (20.6) | 151 (26.1) | 4049 (21.1) | |
| 50–59 | 13,253 (24.6) | 133 (23.0) | 2839 (14.8) | |
| >60 | 6709 (12.4) | 30 (5.2) | 448 (2.3) | |
| <35/Unknown | 890 (1.65) | <11 (0) | 441 (2.3) | |
| Race | ||||
| White | 38,805 (71.9) | 280 (48.4) | 17,611 (91.8) | |
| Black | 11,416 (21.1) | 213 (36.9) | 1002 (5.2) | |
| Other/unknown | 3728 (6.9) | 85 (14.7) | 578 (3.0) | |
| Comorbidities | ||||
| Diabetes | 30,489 (56.5) | 405 (70.1) | 11,601 (60.5) | <0.0001 |
| GERD | 34,441 (63.8) | 321 (55.5) | 11,320 (59.0) | <0.0001 |
| Hyperlipidemia | 34,459 (63.8) | 409 (70.8) | 14,518 (75.7) | <0.0001 |
| Hypertension | 44,240 (82.0) | 562 (97.2) | 17,377 (90.6) | <0.0001 |
| CAD | 8316 (15.4) | 157 (27.2) | 4442 (23.2) | <0.0001 |
| CHF | 5672 (10.5) | 136 (25.5) | 1620 (8.4) | <0.0001 |
| PVD | 1879 (3.5) | 43 (7.4) | 723 (3.8) | <0.0001 |
| COPD | 21,248 (39.4) | 131 (22.7) | 5009 (26.1) | <0.0001 |
| Smoking | 7095 (13.2) | 44 (7.6) | 619 (3.2) | <0.0001 |
| Depression | 22,486 (41.7) | 142 (24.6) | 5168 (26.9) | <0.0001 |
| OSA | 36,187 (67.1) | 345 (59.7) | 12,053 (62.8) | <0.0001 |
| Charlson Comorbidity Index | ||||
| 0 | 11,211 (20.8) | <11 (0) | 4307 (22.4) | <0.0001 |
| 1 | 17,432 (32.3) | 0 | 6923 (36.1) | |
| 2 | 10,670 (19.8) | 97 (16.8) | 3335 (17.4) | |
| 3 | 5938 (11.0) | 122 (21.1) | 2047 (10.7) | |
| 4 | 8698 (16.1) | 344 (59.5) | 2579 (13.4) | |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; ESRD, end stage renal disease; GERD, gastroesophageal reflux disease; PVD, peripheral vascular disease; OSA, obstructive sleep apnea.
Chi‐square test.
Patient preoperative comorbidities are also presented in Table 1. Hypertension was the most common comorbidity, presenting in >80% of patients. This was followed in frequency by hyperlipidemia. Diabetes affected 57% of disabled patients, 70% of ESRD patients, and 61% of elderly patients. 67% of disabled patients, 60% of ESRD, and 63% of elderly patients had a diagnosis of sleep apnea.
3.2. Procedure selection
Overall, 29,194 (40%) patients underwent SG, 37,443 (51%) patients underwent RYGB, 6787 (9%) patients underwent LABG, and 294 (<1%) patients underwent revision of gastric banding. In 2011, 68% of patients in the Medicare population underwent RYGB and 31% LAGB. SG made up 1% of procedures in 2011. Over time, SG overtook RYGB as the most commonly performed procedure in this cohort (Figure 1). LAGB also steadily declined in popularity. In 2014, 61% of patients underwent SG, 37% underwent RYGB, and 1% underwent LAGB. The trend of increased SG and decreasing RYGB and LAGB continued up until the third fiscal quarter of 2015 (corresponding to the adoption of ICD‐10 coding). Revisions of gastric band remain from 0.35%–0.41% of procedures yearly in this cohort. Procedural trend appeared similar between disabled, ESRD, and elderly patients (data not shown).
FIGURE 1.
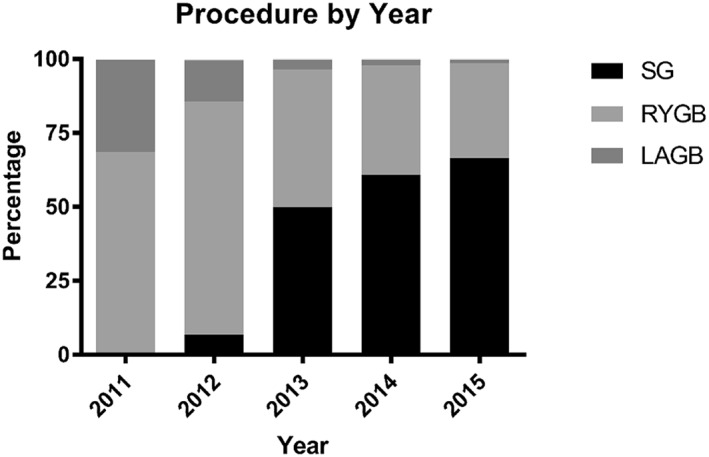
Procedure selection among the Medicare population. Abbreviations: RYGB: Roux‐en‐Y gastric bypass; SG: Sleeve Gastrectomy; LAGB: Laparoscopic Adjustable Gastric Banding
3.3. Length of stay, readmissions, and mortality
Overall in the Medicare population, mean LOS was 2.24 ± 2.62 days with a median of 2 days. A description of LOS trends among procedure types, groups, and over time is found in Table 2A,B. LOS was on average the longest after RYGB (2.54 ± 2.87 days), while LOS after LAGB was the shortest (1.32 ± 1.77 days) The elderly group demonstrated the shortest LOS, with a mean of 2.10 ± 2.12 days. Mean LOS decreased from 2.23 ± 3.10 in 2011 to 2.12 ± 1.98 days in 2015. Further breakdown of these trends can be noted in Table 3A.
TABLE 2.
Outcomes in bariatric surgery among patients of the Medicare population
| A. Length of stay | |||||
|---|---|---|---|---|---|
| All a | Disabled | ESRD | Elderly | p‐value b | |
| All | 2.24 ± 2.62 | 2.28 ± 2.59 | 2.54 ± 2.09 | 2.10 ± 2.12 | <0.0001 |
| SG | 2.06 ± 2.31 | 2.10 ± 2.45 | 2.27 ± 1.35 | 1.94 ± 1.93 | <0.0001 |
| RYGB | 2.54 ± 2.87 | 2.56 ± 2.67 | 3.24 ± 2.93 | 2.46 ± 3.42 | <0.0001 |
| LAGB | 1.32 ± 1.77 | 1.34 ± 1.55 | 1.53 ± 1.30 | 1.21 ± 1.36 | 0.1679 |
| Revision | 2.24 ± 5.89 | 2.33 ± 6.79 | NR | 2.04 ± 3.37 | 0.6929 |
| B. Length of stay All patients a | |
|---|---|
| 2011 | 2.23 ± 3.10 |
| 2012 | 2.39 ± 2.75 |
| 2013 | 2.29 ± 2.83 |
| 2014 | 2.19 ± 2.34 |
| 2015Q3 | 2.12 ± 1.98 |
| C. 30‐day readmission | ||||||||
|---|---|---|---|---|---|---|---|---|
| All a | Disabled | ESRD | Elderly | |||||
| N | % | N | % | N | % | N | % | |
| All b | 5575 | 7.56 | 4446 | 8.24 | 74 | 12.80 | 1055 | 5.50 |
| SG | 1836 | 6.25 | 1410 | 6.73 | 41 | 12.20 | 375 | 4.74 |
| RYGB | 3414 | 9.12 | 2788 | 9.83 | 29 | 14.87 | 597 | 6.71 |
| LAGB | 312 | 4.60 | 232 | 5.22 | NR | NR | 80 | 3.51 |
| D. 30‐day readmission all patients a | ||
|---|---|---|
| N | % | |
| 2011 | 1077 | 8.09 |
| 2012 | 1089 | 8.86 |
| 2013 | 1231 | 7.49 |
| 2014 | 1256 | 6.98 |
| 2015Q3 | 921 | 6.73 |
| E. 30‐day mortality | ||||||||
|---|---|---|---|---|---|---|---|---|
| All | Disabled | ESRD | Elderly | |||||
| N | % | N | % | N | % | N | % | |
| Overall | 174 | 0.24 | 121 | 0.22 | NR | NR | 53 | 0.28 |
| SG | 51 | 0.17 | 31 | 0.15 | NR | NR | 20 | 0.25 |
| RYGB | 115 | 0.31 | 85 | 0.30 | NR | NR | 30 | 0.34 |
| F. 30‐days mortality all patients | ||
|---|---|---|
| N | % | |
| 2011 | 28 | 0.29 |
| 2012 | 32 | 0.26 |
| 2013 | 35 | 0.21 |
| 2014 | 39 | 0.22 |
| 2015Q3 | 32 | 0.23 |
Abbreviations: ESRD, end stage renal disease; LAGB, laparoscopic adjustable gastric banding; RYGB, Roux‐en‐Y gastric bypass; SG, sleeve gastrectomy.
p < 0.0001 comparing the difference among procedures.
p value comparing the difference among procedures.
TABLE 3.
Breakdown of trends for 30‐, 90‐day readmission and mortality by year
| A. Length of stay by procedure (mean [std]) | |||||
|---|---|---|---|---|---|
| All | Disabled | ESRD | Elderly | p‐Value | |
| All | 2.24 (2.62) | 2.28 (2.59) | 2.54 (2.09) | 2.10 (2.12) | <0.0001 |
| SG | 2.06 (2.31) | 2.10 (2.45) | 2.27 (1.35) | 1.94 (1.93) | <0.0001 |
| RYGB | 2.54 (2.87) | 2.56 (2.67) | 3.24 (2.93) | 2.46 (3.42) | <0.0001 |
| LAGB | 1.32 (1.77) | 1.34 (1.55) | 1.53 (1.30) | 1.21 (1.36) | 0.1679 |
| Revision | 2.24 (5.89) | 2.33 (6.79) | NA | 2.04 (3.37) | 0.6929 |
| B. 30‐day readmission by year (N [%]) | |||||
|---|---|---|---|---|---|
| All | Disabled | ESRD | Elderly | p‐value | |
| 2011 | 1077 (8.09) | 900 (9.02) | 11 (13.92) | 166 (5.08) | <0.0001 |
| 2012 | 1089 (8.86) | 866 (9.36) | <11 (>12) | 213 (7.20) | <0.0001 |
| 2013 | 1231 (7.49) | 999 (8.25) | 15 (12.10) | 218 (5.19) | <0.0001 |
| 2014 | 1256 (6.98) | 990 (7.68) | 11 (6.67) | 255 (5.18) | <0.0001 |
| 2015 | 921 (6.73) | 691 (7.11) | 27 (20.61) | 203 (5.28) | <0.0001 |
| C. 90‐day readmission by year (N [%]) | |||||
|---|---|---|---|---|---|
| All | Disabled | ESRD | Elderly | p‐value | |
| 2011 | 1666 (12.51) | 1377 (13.80) | 21 (26.58) | 268 (8.21) | <0.0001 |
| 2012 | 1618 (13.17) | 1292 (13.96) | 19 (16.94) | 307 (10.38) | <0.0001 |
| 2013 | 1867 (11.36) | 1498 (12.37) | 21 (16.94) | 348 (8.28) | <0.0001 |
| 2014 | 1990 (11.07) | 1559 (12.09) | 25 (15.15) | 406 (8.25) | <0.0001 |
| 2015 | 1466 (10.07) | 1107 (11.39) | 36 (27.48) | 323 (8.40) | <0.0001 |
| D. 30‐day mortality by year (N [%]) | |||||
|---|---|---|---|---|---|
| All | Disabled | ESRD | Elderly | p‐value | |
| 2011 | 38 (0.29) | 26 (0.26) | 0 | 12 (0.37) | 0.5445 |
| 2012 | 32 (0.26) | 26 (0.28) | 0 | <11 (<0.21) | 0.6928 |
| 2013 | 35 (0.21) | 22 (0.18) | 0 | 13 (0.31) | 0.2644 |
| 2014 | 39 (0.22) | 28 (0.22) | 0 | 11 (0.22) | 0.8317 |
| 2015 | 32 (0.23) | 19 (0.2) | <11 (<0.8) | <12 (0.29) | 0.2606 |
| E. 90‐day mortality by year (N [%]) | |||||
|---|---|---|---|---|---|
| All | Disabled | ESRD | Elderly | p‐value | |
| 2011 | 56 (0.42) | 42 (0.42) | 0 | 14 (0.43) | 0.8441 |
| 2012 | 45 (0.37) | 34 (0.37) | <11 | <11 | 0.4033 |
| 2013 | 52 (0.32) | 36 (0.30) | 0 | 16 (0.38) | 0.5811 |
| 2014 | 51 (0.28) | 36 (0.28) | <11 | 14 (0.28) | 0.7351 |
| 2015 | 48 (0.35) | 30 (0.31) | <11 (<0.8) | 17 (0.44) | 0.3587 |
Note: p‐value: comparing the difference among groups of disabled, ESRD and elderly. One Way Anova or chi‐square test was used depending on the outcome variable is continuous or categorical variable.
Abbreviations: ESRD, end stage renal disease; LAGB, laparoscopic adjustable gastric banding; RYGB, Roux‐en‐Y gastric bypass; SG, sleeve gastrectomy.
Trends in 30‐day readmission rates are presented in Table 2C,D. Full details of 90‐day readmission rates can be noted in Tables 3, 4 and 5, and demonstrate the same trends. For all years, 8% of disabled patients were readmitted at 30 days and 13% were readmitted at 90 days. For ESRD patients, 13% were readmitted at 30 days and 21% were readmitted at 90 days. For elderly patients, 6% were readmitted at 30 days and 9% at 90 days. Rates of readmission decreased over time, with 8% of patients readmitted in 2011, and 7% of patients in 2015.
TABLE 4.
Breakdown of trends for readmission by group, procedure, and year
| Percentage of patients had at least one readmission with in 30, 60, or 90 days postoperation a | ||||||||
|---|---|---|---|---|---|---|---|---|
| By study group | ||||||||
| Disabled | ESRD | Elder | ||||||
| Readmission | #patients | % | #patients | % | #patients | % | ||
| 30 days | 4446 | 8.24 | 74 | 12.80 | 1055 | 5.50 | ||
| 60 days | 5913 | 10.96 | 98 | 16.96 | 1412 | 7.36 | ||
| 90 days | 6833 | 12.67 | 122 | 21.11 | 1652 | 8.61 | ||
| BY study group and procedure type | ||||||||
| Sleeve | ||||||||
| 30 days | 1410 | 6.73 | 41 | 12.20 | 375 | 4.74 | ||
| 60 days | 1899 | 9.07 | 51 | 15.18 | 501 | 6.33 | ||
| 90 days | 2207 | 10.54 | 63 | 18.75 | 597 | 7.55 | ||
| Bypass | ||||||||
| 30 days | 2788 | 9.83 | 29 | 14.87 | 597 | 6.71 | ||
| 60 days | 3672 | 12.95 | 39 | 20.00 | 796 | 8.95 | ||
| 90 days | 4214 | 14.86 | 48 | 24.62 | 910 | 10.23 | ||
| Banding | ||||||||
| 30 days | 232 | 5.22 | 80 | 3.51 | ||||
| 60 days | 319 | 7.17 | 110 | 4.83 | ||||
| 90 days | 383 | 8.61 | 140 | 6.15 | ||||
| BY study group, year and procedure type | ||||||||
| 2011 | Sleeve | |||||||
| 30 days | NR | NR | NR | |||||
| 60 days | NR | NR | NR | |||||
| 90 days | NR | NR | NR | |||||
| Bypass | ||||||||
| 30 days | 748 | 10.50 | 119 | 6.38 | ||||
| 60 days | 1005 | 14.11 | 13 | 16.46 | 162 | 8.68 | ||
| 90 days | 1134 | 15.92 | 14 | 17.72 | 182 | 9.75 | ||
| Banding | ||||||||
| 30 days | 136 | 4.96 | 40 | 2.96 | ||||
| 60 days | 183 | 6.68 | 59 | 4.37 | ||||
| 90 days | 219 | 7.99 | 75 | 5.56 | ||||
| 2012 | Sleeve | |||||||
| 30 days | 43 | 7.21 | 20 | 9.09 | ||||
| 60 days | 50 | 8.39 | 23 | 10.45 | ||||
| 90 days | 55 | 9.23 | 23 | 10.45 | ||||
| Bypass | ||||||||
| 30 days | 756 | 10.11 | 168 | 7.82 | ||||
| 60 days | 974 | 13.02 | 12 | 15.19 | 215 | 10.01 | ||
| 90 days | 1122 | 15.00 | 16 | 20.25 | 242 | 11.27 | ||
| Banding | ||||||||
| 30 days | 62 | 5.53 | 24 | 4.20 | ||||
| 60 days | 90 | 8.03 | 31 | 5.43 | ||||
| 90 days | 109 | 9.72 | 40 | 7.01 | ||||
| 2013 | Sleeve | |||||||
| 30 days | 449 | 7.46 | 95 | 4.53 | ||||
| 60 days | 603 | 10.02 | 11 | 13.10 | 130 | 6.20 | ||
| 90 days | 670 | 11.13 | 14 | 16.67 | 155 | 7.39 | ||
| Bypass | ||||||||
| 30 days | 529 | 9.22 | NR | 115 | 6.07 | |||
| 60 days | 691 | 12.05 | NR | 148 | 7.81 | |||
| 90 days | 792 | 13.81 | NR | 179 | 9.45 | |||
| Banding | ||||||||
| 30 days | 17 | 5.38 | NR | NR | ||||
| 60 days | 25 | 7.91 | NR | NR | ||||
| 90 days | 30 | 9.49 | NR | NR | ||||
| 2014 | Sleeve | |||||||
| 30 days | 512 | 6.51 | 139 | 4.71 | ||||
| 60 days | 693 | 8.81 | 12 | 9.16 | 180 | 6.10 | ||
| 90 days | 826 | 10.50 | 18 | 13.74 | 219 | 7.42 | ||
| Bypass | ||||||||
| 30 days | 462 | 9.60 | NR | 112 | 6.18 | |||
| 60 days | 613 | 12.74 | NR | 163 | 8.99 | |||
| 90 days | 707 | 14.70 | NR | 180 | 9.93 | |||
| Banding | ||||||||
| 30 days | 14 | 7.87 | NR | NR | ||||
| 60 days | 15 | 8.43 | NR | NR | ||||
| 90 days | 19 | 10.67 | NR | NR | ||||
| 2015Q3 | Sleeve | |||||||
| 30 days | 393 | 6.15 | 24 | 22.22 | 114 | 4.38 | ||
| 60 days | 538 | 8.42 | 28 | 25.93 | 159 | 6.10 | ||
| 90 days | 639 | 10.00 | 30 | 27.78 | 190 | 7.29 | ||
| Bypass | ||||||||
| 30 days | 293 | 9.15 | NR | 83 | 7.07 | |||
| 60 days | 389 | 12.15 | NR | 108 | 9.20 | |||
| 90 days | 459 | 14.33 | NR | 127 | 10.82 | |||
Note: Only included patients who had procedures in inpatient setting.
Abbreviations: ESRD, end stage renal disease; NR, not reported due to N < 11.
Chi‐square test was performed.
TABLE 5.
30‐, 60‐, and 90‐day readmissions
| Percentage of patients had at least one readmission with in 30, 60 or 90 days postoperation a | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| By study group | |||||||||
| Disabled | ESRD | Elder | p‐value b | ||||||
| Readmission | #patients | % | #patients | % | #patients | % | |||
| 30 days | 4446 | 8.24 | 74 | 12.80 | 1055 | 5.50 | <0.0001 | ||
| 60 days | 5913 | 10.96 | 98 | 16.96 | 1412 | 7.36 | <0.0001 | ||
| 90 days | 6833 | 12.67 | 122 | 21.11 | 1652 | 8.61 | <0.0001 | ||
| BY study group and procedure type | |||||||||
| Sleeve | |||||||||
| 30 days | 1410 | 6.73 | 41 | 12.20 | 375 | 4.74 | |||
| 60 days | 1899 | 9.07 | 51 | 15.18 | 501 | 6.33 | |||
| 90 days | 2207 | 10.54 | 63 | 18.75 | 597 | 7.55 | |||
| Bypass | |||||||||
| 30 days | 2788 | 9.83 | 29 | 14.87 | 597 | 6.71 | |||
| 60 days | 3672 | 12.95 | 39 | 20.00 | 796 | 8.95 | |||
| 90 days | 4214 | 14.86 | 48 | 24.62 | 910 | 10.23 | |||
| Banding | |||||||||
| 30 days | 232 | 5.22 | 80 | 3.51 | |||||
| 60 days | 319 | 7.17 | 110 | 4.83 | |||||
| 90 days | 383 | 8.61 | 140 | 6.15 | |||||
| BY study group, year, and procedure type | |||||||||
| 2011 | Sleeve | ||||||||
| 30 days | NR | NR | NR | ||||||
| 60 days | NR | NR | NR | ||||||
| 90 days | NR | NR | NR | ||||||
| Bypass | |||||||||
| 30 days | 748 | 10.50 | 119 | 6.38 | |||||
| 60 days | 1005 | 14.11 | 13 | 16.46 | 162 | 8.68 | |||
| 90 days | 1134 | 15.92 | 14 | 17.72 | 182 | 9.75 | |||
| Banding | |||||||||
| 30 days | 136 | 4.96 | 40 | 2.96 | |||||
| 60 days | 183 | 6.68 | 59 | 4.37 | |||||
| 90 days | 219 | 7.99 | 75 | 5.56 | |||||
| 2012 | Sleeve | ||||||||
| 30 days | 43 | 7.21 | 20 | 9.09 | |||||
| 60 days | 50 | 8.39 | 23 | 10.45 | |||||
| 90 days | 55 | 9.23 | 23 | 10.45 | |||||
| Bypass | |||||||||
| 30 days | 756 | 10.11 | 168 | 7.82 | |||||
| 60 days | 974 | 13.02 | 12 | 15.19 | 215 | 10.01 | |||
| 90 days | 1122 | 15.00 | 16 | 20.25 | 242 | 11.27 | |||
| Banding | |||||||||
| 30 days | 62 | 5.53 | 24 | 4.20 | |||||
| 60 days | 90 | 8.03 | 31 | 5.43 | |||||
| 90 days | 109 | 9.72 | 40 | 7.01 | |||||
| 2013 | Sleeve | ||||||||
| 30 days | 449 | 7.46 | 95 | 4.53 | |||||
| 60 days | 603 | 10.02 | 11 | 13.10 | 130 | 6.20 | |||
| 90 days | 670 | 11.13 | 14 | 16.67 | 155 | 7.39 | |||
| Bypass | |||||||||
| 30 days | 529 | 9.22 | NR | 115 | 6.07 | ||||
| 60 days | 691 | 12.05 | NR | 148 | 7.81 | ||||
| 90 days | 792 | 13.81 | NR | 179 | 9.45 | ||||
| Banding | |||||||||
| 30 days | 17 | 5.38 | NR | NR | |||||
| 60 days | 25 | 7.91 | NR | NR | |||||
| 90 days | 30 | 9.49 | NR | NR | |||||
| 2014 | Sleeve | ||||||||
| 30 days | 512 | 6.51 | 139 | 4.71 | |||||
| 60 days | 693 | 8.81 | 12 | 9.16 | 180 | 6.10 | |||
| 90 days | 826 | 10.50 | 18 | 13.74 | 219 | 7.42 | |||
| Bypass | |||||||||
| 30 days | 462 | 9.60 | NR | 112 | 6.18 | ||||
| 60 days | 613 | 12.74 | NR | 163 | 8.99 | ||||
| 90 days | 707 | 14.70 | NR | 180 | 9.93 | ||||
| Banding | |||||||||
| 30 days | 14 | 7.87 | NR | NR | |||||
| 60 days | 15 | 8.43 | NR | NR | |||||
| 90 days | 19 | 10.67 | NR | NR | |||||
| 2015Q3 | Sleeve | ||||||||
| 30 days | 393 | 6.15 | 24 | 22.22 | 114 | 4.38 | |||
| 60 days | 538 | 8.42 | 28 | 25.93 | 159 | 6.10 | |||
| 90 days | 639 | 10.00 | 30 | 27.78 | 190 | 7.29 | |||
| Bypass | |||||||||
| 30 days | 293 | 9.15 | NR | 83 | 7.07 | ||||
| 60 days | 389 | 12.15 | NR | 108 | 9.20 | ||||
| 90 days | 459 | 14.33 | NR | 127 | 10.82 | ||||
Abbreviation: ESRD, end stage renal disease; NR, not reported due to N < 11.
Only included patients who had procedures in inpatient setting.
Chi‐square test was performed.
In patients with disability, 30‐day and 90‐day readmission rates were 7% and 11% respectively for patients who underwent SG. In these patients, RYGB readmission rates were higher (10% 30‐day readmission and 15% 90‐day readmission) and LAGB were lower at all time points (5% 30‐day readmission and 9% 90‐day readmission). For patients with ESRD, 30‐day and 90‐day readmission rates were 12% and 19%, respectively, for patients who underwent SG. RYGB, in these patients, had readmission rates of 15% at 30‐days and 25% at 90‐days. For elderly patients, SG readmission rates were 5% at 30 days and 8% at 90 days. In this patient cohort, RYGB had higher readmission rates (7% 30 days and 10% 90 days), and LAGB had lower readmission rates (4% 30 days and 6% 90 days).
Multivariable logistic regression analysis was performed to assess for these factors associated with 30‐day readmissions (Table 6). Factors associated with higher 30‐day readmissions included patients with disability (as compared to elderly patients), younger age (18–34 compared to age>65), black or other races (as compared to non‐Hispanic white), BMI ≥ 45, and patients with the comorbidities listed in Table 6, excluding hyperlipidemia. Factors associated with lower likelihood for readmission was SG and LAGB (as compared to RYGB), later year of procedure, and among men, as compared to women.
TABLE 6.
Multivariable logistic regression for factors associated with 30‐day readmission
| Factors associated with 30‐day readmission | ||||
|---|---|---|---|---|
| Characteristic | Odds ratio | 95% odds ratio confidence limits | p value | |
| Procedure | ||||
| RYGB | REF | |||
| SG | 0.73 | 0.675 | 0.79 | <0.0001 |
| LAGB | 0.513 | 0.445 | 0.593 | <0.0001 |
| Revision | 0.951 | 0.565 | 1.598 | 0.8485 |
| Diagnosis year | ||||
| 2011 | REF | |||
| 2012 | 0.939 | 0.849 | 1.038 | 0.2186 |
| 2013 | 0.864 | 0.779 | 0.959 | 0.0059 |
| 2014 | 0.813 | 0.731 | 0.905 | 0.0002 |
| 2015 | 0.792 | 0.705 | 0.891 | <0.0001 |
| Age group | ||||
| ≥65 | REF | |||
| 18–34 | 1.375 | 1.165 | 1.623 | 0.0002 |
| 35–64 | 1.023 | 0.902 | 1.162 | 0.7203 |
| Gender | ||||
| Women | REF | |||
| Men | 0.942 | 0.871 | 1.018 | 0.1308 |
| Race | ||||
| White | REF | |||
| Black | 1.303 | 1.207 | 1.406 | <0.0001 |
| Others | 1.198 | 1.057 | 1.358 | 0.0048 |
| Region | ||||
| Midwest | REF | |||
| Northeast | 1.159 | 1.055 | 1.274 | 0.002 |
| South | 1.035 | 0.956 | 1.121 | 0.396 |
| West | 1.126 | 1.013 | 1.251 | 0.0279 |
| BMI | ||||
| 35–39 | REF | |||
| 40–44 | 1.002 | 0.9 | 1.116 | 0.9711 |
| 45–49 | 1.048 | 0.938 | 1.171 | 0.4026 |
| 50–59 | 1.088 | 0.977 | 1.212 | 0.1226 |
| >60 | 1.177 | 1.041 | 1.332 | 0.0095 |
| <35/Unknown | 0.9 | 0.676 | 1.198 | 0.4697 |
| Comorbidities | ||||
| Diabetics | 1.083 | 1.01 | 1.162 | 0.025 |
| GERD | 1.405 | 1.309 | 1.509 | <0.0001 |
| HLD | 1.004 | 0.933 | 1.08 | 0.9203 |
| HTN | 1.388 | 1.252 | 1.539 | <0.0001 |
| CAD | 1.292 | 1.187 | 1.406 | <0.0001 |
| CHF | 1.456 | 1.329 | 1.595 | <0.0001 |
| PAD | 1.554 | 1.357 | 1.78 | <0.0001 |
| COPD | 1.273 | 1.193 | 1.359 | <0.0001 |
| CRF | 1.859 | 1.706 | 2.026 | <0.0001 |
| RA | 1.243 | 1.102 | 1.403 | 0.0004 |
| Smoking | 1.351 | 1.242 | 1.471 | <0.0001 |
| Depression | 1.479 | 1.387 | 1.578 | <0.0001 |
| Sleep apnea | 1.108 | 1.029 | 1.192 | 0.0065 |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CRF, Chronic renal failure; GERD, gastroesophageal reflux disease; HLD, hyperlipidemia; HTN, hypertension; LAGB, laparoscopic adjustable gastric banding; PAD, peripheral arterial disease; RA, rheumatoid arthritis; RYGB, Roux‐en‐Y gastric bypass; SG, sleeve gastrectomy.
Thirty‐day mortality rates are shown in Table 2E,F. Further breakdown of these groups as well as 90‐day mortality rates may be found in Tables 3E and 7. Because the MedPAR database does not report findings when there are <11 patients, mortality for patients with ESRD were unable to be analyzed. For patients with disability, 30‐day mortality was 0.22% and 90‐day mortality was 0.33%. Patients with disability undergoing SG, mortality was 0.15% at 30‐days and 0.23% at 90‐days. RYGB mortality rate in this group was 0.30% at 30 days and 0.43% at 90 days. For elderly patients, overall mortality was 0.28% at 30 days and 0.37% at 90 days. For elderly patients undergoing SG, mortality was 0.25% at 30 days and 0.33% at 90 days. In this patient cohort, RYGB mortality rate was recorded as 0.34% at 30 days and 0.46% at 90 days. Over time, mortality rates remained similar overall, with a rate of 0.29% in 2011 and 0.23% in 2015; year of procedure was not a significant factor associated with mortality as noted below in the adjusted analysis.
TABLE 7.
30‐, 60‐, and 90‐day mortality
| Motality rate with in 30, 60, or 90 days post‐operation a | ||||||||
|---|---|---|---|---|---|---|---|---|
| By study group | ||||||||
| Disabled | ESRD1 | Elder | p‐value b | |||||
| Death | #patients | % | #patients | % | #patients | % | ||
| 30 days | 121 | 0.22 | NR | . | 53 | 0.28 | 0.2051 | |
| 60 days | 163 | 0.30 | NR | . | 64 | 0.33 | 0.5025 | |
| 90 days | 178 | 0.33 | NR | . | 71 | 0.37 | 0.4136 | |
| BY study group and procedure type1 | ||||||||
| Sleeve | ||||||||
| 30 days | 31 | 0.15 | NR | . | 20 | 0.25 | ||
| 60 days | 45 | 0.21 | NR | . | 25 | 0.32 | ||
| 90 days | 49 | 0.23 | NR | . | 26 | 0.33 | ||
| Bypass | ||||||||
| 30 days | 85 | 0.30 | NR | . | 30 | 0.34 | ||
| 60 days | 111 | 0.39 | NR | . | 36 | 0.40 | ||
| 90 days | 121 | 0.43 | NR | . | 41 | 0.46 | ||
Abbreviation: ESRD, end stage renal disease.
Only included patients who had procedures in inpatient setting.
Chi‐square test was performed.
Multivariable logistic regression was performed to analyze factors associated with 30‐day mortality (Table 8). Factors associated with lower likelihood for mortality included LAGB and SG (as compared to RYGB), lower age, and patients with reflux or depression. While factors which are associated with higher likelihood for 30‐day mortality include men, as compared to women, BMI ≥45, and the comorbidities of congestive heart failure and chronic renal failure.
TABLE 8.
Factors associated with 30‐day mortality
| Factors associated with cost of procedure | ||||
|---|---|---|---|---|
| Characteristic | Odds ratio | Odds ratio 95% CI | p‐value | |
| Procedure | ||||
| RYGB | REF | |||
| SG | 0.87 | 0.86 | 0.88 | <0.0001 |
| LAGB | 0.73 | 0.72 | 0.75 | <0.0001 |
| Revision | 0.89 | 0.83 | 0.95 | 0.0005 |
| Diagnosis year | ||||
| 2011 | REF | |||
| 2012 | 1.03 | 1.02 | 1.05 | <0.0001 |
| 2013 | 1.04 | 1.02 | 1.05 | <0.0001 |
| 2014 | 1.03 | 1.02 | 1.05 | <0.0001 |
| 2015 | 1.05 | 1.04 | 1.07 | <0.0001 |
| Age group | ||||
| ≥65 | REF | |||
| 18–34 | 0.96 | 0.94 | 0.98 | <0.0001 |
| 35–64 | 0.98 | 0.97 | 1 | 0.0493 |
| Gender | ||||
| Women | REF | |||
| Men | 1.03 | 1.02 | 1.04 | <0.0001 |
| Race | ||||
| White | REF | |||
| Black | 1.06 | 1.05 | 1.07 | <0.0001 |
| Others | 1.04 | 1.03 | 1.06 | <0.0001 |
| Region | ||||
| Midwest | REF | |||
| Northeast | 0.96 | 0.94 | 0.97 | <0.0001 |
| South | 0.89 | 0.89 | 0.9 | <0.0001 |
| West | 1.07 | 1.06 | 1.09 | <0.0001 |
| BMI | ||||
| 35–39 | REF | |||
| 40–44 | 1.01 | 1 | 1.02 | 0.0743 |
| 45–49 | 1.03 | 1.02 | 1.04 | <0.0001 |
| 50–59 | 1.03 | 1.02 | 1.04 | <0.0001 |
| >60 | 1.12 | 1.11 | 1.14 | <0.0001 |
| <35/Unknown | 1.12 | 1.08 | 1.16 | <0.0001 |
| Comorbidities | ||||
| Diabetics | 1.03 | 1.02 | 1.04 | <0.0001 |
| GERD | 1.01 | 1 | 1.01 | 0.2073 |
| HLD | 0.99 | 0.98 | 1 | 0.1438 |
| HTN | 1.01 | 1 | 1.02 | 0.1542 |
| CAD | 1.01 | 1 | 1.02 | 0.0464 |
| CHF | 1.08 | 1.07 | 1.1 | <0.0001 |
| PAD | 1.08 | 1.06 | 1.1 | <0.0001 |
| COPD | 1.04 | 1.03 | 1.05 | <0.0001 |
| CRF | 1.12 | 1.11 | 1.14 | <0.0001 |
| RA | 0.99 | 0.97 | 1 | 0.1053 |
| Smoking | 0.99 | 0.98 | 1 | 0.1265 |
| Depression | 1 | 0.99 | 1.01 | 0.9325 |
| Sleep apnea | 1.05 | 1.04 | 1.05 | <0.0001 |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CRF, chronic renal failure; GERD, gastroesophageal reflux disease; HLD, hyperlipidemia; HTN, hypertension; LAGB, laparoscopic adjustable gastric banding; PAD, peripheral arterial disease; RA, rheumatoid arthritis; RYGB, Roux‐en‐Y gastric bypass; SG, sleeve gastrectomy.
3.4. Cost
After controlling for patient's year of surgery, demographics such as age, gender and race/ethnicity, BMI, comorbidities, and hospital regions, the differences in cost of surgery can be seen among the procedure types. Results of adjusted cost ratio analysis are shown in Table 9.
TABLE 9.
Factors associated with adjusted cost ratio for surgical intervention
| Adjusted cost ratio | ||||
|---|---|---|---|---|
| Characteristic | Odds ratio | 95% odds ratio confidence limits | p value | |
| Study group | ||||
| Elderly | REF | |||
| Disabled | 1.01 | 1.03 | 1.00 | 0.0805 |
| ESRD | 0.98 | 1.02 | 0.94 | 0.3588 |
| Procedure | ||||
| RYGB | REF | |||
| SG | 0.87 | 0.87 | 0.86 | <0.0001 |
| LAGB | 0.73 | 0.74 | 0.72 | <0.0001 |
| Revision | 0.85 | 0.90 | 0.81 | <0.0001 |
| Diagnosis year | ||||
| 2011 | REF | |||
| 2012 | 1.03 | 1.04 | 1.02 | <0.0001 |
| 2013 | 1.04 | 1.05 | 1.03 | <0.0001 |
| 2014 | 1.03 | 1.04 | 1.02 | <0.0001 |
| 2015 | 1.05 | 1.06 | 1.03 | <0.0001 |
| Age group | ||||
| ≥65 | REF | |||
| 18–34 | 0.95 | 0.97 | 0.93 | <0.0001 |
| 35–64 | 0.98 | 1.00 | 0.97 | 0.0159 |
| Gender | ||||
| Women | REF | |||
| Men | 1.03 | 1.04 | 1.02 | <0.0001 |
| Race | ||||
| White | REF | |||
| Black | 1.06 | 1.07 | 1.05 | <0.0001 |
| Others | 1.05 | 1.06 | 1.03 | <0.0001 |
| Region | ||||
| Midwest | REF | |||
| Northeast | 0.96 | 0.97 | 0.95 | <0.0001 |
| South | 0.90 | 0.91 | 0.90 | <0.0001 |
| West | 1.07 | 1.08 | 1.05 | <0.0001 |
| BMI | ||||
| 35–39 | REF | |||
| 40–44 | 1.01 | 1.02 | 1.00 | 0.0262 |
| 45–49 | 1.03 | 1.04 | 1.02 | <0.0001 |
| 50–59 | 1.04 | 1.05 | 1.03 | <0.0001 |
| >60 | 1.13 | 1.14 | 1.11 | <0.0001 |
| <35/Unknown | 1.10 | 1.13 | 1.08 | <0.0001 |
| Comorbidities | ||||
| Diabetics | 1.03 | 1.03 | 1.02 | <0.0001 |
| GERD | 1.01 | 1.02 | 1.00 | 0.0024 |
| HLD | 0.99 | 0.99 | 0.98 | 0.0007 |
| HTN | 1.00 | 1.01 | 0.99 | 0.9635 |
| CAD | 1.01 | 1.02 | 1.00 | 0.0797 |
| CHF | 1.11 | 1.12 | 1.10 | <0.0001 |
| PAD | 1.08 | 1.09 | 1.06 | <0.0001 |
| COPD | 1.04 | 1.04 | 1.03 | <0.0001 |
| CRF | 1.12 | 1.14 | 1.11 | <0.0001 |
| RA | 0.98 | 1.00 | 0.97 | 0.028 |
| Smoking | 0.99 | 1.00 | 0.98 | 0.2483 |
| Depression | 1.00 | 1.01 | 1.00 | 0.512 |
| Sleep apnea | 1.03 | 1.04 | 1.03 | <0.0001 |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CRF, chronic renal failure; ESRD, end stage renal disease; GERD, gastroesophageal reflux disease; HLD, hyperlipidemia; HTN, hypertension; LAGB, laparoscopic adjustable gastric banding; PAD, peripheral arterial disease; RA, rheumatoid arthritis; RYGB, Roux‐en‐Y gastric bypass; SG, sleeve gastrectomy.
The procedural cost of SG decreased while the procedural cost of RYGB increased for the Medicare population over the time period of interest (Figure 2). In 2011, mean cost for SG was $24,726; in 2012, mean cost was $13,212; in 2013, mean cost was $11,943; in 2014, mean cost was $11,893; and in 2015, mean cost was $12,149. Mean cost of RYGB was $13,558 in 2011, $13,751 in 2012, $14,236 in 2013, $14,084 in 2014, and $14,365 in 2015. LAGB mean cost was $9263 in 2011, $10,651 in 2012, $10,913 in 2013, $10,593 in 2014, and $10,092 in 2015. The cost of surgery was estimated by using the Payment Amount variable from inpatient claims which are the Medicare reimbursement amount to institutional providers for the entire hospital stay for the surgery.
FIGURE 2.
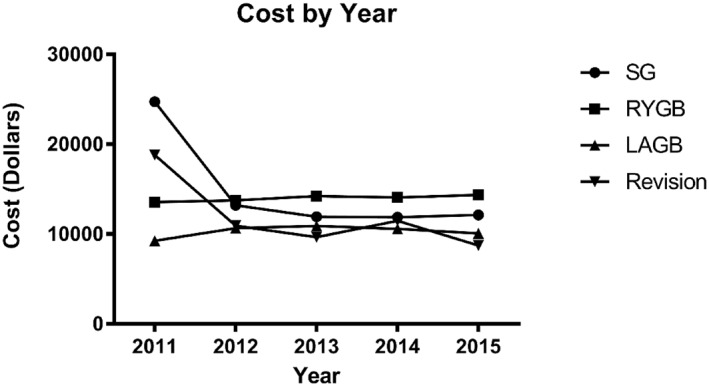
Mean cost per procedure in the Medicare population. Abbreviations: RYGB: Roux‐en‐Y gastric bypass; SG: Sleeve Gastrectomy; LAGB: Laparoscopic Adjustable Gastric Banding
Factors associated with cost of procedure are displayed in Table 9. Factors associated with higher cost included later year of procedure, men, as compared to women, black or other race (compared to non‐Hispanic whites), west region (compared to Midwest), and higher BMI. Factors associated with lower cost include SG, LAGB, or revision of gastric band (compared to RYGB), and northeast or southern region (compared to the Midwest). Cost for SG was 13% lower overall, as compared to RYGB, as noted by an adjusted cost ratio of 0.87 (95% CI, 0.86–0.87).
4. DISCUSSION
In this cohort of Medicare beneficiaries, a shifting trend in procedure selection was noted favoring the SG compared to RYGB from 2011 to the first three calendar quarters of 2015. In this time, readmissions have decreased. Patient specific trends when comparing patients with disability, elderly patients, and patients with ESRD in procedure selection, morbidity, and mortality were characterized. Lastly, there was a decrease in the cost of SG overtime and a concomitant increase the cost of RYGB.
The Medicare population represents an expanding cohort of patients with specific medical challenges and needs. This group currently constitutes 16% of the US population. 2 Reflecting similar trends in the general population, procedure mix has changed significantly from 2011 to 2015, with SG becoming the predominant procedure performed in the year 2013.21, 22 This timing also logically follows the CMS announcement of SG coverage in 2012. 27 RYGB had been the most common procedure as of 2009. 19 SG and RYGB have similar short and medium term outcomes.12, 28 This likely underlies the increase in SG procedures in this population, as complications seem to be lower after the more straightforward SG procedure.29, 30 More recent long‐term studies have demonstrated that T2DM remission and weight loss are likely to be lower in SG than RYGB.31, 32 Benefits to long‐term cardiovascular risk and overall survival have been demonstrated in RYGB however, due to the relatively recent development of this procedure, have not yet been extended to SG. 33 It is unclear if these recent findings will alter procedure trends in the future.
The safety of bariatric surgery in this high‐risk patients is debated. Most single institution studies have identified bariatric surgery to be safe in elderly patients.34, 35 Our findings confirmed this conclusion. A median length of stay of 2 days, and readmission rates of 6% at 30 days and 9% at 90 days are similar to outcomes in the non‐Medicare population.36, 37 However, it is important to note that mortality in elderly patients was found to be 0.28%, which is above the currently reported mortality rates in all patients (most recently reported as 0.1% in SG and 0.15% in RYGB). 38 More in‐depth analysis is necessary to evaluate the efficacy of bariatric surgery, including weight loss and resolution of comorbidity, in the elderly population. However, on the basis of single institution and small database studies, the long‐term benefits of bariatric surgery in this population likely still outweigh the early morbidity after surgery, but thorough patient selection remains important.
This study was the largest evaluation of bariatric surgery in patients with disability and patients with ESRD. Interestingly, the majority of Medicare beneficiaries undergoing bariatric surgery were classified as disabled; however this percentage has decreased since the last report in 2009, with over 75% of the patients being classified as disabled at that time. 19 Although this analysis was unable to evaluate the reasons for disability in these patients, a number of these patients suffer from obesity related comorbidities that can render them disabled. 39 Bariatric surgery may allow many of these patients to enter into the workforce and more robustly impact health care economics. In the present study, patients with disability had a similar LOS and readmission rate as previously published patient groups undergoing bariatric surgery.36, 37 Mortality in this group was similar to the elderly patients. The results of this study predict that bariatric surgery is safe in this group, but again requires careful patient selection and more evaluation to evaluate efficacy of surgically driven weight loss and potentially reenter the workforce.
In patients with obesity and ESRD, bariatric surgery has been proposed as a potential bridge to transplant as obesity is considered a relative contraindication. 40 A single institution study has demonstrated that bariatric surgery is effective in this group and may improve access to transplant. 41 Our study demonstrates that there were significant risks in ESRD patients undergoing bariatric surgery. Approximately 20% of patients were readmitted within 90 days of surgery. While bariatric surgery may provide a bridge to transplant in this population, patient optimization and selection remain important in this high‐risk group.
The cost effectiveness of bariatric surgery as compared to nonsurgical treatment can be controversial; however, a number of studies have demonstrated that long‐term costs are at least equivalent.42, 43 Strategies to decrease the cost of surgery as well as the cost of readmission could improve the economic effectiveness of bariatric surgery and provide further incentive to pursue this treatment in the ever‐expanding Medicare population. The cost of surgery among this patient group appeared similar to those reported in the nationwide inpatient sample as well as from the premier database.21, 44 The cost of SG has decreased over this time period while the cost of RYGB has increased. Both procedure complexity and initial comorbidities are known to increase the cost of bariatric surgery. 45 This may be reflected in the increased cost of RYGB in this population of Medicare beneficiaries.
While this study was the largest review of bariatric surgery in Medicare beneficiaries, there are a number of limitations. Unfortunately, it was not possible to identify preoperative BMI or conversion events. Furthermore, as this is a claims based database, identification of long‐term efficacy, remission of comorbidities, and safety in this patient cohort was not possible. Also, all procedures and conditions were identified using ICD‐9 and CPT codes. While this allows for selection of certain procedures, it was not possible to specifically identify newer procedures including one anastomosis gastric bypass or endoscopic interventions.
Despite these limitations, this was a robust study of bariatric surgery safety, morbidity, mortality, and cost in the Medicare population of the United States. This study provides insight as to the rates of readmission in elderly patients with disability, ESRD, and the elderly. As the Medicare system continues to expand, understanding the cost and safety profile of these procedures in this population allows for evidence based decision making underlying the implementation of health care initiatives.
CONFLICT OF INTERESTS
The institution has received grant support from Medtronic inc. The authors have received statistical support from Medtronic Inc. on this manuscript. Daniel B. Leslie also reports receiving consulting fees from Medtronic Inc.
AUTHOR CONTRIBUTIONS
Keith Wirth*: Manuscript writing and revision, and figure creation. Scott Kizy*: Manuscript writing and revision, and figure creation. Hisham Abdelwahab: Design and manuscript revision. Jianying Zhang: Statistical analysis and manuscript revision. Santosh Agarwal: Statistical analysis and manuscript revision. Sayeed Ikramuddin: Design and manuscript revision, Daniel B. Leslie: Study conceptualization, design, and manuscript revision. *Denotes equal contribution.
INFORMED CONSENT
These data are de‐identified and informed consent does not apply.
ACKNOWLEDGMENT
Keith Wirth is supported by National Institute of Health/National Institute of Diabetes and Digestive Kidney Diseases T32DK108733 (MPI: Yamamoto and Beilman).
Keith Wirth and Scott Kizy contributed equally to the study.
REFERENCES
- 1. Bureau USCUS Census Bureau . The next four decades the older population in the United States : 2010 to 2050. Statistics (Ber). 2010;2011:3‐14. [Google Scholar]
- 2. Centers for Medicare and Medicaid Services . CMS Fast Facts; 2018. Retrieved from https://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/CMS‐Fast‐Facts/index.html
- 3. Musich S, MacLeod S, Bhattarai GR, et al. The impact of obesity on health care utilization and expenditures in a Medicare supplement population. Gerontol Geriatr Med. 2016;2. 233372141562200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hales C, Carroll M, Fryar C, Ogden C. Prevalence of obesity and severe obesity among adults: United States, 2017‐2018. NCHS Data Brief. 2020;360:1‐8. [PubMed] [Google Scholar]
- 5. Doshi JA, Polsky D, Chang VW. Prevalence and trends in obesity among aged and disabled U.S. Medicare beneficiaries, 1997‐2002. Health Aff (Millwood). 2015;26:1111‐1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Magkos F, Fraterrigo G, Yoshino J, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lean ME, Powrie JK, Anderson AS, Garthwaite PH. Obesity, weight loss and prognosis in type 2 diabetes. Diabet Med. 1990;7:228‐233. [DOI] [PubMed] [Google Scholar]
- 8. Williamson DF, Thompson TJ, Thun M, Flanders D, Pamuk E, Byers T. Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care 2000;23:1499‐1504. [DOI] [PubMed] [Google Scholar]
- 9. Wing RR, Hamman RF, Bray GA, et al. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res. 2004;12:1426‐1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Look AHEAD Research Group . Eight‐year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obes (Silver Spring). 2014;22:5‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Purcell K, Sumithran P, Prendergast LA, Bouniu CJ, Delbridge E, Proietto J. The effect of rate of weight loss on long‐term weight management: a randomised controlled trial. Lancet Diabetes Endocrinol. 2014;2:954‐962. [DOI] [PubMed] [Google Scholar]
- 12. Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—5‐year outcomes. N Engl J Med. 2017;376:641‐651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Courcoulas AP, Christian NJ, Belle SH, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. J Am Med Assoc. 2013;15213:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ikramuddin S, Korner J, Lee W‐J, et al. Roux‐en‐Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia. Jama. 2013;309:2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric‐metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow‐up of an open‐label, single‐centre, randomised controlled trial. Lancet (London, England). 2015;386:964‐973. [DOI] [PubMed] [Google Scholar]
- 16. Dorman RB, Abraham AA, Al‐Refaie WB, Parsons HM, Ikramuddin S, Habermann EB. Bariatric surgery outcomes in the elderly: an ACS NSQIP study. J Gastrointest Surg. 2012;16:35‐44. [DOI] [PubMed] [Google Scholar]
- 17. Ibrahim AM, Ghaferi AA, Thumma JR, Dimick JB. Hospital quality and Medicare expenditures for bariatric surgery in the United States. Ann Surg. 2017;266:105‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ibrahim AM, Thumma JR, Dimick JB. Reoperation and Medicare expenditures after laparoscopic gastric band surgery. JAMA Surg. 2017;152:835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Habermann EB, Durham SB, Dorman R, Jarosek S, Virnig BA. Trends in bariatric surgery in Medicare beneficiaries: data points # 17. Data Points Publ Ser. 2011;1‐11. [PubMed] [Google Scholar]
- 20. Center for Medicare and Medicaid Services . Decision Memo for Bariatric Surgery for the Treatment of Morbid Obesity. Woodland, MD: CAG‐00250R; 2006:1‐40. [Google Scholar]
- 21. Kizy S, Jahansouz C, Downey MC, Hevelone N, Ikramuddin S, Leslie D. National trends in bariatric surgery 2012–2015: demographics, procedure selection, readmissions, and cost. Obes Surg. 2017;27:2933‐2939. [DOI] [PubMed] [Google Scholar]
- 22. Abraham A, Ikramuddin S, Jahansouz C, Arafat F, Hevelone N, Leslie D. Trends in bariatric surgery: procedure selection, revisional surgeries, and readmissions. Obes Surg. 2016;26:1371‐1377. [DOI] [PubMed] [Google Scholar]
- 23. Kundi H, Popma JJ, Khabbaz KR, et al. Association of hospital surgical aortic valve replacement quality with 30‐day and 1‐year mortality after transcatheter aortic valve replacement. JAMA Cardiol. 2019;4(1):16‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reames BN, Birkmeyer NJO, Dimick JB, Ghaferi AA. Socioeconomic disparities in mortality after cancer surgery: failure to rescue. JAMA Surg. 2014;149(5):475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brummett CM, England C, Evans‐shields J, et al. Health care burden associated with outpatient opioid use following inpatient or outpatient urgery. J Manag Care Spec Pharm, 2019;25(9):973‐983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Social Security Administration . Survivors Benefits. [WWW document]. https://www.ssa.gov/OP_Home/handbook/handbook.04/handbook-toc04.html
- 27. Centers for Medicare and Medicaid Services Medicaid . Decision Memo for Bariatric Surgery for the Treatment of Morbid Obesity. [WWW document]. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=258&NcaName=Bariatric+Surgery+for+the+Treatment+of+Morbid+Obesity&CoverageSelection=National&KeyWord=obesity&KeyWordLookUp=Title&KeyWordSearchType=And&where=index&nca_id=2
- 28. Carlin AM, Zeni TM, English WJ, et al. The comparative effectiveness of sleeve gastrectomy, gastric bypass, and adjustable gastric banding procedures for the treatment of morbid obesity. Ann Surg. 2013;257:791‐797. [DOI] [PubMed] [Google Scholar]
- 29. Lager CJ, Esfandiari NH, Subauste AR, et al. Roux‐En‐Y gastric bypass vs. sleeve gastrectomy: balancing the risks of surgery with the benefits of weight loss. Obes Surg. 2016;27:154‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Young MT, Gebhart A, Phelan MJ, Nguyen NT. Use and outcomes of laparoscopic sleeve gastrectomy vs laparoscopic gastric bypass: analysis of the American college of Surgeons nsqip. J Am Coll Surg. 2015;220:880‐885. [DOI] [PubMed] [Google Scholar]
- 31. Ignat M, Vix M, Imad I, et al. Randomized trial of Roux‐en‐Y gastric bypass versus sleeve gastrectomy in achieving excess weight loss. Br J Surg. 2017;104:248‐256. [DOI] [PubMed] [Google Scholar]
- 32. Aminian A, Brethauer SA, Andalib A, et al. Can sleeve gastrectomy “cure” diabetes? Long‐term metabolic effects of sleeve gastrectomy in patients with type 2 diabetes. Ann Surg. 2016;264:674‐681. [DOI] [PubMed] [Google Scholar]
- 33. Adams TD, Gress RE, Smith SC, et al. Long‐term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753‐761. [DOI] [PubMed] [Google Scholar]
- 34. Casillas RA, Kim B, Fischer H, Zelada Getty JL, Um SS, Coleman KJ. Comparative effectiveness of sleeve gastrectomy versus Roux‐en‐Y gastric bypass for weight loss and safety outcomes in older adults. Surg Obes Relat Dis. 2017;13:1476‐1483. [DOI] [PubMed] [Google Scholar]
- 35. Navarrete A, Corcelles R, Del Gobbo GD, Perez S, Vidal J, Lacy A. Sleeve gastrectomy in the elderly: a case‐control study with long‐term follow‐up of 3 years. Surg Obes Relat Dis. 2017;13:575‐580. [DOI] [PubMed] [Google Scholar]
- 36. Dorman RB, Miller CJ, Leslie DB, et al. Risk for hospital readmission following bariatric surgery. PLoS One. 2012;7:e32506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Abraham CR, Werter CR, Ata A, et al. Predictors of hospital readmission after bariatric surgery. J Am Coll Surg. 2015;221:220‐227. [DOI] [PubMed] [Google Scholar]
- 38. Sudan R, Nguyen NT, Hutter MM, Brethauer SA, Ponce J, Morton JM. Morbidity, mortality, and weight loss outcomes after reoperative bariatric surgery in the USA. J Gastrointest Surg. 2015;19:171‐178. [DOI] [PubMed] [Google Scholar]
- 39. Anderson WL, Wiener JM, Khatutsky G, Armour BS. Obesity and people with disabilities: the implications for health care expenditures. Obes (Silver Spring). 2013;21:E798‐E804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Al‐Bahri S, Fakhry TK, Gonzalvo JP, Murr MM. Bariatric surgery as a bridge to renal transplantation in patients with end‐stage renal disease. Obes Surg. 2017;27:2951‐2955. [DOI] [PubMed] [Google Scholar]
- 41. Jamal MH, Corcelles R, Daigle CR, et al. Safety and effectiveness of bariatric surgery in dialysis patients and kidney transplantation candidates. Surg Obes Relat Dis;11:419‐423. [DOI] [PubMed] [Google Scholar]
- 42. Keating C, Neovius M, Sjöholm K, et al. Health‐care costs over 15 years after bariatric surgery for patients with different baseline glucose status: results from the Swedish Obese Subjects study. Lancet Diabetes Endocrinol. 2015;3:855‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim DD, Arterburn DE, Sullivan SD, Basu A. Economic value of greater access to bariatric procedures for patients with severe obesity and diabetes. Med Care. 2018;56:583‐588. [DOI] [PubMed] [Google Scholar]
- 44. Khorgami Z, Aminian A, Shoar S, et al. Cost of bariatric surgery and factors associated with increased cost: an analysis of national inpatient sample. Surg Obes Relat Dis. 2017;13:1284‐1289. [DOI] [PubMed] [Google Scholar]
- 45. Khorgami Z, Aminian A, Shoar S, et al. Cost of bariatric surgery and factors associated with increased cost: an analysis of national inpatient sample. Surg Obes Relat Dis. 2017;13:1284‐1289. [DOI] [PubMed] [Google Scholar]


