Abstract
Background
COVID-19 pandemic resulted in an unprecedented number of hospitalizations in general wards and intensive care units (ICU). Severe and critical COVID-19 patients suffer from extensive pneumonia; therefore, long-term respiratory sequelae may be expected.
Research question
We conducted a cohort study to determine respiratory sequelae in patients with severe and critical COVID-19. We aimed at evaluating the proportion of patients with persisting respiratory symptoms and/or abnormalities in pulmonary function tests (PFT) or in lung imaging.
Study design
and methods: This is a single center cohort study including COVID-19 survivors who underwent a three-month follow-up with clinical evaluation, PFT and lung high-resolution computed tomography (HRCT). All clinical, functional, and radiological data were centrally reviewed. Multiple linear regression analysis was performed to identify factors associated with residual lesions on HRCT.
Results
Full clinical evaluation, PFT and lung HRCT were available for central review in 126, 122 and 107 patients, respectively. At follow-up, 25% of patients complained from dyspnea and 35% from fatigue, lung diffusion capacity (DLCO) was decreased in 45%, 17% had HRCT abnormalities affecting more than 5% of their lung parenchyma while signs of fibrosis were found in 21%. In multiple linear regression model, number of days in ICU were related to the extent of persisting lesions on HRCT, while intubation was associated with signs of fibrosis at follow-up (P = 0.0005, Fisher's exact test). In contrast, the severity of lung imaging or PFT changes were not predictive of fatigue and dyspnea.
Interpretation
Although most hospitalized COVID-19 patients recover, a substantial proportion complains from persisting dyspnea and fatigue. Impairment of DLCO and signs suggestive of fibrosis are common but are not strictly related to long-lasting symptoms.
Keywords: COVID19, Follow-up, Pulmonary function tests, Lung fibrosis, Lung HRCT, Long COVID
1. Introduction
The first wave of COVID-19 pandemic affected most western countries between March and June 2020, resulting in an unprecedented burden on healthcare systems [1] and death excess [2]. This new virus mostly affects the elderly [3] and people suffering from chronic cardiovascular comorbidities, obesity, and diabetes are at higher risk [4]. Overall mortality is about 1.5% [5,6]. In-hospital mortality from COVID-19 is estimated to be between 15 and 20% [7], which is substantially higher than from other respiratory viral infections like flu [6]. In Belgium, the first wave peaked in April 2020, with more than 5700 hospitalized patients including nearly 1300 patients in intensive care units (ICU), most of them requiring mechanical ventilation (www.sciensano.be). Whether people who survive from severe (viral pneumonia and oxygen requirement) or critical (requiring mechanical ventilation) COVID-19 will completely recover or not is crucial both on healthcare and on socio-economic perspectives [8]. Like with severe acute respiratory syndrome coronavirus (SARS-Cov), Middle East Respiratory Coronavirus (MERS-Cov) and flu [9,10], long-term consequences are expected for the most affected patients, including residual lung opacities, fibrosis and pulmonary function impairment. However, objective information on respiratory follow-up of COVID-19 patients is scarce [11,12]. Therefore, we aimed at determining in severe and critical COVID-19 survivors the proportion of respiratory sequelae in clinical outcomes, pulmonary function tests (PFT) and lung high-resolution computed tomography (HRCT) at three-month follow-up. We also looked for interactions between analyzed variables. We hypothesized that more severe patients, namely with a greater extent of lesions at baseline and/or admitted in ICU, would display more sequelae at follow-up.
2. Material and methods
2.1. Study population
This is a single center cohort study. We included severe and critical (according to NHS definition, https://www.covid19treatmentguidelines.nih.gov/overview/) COVID-19 patients (diagnosis by confirmed positive PCR on nasopharyngeal swab and lung infiltrates on lung HRCT or chest X-ray at admission) admitted between March 10 and June 30, 2020, who survived and underwent a three-month follow-up in our hospital, consisting of [1] a clinical assessment [2], PFT and [3] lung HRCT. We included all patients for whom at least two out of three (clinical, PFT, HRCT) follow-up evaluations were available. We excluded patients for whom the delay between admission and follow-up evaluation was lower than 60 days or exceeded 120 days. All data (clinical, PFT, HRCT) were centrally reviewed.
We applied the STROBE criteria for observational studies (https://www.strobe-statement.org) and provide our checklist as a supplemental file.
2.2. Clinical assessment
A specialized physician in pulmonology or general internal medicine and infectious diseases evaluated included patients during outpatient clinics. Beside demographic and health data, we collected data at follow-up on respiratory symptoms (dyspnea, cough, chest tightness), general symptoms (fatigue, fever) and modified medical research council (mMRC) dyspnea scale. Only medically confirmed comorbidities (and not self-reported) diseases were considered.
2.3. Lung function assessment
Patients underwent spirometry, including measures of forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), forced expiratory flow at 25–75% of FVC (FEF25-75%) and diffusing capacity of the lung (DLCO). All pulmonary function tests (PFT) data were centrally reviewed (AF and GL). Results were expressed in percentage of the expected value, based on the Global Lung Function Initiative (GLI) references values [13,14].
2.4. Radiological assessment
State-of-the-art HRCT were obtained on supine position in full-inspiration using a 256-detector row CT (Ingenuity CT, Philips Healthcare, Cleveland, OH, USA) or a 128-detector row CT (Definition CT, Siemens Healthineers, Forchheim, Germany). Follow-up CT examination also included a full end-expiration acquisition. HRCT performed at follow-up were compared to baseline HRCT. A central review of both baseline and follow-up imaging was performed in consensus by one junior (AM) and two senior radiologists (BG, EC) with 2, 30 and 32 years of experience in cardio-thoracic CT, respectively. We also obtained a quantitative analysis of the extension of lung lesions on admission and follow-up HRCT with a commercially available software (CT Pneumonia Analysis software, Siemens Healthineers, Forchheim, Germany [15]) and obtained a percentage opacity score, defined as the percentage of volume of lung abnormalities compared to the total lung volume, as previously described [16]. The software provides a percentage of any abnormal lung parenchymal lesion without distinction between ground glass, consolidations or reticulations. Detection of those specific features was performed by readers in a qualitative analysis.
For some analysis, patients were stratified according to the extent of lesions (0–5%, ≥5%, ≥10%, ≥20%).
2.5. Statistics
Descriptive statistics consist of median and interquartile range (IQR), unless specified. For multiple variables analysis, we used a multiple linear regression model with least squares method. We sought to determine determinants of the extent of HRCT lesions at follow-up (dependent variable) among the following independent variables (explanatory variables): age, sex, baseline CRP, baseline HRCT extent, number of days in hospital, number of days in ICU, intubation, maximum oxygen flow (L/min) during hospitalization, lung diffusion capacity at follow-up. Multivariate analysis allowed to take potentially interdependent variables into account.
Single associations between categorical variables were assessed with Fisher's exact test. Statistic tests were performed using GraphPad Prism 9.0 (San Diego, CA, USA) and a P-value below 0.05 was considered significant.
Statistical consultancy was provided by the Support en Méthodologie et Calcul Statistique (SMCS), UCLouvain.
2.6. Ethical considerations
This study was approved by our local ethics committee (Comité d’Ethique Hospitalo-facultaire, Cliniques universitaires Saint-Luc), registration numbers 2020/06AVR/200 and 2020/06AVR/201. Patients provided informed consent.
3. Results
3.1. Study population and baseline characteristics
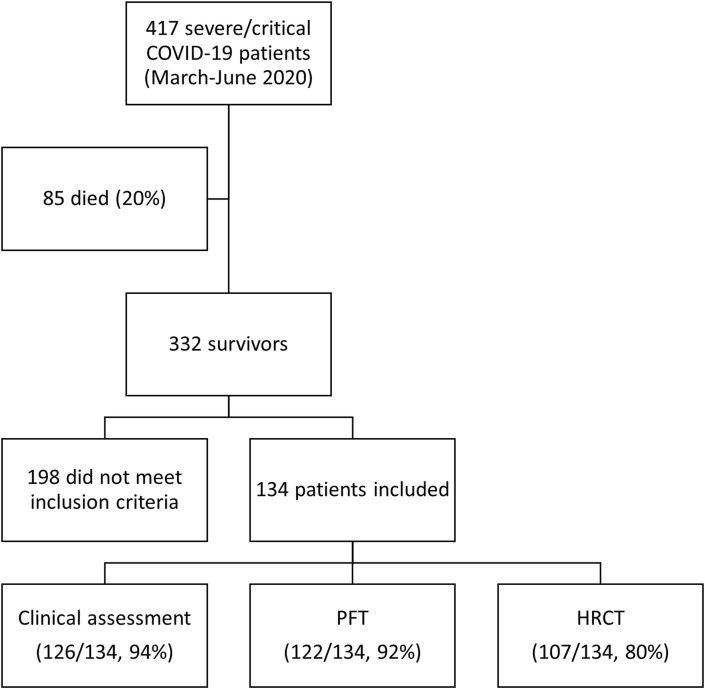
Four hundred seventeen patients were hospitalized between March 10th and June 30th, 2020 for severe or critical COVID-19, according to the NIH definition. Eighty-five of those patients died during their hospital stay (20%). One hundred thirty-four patients met the inclusion criteria (Fig. 1 ). Due to practical issues (missed appointment, patients’ refusal and/or inability to undergo HRCT), clinical, lung function and HRCT assessments were possible in 126 patients (94%), 122 patients (91%) and 107 patients (80%), respectively (see Fig. 2).
Fig. 1.
Study flowchart.
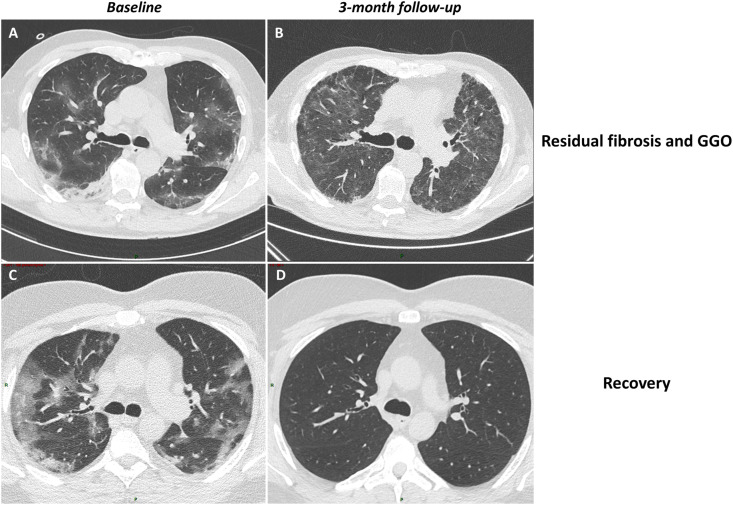
Fig. 2.
Two examples of three-month follow-up HRCT. Patient 1 shows ground-glass opacities (GGO) and consolidations (lesions extent 25.2% of lung parenchyma) at baseline (A) resulting in combined extensive GGO and signs of fibrosis, namely traction bronchiectasis and reticulations (total lesions extent 88.47%) at three-month follow-up (B). Patient 2 shows GGO and consolidations (lesions extent 44.92%) at baseline (C) followed by almost complete recovery (lesions extent 0.7%) at follow-up (D). As described in the methods, the extension of lesions is provided by the software while radiologists perform qualitative assessment of lesions.
The main baseline characteristics of our cohort are shown in Table 1 : the median age was 60 (IQR 53–68), 59% were men. Thirty patients (22%) were current or former smokers and 25 (19%) suffered from a confirmed chronic respiratory condition, namely asthma (N = 12), chronic obstructive pulmonary disease (COPD) with or without emphysema (N = 5), lung cancer (N = 3), interstitial lung disease (N = 2), chronic pulmonary embolism (N = 2) and cystic fibrosis (N = 1). Confirmed non-respiratory comorbidities consisted of overweight, defined by a body mass index (BMI) ≥25 (N = 84, 63%), obesity, defined by a BMI ≥30 (N = 37, 28%), diabetes (N = 29, 22%), hypercholesterolemia (N = 56, 42%) and hypertension (N = 63, 47%). Of note, 39 patients (29%) were treated with an angiotensin-converting-enzyme inhibitor at baseline. Baseline CRP (at admission) was 78.70 (39.00–153.90) mg/dL (median, IQR). Thirty patients (22%) were admitted in ICU, 15 were intubated. Median duration of ICU stay was 12 days (range 1–110). The median length of hospital stay in the whole cohort was 11 days (range 1–183).
Table 1.
Demographic and clinical characteristics of COVID-19 survivors.
| N = 134 | |
|---|---|
| Age (years, median, IQR) | 60 (53–68) |
| Male sex (N, %) | 79 (59) |
| Current or ex-smoker (N, %) | 30 (22) |
| Overweight (BMI≥25 kg/m2, N, %) | 84 (63) |
| Documented respiratory condition (N, %) | 25 (19) |
| Diabetes (N, %) | 29 (22) |
| Hypertension (N, %) | 63 (47) |
| Hypercholesterolemia (N, %) | 56 (42) |
IQR: interquartile range.
3.2. Three-month follow-up clinical assessment
A comprehensive clinical assessment was obtained in 126 patients (94%). At three-month follow-up (median follow-up interval of 95 days, IQR 86–107), 32 patients (25%) reported ongoing fatigue and 45 (35%) were still suffering from dyspnea (N = 26 with grade 1, N = 14 with grade 2, N = 5 with grade 3 mMRC). Four patients required oxygen supplementation at exercise.
Other respiratory complains consisted of chronic dry cough in 14 patients (10%) and chest oppression in six patients (4%).
Twenty-five patients (19%) had participated in a rehabilitation program following their hospitalization.
3.3. Pulmonary function assessment at three-month follow-up
One hundred twenty-two patients (91%) underwent spirometry and DLCO assessment at three-month follow-up (median interval 97 days, IQR 84–110). Table 2 contains PFT median values of the cohort: in brief, little impact of COVID-19 was found on volumes, with median forced vital capacity (FVC) and forced expired volume in 1 s (FEV1) close to normal (median FVC 88%, IQR 78–98, median FEV1 91%, IQR 81–102 and median FEV1/FVC ratio 98%, IQR 83–106). In investigation of small airways involvement, only five patients (4%) had a Z-score below 2 standard deviations for FEF25-75%.
Table 2.
Pulmonary function tests results at three-month follow-up.
| N = 122 | |
|---|---|
| FEV1/FVC ratio (%, median, IQR) | 96 (83–106) |
| FVC (% predicted values, median, IQR) | 88 (78–98) |
| N patients with impaired FVC (Z-score ≤ −2) (N, %) | 24 (19) |
| FEV1 (% predicted values, median, IQR) | 91 (81–102) |
| N patients with impaired FEV1 (Z-score ≤ −2) (N, %) | 19 (15) |
| N patients with impaired FEF25-75 (Z score ≤ −2) (N, %) | 5 (3.73) |
| DLCO (% predicted values, median, IQR) | 74 (61–89) |
| N patients with impaired DLCO (Z-score ≤ −2) (N, %) | 58 (46) |
FEV1: forced expired volume in 1 s; FVC: forced vital capacity; DLCO: lung diffusion capacity; FEF25-75: forced expiratory flow at 25–75% of forced vital capacity.
In contrast, median lung DLCO was lower than normal values (74%, IQR 61.25–88.75). Fifty-eight patients (47%) had an impaired DLCO according to GLI reference values and 33 (27%) had a DLCO <60% of predicted values (moderate to severe DLCO impairment).
3.4. Radiological assessment at three-month follow-up
One hundred and seven patients (80%) underwent HRCT at three-month follow-up (median interval 103 days, IQR 85–114), which was compared to baseline HRCT.
At baseline (N = 113 HRCT available for central review), median extent of lung involvement was 15.79% (IQR 7.32–29.42). HRCT lesions mostly consisted in ground glass opacities (N = 104, 92%) and consolidations (N = 83, 73%). Reticulations and signs suggestive of fibrosis (combining reticulations and traction bronchiolectasis) were rare at baseline (N = 3, 3% and 2, 2% respectively). When stratifying patients for lesions extent as measured by the CT Pneumonia Analysis software, 96 patients (85%) had baseline lesions extent ≥5%, 75 (66%) ≥10% and 42 (37%) ≥20% of lung parenchyma.
At three-month follow-up HRCT, median extent of lung involvement was 0.26% (IQR 0.02–2.62). Persisting lung lesions consisted of ground glass opacities in 73 patients (67%) and consolidations in eight patients (7%). When compared to baseline, most patients had a decrease of the extent of ground glass opacities (complete disappearance in 36, decreased in 63, similar in 2 and increased in 8) and consolidations (complete disappearance in 75, decreased in 4, similar in 1 and increased in 3). Isolated reticulations were found in 26 patients (24%) and signs of fibrosis (combination of reticulations, traction bronchi(ol)ectasis with or without honeycombing) were present in 22 patients (20%). Among them, 18 (82%) had an extent of fibrosis <10%. When considering all HRCT lesions at follow-up, median lesions extent at three months was 0.26% (IQR 0.02–2.62). When stratifying patients for all-type lesions extent, 18 patients (17%) had remaining lesions extent ≥ 5%, 15 (14%) ≥10% and 8 (7%) ≥20% of lung parenchyma.
Median radiation dose delivered to patients, expressed in milliGrays.Centimeter (mGy.cm), was 145.9, 147.4 and 125.1 for the baseline, follow-up in inspiration and follow-up in expiration HRCT, respectively.
3.5. Associations between baseline data and follow-up sequelae
We built a model to explain the extent of HRCT lesions at three-month follow-up (dependent variable) based on age, sex, baseline CRP, baseline HRCT extent, number of days in hospital, intubation, and PFT at follow-up. The R-squared of this model was 0.31. The number of days spent in hospital was significantly associated with HRCT extent at follow-up when controlling for the other explanatory variables, with an estimated coefficient of 0.31 (P < 0.001). This means that our model could explain 31% of the variance of the extent of lesions on follow-up HRCT and that for every supplemental day in hospital, the mean lesions extent on HRCT rises by 0.31% points. The number of days in hospital and the number of days in ICU are highly interdependent, with a variance inflation factor >8 in a simulation integrating age, sex, baseline CRP, baseline HRCT extent, number of days in hospital, number of days in ICU, intubation, and PFT at follow-up). Thus, we built a similar model using the number of days in ICU instead of the number of days in hospital. This model explained even more variability in the dependent variable, with an effect mainly driven by the number of days in ICU (R-squared 0.41, P < 0.001, and estimate of 0.58 for number of days in ICU). In other words, this model explains 41% of the variance of extent of HRCT lesions at follow-up and for every supplemental day in ICU, the mean lesions extent on HRCT rises by 0.58% points. Another multivariate model considering age, sex, baseline CRP, baseline HRCT extent and intubation allowed us to determine that intubation was significantly linked to the extent of lesions on follow-up HRCT (R-squared 0.36, P < 0.0001).
We confirmed the effect of ICU and mechanical ventilation on extent of lesions with a contingency analysis: admission in ICU was significantly associated (P = 0.0005, Fisher's exact test) with an extent of HRCT lesions at three-month follow-up of at least five percent, with an odds ratio (OR) of 7.31 (95% CI 2.45–19.69). Intubation was significantly associated (P < 0.0001, Fisher's exact test) with signs suggestive of fibrosis at follow-up (OR 7.31, 95% CI 3.70–41.59).
Using similar method, we only found a very weak link between DLCO at follow-up and the extent of HRCT lesions (R-squared 0.06, P = 0.02). Similarly, none of the explanatory variables (age, sex, extent of disease on baseline and/or follow-up HRCT, lung function parameter) was significantly linked to fatigue or dyspnea at follow-up on a multiple linear regression.
3.6. Patients clustering based on symptoms and HRCT extent or DLCO defect
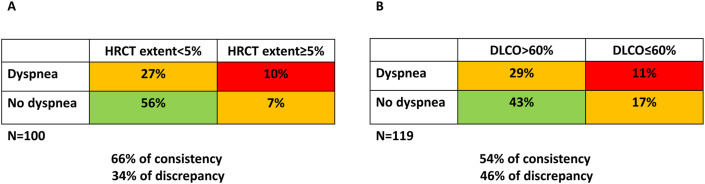
We present, in Fig. 3 , two different methods of clustering considering dyspnea versus HRCT extent (N = 100 patients, Fig. 3A) and dyspnea versus mild or severe DLCO impairment (N = 119 patients, Fig. 3B). The data show that an apparent discrepancy between absence/presence of dyspnea and substantial respiratory impairment (HRCT extent >5% at follow-up in Fig. 3A, DLCO <60% at follow-up in Fig. 3B) concerns 34% of patients when considering HRCT and 46% when considering DLCO.
Fig. 3.
Patients clustering based on symptoms versus HRCT (3A) and symptoms versus DLCO (3B).
4. Discussion
This cohort study addressing [1] respiratory sequelae at three-month follow-up on clinical, functional, and radiological aspects, and [2] the associated risk-related factors in 134 severe and critical COVID-19 survivors shows that whereas most patients have no significant sequelae, a substantial proportion suffers from long lasting symptoms and respiratory impairment. In this cohort, about 25% of patients complained from persistent fatigue at three-month follow-up and about 35% remained short of breath. Whereas many patients had an almost complete recovery of lesions on lung HRCT, 20% had signs suggestive of fibrosis and 17% had persistent lesions affecting more than 5% of their lung parenchyma. In our multivariate analysis, the main factor that explained the variance of HRCT lesions at follow-up was the duration of stay in ICU, i.e. every supplemental day was associated with an increase of mean extent of HRCT lesions. Mechanical ventilation was even associated with a higher risk of lung fibrosis.
Interestingly, none of the factors tested, namely baseline features of the disease, extent of lesions on follow-up HRCT, and PFT were able to explain the persistence of subjective symptoms like dyspnea and fatigue.
To the best of our knowledge, this work constitutes the largest integrative respiratory assessment at follow-up of patients hospitalized for severe or critical COVID-19, which combined clinical evaluation, PFT and HRCT analysis.
Other recent studies evaluated patients 3 months after COVID-19 infection: the study by van der Borst et al. performed a comprehensive health assessment of 124 patients also showing residual lesions on imaging and impaired DLCO in a large proportion of patients [17]. In contrast to our study, however, DLCO and lesions extent were mildly correlated in that study that did not include multivariate analysis. In addition, both hospitalized and ambulatory patients (thus also including patients with a mild disease) were included in that study. Similarly, up to 45% of ambulatory patients still displayed general and respiratory symptoms several weeks after their infection [18].
In our cohort of discharged COVID-19 patients, we found that about a third of patients still complained from fatigue and/or dyspnea despite only mild impairment of their lung function and an almost complete disappearance of their HRCT lesions. This indicates that objective functional and radiological assessment failed to capture all underlying pathophysiological processes at work at this stage of the disease. This point is also illustrated by our clustering of patients, showing an apparent discrepancy between the presence/absence of dyspnea and an objective respiratory impairment, either on HRCT or PFT (Fig. 3). This is consistent with the recent work by Nehme et al. who demonstrated the existence of a large COVID-19 patients population displaying impairment of their functional status and quality of life at three-month follow-up [19]. Another work conducted in Austria also demonstrated a significant proportion of patients with lung function impairment and reticulations on HRCT at follow-up [20]. The absence of significant link between dyspnea, fatigue, and measurable variables (PFT, extent of lesions on HRCT) suggests that those symptoms may be related, at least partially, to deconditioning. Although evidence on this specific point is still lacking, this supports the setting up of exercise training and rehabilitation programs for COVID-19 patients.
Altogether, in our study and the others [20], most of lesions tended to reduce with time. This might indicate that only fibrotic lesions should remain at long-term follow-up (6 months, 12 months or more), although this point will have to be confirmed in the future.
We found HRCT signs suggestive of residual fibrosis in one out of five patients. This proportion is higher than what is usually found for flu (about 10% [9]) and similar to the findings in MERS-Cov (about 30% [10]). The main determinants for fibrosis were the duration of stay in ICU, with mechanical ventilation constituting a strong risk factor. Lung fibrosis is a known consequence of prolonged mechanical ventilation [21], as high pressure triggers mechanical stress and the activation of pro-fibrotic transcription factors triggered by mechanical constrains, such as YAP/TAZ pathway [22]. As critical COVID-19 is associated with prolonged mechanical ventilation [23], whether pulmonary fibrosis is a non-specific consequence of mechanical ventilation or is directly related to the nature of the virus remains elusive. Similarly, the progressive nature (or not) of the disease should be studied and accordingly, antifibrotics like nintedanib could be considered in selected patients [24].
Our real-life study has some limitations: firstly, all patients did not undergo all planned exams, either because they missed their appointment or because they refused. However, we managed to obtain more than a hundred patients for each domain (radiology, clinical assessment, and pulmonary function tests). Secondly, functional tests like 6 min walking distance test were not performed, which precludes to validate our hypothesis that persisting dyspnea could relate to deconditioning. Finally, we may have missed some pre-existing comorbidities and baseline PFT were not available. Third, no pathologic proof of fibrosis was obtained in any patients and we based our definition of fibrosis on state-of-the-art HRCT standards. Whether some lung lesion could still further regress on long-term follow-up should be investigated further on. Finally, whether some remaining abnormalities were linked to the decubitus position was not explored by performing a prone position acquisition in order not to increase total radiation dose. Nonetheless, the vast majority of lung lesions didn't meet the characteristics of dependent artifactual abnormalities.
In conclusion, our study shows that severe and critical COVID-19 survivors may have an almost complete recovery, with PFT and HRCT close to normal, but also that a substantial proportion of patients display persisting and/or signs suggestive of pulmonary fibrosis, in most cases not extensive. We believe that these results significantly improve the knowledge about COVID-19 follow-up and warrant patients’ follow-up after the acute phase of the disease.
Funding
This work is supported by a grant from the Fondation Louvain (grant number M1.21221.004), Université catholique de Louvain, Belgium.
Authors contribution
AF designed the study, analyzed data, and wrote the manuscript.
BG supervised lung CT-scanner review and analyzed data.
JCY designed the study and participated to data interpretation.
AM, BG and EC centrally reviewed lung CT-scanner and analyzed radiological data.
GL centrally reviewed pulmonary function tests according to GLI guidelines.
AB provided council on the design and interpretation of statistics.
JDG, LB, LP, HY, GR, LG, SG, FA, BM, and CP provided clinical data on patients.
SK centralized clinical data.
AF and BG take full responsibility for the integrity of data and their interpretation.
All authors reviewed and approved the manuscript.
Declaration of competing interest
The authors have no conflict of interest in relationship with the present work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmed.2021.106383.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Tyrrell C.S.B., Mytton O.T., Gentry S.V., Thomas-Meyer M., Allen J.L.Y., Narula A.A., et al. Managing intensive care admissions when there are not enough beds during the COVID-19 pandemic: a systematic review. Thorax. 2021;76:302–312. doi: 10.1136/thoraxjnl-2020-215518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kontis V., Bennett J.E., Rashid T., Parks R.M., Pearson-Stuttard J., Guillot M., et al. Magnitude, demographics and dynamics of the effect of the first wave of the COVID-19 pandemic on all-cause mortality in 21 industrialized countries. Nat. Med. 2020;26(12):1919–1928. doi: 10.1038/s41591-020-1112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salimi S., Hamlyn J.M. COVID-19 and crosstalk with the hallmarks of aging. J. Gerontol. A Biol. Sci. Med. Sci. 2020;75(9):e34–e41. doi: 10.1093/gerona/glaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verity R., Okell L.C., Dorigatti I., Winskill P., Whittaker C., Imai N., et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect. Dis. 2020;20(6):669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piroth L., Cottenet J., Mariet A.S., Bonniaud P., Blot M., Tubert-Bitter P., et al. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir. Med. 2020;9(3):251–259. doi: 10.1016/S2213-2600(20)30527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenthal N., Cao Z., Gundrum J., Sianis J., Safo S. Risk factors associated with in-hospital mortality in a US national sample of patients with COVID-19. JAMA Netw. Open. 2020;3(12) doi: 10.1001/jamanetworkopen.2020.29058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nurchis M.C., Pascucci D., Sapienza M., Villani L., D'Ambrosio F., Castrini F., et al. Impact of the burden of COVID-19 in Italy: results of disability-adjusted life years (DALYs) and productivity loss. Int. J. Environ. Res. Publ. Health. 2020;17(12) doi: 10.3390/ijerph17124233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J., Wu J., Hao S., Yang M., Lu X., Chen X., et al. Long term outcomes in survivors of epidemic Influenza A (H7N9) virus infection. Sci. Rep. 2017;7(1):17275. doi: 10.1038/s41598-017-17497-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das K.M., Lee E.Y., Singh R., Enani M.A., Al Dossari K., Van Gorkom K., et al. Follow-up chest radiographic findings in patients with MERS-CoV after recovery. Indian J. Radiol. Imag. 2017;27(3):342–349. doi: 10.4103/ijri.IJRI_469_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogliani P., Calzetta L., Coppola A., Puxeddu E., Sergiacomi G., D'Amato D., et al. Are there pulmonary sequelae in patients recovering from COVID-19? Respir. Res. 2020;21(1):286. doi: 10.1186/s12931-020-01550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Y.M., Shang Y.M., Song W.B., Li Q.Q., Xie H., Xu Q.F., et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quanjer P.H., Stanojevic S., Cole T.J., Baur X., Hall G.L., Culver B.H., et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur. Respir. J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanojevic S., Graham B.L., Cooper B.G., Thompson B.R., Carter K.W., Francis R.W., et al. Official ERS technical standards: global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur. Respir. J. 2017;50(3) doi: 10.1183/13993003.00010-2017. [DOI] [PubMed] [Google Scholar]
- 15.Homayounieh F., Rockenbach MA Bezerra Cavalcanti, Ebrahimian S., Doda Khera R., Bizzo B.C., Buch V., et al. Multicenter assessment of CT pneumonia analysis prototype for predicting disease severity and patient outcome. J. Digit. Imag. 2021:1–10. doi: 10.1007/s10278-021-00430-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Froidure A., Mahieu M., Hoton D., Laterre P.F., Yombi J.C., Koenig S., et al. Short telomeres increase the risk of severe COVID-19. Aging (Albany NY) 2020;12(20):19911–19922. doi: 10.18632/aging.104097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van den Borst B., Peters J.B., Brink M., Schoon Y., Bleeker-Rovers C.P., Schers H., et al. Comprehensive health assessment three months after recovery from acute COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1750. ciaa1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tenforde M.W., Kim S.S., Lindsell C.J., Billig Rose E., Shapiro N.I., Files D.C., et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network - United States, March-June 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69(30):993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nehme M., Braillard O., Alcoba G., Aebischer Perone S., Courvoisier D., Chappuis F., et al. COVID-19 symptoms: longitudinal evolution and persistence in outpatient settings. Ann. Intern. Med. 2020 doi: 10.7326/M20-5926. M20-5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonnweber T., Sahanic S., Pizzini A., Luger A., Schwabl C., Sonnweber B., et al. Cardiopulmonary recovery after COVID-19 - an observational prospective multi-center trial. Eur. Respir. J. 2020;10 doi: 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cabrera-Benitez N.E., Laffey J.G., Parotto M., Spieth P.M., Villar J., Zhang H., et al. Mechanical ventilation-associated lung fibrosis in acute respiratory distress syndrome: a significant contributor to poor outcome. Anesthesiology. 2014;121(1):189–198. doi: 10.1097/ALN.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu F., Lagares D., Choi K.M., Stopfer L., Marinkovic A., Vrbanac V., et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;308(4):L344–L357. doi: 10.1152/ajplung.00300.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Botta M., Tsonas A.M., Pillay J., Boers L.S., Algera A.G., Bos L.D.J., et al. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): a national, multicentre, observational cohort study. Lancet Respir. Med. 2020;9(2):139–148. doi: 10.1016/S2213-2600(20)30459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flaherty K.R., Wells A.U., Cottin V., Devaraj A., Walsh S.L.F., Inoue Y., et al. Nintedanib in progressive fibrosing interstitial lung diseases. N. Engl. J. Med. 2019;381(18):1718–1727. doi: 10.1056/NEJMoa1908681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.