Abstract
Membranous nephropathy is a pathological type of nephrotic syndrome. Current treatments including supportive therapy, corticosteroids, immunosuppressive agents are not effective for all patients. New therapies are needed to treat the disease safely and effectively. Gut microbiota may contribute to the pathogenesis of this disease. Fecal microbiota transplantation (FMT) has made achievements in many diseases. Here, we report a case in which FMT is used to treat a patient with membranous nephropathy and chronic diarrhea, whose symptoms ameliorated and renal function improved.
Keywords: Membranous nephropathy, Fecal microbiota transplantation, Gut microbiota
Introduction
Membranous nephropathy is the most common cause of adult idiopathic nephrotic syndrome which mainly manifests severe proteinuria, hypoalbuminemia and edema. Studies in recent years have indicated that the autoimmune antibody, infection, malignancy, drug (like NSAIDs) and other factors are involved in the pathogenesis of membranous nephropathy. Antibodies to the M-type phospholipase A2 receptor (anti-PLA2R) was identified in about 80% of patients and the use of anti-PLA2R as a diagnostic and prognostic biomarker was widely accepted [1]. The current treatment includes supportive treatment like blood pressure control, minimize proteinuria, salt restriction and a low protein diet, corticosteroids and immunosuppressive drugs [2]. The rituximab is a new option for this disease as aims at B cell which plays a critical part in the pathogenesis. Whereas a large observational cohort study of rituximab for membranous nephropathy showed a 35% failure rates with up to 27% rate of recurrence [3], a random-controlled trial suggested up to 40% failure rates at 24 months following treatment with rituximab [4]. Therefore, a new alternative therapy needs to be developed.
Recent investigations have revealed that there has gut microbiota dysbiosis in patients with chronic kidney diseases and it may contribute to the pathogenesis [5]. The gut microbiota may have a close relationship with chronic kidney disease. Compared to healthy controls, the patients with membranous nephropathy have unique features in gut microbiota [6]. Fecal microbiota transplantation (FMT) have made much progress in the treatment of C. difficile infections and inflammatory bowel diseases [7, 8], however, the treatment of membranous nephropathy by FMT has not been reported. Here, we report a case of FMT treatment in an adult patient with membranous nephropathy.
Case report
A 31-year-old man was admitted to our hospital with abnormal urine test. He had a positive urine protein test for 3 years and was diagnosed as membranous nephropathy based on pathology for 2 years. Tacrolimus was effective initially but relapsed with extenuation. The physical examination had no abnormities except both lower extremities edema. The blood tests showed serum albumin was 20.5 g/L, total protein was 39.5 g/L, urea was 5.48 mmol/L, creatinine was 77.9 μmol/L and low-density lipoprotein was 5.83 mmol/L. The 24 h urine proteins were 3.1 g and blood phospholipase A2 receptor antibody (PLA2R-Ab) titer was 19.26 RU/mL. ANA/ENA and allergen tests are negative while the C-13 test for Helicobacter pylori urease was positive. Ultrasound of kidneys showed no abnormities. We treated him with anti-pylori therapy, limited salt consumption, angiotensin receptor blocker to minimize proteinuria for a week and statin drugs to hyperlipidemia for a week, his symptoms ameliorated and then discharged.
Fourteen months ago, the patient developed fever, cough and diarrhea. The mycoplasma pneumoniae test was positive and sputum smear showed massive gram-positive coccus. He received cefepime and azithromycin therapy. Then, he was admitted to our hospital. The blood tests showed serum albumin was 16.8 g/L, total protein was 31.1 g/L, urea was 4.31 mmol/L, creatinine was 84.2 μmol/L and PLA2R-Ab titer was 62.70 RU/mL. The 24 h urine proteins became 4.12 g. The upper and lower gastrointestinal endoscopy showed no erythema, erosion and ulcer, except mild edema on his colonic mucosa. The patient received twice rituximab instillation (550 mg, respectively) 13 months ago. After treatment, the CD20+ cell was below detection limit while serum albumin was 18.3 g/L, total protein was 29.9 g/L, urea was 4.16 mmol/L, creatinine was 83.3 μmol/L and PLA2R-Ab titer was 31.19 RU/mL. The 24 h urine proteins became 5.9 g. 12 months ago, he received 12 mg methylprednisolone each day in consecutive 3 days, then the amount decreased to 8 mg. He took 8 mg methylprednisolone all the time before and after each FMT treatment. The patient had chronic diarrhea history. His nephropathy condition will reoccur or deteriorate when his diarrhea appeared. However, his stool routine exam was normal without any white cell or other abnormal thing and has no mucopurulent bloody stool. Combined with his gastrointestinal endoscopy results, the patient was not diagnosed as inflammatory bowel disease. In consideration of the patient with chronic diarrhea, we introduced the indications, adverse reactions and previous applications of FMT in clinical trials, the patient completely understood FMT and asked for using FMT therapy and signed the consent form. Ethical approval for refractory intestinal inflammatory disease: S2014-117-01.
Before the FMT treatment, the supplementation of probiotics was stopped for at least 30 days, while the patient still received basic pharmaceutical treatment, ARB drugs, statin, febuxostat and 8 mg methylprednisolone.
Our previous method was referred to the selection of donor and implementation of FMT [8, 9]. Healthy volunteers (between 10 and 40 years old) received a preliminary screening by a questionnaire and then took laboratory examinations for common pathogens, including human immunodeficiency virus, hepatitis A, B, and C virus, enteropathogenic Escherichia coli (EPEC), Shigella, Salmonella, C. difficile toxin, Epstein–Barr virus as well as fungi, ova, cysts, and parasites, etc. Donors were not allowed to have used antibiotics, probiotics, or other agents that influence intestinal flora before 4 weeks of donating their feces.
The fecal microbiota suspension was prepared from a 14-year-old male eligible donor. FMT was endoscopically administered to the patient’s small intestine with 150 mL of fecal suspension and to the colon with 350 mL under anesthesia.
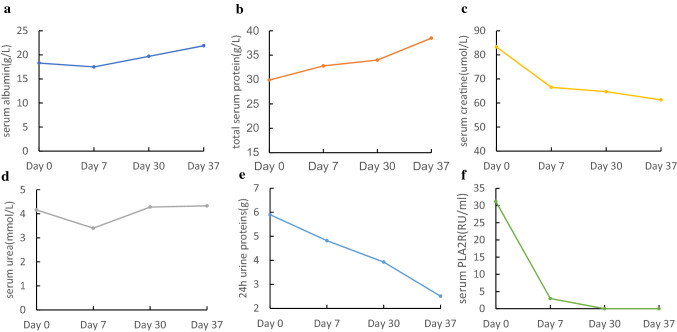
After the first FMT Procedure, the patient appeared low fever for 2 days with the highest temperature 38.3 °C and recovered after physical cooling. The reassessment results after 7 days showed that serum albumin and total protein increased to 17.5 g/L and 32.8 g/L, urea and creatine declined to 3.4 mmol/L and 66.5 μmol/L. The blood PLA2R-Ab titer was 2.95 RU/mL and the 24 h urine proteins became 4.82 g (Fig. 1a–f). On 28 days from the first FMT treatment, the patient received the repeated FMT. Before the second FMT treatment, his blood tests displayed that serum albumin was 19.7 g/L, total protein was 34.0 g/L, urea was 4.28 mmol/L, creatinine was 64.7 μmol/L. The PLA2R-Ab titer was below detection limit with < 2 RU/mL and the 24 h urine proteins decreased to 3.93 g. At 8 days following the second FMT therapy, serum albumin and total protein improved to 21.9 g/L and 32.8 g/L, PLA2R-Ab titer decreased to below detection limit with < 2 RU/mL, and 24 h urine proteins were down to 2.51 g. The process of FMT treatment did not cause other adverse problems.
Fig. 1.
Some related laboratory test results’ changes before and after twice FMT treatments. a Serum albumin; b total serum protein; c serum creatine; d serum urea; e 24 h urine proteins; f serum PLA2R. Day 0: before the first FMT; Day 7: after the first FMT; Day 30: before the second FMT; Day 37: after the second FMT
Discussion
The pathogenesis of membranous nephropathy remains unclear and the patients with this disease might appear diarrhea. Edema happening on gastrointestinal mucosa might impair gut barrier causing diarrhea. Weakened immunity by immunosuppressive agents also may contribute to dysbiosis and diarrhea.
Previous studies have reported the differences of gut microbiota between patients with membranous nephropathy and healthy controls [6]. At the phylum level, the abundance of Proteobacteria increased whereas that of Synergistetes decreased. At the genus level, Escherichia-Shigella and Streptococcus are more abundant in patients’ gut while Lachnospira and Veillonella are less. The accumulation in Escherichia-Shigella population may intensify gut permeability by decreasing butyrate biosynthesis and increasing oxidative stress to harm the intestinal epithelial barrier [10], while the butyrate-producing bacteria Lachnospira modulate immunity through Foxp3 gene and Treg cells [11].
In this case, the patient with membranous nephropathy had a chronic diarrhea history. Initially, the patient received tacrolimus for nephropathy and it was effective for him, whereas the disease relapsed with dosage reduction. Following treatment with rituximab did not obtain desirable results. The treatment for him was getting into an intractable status. After two times of FMT, the level of serum albumin and total protein elevated, while the creatine and 24 h urine protein decreased, together with the PLA2R antibody titer, besides, symptoms of edema and diarrhea disappeared, the surprising effect of FMT on this case suggests that gut microbiota plays a significant role in both diarrhea and membranous nephropathy, besides, it indicates that the FMT is a promising biotherapy for the membranous nephropathy. Certainly further studies are needed to validate its efficacy and underlying mechanism.
Compliance with ethical standards
Conflict of interest
All the authors have declared no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee at which the studies were conducted and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from the patient included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Di Wu, Email: 13213875@qq.com.
Yunsheng Yang, Email: sunnyddc@plagh.org.
References
- 1.van de Logt AE, Fresquet M, Wetzels JF, Brenchley P. The anti-PLA2R antibody in membranous nephropathy: what we know and what remains a decade after its discovery. Kidney Int. 2019;96(6):1292–1302. doi: 10.1016/j.kint.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Couser WG. Primary membranous nephropathy. Clin J Am Soc Nephrol. 2017;12(6):983–997. doi: 10.2215/CJN.11761116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruggenenti P, Cravedi P, Chianca A, Perna A, Ruggiero B, Gaspari F, et al. Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol. 2012;23(8):1416–1425. doi: 10.1681/ASN.2012020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fervenza FC, Appel GB, Barbour SJ, Rovin BH, Lafayette RA, Aslam N, et al. Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med. 2019;381(1):36–46. doi: 10.1056/NEJMoa1814427. [DOI] [PubMed] [Google Scholar]
- 5.Ondrussek-Sekac M, Navas-Carrillo D, Orenes-Pinero E. Intestinal microbiota alterations in chronic kidney disease and the influence of dietary components. Crit Rev Food Sci Nutr. 2020 doi: 10.1080/10408398.2020.1761771. [DOI] [PubMed] [Google Scholar]
- 6.Sun S, Dong R, Bai M, et al. A Comparative study of the gut microbiota associated with immunoglobulin A nephropathy and membranous nephropathy. 2020. 10.21203/rs.2.24727/v1. https://www.researchgate.net/publication/340188456_A_Comparative_Study_of_the_Gut_Microbiota_Associated_with_Immunoglobulin_A_Nephropathy_and_Membranous_Nephropathy. [DOI] [PMC free article] [PubMed]
- 7.Quraishi MN, Shaheen W, Oo YH, Iqbal TH. Immunological mechanisms underpinning faecal microbiota transplantation for the treatment of inflammatory bowel disease. Clin Exp Immunol. 2020;199(1):24–38. doi: 10.1111/cei.13397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao H, Shi Y, Luo X, Peng L, Yang Y, Zou L. The effect of fecal microbiota transplantation on a child with tourette syndrome. Case Rep Med. 2017;2017:6165239. doi: 10.1155/2017/6165239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren R, Sun G, Yang Y, et al. A pilot study of treating ulcerative colitis with fecal microbiota transplantation. Zhonghua Nei Ke Za Zhi. 2015;54(5):411–415. [PubMed] [Google Scholar]
- 10.Croxen MA, Law RJ, Scholz R, Keeney KM, Wlodarska M, Finlay BB. Recent advances in understanding enteric pathogenic Escherichia coli. Clin Microbiol Rev. 2013;26(4):822–880. doi: 10.1128/CMR.00022-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]