Abstract
Aims
This meta-analysis aimed to assess the prognostic value of fasting hyperglycemia in patients with COVID-19.
Methods
A systematic literature search on PubMed, Embase, and Scopus were performed up until February 18, 2021. Fasting hyperglycemia was defined as fasting plasma glucose level above the reference value. The outcome of interest was poor outcome, which was a composite of mortality and severe COVID-19. The effect estimate was in odds ratio (OR).
Results
There were 9045 patients from 12 studies included in this systematic review and meta-analysis. The prevalence of fasting hyperglycemia was 29%. The incidence of poor outcome was 15%. Fasting hyperglycemia was associated with poor outcome in COVID-19 (OR 4.72 [3.32, 6.72], p < 0.001; I2: 69.8%, p < 0.001). Subgroup analysis in patients without prior history of diabetes showed that fasting hyperglycemia was associated with poor outcome in COVID-19 (OR 3.387 [2.433, 4.714], p < 0.001; I2: 0, p = 0.90). Fasting hyperglycemia has a sensitivity of 0.57 [0.45, 0.68], specificity of 0.78 [0.70, 0.84], PLR of 2.6 [2.0, 3.3], NLR of 0.55 [0.44, 0.69], DOR of 5 [3, 7], and AUC of 0.74 [0.70, 0.78] for predicting poor outcome. In this pooled analysis, fasting hyperglycemia has a 32% post-test probability for poor outcome, and absence of fasting hyperglycemia confers to a 9% post-test probability. Meta-regression and subgroup analysis showed that the sensitivity and specificity varies by chronic kidney disease but not by age, male (gender), hypertension, and chronic kidney disease.
Conclusion
Fasting hyperglycemia was associated with mortality in COVID-19 patients, with or without diabetes.
Prospero
CRD42021237997.
Keywords: Coronavirus, Glucose, Mortality, Severity, SARS-CoV-2
1. Introduction
Coronavirus disease 2019 (COVID-19) has affected hundreds of millions people worldwide and caused a considerable number of deaths (World Health Organization, 2021). Most patients with COVID-19 have mild-moderate symptoms, however, a considerable number experienced end-organ complications that resulted in death (Lim et al., 2020a). Risk stratification by considering comorbidities and biomarkers are crucial in a decision-making process in order to facilitate an efficient resource allocation (Huang et al., 2020b; July and Pranata, 2020; Martha et al., 2021; Pranata et al., 2021a, 2021b; Raymond Pranata et al., 2020b; Tuty Kuswardhani et al., 2020).
Diabetes, especially newly diagnosed diabetes (Sathish et al., 2021a), has been shown to increase mortality in patients with COVID-19 (Huang et al., 2020a; Shrestha et al., 2021) Individual studies have studied the association between fasting hyperglycemia and poor outcomes in COVID-19 patients (Cai et al., 2020; Chen et al., 2021; Coppelli et al., 2020; Li et al., 2020; Liu et al., 2020; Wang et al., 2020); most showing a positive association, while a few have not (Gastélum-Cano et al., 2021). In patients with diabetes, hyperglycemia may also represent a glycemic poor control, in addition to the acute stress caused by infection. In this study, we aimed to quantity the association by pooling data from these studies using a meta-analysis approach.
2. Methods
This meta-analysis follows the recommendation from Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). This study is registered in PROSPERO (CRD42021237997).
2.1. Eligibility criteria
We included studies that fulfill all of these criteria: 1) observational prospective and retrospective studies, 2) reporting patients with COVID-19, 3) fasting plasma glucose, 4) hyperglycemia versus without hyperglycemia, and 5) mortality/severe COVID-19.
We excluded the following studies: 1) conference abstracts, 2) preprints, 3) case reports, 4) review articles, and 5) non-English language studies.
2.2. Search strategy and study selection
A systematic literature search on PubMed, Embase, and Scopus with keywords (COVID-19 OR SARS-COV-2 OR Coronavirus Disease) AND (hyperglycemia OR high blood glucose OR fasting plasma glucose) was performed from the inception of the database up until February 18, 2021. The search strategy can be seen in Supplementary Table 1. The duplicates were then removed after screening of the title/abstracts by two independent authors. Discrepancies among the reviewers were resolved by discussion.
2.3. Data extraction
Two authors performed data extraction of the included studies independently. The data of interest includes the author, baseline characteristics, design of the study, cut-off points for fasting hyperglycemia, and the outcome of interest. Discrepancies among the reviewers were resolved by discussion.
2.4. Exposure and outcome
Fasting hyperglycemia was defined as fasting plasma glucose level above the reference value defined by each studies. The outcome of interest was poor outcome, which was a composite of mortality and severe COVID-19. Severe COVID-19 was defined severe pneumonia or need for intensive unit care (ICU)/invasive mechanical ventilation (IMV). The pooled effect estimate in this study was in odds ratio (OR). Important diagnostic parameters 1) sensitivity, 2) specificity, 3) positive and negative likelihood ratio (PLR & NLR), 4) diagnostic odds ratio (DOR), and 5) area under the curve (AUC) were generated.
2.5. Risk of bias assessment
Two independent authors performed risk of bias assessment using the Newcastle-Ottawa Scale (NOS) (Li et al., 2020). Discrepancies were resolved by discussion.
2.6. Statistical analysis
Prevalence of hyperglycemia and poor outcome was synthesized using the meta-analysis of proportion method. The pooled effect estimate was generated using the DerSimonian-Laird random-effects model. P-values <0.05 were considered statistically significant. The heterogeneity of the included studies was assessed using the I-squared (I2) and Cochran Q test, value of <50% or p < 0.10 indicates heterogeneity. To evaluate the prognostic value of hyperglycemia, sensitivity, specificity, PLR, NLR, DOR, and AUC were calculated. Funnel-plot analysis and Egger's test were performed to evaluate the presence of small-study effects and publication bias. The association between hyperglycemia and poor outcome was then assessed using Restricted-maximum likelihood (REML) meta-regression, using baseline characteristics as covariates. Subgroup analysis was performed in patients without history of diabetes. STATA 16 (Stata Corp) was used to perform the analyses.
3. Results
There were 9045 patients from 12 studies included in this systematic review and meta-analysis [Fig. 1 ] (Cai et al., 2020; Chen et al., 2021; Coppelli et al., 2020; Deng et al., 2020; Gastélum-Cano et al., 2021; Huh et al., 2020; Li et al., 2020; Liu et al., 2020; Sardu et al., 2020; Sun et al., 2020; Wang et al., 2020; Zhang et al., 2020). Baseline characteristics of the included studies are presented in Table 1 . The prevalence of fasting hyperglycemia was 29% [23%–35%]. The incidence of poor outcome was 15% [11%–20%].
Fig. 1.
Prisma flowchart.
Table 1.
Baseline Characteristics of Samples included in Analysis.
| First Authors | Design | Samples | Hyperglycemia Cut-off | Age (years) | Male (%) | Diabetes (%) | Hypertension (%) | CKD (%) | Outcome | NOS |
|---|---|---|---|---|---|---|---|---|---|---|
| Cai Y 2020 | RC | 941 | 7.0 mmol/L | 57 | 48.2 | 13.1 | 28.9 | 4.7 | Mortality | 8 |
| Chen L 2021 | RC | 709 | 7.7 mmol/L | 55 | 35.3 | 18.1 | 27.5 | 4.7 | Mortality | 8 |
| Coppelli A 2020 | RC | 271 | 7.78 mmol/L | 72 | 66.8 | No Diabetes | NR | NR | Mortality | 7 |
| Deng M 2020 | RC | 65 | 6.1 mmol/L | 33.8 | 55.4 | 3.1 | 4.6 | NR | Severity | 7 |
| Gastélum-Cano J 2021 | RC | 82 | 7.0 mmol/L | 61.2 | 58.5 | 31.7 | NR | NR | Mortality | 5 |
| Huh K 2020 | RC | 2231 | 7.0 mmol/L | Stratified | 39 | 33.9 | 32.7 | 8.3 | Severity and Mortality | 9 |
| Li H 2020 | RC | 261 | 7.0 mmol/L | 54.7 | 46.7 | No Diabetes | 22.2 | 1.1 | Mortality | 8 |
| Liu S 2020 | RC | 255 | 7.0 mmol/L | 64 | 53.3 | 20 | 39.6 | NR | ICU | 8 |
| Sardu C 2020 | RC | 59 | 7.7 mmol/L | 67.4 | 81.4 | 44 | 74.6 | NR | Severity and Mortality | 6 |
| Sun Y 2020 | RC | 3400 | 7.0 mmol/L | 61 | 48.5 | 21.6 | 52.4 | 2.2 | Mortality | 8 |
| Wang S 2020 | RC | 605 | 7.0 mmol/L | 59 | 53.2 | No Diabetes | 25.6 | NR | Mortality | 8 |
| Zhang Y 2020 | RC | 166 | 7.0 mmol/L | 62.7 | 51.2 | No Diabetes | 45.8 | 5.4 | Severity | 6 |
CKD: Chronic Kidney Disease; ICU: Intensive Care Unit; RC: Retrospective Cohort; NOS: Newcastle-Ottawa Scale; NR: Not Reported.
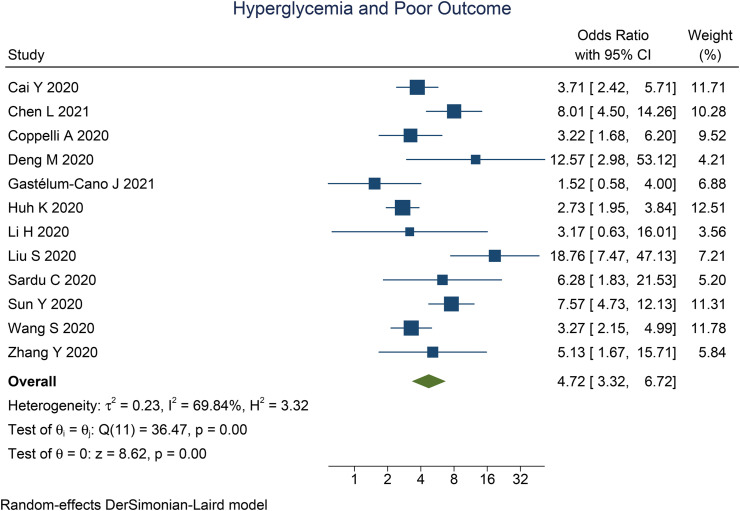
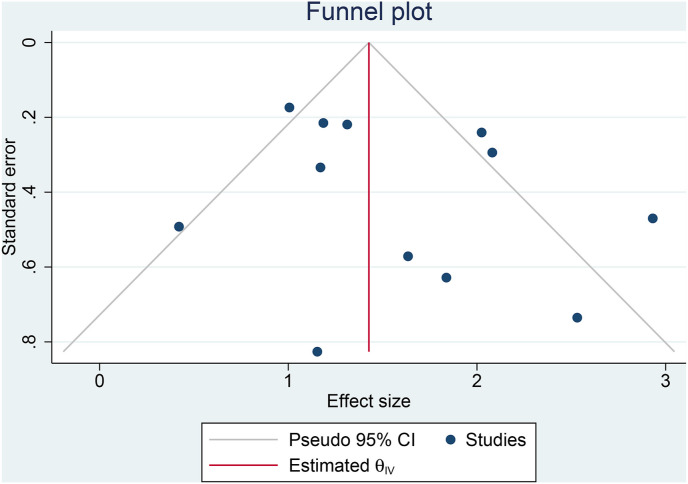
Fasting hyperglycemia was associated with poor outcome in COVID-19 (OR 4.72 [3.32, 6.72], p < 0.001; I2: 69.8%, p < 0.001) [Fig. 2 ]. The funnel-plot was symmetrical [Fig. 3 ], and there is no indication of significant small-study effects (p = 0.419). The association between fasting hyperglycemia and poor outcome was affected by chronic kidney disease (OR 0.87 [0.76, 0.99], p = 0.033), but not age (p = 0.418), male (gender) (p = 0.860), and hypertension (p = 0.657).
Fig. 2.
Fasting hyperglycemia and poor outcome.
Fig. 3.
Publication bias. Funnel-plot analysis.
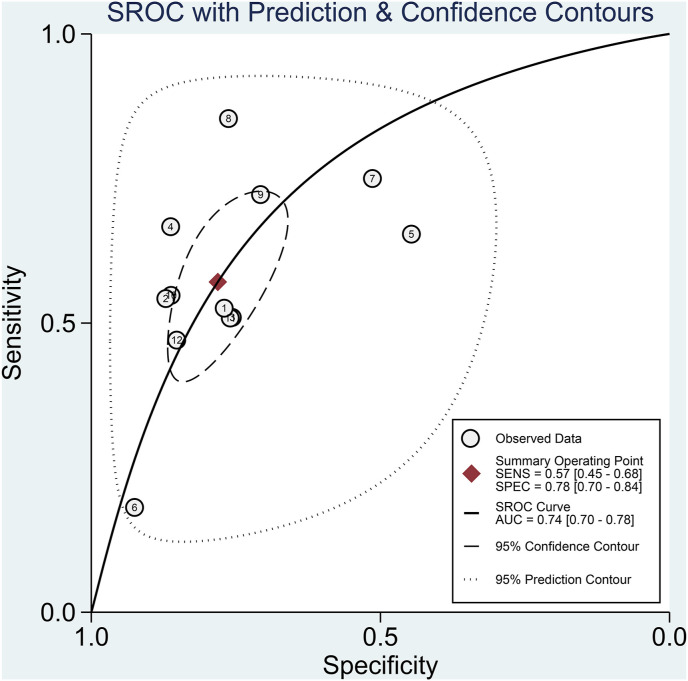
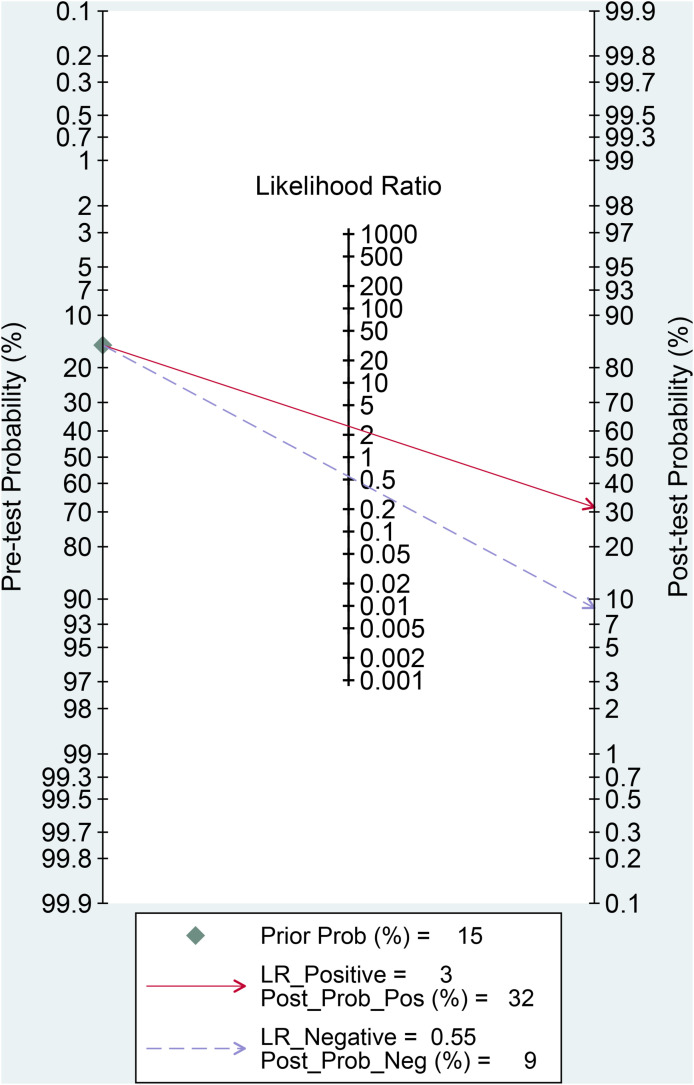
Fasting hyperglycemia has a sensitivity of 0.57 [0.45, 0.68], specificity of 0.78 [0.70, 0.84], PLR of 2.6 [2.0, 3.3], NLR of 0.55 [0.44, 0.69], DOR of 5 [3, 7], and AUC of 0.74 [0.70, 0.78] for predicting poor outcome [Fig. 4 ]. In this pooled analysis, fasting hyperglycemia has a 32% post-test probability for poor outcome, and absence of fasting hyperglycemia confers to a 9% post-test probability [Fig. 5 ]. Meta-regression and subgroup analysis showed that the sensitivity and specificity varies by chronic kidney disease but not by age, male (gender), and hypertension.
Fig. 4.
Sroc curve.
Fig. 5.
Fagan's nomogram.
3.1. Subgroup analysis
Subgroup analysis in patients without prior history of diabetes showed that fasting hyperglycemia was associated with poor outcome in COVID-19 (OR 3.387 [2.433, 4.714], p < 0.001; I2: 0, p = 0.90). Fasting hyperglycemia has a sensitivity of 0.54 [0.42, 0.65], specificity of 0.73 [0.60, 0.83], PLR of 2.0 [1.4, 2.7], NLR of 0.63 [0.53, 0.76], DOR of 3 [2, 5], and AUC of 0.65 [0.61, 0.69] for predicting poor outcome.
4. Discussion
This meta-analysis showed that fasting hyperglycemia was associated with poor outcome in patients with COVID-19, with a 57% sensitivity, 78% specificity, and AUC of 0.74. Our meta-analysis indicates that fasting hyperglycemia increases the risk of poor outcome in patients with and without diabetes.
Inflammation triggered by COVID-19 infection may impair insulin signaling which causes reduced glycogen synthesis, glucose uptake, and lipogenesis; thereby causing hyperglycemia and insulin resistance, which may manifest as new-onset diabetes (Sathish et al., 2021b). An observational study on patients without diabetes indicates a significantly higher glycemic fluctuation in previously normoglycemic patients with COVID-19, compared to controlled match (Shen et al., 2021).
Increased severity in diabetic patients with COVID-19 has been described previously, in which one of them is due to dysfunctional pro-inflammatory cytokine responses (Maddaloni and Buzzetti, 2020; Pal and Bhansali, 2020). Higher pro-inflammatory cytokines are observed in diabetic patients. Inflammatory markers such as C-reactive protein and D-dimer are more pronounced in diabetic COVID-19 patients (Maddaloni and Buzzetti, 2020). These factors are associated with severity and mortality in COVID-19 patients (Huang et al., 2020b). These may further aggravate COVID-19 induced cytokine storms, causing more severe disease (Mehta et al., 2020; Pal and Bhansali, 2020).
The proportion of patients with diabetes might cause heterogeneity. In the subgroup analysis of patients without diabetes, the heterogeneity was 0%. In patients without diabetes, the increased mortality due to hyperglycemia might indicate acute blood-glucose disorder due to stress hyperglycemia (Bar-Or et al., 2019). Critically ill patients are also likely to develop acute insulin resistance, which manifests as hyperglycemia and hyperinsulinaemia; this can be found in sepsis and other pathologies, irrespective of diabetes (Bar-Or et al., 2019; McAlister et al., 2005). These mechanisms may also exacerbate hyperglycemia in diabetic patients.
Meta-regression analysis showed that chronic kidney disease reduces the association between hyperglycemia and poor outcome. Sardu et al. demonstrate that patients with hyperglycemia receiving insulin during hospitalization have lower mortality (Sardu et al., 2020), patients with chronic kidney disease are more likely to be given insulin because of its safety profile in renal insufficiency. Thus, chronic kidney disease may seem to reduce mortality. Sardu et al. study also provides evidence that optimal glycemic control during hospitalization is of paramount importance to ensure the best outcome. Another possibility is because of renal insufficiency association with mortality in COVID-19 patients (Lim et al., 2020b; Raymond Pranata et al., 2020c), hyperglycemia as a factor for mortality in these patients might be weaker than chronic kidney disease itself.
There are several limitations in our meta-analysis, first is that all studies were retrospective. Then other essential parameters such as HbA1C, specifics of antidiabetic medications (Lukito et al., 2020), and other comorbidities were inadequately reported, thus, meta-regression of these variables were not feasible. Variables such as cardiovascular disease are not reported uniformly; i.e. it might be designated as “cardiovascular disease” or “coronary heart disease” or “chronic heart disease” which are different per definition. Comorbidities such as obesity are also inadequately reported. These variables have been shown to increase mortality in COVID-19 patients (R. Pranata et al., 2020d; Raymond Pranata et al., 2020a). The cut-off point for fasting hyperglycemia also varies between studies.
5. Conclusion
Fasting hyperglycemia was associated with mortality in COVID-19 patients, with or without diabetes.
Funding statement
None.
Ethical approval
Not Applicable.
Informed consent
Not Applicable.
Data availability
Data are available on reasonable request.
CRediT authorship contribution statement
Dewi Ratih Handayani: Data curation, Investigation, Writing – original draft. Henny Juliastuti: Conceptualization, Data curation, Investigation, Writing – review & editing, Supervision. Eka Noneng Nawangsih: Investigation, Writing – review & editing. Yudith Yunia Kusmala: Investigation, Writing – review & editing. Iis Inayati Rakhmat: Investigation, Writing – review & editing. Arief Wibowo: Investigation, Writing – review & editing. Raymond Pranata: Conceptualization, Methodology, Data curation, Formal analysis, Investigation, Validation, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declared no conflict of interest.
Acknowledgement
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.obmed.2021.100333.
Abbreviations
- COVID-19
Coronavirus Disease 2019
- ICU
Intensive Care Unit
- IMV
Invasive Mechanical Ventilation
- NLR
Negative Likelihood Ratio
- OR
Odds Ratio
- PLR
Positive Likelihood Ratio
- SROC
Summary Receiver Operating Characteristic
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- Bar-Or D., Rael L.T., Madayag R.M., Banton K.L., Tanner A., Acuna D.L., Lieser M.J., Marshall G.T., Mains C.W., Brody E. Stress hyperglycemia in critically ill patients: insight into possible molecular pathways. Front. Med. 2019;6 doi: 10.3389/fmed.2019.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Shi S., Yang F., Yi B., Chen X., Li J., Wen Z. Fasting blood glucose level is a predictor of mortality in patients with COVID-19 independent of diabetes history. Diabetes Res. Clin. Pract. 2020;169 doi: 10.1016/j.diabres.2020.108437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Sun W., Liu Y., Zhang L., Lv Y., Wang Q. Association of early-phase in-hospital glycemic fluctuation with mortality in adult patients with coronavirus disease 2019 2019. 2021. 1-9. [DOI] [PubMed]
- Coppelli A., Giannarelli R., Aragona M., Penno G., Falcone M., Tiseo G., Ghiadoni L., Barbieri G., Monzani F., Virdis A., Menichetti F., Prato S. Del. Hyperglycemia at hospital admission is associated with severity of the prognosis in patients hospitalized for COVID-19: the pisa COVID-19 study. Diabetes Care. 2020;43:2345–2348. doi: 10.2337/dc20-1380. [DOI] [PubMed] [Google Scholar]
- Deng M., Qi Y., Deng L., Wang H., Xu Y., Li Z., Meng Z., Tang J., Dai Z. Obesity as a potential predictor of disease severity in young COVID-19 patients: a retrospective study. Obesity. 2020;28:1815–1825. doi: 10.1002/oby.22943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastélum-Cano J.M., Islas-Osuna M.A., Arízaga-Berber J.A. Higher values of fasting blood glucose and glycated hemoglobin are not associated with mortality in Covid-19 Mexican patients. Prim. Care Diabetes. 2021;15:1–3. doi: 10.1016/j.pcd.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia – a systematic review, meta-analysis, and meta-regression: diabetes and COVID-19. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang I., Pranata R., Lim M.A., Oehadian A., Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther. Adv. Respir. Dis. 2020;14 doi: 10.1177/1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh K., Lee R., Ji W., Kang M., Hwang I.C., Lee D.H., Jung J. Impact of obesity, fasting plasma glucose level, blood pressure, and renal function on the severity of COVID-19: a matter of sexual dimorphism? Diabetes Res. Clin. Pract. 2020;170 doi: 10.1016/j.diabres.2020.108515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- July J., Pranata R. Prevalence of dementia and its impact on mortality in patients with coronavirus disease 2019: a systematic review and meta-analysis. Geriatr. Gerontol. Int. (GGI) 2020:14107. doi: 10.1111/ggi.14107. [DOI] [PubMed] [Google Scholar]
- Li H., Tian S., Chen T., Cui Z., Shi N., Zhong X., Qiu K., Zhang J., Zeng T., Chen L., Zheng J. Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID-19. Diabetes Obes. Metabol. 2020;22:1897–1906. doi: 10.1111/dom.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim M.A., Pranata R., Huang I., Yonas E., Soeroto A.Y., Supriyadi R. Multiorgan failure with emphasis on acute kidney injury and severity of COVID-19: systematic review and meta-analysis. Can. J. Kidney Health Dis. 2020;7 doi: 10.1177/2054358120938573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim M.A., Pranata R., Huang I., Yonas E., Soeroto A.Y., Supriyadi R. Multiorgan failure with emphasis on acute kidney injury and severity of COVID-19: systematic review and meta-analysis. Can. J. Kidney Health Dis. 2020;7 doi: 10.1177/2054358120938573. 205435812093857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. ping, Zhang Q., Wang W., Zhang M., Liu C., Xiao X., Liu Z., Hu W. mu, Jin P. Hyperglycemia is a strong predictor of poor prognosis in COVID-19. Diabetes Res. Clin. Pract. 2020;167:108338. doi: 10.1016/j.diabres.2020.108338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukito A.A., Pranata R., Henrina J., Lim M.A., Lawrensia S., Suastika K. The effect of metformin consumption on mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:2177–2183. doi: 10.1016/j.dsx.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddaloni E., Buzzetti R. Covid-19 and diabetes mellitus: unveiling the interaction of two pandemics. Diabetes. Metab. Res. Rev. 2020:e33213321. doi: 10.1002/dmrr.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martha J.W., Wibowo A., Pranata R. Prognostic value of elevated lactate dehydrogenase in patients with COVID-19: a systematic review and meta-analysis. Postgrad. Med. 2021 doi: 10.1136/postgradmedj-2020-139542. postgradmedj-2020-139542. [DOI] [PubMed] [Google Scholar]
- McAlister F.A., Majumdar S.R., Blitz S., Rowe B.H., Romney J., Marrie T.J. The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community-acquired pneumonia. Diabetes Care. 2005;28:810–815. doi: 10.2337/diacare.28.4.810. [DOI] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Bhansali A. COVID-19, diabetes mellitus and ACE2: the conundrum. Diabetes Res. Clin. Pract. 2020;162:108132. doi: 10.1016/j.diabres.2020.108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata R., Henrina J., Lim M.A., Lawrensia S., Yonas E., Vania R., Huang I., Lukito A.A., Suastika K., Kuswardhani R.A.T., Setiati S. Clinical frailty scale and mortality in COVID-19: a systematic review and dose-response meta-analysis: clinical Frailty Scale in COVID-19. Arch. Gerontol. Geriatr. 2021;93:104324. doi: 10.1016/j.archger.2020.104324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata Raymond, Huang I., Lim M.A., Wahjoepramono E.J., July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19–systematic review, meta-analysis, and meta-regression. J. Stroke Cerebrovasc. Dis. 2020;29:104949. doi: 10.1016/j.jstrokecerebrovasdis.2020.104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata Raymond, Lim M.A., Huang I., Raharjo S.B., Lukito A.A. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. (JRAAS) J. Renin-Angiotensin-Aldosterone Syst. 2020;21 doi: 10.1177/1470320320926899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata R., Lim M.A., Yonas E., Huang I., Nasution S.A., Setiati S., Alwi I., Kuswardhani R.A.T. Thrombocytopenia as a prognostic marker in COVID-19 patients: diagnostic test accuracy meta-analysis. Epidemiol. Infect. 2021;149:e40. doi: 10.1017/S0950268821000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata R., Lim M.A., Yonas E., Vania R., Lukito A.A., Siswanto B.B., Meyer M. Body mass index and outcome in patients with COVID-19: a dose–response meta-analysis. Diabetes Metab. 2020 doi: 10.1016/j.diabet.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata Raymond, Supriyadi R., Huang I., Permana H., Lim M.A., Yonas E., Soetedjo N.N.M., Lukito A.A. The association between chronic kidney disease and new onset renal replacement therapy on the outcome of COVID-19 patients: a meta-analysis. Clin. Med. Insights Circulatory, Respir. Pulm. Med. 2020;14 doi: 10.1177/1179548420959165. 1179548420959165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardu C., D'Onofrio N., Balestrieri M.L., Barbieri M., Rizzo M.R., Messina V., Maggi P., Coppola N., Paolisso G., Marfella R. Outcomes in patients with hyperglycemia affected by COVID-19: can we do more on glycemic control? Diabetes Care. 2020;43:1408–1415. doi: 10.2337/dc20-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathish T., de Mello G.T., Cao Y. Is newly diagnosed diabetes a stronger risk factor than pre-existing diabetes for COVID-19 severity? J. Diabetes. 2021;13:177–178. doi: 10.1111/1753-0407.13125. [DOI] [PubMed] [Google Scholar]
- Sathish T., Tapp R.J., Cooper M.E., Zimmet P. Potential metabolic and inflammatory pathways between COVID-19 and new-onset diabetes. Diabetes Metab. 2021;47:101204. doi: 10.1016/j.diabet.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Zhang L., Fan X., Zhou J. Glycemic fluctuations caused by COVID-19: results from continuous glucose monitoring. Obes. Med. 2021;22:100328. doi: 10.1016/j.obmed.2021.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha E., Charkviani M., Musurakis C., Kansakar A.R., Devkota A., Banjade R., Pudasainee P., Chitrakar S., Sharma A., Sous M., Padhamanbhan S., Friedman H.J., Nava G.R. Type 2 diabetes is associated with increased risk of critical respiratory illness in patients COVID-19 in a community hospital. Obes. Med. 2021 doi: 10.1016/j.obmed.2020.100316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Guan X., Jia L., Xing N., Cheng L., Liu B., Zhang S., He K. Independent and combined effects of hypertension and diabetes on clinical outcomes in patients with COVID-19: a retrospective cohort study of Huoshen Mountain Hospital and Guanggu Fangcang Shelter Hospital. J. Clin. Hypertens. 2020:1–14. doi: 10.1111/jch.14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuty Kuswardhani R.A., Henrina J., Pranata R., Anthonius Lim M., Lawrensia S., Suastika K. Charlson comorbidity index and a composite of poor outcomes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:2103–2109. doi: 10.1016/j.dsx.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Ma P., Zhang S., Song S., Wang Z., Ma Y., Xu J., Wu F., Duan L., Yin Z., Luo H., Xiong N., Xu M., Zeng T., Jin Y. Fasting blood glucose at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes: a multi-centre retrospective study. Diabetologia. 2020;63:2102–2111. doi: 10.1007/s00125-020-05209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO COVID-19 Epidemiological Updates; Geneva: 2021. Weekly Epidemiological Update - 9 February 2021. [Google Scholar]
- Zhang Y., Li H., Zhang Jian, Cao Y., Zhao X., Yu N., Gao Y., Ma J., Zhang H., Zhang Junqing, Guo X., Liu X. The clinical characteristics and outcomes of patients with diabetes and secondary hyperglycaemia with coronavirus disease 2019: a single-centre, retrospective, observational study in Wuhan. Diabetes Obes. Metabol. 2020;22:1443–1454. doi: 10.1111/dom.14086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request.