Abstract
This study aimed to characterize the clinical profile of patients with brief psychotic disorders (BPD) triggered by the psychosocial distress derived from the COVID-19 crisis. A multicenter study was conducted from March 14 to May 14, 2020 (the peak weeks of the pandemic in Europe). All consecutive patients presenting non-affective psychotic episodes with a duration of untreated psychosis of less than 1 month and whose onset was related to the COVID-19 crisis were recruited, but only those patients meeting Diagnostic Statistical Manual 5th edition (DSM-5) criteria for “BPD with marked stressors” (DSM-5 code: 298.8) during follow-up were finally included. Patients' sociodemographic and clinical characteristics were collected at baseline and summarized with descriptive statistics. During the study period, 57 individuals with short-lived psychotic episodes related to the emotional stress of the COVID-19 pandemic were identified, of whom 33 met DSM-5 criteria for “BPD with marked stressors”. The mean age was 42.33 ± 14.04 years, the gender distribution was almost the same, and the majority were rated as having good premorbid adjustment. About a quarter of the patients exhibited suicidal symptoms and almost half presented first-rank schizophrenia symptoms. None of them were COVID-19 positive, but in more than half of the cases, the topic of their psychotic features was COVID-19-related. The coronavirus pandemic is triggering a significant number of BPD cases. Their risk of suicidal behavior, their high relapse rate, and their low temporal stability make it necessary to closely monitor these patients over time.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00406-021-01256-w.
Keywords: Stress, Psychoses, Suicide, Schizophrenia, COVID-19
Introduction
The COVID-19 pandemic, caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), in just a few months has become the most severe health crisis of our time with more than 37 million confirmed cases and about one million deaths worldwide as of October 12, 2020 [1]. Almost every country in the world is suffering under the negative consequences of the pandemic, which is breaking up the normal functioning of our society, pushing healthcare systems to the limit, and becoming a serious threat to the entire global economy [2]. This dramatic situation is the worst since World War II and its impact on the global population is raising mental health concerns around the world [3, 4]. Beyond the acute neuropsychiatric manifestations of COVID-19 infection [5–8], fear of contagion, loss of loved ones, mandatory quarantine imposed by governments to avoid spread of the virus and the enormous socioeconomic uncertainty derived from this crisis are causing an increase in the incidence of mental disorders, such as stress-related psychosis, in the general population [3, 4, 9].
Reactive, or psychogenic, psychoses are defined as those that appear in response to psychological distress [10, 11]. This classical diagnostic concept, which originated from Jasper's 'true reaction' and was further developed in Scandinavia by Wimmer, Faergeman and Strömgren [12], encompasses a group of short-lived, acute psychotic episodes in reaction to a stressful situation, with symptomatology usually related to mental trauma [10]. Although the diagnostic validity of reactive psychosis is limited [13], its clinical picture has been captured, to a greater or lesser extent, in major international psychiatric classification systems [11]. Thus, while in the third edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-III) diagnosis of “brief reactive psychosis” included precipitating stress as a mandatory criterion, in the DSM-IV and DSM-5 that criterion was removed, the condition was renamed “brief psychotic disorder” (BPD; DSM-5 code: 298.8), and the presence of “marked stressors” was considered only a specifier [11, 14]. Similarly, while the 8th and 9th revisions of the International Classification of Diseases (ICD) classified reactive psychoses within the category “other non-organic psychosis” as psychotic conditions attributable to a recent stressful event, ICD-10 subsumed them within the composite category “acute and transient psychotic disorders” (ATPD; ICD-10 code F23) under the specifier “with associated acute stress” (F23. × 1) but avoiding any aetiological implication, and in the forthcoming ICD-11 that specifier will no longer be available in the new ATPD category [11, 15, 16]. Furthermore, it is noteworthy that there is a diagnostic and prognostic overlap between BPD/ATPD and other operationalizations of short-lived psychotic episodes used in the Clinical High Risk for Psychosis (CHR-P) paradigm such as “Brief Limited Intermittent Psychotic Symptoms” (BLIPS) and “Brief Intermittent Psychotic Symptoms” (BIPS) [17–19]. According to the diathesis-stress model [20], individuals with brief reactive psychosis have a latent psychological vulnerability (such as heightened emotional reactivity) that makes them more vulnerable to psychotic symptoms when faced with stressful environmental factors [13]. However, the aetiopathology of these psychotic episodes, which share a multifactorial genetic and environmental pathogenesis, has not been established [21, 22]. The epidemiology of brief reactive psychoses is not well known owing to the varying criteria used to define them in diagnostic classification systems for mental disorders, even though they could represent 1.6–11% of all psychiatric first admissions [10]. Middle-aged women seem to be more prone to these brief reactive psychotic episodes, which are typically precipitated by loss, isolation, and disaster, as in the ongoing coronavirus pandemic [11, 23]. Notwithstanding, these findings have not been consistently replicated and there is uncertainty about predisposing factors.
There is a growing body of the literature on the role of the COVID-19 crisis in the genesis of psychotic symptoms in both the general population and in patients with preexisting mental disorders [5, 24–27]. Many reports of individuals experiencing psychosis in response to the coronavirus were published during the first months of the pandemic [9, 28–39]. However, the majority of these reports were based on single-center case series and spanned heterogeneous acute psychotic conditions (such as organic, affective, and non-affective psychoses) (see Table 1 for further details of the systematic review [40]). As a result, there is as yet no clear picture of the clinical and sociodemographic profile of individuals presenting reactive psychosis triggered by the psychosocial stress associated with COVID-19.
Table 1.
Summary of published reports on the onset of psychotic disorders in relation to the COVID-19 pandemic during the first months of the outbreak: results of a scoping review
| Authors | Date of publication | Country | Design | Sample size | BPD according to DSM criteria | Observations |
|---|---|---|---|---|---|---|
| Bernard-Valnet et al. [28] | April 2020 | Switzerland | Case report | 2 | No | Two COVID-19-positive women were diagnosed with acute meningoencephalitis. One of them developed psychotic symptoms |
| Colizzi et al. [32] | April 2020 | Italy | Case report | 1 | No | A 16-year-old male with somatic symptom disorder experienced brief psychotic symptoms triggered by the fear of having COVID-19 |
| Finatti et al. [33] | May 2020 | Italy | Case series | 3 | Yes | Two men and one woman with no past history of psychiatric disorders had BPD in the context of the mandatory nationwide quarantine. One of them had suicidal symptoms |
| Fischer et al. [34] | April 2020 | Germany | Case report | 1 | No | A male patient showed acute exacerbation of schizophrenia with psychotic content related to COVID-19 |
| Hu et al. [35] | February 2020 | China | Observational study | N/S | N/A | This preprint reported an increase in incidence of first-episode schizophrenia in elderly adults during the early stages of the pandemic in China |
| Huarcaya et al. [36] | April 2020 | Perú | Case report | 1 | No | A 38-year-old woman with no psychiatric history developed acute psychotic symptoms in response to her fear of COVID-19 contagion |
| Martin EB Jr [37] | March 2020 | USA | Case series | 3 | N/S | Three women employed at the same hospital presented brief stress-related psychoses related to the COVID-19 healthcare crisis. One of them developed suicidal symptoms in the context of her delusional beliefs |
| Mehra et al. [38] | April 2020 | India | Case report | 2 | No | Two elderly patients (a 72-year-old man and a 60-year-old woman) experienced a recurrence of depression triggered by the fear of contracting COVID-19. The woman's depression had psychotic features |
| Ovejero et al. [39] | April 2020 | Spain | Case report | 1 | No | A 41-year-old woman with bipolar disorder had a manic episode with psychotic symptoms in which COVID-19 infection was a delusional topic |
| Rentero et al. [29] | May 2020 | Spain | Case series | N/S | N/S | Clinicians from a consultation-liaison psychiatry unit reported that several patients from their hospital were experiencing psychotic symptoms as an acute neuropsychiatric manifestation of COVID-19 infection |
| Valdés-Florido et al. [9] | April 2020 | Spain | Case series | 4 | Yes | Two men and two women, one of whom had a past history of BPD, showed reactive psychoses attributed to the psychological distress caused by the COVID-19 healthcare and economic crisis. Two of these patients were suicidal at the time of evaluation |
| Weise et al. [30] | April 2020 | Germany | Case report | 1 | No | Psychotic symptoms and severe suicidal behavior of a woman in her mid-60 s with delusional disorder worsened as a result of the coronavirus pandemic |
| Zulfiki et al. [31] | February 2020 | Malaysia | Case report | 1 | Yes | A 31-year-old man with no past history of psychiatric disorders had a BPD caused by psychosocial stress from the COVID-19 pandemic |
BPD Brief psychotic disorder; DSM Diagnostic and Statistical Manual of Mental Disorders; N/A Not Applicable; N/S Not Specified; USA United States of America
Note: To identify articles matching the scope of this review, a literature search was conducted in PubMed/Medline, Web of Science and Scopus databases from inception to 14 May 2020 using the following terms and strategy: (COVID-19 OR SARS-Cov-2 OR 2019-ncov OR coronavirus) AND (psychosis OR psychotic OR schizophrenia). The systematic search followed PRISMA guidelines [40] (see flowchart in Supplementary appendix)
The aim of this prospective observational study was to characterize the profile of patients with brief reactive psychosis related to the coronavirus crisis who were attended by the mental health services of several hospitals in southern Spain during the peak 8 weeks of the pandemic. To our knowledge, this is the first characterization study to involve patients from a large geographical area of one of the countries most affected by the COVID-19 crisis.
Methods
Study population and inclusion/exclusion criteria
This multicenter case-register study included all consecutive patients aged 18–65 diagnosed with brief reactive psychosis as a result of stress from the current COVID-19 pandemic. The 2-month study period, from March 14, 2020 (date when the state of emergency and national confinement were imposed in Spain) to May 14, 2020 (when social distancing enforcement measures started to be relaxed), captured the peak weeks of the pandemic in the country. The sample was recruited from individuals attending the Mental Health Services (emergency, inpatient and outpatient settings) of 10 public hospitals in Andalusia (southern Spain) covering an epidemiological catchment area of 4.3 million people (2.8 million aged between 18 and 65 years old). The main outcome of our study was determination of the sociodemographic and clinical profile of patients with brief reactive psychosis (DSM-5 code: 298.8) related to the COVID-19 crisis. The STROBE statement guidelines for reporting observational studies was followed (see checklist in Supplementary appendix) [41]. All patients gave their informed consent to participate in this research, which was approved by the Andalusian Biomedical Research Ethics Committee.
All patients who presented non-affective psychotic episodes with a duration of untreated psychosis (DUP) of less than 1 month (i.e., with a provisional diagnosis of BPD) and whose symptom onset was related to the psychosocial distress from the COVID-19 pandemic (such as fear of contagion, the mandatory home-confinement measures or concerns about the social and economic effects) were initially considered eligible. However, only those patients with a confirmed DSM-5 diagnosis of ‘BPD with marked stressors’ (DSM-5 code: 298.8) during the follow-up were finally included in the study [14]. For more robust diagnoses, clinical interviews were supplemented, when available, by additional information from family members and by reviewing all available medical records. Schizophrenia, schizophreniform disorder, schizoaffective disorder, delusional disorder, psychotic disorder not otherwise specified, bipolar disorder, major depressive disorder with psychotic features, substance/medication‐induced psychotic disorder, and psychotic disorder due to another medical condition were excluded following DSM-5 criteria [14], as were those brief reactive psychoses with stressors which, at the clinician’s criteria, were not directly related to the coronavirus pandemic or its consequences. Other exclusion criteria were intellectual disability or autism spectrum disorders, severe or unstable medical condition, history of traumatic brain injury, cognitive impairment or major neurological diseases, and not speaking Spanish or English well enough for clinical assessment.
Selected demographic, clinical and psychopathological variables
Demographic, clinical, and psychopathological features were systematically collected at baseline using a clinician-administered questionnaire designed by the authors which was included in the case-record form. Sociodemographic characteristics of patients included age, gender, ethnicity (European Caucasian or other), marital status (married/partnership or unmarried), cohabitation (alone or with others), education (higher education, complete or incomplete secondary education), and occupation (employed or unemployed or student). Clinical characteristics collected at the onset of the disorder were as follows: (a) Premorbid psychosocial adjustment (dichotomized into good or poor, using a methodology similar to other studies [42–44]); (b) Past psychiatric records; (c) History of harmful or hazardous substance use (alcohol, cannabis or other illicit drugs); (d) First-degree family history of psychosis; (e) First-episode or recurrent BPD episode; (f) Type of onset of psychotic symptoms (dichotomized into abrupt or non-abrupt, depending on whether the change from a non-psychotic to a clearly psychotic state occurred within 48 h or not); (g) DUP following the methodology outlined in the Nottingham Onset Schedule [45]; (h) Presence of suicidal symptoms (including suicidal ideation, plans or attempts [46, 47]); (i) Psychotic psychopathological symptoms; (j) COVID-19 screening results as determined by reverse transcriptase–polymerase chain reaction (RT-PCR) tests; (k) Inpatient or outpatient status; and (l) Duration of the psychotic episode to clinical remission.
The presence of marked stressors related to the coronavirus crisis and the onset of psychosis was assessed by clinical interview and review of medical records to explore whether the appearance of psychotic symptoms was from fear of contagion (for themselves or loved ones), grief due to loss of family members, distress derived from enforced home-confinement, or socioeconomic consequences of the national lockdown (such as job loss or the fear of job loss). The possibility of bias in assessing whether stress due to COVID-19 precipitated the onset of psychotic symptoms or vice-versa was minimized by asking key informants about how the pandemic and its associated socioeconomic consequences adversely affected patients' mental health prior to psychosis onset. Any ambiguities during this task were resolved by the consensus of two senior psychiatrists (ÁL-D and MR-V).
Premorbid adjustment was rated as good or poor following the same criteria as in other previous studies [42–44]. According to this methodology, the sum of the following sociodemographic variables was used as an indicator of poor premorbid adjustment: being unmarried, unemployed, and having a secondary or lower education. Suicidal symptoms (ideation, plans or attempts) were evaluated following the methodology outlined in the Paykel Suicide Scale [48]. Psychopathological characteristics were assessed using a methodology similar to previous studies [42, 49, 50]. An ad hoc checklist based on DSM-5 BPD symptom criteria was used to screen for psychotic features during the episode [14]. These included the presence or absence of delusions, hallucinations, disorganized speech, and grossly disorganized or catatonic behavior. In addition, the presence or absence of first-rank schizophrenia symptoms was assessed using a symptom checklist that included: (a) thought withdrawal, insertion and interruption; (b) thought broadcasting; (c) hallucinatory voices giving a running commentary on the patient’s behavior, or discussing the patient among themselves; (d) somatic hallucinations; (e) feelings or actions experienced as made or influenced by external agents; and (f) delusional perception [51]. Finally, whether the content of psychotic experiences was related to the COVID-19 pandemic or not was also recorded.
Data analysis
Descriptive statistics were calculated to characterize the sample recruited for the study. Categorical variables were summarized using percentages and continuous variables by means or medians with their standard deviation (SD) or interquartile range (IQR) as appropriate. The normality of the distribution for continuous variables was assessed by the Shapiro–Wilk test. Missing cases were excluded from the data analysis. Statistical analyses were performed using SPSS Statistics (ver. 24, IBM Corp., Armonk, USA).
Results
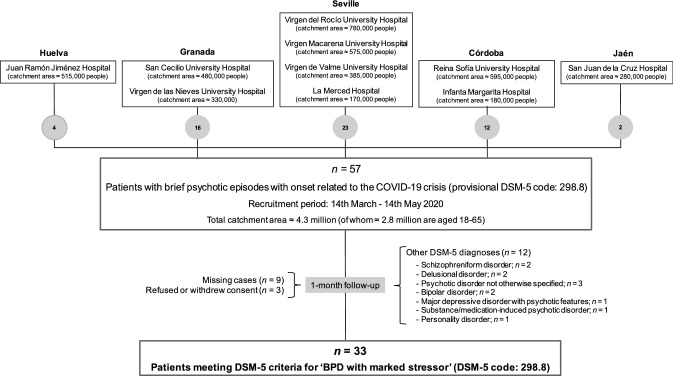
The figure shows the patient recruitment diagram. From March 14, 2020 to May 14, 2020, 57 patients were identified who presented non-affective psychotic episodes with a DUP shorter than 1 month (i.e., a provisional diagnosis of BPD) and whose onset was related to the coronavirus crisis. Of these cases, 33 completely remitted within one month and met the DSM-5 criteria for BPD with marked stressors (DSM-5 code: 298.8). The rest were excluded from the study based on the exclusion criteria described under Methods (Fig. 1). The final sample, therefore, consisted of 33 patients with brief reactive psychoses triggered by stress derived from the COVID-19 pandemic, which accounted for 57.9% of all coronavirus-related psychosis initially identified.
Fig. 1.
Patient recruitment diagram
Table 2 illustrates the sociodemographic, clinical and psychopathological characteristics of the sample (n = 33). The mean age was 42.33 years (SD ± 14.04, range 19–65), 54.5% (n = 18) were women and the majority (84.8%, n = 28) were European Caucasian. Other frequent sociodemographic factors were: married or with partner (57.6%, n = 19), living with others (84.8%, n = 28), completed secondary education (45.5%, n = 15) and employed (n = 16, 48.5%). The majority of the cohort (81.8%, n = 27) were assessed as having good premorbid psychosocial adjustment. More than half of the patients (57.6%, n = 19) had past psychiatric records (see Table 2 for further details) and 15.2% (n = 5) had a concurrent history of harmful or hazardous substance use. A first-degree family history of psychosis was positive in 21.2% (n = 7) of the sample. For the majority of the patients (63.6%, n = 21), it was their first episode of BPD, while for the remaining 36.4% (n = 12), it was a recurrent BPD episode. The onset of the psychotic symptoms was abrupt (< 48 h) in 42.4% of patients (n = 14), and the median DUP was 5 days (IQR 3.75–11.5). Suicidal symptoms were observed in 24.2% (n = 8) of the cases. Psychotic psychopathological features present included delusions and hallucinations in 84.8% (n = 28) and 42.4% (n = 14) of the sample, respectively, while disorganized speech was observed in 39.4% (n = 13), and 45.5% (n = 15) had grossly disorganized or catatonic behaviors. First-rank symptoms of schizophrenia were identified in 45.5% (n = 15) of the cohort, and in 57.6% (n = 19) of the cases the content of psychotic experiences was related to the coronavirus pandemic. None of the patients tested positive for COVID-19. Most of the sample (84.8%, n = 28) required hospitalization for control of symptoms. The median duration of the psychotic episode to clinical remission was 15 days (IQR 7.75–25.75).
Table 2.
Sociodemographic, clinical and psychopathological characteristics
| Total sample (n = 33) | |
|---|---|
| Sociodemographic characteristics | |
| Age (years), mean ± SD | 42.33 ± 14.04 |
| Gender (male), n (%) | 15 (45.5%) |
| Ethnicity (European Caucasian), n (%) | 28 (84.8%) |
| Marital status (unmarried), n (%) | 14 (42.4%) |
| Cohabitation (living alone), n (%) | 5 (15.2%) |
| Education, n (%) | |
| Higher education | 9 (27.3%) |
| Complete secondary education | 15 (45.5%) |
| Incomplete secondary education | 9 (27.3%) |
| Occupation (unemployed), n (%) | 15 (45.5%) |
| Clinical characteristics | |
| Premorbid psychosocial adjustment †, n (%) | |
| Good premorbid adjustment | 27 (81.8%) |
| Poor premorbid adjustment | 6 (18.2%) |
| Past psychiatric records, n (%) | |
| BPD | 12 (36.4%) |
| Depressive disorder | 3 (9.1%) |
| Anxiety disorder | 3 (9.1%) |
| Hoarding disorder | 1 (3%) |
| History of substance use, n (%) | 5 (15.2%) |
| Family history of psychosis, n (%) | 7 (21.2%) |
| First-episode psychosis, n (%) | 21 (63.6%) |
| Abrupt onset (< 48 h) of psychotic symptoms, n (%) | 14 (42.4%) |
| DUP (days), median (IQR) | 5 (3.75–11.5) |
| Suicidal symptoms, n (%) | 8 (24.2%) |
| COVID-19 screening results (negative), n (%) | 33 (100%) |
| Inpatient status, n (%) | 28 (84.8%) |
| Duration of the psychotic episode until clinical remission (days), median (IQR) | 15 (7.75–25.75) |
| Psychotic psychopathological features | |
| Delusions, n (%) | 28 (84.8%) |
| Hallucinations, n (%) | 14 (42.4%) |
| Disorganized speech, n (%) | 13 (39.4%) |
| Grossly disorganized or catatonic behavior, n (%) | 15 (45.5%) |
| First-rank symptoms of schizophrenia n (%) | 15 (45.5%) |
| Content of psychotic symptoms related to the COVID-19 pandemic, n (%) | 19 (57.6%) |
BPD brief psychotic disorder; IQR interquartile range; SD standard deviation; DUP duration of untreated psychosis
†The sum of the following sociodemographic variables was used as an indicator of poor premorbid adjustment: being unmarried, unemployed, and low education
Discussion
The aim of this study was to characterize the sociodemographic and clinical profile of patients diagnosed with brief reactive psychosis related to the current COVID-19 crisis during the first two months (March 14 to May 14, 2020) of the pandemic in Spain. During the period of study, 33 cases were identified as BPD triggered by the coronavirus outbreak. The cohort was characterized by having a relatively old mean age, almost the same gender distribution and good premorbid psychosocial functioning. Almost half of the patients had first-rank symptoms of schizophrenia and high prevalence of suicidal symptoms was observed during the acute phase of psychosis. Although there are several other case reports of short-lived psychotic episodes in the context of the coronavirus pandemic [9, 31–33, 36, 37], to the best of our knowledge, this is the first characterization study of patients with BPD associated with the fear and stress of the COVID-19 crisis and the largest cohort published to date.
The number of patients with brief reactive psychosis (n = 33; 21 first episodes and 12 recurrent BPD episodes) identified in our catchment area (4.3 million people, of whom 2.8 million were aged 18–65) during the study period (from March 14, 2020 to May 14, 2020), was higher than expected compared to other large-scale epidemiological studies on short-lived psychotic disorders [52, 53]. In these studies, carried out in Scotland [52] and Denmark [53] (both countries with a population size slightly larger than our catchment area), the epidemiology of ATPDs was roughly similar to our study even though the DSM-5 BPD diagnosis is a less frequent psychotic condition than ICD-10 ATPD, and of that, brief reactive psychosis represents about 50% of total BPDs [10, 23]. Moreover, it is worth mentioning that an alarming reduction in the number of patients attending Mental Health Services was observed during the initial stages of the pandemic [54, 55], and therefore, there may have been more cases in the population than actually detected. Contamination phobia, death anxiety, grief, social deprivation, economic stress, excessive media exposure and misinformation, stigma, changes in patterns of interpersonal interaction, and loss of cultural traditions and their personal meanings could be effects of the COVID-19 crisis predisposing prone individuals to a psychotic process [9, 56]. Therefore, we believe that the high psychological distress derived from the current COVID-19 pandemic and its socioeconomic consequences are profoundly impacting on the population's mental health and are leading to a heightened incidence of these brief stress-related psychotic episodes. In fact, preliminary evidence suggests that we are witnessing an increase in incident cases of psychosis both in the general population and in healthcare professionals working on the front line against COVID- 19 [24, 57].
The sociodemographic and clinical characteristics of our sample were generally congruent with previous literature, although some findings are worth a more detailed discussion [10, 23]. The mean age of our cohort (42.33 ± 14.04) was higher than in other studies on BPD [58–61]. However, it should be recalled that our study included both first-episode patients and those with recurrent BPD. Although short-lived psychotic episodes are more frequent in women [23], the nearly equal gender distribution (54.5% women and 45.5% men) in our sample was similar to other studies [61, 62]. The ethnicity of our cohort was 15.2% non European Caucasian. Along this line, although higher rates of brief psychotic episodes have been reported in migrant populations [23], percentages similar to ours have been published in other studies on short-lived psychotic disorders occurring in Spain [44, 63]. Most of our patients (81.8%) were rated as having had good premorbid adjustment at psychosis onset, which is common in brief psychotic episode cohorts and is considered a factor predicting good prognosis [22, 23]. About a fifth of the patients in the sample (21.2%) had family antecedents of psychosis, a proportion similar to previous studies [44, 58], which is associated with greater vulnerability to psychosis due to impaired tolerance to stress [10, 22, 23]. Harmful or hazardous substance use, i.e., alcohol, cannabis or other illicit drugs, by our patients was only 15.2%. This is lower than observed in FEP cohorts [64], but in agreement with what has been reported for short-lived psychotic disorders [65]. On the contrary, the proportion of participants with suicidal symptoms in our study (24.2%) was higher than traditionally reported in individuals with FEP [66] and similar to what is observed in subjects with schizophrenia [67], a high prevalence, which again, warns of risk of suicide by patients with brief psychotic episodes, especially in the acute phase of the disorder [65, 68, 69]. Finally, concerning psychopathological features, the rate of hallucinations (42.4%), grossly disorganized or catatonic behavior (45.5%) and first-rank symptoms of schizophrenia (45.5%) observed in our sample was substantially lower than reported by Marneros et al. in the ‘Halle study on brief and acute psychoses’ (HASBAP) [49]. However, proportions similar to ours have also been published [42].
It should be recalled that, in this study, we used the concept of brief reactive psychosis defined in the DSM-5 [14]. As explained in the Introduction, although this same concept of brief stress-related psychosis is also outlined in the ICD-10 [15], there are some important differences, especially with respect to temporal criteria. Thus, while in the DSM, the maximum duration of BPD with marked stressor(s) should be no longer than one month, ATPDs with associated acute stress in the ICD-10 may last up to 3 months if they do not have schizophrenic features [14, 15]. Another difference to consider is the temporal relationship between stressful life events and the onset of psychotic symptoms, since in the ICD-10, these must appear within 2 weeks of acute stressor exposure, whereas there is no such restrictive temporal criterion in the DSM. Nevertheless, in spite of these differences, there is close agreement between the two brief stress-related psychotic syndromes, which means that they can be considered analogous disorders [70].
Other characteristics which these short-lived psychotic episodes have in common are the high relapse rates (53–54%) and diagnostic transition (52–60%) to serious chronic mental disorders such as schizophrenia and bipolar disorder [12, 71]. Male gender, younger age at onset, poor premorbid psychosocial adjustment, non-abrupt onset of the psychotic symptoms, the presence of hallucinations, and Schneiderian first-rank symptoms, although not consistently replicated, seem to be factors predictive of poor prognosis [42, 44, 50, 52, 60, 72–74]. In addition, some studies have suggested that longer DUP, long hospitalizations, the need for high dosages of antipsychotics for symptom control, and the appearance of psychotic recurrences are variables that could be associated with an increased risk of developing persistent psychotic conditions during follow-up [72, 75–77]. Therefore, preventive approaches and long-term follow-ups are recommended for individuals with reactive psychosis, since almost half of BPD cases could be the first manifestation of a severe mental disorder [9]. In this regard, as the treatment requirements of individuals with BPD and ATPD are currently unmet in routine practice [78], and since their diagnosis and prognosis overlap with BLIPS and BIPS concepts [17], some authors suggest that this population should be included in CHR-P programs for preventive intervention instead of receiving standard care in general mental health services [79].
Methodological limitations, clinical implications, and future directions for research
The findings of our study should be interpreted in the light of certain limitations. First, limitations derived from the short recruitment period and the consequent small sample size. Second, the limitations of the descriptive nature of our research, which did not allow us to make any inferences about causality or verification of hypotheses. Third, as this study was conducted under routine clinical conditions, the lack of structured interviews for psychiatric diagnoses, and standardized psychometric tools for assessing the severity of psychotic symptoms were also a limitation. Despite these limitations, the study has relevant implications, as it warns clinicians of the possible increase in the incidence of reactive psychosis, and would serve as orientation for therapeutic management of these patients, both in the acute phase and during later follow-up. Future research should be directed at long-term international epidemiological studies examining the generalizability of our findings, and the impact that the COVID-19 pandemic has had on the incidence of psychosis and the course of these short-lived psychotic disorders over time.
Summarizing, we conclude that reactive psychoses derived from the psychosocial stress from the COVID-19 crisis could become a prevalent psychotic condition during the current pandemic. The clinical and sociodemographic profile of these individuals is similar to those reported in the literature and confirms once more the high risk of suicide associated with these short-lived psychotic episodes. Their high rate of recurrence and strong likelihood of transition to chronic psychotic disorders makes preventive approaches and long-term longitudinal follow-ups necessary in this population with brief reactive psychosis.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank all participants who agreed to participate in this study.
Author contributions
ÁL-D and MR-V devised the study concept and design. MJV-F, ÁL-D, and FJP-Z carried out data analysis and produced the figures and tables. MJV-F, ÁL-D, FJP-Z, and MR-V prepared the first draft of the manuscript, which was critically revised by LG-R and BC-F. All authors recruited patients, collected data, read the final manuscript, and approved it to be submitted for publication.
Funding
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declartions
Conflict of interest
The authors declare that there are no conflicts of interest in relation to the subject of this study.
Footnotes
María José Valdés-Florido and Álvaro López-Díaz Joint first authors.
References
- 1.World Health Organization Coronavirus disease (COVID-2019) situation reports. Situat Rep. 2020;1:207–210. [Google Scholar]
- 2.Kentikelenis A, Gabor D, Ortiz I, et al. Softening the blow of the pandemic: will the International Monetary Fund and World Bank make things worse? Lancet Glob Heal. 2020;8:e758–e759. doi: 10.1016/S2214-109X(20)30135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vieta E, Pérez V, Arango C. Psychiatry in the aftermath of COVID-19. Rev Psiquiatr Salud Ment. 2020;13:105–110. doi: 10.1016/j.rpsm.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torales J, O’Higgins M, Castaldelli-Maia JM, Ventriglio A. The outbreak of COVID-19 coronavirus and its impact on global mental health. Int J Soc Psychiatry. 2020;66:317–320. doi: 10.1177/0020764020915212. [DOI] [PubMed] [Google Scholar]
- 5.Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7:611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Losilla-Rodríguez B, Maldonado N, Moreno-Mellado E, López-Díaz A. COVID-19 natural herd immunity and risk of neuropsychiatric disorders. Rev Psiquiatr Salud Ment. 2020;13:228–229. doi: 10.1016/j.rpsm.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;2:1–8. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salazar de Pablo G, Vaquerizo-Serrano J, Catalan A, et al. Impact of coronavirus syndromes on physical and mental health of health care workers: Systematic review and meta-analysis. J Affect Disord. 2020;275:48–57. doi: 10.1016/j.jad.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valdés-Florido MJ, López-Díaz Á, Palermo-Zeballos FJ, et al. Reactive psychoses in the context of the COVID-19 pandemic: clinical perspectives from a case series. Rev Psiquiatr Salud Ment. 2020;13:90–94. doi: 10.1016/j.rpsm.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zielasek J, Gaebel W. Brief reactive psychoses. In: Malhi G, Bhugra D, editors. Troublesome disguises Managing challenging disorders in psychiatry. Wiley-Blackwell; 2015. pp. 27–43. [Google Scholar]
- 11.Opjordsmoen S. Reactive psychosis and other brief psychotic episodes. Curr Psychiatry Rep. 2001;3:338–341. doi: 10.1007/s11920-001-0031-0. [DOI] [PubMed] [Google Scholar]
- 12.Fusar-Poli P, Cappucciati M, Bonoldi I, et al. Prognosis of brief psychotic episodes. JAMA Psychiat. 2016;73:211–220. doi: 10.1001/jamapsychiatry.2015.2313. [DOI] [PubMed] [Google Scholar]
- 13.Castagnini AC, Galeazzi GM. Acute and transient psychoses: clinical and nosological issues. BJPsych Adv. 2016;22:292–300. doi: 10.1192/apt.bp.115.015198. [DOI] [Google Scholar]
- 14.American Psychiatric Association . Diagnostic and statistical manual of mental disorders, (DSM-V) American Psychiatric Press; 2013. [Google Scholar]
- 15.WHO . The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. World Health Organization; 1992. [Google Scholar]
- 16.WHO . The international classification of diseases 11th Revision. WHO; 2019. [Google Scholar]
- 17.Fusar-Poli P, Cappucciati M, De MA, et al. Diagnostic and prognostic significance of brief limited intermittent psychotic symptoms (BLIPS ) in individuals at ultra high risk. Schizophr Bull. 2017;43:48–56. doi: 10.1093/schbul/sbw151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fusar-Poli P, De Micheli A, Chalambrides M, et al. Unmet needs for treatment in 102 individuals with brief and limited intermittent psychotic symptoms (BLIPS): implications for current clinical recommendations. Epidemiol Psychiatr Sci. 2020;29:e67. doi: 10.1017/S2045796019000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fusar-Poli P, De Micheli A, Signorini L, et al. Real-world long-term outcomes in individuals at clinical risk for psychosis: The case for extending duration of care. EClinicalMedicine. 2020;28:100578. doi: 10.1016/j.eclinm.2020.100578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor SF, Grove TB, Ellingrod VL, Tso IF. The fragile brain: Stress vulnerability, negative affect and gabaergic neurocircuits in psychosis. Schizophr Bull. 2019;45:1170–1183. doi: 10.1093/schbul/sbz046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zwicker A, Denovan-Wright E, Uher R. Gene–environment interplay in the etiology of psychosis. Psychol Med. 2018;48:1925–1936. doi: 10.1017/S003329171700383X. [DOI] [PubMed] [Google Scholar]
- 22.Malhotra S, Sahoo S, Balachander S. Acute and transient psychotic disorders: newer understanding. Curr Psychiatry Rep. 2019;21:113. doi: 10.1007/s11920-019-1099-8. [DOI] [PubMed] [Google Scholar]
- 23.Castagnini AC, Fusar-Poli P. Diagnostic validity of ICD-10 acute and transient psychotic disorders and DSM-5 brief psychotic disorder. Eur Psychiatry. 2017;45:104–113. doi: 10.1016/j.eurpsy.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 24.Brown E, Gray R, Lo Monaco S, et al. The potential impact of COVID-19 on psychosis: a rapid review of contemporary epidemic and pandemic research. Schizophr Res. 2020;222:79–87. doi: 10.1016/j.schres.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Agostino A, D’Angelo S, Giordano B, et al. Brief Psychotic Disorder during the national lockdown in Italy: an emerging clinical phenomenon of the coronavirus pandemic. Schizophr Bull. 2020;47:15–22. doi: 10.1093/schbul/sbaa112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dinakaran D, Manjunatha N, Naveen Kumar C, Suresh BM. Neuropsychiatric aspects of COVID-19 pandemic: a selective review. Asian J Psychiatr. 2020;53:102188. doi: 10.1016/j.ajp.2020.102188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fierini F, Moretti D, Ballerini A. Psychosis spectrum disorders during and after the COVID-19 pandemic: warning signs of “stress incubation”. Psychiatry Res. 2020;291:113291. doi: 10.1016/j.psychres.2020.113291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernard-Valnet R, Pizzarotti B, Anichini A, et al. Two patients with acute meningoencephalitis concomitant with SARS-CoV-2 infection. Eur J Neurol. 2020;27:e43–e44. doi: 10.1111/ene.14298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rentero D, Juanes A, Losada CP, et al. New-onset psychosis in COVID-19 pandemic: a case series in Madrid. Psychiatry Res. 2020;290:113097. doi: 10.1016/j.psychres.2020.113097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weise J, Schomerus G, Speerforck S. The SARS-CoV-2 pandemic and an attempted suicide of a patient with delusional disorder. Psychiatr Prax. 2020;47:218–220. doi: 10.1055/a-1158-1745. [DOI] [PubMed] [Google Scholar]
- 31.Zulkifli NA, Sivapatham S, Guan NC. Brief psychotic disorder in relation to coronavirus, COVID-19 outbreaks: a case report. Malaysian J Psychiatry. 2020;29:1. [Google Scholar]
- 32.Colizzi M, Bortoletto R, Silvestri M, et al. Medically unexplained symptoms in the times of COVID-19 pandemic: a case-report. Brain, Behav Immun Heal. 2020;5:100073. doi: 10.1016/j.bbih.2020.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finatti F, Pigato G, Pavan C, et al. Psychosis in patients in COVID-19–related quarantine: a case series. Prim Care Companion CNS Disord. 2020;22:2010–2640. doi: 10.4088/PCC.20l02640. [DOI] [PubMed] [Google Scholar]
- 34.Fischer M, Coogan AN, Faltraco F, Thome J. COVID-19 paranoia in a patient suffering from schizophrenic psychosis - a case report. Psychiatry Res. 2020;288:113001. doi: 10.1016/j.psychres.2020.113001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu W, Su L, Qiao J, et al. COVID-19 outbreak increased risk of schizophrenia in aged adults. PsyChinaXiv. 2020;1:2–4. [Google Scholar]
- 36.Huarcaya-Victoria J, Herrera D, Castillo C. Psychosis in a patient with anxiety related to COVID-19: a case report. Psychiatry Res. 2020;289:113052. doi: 10.1016/j.psychres.2020.113052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin EB., Jr Brief psychotic disorder triggered by fear of coronavirus? Psychiatr Times. 2020;37:5. [Google Scholar]
- 38.Mehra A, Rani S, Sahoo S, et al. A crisis for elderly with mental disorders: relapse of symptoms due to heightened anxiety due to COVID-19. Asian J Psychiatr. 2020;51:102114. doi: 10.1016/j.ajp.2020.102114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ovejero S, Baca-García E, Barrigón ML. Coronovirus infection as a novel delusional topic. Schizophr Res. 2020;222:541–542. doi: 10.1016/j.schres.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:332–336. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 42.López-Díaz Á, Fernández-González JL, Lara I, et al. The prognostic role of catatonia, hallucinations, and symptoms of schizophrenia in acute and transient psychosis. Acta Psychiatr Scand. 2019;140:574–585. doi: 10.1111/acps.13092. [DOI] [PubMed] [Google Scholar]
- 43.van Os J, Takei N, Castle DJ, et al. Premorbid abnormalities in mania, schizomania, acute schizophrenia and chronic schizophrenia. Soc Psychiatry Psychiatr Epidemiol. 1995;30:274–278. doi: 10.1007/BF00805794. [DOI] [PubMed] [Google Scholar]
- 44.López-Díaz Á, Fernández-González JL, Lara I, Ruiz-Veguilla M. Predictors of diagnostic stability in acute and transient psychotic disorders: validation of previous findings and implications for ICD-11. Eur Arch Psychiatry Clin Neurosci. 2020;270:291–299. doi: 10.1007/s00406-019-01014-z. [DOI] [PubMed] [Google Scholar]
- 45.Singh SP, Cooper JE, Fisher HL, et al. Determining the chronology and components of psychosis onset: the Nottingham Onset Schedule (NOS) Schizophr Res. 2005;80:117–130. doi: 10.1016/j.schres.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 46.Silverman MM, Berman AL, Sanddal ND, et al. Rebuilding the Tower of Babel: a revised nomenclature for the study of suicide and suicidal behaviors part 2: suicide-related ideations, communications, and behaviors. Suicide Life-Threatening Behav. 2007;37:264–277. doi: 10.1521/suli.2007.37.3.264. [DOI] [PubMed] [Google Scholar]
- 47.Silverman MM, Berman AL, Sanddal ND, et al. Rebuilding the tower of babel: a revised nomenclature for the study of suicide and suicidal behaviors part 1: background, rationale, and methodology. Suicide Life-Threat Behav. 2007;37:248–263. doi: 10.1521/suli.2007.37.3.248. [DOI] [PubMed] [Google Scholar]
- 48.Paykel ES, Myers JK, Lindenthal JJ, Tanner J. Suicidal feelings in the general population: a prevalence study. Br J Psychiatry. 1974;124:460–469. doi: 10.1192/bjp.124.5.460. [DOI] [PubMed] [Google Scholar]
- 49.Marneros A, Pillmann F, Haring A, et al. Features of acute and transient psychotic disorders. Eur Arch Psychiatry Clin Neurosci. 2003;253:167–174. doi: 10.1007/s00406-003-0420-y. [DOI] [PubMed] [Google Scholar]
- 50.Rusaka M, Rancāns E. First-episode acute and transient psychotic disorder in Latvia: a 6-year follow-up study. Nord J Psychiatry. 2014;68:24–29. doi: 10.3109/08039488.2012.761726. [DOI] [PubMed] [Google Scholar]
- 51.Soares-Weiser K, Maayan N, Bergman H, et al. First rank symptoms for schizophrenia. Cochrane Database Syst Rev. 2015;1:CD010653. doi: 10.1002/14651858.CD010653.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Queirazza F, Semple DM, Lawrie SM. Transition to schizophrenia in acute and transient psychotic disorders. Br J Psychiatry. 2014;204:299–305. doi: 10.1192/bjp.bp.113.127340. [DOI] [PubMed] [Google Scholar]
- 53.Castagnini AC, Foldager L. Variations in incidence and age of onset of acute and transient psychotic disorders. Soc Psychiatry Psychiatr Epidemiol. 2013;48:1917–1922. doi: 10.1007/s00127-013-0726-7. [DOI] [PubMed] [Google Scholar]
- 54.Hoyer C, Ebert A, Szabo K, et al. Decreased utilization of mental health emergency service during the COVID-19 pandemic. Eur Arch Psychiatry Clin Neurosci. 2020;1:1–3. doi: 10.1007/s00406-020-01151-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kølbæk P, Kølbæk P, Nørremark B, et al. Forty percent reduction in referrals to psychiatric services during the COVID-19 pandemic. Psychother Psychosom. 2020;90:67–68. doi: 10.1159/000509575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chandra PS, Shiva L, Nagendrappa S, et al. COVID 19 related Psychosis as an interface of fears, socio-cultural issues and vulnerability- case report of two women from India. Psychiatry Res. 2020;290:113136. doi: 10.1016/j.psychres.2020.113136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Burgos-Berdud I, Valdés-Florido MJ, López-Díaz Á. Are healthcare workers during the COVID-19 pandemic at risk of psychosis? Findings from a scoping review. Gen Hosp Psychiatry J. 2020;29:1136. doi: 10.1016/j.genhosppsych.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ayesa-Arriola R, Rodríguez-Sánchez JM, Suero ES, et al. Diagnosis and neurocognitive profiles in first-episode non-affective psychosis patients. Eur Arch Psychiatry Clin Neurosci. 2016;266:619–628. doi: 10.1007/s00406-015-0667-0. [DOI] [PubMed] [Google Scholar]
- 59.Korver-Nieberg N, Quee PJ, Boos HB, et al. The validity of the DSM-IV diagnostic classification system of non-affective psychoses. Aust N Z J Psychiatry. 2011;45:1061–1068. doi: 10.3109/00048674.2011.620562. [DOI] [PubMed] [Google Scholar]
- 60.Lee EHM, Hui CLM, Chang WC, et al. Comparison of cognitive functions, pre-morbid conditions and clinical characteristics between brief psychotic disorder and schizophrenia. Psychol Med. 2016;46:2011–2013. doi: 10.1017/S0033291716000623. [DOI] [PubMed] [Google Scholar]
- 61.Lyne J, O’Donoghue B, Owens E, et al. Prevalence of item level negative symptoms in first episode psychosis diagnoses. Schizophr Res. 2012;135:128–133. doi: 10.1016/j.schres.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 62.Castagnini AC, Bertelsen A, Berrios GE. Incidence and diagnostic stability of ICD-10 acute and transient psychotic disorders. Compr Psychiatry. 2008;49:255–261. doi: 10.1016/j.comppsych.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 63.Pina-Camacho L, Parellada M, García-Prieto J, et al. Predictors of diagnosis of nonaffective acute remitting psychosis in patients with early-onset first episodes of psychosis. Eur Neuropsychopharmacol. 2012;22:S418–S419. doi: 10.1016/S0924-977X(12)70656-9. [DOI] [Google Scholar]
- 64.Wisdom J, Manuel J, Drake R. Substance use disorder among people with first-episode psychosis: a systematic review of course and treatment. Psychiatr Serv. 2011;62:1007–1012. doi: 10.1176/ps.62.9.pss6209_1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.López-Díaz Á, Lorenzo-Herrero P, Lara I, et al. Acute stress and substance use as predictors of suicidal behaviour in acute and transient psychotic disorders. Psychiatry Res. 2018;269:414–418. doi: 10.1016/j.psychres.2018.08.036. [DOI] [PubMed] [Google Scholar]
- 66.Challis S, Nielssen O, Harris A, Large M. Systematic meta-analysis of the risk factors for deliberate self-harm before and after treatment for first-episode psychosis. Acta Psychiatr Scand. 2013;127:442–454. doi: 10.1111/acps.12074. [DOI] [PubMed] [Google Scholar]
- 67.Lu L, Dong M, Zhang L, et al. Prevalence of suicide attempts in individuals with schizophrenia: a meta-analysis of observational studies. Epidemiol Psychiatr Sci. 2019;29:e39. doi: 10.1017/S2045796019000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pillmann F, Balzuweit S, Haring A, et al. Suicidal behavior in acute and transient psychotic disorders. Psychiatry Res. 2003;117:199–209. doi: 10.1016/S0165-1781(03)00023-4. [DOI] [PubMed] [Google Scholar]
- 69.Sher L. Brief psychotic disorder and suicidal behavior. Aust N Z J Psychiatry. 2015;49:578. doi: 10.1177/0004867415569804. [DOI] [PubMed] [Google Scholar]
- 70.Pillmann F, Haring A, Balzuweit S, et al. The concordance of ICD-10 acute and transient psychosis and DSM-IV brief psychotic disorder. Psychol Med. 2002;32:525–533. doi: 10.1017/S0033291702005408. [DOI] [PubMed] [Google Scholar]
- 71.Fusar-Poli P, Cappucciati M, Rutigliano G, et al. Diagnostic stability of ICD/DSM first episode psychosis diagnoses: meta-analysis. Schizophr Bull. 2016;42:1395–1406. doi: 10.1093/schbul/sbw020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Subramaniam M, Pek E, Verma S, et al. Diagnostic stability 2 years after treatment initiation in the early psychosis intervention programme in Singapore. Aust N Z J Psychiatry. 2007;41:495–500. doi: 10.1080/00048670701332276. [DOI] [PubMed] [Google Scholar]
- 73.Castagnini AC, Foldager L, Bertelsen A. Long-term stability of acute and transient psychotic disorders. Aust New Zeal J Psychiatry. 2013;47:59–64. doi: 10.1177/0004867412461692. [DOI] [PubMed] [Google Scholar]
- 74.Castagnini AC, Foldager L. Epidemiology, course and outcome of acute polymorphic psychotic disorder: Implications for ICD-11. Psychopathology. 2014;47:202–206. doi: 10.1159/000357784. [DOI] [PubMed] [Google Scholar]
- 75.Sajith SG, Chandrasekaran R, Sadanandan Unni KE, Sahai A. Acute polymorphic psychotic disorder: diagnostic stability over 3 years. Acta Psychiatr Scand. 2002;105:104–109. doi: 10.1034/j.1600-0447.2002.01080.x. [DOI] [PubMed] [Google Scholar]
- 76.Wang HY, Guo WJ, Li XJ, et al. Higher required dosage of antipsychotics to relieve the symptoms of first-onset acute and transient psychotic disorder (ATPD) predicted the subsequent diagnostic transition to schizophrenia: a longitudinal study. Schizophr Res. 2018;193:461–462. doi: 10.1016/j.schres.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 77.Poon JYK, Leung CM. Outcome of first-episode acute and transient psychotic disorder in Hong Kong Chinese: a 20-year retrospective follow-up study. Nord J Psychiatry. 2017;71:139–144. doi: 10.1080/08039488.2016.1252426. [DOI] [PubMed] [Google Scholar]
- 78.Minichino A, Rutigliano G, Merlino S, et al. Unmet needs in patients with brief psychotic disorders: too ill for clinical high risk services and not ill enough for first episode services. Eur Psychiatry. 2019;57:26–32. doi: 10.1016/j.eurpsy.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 79.Rutigliano G, Merlino S, Minichino A, et al. Long term outcomes of acute and transient psychotic disorders: The missed opportunity of preventive interventions. Eur Psychiatry. 2018;52:126–133. doi: 10.1016/j.eurpsy.2018.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.