Dear editor,
We read with interest the paper by Moreno-Pérez et al.1 which outlines post-acute COVID-19 syndrome in 279 survivors at 77 days post disease onset. Symptoms were detected in half of patients and no baseline clinical features were predictive of development. Abnormal liver blood tests are common in the acute phase of COVID-19 and are associated with adverse outcomes,2 however we note that these were not evaluated as a risk factor in this study. Furthermore, the persistence of liver abnormalities beyond the acute phase was not explored. Given other aetiologies of liver dysfunction can move from acute to chronic phases it is important to delineate if this is the case with SARS-CoV-2 infection. Reported data on liver dysfunction is limited to 30 days post infection and is conflicting. A small subgroup suggested abnormalities return to baseline,3 whilst a larger population reported persistent abnormalities in a proportion,4 although precise details on numbers and severity were not described.
To further characterise persistent liver blood test abnormalities and patterns which may not rapidly improve, we included consecutive patients tested for SARS-CoV-2 infection using reverse-transcriptase polymerase chain reaction assays of respiratory tract samples between 25th February to 31st March 2020 at King's College Hospital London. Patients not admitted to hospital and those with known chronic liver disease were excluded. We collected data on demographics, clinical variables and clinical outcomes. Included patients were followed up until death or 20th January 2021. The definition of abnormal liver blood test was aspartate aminotransferase (AST) or alanine aminotransferase (ALT) above the upper limit of normal (ULN) for our laboratory (AST 50 IU/L, ALT 55 IU/L). ULN for alkaline phosphatase (ALP) was 130 IU/L, and gamma-glutamyl transferase (GGT) 55 U/L. The end point of normalisation of liver blood tests was defined as all measured liver blood tests within the normal range. This was conducted under London South East Research Ethics Committee (18/LO/2048) approval granted to the King's Electronic Records Research Interface (KERRI).
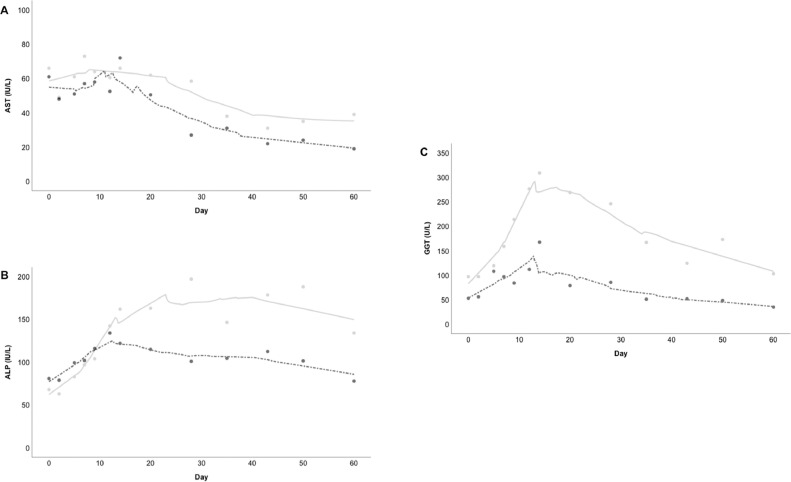
We included 564 patients with SARS-CoV-2 infection. The baseline characteristics of the study population and univariate analysis of the 312 (56.4%) patients with abnormal AST/ALT are outlined in Table 1 . Of 397 patients who survived to 60 days, a subgroup of 216 had abnormal liver blood tests. Repeat blood tests at day 60 were performed in 43 of these patients, and a persistent abnormality in liver blood tests was detected in 24/43 patients (55.8%), predominantly in a cholestatic pattern: 3/38 (7.9%) AST abnormal; 12/43 (27.9%) ALP abnormal; and 23/43 (53.5%) GGT abnormal. The dynamic patterns of liver enzymes through to day 60 are represented in Fig. 1 . On univariate analysis only peak GGT and peak ALP were significantly associated with persistently abnormal liver blood tests at day 60. On backwards elimination multivariate analysis only peak GGT was independently associated with persistently abnormal liver blood tests (OR 1.0048; 95% CI, 1.001–1.008, P = 001). On ROC curve analysis peak GGT had an AUROC of 0.794 (95% CI, 0.676–0.912, P < .001). A criterion of >254 U/L had: sensitivity 73% (95% CI, 56–85), specificity 80% (95% CI, 59–93), positive likelihood ratio 3.6 (1.6–8.1) and negative likelihood ratio 0.3 (0.2–0.6). A smaller subgroup of 14 patients with abnormal liver blood tests at day 60 had follow-up liver blood tests at more than 6 months following admission (median 277 days [IQR, 221.8–303]). In 9/14 (64.3%) a persistent cholestatic pattern of abnormality was identified: 1/14 (7.1%) AST/ALT abnormal; 5/14 (35.7%) ALP abnormal; and 9/14 (64.3%) GGT abnormal.
Table 1.
Clinical characteristics of SARS-CoV-2 patients with normal and abnormal AST/ALT.
| Normal | Abnormal | Total | P value | |
|---|---|---|---|---|
| Number (%) | 241 (43.6%) | 312 (56.4%) | 553 | |
| Age, median (IQR), years | 73.2 (57.2–84.5) | 63.75 (52.6–79.3) | 67.7 (53.5–81.7) | <0.001 |
| Sex, male | 116 (48.1%) | 190 (60.9%) | 306 (55.3%) | .003 |
| Ethnic minority | 77/217 (35.5%) | 171/282 (60.6%) | 248 (49.7%) | <0.001 |
| Hypertension | 120 (49.8%) | 160 (51.3%) | 280 (50.6%) | .73 |
| Diabetes | 76 (31.5%) | 104 (33.3%) | 180 (32.5%) | .66 |
| BMI | 25.2 (21.9–30.5) | 27.1 (23.6–32.0) | 26.3 (22.9–31.5) | .005 |
| Chronic heart disease | 24 (10%) | 30 (9.6%) | 54 (9.8%) | .89 |
| Chronic respiratory disease | 34 (14.1%) | 36 (11.5%) | 70 (12.7%) | .37 |
| Chronic kidney disease | 33 (13.7%) | 42 (13.5%) | 75 (13.6%) | .94 |
| AST, median (IQR), IU/L | 34 (26–43) | 99 (69–176) | 71 (44–127) | <0.001 |
| ALT, median (IQR), IU/L | 27 (19–40) | 84 (47–147) | 42 (25–84) | <0.001 |
| ALP, median (IQR), IU/L | 84 (66–117) | 113 (76–199) | 94 (70–161) | <0.001 |
| GGT, median (IQR), U/L | 44 (26–80) | 115 (57–286) | 72 (36–183) | <0.001 |
| Bilirubin, median (IQR), umol/L | 8 (6–11) | 11 (8–18) | 10 (7–15) | <0.001 |
| WBC, median (IQR), x109/L | 6.7 (5.3–9.0) | 7.0 (5.2–9.8) | 6.9 (5.2–9.4) | .32 |
| Neutrophils, median (IQR), x109/L | 5.0 (3.7–7.1) | 5.5 (3.7–8.0) | 5.3 (3.7–7.5) | .11 |
| Lymphocytes, median (IQR), x109/L | 1.0 (0.7–1.4) | 1.0 (0.7–1.4) | 1.0 (0.7–1.4) | .65 |
| Haemoglobin, median (IQR), g/L | 124 (111–141) | 133 (118–144) | 130 (114–142) | .002 |
| Platelets, median (IQR), x109/L | 218 (170–281) | 201 (153–264) | 208 (164–269) | .02 |
| C-reactive protein, median (IQR), mg/L | 116 (43–209) | 195 (100–321) | 156 (71–275) | <0.001 |
| Creatinine, median (IQR), umol/L | 87 (66–117) | 95 (71–128) | 91 (69–120) | .02 |
| ICU admission | 7 (2.9%) | 80 (25.6%) | 87 (15.7%) | <0.001 |
| Death | 65 (27.0%) | 96 (30.8%) | 161 (29.1%) | .33 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); AST, aspartate aminotransferase; ULN, upper limit of normal; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transferase; WBC, white blood count; ICU, intensive care unit.
Fig. 1.
Locally weight scatterplot smoothing (LOESS) for median values of (A) AST, (B) ALP and (C) GGT; in patients with abnormal AST/ALT and day 60 liver blood tests, with normalised (black dashed line) and not-normalised (grey solid line) groups.
Abbreviations: AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transferase; ALT, alanine aminotransferase.
Liver blood test abnormalities were previously described as self-limiting,3 however we provide early data on the potential persistence of abnormalities and predictive factors for persistence in a small subgroup of patients at 60 days. The predominant pattern of abnormality is cholestatic with ALP/GGT elevation at follow-up, and a predictor of this is an elevated GGT above 254 U/L during the acute phase of SARS-CoV-2 infection. Our data also suggests that in a subgroup of patients with abnormal liver blood tests at 60 days, ongoing ALP/GGT elevations may be still present at 6 months.
A variety of causes of liver injury may exist in SARS-CoV-2 infection,5 including cytotoxic effect from the virus,6 bystander liver injury from systemic infection,7 and secondary sclerosing cholangitis of the critically ill patient.8 Of relevance is that SARS-CoV-2 utilises the angiotensin converting enzyme 2 (ACE2) receptor for host cell entry,9 and ACE2 receptors are widely expressed in liver cholangiocytes. Data from human liver ductal organoids infected with SARS-CoV-2, suggests impairment of tight junctions and bile acid transportation may result in direct cholangiocyte damage and consequent bile acid accumulation.10 Although GGT is a non-specific marker of liver injury, our study supports the concept that liver damage in COVID-19 patients might result from direct cholangiocyte injury and consequent bile acid accumulation induced by viral infection.
There are limitations to our study. Patients were not systemically investigated for aetiology of liver abnormalities during the acute phase or the presence of underlying chronic liver disease, so abnormalities may be related to undiagnosed chronic liver disease and not SARS-CoV-2 liver involvement. Although a significant proportion of our study population had components of the metabolic syndrome and may have unappreciated non-alcoholic fatty liver disease, these factors were not associated with persistent abnormalities in our cohort. The follow-up liver blood tests were clinically driven by specific care-givers rather than protocolised and there is a risk of selection bias for those who underwent repeat testing.
In conclusion, we demonstrate an important novel finding that a proportion of patients with SARS-CoV-2 infection do not normalise their liver blood tests during follow-up, particularly those with elevated GGT above 254 U/L. Whilst these findings need to be validated in an independent cohort, we recommend that a plan for longitudinal assessment of liver blood tests should be made at discharge, with a focus on those with elevated GGT in addition to AST/ALT. In patients with a persistent abnormality, radiological imaging and liver histology may help elucidate the underlying pathophysiology and the development of a chronic biliary injury.
References
- 1.Moreno-Pérez O., Merino E., Leon-Ramirez J.M., Andres M., Ramos J.M., Arenas-Jiménez J., et al. Post-acute COVID-19 Syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect. 2021 doi: 10.1016/j.jinf.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skevaki C., Fragkou P.C., Cheng C., Xie M., Renz H. Laboratory characteristics of patients infected with the novel SARS-CoV-2 virus. J Infect. 2020;81(2):205–212. doi: 10.1016/j.jinf.2020.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romana Ponziani F., Del Zompo F., Nesci A., Santopaolo F., Ianiro G., Pompili M., et al. Liver involvement is not associated with mortality: results from a large cohort of SARS-CoV-2 positive patients. Aliment Pharmacol Ther. 2020;52(6):1060–1068. doi: 10.1111/apt.15996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yip T.C., Lui G.C., Wong V.W., Chow V.C., Ho T.H., Li T.C., et al. Gut; 2020. Liver Injury is Independently Associated With Adverse Clinical Outcomes in Patients With COVID-19. [DOI] [PubMed] [Google Scholar]
- 5.Nardo A.D., Schneeweiss-Gleixner M., Bakail M., Dixon E.D., Lax S.F., Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41(1):20–32. doi: 10.1111/liv.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y., Liu S., Liu H., Li W., Lin F., Jiang L., et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73(4):807–816. doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bangash M.N., Patel J.M., Parekh D., Murphy N., Brown R.M., Elsharkawy A.M., et al. SARS-CoV-2: is the liver merely a bystander to severe disease? J Hepatol. 2020;73(4):995–996. doi: 10.1016/j.jhep.2020.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roth N.C., Kim A., Vitkovski T., Xia J., Ramirez G., Bernstein D., et al. Post-COVID-19 cholangiopathy: a novel entity. Am J Gastroenterol. 2021 doi: 10.14309/ajg.0000000000001154. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao B., Ni C., Gao R., Wang Y., Yang L., Wei J., et al. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;17:1–5. doi: 10.1007/s13238-020-00718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]