Abstract
Objective
As severity of outcome in COVID‐19 is disproportionately higher among individuals with obesity, smokers, patients with hypertension, kidney disease, chronic pulmonary disease, coronary heart disease (CHD), and/or type 2 diabetes (T2D), serum levels of ACE2, the cellular entry point for the coronavirus SARS‐CoV‐2, were examined in these high‐risk groups.
Methods
Associations of ACE2 levels to smokers and patients with hypertension, T2D, obesity, CHD, or COPD were investigated in a single center population‐based study of 5457 Icelanders from the Age, Gene/Environment Susceptibility Reykjavík Study (AGES‐RS) of the elderly (mean age 75 ± 6 years), using multiple linear regression analysis.
Results
Serum levels of ACE2 were higher in smokers and individuals with T2D and/or obesity while they were unaffected in the other patient groups.
Conclusion
ACE2 levels are higher in some patient groups with comorbidities linked to COVID‐19 including obesity and T2D and as such may have an emerging role as a circulating biomarker for severity of outcome in the disease.
1. INTRODUCTION
The current coronavirus disease 2019 (COVID‐19) pandemic is caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 1 SARS‐CoV‐2 interacts with the receptor ACE2, 2 to enter the host cell, binding the receptor with a 10‐ to 20‐fold higher affinity than other SARS coronaviruses do. 3 COVID‐19 is associated with major respiratory failure and greatest adverse outcomes from the disease are among the elderly, smokers, and/or individuals with an underlying disease including but not limited to coronary heart disease (CHD), hypertension, kidney disease, chronic pulmonary disease, type 2 diabetes (T2D), and obesity (body mass index, BMI > 30 kg/m2).4, 5, 6, 7, 8, 9, 10
While lower survival in COVID‐19 might be attributed to the frailty and vulnerabilities of the high‐risk patient groups mentioned above, other underlying factors could play role. For instance, variable levels and/or activity of the membrane bound receptor ACE2, 2 and its soluble counterpart, may contribute to this susceptibility. Just how the balance between the levels of soluble ACE2 and its membrane‐bound receptor counterpart is maintained, has still to be fully defined. We postulate that SARS‐CoV‐2 infectivity may vary among individuals depending on their endogenous levels of circulating ACE2 reflecting its levels in solid tissues. ACE2 levels in serum from individuals with hypertension, kidney disease, obesity, T2D, COPD, or CHD were examined in a large deeply phenotyped population‐based study of the elderly.
2. METHODS
2.1. Study population and measurements
Participants aged 66 through 96 were from the Age, Gene/Environment Susceptibility Reykjavík Study (AGES‐RS) cohort, 11 a single‐center prospective population‐based study of deeply phenotyped subjects (5764, mean age 75 ± 6 years) and who are the surviving subset of the 40‐years long prospective Reykjavík study (n ∼ 18,000), an epidemiologic study aimed to understand aging in the context of gene/environment interaction by focusing on four biologic systems: vascular, neurocognitive (including sensory), musculoskeletal, and body composition/metabolism. The AGES‐RS was approved by the NBC in Iceland (approval number VSN‐00‐063), and by the National Institute on Aging Intramural Institutional Review Board (The United States) and the Data Protection Authority in Iceland.
The following disease traits and characteristics were investigated in multiple regression analysis for an effect on serum ACE2 levels: body mass index (BMI) (kg/m2) categories, type 2 diabetes (fasting serum glucose >7.0 mmol/L or self‐reported history of diabetes or the use of insulin or oral glucose‐lowering drugs), hypertension (systolic blood pressure >140 or diastolic blood pressure >90, use of antihypertension medications), former, and current smoking, CHD (ascertained using hospital records and/or cause of death information). A CHD event was defined as any occurrence of myocardial infarction, ICD‐10 codes: I21–I25, coronary revascularization (either CABG‐surgery or PTCA intervention) or death from CHD. Chronic obstructive pulmonary disease (COPD) was defined according to hospital records ICD‐10 code: J44, medications, and questionnaire. Estimated glomerular filtration rage (eGFR) was calculated from age, sex, and serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation. 12 Means and standard deviations were calculated for continuous characteristics and numbers and percentages for categorical characteristics.
2.2. Protein measurements
Serum protein levels of ACE2 were measured in 5457 individuals from the AGES‐RS using the slow‐off rate modified aptamer (SOMAmer)‐based protein profiling technology,13, 14, 15 performed at SomaLogic Inc. (Boulder, The United States). Blood samples were collected at the AGES‐Reykjavik baseline after an overnight fast. Serum was prepared using a standardized protocol, 16 stored in 0.5 ml aliquots at −80°C and serum samples that had not been previously thawed were used for the protein measurements. The order of sample collection and processing for protein measurements (4782 proteins measured including ACE2) were randomized and all samples run as a single set.
All SOMAmers that passed quality control had median intra‐ and inter‐assay coefficient of variation (CV) less than 5% similar to that reported on variability in the SOMA scan assays. 17 Various metrics related to the performance of the proteomic platform were applied, including both direct assessment of aptamer specificity via tandem mass spectrometry analysis as well as inferential support using proteogenomics analysis, indicating consistent target specificity across the platform. 13 Hybridization controls were used to correct for systematic variability in detection and calibrator samples of three dilution sets (40%, 1%, and 0.005%) were included so that the degree of fluorescence was a quantitative reflection of protein concentration.
ACE2 was investigated on a log2 scale, where a one‐unit increase represents a doubling in levels on the RFU scale. Multiple linear regression analysis was used to study the association between two or more independent variables (predictors) to a single continuous dependent variable (outcome). 18 In our case, the independent risk predictors included a priori selected set of comorbidities known to be associated with severity of outcome in COVID‐19 (see Table 1), while the outcome variable was ACE2 levels in serum. Also, the model included adjustment for important confounding variables such as age and sex.
TABLE 1.
Results from a multiple linear regression analysis with ACE2 levels (log2) as the dependent variable
| Characteristic a | β coefficient | Std. error | 95% CI | t value | p‐value | |
|---|---|---|---|---|---|---|
| Age (years) | −0.0014 | 0.0008 | −1.90 | −0.0029 | 0.0000 | 0.057 |
| Sex (females reference) | −0.0385 | 0.0084 | 4.60 | 0.0221 | 0.0550 | <0.001 |
| BMI (kg/m2) (<25 reference) | 0.022 b | |||||
| Overweight, 25–30 | 0.0259 | 0.0090 | 2.88 | 0.0083 | 0.0435 | 0.004 |
| Obese, 30–35 | 0.0246 | 0.0118 | 2.10 | 0.0016 | 0.0477 | 0.036 |
| Severely obese, >35 | 0.0331 | 0.0193 | 1.71 | −0.0048 | 0.0710 | 0.087 |
| T2D status (glucose <5.6 mmol/L reference) | <0.001 b | |||||
| Impaired fasting glucose 5.6–6.9 mmol/L | 0.0295 | 0.0085 | 3.45 | 0.0127 | 0.0462 | <0.001 |
| T2D | 0.0377 | 0.0128 | 2.94 | 0.0126 | 0.0629 | 0.003 |
| Hypertension | ||||||
| Systolic bp. >140 or diastolic bp. >90 | −0.0085 | 0.0078 | −1.10 | −0.0237 | 0.0067 | 0.273 |
| ARBs | −0.0344 | 0.0124 | −2.78 | −0.0586 | −0.0102 | 0.005 |
| ACE inhibitors | −0.0156 | 0.0127 | −1.22 | −0.0405 | 0.0093 | 0.221 |
| Other hypertension medication | −0.0089 | 0.0094 | −0.94 | −0.0274 | 0.0096 | 0.346 |
| Smoking status (never reference) | 0.044 b | |||||
| Former | 0.0137 | 0.0086 | 1.59 | −0.0032 | 0.0305 | 0.111 |
| Current | 0.0299 | 0.0127 | 2.36 | 0.0051 | 0.0549 | 0.018 |
| CHD | −0.0040 | 0.0098 | −0.41 | −0.0232 | 0.0152 | 0.685 |
| COPD | −0.0186 | 0.0121 | −1.54 | −0.0422 | 0.0051 | 0.123 |
| eGFR (per 10 ml/min/1.73 m2) | 0.0024 | 0.0024 | 1.00 | −0.0023 | 0.0071 | 0.315 |
Phenotypes definitions are provided in Section 2. Age in years 76.6(5.6); 57.3% females; impaired fasting glucose 5.6–6.9 (n = 1983); mean BMI 27.1 (4.4), T2D (n = 660); hypertension (n = 2739); ARBs users (n = 753), ACEIs users (n = 699); other antihypertensive users (n = 1991); never smoker (n = 2253), former smoker (n = 2405), current smoker (n = 655); CHD (n = 1217); COPD (n = 644); eGFR ml/min/1.73 m2 = 75.0 (17.3).
p‐value from a F‐test.
3. RESULTS
Results from multiple linear regression analysis with ACE2 levels (log2) as the outcome are highlighted in Table 1. Serum ACE2 levels were higher in individuals with overweight (β = 0.0259, p = 0.004) and obesity (β = 0.0246, p = 0.036) when compared with lean (BMI < 25) individuals. ACE2 levels measured higher in persons with severe obesity (β = 0.0331, p = 0.087), but did not reach significance perhaps due to the relatively few individuals with severe obesity in this cohort of old people. The violin plots in Figure 1 demonstrate elevated levels (log2 scale) of ACE2 in serum in response to increasing adiposity presented by different BMI categories. Furthermore, both individuals with impaired fasting glucose levels (β = 0.0295, p < 0.001) and those with established T2D (β = 0.0377, p = 0.003) showed higher ACE2 levels compared with those with no T2D or with normal glucose levels (Table 1). Serum levels were significantly increased in current smokers compared with individuals who never smoked (β = 0.0299, p = 0.018) and with sex (β = −0.0385, p < 0.001) (Table 1). In contrast, ACE2 levels were not significantly associated with age, eGFR, COPD, hypertension, or CHD (Table 1).
FIGURE 1.
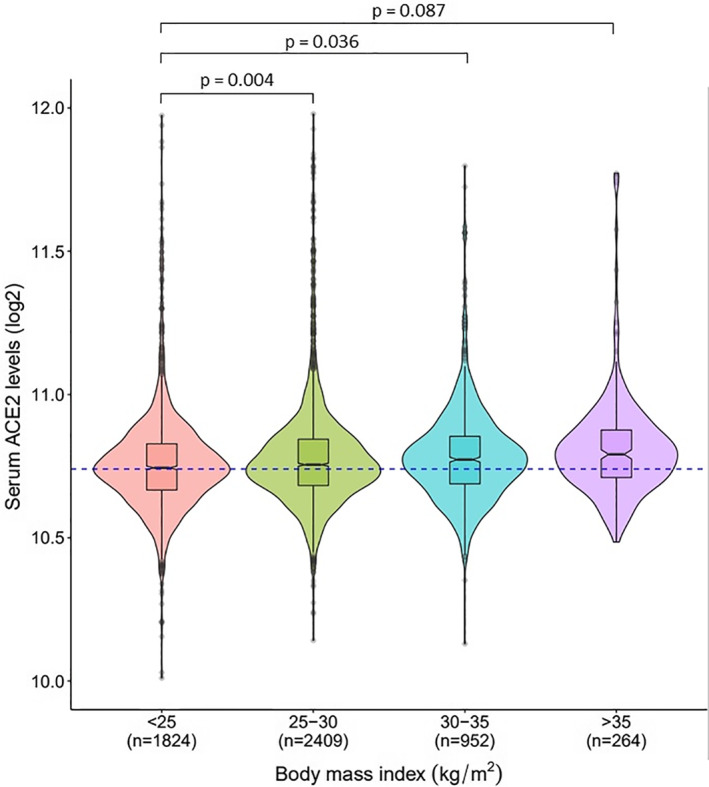
The y‐axis represents log2‐transformed distribution of ACE2 levels in the serum while the x‐axis shows different categories of BMI (kg/m2), also expressed in Table 1. The broken line (blue) going through the median in ACE2 levels of the lean group (BMI < 25), enables better comparison to the other BMI categories. The statistical significance of the comparison between individuals with overweight, obesity, or severe obesity to that of individuals who are lean was obtained from the multiple regression analysis presented in Table 1
4. DISCUSSION
The severity of outcome in COVID‐19 is greatly influenced by various comorbidities and unhealthy lifestyle.8, 9, 10 Higher circulating levels of ACE2 were observed in some of these groups including smokers and patients with obesity and/or T2D. The observation that ACE2 levels are elevated in smokers agrees well with a study showing upregulation of ACE2 mRNA in the respiratory tract of smokers as well as rodents exposed to cigarette smoke. 19 Further, the finding that ACE2 levels are higher in both diabetics and those with impaired fasting glucose (Table 1) is of interest given recent reports showing that risk of critical admissions and mortality in COVID‐19 is two to three times greater in patients with diabetes.20, 21 There can be many reasons for the increased susceptibility to COVID‐19 among the obese and diabetics including for instance impaired immune response in these patient groups. 22 However, the adipose tissue contains as many ACE2 receptors as pulmonary tissues, 23 and ACE2 expression in adipose tissue of mice is increased by high fat diet. 24 We postulate that higher circulating levels of ACE2 in patients with obesity and/or T2D may reflect increased abundance of the membrane bound ACE2 receptor and/or activity in solid tissues, that in turn may reflect higher viral load among these high‐risk groups leading to increased susceptibility to adverse outcomes in COVID‐19. Thus in addition to altered ACE2 levels, obesity and T2D are associated with chronic inflammation that can aggravate inflammation in pulmonary tissues, 25 which combined may influence the severity of outcome in COVID‐19.
ACE2 is a membrane‐bound ectoenzyme that is released into the circulation via ectodomain shedding. 26 Variable levels of ACE2 in serum are likely a result of genetic factors, differential gene expression, and/or ectodomain shedding influenced by disease or administration of drugs via negative or positive feedback loops of the renin–angiotensin system. 26 As proteins in circulation emanate from virtually every tissue of the body, 13 it cannot easily be determined how much each tissue contributes to ACE2 release into blood. For instance, high serum ACE2 levels may not mirror higher activity or abundances of its membrane bound counterpart in pulmonary tissues. Finally, soluble ACE2 as well as ACE2 receptors attached to small extracellular vesicles may function as decoys via competitive inhibition of SARS‐CoV‐2 binding to ACE2 located at the host cells. 27
There are several limitations of the present study including: This work does not show that higher ACE2 serum levels in smokers and patients with obesity and/or T2D cause worse clinical outcomes in COVID‐19, although that is a possibility. The results presented may be limited to that of serum and may not reflect the levels and activity of the membrane bound ACE2 receptor in pulmonary tissues or in other solid tissues. All participants of the AGES‐RS are white (Caucasians) which may limit the transferability and generalizability of the results. Finally, although statistically significant, the effect sizes for higher ACE2 levels in patients with obesity or T2D in Table 1 are relatively small, thus the estimates for these patient groups may not be of great clinical importance. In summary, serum levels of ACE2 are raised in patients with obesity or T2D that are common comorbidities associated with severity of outcome in COVID‐19, and that may signal enhanced severity of outcome in COVID‐19 for these individuals.
CONFLICT OF INTEREST
John R. Lamb and Lori L. Jennings was and is, respectively, employee and stockholder of Novartis. All other authors declare they have no competing interests.
AUTHOR CONTRIBUTIONS
Designed the study and supervised the project: Valur Emilsson and Vilmundur Gudnason. Performed data analysis: Alexander Gudjonsson, Lenore J. Launer, Valur Emilsson, Valborg Gudmundsdottir, Elias F Gudmundsson, Brynjolfur G Jonsson and Thor Aspelund. Provided expertise on proteomics data and contributed to discussion: John R Lamb and Lori L Jennings. Wrote the first draft of the manuscript, with all coauthors contributing to data interpretation, manuscript editing, and revision: Valur Emilsson.
ACKNOWLEDGMENTS
We thank the staff of the Icelandic Heart Association for their contribution to the AGES‐Reykjavik study and all study members of AGES‐Reykjavik cohort for their participation.
Contributor Information
Valur Emilsson, Email: valur@hjarta.is, Email: v.gudnason@hjarta.is.
Vilmundur Gudnason, Email: valur@hjarta.is, Email: v.gudnason@hjarta.is.
REFERENCES
- 1. Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92(4):401‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade‐long structural studies of SARS coronavirus. J Virol. 2020;94(7):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wrapp D, Wang N, Corbett KS, et al. Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wise J. A third of covid‐19 patients admitted to UK hospitals die. BMJ. 2020;369:m1794. [DOI] [PubMed] [Google Scholar]
- 5. Williamson EJ, Walker AJ, Bhaskaran K, et al. OpenSAFELY: factors associated with COVID‐19 death in 17 million patients. Nature. 2020;584:430‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stefan N, Birkenfeld AL, Schulze MB, Ludwig DS. Obesity and impaired metabolic health in patients with COVID‐19. Nat Rev Endocrinol. 2020;16(7):341‐342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Docherty AB, Harrison EM, Green CA, et al. Features of 20,133 UK patients in hospital with covid‐19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York city area. JAMA. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 ‐ United States, February 12‐March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):382‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harris TB, Launer LJ, Eiriksdottir G, et al. Age, gene/environment susceptibility‐Reykjavik study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165(9):1076‐1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Emilsson V, Ilkov M, Lamb JR, et al. Co‐regulatory networks of human serum proteins link genetics to disease. Science. 2018;361(6404):769‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gold L, Ayers D, Bertino J, et al. Aptamer‐based multiplexed proteomic technology for biomarker discovery. PLoS ONE. 2010;5(12):e15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lamb JR, Jennings LL, Gudmundsdottir V, Gudnason V, Emilsson V. It's in our blood: a glimpse of personalized medicine. Trends Mol Med. 2020;S1471‐491(20):30220–30223. 10.1016/j.molmed.2020.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tuck MK, Chan DW, Chia D, et al. Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. J Proteome Res. 2009;8(1):113‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hathout Y, Brody E, Clemens PR, et al. Large‐scale serum protein biomarker discovery in Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2015;112(23):7153‐7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eberly LE. Multiple linear regression. Methods Mol Biol. 2007;404:165‐187. [DOI] [PubMed] [Google Scholar]
- 19. Smith JC, Sausville EL, Girish V, et al. Cigarette smoke exposure and inflammatory signaling increase the expression of the SARS‐CoV‐2 receptor ACE2 in the respiratory tract. Dev Cell. 2020;53(5):514‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID‐19 pneumonia ‐ a systematic review, meta‐analysis, and meta‐regression. Diabetes Metab Syndr. 2020;14(4):395‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID‐19 cases: a systematic literature review and meta‐analysis. J Infect. 2020;81(2):e16‐e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Frasca D, Blomberg BB, Paganelli R. Aging, obesity, and inflammatory age‐related diseases. Front Immunol. 2017;8:1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sattar N, McInnes IB, McMurray JJV. Obesity is a risk factor for severe COVID‐19 infection: multiple potential mechanisms. Circulation. 2020;142(1):4‐6. [DOI] [PubMed] [Google Scholar]
- 24. Gupte M, Boustany‐Kari CM, Bharadwaj K, et al. ACE2 is expressed in mouse adipocytes and regulated by a high‐fat diet. Am J Physiol Regul Integr Comp Physiol. 2008;295(3):R781‐R788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860‐867. [DOI] [PubMed] [Google Scholar]
- 26. Patel SK, Velkoska E, Freeman M, Wai B, Lancefield TF, Burrell LM. From gene to protein‐experimental and clinical studies of ACE2 in blood pressure control and arterial hypertension. Front Physiol. 2014;5:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Inal JM. Decoy ACE2‐expressing extracellular vesicles that competitively bind SARS‐CoV‐2 as a possible COVID‐19 therapy. Clin Sci (Lond). 2020;134(12):1301‐1304. [DOI] [PMC free article] [PubMed] [Google Scholar]


