Abstract
Posttraumatic Stress Disorder (PTSD) is a debilitating condition often associated with difficulty in emotion regulation, including reappraising negative emotions. This study assessed neural mechanisms associated with emotion regulation in veterans prior to and following treatment for PTSD. Participants with PTSD and combat exposed controls (CC) completed diagnostic evaluation and underwent fMRI scanning while completing Emotion Regulation Task (ERT) and Emotional Faces Assessment Task (EFAT). Participants with PTSD were randomly assigned to Prolonged Exposure plus placebo (PE+PLB), Sertraline plus enhanced medication management (SERT+EMM), or PE plus SERT (PE+SERT) and repeated diagnostic evaluation and MRI scanning following treatment. The amygdala, dmPFC, and dlPFC were examined as regions of interest. On ERT, veterans with PTSD showed significantly less dmPFC activation than CCs during reappraisal vs emotional maintenance. Within the PTSD group, results demonstrated a significant association between less activation in the dmPFC during emotion reappraisal vs maintenance trials before treatment and greater reductions in symptoms from pre- to post-treatment. During the EFAT, there were no group differences between participants with PTSD and CCs in brain activation, and no relationships between brain function and PTSD symptoms. These findings suggest that less emotional reactivity might potentially reflect less need for recruitment of prefrontal regions when reappraising negative emotion, and is an individual factor associated with better treatment outcome.
Keywords: Amygdala, Dorsomedial prefrontal cortex, Medial prefrontal cortex, Veterans, fMRI, Prolonged Exposure, SSRI
1. Introduction
PTSD is a debilitating disorder that can arise following exposure to a traumatic event characterized by symptoms of negative affect, re-experiencing the traumatic memory, hyperarousal, and avoidance of trauma reminders (American Psychiatric Association, 2013). Military veterans with combat exposure from Afghanistan and Iraq (Operation Enduring Freedom/Operation Iraqi Freedom/Operation New Dawn; OEF/OIF/OND) show prevalence rates of PTSD between 6–16% (Hines et al., 2014; Richardson et al., 2011). The VA/DOD Clinical Practice Guideline for PTSD (2017) includes several effective interventions including Prolonged Exposure therapy (PE) and Selective Serotonin Reuptake Inhibitors (SSRIs; including sertraline; Rauch et al., 2018).
Recent research on PTSD has sought to examine neural correlates of psychological processes associated with PTSD symptoms and treatment response. One consistent finding is that people with PTSD demonstrate exaggerated emotional reactivity compared to controls, indexed by greater activation in the amygdala (Garfinkel et al., 2014; Rauch et al., 2000; Rougemont-Bücking et al., 2011). In particular, symptoms such as hyperarousal and persistence of traumatic memory are thought to be mediated by hyperresponsivity in the amygdala (Girgenti et al., 2017). Indeed, greater PTSD symptoms (hyperarousal in particular) have been associated with greater activation in the amygdala while viewing negative (e.g. fearful, angry) compared to neutral faces (Lieberman et al., 2017; Stevens et al., 2013).
Diminished top-down control of the amygdala by medial prefrontal regions (mPFC) is thought to contribute to overactive emotional reactivity (Fitzgerald et al., 2017; Javanbakht et al., 2015), and difficulty inhibiting responses to trauma-related stimuli (Heroux et al., 2017). The regulatory relationship between prefrontal executive control regions and the amygdala has been of particular interest in understanding emotional reactivity and reappraisal in PTSD. Previous literature on neural activation during emotional reappraisal in healthy adults (Kompus et al., 2009) have demonstrated increased neural activation in brain regions associated with emotion regulation such as the dmPFC, dlPFC, and anterior cingulate cortex (ACC), and decreased activation in centers of emotional reactivity such as the amygdala, in veterans with and without PTSD (Fitzgerald et al., 2016). Behavioral treatment studies examining neural activation prior to and following PE have demonstrated relationships between greater symptom reduction following PE and greater prefrontal activation and less amygdala activation at baseline during emotion regulation tasks (Fonzo et al., 2017). Although prior treatment studies have demonstrated effects on brain function in regions associated with emotion processing and regulation, no studies to date have compared the neural impact of psychotherapy and pharmacotherapy, and their combination.
The literature examining emotion processing in individuals with PTSD suggests both overactive emotion reactivity to negative stimuli and difficulty regulating emotion, which have been linked to aberrant neural activation in regions involved in emotion processing (i.e. reactivity to emotional faces and regulation (Lieberman et al., 2017; Rauch et al., 2018). Moreover, people who experienced symptom reductions following PTSD treatment had less activation in emotion processing regions like amygdala and more activation in regulatory regions like PFC (Fonzo et al., 2017). Thus, in order to fully identify neural mechanisms associated with treatment response in PTSD, tasks that probe both emotion processing and emotion regulation are needed.
The present study used two well-established tasks to assess neural function associated with emotion processing and emotion regulation. We assessed neural function during emotion processing and regulation in participants with PTSD before and after assignment to evidence-based treatment groups [Prolonged Exposure plus placebo (PE+PLB), Sertraline plus enhanced medication management (SERT+EMM), or PE plus SERT (PE+SERT)]. Trauma-exposed (not meeting criteria for PTSD) combat controls (CC) were assessed at pre-treatment to serve as a comparison group. Using both the Emotion Regulation Task (ERT; Rabinak et al., 2014) and Emotional Face Assessment Task (EFAT; Hariri et al., 2002), we examined three primary questions. First, we examined differences in neural activation between PTSD and CC. We predicted that during emotion regulation on the ERT, participants with PTSD would show greater amygdala activation and less dmPFC and dlPFC activation during reappraisal vs. emotional maintenance than CC. Similarly, on the EFAT, we predicted that participants with PTSD would show hyperreactivity in the right and left amygdalae and hypoactivation in the mPFC during emotional compared to neutral faces.
Second, we examined relationships between pre-treatment neural activation and PTSD symptoms (both pre-treatment symptoms and change in symptoms from pre to post treatment) in participants with PTSD. We expected that more severe pre-treatment symptoms would be associated with reduced recruitment of regulatory regions (i.e. dmPFC and dlPFC) during cognitive reappraisal on the ERT and negative faces on EFAT. We hypothesized that greater differences in symptoms from pre to post treatment would be associated with greater recruitment of these regulatory regions during cognitive reappraisal on the ERT and negative faces on EFAT.
Third, we examined relationships between change in activation from pre to post treatment and PTSD symptoms from pre to post treatment in participants with PTSD. We hypothesized that symptom improvement would be associated with decreased amygdala activation and greater PFC activation during reappraisal for ERT and emotional faces on EFAT from pre to post treatment. Additional secondary aims for both tasks included task-based connectivity using a generalized psychophysiological interaction analysis (gPPI) to examine how connectivity between amygdala and PFC differed between PTSD and CC groups, and whether it was predictive of treatment response.
2. Method
2.1. Participants
The fMRI study was a part of a multisite randomized controlled treatment trial for PTSD, funded by the Department of Defense between 2011 and 2016 (PROlonGed ExpoSure Sertraline Trial [PROGrESS]; see Rauch et al., 2018, 2019, for further details on the larger study). fMRI data from 75 OEF/OIF/OND veterans (51 with PTSD and 24 CCs ranging in age from 20 to 57 years (Mean (M)PTSD= 33.07, standard deviation (SD)PTSD= 8.70; MCC= 35.73, SDCC= 8.32)) collected prior to treatment were included in these analyses. Due to attrition over the course of treatment and unusable data, data from 26 participants with PTSD (Mage = 34.46, SDage = 9.02) were retained from pre to post treatment and were included in the final analyses (See Tables 1 and 2 for task-based sample demographics). The Prolonged Exposure and Sertraline Trial (PROGrESS) is a randomized clinical trial approved by the institutional review boards at the Veterans Affairs Ann Arbor Healthcare System, University of Michigan Healthcare System, the Veterans Affairs San Diego Healthcare System, the Ralph H. Johnson Veterans Affairs Medical Center, and the Massachusetts General Hospital Home Base Veterans Program and the Department of Defense Human Research Protection Office.
Table 1.
ERT demographic data.
| Group | Time Point | N | Age (Mean) | Gender | PE + PLB | SERT/EMM | PE + SERT |
|---|---|---|---|---|---|---|---|
| PTSD | Pretreatment | 51 | 33.07 | 46M/5F | 19 | 9 | 20 |
| CC | 26 | 35.73 | 26M/0F | ||||
| PTSD | Pre - Post Treatment | 26 | 34.46 | 22M/4F | 12 | 3 | 11 |
Table 2.
EFAT demographic data.
| Group | Time Point | N | Age (Mean) | Gender | PE + PLB | SERT/EMM | PE + SERT |
|---|---|---|---|---|---|---|---|
| PTSD | Pretreatment | 46 | 32.69 | 39M/5F | 16 | 8 | 17 |
| CC | 25 | 35.22 | 25M/0F | ||||
| PTSD | Pre - Post Treatment | 23 | 32.68 | 20M/3F | 11 | 2 | 9 |
2.1.1. Inclusion/Exclusion criteria
OEF/OIF/OND Veterans with combat-related PTSD with significant impairment (CAPS ≥ 50) lasting at least three months were recruited. Veterans and active duty service members obtaining care at Veterans Affairs Ann Arbor Healthcare System at the University of Michigan, VA San Diego Healthcare System, Charleston VA Medical Center, and Massachusetts General Hospital were eligible for participation. Exclusion criteria included imminent risk of suicide, active psychosis, alcohol or substance dependence in the past 8 weeks, inability to attend appointments, prior intolerance or failure of adequate trial of PE or Sertraline (SERT; defined as at least 2 months of SERT at least 100 mg/day), medical illness likely to result in hospitalization or for which treatments are contraindicated, or cognitive impairment. Inclusion and exclusion criteria for CCs were the same as for the PTSD patient group. However, potential CC participants were excluded if they endorsed a history of PTSD symptoms (CAPS > 20), related to any type of trauma. fMRI specific exclusion criteria include: left-handedness, ferrous containing metals within the body (e.g., aneurysm clips, shrapnel/retained particles), inability to tolerate small, enclosed spaces, and patient girth exceeding fMRI machine dimensions.
2.2. Procedures
2.2.1. Clinical assessment
The Clinician Administered PTSD Scale for DSM-IV (CAPS-IV; Blake et al., 1995) was the primary outcome measure to assess PTSD symptoms pre-treatment (Week 0) and post-treatment (Week 24). The CAPS-IV was administered for all participants by treatment-blind, CAPS-trained, independent evaluators with at least Master’s level clinical training (See Fig. 1 for timeline of treatment administration; Rauch et al., 2018). In addition to assessing total symptom scores, scores were also collected for individual symptom clusters. Intrusion (Cluster B) symptoms included unwanted memories, nightmares, flashbacks and distress surrounding reminders of traumatic events. Avoidance (Cluster C) symptoms included avoidance of thoughts, feelings, or external reminders related to trauma. Negative alterations in cognitions or mood (Cluster D) include inability to remember features of trauma, negative thoughts about oneself, others, or the world, blame of self or others for the trauma, decreased interest in activities, and overall negative affect.
Fig. 1.
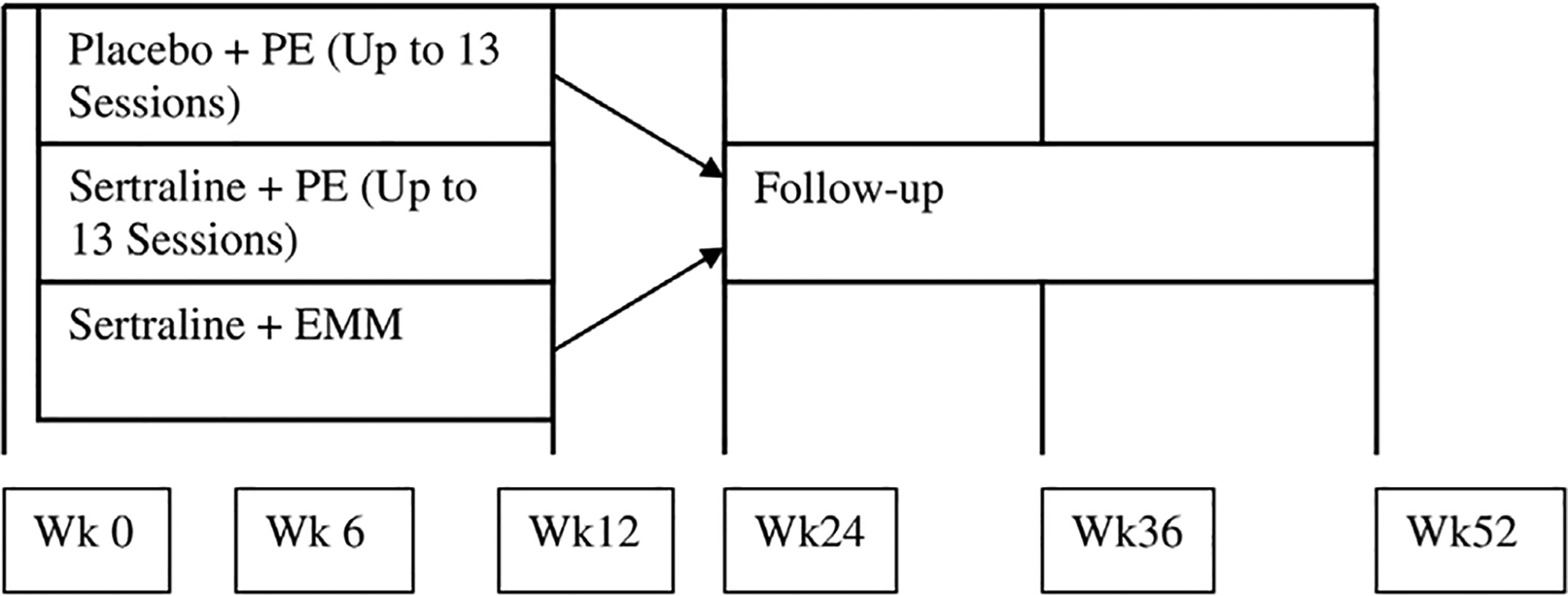
Timeline for administration of treatment in each treatment group (Rauch et al., 2018).
2.2.2. PTSD treatment
Participants with PTSD completed their randomly assigned treatment protocol over 24 weeks. Blinded clinical interviews were conducted prior to and following treatment. Participants received 1) Prolonged Exposure with a placebo pill (PE+PLB), 2) Sertraline with 30 min of Enhanced Medication Management (psychoeducation about PTSD and support to enhance medication compliance; see Rauch et al., 2018 for full details) for the first twelve weeks to balance time spent with a therapist/psychiatrist (SERT/EMM), or 3) both PE and SERT (PE +SERT; See Tables 1 and 2 for sample size of treatment groups by task). Data from the CC group were collected at baseline only. All participants received sertraline or a placebo pill in a double-blinded design. Participant attrition was similar across treatment groups, but contributed to unequal and underpowered treatment groups. Furthermore, in concert with results from the larger PROGrESS study (Rauch et al., 2019), we found no differences in treatment response between the three treatment arms, (F(1, 137)= 0.18, p = 0.68). Thus, fMRI analyses were not conducted on treatment group differences related to neural function during ERT and EFAT.
2.2.3. fMRI tasks
For additional details regarding the Emotion Regulation Task (ERT) and Emotional Face Assessment Task (EFAT), see (Rauch et al., 2018). Brief descriptions of the tasks are as follows.
2.2.3.1. Emotion regulation task.
Participants completed three runs of the ERT during fMRI scanning. The task consisted of 80 aversive and 80 neutral pictures from the International Affective Picture System (IAPS; Lang and Cuthbert, 1997) presented in block format (5 images over 20 s). Prior to each block, participants saw a crosshair, followed by an instruction (Look, Maintain, Reappraise; Fig. 2A). Maintain and Reappraise instructions appeared prior to blocks of negative images, while Look instructions appeared for neutral images. For “Maintain” blocks, participants were instructed to passively view images and experience emotions evoked by the images; for “Reappraise” blocks, participants were instructed to use the cognitive strategy of reframing negative images to decrease the intensity of their negative emotional responses (e.g., reinterpret a woman crying outside of a church as expressing tears of joy at a wedding). Participants also viewed neutral images and were asked to simply “Look” at the images. The key contrast of interest was between trials of reappraisal and emotional maintenance, although secondary analyses were also conducted on Reappraise vs Look and Maintain vs Look contrasts.
Fig. 2.
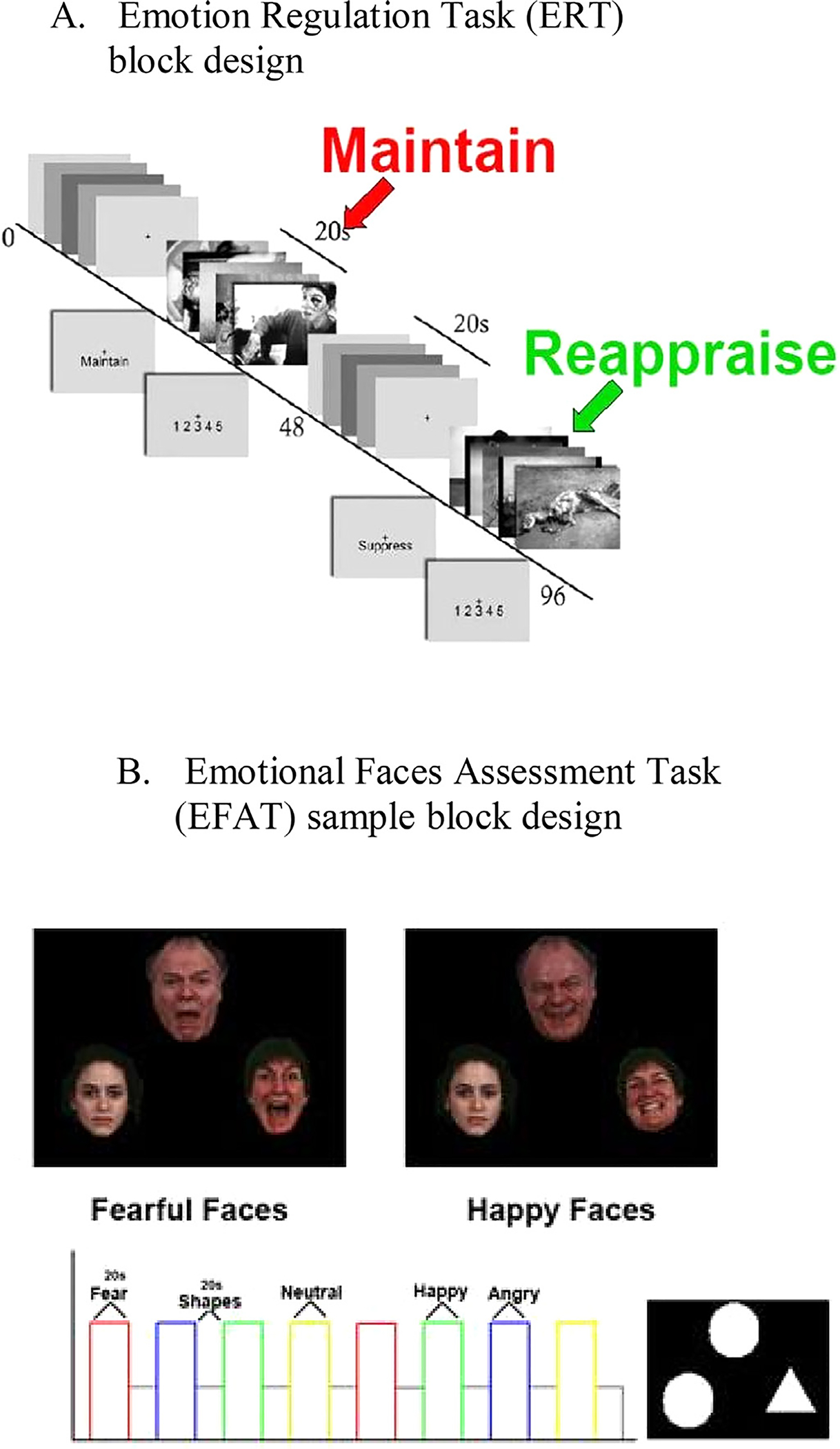
Task block designs of emotion regulation and appraisal tasks.
2.2.3.2. Emotional faces assessment task.
Participants completed two runs of the EFAT during fMRI scanning. Each run consisted of twelve blocks during which participants viewed a trio of faces and were instructed to match one of the two faces (bottom) that expressed the same emotion (e.g. angry, fearful, happy, neutral) as the target face (top; Fig. 2B). Participants also completed twelve baseline blocks during which they matched simple geometric shapes (e.g. circles, rectangles, triangles). The key contrast of interest for all analyses on this task was negative (e.g. fearful and angry face trials) compared to neutral faces.
2.2.4. MRI data collection
A Philips 3-T Achieva X-series MRI scanner (Philips Medical Systems, Andover, MA) with a SENSE 8 channel head coil was used for data collection at the Ann Arbor Veterans Affairs Hospital. The scanning parameters and analysis methods are consistent with those previously reported by our lab (Duval et al., 2018; Liberzon et al., 2015) and other fMRI aims in this study (to be reported elsewhere). A 3D FFE-TFE sequence (field of view (FOV)= 256 × 256 mm, slice thickness= 1 mm, 0 mm gap) was used to acquire T1-weighted anatomic images and slice localization, transformation, and co-registration were conducted using axial slices aligned with the AC-PC plane. EPI single shot sequence was used (EPI factor= 43, repetition time/echo time (TR/TE)= 2000/25 ms, flip angle= 90°, field of view (FOV)= 220 × 220 mm, slice thickness= 2.8 mm, 0 mm gap, 42 data points, 150 dynamic scan). Gradient echo blood oxygen level dependent (BOLD) scans were used to acquire functional data.
2.2.5. Data scoring and analysis
MRI data were analyzed using Statistical Parametric Mapping (SPM8) for MATLAB. Pre-processing of functional images included slice-time correction, realignment, and co-registration to structural images, normalization to the Montreal Neurological Institute (MNI) standard brain template and smoothing. Runs with greater than 3 mm of motion in any of the six planes (x, y, z, pitch, roll, yaw) were excluded from subsequent analyses.
2.2.6. Regions of interest
Based on prior literature using EFAT and ERT to examine regions involved in emotion processing and regulation in PTSD, we hypothesized that activation in dmPFC, dlPFC, and amygdala would be associated with performance on both EFAT and ERT (Javanbakht et al., 2015; Kim et al., 2013). In order to define regions of interest (ROIs) based on task-related activation in our sample, we first created contrasts (reappraise vs look on ERT, all faces vs all shapes on EFAT) to examine general task-related brain activation at pre-treatment, across all participants (PTSD and CC combined), independent of group membership, symptom severity, or treatment arm. The reappraise vs look contrast was used on the ERT, as we wanted to isolate regions involved in emotional reappraisal, while controlling for passive viewing of neutral images without emotional content or efforts to regulate (Fitzgerald et al., 2017). On the EFAT, all faces trials were expected to recruit the greatest activation in regions previously associated with emotion processing, and the shapes trials allowed us to establish a baseline for matching non-emotional stimuli (Klumpp et al., 2013). For all ROIs, we used the Montreal Neurological Institute (MNI) atlas to determine that our regions of activation fell within the hypothesized regions based on prior literature Fig. 3. Results revealed significant task-based activation in dmPFC and dlPFC on ERT, and in dmPFC and amygdala on EFAT. We then created 3 mm radius spheres around the activation peak for right and left amygdala, and 5 mm radius spheres for the mPFC and dlPFC. The average beta weight from each ROI was then extracted and submitted to analyses described below to examine group differences in brain function and relationships between brain function and treatment-related symptom change. Since we did not expect laterality effects (Blair et al., 2007; Kim et al., 2013), we averaged across both left and right sides to generate average beta weights in the three ROIs (amygdala, dmPFC, dlPFC).
Fig. 3.
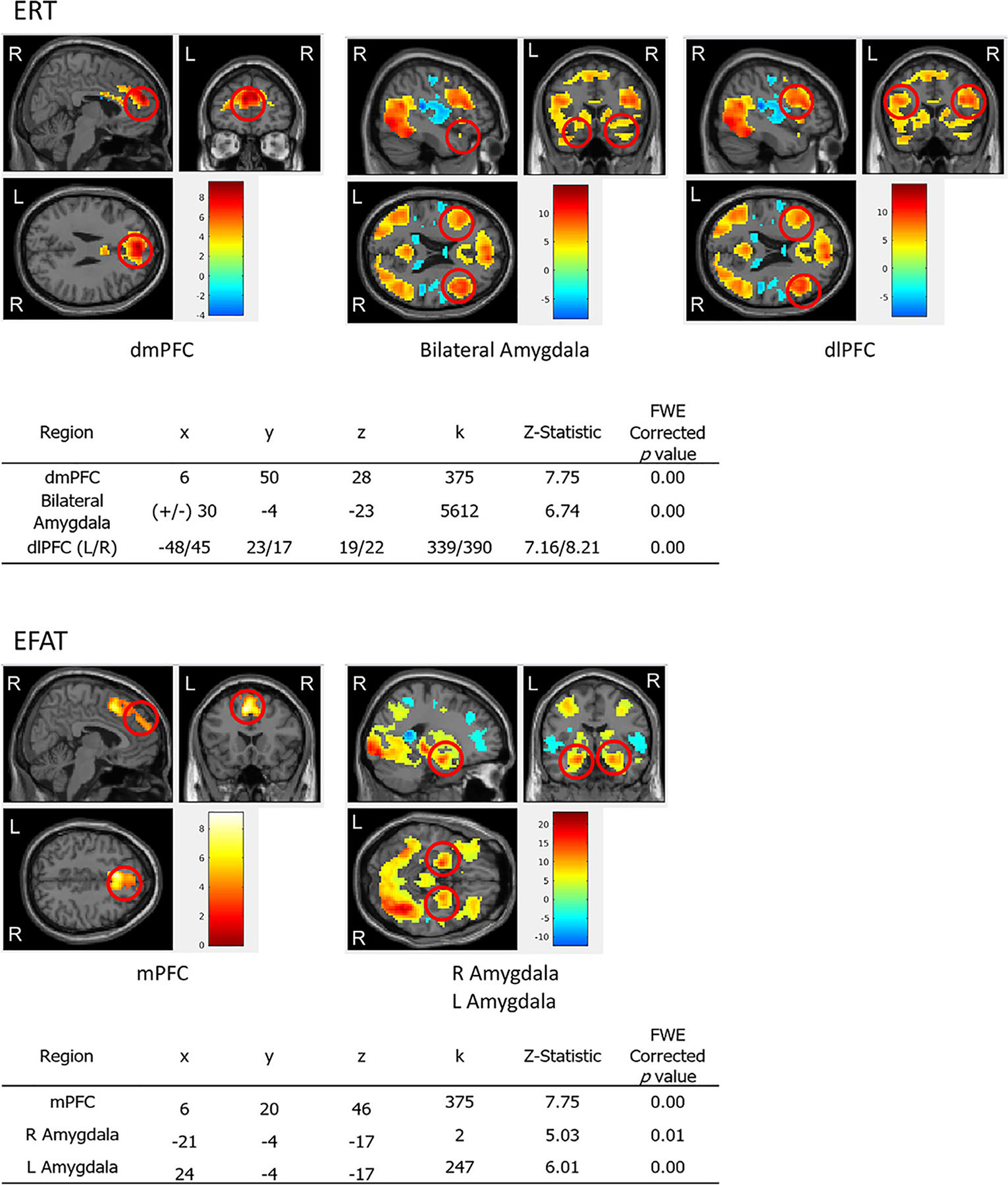
ROIs with coordinates (x, y, z), # of voxels (k), z statistics, and FWE corrected p-values for ERT and EFAT.
2.2.7. Statistical analyses
Primary analyses were performed in R (R Development Core Team, 2013; version 3.5.1) to examine 1) pre-treatment differences between PTSD and CC groups; 2) relationships between pre-treatment neural activation and PTSD symptoms prior to treatment and from pre to post treatment; and 3) relationships between change in activation from pre to post treatment and change in PTSD symptoms from pre to post treatment. Independent t-test analyses were conducted to examine pre-treatment group differences while forced entry linear regression models were used to examine pre-treatment brain function as predictors of total and subscale PTSD symptoms at pre and post treatment. Linear regressions were also used to examine change from pre to post treatment brain function as predictors of total and subscale PTSD symptoms from pre to post treatment. Secondary analyses utilized generalized psychophysiological interaction analysis (gPPI) in MATLAB using SPM 8, in order to assess relationships between neural connectivity during both the ERT and EFAT and PTSD symptom severity. Each of the ROIs from analyses of task-based activation were included as a seed for the gPPI analyses; connectivity between ROI seeds and all other voxels in the brain were examined in PTSD compared to CC groups and relationships between connectivity and symptom change over time were assessed. All analyses were FWE corrected at p < 0.05 at the whole brain level (after initial thresholding at p < 0.001 uncorrected).
3. Results
As expected, participants with PTSD scored significantly higher on the CAPS (MERT = 75.16, SDERT = 15.37; MEFAT= 73.96, SDEFAT= 15.59) compared to CC (MERT= 1.75, SDERT= 3.68; t(60.99) = −32.20, p < 0.001; MEFAT= 1.96, SDEFAT= 3.76; t(53.98) = −29.78, p < 0.001). Average age did not differ between PTSD and CC groups for either task (p > 0.05).
3.1. Emotion-regulation task (ERT)
3.1.1. Tests of normality
Shapiro-Wilks Tests of normality were conducted on variables of interest for analysis. Pre-treatment symptom scores (W = 0.83, p < 0.01) were not normally distributed; however, difference scores between pre-treatment and post-treatment symptoms (W = 0.99, p = 0.98) did follow a normal distribution. Beta weights extracted from dmPFC during Reappraise vs. Baseline (W = 0.99, p = 0.59) and Maintain vs. Baseline (W = 0.99, p = 0.77) followed a normal distribution. However, beta weights extracted from the bilateral amygdala did not follow a normal distribution for Reappraise vs. Baseline (W = 0.96, p = 0.01) or Maintain vs. Baseline (W = 0.96, p = 0.02). Although beta weights extracted from dlPFC during Maintain vs. Baseline were normally distributed (W = 0.98, p = 0.40), beta weights extracted for dlPFC during Reappraise vs. Baseline (W = 0.96, p = 0.03) were not normally distributed. Analyses were conducted using log transformed values for variables that were not normally distributed.
3.1.2. Pre-treatment group differences in activation
There was a marginally significant group difference in dmPFC activation with CC demonstrating greater activation for the Reappraise vs. Maintain contrast (M = 0.24, SD= 0.43) compared to participants with PTSD (M = 0.04, SD= 0.38; t(51.89) = 2.01, p = 0.05; Fig. 4). There were no significant group differences for other ROIs during Reappraise compared to Maintain trials.
Fig. 4.
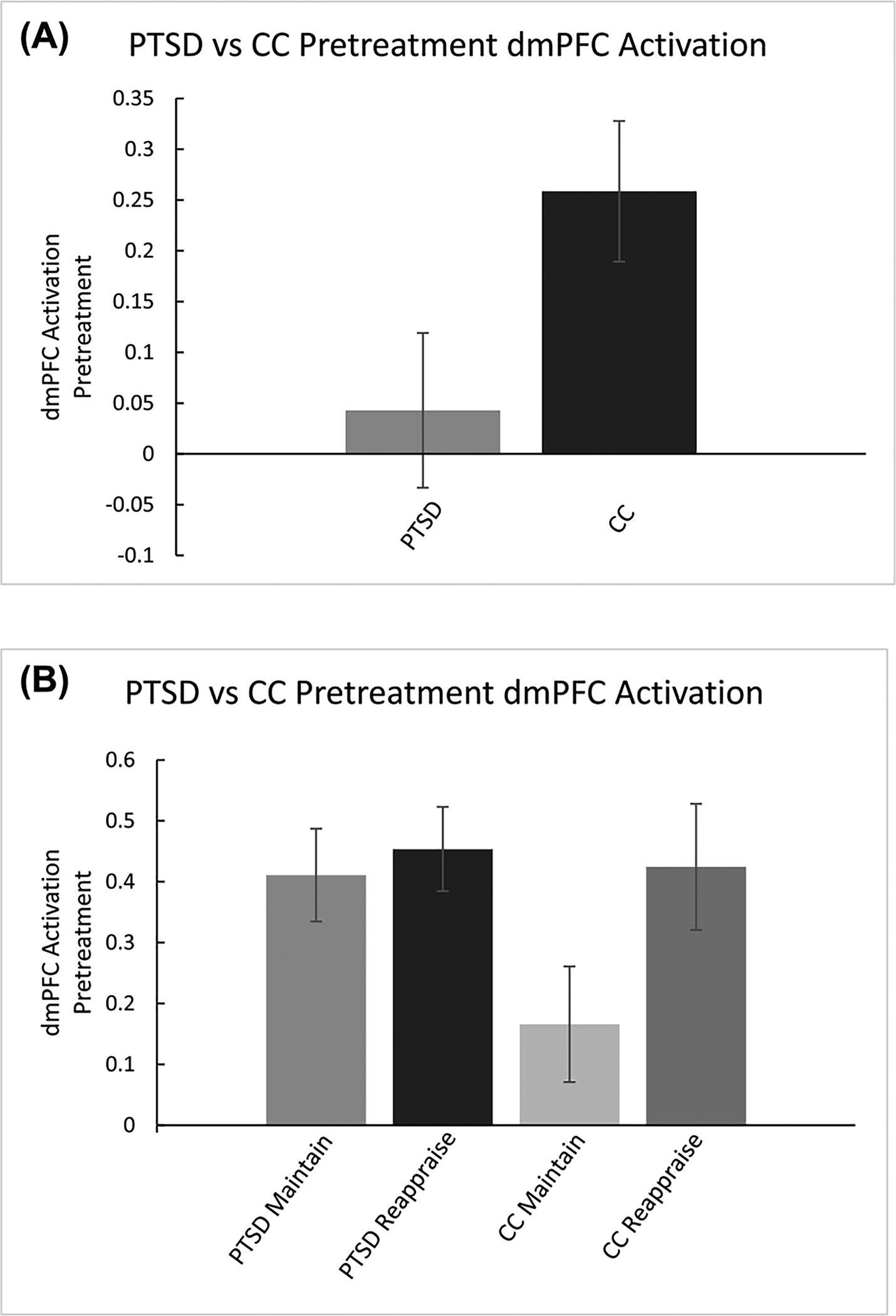
A. Pretreatment activation on Reappraise vs. Maintain trials was greater in the PTSD group compared to CC group. B. A further breakdown of average activation across Reappraise and Maintain trials for both PTSD and CC is provided to clarify direction of relationships. Error bars are based on standard error of the mean.
3.1.3. Pre-treatment neural activation associated with pre-treatment PTSD symptoms
A linear regression model revealed a trending relationship between pre-treatment neural activation in the ROIs (amygdala, mPFC, dlPFC) and pre-treatment PTSD symptoms during Reappraise vs. Maintain trials (F(3, 47)= 2.39, p = 0.08, R2= 0.08). DmPFC was a significant contributor to the model (β= −0.42, p = 0.01), suggesting that greater pre-treatment dmPFC activation for Reappraise compared to Maintain trials was associated with fewer pre-treatment PTSD symptoms.
3.1.4. Pre-Treatment neural activation associated with change in PTSD symptoms
We found a significant relationship between pre-treatment neural activation in the ROIs (amygdala, mPFC, dlPFC) during Reappraise vs. Maintain trials, and change in symptoms from pre to post treatment, even when pre-treatment symptoms were controlled for in the model (F (4, 35)= 3.22, p = 0.02, R2= 0.19) Fig. 5. Specifically, less pre-treatment dmPFC activation was associated with greater symptom change (β = −0.50 p = 0.002). Amygdala and dlPFC activations did not significantly contribute to the model individually (β= −0.22, p = 0.14; β= −0.02, p = 0.90; Fig. 4a–c). When this model was repeated with ROIs predicting CAPS symptom clusters, it was only significant with re-experiencing symptoms. This model revealed a significant main effect of pre-treatment activation on change in re-experiencing symptom scores from pre to post treatment (F(3, 29)= 3.85, p = 0.02, R2= 0.21). Individual predictors were examined further and revealed a significant relationship between dmPFC activation and change in cluster B (re-experiencing) symptom scores (β= −0.52, p = 0.02). There were no significant main effects of PTSD clusters C (avoidance) or D (negative alterations in cognition and mood), all p’s > 0.05.
Fig. 5.
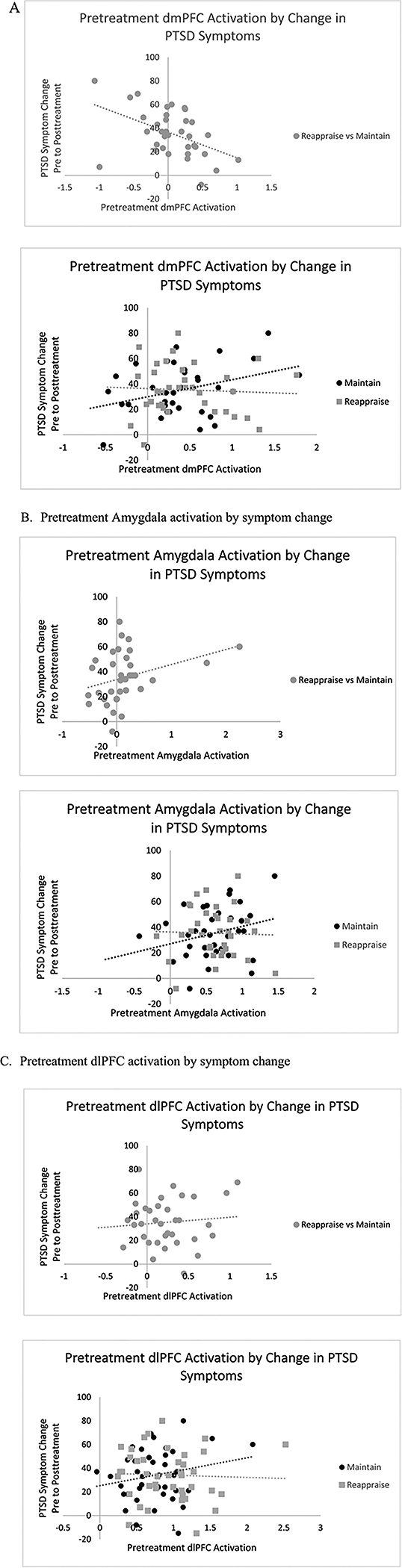
Less pretreatment neural activation for participants with PTSD in A. dmPFC, B. bilateral amygdala, and C. dlPFC was associated with greater symptom reduction over time. Reappraise vs. Maintain Contrast plotted against symptom change, with further breakdown of Reappraise and Maintain trials by symptom change to clarify direction of relationships.
3.1.5. Change in neural activation and change in PTSD symptoms
We found no significant relationships between change (defined as a difference between Pre-treatment activation-Post-treatment activation) in neural activation in the ROIs (amygdala, mPFC, dlPFC) for any contrasts and change (Pre-Post) in PTSD symptoms, all p’s > 0.05.
3.1.6. Neural connectivity differences between groups and PTSD symptoms
Results from gPPI analyses revealed no significant pretreatment differences in functional connectivity between PTSD and CC groups in the three seed regions from primary ERT analyses, all p’s > 0.05.
3.2. Emotional faces assessment task (EFAT)
There were no significant group differences in pre-treatment activation in primary ROIs for EFAT (amygdala, mPFC; all p’s > 0.05). Furthermore, there were no significant relationships between pre-treatment activation or change in neural activation and pre-treatment or change in PTSD symptoms in primary ROIs. In addition, results showed no significant relationships between neural activation and discrete symptom clusters in ROIs. Finally, results from gPPI analyses revealed no significant group differences in functional connectivity between the seed regions identified in primary analyses.
4. Discussion
We examined group differences in neural activation during emotion processing and regulation between people with PTSD and CCs. We also assessed relationships between neural activation and the change in PTSD symptoms before treatment and from pre- to post-treatment. ERT was used to assess brain function during emotional maintenance and reappraisal while EFAT was used to examine neural activation during processing of emotional faces. Somewhat surprisingly, our findings suggest that better treatment outcome is related to less recruitment of prefrontal regions during reappraisal of negative emotions (i.e. less activation in the dmPFC during cognitive reappraisal).
We expected that activation in ROIs associated with emotion processing and regulation (amygdala, mPFC, dlPFC) would be associated with PTSD symptoms both at pre-treatment and pre- to post-treatment. Based on prior literature (Fitzgerald et al., 2016; Kompus et al., 2009), we also hypothesized that we would observe differences in both emotion processing (amygdala) and regulation ROIs (dmPFC, dlPFC) during these tasks in participants with PTSD compared to CCs. Our hypotheses that there would be differences in activation between PTSD and CC groups in the amygdala and dlPFC were not supported, nor did this study provide evidence for relationships between change in activation in these regions and change in symptoms. However, present findings partially supported our hypotheses regarding relationships between pre-treatment activation in the dmPFC and symptom change from pre- to post-treatment in participants with PTSD.
Less dmPFC activation during reappraisal was associated with better treatment response in participants with PTSD in the current sample. These results are in contrast with previous literature suggesting that greater symptom reduction following treatment is associated with less activation in emotion processing regions like amygdala and more activation in regulatory regions like PFC (Fonzo et al., 2017; MacNamara et al., 2016).
One possible explanation of our findings is that less dmPFC activation was required to regulate emotional response in participants that went on to have better outcomes, and could be associated with less emotional reactivity and/or less effort to modulate emotions during reappraisal. Reduced prefrontal regulation during reappraisal of negative information prior to treatment will be associated with more successful treatment response. Notably, also in contrast to prior literature suggesting that greater prefrontal cortex activation was associated with less re-experiencing of emotional autobiographical memory (Denkova et al., 2015), less dmPFC activation was a significant predictor of reductions in re-experiencing symptoms (e.g. nightmares, flashbacks) in our sample. Our finding suggests that less prefrontal activation may be required, or less effort to regulate emotional responses may occur, in people who response better to treatment. Given that a significant model including less activation in the dmPFC, dlPFC, and amygdala predicted symptom reduction, it is possible that decreased emotional reactivity may also contribute to decreased need for prefrontal regulation. Furthermore, congruent patterns of activation between the dmPFC and amygdala during maintenance of emotion mirror previous findings suggesting that greater coupling between these regions may be required for sustained emotional states, like anxiety (Andreatta et al., 2015; Vytal et al., 2014).
The dlPFC has previously been implicated in emotion regulation models and has demonstrated greater activation in participants with PTSD undergoing cognitive reappraisal tasks (Sheynin and Liberzon, 2017). Although clinical populations (e.g. participants with anxiety, PTSD) have exhibited reduced activation in the dlPFC during cognitive reappraisal (Kim et al., 2013; Pico-Perez et al., 2017; Zilverstand et al., 2016), some literature has suggested that the process of reappraisal reflects mind wandering states or internal self-referential states typically reflective of neural function at rest (Deak et al., 2017). Therefore, it is possible that the ERT does not specifically probe dlPFC activation, which is typically associated with external, task-based thought (Aupperle et al., 2012).
Analyses of brain function during EFAT did not yield significant differences between PTSD and CC groups, nor significant correlations between neural activation and symptom change from pre- to post-treatment. It is possible that, at least in our sample, processes of emotion regulation assessed on the ERT are more sensitive in predicting treatment response than more emotion-processing focused tasks like EFAT.
Several limitations of this study should be considered when interpreting our results. First, statistical power was reduced due to participant dropout and therefore, results were at a risk of false negatives. Furthermore, the CC group did not return for a follow-up MRI scan, as the primary focus of this study was to examine predictors of treatment outcome in PTSD. However, due to this design decision, we cannot rule out the possibility that changes from pre- to post-treatment were due to practice/habituation effects rather than treatment effects. Future studies should include “pre” and “post” scans in both PTSD and control groups. We were underpowered to detect differences in neural function between the three treatment modalities. Thus, we are not able to determine whether different treatments (PE vs SERT vs. the combination) impact neural function differently. Future studies with larger samples should continue to investigate treatment specific changes in neural function from pre- to post-treatment. On the ERT, due to the block design of the task, it is possible that the BOLD signal did not adequately capture the renewed process of reappraisal for each trial, and future studies may need to use event related designs and/or vary trial length to fully capture neural activation during reappraisal. Furthermore, although previous research has demonstrated the utility of EFAT in eliciting amygdala activation, this task may not have enough sensitivity to elicit specific neural differences in prefrontal regions. Future research using this task would also benefit from an implicit baseline (e.g. crosshair), in addition to the baseline of shape trials.
Although previous research has examined associations between treatment outcomes for PTSD and neural function underlying emotion processing and regulation, this is one of the first studies to examine the relationship between treatment response and brain function associated with emotion regulation and appraisal in veterans with and without PTSD. Overall, our findings suggest that less activation in dmPFC might reflect less need for recruitment of prefrontal regions when reappraising negative emotion, and is an individual factor associated with better treatment response.
Acknowledgments
We would like to thank all members of the PROGrESS study team, especially Murray Stein, MD, MPH for assistance with project development and execution, Margaret Venners, MPH, MSW for project management, Nita Patel BS, for MRI scanning, Sean Ma, PhD for data collection and organization, Mike Angstadt, MS, for MRI analysis consultation and support, and Mariam Reda BS, for assistance with imaging processing. We thank all of the veterans who participated in this study. Symposium presented at the Anxiety and Depression Association of America Annual Meeting, Chicago, IL (2019). Poster presented at the Association of Psychological Sciences Annual Meeting, Washington, DC (2019).
This work was supported by the U.S. Department of Defense through the U.S. Army Medical Research and Materiel Command (MRMC; Randomized Controlled Trial of Sertraline, Prolonged Exposure Therapy, and Their Combination in OEF/OIF Combat Veterans with PTSD; Grant #W81XWH-11-1-0073); the National Center for Advancing Translational Sciences of the National Institutes of Health (Grant #UL1TR000433). This material is also the result of work supported with resources and the use of facilities at Massachusetts General Hospital, the VA Ann Arbor Healthcare System, Ralph H. Johnson VA Medical Center, and VA San Diego Healthcare System.
Declaration of Competing Interest
Dr. Duval reports in past 24 months funding from NIH, the Michigan Institute for Clinical Health Research, Cohen Veterans Bioscience, and One Mind Institute. Dr. King reports in past 24 months funding from NIH and the Michigan Institute for Clinica Health Research. Dr. Liberzon reports in past 24 months speaking or consulting with: Department of Defense, NIH, VA, Cohen Veteran Bioscience, ARMGO Pharma Inc., Sunovion Pharmaceuticals Inc. Aptinyx Inc., Heptares Therapeutics Ltd., Nobilis Therapeutics, Inc., Astrotide, Inc. Dr. Rauch reports in past 24 months speaking or consulting with Department of Defense, NIH, VA, Woodruff Foundation, McCormick Foundation, Wounded Warrior Project, Cohen Veteran Bioscience, and The Pennsylvania State University. Dr. Rauch receives royalties from Oxford University Press. Ms. Joshi and Drs. Sheynin, Phan, Martis, and Porter report no disclosures. The views expressed in this article are solely those of the authors and do not reflect an endorsement by or the official policy of the Department of Veterans Affairs, or the U.S. Government.
Footnotes
References
- American Psychiatric Association. (2013). In Diagnostic and statistical manual of mental disorders (5th ed.). Trauma-and Stressor-Related Disorders; 10.1176/appi.books.9780890425596.dsm07. [DOI] [Google Scholar]
- Andreatta M, Glotzbach-Schoon E, Muhlberger A, Schulz SM, Wiemer J, Pauli P, 2015. Initial and sustained brain responses to contextual conditioned anxiety in humans. Cortex 63, 352–363. 10.1016/j.cortex.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Aupperle RL, Allard CB, Grimes EM, et al. , 2012. Dorsolateral prefrontal cortex activation during emotional anticipation and neuropsychological performance in posttraumatic stress disorder. Arch. Gen. Psychiatry 69 (4), 360–371. 10.1001/archgenpsychiatry.2011.1539. [DOI] [PubMed] [Google Scholar]
- Blair KS, Smith BW, Mitchell DG, Morton J, Vythilingam M, Pessoa L, … Blair RJ, 2007. Modulation of emotion by cognition and cognition by emotion. Neuroimage 35 (1), 430–440. 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM, 1995. The development of a clinician-administered PTSD scale. J. Trauma Stress 8, 75–90. 10.1002/jts.2490080106. [DOI] [PubMed] [Google Scholar]
- Deak A, Bodrogi B, Biro B, Perlaki G, Orsi G, Bereczkei T, 2017. Machiavellian emotion regulation in a cognitive reappraisal task: an fMRI study. Cogn. Affect. Behav. Neurosci 17, 528. 10.3758/s13415-016-0495-3. [DOI] [PubMed] [Google Scholar]
- Denkova E, Dolcos S, Dolcos F, 2015. Neural correlates of “distracting” from emotion during autobiographical recollection. Soc. Cogn. Affect. Neurosci 219–230. 10.1093/scan/nsu039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval ER, Joshi SA, Block SR, Abelson JL, Liberzon I, 2018. Insula activation is modulated by attention shifting in social anxiety disorder. J. Anxiety Disord 56, 56–62. 10.1016/j.janxdis.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JM, MacNamara A, Kennedy AE, Rabinak CA, Rauch SA, Liberzon I, Phan KL, 2016. Individual differences in cognitive reappraisal use and emotion regulatory brain function in combat-exposed veterans with and without PTSD. Depress. Anxiety 34 (1), 79–88. 10.1002/da.22551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JM, Phan KL, Kennedy AE, Shankman SA, Langenecker SA, Klumpp H, 2017. Prefrontal and amygdala engagement during emotional reactivity and regulation in generalized anxiety disorder. J. Affect. Disord 218, 398–406. 10.1016/j.jad.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo GA, Goodkind MS, Oathes DJ, Zaiko YV, Harvey M, Peng KK, Weiss ME, Thompson AL, Zack SE, Lindley SE, Arnow BA, Jo B, Gross JJ, Rothbaum BO, Etkin A, 2017. PTSD psychotherapy outcome predicted by brain activation during emotional reactivity and regulation. Am.n J. Psychiatry 174 (12), 1163–1174. 10.1176/appi.ajp.2017.16091072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel SN, Abelson JL, King AP, Sripada RK, Wang X, Gaines LM, Liberzon I, 2014. Impaired contextual modulation of memories in PTSD: an fMRI and psychophysiological study of extinction retention and fear renewal. J. Neurosci 34 (40), 13435–13443. 10.1523/JNEUROSCI.4287-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgenti MJ, Hare BD, Ghosal S, Duman RS, 2017. Molecular and cellular effects of traumatic stress: implications for PTSD. Curr. Psychiatry Rep 19, 85. 10.1007/s11920-017-0841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR, 2002. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage 17, 317–323. 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Heroux NA, Robinson-Drummer PA, Sanders HR, Rosen JB, Stanton ME, 2017. Differential involvement of the medial prefrontal cortex across variants of contextual fear conditioning. Learn. Mem 24 (8), 322–330. 10.1101/lm.045286.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines LA, Sundin J, Rona RJ, Wessely S, Fear NT, 2014. Posttraumatic stress disorder post Iraq and Afghanistan: prevalence among military subgroups. Can. J. Psychiatry 59 (9), 468–479. 10.1177/070674371405900903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javanbakht A, King AP, Evans GW, Swain JE, Angstadt M, Phan KL, Liberzon I, 2015. Childhood poverty predicts adult amygdala and frontal activity and connectivity in response to emotional faces. Front. Neurosci 9 (154). 10.3389/fnbeh.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Evans GW, Angstadt M, Ho SS, Sripada CS, Swain JE, … Phan KL, 2013. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. PNAS 110 (46), 18442–18447. 10.1073/pnas.1308240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Post D, Angstadt M, Fitzgerald DA, Phan KL, 2013. Anterior cingulate cortex and insula response during indirect and direct processing of emotional faces in generalized social anxiety disorder. Biol. Mood Anx. Disord 3, 7. 10.1186/2045-5380-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kompus K, Hugdahl K, Öhman A, Marklund P, Nyberg L, 2009. Distinct control networks for cognition and emotion in the prefrontal cortex. Neurosci. Lett 467 (2), 76–80. 10.1016/j.neulet.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Lang MM, Cuthbert BN, 1997. International Affective Picture System (IAPS): Technical Manual and Affective Ratings the University of Florida. The Center for Research in Psychophysiology, Gainesville. [Google Scholar]
- Liberzon I, Ma ST, Okada G, Ho SS, Swain JE, Evans GW, 2015. Childhood poverty and recruitment of adult emotion regulatory neurocircuitry. Soc. Cogn. Affect. Neurosci 10 (11), 1596–1606. 10.1093/scan/nsv045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman L, Gorka SM, DiGangi JA, Frederick A, Phan KL, 2017. Impact of posttraumatic stress symptom dimensions on amygdala reactivity to emotional faces. Prog. Neuropsychopharmacol. Biol. Psychiatry 79, 401–407. 10.1016/j.pnpbp.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A, Rabinak CA, Kennedy AE, Fitzgerald DA, Liberzon I, Stein MB, Phan KL, 2016. Emotion regulatory brain function and SSRI treatment in PTSD: neural correlates and predictors of change. Neuropsychopharmacology 41 (2), 611–618. 10.1038/npp.2015.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pico-Perez M, Radua J, Steward T, Menchon JM, Soriano-Mas C, 2017. Emotion regulation in mood and anxiety disorders: a meta-analysis of fMRI cognitive reappraisal studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 79, 96–104. 10.1016/j.pnpbp.2017.06.001. [DOI] [PubMed] [Google Scholar]
- R Core Team (2013). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/. [Google Scholar]
- Rabinak CA, MacNamara A, Kennedy AE, Angstadt M, Stein MB, Liberzon I, Phan KL, 2014. Focal and aberrant prefrontal engagement during emotion regulation in veterans with posttraumatic stress disorder. Depress Anxiety 31 (10), 851–861. 10.1002/da.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK, 2000. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol. Psychiatry 47 (9), 769–776. 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Rauch SAM, Simon NM, Kim HM, Acierno R, King AP, Norman SB, Venners MR, Porter K, Phan KL, Tuerk PW, 2018. Integrating biological treatment mechanisms into randomized clinical trials: design of progress (PROlonGed exposure and sertraline trial). Contemp. Clin. Trials 64, 128–138. 10.1016/j.cct.2017.10.013. [DOI] [PubMed] [Google Scholar]
- Rauch SAM, Kim HM, Powell C, … , Hoge CW, 2019. Efficacy of prolonged exposure therapy, sertraline hydrochloride, and their combination among combat veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry 76 (2), 117–126. 10.1001/jamapsychiatry.2018.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson LK, Frueh BC, Acierno R, 2011. Prevalence estimates of combat-related PTSD: a critical review. Aust. NZJ Psychiatry 44 (1), 4–19. 10.3109/00048670903393597.Prevalence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougemont-Bücking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J, Rauch SL, Pitman RK, Milad MR, 2011. Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS Neurosci. Ther 17 (4), 227–236. 10.1111/j.1755-5949.2010.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheynin J, Liberzon I, 2017. Circuit dysregulation and circuit-based treatments in posttraumatic stress disorder. Neurosci. Lett 649, 133–138. 10.1016/j.neulet.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Jovanovic T, Fani N, et al. , 2013. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J. Psychiatr. Res 47 (10), 1469–1478. 10.1016/j.jpsychires.2013.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vytal KE, Overstreet C, Charney DR, Robinson OJ, Grillon C, 2014. Sustained anxiety increases amygdala-dorsomedial prefrontal coupling: a mechanism for maintaining an anxious state in healthy adults. JPN 39 (5), 321–329. 10.1503/jpn.130145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilverstand A, Parvaz MA, Goldstein RZ, 2016. Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review. Neuroimage. 10.1016/j.neuroimage.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


