Abstract
Background
Insulin resistance has been demonstrated to be involved in the pathogenesis of atherosclerotic cardiovascular diseases (ASCVDs). This study evaluated the association between the triglyceride–glucose (TyG) index, a novel surrogate indicator of insulin resistance, and the incidence of ASCVDs in people without ASCVDs at baseline by performing a meta-analysis.
Methods
Cohort studies reporting the multivariate-adjusted association between the TyG index and the incidence of ASCVDs were obtained by searching the PubMed and Embase databases. A random-effects model incorporating intra-study heterogeneity was applied to combine the results.
Results
Eight cohort studies comprising 5,731,294 participants were included in this meta-analysis. The results showed that compared to those with the lowest TyG index category, participants with the highest TyG index category were independently associated with a higher risk of ASCVDs [hazard ratio (HR): 1.61, 95% confidence interval (CI) 1.29–2.01, I2 = 80%, P < 0.001]. This finding was consistent with the meta-analysis results with the TyG index analyzed as a continuous variable (HR per 1-unit increment of the TyG index: 1.39, 95% CI 1.18–1.64, I2 = 89%, P < 0.001). Subgroup analyses suggested that the age, sex, and diabetic status did not significantly affect the association (for subgroup analyses, all P > 0.05). Moreover, participants with the highest TyG index category were independently associated with a higher risk of coronary artery disease [(CAD), HR: 1.95, 95% CI 1.47–2.58, I2 = 92%, P < 0.001] and stroke (HR: 1.26, 95% CI 1.23–1.29, I2 = 0%, P < 0.001).
Conclusions
A higher TyG index may be independently associated with a higher incidence of ASCVDs, CAD, and stroke in people without ASCVDs at baseline.
Keywords: Triglyceride-glucose index, Insulin resistance, Atherosclerotic cardiovascular diseases, Coronary artery disease, Meta-analysis
Background
Despite significant advances in the prevention and treatment of cardiovascular diseases, atherosclerotic cardiovascular diseases (ASCVDs), which mainly include coronary artery disease (CAD) and stroke, remain one of the leading causes of death worldwide [1, 2]. Established risk factors of ASCVDs include age, the male sex, family history of ASCVDs, obesity, hypertension, hypercholesteremia, and diabetes [3, 4]. However, more recent studies have demonstrated that some patients without these risk factors may still develop ASCVDs, thus highlighting the importance of identifying novel risk factors for ASCVDs in the general population [5–7]. Previous studies have suggested that insulin resistance, which is not only prevalent in patients with type 2 diabetes mellitus but also in people with obesity or metabolic syndrome, may also be involved in the pathogenesis of ASCVDs [8–10]. Classically, the “gold standard” method for the evaluation of insulin sensitivity is the hyperinsulinemic-euglycemic clamp test [11]. However, this method is time consuming and expensive, which limit its use in clinical settings [12]. Interestingly, the triglyceride–glucose (TyG) index, a parameter derived from the fasting blood glucose and triglyceride levels, has been proposed as a convincing indicator of insulin resistance [13]. Observational studies have shown that a higher TyG index is associated with the prevalence of ASCVDs in the general population [14]. Nevertheless, these studies mostly consist of a cross-sectional design [15–17]. Recently, accumulating cohort studies evaluating the association between the TyG index at baseline and the subsequent incidence of ASCVDs in the general population have been published [18–25]. Accordingly, the aim of this study was to summarize the potential independent association between the TyG index and the risk of ASCVDs in participants without ASCVDs at baseline.
Methods
The Meta-analysis of Observational Studies in Epidemiology [26] Statement and Cochrane’s Handbook [27] were followed for the design, performance, and reporting of this meta-analysis.
Literature search
Electronic databases including PubMed and Embase were searched with the combination of the following terms: (1) “TyG index” OR “triglyceride-glucose index” OR “triglyceride and glucose index” OR “triglyceride glucose index” OR “triacylglycerol glucose index;” and (2) “atherosclerotic cardiovascular disease” OR “ASCVD” OR “cardiovascular events” OR “MACE” OR “cardiovascular” OR “coronary artery disease” OR “CAD” OR “CHD” OR “stroke.” Filters were applied so that only studies conducted in humans and published in English or Chinese were considered. The reference lists of related original and review articles were manually searched for potential eligible studies. The final literature search was performed on January 5, 2021.
Study selection
Studies fulfilling all of the following criteria were included: (1) cohort studies published as full-length articles; (2) included an adult population without ASCVDs at baseline; (3) TyG index was measured at baseline; (4) the outcome of interest was CAD, stroke, or a composite outcome of ASCVD; and (5) reported the relative risk for the association after adjustment of potential confounding factors. The TyG index was calculated as ln[TG (mg/dL) × FPG (mg/dL)/2] [28]. A composite outcome of ASCVDs was defined as the incidence of CAD, stroke, and peripheral artery disease (PAD). The diagnosis of CAD or stroke was consistent with the criteria of the original studies. Typically, CAD was defined as acute myocardial infarction, angina pectoris, and other ischemic heart disease, both fatal and non-fatal. Stroke was defined as fatal and non-fatal ischemic stroke.
Data extraction and quality evaluation
The literature search, data extraction, and quality assessment of the included studies were performed by two authors independently, according to the predefined criteria. Discrepancies were resolved by consensus. The extracted data were as follows: (1) name of the first author, publication year, and country; (2) study design characteristics; (3) participant characteristics, including health status, sample size, age, sex, and proportion of patients with diabetes; (4) patterns for TyG index analysis; (5) follow-up duration; (6) outcomes reported and methods for outcome validation; and (7) confounding factors adjusted in the multivariate analyses. The quality of each study was evaluated using the Newcastle–Ottawa Scale [29]. This scale ranges from 1 to 9 in total and judges the quality of cohort studies according to the selection of the study groups, comparability of the groups, and ascertainment of the outcome of interest.
Statistical analyses
Hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs) were applied as the general measure for the association between the TyG index and ASCVDs, CAD, and stroke in an adult population without ASCVDs at baseline. For studies with the TyG index analyzed as a categorical variable, the HRs of the ASCVD incidence in participants with the highest TyG index level compared to those with the lowest TyG index level were extracted. For studies with the TyG index analyzed as a continuous variable, the HRs of the ASCVD incidence per 1-unit increment of the TyG index was extracted. Data of the HRs and their standard errors were calculated from the 95% CIs or P values; then they were logarithmically transformed for variance stabilization and distribution normalization [27]. Cochran’s Q test was used to evaluate the heterogeneity among the included cohort studies as well as to estimate the I2 statistic [30]. If I2 > 50%, a significant heterogeneity was considered. A random-effects model was used to synthesize the HR data because this model is considered as a more generalized method to incorporate the potential heterogeneity among included studies [27]. Sensitivity analyses, which excluded one individual study at a time, were performed to test the stability of the results [31]. Predefined subgroup analyses were carried out to evaluate the influences of the study characteristics, including age, sex, and diabetic status, on the association between the TyG index and the ASCVD risk. The potential publication bias was assessed by visual inspection of the funnel plots for symmetry as well as by Egger’s regression asymmetry test [32]. RevMan (Version 5.1; Cochrane Collaboration, Oxford, UK) and STATA (Version 12.0) software were used to perform the meta-analysis and statistical analysis.
Results
Literature search
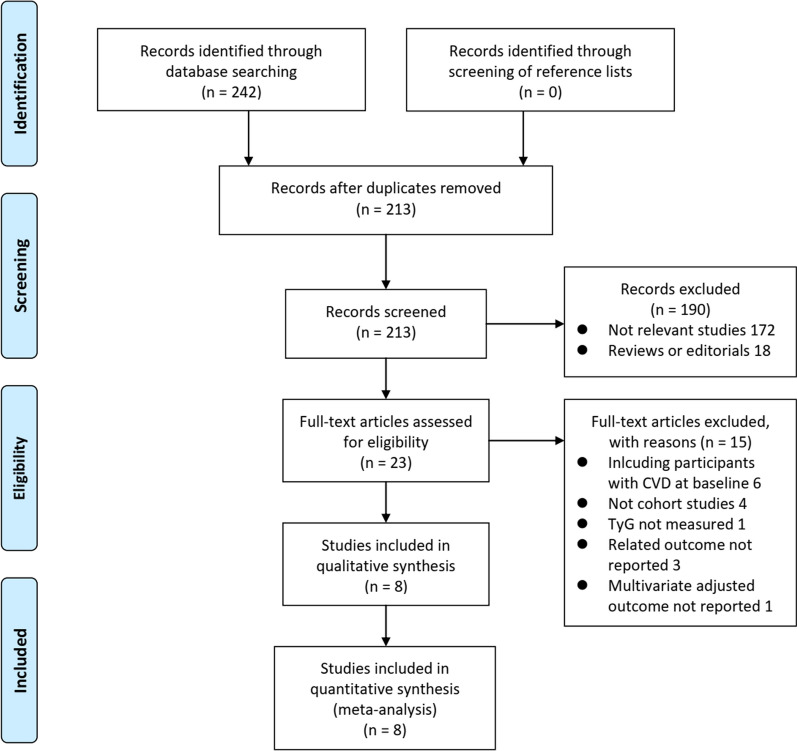
Figure 1 shows the process of the database search. Briefly, 213 articles were obtained via the initial literature search of the PubMed and Embase databases after excluding the duplications. Among them, 190 articles were excluded through screening of the titles and abstracts for irrelevance. Subsequently, 23 articles underwent full-text review. Of these, 15 articles were further excluded for the reasons listed in Fig. 1. Finally, eight cohort studies were obtained for this meta-analysis [18–25].
Fig. 1.
Flowchart of the database search and study identification
Study characteristics and quality evaluation
The characteristics of the included studies are summarized in Table 1. Overall, eight cohort studies [18–25] comprising a total of 5,731,298 participants who did not have any ASCVDs at baseline were included in this meta-analysis. The studies were performed in Spain [19], Argentina [20], China [21, 25], Korea [23, 24], and Iran [22]. Populations such as first-time attendee outpatients to an internal medicine department [19], participants in a routine health check-up program [18], community populations [20, 22–25], and diabetes mellitus patients without any ASCVDs [21] were included. The sample sizes of the included studies varied between 723 and 5,593,134. The mean ages of the included participants varied from 46 to 71 years old, with the proportion of male participants ranging from 32 to 80%. The baseline TyG index was analyzed as a categorical variable in four studies [19, 22–25], as a continuous variable in one study [21], and as both in three studies [18, 20, 22]. The follow-up durations varied between 2.4 and 16.1 years. The incidence of ASCVD outcomes was validated by medical record review in one study [21], by clinical evaluation in two studies [20, 22], and by International Classification of Diseases 10 codes in the other five studies [18, 19, 21, 24, 25]. Age, sex, body mass index, smoking status, blood pressure, serum total cholesterol or low-density lipoprotein cholesterol, diabetic status, and concurrent antihypertensive or lipid-lowering medications were adjusted to a varying degree when the association between the TyG index and the ASCVD risk was reported. The Newcastle–Ottawa Scale score was nine for all of the included studies, indicating good study quality (Table 2).
Table 1.
Characteristics of the included cohort studies
| Study | Country | Design | Characteristics of participants | Number of participants | Mean age (years) | Male (%) | DM (%) | TyG index analysis | Follow-up duration (years) | Outcome validation | Outcomes reported | Variables adjusted |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sanchez-Inigo [19] | Spain | PC | First-time attendee outpatients to an internal medicine department without ASCVDs | 5,014 | 54.4 | 61.2 | 5.2 | Q5:Q1 | 8.8 | ICD-10 | Composite ASCVDs (505), CAD (233), stroke (157), and PAD (74) | Age, sex, BMI, smoking, alcohol intake, lifestyle pattern, HTN, T2DM, antiplatelet therapy, HDL-C, and LDL-C |
| Salazar [20] | Argentina | PC | Community population without DM or ASCVDs | 723 | 50.3 | 32.8 | 0 | Q4:Q1–Q3; Continuous | 8.2 | Clinical evaluation | Composite ASCVDs (42) | Age, sex, smoking, LDL-C, BMI, and aspirin, antihypertensive and lipid-lowering drug use |
| Su [21] | China | RC | T2DM patients without previous ASCVDs | 3,524 | 61.7 | 49.1 | 100 | Continuous | 5.9 | Medical record review | Composite ASCVDs (215) | Age, sex, HTN, BMI, HDL-C, eGFR, antihypertensive and lipid-lowering drug use |
| Li [18] | China | RC | Participants aged over 60 years without previous ASCVDs who participated in a routine health check-up program | 6,078 | 70.5 | 53.1 | 11.8 | Q4:Q1; Continuous | 5.5 | ICD-10 | Composite ASCVDs (705), CAD (500), and stroke (234) | Age, sex, living alone, current smoker, alcohol consumption, exercise, BMI, SBP, HDL-C, LDL-C, and T2DM |
| Hong [23] | Korea | RC | Community population without ASCVDs | 5,593,134 | 53.0 | 50.5 | 3.7 | Q4:Q1 | 8.2 | ICD-10 | Composite ASCVDs (146,744), CAD (62,577), and stroke (89,120) | Age, sex, smoking, alcohol consumption, regular physical activity, low socioeconomic status, BMI, HTN, and TC |
| Park [24] | Korea | PC | Community population without DM or ASCVDs | 16,455 | 46.1 | 51.2 | 0 | Q4:Q1 | 2.4 | ICD-10 | CAD (322) | Age, sex, BMI, smoking status, alcohol intake, physical activity, mean arterial BP, hs-CRP, CKD, and hypertension medication |
| Barzegar [22] | Iran | PC | Community population without ASCVDs | 7,521 | 46.7 | 44.7 | 13.2 | Q4:Q1; Continuous | 16.1 | Clinical evaluation | Composite ASCVDs (1084), and CAD (924) | Age, sex, WC, BMI, educational level, smoking status, physical activity, family history of CVD, T2DM, HTN, LDL-C, HDL-C, and lipid-lowering drugs |
| Tian [25] | China | PC | Community population without ASCVDs | 98,849 | 51.8 | 79.8 | 3.07 | Q4:Q1 | 11.0 | ICD-10 | CAD (1555) | Age, sex, education, income, smoking, alcohol abuse, physical activity, BMI, SBP, DBP, HTN, DM, dyslipidemia, antidiabetic drugs, lipid-lowering drugs, antihypertensive drugs, and HDL-C, LDL-C, and hs-CRP levels at baseline |
TyG: triglyceride–glucose; DM: diabetes mellitus; PC: prospective cohort; RC: retrospective cohort; T2DM: type 2 diabetes mellitus; ASCVDs: atherosclerotic cardiovascular disease; CVD: cardiovascular disease; ICD-10: International Classification of Diseases, tenth edition; CAD: coronary artery disease; PAD: peripheral artery disease; HTN: hypertension; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; BMI: body mass index; eGFR: estimated glomerular filtrating rate; SBP: systolic blood pressure; BP: blood pressure; DBP: diastolic blood pressure; TC: total cholesterol; hs-CRP: high-sensitivity C-reactive protein; CKD: chronic kidney disease
Table 2.
Details of quality evaluation via the Newcastle–Ottawa Scale
| Study | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Outcome not present at baseline | Control for age | Control for other confounding factors | Assessment of outcome | Sufficient follow-up duration | Adequacy of follow-up of cohorts | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Sanchez-Inigo [19] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Salazar [20] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Su [21] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Li [18] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Hong [23] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Park [24] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Barzegar [22] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Tian [25] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
TyG index and the incidence of ASCVDs
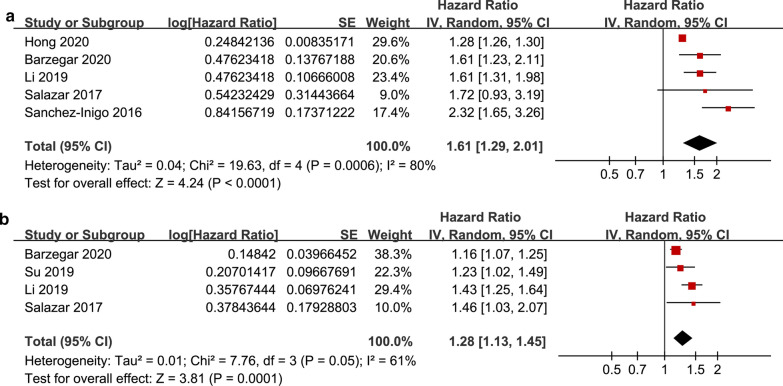
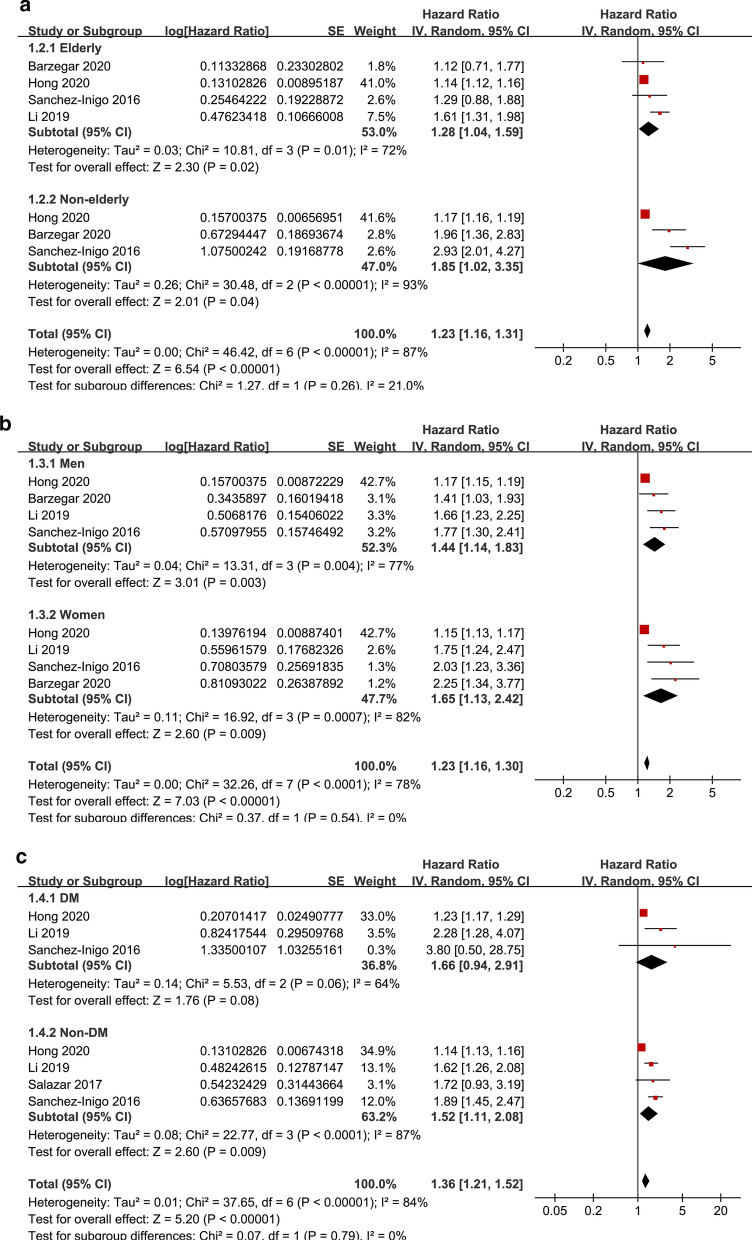
The pooled results of five studies [18–20, 22, 23] showed that compared to participants with the lowest TyG index category at baseline, the participants with the highest TyG index category had a significantly increased risk of ASCVD during follow-up (HR: 1.61, 95% CI 1.29–2.01, I2 = 80%, P < 0.001; Fig. 2a). This finding was consistent with the meta-analysis results with the TyG index analyzed as a continuous variable (four studies [18–22], HR per 1-unit increment of the TyG index: 1.28, 95% CI 1.13–1.45, I2 = 61%, P < 0.001; Fig. 2b). The sensitivity analyses by excluding one study at a time showed similar results (HRs for the TyG index analyzed as a categorical variable: 1.46–1.74, all P < 0.05; HRs for the TyG index analyzed as a continuous variable: 1.18–1.37, all P < 0.05). The subgroup analyses showed that the participants with the highest TyG index category had a significantly increased risk of ASCVDs compared to those with the lowest category and were independent of the age, sex, or diabetic status of the participants (for subgroup analyses, all P > 0.05; Fig. 3a–c).
Fig. 2.
Forest plots for the meta-analysis of the association between the TyG index and the risk of ASCVDs. a Meta-analysis with the TyG index analyzed as a categorical variable. b Meta-analysis with the TyG index analyzed as a continuous variable
Fig. 3.
Subgroup analyses for the association between the TyG index analyzed as a categorical variable and the risk of ASCVDs. a Subgroup analysis according to the age of the participants. b Subgroup analysis according to the sex of the participants. c Subgroup analysis according to the diabetic status of the participants
TyG index and the incidence of CAD and stroke
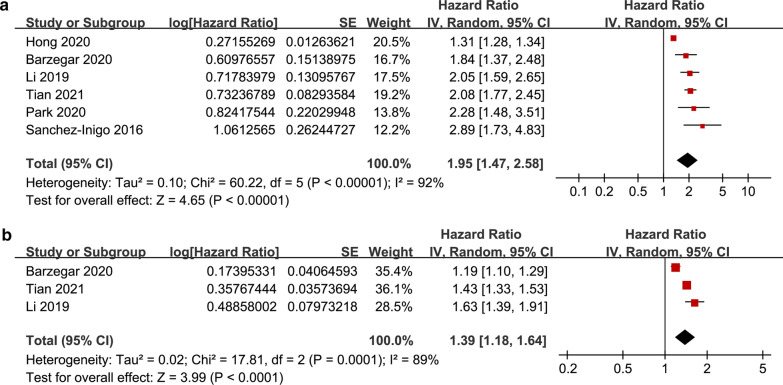
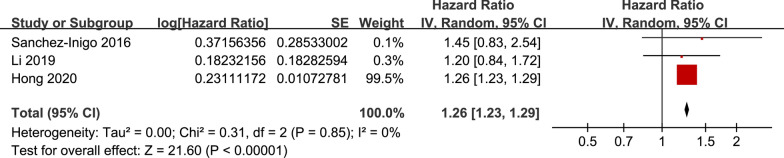
The pooled results of six studies [18, 19, 22–25] showed that the participants with the highest TyG index category had a significantly increased risk of CAD during follow-up compared to those with the lowest TyG category (HR: 1.95, 95% CI 1.47–2.58, I2 = 92%, P < 0.001; Fig. 4a). These findings were consistent with the meta-analysis results with the TyG index analyzed as a continuous variable (three studies [18, 22, 25], HR per 1-unit increment of the TyG index: 1.39, 95% CI 1.18–1.64, I2 = 89%, P < 0.001; Fig. 4b). The sensitivity analyses by excluding one study at a time showed similar results (HRs for the TyG index analyzed as a categorical variable: 1.84–2.08, all P < 0.05; HRs for the TyG index analyzed as a continuous variable: 1.31–1.50, all P < 0.05). Moreover, the pooled results of three studies [18, 19, 23] showed that the participants with the highest TyG index category had a significantly increased risk of stroke during follow-up compared to those with the lowest TyG category (HR: 1.26, 95% CI 1.23–1.29, I2 = 0%, P < 0.001; Fig. 5).
Fig. 4.
Forest plots for the meta-analysis of the association between the TyG index and the risk of CAD. a Meta-analysis with the TyG index analyzed as a categorical variable. b Meta-analysis with the TyG index analyzed as a continuous variable
Fig. 5.
Forest plots for the meta-analysis of the association between the TyG index analyzed as a categorical variable and the risk of stroke
Publication bias
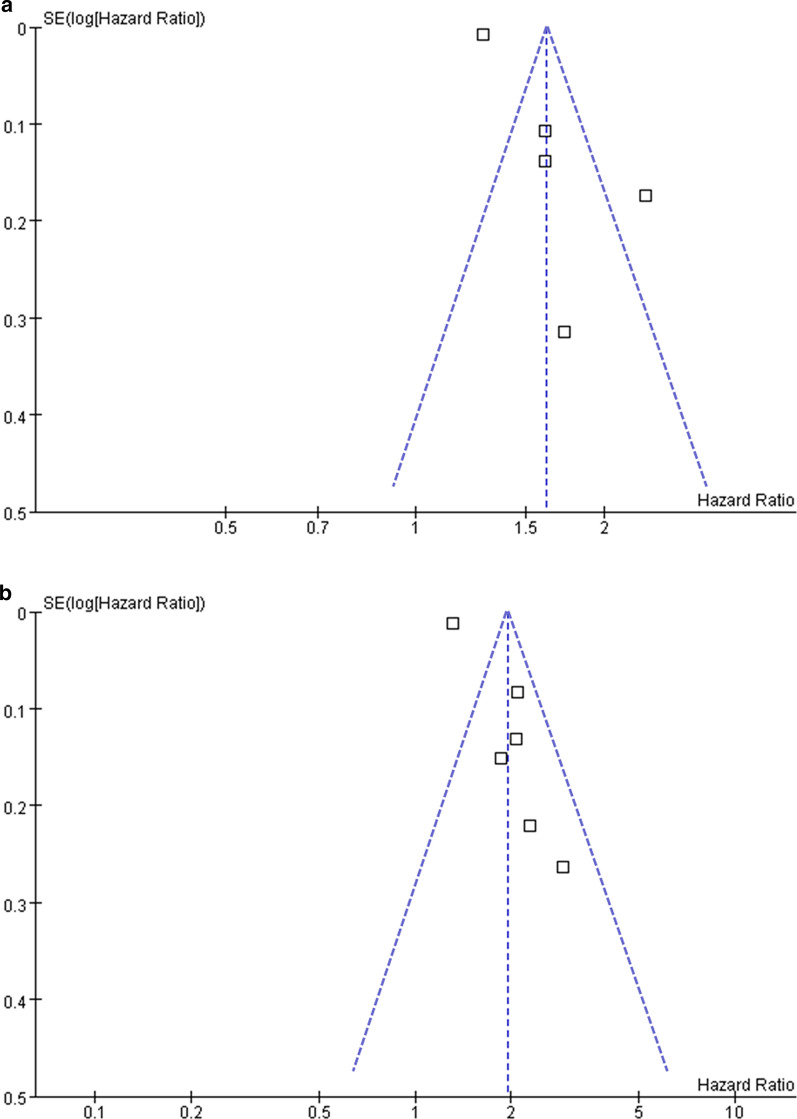
The funnel plots depicting the association between the serum TyG index analyzed as a categorical variable and ASCVD and CAD are shown in Fig. 6. The funnel plots were symmetric on visual inspection, suggesting a low risk of publication bias. Publication bias for the meta-analysis of the other outcomes was difficult to estimate because a limited number of datasets was included. In addition, Egger’s regression tests were unable to perform, since fewer than ten datasets were available for each outcome.
Fig. 6.
Funnel plots for the publication bias underlying the meta-analysis of the association between the TyG index analyzed as a categorical variable and ASCVD and CAD. a Funnel plots for the meta-analysis of the TyG index and ASCVD risk. b Funnel plots for the meta-analysis of the TyG index and CAD risk
Discussion
In this meta-analysis of cohort studies, compared to patients with the lowest TyG index category, those with the highest category were independently associated with an increased incidence of ASCVDs, CAD, and stroke. For ASCVDs, subgroup analyses showed that the association between the TyG index and the subsequent incidence of an ASCVD was not significantly affected by the age, sex, or diabetic status of the participants. Moreover, meta-analysis with the TyG index analyzed as a continuous variable also showed that a higher TyG index at baseline was independently associated with an increased risk of the subsequent incidence of ASCVD or CAD. Taken together, these results suggested that a higher TyG index may be an independent predictor of an increased risk of ASCVD incidence in a population without ASCVDs at baseline.
To the best of our knowledge, this study is the first meta-analysis to summarize the association between the TyG index and the ASCVD incidence in a general population without ASCVDs at baseline. Only cohort studies were included; therefore, the potential recall bias associated with studies having a cross-sectional design was avoided. In addition, only studies with multivariate analyses were included. The results indicated a potential independent association between a higher TyG index and an increased risk of ASCVDs. Moreover, meta-analyses were performed separately with the TyG index analyzed as a categorical variable as well as a continuous variable, and similar results were obtained, thus confirming the robustness of the findings. Furthermore, multiple sensitivity and subgroup analyses were performed to confirm the stability of the findings, which were not driven by a single study or affected by participant characteristics such as age, sex, or diabetic status. Pathophysiologically, the current results reflect the previously suggested role of insulin resistance in the pathogenesis of atherosclerosis [8, 10]. It has been hypothesized that insulin resistance is associated with persistent, low-degree inflammation [33, 34], which is considered to play a key role in the pathogenesis of ASCVDs [9]. Besides, insulin resistance may directly lead to endothelial dysfunction [35], a key pathophysiological process in the initiation and progression of atherosclerosis. Additionally, insulin resistance has been associated with increased activity of the sympathetic nervous system [36] and impaired cardiac autonomic function [37], which have been implicated in the pathogenesis of ASCVDs.
The current study supports the potential of the TyG index to be used as an indicator of ASCVD risk in a general population without ASCVDs. Methodologically, the TyG index can be easily calculated in real-world clinical practice based on routine blood biochemical tests in a cost-effective manner. A previous study has shown that the TyG index is highly sensitive (96.5%) and specific (85.0%) for the detection of insulin resistance, compared to the hyperinsulinemic-euglycemic clamp test [38]. Furthermore, the TyG index has been demonstrated to confer a better performance than homeostatic model assessment for measuring insulin resistance [39]. However, further studies are needed to determine whether the addition of the TyG index to conventional ASCVD risk prediction tools, such as the Framingham risk score, can improve the predictive efficacy in the general population.
Despite the above strengths and potential clinical implications, this meta-analysis has some limitations that should be considered when interpreting the results. First, a limited number of studies was available for this meta-analysis, and significant heterogeneity was detected among them. Additional studies are needed to determine whether other study characteristics can affect the results, such as ethnicity and comorbidities of the participants, follow-up duration, and concurrent medications. In addition, the sample sizes of the included studies varied significantly. For example, for the outcome of stroke, the weight of the study by Hong 2000 is significantly larger than the other two, and the result of the meta-analysis was mainly driven by the result of this study. Second, it remains unknown whether the association between the TyG index and an increased risk of ASCVDs is linear and what the optimal cut-off value of the TyG index is for the prediction of future risk of ASCVDs. Third, residual confounding factors possibly affecting the association between the TyG index and the ASCVD risk, such as the dietary and nutritional factors that may affect the TyG index, could not be excluded [40]. Finally, this meta-analysis was based on cohort studies; thus, a causative association between the TyG index and the incidence of ASCVDs cannot be implied.
Conclusions
In conclusion, the existing evidence from cohort studies suggests that a higher TyG index may be an independent predictor of ASCVD risk in people without ASCVDs at baseline. Due to the convenience of measuring the TyG index in clinical settings, future studies are needed to determine whether incorporation of the TyG index on top of the ASCVD risk prediction tools currently used can improve their predictive efficacies.
Acknowledgements
None
Abbreviations
- ASCVDs
Atherosclerotic cardiovascular diseases
- CAD
Coronary artery disease
- TyG
Triglyceride–glucose
- PAD
Peripheral artery disease
- HRs
Hazard ratios
- Cis
Confidence intervals
Authors' contributions
XD, XW, JW and MC conceived and designed research; XD, XW and MZ collected data and conducted research; XD, JW and MC analyzed and interpreted data; XD and XW wrote the initial paper; XW and MC revised the paper; MC had primary responsibility for final content. All authors read and approved the final manuscript.
Funding
This work was supported by Research Fund of the First Hospital of Jilin University (04020940001).
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaobo Ding, Xiaozhen Wang authors contributed equally to this work
References
- 1.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Barquera S, Pedroza-Tobias A, Medina C, Hernandez-Barrera L, Bibbins-Domingo K, Lozano R, et al. Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch Med Res. 2015;46:328–338. doi: 10.1016/j.arcmed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Rosenblit PD. Extreme atherosclerotic cardiovascular disease (ASCVD) risk recognition. Curr Diab Rep. 2019;19:61. doi: 10.1007/s11892-019-1178-6. [DOI] [PubMed] [Google Scholar]
- 4.Choi S. The potential role of biomarkers associated with ASCVD risk: risk-enhancing biomarkers. J Lipid Atheroscler. 2019;8:173–182. doi: 10.12997/jla.2019.8.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahtta D, Khalid U, Misra A, Samad Z, Nasir K, Virani SS. Premature atherosclerotic cardiovascular disease: what have we learned recently? Curr Atheroscler Rep. 2020;22:44. doi: 10.1007/s11883-020-00862-8. [DOI] [PubMed] [Google Scholar]
- 6.Vikulova DN, Grubisic M, Zhao Y, Lynch K, Humphries KH, Pimstone SN, et al. Premature atherosclerotic cardiovascular disease: trends in incidence, risk factors, and sex-related differences, 2000 to 2016. J Am Heart Assoc. 2019;8:e012178. doi: 10.1161/JAHA.119.012178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayman LL. Prevention of atherosclerotic cardiovascular disease in childhood. Curr Cardiol Rep. 2020;22:86. doi: 10.1007/s11886-020-01332-y. [DOI] [PubMed] [Google Scholar]
- 8.Di Pino A, DeFronzo RA. Insulin resistance and atherosclerosis: implications for insulin-sensitizing agents. Endocr Rev. 2019;40:1447–1467. doi: 10.1210/er.2018-00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beverly JK, Budoff MJ. Atherosclerosis: pathophysiology of insulin resistance, hyperglycemia, hyperlipidemia, and inflammation. J Diabetes. 2020;12:102–104. doi: 10.1111/1753-0407.12970. [DOI] [PubMed] [Google Scholar]
- 10.Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14:575–585. doi: 10.1016/j.cmet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bloomgarden ZT. Measures of insulin sensitivity. Clin Lab Med. 2006;26(611–33):vi. doi: 10.1016/j.cll.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Cersosimo E, Solis-Herrera C, Trautmann ME, Malloy J, Triplitt CL. Assessment of pancreatic beta-cell function: review of methods and clinical applications. Curr Diabetes Rev. 2014;10:2–42. doi: 10.2174/1573399810666140214093600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan SH, Sobia F, Niazi NK, Manzoor SM, Fazal N, Ahmad F. Metabolic clustering of risk factors: evaluation of Triglyceride-glucose index (TyG index) for evaluation of insulin resistance. Diabetol Metab Syndr. 2018;10:74. doi: 10.1186/s13098-018-0376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alizargar J, Bai CH, Hsieh NC, Wu SV. Use of the triglyceride-glucose index (TyG) in cardiovascular disease patients. Cardiovasc Diabetol. 2020;19:8. doi: 10.1186/s12933-019-0982-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee EY, Yang HK, Lee J, Kang B, Yang Y, Lee SH, et al. Triglyceride glucose index, a marker of insulin resistance, is associated with coronary artery stenosis in asymptomatic subjects with type 2 diabetes. Lipids Health Dis. 2016;15:155. doi: 10.1186/s12944-016-0324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thai PV, Tien HA, Van Minh H, Valensi P. Triglyceride glucose index for the detection of asymptomatic coronary artery stenosis in patients with type 2 diabetes. Cardiovasc Diabetol. 2020;19:137. doi: 10.1186/s12933-020-01108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi W, Xing L, Jing L, Tian Y, Yan H, Sun Q, et al. Value of triglyceride-glucose index for the estimation of ischemic stroke risk: insights from a general population. Nutr Metab Cardiovasc Dis. 2020;30:245–253. doi: 10.1016/j.numecd.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Guo B, Chen H, Shi Z, Li Y, Tian Q, Shi S. The role of the triglyceride (triacylglycerol) glucose index in the development of cardiovascular events: a retrospective cohort analysis. Sci Rep. 2019;9:7320. doi: 10.1038/s41598-019-43776-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanchez-Inigo L, Navarro-Gonzalez D, Fernandez-Montero A, Pastrana-Delgado J, Martinez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. 2016;46:189–197. doi: 10.1111/eci.12583. [DOI] [PubMed] [Google Scholar]
- 20.Salazar MR, Carbajal HA, Espeche WG, Aizpurua M, Dulbecco CA, Reaven GM. Comparison of two surrogate estimates of insulin resistance to predict cardiovascular disease in apparently healthy individuals. Nutr Metab Cardiovasc Dis. 2017;27:366–373. doi: 10.1016/j.numecd.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Su WY, Chen SC, Huang YT, Huang JC, Wu PY, Hsu WH, et al. Comparison of the effects of fasting glucose, hemoglobin A1c, and triglyceride-glucose index on cardiovascular events in type 2 diabetes mellitus. Nutrients. 2019;11:2838. doi: 10.3390/nu11112838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barzegar N, Tohidi M, Hasheminia M, Azizi F, Hadaegh F. The impact of triglyceride-glucose index on incident cardiovascular events during 16 years of follow-up: Tehran lipid and glucose study. Cardiovasc Diabetol. 2020;19:155. doi: 10.1186/s12933-020-01121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong S, Han K, Park CY. The triglyceride glucose index is a simple and low-cost marker associated with atherosclerotic cardiovascular disease: a population-based study. BMC Med. 2020;18:361. doi: 10.1186/s12916-020-01824-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park B, Lee YJ, Lee HS, Jung DH. The triglyceride-glucose index predicts ischemic heart disease risk in Koreans: a prospective study using National Health Insurance Service data. Cardiovasc Diabetol. 2020;19:210. doi: 10.1186/s12933-020-01186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian X, Zuo Y, Chen S, Liu Q, Tao B, Wu S, et al. Triglyceride-glucose index is associated with the risk of myocardial infarction: an 11-year prospective study in the Kailuan cohort. Cardiovasc Diabetol. 2021;20:19. doi: 10.1186/s12933-020-01210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 27.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. The cochrane collaboration. 2011; www.cochranehandbook.org.
- 28.Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6:299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- 29.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2010; http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 30.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 31.Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37:1148–1157. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beddhu S. The body mass index paradox and an obesity, inflammation, and atherosclerosis syndrome in chronic kidney disease. Semin Dial. 2004;17:229–232. doi: 10.1111/j.0894-0959.2004.17311.x. [DOI] [PubMed] [Google Scholar]
- 34.Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399–409. doi: 10.1038/nrcardio.2009.55. [DOI] [PubMed] [Google Scholar]
- 35.Wheatcroft SB, Williams IL, Shah AM, Kearney MT. Pathophysiological implications of insulin resistance on vascular endothelial function. Diabet Med. 2003;20:255–268. doi: 10.1046/j.1464-5491.2003.00869.x. [DOI] [PubMed] [Google Scholar]
- 36.Kaaja RJ, Poyhonen-Alho MK. Insulin resistance and sympathetic overactivity in women. J Hypertens. 2006;24:131–141. doi: 10.1097/01.hjh.0000194121.19851.e5. [DOI] [PubMed] [Google Scholar]
- 37.Poon AK, Whitsel EA, Heiss G, Soliman EZ, Wagenknecht LE, Suzuki T, et al. Insulin resistance and reduced cardiac autonomic function in older adults: the atherosclerosis risk in communities study. BMC Cardiovasc Disord. 2020;20:217. doi: 10.1186/s12872-020-01496-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, Martinez-Abundis E, Ramos-Zavala MG, Hernandez-Gonzalez SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95:3347–3351. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 39.Vasques AC, Novaes FS, de Oliveira MS, Souza JR, Yamanaka A, Pareja JC, et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93:e98–e100. doi: 10.1016/j.diabres.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 40.Shahavandi M, Djafari F, Shahinfar H, Davarzani S, Babaei N, Ebaditabar M, et al. The association of plant-based dietary patterns with visceral adiposity, lipid accumulation product, and triglyceride-glucose index in Iranian adults. Complement Ther Med. 2020;53:102531. doi: 10.1016/j.ctim.2020.102531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.