Abstract
We evaluated 79 patients with multiple myeloma (MM) ≥ 70 years referred to our blood and marrow transplant clinic, within 1 year of diagnosis from 2010–19, for consideration of autologous stem cell transplant (ASCT). Thirty-eight (48%) of 79 patients underwent ASCT. ASCT was not pursued in 41 (52%) patients due to: patient or physician preference in 80% (n=33) or ineligibility in 20% (n=8). Baseline characteristics of patients in the 2 groups were similar. Median PFS from treatment start amongst patients undergoing ASCT (n=38) vs. not (n=41) was 41 months vs. 33 months, p=0.03. There was no difference in OS, with estimated 5-year OS of 73% vs. 83%, respectively (p=0.86). Day +100 transplant related mortality was 0%. ASCT was an independent favorable prognostic factor for PFS in multivariate analysis, after accounting for HCT-CI score, performance status, hematologic response and maintenance. Finally, patients ≥ 70 years undergoing ASCT had similar PFS compared to a contemporaneous institutional cohort of patients<70 years (n=631) (median PFS from transplant: 36 vs. 47 months, p=0.25). In this retrospective analysis, ASCT was associated with low TRM and better PFS in fit older adults with MM compared to non-transplant therapy, with comparable benefits as seen in younger patients.
Keywords: multiple myeloma, elderly, stem cell transplant, 70 years
Introduction
High-dose chemotherapy followed by autologous stem cell transplantation (ASCT) has been considered the standard of care first-line therapy for eligible multiple myeloma (MM) patients for more than three decades1–4. Multiple myeloma is mainly diagnosed in older patients with a median age at diagnosis of 70 years old1. With an expected MM incidence rising by 57% from 2010 to 20302, the number of older patients being considered for ASCT will continue to increase. Unfortunately, most randomized studies evaluating ASCT in MM only included patients <65 years old3, 4. However, retrospective studies have shown that patients over 65 years old who undergo ASCT have good survival outcomes with acceptable toxicities5–18. Patient selection remains one of the crucial aspects of ASCT.
The main advantage of transplant in the era of novel agents is improved progression-free survival (PFS)19, 20. There has been no prospective randomized study amongst patients ≥ 70 years old with MM comparing ASCT to non-ASCT approaches. The objective of this single-center retrospective study was to compare the safety and efficacy of ASCT to non-ASCT treatment in patients ≥ 70 years old with MM.
Methods
Study design
We conducted a single center, retrospective study examining outcomes of ASCT versus non-ASCT based treatment in patients with MM who were ≥ 70 years old. Patients who were ≥ 70 years old, with a diagnosis of multiple myeloma based on the International Myeloma Working Group consensus criteria21 and referred within 1 year after their diagnosis for ASCT for a transplant consult at Stanford University Medical Center between January 1, 2010, and November 1, 2019 were included in this study. We excluded patients who received a second or a tandem autologous transplantation. Patients who underwent transplant were treated with a high dose melphalan (140–200 mg/m2) +/− BCNU (300–350 mg/m2) preparative regimen. ASCT was primarily done as an outpatient transplant per institutional guidelines. This study was approved by the institutional review board.
High-risk cytogenetics was defined by detection of t(4;14), t(14:16), t(14:20), del 17p or amplification 1q by fluorescence in situ hybridization (FISH). Treatment response at time of transplantation was evaluated based on the International uniform response criteria for multiple myeloma22. The International Staging System (ISS)23 for multiple myeloma prognosis and the Hematopoietic cell transplantation comorbidity index (HCT-CI)24, 25 were calculated for each patient with the available data. Patients were divided into three HCT-CI risk groups: low (score 0), intermediate (score 1–2) and high risk (score ≥ 3). Reasons not to undergo transplant were either transplant ineligibility, patient choice or physician recommendation despite patient being deemed eligible. Time to next treatment (TTNT) was defined as the time from start of treatment to start of next treatment or death, whichever occurred earlier. Progression-free survival was defined as the time from start of treatment to disease progression or death, regardless of the cause of death. Overall survival (OS) was defined as the time from start of treatment to death from any cause. Data for both OS and PFS were censored at the time of last follow-up in patients who had not experienced events. We defined transplant related mortality (TRM) as death from any cause related to the transplant.
Finally, progression free survival outcomes from transplant of patients 70 years or above undergoing ASCT were compared to a contemporaneous cohort of patients <70 years (n=631) who underwent ASCT for myeloma at our institution from 2010–19. Inclusion criteria for selecting this cohort of younger patients were similar to that described above and patients were within one year of myeloma diagnosis.
Statistical Analysis
Statistical analyses were run using the JMP pro 14 software. Univariate analysis was done using Chi-Square and Fischer’s exact tests for categorical variables and Wilcoxon Rank Sum/Kruskal Wallis test for continuous variables. TTNT, PFS and OS were calculated with the Kaplan-Meier method. The log-rank test was used to evaluate the differences between groups. PFS and OS were adjusted for the following factors with Cox proportional hazard method: HCT-CI, high-risk cytogenetics, hematologic response at the time of BMT consultation and use of maintenance as these factors are known predictors of outcomes in patients undergoing transplant24, 26, 27. Statistical significance was considered at two-sided p < 0.05.
Results
Between January 1, 2010, and November 1, 2019, 79 patients ≥ 70 years old were evaluated for stem cell transplantation within 1 year after their diagnosis at the Stanford Blood and Marrow Transplantation Clinic. Thirty-eight patients (48%) proceeded to ASCT. ASCT was not pursued in 41 (52%) patients, despite most patients (80%, n=33) being deemed eligible for a transplant. Reasons for deferring ASCT in eligible patients included patient preference (52%, n=17) and physician preference (48%, n=16).
Baseline Characteristics:
There was no difference in baseline characteristics among the patients categorized by whether they received ASCT or non-ASCT therapy other than sex distribution (Table 1). Median age was 71.5 years (range 70–78) for the ASCT cohort and 71 years (range 70–77) for the non-ASCT cohort (p=0.90). High-risk cytogenetics were observed in 45% and in 34% of the two cohorts, respectively (p=0.47).
Table 1:
Baseline and treatment characteristics of patients aged 70 years and older evaluated for transplant within one year of diagnosis
| ASCT cohort N (%) | Non-ASCT cohort N (%) | P-value | |
|---|---|---|---|
| Total number per group | 38 | 41 | |
| Sex | 0.02 | ||
| ISS, (n=61) | n=28 | n=32 | 0.16 |
| High risk cytogenetics, (N=71) | n=33 | n=38 | 0.47 |
| Median age, years (range) | 71.5 (70–78) | 71 (70–77) | 0.90 |
| HCT-CI | 0.38 | ||
| Status at BMT consultation | 0.11 | ||
| KPS | 0.12 | ||
| Disease status at transplantation evaluation | 0.38 | ||
| Treatment | 0.72 | ||
| Conditioning regimen | - | ||
| Maintenance received | 21(55) | 20(49) | 0.65 |
BCNU: carmustine
Cy: cyclophosphamide
HCT-CI= hematopoietic cell transplantation comorbidity index
ISS= International staging system
IMiD: Immunomodulatory drugs
KPS= Karnofsky performance status
PI: Proteasome inhibitor
ISS stage at diagnosis was similar in both groups (p=0.16). Distribution of patients by ISS stage in this ASCT cohort was as follows: Stage I: 32% (n=9), Stage II: 21% (n=6) and Stage III: 47% (n=13). In the non-ASCT cohort, the distribution patients by the ISS was: Stage I: 34% (n=11), Stage II: 41% (n=13) and Stage III: 25% (n=8). HCT-CI comorbidity score was similar in the ASCT vs. non-ASCT groups, with low risk score (score 0) observed in 29% vs. 44% of patients and high-risk score (score >2) observed in 29% vs. 24% of patients in the two groups, respectively, p=0.38.
Treatment Regimens:
All patients received novel agent based therapy as described in Table 1.
In the transplant cohort, the induction regimen consisted of a doublet in 8 (21%) patients and a triplet in 30 (79%) patients. Doublet therapy included a proteasome inhibitor (PI) doublet in 4 (11%) patients, immunomodulatory drug (IMiD) doublet in 3 (8%) patients and daratumumab doublet in 1 (2%) patient, respectively. In the triplet group, the regimen was PI+IMiD triplet in 20 (53%) patients, PI+cyclophosphamide triplet in 9 (24%) patients and daratumumab triplet in 1 (2%) patient. The conditioning regimen before stem cell infusion was as follows: melphalan 140 mg/m2 in 9 (24%), melphalan 200 mg/m2 in 12 (32%) and melphalan 200 mg/m2 + BCNU 300–350 mg/m2 in 17 (44%) patients. Overall, the melphalan dosage in the conditioning regimen was 140 mg/m2 in 26% and 200 mg/m2 in 74% of patients. Twenty-one (55%) patients received maintenance therapy after ASCT for a median duration of 7 months (range 1–44) from start of maintenance at last follow-up. Maintenance included an IMiD in the majority of patients (n=14, 67%), followed by PI in 5 patients (24%) and both an IMiD and a PI in 2 patients (9%).
The treatment regimen in the non-transplant cohort included a doublet in 6 (15%) patients and a triplet regimen in 35 (85%) patients. Doublet therapy included a proteasome inhibitor (PI) doublet in 4 (10%) patients, immunomodulatory drug (IMiD) doublet in 1 (2%) patient and daratumumab doublet in 1 (2%) patient, respectively. In the triplet group, the regimen was PI+IMiD triplet in 27 (66%) patients, PI+cyclophosphamide triplet in 6 (15%) patients and daratumumab triplet in 2 (5%) patients. The median duration of initial therapy (excluding maintenance) was 8.5 months (range 1–29). Twenty (49%) patients in the non-ASCT cohort received maintenance therapy for a median duration of 22.5 months (range 3–60) from start of maintenance. Maintenance therapy was with an IMiD in 13 patients, PI in 6 patients and daratumumab in 1 patient.
There was no difference when comparing the treatment regimen category (i.e. receiving doublets vs. triplets) in the ASCT cohort to the non-ASCT cohort (p=0.56), with the majority of patients receiving triplets in both groups (79% vs. 85%). The proportion of patients receiving maintenance therapy (55% vs. 49%) was also comparable in both cohorts (p=0.65).
Transplant related toxicity:
Complications in the ASCT cohort (n=38) included: arrhythmia (n=7, 18%), infection (n=5, 13%), bacteremia (n=2, 5%) and BCNU pneumonitis (n=2, 12%). One patient required intensive care unit level care. The median times to neutrophil and platelet engraftment were 11 days (range 10–22) and 17 days (range 8–91), respectively. Twenty-six (68%) patients required hospitalization with a median length of stay of 8 days (range 2–18). Between day 30 and day 90 post-transplant, 4 (11%) patients required an admission with a median length of stay of 5.5 days (range 3–12).
Survival Outcomes and TTNT:
Median follow-up of this cohort from start of treatment was 40 months [95% confidence interval (CI): 29–46], with no difference in follow-up amongst patients undergoing transplant vs. not. Median PFS from start of treatment in patients ≥ 70 years undergoing ASCT was 41 months compared to 33 months in the non-ASCT cohort, p=0.03 (Figure 1A). Overall survival was similar in both groups, with median OS not reached in either group. Estimated 5-year OS was not significantly different with 73% in the ASCT group and 82% in the non-ASCT group, p=0.86 (Median OS not reached) (Figure 1B). TRM at D+100 was 0% in both groups. Main cause of death was progressive disease in 83% of ASCT cohort and 86% of non-ASCT cohort. No difference was noted based on the time period (2016–20 vs. 2010–15).
Figure 1:
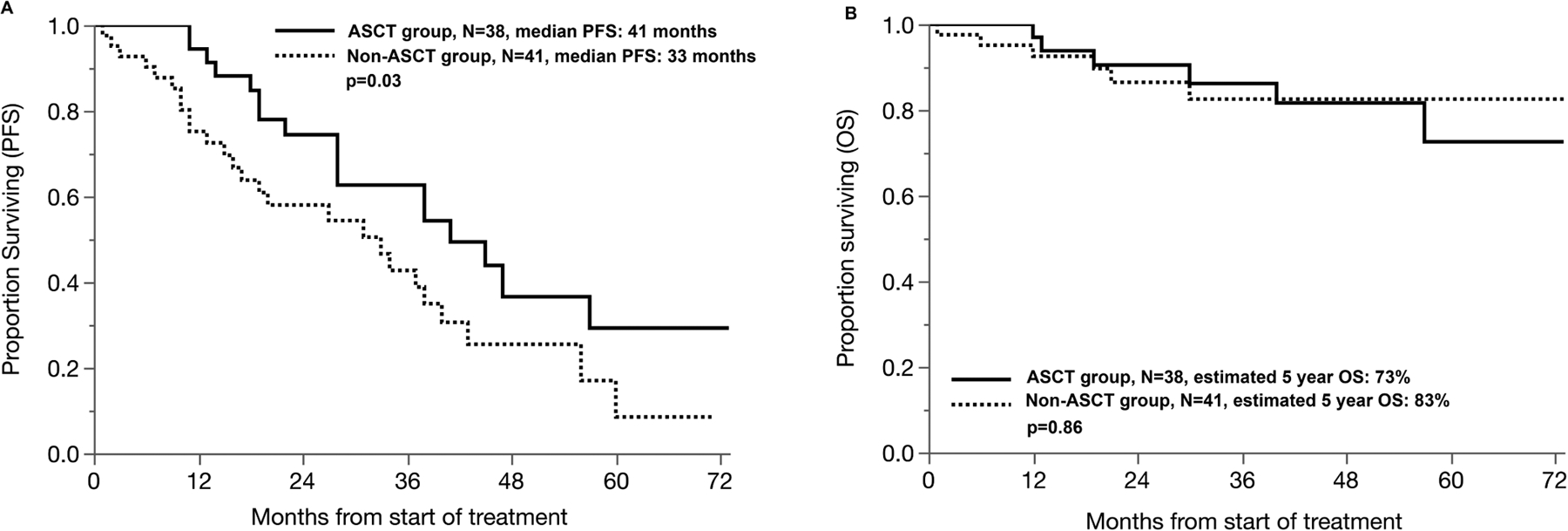
Progression-free survival (A) and overall survival (B) in patients 70 years and above undergoing ASCT versus non-ASCT based treatment.
When comparing sub-groups of patients based on cytogenetic risk, the difference did not reach statistical significance in patients with high-risk disease (median PFS: 45 vs. 33 months, p=0.57), though sample size of the group was small (n=28).
TTNT:
The time to next treatment was significantly longer for the ASCT cohort with a median time of 44 months compared to 34 months in the non-ASCT cohort, p=0.029. There was no difference in the number of lines of therapy received within 3 years after the initial diagnosis with a median of 1.5 (range 1–5) lines in the ASCT cohort compared to 2 (1–7) in the non-ASCT cohort, p=0.67. No patient in the non-ASCT cohort received an autologous transplant at relapse.
Multivariate analysis:
On multivariate analysis using Cox proportional hazards, ASCT was associated with improved PFS in patients 70 years or older, even after adjusting for HCT-CI score, performance status (KPS), response at transplant evaluation (at least partial response vs. not), use of maintenance and cytogenetic risk. (Table 2). Hazard ratio for PFS for ASCT vs. non-ASCT treatment was: 0.30 (95% CI 0.15–0.63, p=0.002). As expected, patients in the low risk HCT-CI group (score:0) also had better PFS (HR:0.31, 95% CI 0.15–0.66, p=0.002). Maintenance was also associated with better PFS (HR:0.50, 95% CI 0.25–0.97, p=0.042).
Table 2:
Multivariate Analysis for Progression Free Survival (PFS) amongst patients aged 70 years and older evaluated for transplant within one year of diagnosis
| Hazard ratio with 95% CI | P-value | |
|---|---|---|
| ASCT vs not | 0.30 (0.15–0.63) | 0.002 |
| At least a partial response vs. not at the time of transplant consultation | 0.08 (0.02–0.35) | <0.001 |
| HCT-CI score: 0 vs. not | 0.31 (0.15–0.66) | 0.002 |
| High-risk cytogenetics: present vs. not | 1.76 (0.84–3.70) | 0.13 |
| Use of maintenance | 0.50 (0.25–0.97) | 0.042 |
CI: Confidence interval
ASCT= Autologous stem cell transplant
HCT-CI= hematopoietic cell transplantation comorbidity index
Survival outcomes compared to patients < 70 years:
We then compared outcomes of patients ≥ 70 years undergoing ASCT to a contemporaneous cohort of patients <70 years (n=631) undergoing ASCT for myeloma our institution from 2010–19. The median PFS from transplant in patients ≥ 70 years vs. < 70 years was 36 vs. 47 months, p=0.25, respectively. (Figure 2) TRM at 3 months was at 0% and 1% in the two groups, respectively.
Figure 2:
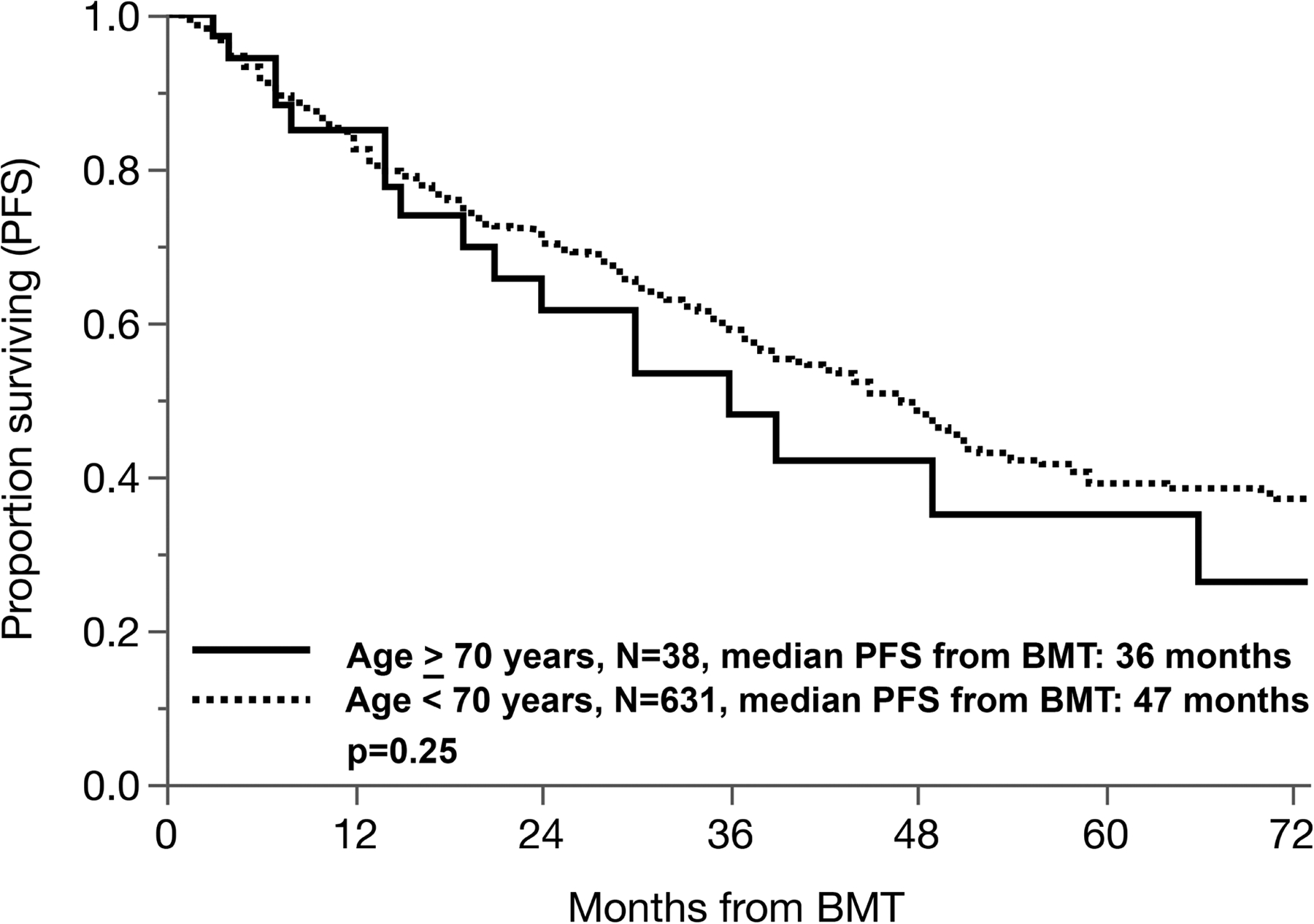
Progression-free survival in patients 70 years or above undergoing ASCT compared with a younger contemporaneous cohort (< 70 years) of patients undergoing ASCT.
Discussion
Autologous stem cell transplantation is a safe and effective treatment with durable outcomes in appropriately selected older patients. Moreover, amongst older patients with MM referred for transplant, patients who ultimately underwent transplant were observed to have longer PFS compared to those who did not undergo transplant.
Several retrospective and few prospective studies have confirmed that ASCT is an effective and safe treatment for older patients with MM5–18. However, there is limited data comparing ASCT and non-ASCT based treatment approaches in patients with MM above the age of 70 years. Our study provides comparative data on outcomes with transplant vs. non-transplant-based therapy in older patients who were deemed fit enough to be referred for a transplant consult by their hematologist. Our findings of improved PFS in older adults are similar to those observed in the IFM 2009 clinical trial of early vs. deferred transplant, which included patients up to the age of 65 years20. Older adults above 70 years of age with MM were found to have a comparable magnitude of benefit with transplant vs. no transplant (PFS 41 vs. 33 months) in our study as seen in younger patients. About half of the patients in the transplant and non-transplant groups received maintenance, though duration of maintenance was only 7 months (range 1–44) in the transplant group compared to 22.5 months (range 3–60) in the non-transplant group, p=0.004. The PFS benefit of transplant persisted even after adjusting for known prognostic factors including the HCT-CI score, performance status, use of maintenance and response at the time of transplant consultation. As expected, a low HCT-CI score was also an independent favorable prognostic factor for superior PFS. HCT-CI developed by Sorror et al.25 for the evaluation of transplantation-related mortality (TRM) in patients undergoing allogeneic transplantation has become an important tool for patient selection28. Subsequent studies have also validated HCT-CI in patients with multiple myeloma24, 29–31. Comprehensive geriatric assessment in older patients with MM has been proven helpful in predicting survival and toxicity and should be considered when evaluating older adults with myeloma in clinic32. Similar to the results of the IFM-2009 study, there was no difference in overall survival with transplant vs. not in our cohort of older adults with MM, with the current median follow-up of 40 months. This may be attributable to the duration of follow-up as over 75% patients were still alive at the 5-year time-point.
Multiple myeloma treatment landscape has evolved significantly over the last decade during which patients included in this study were evaluated and treated. The major practice change has been with the widespread use of triplet regimens as the standard of care over doublets, given the PFS and OS advantage seen with triplets in randomized trials33. There is equipoise among the different triplet regimens, with no observed PFS or OS benefit seen with one regimen over another in prospective trials. Recent data from the ENDURANCE trial reported similar PFS in patients receiving VRD (bortezomib, lenalidomide and dexamethasone) and KRD (carfilzomib, lenalidomide and dexamethasone), even though deeper responses were seen in the KRD cohort34. Similarly, deeper responses have been observed with VTD (bortezomib, thalidomide and dexamethasone) induction before transplant compared VCD (bortezomib, cyclophosphamide and dexamethasone) induction, though follow-up survival data has not been published35. Recently, daratumumab based regimens have become another attractive treatment option in the upfront setting, especially in older adults given the relatively favorable toxicity profile36. The role of maintenance has also been firmly established, with both PFS and OS benefit with maintenance therapy following transplant37. There is limited data to support maintenance in non-transplant eligible patients, though it is now frequently used in clinical practice as continuous therapy has shown to offer PFS advantage over limited duration therapy as seen in the FIRST trial38. The treatment options available at time of relapse have also exponentially increased, with several new therapies being approved39. The ever changing treatment landscape of myeloma over the last decade resulted in a wide variety of regimens being used in our study cohort. However, it was reassuring to see a similar proportion of patients in both groups receiving triplet regimens compared to doublets and as well as similar number of patients receiving maintenance therapy, thereby minimizing any confounding of outcomes based on the heterogenous treatment landscape.
Our study also demonstrates the low toxicity of ASCT in appropriately selected patients, with day 100 TRM of 0% despite having used a more intensive regimen (Melphalan 200 mg/m2+BCNU) in 17 patients and melphalan dose of 200 mg/m2 used in 76% patients overall. Various retrospective studies have shown TRM between 0% and 18% with the use of the standard 200 mg/m2 in older patients11, 16, 40. Melphalan 200 mg/m2 remains the gold standard conditioning regimen for MM. Reduction of the conditioning regimen dose to 100 mg/m2 is associated with significantly worse outcomes41. In patients ≥ 65–70 years old, the best melphalan conditioning dosage is still unknown. Reduction of the dose to 140 mg/m2 is common when patients have renal impairment, heart failure or increase frailty. Retrospective studies in older patients have reported a use of different reduced melphalan doses, with 2–45% patients receiving a reduced dose in prior reports14, 18, 42. A recent EBMT registry study compared melphalan 200 mg/m2 to 140 mg/m2 and found no difference in terms of efficacy in the older patient group43. However, a significant number of patients (37%) received the reduced dosage primarily for renal impairment, where the total marrow exposure with a reduced dose is expected to be similar to full dose melphalan due to impaired metabolism. Our numbers are too small to compare outcomes with the two doses. Therefore, until additional data is available, melphalan 200 mg/m2 should be the recommended conditioning regimen in fit patients, including those ≥ 70 years old. Dose reduction may be considered on a case by case basis, accounting for renal function and other patient factors.
Our study has limitations. First, there is a referral bias among our patients. Patients included in this study needed to be fit enough to be referred for consultation by their primary hematologist. However, most patients who were evaluated for transplant and did not receive ASCT were deemed fit enough to undergo transplant and the decision of not proceeding with transplant was due to physician/patient preference. Therefore, this comparison is apt in patients who are referred for transplant. Secondly, our study includes patients treated over the past decade with heterogenous induction regimens and variable use of maintenance therapy, thereby limiting the treatment homogeneity. However, all patients received novel regimens and the proportion of patients receiving triplets vs. doublets and maintenance therapy was similar between both groups. Thirdly, the retrospective design of the study is by itself a limitation. Retrospective analyses are subject to unmeasured confounding differences between groups, and it is possible that the better outcomes seen in the transplant group reflect the identification of better-risk patients for transplant. Quality of life (QoL) data remains an important aspect of transplant in that population that we were not able to evaluate. Future studies should evaluate QoL in this context. The power of the study is also a limitation, thereby limiting sub-group analyses. Despite these limitations, our study provides valuable data and is one of the only contemporary studies comparing ASCT and non-ASCT for fit older patients.
In conclusion, fit patients ≥ 70 years old with MM undergoing ASCT have a better PFS compared to patients with similar characteristics who do not undergo ASCT in this retrospective analysis. When patients are well selected, ASCT is safe in this older population and outcomes are similar when compared to younger patients with MM undergoing ASCT.
ACKNOWLEDGEMENTS:
Stanford BMT Database Management team
Footnotes
Conflict of interest: The authors declare no competing financial interests other than: SS: Consultancy: Janssen. AR: Research Support, Pharmacyclics; one-time advisory boards for Nohla and Kaleido; expert witness, U.S. Department of Justice; brother employed by Johnson & Johnson.
REFERENCES:
- 1.Palumbo A, Bringhen S, Ludwig H, Dimopoulos MA, Blade J, Mateos MV et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: a report of the European Myeloma Network (EMN). Blood 2011; 118(17): 4519–4529. e-pub ahead of print 2011/08/16; doi: 10.1182/blood-2011-06-358812 [DOI] [PubMed] [Google Scholar]
- 2.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2009; 27(17): 2758–2765. e-pub ahead of print 2009/05/01; doi: 10.1200/JCO.2008.20.8983 [DOI] [PubMed] [Google Scholar]
- 3.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. The New England journal of medicine 1996; 335(2): 91–97. e-pub ahead of print 1996/07/11; doi: 10.1056/NEJM199607113350204 [DOI] [PubMed] [Google Scholar]
- 4.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. The New England journal of medicine 2003; 348(19): 1875–1883. e-pub ahead of print 2003/05/09; doi: 10.1056/NEJMoa022340 [DOI] [PubMed] [Google Scholar]
- 5.Ozaki S, Harada T, Saitoh T, Shimazaki C, Itagaki M, Asaoku H et al. Survival of multiple myeloma patients aged 65–70 years in the era of novel agents and autologous stem cell transplantation. A multicenter retrospective collaborative study of the Japanese Society of Myeloma and the European Myeloma Network. Acta Haematol 2014; 132(2): 211–219. e-pub ahead of print 2014/03/26; doi: 10.1159/000357394 [DOI] [PubMed] [Google Scholar]
- 6.Merz M, Neben K, Raab MS, Sauer S, Egerer G, Hundemer M et al. Autologous stem cell transplantation for elderly patients with newly diagnosed multiple myeloma in the era of novel agents. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2014; 25(1): 189–195. e-pub ahead of print 2013/12/21; doi: 10.1093/annonc/mdt509 [DOI] [PubMed] [Google Scholar]
- 7.Gay F, Magarotto V, Crippa C, Pescosta N, Guglielmelli T, Cavallo F et al. Bortezomib induction, reduced-intensity transplantation, and lenalidomide consolidation-maintenance for myeloma: updated results. Blood 2013; 122(8): 1376–1383. e-pub ahead of print 2013/06/19; doi: 10.1182/blood-2013-02-483073 [DOI] [PubMed] [Google Scholar]
- 8.Bashir Q, Shah N, Parmar S, Wei W, Rondon G, Weber DM et al. Feasibility of autologous hematopoietic stem cell transplant in patients aged >/=70 years with multiple myeloma. Leuk Lymphoma 2012; 53(1): 118–122. e-pub ahead of print 2011/07/26; doi: 10.3109/10428194.2011.606942 [DOI] [PubMed] [Google Scholar]
- 9.Kumar SK, Dingli D, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK et al. Autologous stem cell transplantation in patients of 70 years and older with multiple myeloma: Results from a matched pair analysis. Am J Hematol 2008; 83(8): 614–617. e-pub ahead of print 2008/04/23; doi: 10.1002/ajh.21191 [DOI] [PubMed] [Google Scholar]
- 10.Gertz MA, Lacy MQ, Dispenzieri A, Hayman SR, Kumar S, Leung N et al. Impact of age and serum creatinine value on outcome after autologous blood stem cell transplantation for patients with multiple myeloma. Bone marrow transplantation 2007; 39(10): 605–611. e-pub ahead of print 2007/03/21; doi: 10.1038/sj.bmt.1705627 [DOI] [PubMed] [Google Scholar]
- 11.Jantunen E, Kuittinen T, Penttila K, Lehtonen P, Mahlamaki E, Nousiainen T. High-dose melphalan (200 mg/m2) supported by autologous stem cell transplantation is safe and effective in elderly (>or=65 years) myeloma patients: comparison with younger patients treated on the same protocol. Bone marrow transplantation 2006; 37(10): 917–922. e-pub ahead of print 2006/05/04; doi: 10.1038/sj.bmt.1705360 [DOI] [PubMed] [Google Scholar]
- 12.Reece DE, Bredeson C, Perez WS, Jagannath S, Zhang MJ, Ballen KK et al. Autologous stem cell transplantation in multiple myeloma patients <60 vs >/=60 years of age. Bone marrow transplantation 2003; 32(12): 1135–1143. e-pub ahead of print 2003/12/04; doi: 10.1038/sj.bmt.1704288 [DOI] [PubMed] [Google Scholar]
- 13.Muta T, Miyamoto T, Fujisaki T, Ohno Y, Kamimura T, Kato K et al. Evaluation of the feasibility and efficacy of autologous stem cell transplantation in elderly patients with multiple myeloma. Internal medicine (Tokyo, Japan) 2013; 52(1): 63–70. e-pub ahead of print 2013/01/08; doi: 10.2169/internalmedicine.52.8390 [DOI] [PubMed] [Google Scholar]
- 14.Wildes TM, Finney JD, Fiala M, Gao F, Vij R, Stockerl-Goldstein K et al. High-dose therapy and autologous stem cell transplant in older adults with multiple myeloma. Bone marrow transplantation 2015; 50(8): 1075–1082. e-pub ahead of print 2015/05/12; doi: 10.1038/bmt.2015.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merz M, Jansen L, Castro FA, Hillengass J, Salwender H, Weisel K et al. Survival of elderly patients with multiple myeloma-Effect of upfront autologous stem cell transplantation. Eur J Cancer 2016; 62: 1–8. e-pub ahead of print 2016/05/18; doi: 10.1016/j.ejca.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 16.Sirohi B, Powles R, Treleaven J, Mainwaring P, Kulkarni S, Pandha H et al. The role of autologous transplantation in patients with multiple myeloma aged 65 years and over. Bone marrow transplantation 2000; 25(5): 533–539. e-pub ahead of print 2000/03/14; doi: 10.1038/sj.bmt.1702188 [DOI] [PubMed] [Google Scholar]
- 17.Auner HW, Szydlo R, Hoek J, Goldschmidt H, Stoppa AM, Morgan GJ et al. Trends in autologous hematopoietic cell transplantation for multiple myeloma in Europe: increased use and improved outcomes in elderly patients in recent years. Bone marrow transplantation 2015; 50(2): 209–215. e-pub ahead of print 2014/11/12; doi: 10.1038/bmt.2014.255 [DOI] [PubMed] [Google Scholar]
- 18.Garderet L, Beohou E, Caillot D, Stoppa AM, Touzeau C, Chretien ML et al. Upfront autologous stem cell transplantation for newly diagnosed elderly multiple myeloma patients: a prospective multicenter study. Haematologica 2016; 101(11): 1390–1397. e-pub ahead of print 2016/11/02; doi: 10.3324/haematol.2016.150334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koreth J, Cutler CS, Djulbegovic B, Behl R, Schlossman RL, Munshi NC et al. High-dose therapy with single autologous transplantation versus chemotherapy for newly diagnosed multiple myeloma: A systematic review and meta-analysis of randomized controlled trials. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2007; 13(2): 183–196. e-pub ahead of print 2007/01/24; doi: 10.1016/j.bbmt.2006.09.010 [DOI] [PubMed] [Google Scholar]
- 20.Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. The New England journal of medicine 2017; 376(14): 1311–1320. e-pub ahead of print 2017/04/06; doi: 10.1056/NEJMoa1611750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.International Myeloma Working G. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol 2003; 121(5): 749–757. e-pub ahead of print 2003/06/05; [PubMed] [Google Scholar]
- 22.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K et al. International uniform response criteria for multiple myeloma. Leukemia 2006; 20(9): 1467–1473. e-pub ahead of print 2006/07/21; doi: 10.1038/sj.leu.2404284 [DOI] [PubMed] [Google Scholar]
- 23.Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J et al. International staging system for multiple myeloma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2005; 23(15): 3412–3420. e-pub ahead of print 2005/04/06; doi: 10.1200/JCO.2005.04.242 [DOI] [PubMed] [Google Scholar]
- 24.Saad A, Mahindra A, Zhang MJ, Zhong X, Costa LJ, Dispenzieri A et al. Hematopoietic cell transplant comorbidity index is predictive of survival after autologous hematopoietic cell transplantation in multiple myeloma. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2014; 20(3): 402–408 e401. e-pub ahead of print 2013/12/18; doi: 10.1016/j.bbmt.2013.12.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005; 106(8): 2912–2919. e-pub ahead of print 2005/07/05; doi: 10.1182/blood-2005-05-2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015; 33(26): 2863–2869. e-pub ahead of print 2015/08/05; doi: 10.1200/JCO.2015.61.2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 2003; 78(1): 21–33. e-pub ahead of print 2003/01/17; doi: 10.4065/78.1.21 [DOI] [PubMed] [Google Scholar]
- 28.Majhail NS, Brunstein CG, McAvoy S, DeFor TE, Al-Hazzouri A, Setubal D et al. Does the hematopoietic cell transplantation specific comorbidity index predict transplant outcomes? A validation study in a large cohort of umbilical cord blood and matched related donor transplants. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2008; 14(9): 985–992. e-pub ahead of print 2008/08/30; doi: 10.1016/j.bbmt.2008.06.008 [DOI] [PubMed] [Google Scholar]
- 29.Berro M, Arbelbide JA, Rivas MM, Basquiera AL, Ferini G, Vitriu A et al. Hematopoietic Cell Transplantation-Specific Comorbidity Index Predicts Morbidity and Mortality in Autologous Stem Cell Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2017; 23(10): 1646–1650. e-pub ahead of print 2017/07/04; doi: 10.1016/j.bbmt.2017.06.014 [DOI] [PubMed] [Google Scholar]
- 30.Labonte L, Iqbal T, Zaidi MA, McDiarmid SA, Huebsch LB, Tay J et al. Utility of comorbidity assessment in predicting transplantation-related toxicity following autologous hematopoietic stem cell transplantation for multiple myeloma. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2008; 14(9): 1039–1044. e-pub ahead of print 2008/08/30; doi: 10.1016/j.bbmt.2008.06.019 [DOI] [PubMed] [Google Scholar]
- 31.Jaglowski SM, Ruppert AS, Hofmeister CC, Elder P, Blum W, Klisovic R et al. The hematopoietic stem cell transplant comorbidity index can predict for 30-day readmission following autologous stem cell transplant for lymphoma and multiple myeloma. Bone marrow transplantation 2014; 49(10): 1323–1329. e-pub ahead of print 2014/07/30; doi: 10.1038/bmt.2014.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palumbo A, Bringhen S, Mateos MV, Larocca A, Facon T, Kumar SK et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood 2015; 125(13): 2068–2074. e-pub ahead of print 2015/01/30; doi: 10.1182/blood-2014-12-615187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durie BGM, Hoering A, Abidi MH, Rajkumar SV, Epstein J, Kahanic SP et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. The Lancet 2017; 389(10068): 519–527. doi: 10.1016/s0140-6736(16)31594-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar S, Jacobus SJ, Cohen AD, Weiss M, Callander NS, Singh AA et al. Carfilzomib, lenalidomide, and dexamethasone (KRd) versus bortezomib, lenalidomide, and dexamethasone (VRd) for initial therapy of newly diagnosed multiple myeloma (NDMM): Results of ENDURANCE (E1A11) phase III trial. Journal of Clinical Oncology 2020; 38(18_suppl): LBA3–LBA3. doi: 10.1200/JCO.2020.38.18_suppl.LBA3 [DOI] [Google Scholar]
- 35.Moreau P, Hulin C, Macro M, Caillot D, Chaleteix C, Roussel M et al. VTD is superior to VCD prior to intensive therapy in multiple myeloma: results of the prospective IFM2013-04 trial. Blood 2016; 127(21): 2569–2574. e-pub ahead of print 2016/03/24; doi: 10.1182/blood-2016-01-693580 [DOI] [PubMed] [Google Scholar]
- 36.Facon T, Kumar S, Plesner T, Orlowski RZ, Moreau P, Bahlis N et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. The New England journal of medicine 2019; 380(22): 2104–2115. e-pub ahead of print 2019/05/30; doi: 10.1056/NEJMoa1817249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCarthy PL, Holstein SA, Petrucci MT, Richardson PG, Hulin C, Tosi P et al. Lenalidomide Maintenance After Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: A Meta-Analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2017; 35(29): 3279–3289. e-pub ahead of print 2017/07/26; doi: 10.1200/JCO.2017.72.6679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benboubker L, Dimopoulos MA, Dispenzieri A, Catalano J, Belch AR, Cavo M et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. The New England journal of medicine 2014; 371(10): 906–917. e-pub ahead of print 2014/09/04; doi: 10.1056/NEJMoa1402551 [DOI] [PubMed] [Google Scholar]
- 39.Botta C, Ciliberto D, Rossi M, Staropoli N, Cuce M, Galeano T et al. Network meta-analysis of randomized trials in multiple myeloma: efficacy and safety in relapsed/refractory patients. Blood Adv 2017; 1(7): 455–466. e-pub ahead of print 2018/01/04; doi: 10.1182/bloodadvances.2016003905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siegel DS, Desikan KR, Mehta J, Singhal S, Fassas A, Munshi N et al. Age is not a prognostic variable with autotransplants for multiple myeloma. Blood 1999; 93(1): 51–54. e-pub ahead of print 1998/12/24; [PubMed] [Google Scholar]
- 41.Palumbo A, Bringhen S, Bruno B, Falcone AP, Liberati AM, Grasso M et al. Melphalan 200 mg/m(2) versus melphalan 100 mg/m(2) in newly diagnosed myeloma patients: a prospective, multicenter phase 3 study. Blood 2010; 115(10): 1873–1879. e-pub ahead of print 2009/12/08; doi: 10.1182/blood-2009-09-241737 [DOI] [PubMed] [Google Scholar]
- 42.Muchtar E, Dingli D, Kumar S, Buadi FK, Dispenzieri A, Hayman SR et al. Autologous stem cell transplant for multiple myeloma patients 70 years or older. Bone marrow transplantation 2016; 51(11): 1449–1455. e-pub ahead of print 2016/11/03; doi: 10.1038/bmt.2016.174 [DOI] [PubMed] [Google Scholar]
- 43.Auner HW, Iacobelli S, Sbianchi G, Knol-Bout C, Blaise D, Russell NH et al. Melphalan 140 mg/m(2) or 200 mg/m(2) for autologous transplantation in myeloma: results from the Collaboration to Collect Autologous Transplant Outcomes in Lymphoma and Myeloma (CALM) study. A report by the EBMT Chronic Malignancies Working Party. Haematologica 2018; 103(3): 514–521. e-pub ahead of print 2017/12/09; doi: 10.3324/haematol.2017.181339 [DOI] [PMC free article] [PubMed] [Google Scholar]


