Abstract
Purpose
Prolactin-releasing peptide (PrRP) is a neuropeptide that suppresses food intake and increases body temperature when delivered to the forebrain ventricularly or parenchymally. However, PrRP’s receptor GPR10 is widely distributed throughout the brain with particularly high levels found in the dorsomedial hindbrain. Thus, we hypothesized that hindbrain-directed PrRP administration would affect energy balance and motivated feeding behavior.
Methods
To address this hypothesis, a range of behavioral and physiologic variables were measured in Sprague-Dawley rats that received PrRP delivered to the fourth ventricle (4V) or the nucleus of the solitary tract (NTS) at the level of the area postrema (AP).
Results
4V PrRP delivery decreased chow intake and body weight, in part, through decreasing meal size in ad libitum maintained rats tested at dark onset. PrRP inhibited feeding when delivered to the nucleus tractus solitarius (NTS), but not to more ventral hindbrain structures. In addition, 4V as well as direct NTS administration of PrRP increased core temperature. By contrast, 4V PrRP did not reduce ad libitum intake of highly palatable food or the motivation to work for or seek palatable foods.
Conclusions
The dorsomedial hindbrain and NTS/AP, in particular, are sites of action in PrRP/GPR10-mediated control of chow intake, core temperature, and body weight.
Keywords: NTS, Body temperature, Reward, Food intake, Hyperthermia
Introduction
Prolactin-releasing peptide (PrRP) is a neuropeptide synthesized by a subset of neurons in the dorsomedial hypothalamus (DMH), ventrolateral medulla (VLM), area postrema (AP), and nucleus tractus solitarius (NTS) and serves as the ligand for the G-protein-coupled receptor GPR10 (Hinuma et al. 1998; Chen et al. 1999; Maruyama et al. 1999; Ibata et al. 2000; Lee et al. 2000). PrRP modulates a range of physiologic functions including energy balance (Takayanagi and Onaka 2010; Dodd and Luckman 2013), stress (Matsumoto et al. 2000; Maruyama et al. 2001; Samson et al. 2003; Mera et al. 2006), and cardiovascular responses (Samson et al. 2000; Horiuchi et al. 2002; Yamada et al. 2009). Endogenous PrRP is highly relevant to energy balance control. Whole body knockout of either GPR10 (Gu et al. 2004; Bechtold and Luckman 2006; Bjursell et al. 2007) or PrRP (Takayanagi et al. 2008; Dodd et al. 2014) results in obesity that is driven primarily from hyperphagia, as does a naturally occurring GPR10 mutation (Watanabe et al. 2005). Energy status acutely regulates PrRP mRNA expression in both rats and mice such that levels are low during energy deficit and high during energy surfeit (Lawrence et al. 2000; Dodd et al. 2014), and PrRP neurons in the NTS activate in response to food ingestion (Takayanagi et al. 2008; Kreisler et al. 2014).
Forebrain ventricular delivery (lateral or third ventricle) of PrRP reduces chow intake and body weight in food-deprived as well as ad libitum maintained rats (Lawrence et al. 2000, 2002, 2004; Ellacott et al. 2002). Supporting the central (CNS) mediation of these effects, other data show that peripheral administration of lipidized PrRP that penetrates the blood-brain barrier reduces food intake and body weight while non-penetrant, non-lipidized PrRP has no energy balance effects (Maletínská et al. 2015; Mikulášková et al. 2015). Additionally, the obese phenotype of whole body PrRP knockout can be rescued when PrRP expression is selectively restored in the brain (Dodd et al. 2014) and targeted agonism in the DMH, a GPR10-expressing site in the forebrain, is sufficient to reduce food intake in 24 h fasted rats (Seal et al. 2001). Interestingly, the weight loss-promoting effects of central PrRP are not limited to inhibitory action on food intake, as administering PrRP into the forebrain also induces hyperthermia (Lawrence et al. 2000; Ellacott et al. 2003).
To date, all of the literature on PrRP’s contribution to energy balance control has focused exclusively on forebrain sites of action. However, GPR10 and PrRP expressions are anatomically distributed across all levels of the brain, with particularly high levels found in the hindbrain (Fujii et al. 1999; Iijima et al. 1999; Roland et al. 1999; Ibata et al. 2000; Lee et al. 2000; Kataoka et al. 2001). Despite the significant levels of GPR10 and PrRP found in this region, there has been no examination of hindbrain GPR10 as a site of action for PrRP’s energy balance effects. In addition to the need to better define central sites of action, the literature has not examined whether PrRP’s intake-inhibitory effects on food intake extend to appetitive and motivational aspects of feeding or to the intake of highly palatable foods. This question is important as the availability and intake of high fat, high sugar, and high reward foods is a key driver of the obesity epidemic (Stice et al. 2013).
For these reasons, the present studies addressed several novel hypotheses related to sites of action and functional effects of targeted PrRP delivery. We predicted that (1) ventricular PrRP directed at the hindbrain reduces food intake via interaction with satiation and satiety processes (e.g. reduced meal size or number) and also with hedonic and appetitive aspects of feeding control (e.g. motivated intake of palatable food, food-seeking), (2) PrRP acting on hindbrain GPR10 elevates core temperature, and (3) the dorsomedial hindbrain is a site of action for hindbrain PrRP/GPR10’s effects on food intake and core temperature.
Materials and methods
Subjects
Adult male Sprague-Dawley rats (250–265 g upon arrival, Charles River Laboratories, Wilmington, MA) were, unless otherwise noted, individually housed in hanging wire-bottom metal cages and had ad libitum access to pelleted chow (Purina Rodent Chow 5001, St. Louis, MO) and water. Animals were maintained on a 12:12 h reversed light-dark cycle.
Surgeries
For all surgeries, rats received an intraperitoneal anesthetic [ketamine (90 mg/kg; Henry Schein Animal Health Supply, Dublin, OH), xylazine (2.7 mg/kg; Anased, Shenandoah, IA), and acepromazine (0.64 mg/kg; Henry Schein Animal Health Supply)] and subcutaneous analgesia (2.0 mg/kg Meloxicam and 0.1 mg/kg buprenorphine; Midwest Veterinary Supply, Norristown, PA).
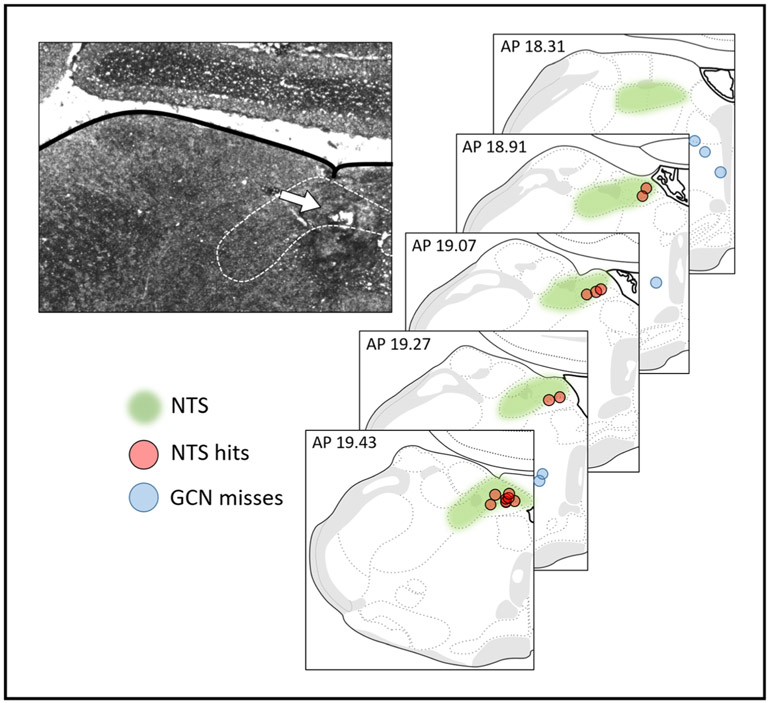
For craniotomies, rats were positioned in a stereotaxic device. For experiments involving fourth ventricle (4V) drug delivery, rats were implanted with unilateral guide cannulae (26 gauge; Plastics One, Roanoke, VA) (coordinates: on midline, 2.5 mm anterior to occipital suture, 5.2 mm ventral to skull). For experiments involving NTS drug delivery, rats were implanted with bilateral guide cannulae [coordinates: ± 0.5 mm lateral to midline, 1.7 mm anterior to occipital suture, 6.6 mm ventral from skull, at a 15° angle (anterior to posterior)]. 4V cannula placements were verified by assessing the sympathoadrenal-mediated glycemic response to cytoglucopenia induced by 5-thio-d-glucose (210 μg in 2 μl artificial cerebral spinal fluid) (Ritter et al. 1981). A post-injection increase in blood glucose level of 100% or greater from baseline within 1 h (assessed via tail vein laceration) was required for subject inclusion. NTS cannula placements were histologically evaluated postmortem with a 200 nl injection of 2% Pontamine Sky Blue. A summary of histological results and representative photomicrograph are seen in Fig. 1.
Fig. 1.
Representative photomicrograph and schematic depicting injection sites in the medulla. White arrow designates an injection site. Brain maps are derived from Swanson, LW (2018) Brain maps 4.0—Structure of the rat brain: An open access atlas with global nervous system nomenclature ontology and flatmaps. J Comp Neuro 526:6
For implantation of telemetric transponders (experiment 2a: model HRC 4000, experiment 2b: model G2; STARR life sciences, Holliston, MA), the transponders were inserted into the abdomen of anesthetized rats. For HR transponders, heart rate leads were positioned subcutaneously and secured to the chest muscles on either side of the heart with sutures as described in Skibicka and Grill 2008.
Experimental procedures
All experiments with the exception of conditioned place preference (CPP) were conducted using within-subjects counterbalanced designs, with at least 48 h elapsing between experimental conditions. CPP was conducted using a between-subjects design with groups matched for their baseline chamber preference. The same cohort of n = 15 4V-cannulated animals was used in experiments 1a and 3–5.
For all experiments, Rat PrRP-31 (Phoenix Pharmaceuticals, Inc., Burlingame, CA) was dissolved in sterile saline. Drugs were administered with a microsyringe at a volume of either 1 μl (4V) manually over 1–2 s or 100 nl (NTS) using a Harvard Apparatus PHD 2000 infusion pump at a delivery rate of 2.6 μl/min. Injectors were left in place for 30 s after injections to allow for drug diffusion and then removed. Injectors extending 2 mm below the guide cannula were used. Rats were habituated to handling and to the restraint associated with having their cannula manipulated during injection several times prior to the start of data gathering.
Experiment 1a: ad libitum chow intake with 4V PrRP administered at dark onset
To test the effects of hindbrain ventricular (4V) PrRP delivered at dark onset on chow intake, associated meal parameters (meal size, meal number), and body weight, naïve ad libitum maintained rats (n = 14; one cannulated rat was not included as there were not enough chambers in the apparatus) were housed individually in a custom-made automated food intake monitoring system. A meal was defined as intake of ≥ 0.25 g with ≥ 10 min elapsing between feeding bouts. These criteria were chosen based on prior work (Zheng 2005; Hayes et al. 2011; Kanoski et al. 2012). Rats had access through a port in the cage wall to a cup of powdered chow that rested on a load cell on the front wall of the cage. The weight of the food cup was electronically monitored using LabVIEW (National Instruments, Austin, TX). This automated feeding system tracks food intake every 10 s over a 24-h period without disturbing the natural feeding cycle of the rats by eliminating experimenter interruption. For analysis, cumulative and non-cumulative (interval) chow intake and meal parameters were assessed. After habituation to being restrained and having their cannula manipulated, rats were infused with 0 (vehicle), 0.05, 0.1, 0.5, or 5 nmol PrRP into the 4V immediately before dark onset using a within-subjects design. Doses were delivered in counterbalanced order and were selected based on published reports on the intake-inhibitory effects of ventricular PrRP in rats. Additionally, as PrRP neurons can be endogenously activated by stress (Maniscalco et al. 2012), rats underwent a sham condition where they were handled but not injected. All animals received the sham handling on the same calendar day (between injection days 3–4 of the five injection conditions) in order to minimize stress; therefore, the sham condition was not included in the counterbalancing of the injection conditions. Body weight was measured before and after each condition.
Experiment 1b: ad libitum cumulative chow intake with NTS PrRP administered at dark onset
The general procedures of experiment 1a were applied to n = 24 naïve NTS-cannulated rats housed in the automated food intake monitoring system using doses of 0 (vehicle) and 0.1 nmol PrRP. This dose was selected from the data obtained in experiment 1a for its lack of effect (subthreshold) on chow intake when administered to the 4V. Rats (n = 6) whose injection site was confirmed to be located within the NTS via postmortem histological examination were included in NTS analyses.
Experiment 2a: effects of 4V PrRP on core temperature, heart rate, and activity
To examine the autonomic and energetic effects of 4V PrRP, naïve rats (n = 10) with 4V cannulae and telemetric transponders were individually housed in microisolator cages. The cages were placed on receivers that were connected to a computer that collected measurements. For each counterbalanced condition, rats received vehicle or 5 nmol PrRP into the 4V 3 h after lights on (early light phase). Food was removed in order to eliminate feeding-related thermic effects; note that normally in this phase of the light cycle, spontaneous chow intake is minimal. Baseline core temperature, heart rate, and activity measurements began 1 h prior to injections. Measurements were taken every 5 min for temperature and activity (recorded by the Emitter Telemetry System as the summation of the intensity and frequency of the animal’s overt motor movement) and every minute for heart rate. Food was returned immediately prior to dark onset, which occurred 9 h post-injection. Change from each animal’s average baseline measurement was calculated and averaged in 2 h bins for analysis. After sacrifice, one animal’s electrical leads for recording heart rate were found to have migrated over the course of the experiment; therefore, heart rate measurements include n = 9 out of ten animals.
Experiment 2b: effects of NTS PrRP on core temperature and activity
To examine the autonomic and energetic effects of intraparenchymal NTS PrRP delivery, naïve rats (n = 12) with NTS cannulae and telemetric transponders were subjected to the same procedures as in experiment 2a using doses of 0 (vehicle) and 0.05 nmol PrRP. This dose was selected from the data obtained in experiment 1a for its lack of effect (subthreshold) on intake as well as 24 h body weight when administered to the 4V. Telemeters without heart rate leads were used in this experiment due to the lack of effect on heart rate of 4V PrRP in experiment 2a. Rats (n = 8) whose injection site was confirmed to be located within the NTS via postmortem histological examination were included in NTS analyses.
Experiment 3: PR operant responding for sucrose following 4V PrRP treatment
To test whether 4V PrRP affected the motivation to work to obtain and consume palatable food (sucrose pellets), rats (n = 15) underwent operant training where they learned to lever press for these pellets. For all procedures, food was removed at dark onset and the session took place 2 h later in a separate room. Chow was returned immediately after the session. Training was carried out over 7 days with a 1-h session each day in operant conditioning chambers (Med Associates, Fairfax, VT). The right lever was designated the “active” lever; a left “inactive” lever served as a control for non-conditioned responses. During the first 3 days, a fixed ratio 1 (FR1) procedure was used where each lever press earned a 45 mg sucrose pellet (Bio-Serv, Flemington, NJ). This was followed by 2 days of an FR3 schedule, 2 days of an FR5 schedule, and then 14 days of a progressive ratio (PR) schedule where the response requirements increased progressively as previously described (Davis et al. 2011; Kanoski et al. 2013) using the following formula: F(i) = 5e0.2i−5, where F(i) is the number of lever presses required for next pellet at i = pellet number. The PR session ended when 20 min passed without earning a reinforcer, after which animals were immediately returned to their home cages. After training, rats were given two tests where vehicle or 5 nmol PrRP was injected into the 4V at dark onset with testing commencing 2 h later for a duration of approximately 1 h, a time period wherein 4V PrRP was shown to reduce feeding in experiment 1a. Seven days intervened between tests, during which PR sessions were conducted without injections as in training. Rats reliably consumed all of the sucrose pellets that they earned during PR sessions.
Experiment 4: CPP for HFD following 4V PrRP treatment
To test 4V PrRP’s effects on palatable food seeking, we examined the expression of an established CPP for high-fat diet (HFD) vs. chow after PrRP or vehicle administration. Procedures were conducted in a dimly lit room with a white noise generator. The CPP chamber consisted of two conjoined Plexiglas compartments (each 74 cm long, 57.4 cm wide, 24.7 cm height) with a removable divider wall in the center and a removable clear Plexiglas cover. The two contexts were made distinguishable by varying wall color (white vs. black Plexiglas), floor texture (fine vs. coarse steel mesh), and orientation of wall stripes (horizontal vs. vertical adhesive tape). A video camera mounted above the chambers recorded the rat’s movement during baseline and test sessions. The recorded videos were subsequently analyzed using ANY-maze behavioral tracking software (Stoelting Co., Wood Dale, IL) by an experimenter blind to the group assignments.
For all procedures, food was removed from home cages at dark onset and CPP sessions took place 2 h later. Food was returned immediately after each session. Prior to training, a baseline context (aka place) preference test was conducted during which n = 15 rats were placed in CPP chamber with the divider wall removed and freely explored both contexts while their position was videotaped for a 15-min period. For each rat, the context that the rat spent less time in was associated with access to HFD for subsequent training, whereas the context that the rat spent more time in was associated with access to chow. CPP training consisted of 30 min sessions (one session per day for 20 continuous days, alternating between HFD-paired context and chow-paired context): ten sessions where the rat was isolated in the HFD-paired context and ten sessions where the rat was isolated in the chow-paired context. During HFD-paired training sessions, a total of 5 g of a high-fat/high-sucrose diet (60% kcal from fat; Research Diets D12492) was divided into ten aliquots and scattered throughout the initially non-preferred context. During chow-paired training sessions, a calorie-matched total of 8 g of the animals’ normal chow was divided into ten aliquots and scattered throughout the initially preferred context. Place preference testing occurred 1 day after the last training session using a between-subjects design. Rats were assigned to groups matched for baseline context preference (n = 7 for vehicle, n = 8 for PrRP). A 4V injection of either vehicle or 5 nmol PrRP was given just before dark onset and 2 h later; rats were placed in the CPP chamber with the divider wall removed and without HFD or chow provided. They were allowed to freely explore the chamber for 15 min. The dependent variable used for analysis was the percentage of time spent in the context that was less preferred during habituation.
Experiment 5: deprivation-induced and palatability-motivated intake under sated conditions
To test whether 4V PrRP affected chow feeding in food deprived rats as well as, separately, whether PrRP would affect palatability-motivated feeding after refeeding on chow, we used a paradigm described in (Choi et al. 2012). Several days before the start of the experiment, n = 15 rats were given a small amount of HFD (32% kcal from fat; Research Diets D12266B) in their home cages to habituate them to this palatable, energy-dense food. This HFD was of a different color, size, energy composition, and texture than the HFD used in experiment 4. Next, rats were deprived of maintenance chow but had access to water for 24 h. At dark onset at the end of the fast, rats were injected with either vehicle or 5 nmol PrRP and their normal chow food hoppers were returned. Chow intake was measured at 60 and 120 min after dark onset. After 120 min of chow access, a second food hopper containing HFD was provided. Intake from both chow and HFD hoppers was measured 60 min later. Seven days intervened between tests, during which animals were maintained on ad libitum chow. Two animals in this experiment were administered treatment using injector tips of the wrong length; therefore, analyses include n = 13/15 animals.
Data and statistical analyses
Data for each experiment were analyzed separately using PASW Statistics 18 (SPSS Inc., Chicago, IL) and expressed in the figures as mean ± SEM. Data were analyzed by using repeated measures analysis of variance (ANOVA). Significant interactions were followed up with planned comparisons between the levels of the variable. Alpha was set as p < .05 for all analyses.
Results
Experiment 1a: ad libitum chow intake with 4V PrRP administered at dark onset
4V PrRP administration at dark onset significantly reduced 24 h body weight gain compared to vehicle (main effect of treatment; F(5,65) = 6.48, p < .0001) at doses of 0.1 nmol (p = .026), 0.5 nmol (p = .048) and 5 nmol (p < .0001) (Fig. 2a). Sham handling resulted in greater 24 h weight gain compared to every injection condition, including vehicle (main effect of treatment; vs. vehicle p = .046, vs. 0.05 nmol p = .036, vs. 0.1 nmol p = .002, vs. 0.5 nmol p = .021, vs. 5 nmol p < .0001).
Fig. 2.
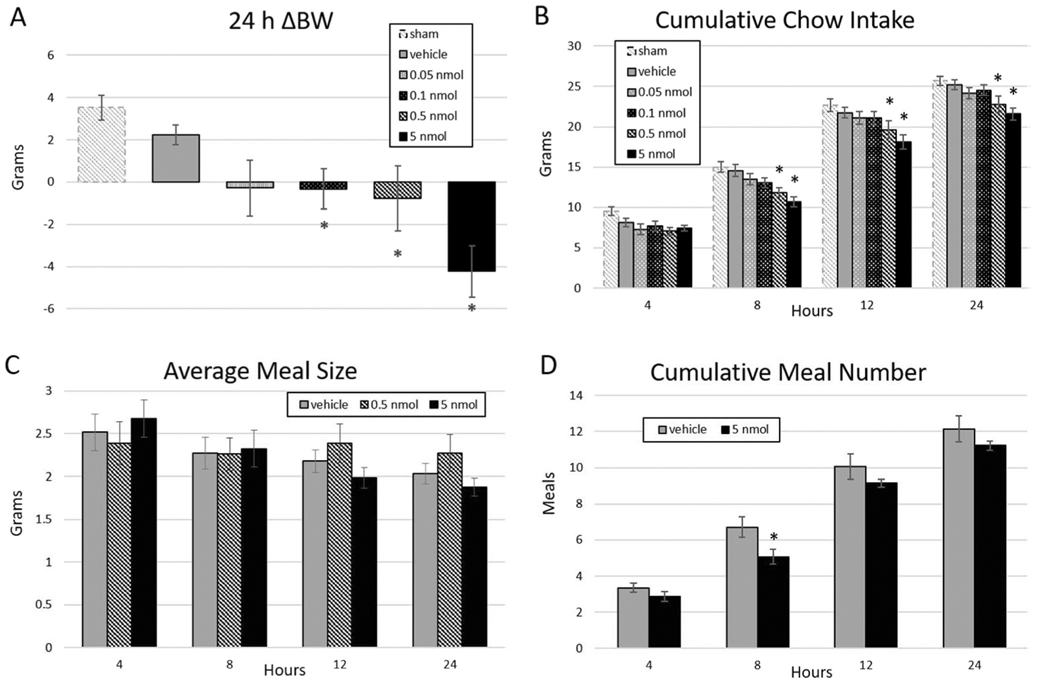
Dose-dependent effect of hindbrain (4V) ventricle delivery of PrRP on 24 h body weight and ad libitum chow intake in n =14 animals. a PrRP reduced 24 h body weight change at doses of 0.1, 0.5, and 5 nmol relative to vehicle. b PrRP at the 0.5 and 5 nmol doses decreased cumulative chow intake at 8, 12, and 24 h. These effects were mediated by interactions between treatment and time for c average meal size and d cumulative meal number. * = p < .05; error bars represent SEM
4V PrRP dose-dependently reduced ad libitum cumulative chow intake (Fig. 2b). There was a main effect of treatment on intake (F(4,52) = 6.35, p < .0001), where PrRP at 5 nmol (p < .001) and 0.5 nmol (p = .003) significantly reduced chow intake versus vehicle (Fig. 2b). There was also a significant treatment × time interaction (F(12,156) = 2.63, p = .003), where, compared to vehicle, the intake-inhibitory effects of 0.5 and 5 nmol PrRP on intake began at 8 h and lasted through the final 24 h measurement (5 nmol: 8 h p < .001, 12 h p = .001, 24 h p < .001; 0.5 nmol: 8 h p = .002, 12 h p = .027, 24 h p = .011). Sham handling resulted in greater chow intake compared to every PrRP dose (main effect of treatment; vs. 0.05 nmol p = .003 vs. 0.1 nmol p = .002 vs. 0.5 and 5 nmol p < .0001). Examination of meal parameters showed a treatment × time interaction for average meal size (F(12,156) = 2.44, p = .006), where vehicle decreased average meal size over time (p = .005) but 0.5 nmol PrRP did not (treatment × time interaction p = .028) and 5 nmol PrRP caused reduced average meal size more robustly than vehicle (treatment × time interaction p = .027; main effect of time for 5 nmol PrRP p < .000) (Fig. 2c). There was also a treatment × time interaction for meal number (F(12,156) = 2.07, p = .022), where 5 nmol PrRP significantly reduced the total number of meals taken as compared to vehicle (5 nmol main effect of treatment p = .047; treatment × time interaction p = .041) (Fig. 2d).
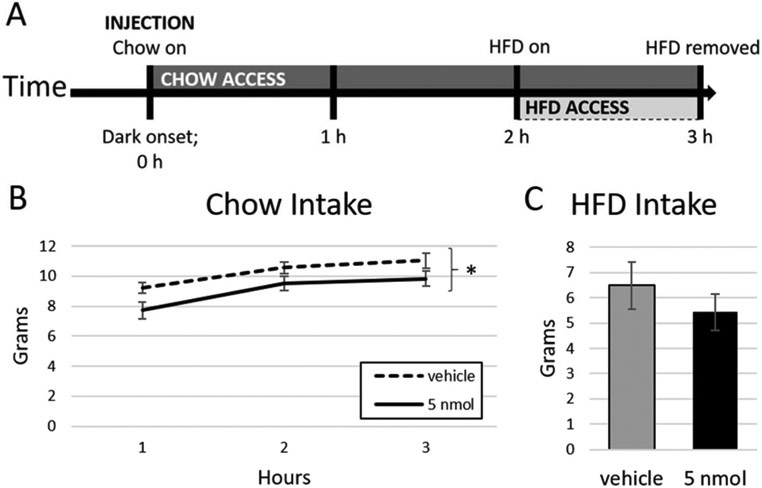
To more precisely assess the latency at which 4V PrRP inhibits food intake, we examined the effects of vehicle versus 5 nmol PrRP on non-cumulative intake by measuring the food intake that took place within discrete hourly time periods. This analysis revealed that 5 nmol PrRP treatment first decreased cumulative intake in the intervals 2–3 h (p = .006; Fig. 3); thus, this time period was chosen as the latency between injection and testing in subsequent experiments. Finally, we tested for evidence of tolerance to PrRP-mediated intake inhibition over repeated injections by limiting our analyses to animals who received 5 nmol PrRP in the last and second-to-last injection conditions. Indeed, we found that the reduced intake effect of 5 nmol PrRP persisted in this subgroup (F(1,6) = 7.91, p = .036).
Fig. 3.
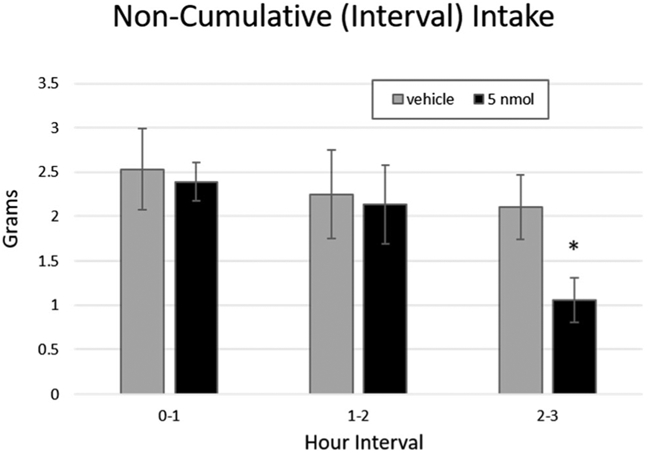
4V PrRP decreases interval chow intake beginning at 2–3 h post-injection in n = 14 animals. * = p < .05; error bars represent SEM
Experiment 1b: ad libitum cumulative chow intake with NTS PrRP administered at dark onset
In rats whose cannulae were histologically confirmed to be in the NTS, there was a strong trend towards a main effect of treatment on intake (F(1,5) = 6.38, p = .053) where 0.1 nmol PrRP (a dose subthreshold for effect on chow intake in the 4V) reduced cumulative chow intake versus vehicle when injected into the NTS (Fig. 4a). When individual timepoints were examined, PrRP reduced intake at 4 (t(1,5) = 3.40, p = .019) and 8 h (t(1,5) = 4.06 p = .01). To compare the effects of PrRP administered to the NTS with PrRP administered to a medullary site outside of the NTS, the treatment effect was examined in an additional n = 6 rats whose cannula were revealed to reside ventral to the NTS in the gigantocellular nucleus (GCN). In contrast to NTS delivery, GCN delivery of PrRP did not affect cumulative chow intake (Fig. 4b).
Fig. 4.
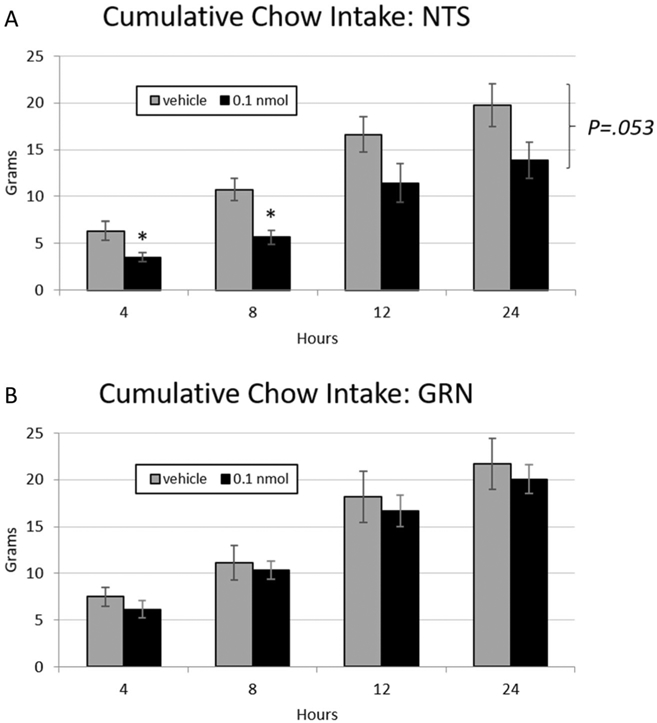
a PrRP (0.1 nmol) delivered to the NTS at dark onset exhibited a strong trend towards decreasing ad libitum chow intake relative to vehicle values in n =6 animals, * = p < .05. b PrRP injected to the GCN did not affect chow intake in a separate cohort of n = 6 animals. Error bars represent SEM
Experiment 2a: effects of 4V PrRP on core temperature, activity, and heart rate
Baseline core temperature was M = 36.3 °C SD = 0.7 in the PrRP condition and M = 36.6 °C SD = 0.6 in the vehicle condition. There was a significant treatment × time interaction where 4V PrRP administration significantly increased core temperature relative to vehicle (F(9, 81 = 2.15, p = .034). This thermogenic effect was significant for the period between 2 and 4 h after injection (t(9) = 2.59, p = .029; Fig. 5a) and trends were noted between 4 and 6 h (t(9) = 1.96, p = .082) and 6–8 h (t(9) = 2.15, p = .06) after injection. 4V PrRP treatment did not affect physical activity (Baseline activity PrRP M = 15.5 SD 10.6, vehicle M = 30.1 SD = 23.9; Fig. 5b) or heart rate (baseline heart rate PrRP M = 342.9 SD 19.1, vehicle M = 346.7 SD = 28.8; Fig. 5c).
Fig. 5.
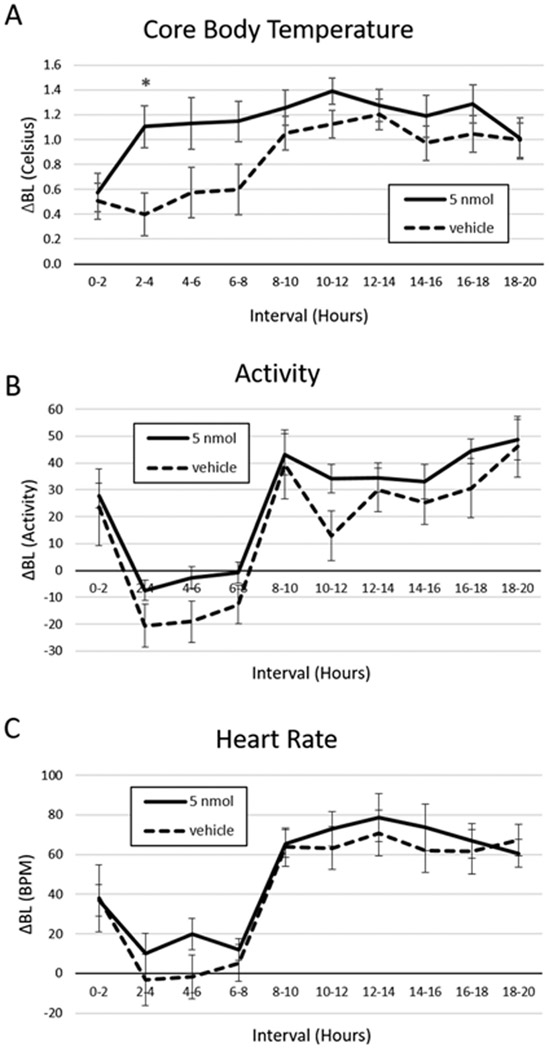
4V PrRP delivered in the light phase increased core temperature but not physical activity or heart rate. a Injection of 5 nmol PrRP increased core body temperature 2–4 h after injection relative to vehicle. In contrast, 4V PrRP had no effect on b physical activity or c heart rate. * = p < 0.05; error bars represent SEM
Experiment 2b: energy of NTS PrRP on core temperature and activity
Baseline core temperature was M = 36.6 °C SD = 0.5 in the PrRP condition and M =36.2 °C SD = 1.8 in the vehicle condition. NTS PrRP administration of 0.05 nmol PrRP (a dose subthreshold for effect on body weight in the 4V) significantly increased core temperature relative to vehicle (main effect of treatment; F(1,7) = 9.28, p = .019); Fig. 6a). The effect on body temperature first reached significance from 4 to 6 h post-injection. In contrast, NTS PrRP treatment did not affect physical activity (baseline activity PrRP M = 22.9 SD = 16.4, vehicle M = 15.8 SD = 8.2; Fig. 6b).
Fig. 6.
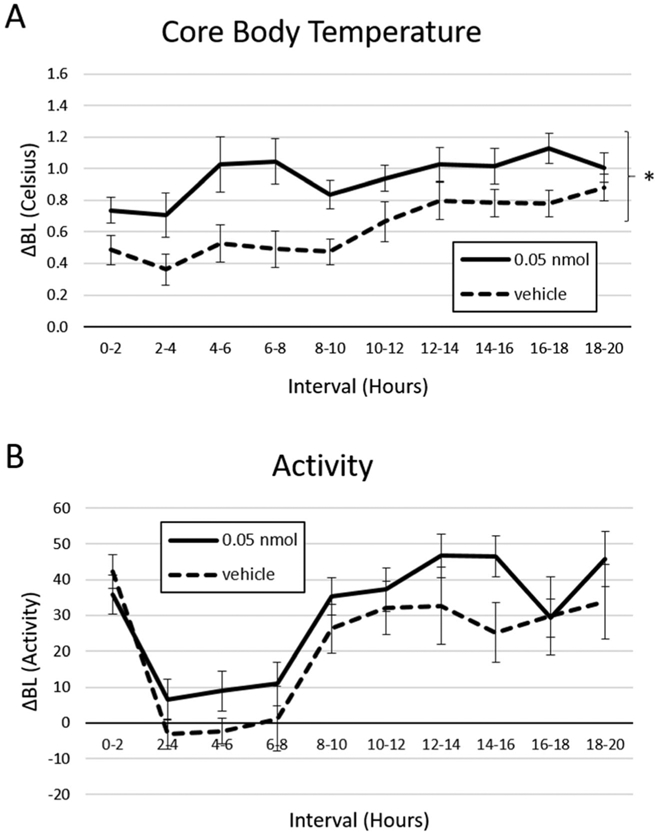
NTS PrRP delivered in the light phase increased core temperature but not physical activity in n = 8 animals. a Injection of 0.05 nmol PrRP increased core body temperature relative to vehicle. In contrast, 4V PrRP had no effect on b physical activity. * = p < 0.05; error bars represent SEM
Experiment 3: PR operant responding for sucrose following 4V PrRP treatment
Compared to vehicle-injected rats, 4V PrRP administration did not significantly alter the number of presses on the active or inactive levers (active lever: PrRP M = 152.9 SD = 112.5, vehicle M = 198.9 SD = 117.9; Inactive lever: PrRP M = 2.6 SD = 2.9, vehicle M = 3.9, SD = 3.7), breakpoint (PrRP M = 33.2 SD = 21.7; vehicle M = 40.4 SD = 21.3) or the number of pellets earned (PrRP M = 9.5 SD = 2.8, vehicle M = 10.4 SD = 2.8). PrRP or vehicle administration also did not significantly alter PR responding as compared to the last day of training (active lever 139.2 SD = 83.1; inactive lever M = 5.1 SD = 4.8; breakpoint M = 30.4 SD = 14.5; pellets earned M = 9.4 SD = 2.2).
Experiment 4: CPP for HFD versus chow following 4V PrRP treatment
At baseline place preference testing prior to training, all n = 15 rats spent more time in the black Plexiglas chamber than the white one. There was a main effect of training on context preference, where after associating it with the availability of HFD, rats spent more time in the initially less-preferred chamber on test day (F(1,13) = 4.74, p = .002; Fig. 7). There was however no main effect of, or interactions with, treatment, even when analyses were limited to the first 3 min of the test when the rats first discover that there is no food in the arena; thus, administration of 4V PrRP vs. vehicle did not affect the training-mediated shift in preference towards the HFD-paired side. Additionally, there was no effect of PrRP vs. vehicle on distance traveled (PrRP M = 1.4 SD = 0.6; vehicle M = 1.4 SD = 0.3), number of entries into the HFD-paired chamber (PrRP M = 24.5 SD = 6.3; vehicle M = 32.3 SD = 14.4), or total time active (PrRP M = 500.6 SD = 113.2; vehicle M = 412.8 SD = 181.9). Note that this dose of PrRP in the 4V significantly reduced non-cumulative chow intake in experiment 1a during the same time interval (2–3 h post-injection) as used in this experiment.
Fig. 7.
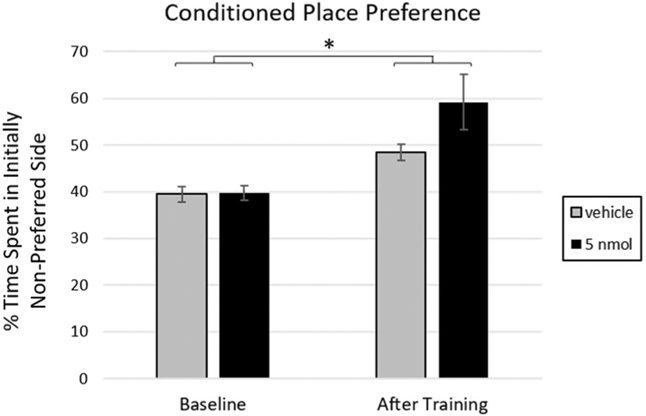
Rats shifted their preference to the HFD-paired side (that was initially non-preferred) after CPP training (* = p < .05). Delivery of 5 nmol PrRP to the 4V prior to the test session did not significantly affect this shift in preference. N = 15 animals; error bars represent SEM
Experiment 5: deprivation-induced and palatable food-motivated intake under sated conditions
4V PrRP significantly and rapidly inhibited chow intake after a 24 h fast in n = 13 animals (main effect of treatment; F(1,12) = 6.82, p = .023; Fig. 8a, b). There was no drug × time interaction, indicating that PrRP was efficacious at inhibiting chow intake for the entire 3 h interval of measurement. However, there was no effect of drug on the intake of HFD when it was presented between hours 2–3 (Fig. 8c). During the third hour in which HFD and chow were simultaneously available, animals reliably consumed less than half a gram of chow (PrRP M = 0.3 g SD = 0.7; vehicle M = 0.5 g SD = 1.0); additionally, during this period, there was no significant effect of treatment on total caloric intake (HFD plus chow) (PrRP M = 25.0 kcal SD = 10.2; vehicle M = 30.2 kcal SD = 13.7).
Fig. 8.
4V PrRP inhibited chow intake after 24 h fast in n = 13 animals. a Schematic of refeed on chow followed by HFD access. b After a 24 h fast, PrRP decreased chow intake for the entire 0–3 h refeeding interval (* = p < .05). b There was no effect of drug on the intake of HFD presented concurrently with hour 3 of chow. Error bars represent SEM
Discussion
When administered to the forebrain (ventricle or DMH parenchyma), PrRP decreases body weight by suppressing chow intake and increasing energy expenditure (e.g., hyperthermia) (Takayanagi and Onaka 2010; Dodd and Luckman 2013). Despite GPR10 receptors being highly expressed in the hindbrain (Fujii et al. 1999; Roland et al. 1999; Ibata et al. 2000), the current study is the first to examine the energy balance effects of hindbrain PrRP administration/GPR10 activation. Additionally, to date, no investigation has addressed whether CNS PrRP suppresses the motivation to work for, seek out, or consume highly palatable food; this information is directly relevant to the potential development of anti-obesity pharmacotherapies targeting the central PrRP system. Here we show that when administered to the hindbrain ventricle (4V) where rostro-caudal cerebrospinal fluid flow limits its action, PrRP (1) decreased ad libitum daily chow intake, (2) increased core temperature, and (3) reduced body weight. The same treatment failed to affect palatable food seeking, motivation to obtain palatable food, or palatable food intake following chow refeed. Furthermore, injection of doses of PrRP that were without effect when administered to the 4V increased core temperature and exhibited a strong trend towards reducing chow intake when injected into the GPR10-expressing NTS. These findings indicate a role of hindbrain GPR10 in the inhibitory control of chow feeding under ad libitum conditions.
Hindbrain ventricular PrRP administration triggered body weight loss that was associated with its effects on both energy intake and core temperature. Injection of PrRP directly into the 4V decreased ad libitum dark cycle onset-associated chow intake, recapitulating the intake-inhibitory effect of forebrain PrRP infusion (Lawrence et al. 2000). This attenuation of chow intake first manifests at 2–3 h post-injection and was mediated by decreased average meal size as well as meal frequency, suggesting that PrRP amplifies both satiation and satiety processing (Smith 2000; Cummings and Overduin 2007; Moran and Dailey 2011). Previously, forebrain ventricular application of a monoclonal anti-PrRP antibody (serving to antagonize GPR10) was shown to increase meal size without affecting meal frequency in rats (Takayanagi et al. 2008), perhaps indicating differential effects of endogenous vs. exogenous stimulation of the PrRP system on meal patterns. We were also able to reproduce prior work showing that PrRP attenuates deprivation-induced chow intake with a shorter latency (Lawrence et al. 2000). As both ad libitum and deprivation-induced feeding manipulations occurred at dark onset, we do not believe the difference in latency is due to a circadian effect. Rather, it is more likely that chow intake under non-deprived conditions incurs a floor effect where relatively subtle augmentations in satiation processing do not begin to measurably accumulate until later in time. This is in line with the putative role of PrRP as a satiety peptide, as PrRP neurons in the NTS are progressively recruited as a function of meal size (Kreisler et al. 2014) and PrRP mRNA is downregulated in states of negative energy balance (Ellacott et al. 2002). Importantly, i.c.v. administration of PrRP does not affect water intake in either ad libitum-fed or 24 h-fasted rats (Lawrence et al. 2000), demonstrating that PrRP does not non-specifically inhibit all ingestive behavior. Additionally, while PrRP activates neurons in many of the same brain regions as lithium chloride and supraphysiological doses of cholecystokinin (CCK), unlike treatments with these latter substances, PrRP does not produce a conditioned taste aversion (Lawrence et al. 2002). Thus, PrRP’s intake-inhibitory effects do not appear to be due to visceral malaise.
As the overconsumption of highly palatable food is the primary contributor to the obesity epidemic, in the search for novel and effective pharmacotherapies for obesity, it is important to address whether any treatment that reduces chow feeding also modifies behavioral responses to palatable foods. Previous studies have not examined whether central PrRP administration affects motivational and appetitive feeding or the intake of palatable food. Additionally, the vast majority of the extant literature on PrRP’s effects on food intake examines effects on chow intake in food-deprived animals, which do not reflect the energy replete state of eating in the absence of homeostatic need. We found that 4V PrRP failed to affect food seeking (CPP), motivation to work for food (PR), or palatable food intake after chow refeed. These data stand in contrast to some results from hindbrain administration of other intake inhibitory agents including leptin and the GLP-1 agonist exendin-4 (Alhadeff and Grill 2014; Kanoski et al. 2014). One potential explanation is that in the hindbrain/NTS, the receptors for leptin and GLP-1 are expressed on, or at least engage, common neural substrates that affect reward processing while GPR10 do not. In rats, neurons that produce GLP-1 do not express leptin receptors (Huo et al. 2008). This opens the possibility that receptors for leptin and GLP-1 may be co-expressed on the same non-GLP-1-producing neurons. Additionally, in rats, application of the GLP-1R antagonist exendin-9 prevents stress-induced activation of PrRP neurons but not GLP-1-producing neurons (Maniscalco et al. 2015), supporting the idea that these ligands engage different subpopulations of NTS neurons that may ultimately culminate in differing behavioral responses in palatability-driven feeding.
More general explanations may underlie the null effects we observed in PrRP’s action on food reward. For example, four of the present experiments used the same cohort of 4V-cannulated animals, and tolerance to repeated i.c.v. injections of PrRP has previously been reported (Ellacott et al. 2003). However, the protocol in that study injected PrRP twice daily for 5 days, and tolerance to PrRP’s anorectic effects only became apparent in the fourth day of this rigorous administration schedule. In contrast, in our paradigm, injections occurred once a day with conditions separated by at least 48 h, and limiting our ad libitum chow analysis to only the animals that received 5 nmol PrRP in the last injection conditions still resulted in significant intake reduction. Each of our food reward tests also used distinct palatable food stimuli so as to minimize potential carryover associations with other foods used in previous tests. Another potential concern is that in comparison to some prior findings in our lab (Alhadeff and Grill 2014; Kanoski et al. 2014; Alhadeff et al. 2017), the effect size of the CPP for HFD in the present study appears mild. We believe that the smaller effect size results from a change in paradigm. The prior work tested the acquisition of a CPP for HFD-paired environment vs. a no-food paired environment. Here we changed our paradigm to examine a HFD-paired vs. a chow-paired environment to specifically assess palatable food seeking rather than food seeking in general. Finally, the magnitude of chow intake suppression resulting from hindbrain PrRP delivery appears more mild than that of other anorexigenic peptides tested in our lab (Hayes et al. 2011; Kanoski et al. 2014). Thus, it remains a possibility that the measures we utilized are not sensitive enough to detect potentially subtle effects of 4V PrRP on motivational and appetitive aspects of feeding.
The caudal brainstem produces endogenous PrRP ligand in the NTS, AP, and VLM, and expresses GPR10 in the NTS, AP, and spinal vestibular nucleus (Fujii et al. 1999; Maruyama et al. 1999; Roland et al. 1999; Ibata et al. 2000). We found that a ventricle-subthreshold dose of PrRP directly injected into the NTS triggered intake-inhibitory and hyperthermia-inducing effects also seen with 4V PrRP delivery. Importantly, the intake reduction observed following PrRP delivery to NTS cannula was not observed when the injection target was the non-GPR10-expressing GCN ventral to the NTS. As GPR10 is also expressed in the AP directly dorsal to the NTS, we cannot functionally distinguish between the contributions of these two sites at this time. NTS and AP neurons are projection targets of vagal afferent neurons transmitting feeding-related sensory inputs such as gastrointestinal (GI) signals (Grill 2006). PrRP cell bodies, fibers and GPR10 alike are highly expressed in the NTS (Dodd and Luckman 2013), as are receptors for other peptides with energy status-related effects such as GLP-1, leptin, oxytocin, and melanocortin (Grill and Hayes 2012; Olszewski et al. 2016). Furthermore, PrRP neurons in the NTS are co-expressed within a subpopulation of A2 catecholaminergic cells (Chen et al. 1999). These A2 neurons are activated by CCK and food intake and are known to project both locally within the caudal hindbrain as well as distally to areas such as the hypothalamus and contribute to satiety-induced intake inhibition (Rinaman 2011; Maniscalco et al. 2012). Thus, our working model is that vagal afferent neurons stimulated by GI satiation signals resulting from food ingestion make synaptic contact with NTS neurons expressing PrRP as well as tyrosine hydroxylase (Maniscalco et al. 2012; Kreisler et al. 2014), activating PrRP gene expression and release at local NTS GPR10-expressing sites as well as at distal ones. This model is analogous to one our lab applies to experiments investigating functional responses to the application of GLP1-R agonists to NTS neurons (Hayes et al. 2009). Our finding that the NTS/AP is a site of action for the intake-inhibitory effects of PrRP is in line with the known role of this region as a critical interface between gastrointestinal satiation signals and the distributed CNS control of feeding behavior. PrRP gene is also expressed in VLM and DMH neurons that are known to project to the NTS and therefore may also be sources of endogenous ligand release (Loewy et al. 1981; Thompson et al. 1996) of relevance to physiological NTS/AP GPR10 signaling.
We report that 4V and NTS PrRP administration increased core temperature. Previous studies have observed a hyperthermic effect of PrRP when administered to the lateral ventricle (Lawrence et al. 2000, 2004; Ellacott et al. 2002, 2003), an effect that may be mediated through increased expression of uncoupling protein-1 (UCP-1) mRNA in brown adipose tissue (Ellacott et al. 2003). Data from pair-fed animals demonstrate that PrRP administration reduces body weight to a greater extent than explained by reduced intake alone, implicating an additive effect of decreased feeding behavior and increased autonomic activity on PrRP-induced weight loss (Ellacott et al. 2003). Additionally, while PrRP administration into both the ventricle as well as the VLM parenchyma has cardiovascular effects (Samson et al. 2000; Horiuchi et al. 2002; Yamada et al. 2009), we were unable to detect an alteration in heart rate following 4V-injected PrRP targeting the dorsomedial medulla. This outcome is consistent with a prior observation that PrRP does not increase heart rate when injected into the NTS or AP (Horiuchi et al. 2002). Interestingly, the intake-inhibitory and cardiovascular but not hyperthermia-inducing effects of PrRP administration are mediated by corticotropin-releasing hormone (CRH) receptors (Lawrence et al. 2004; Yamada et al. 2009), suggesting that the sympathetic, thermogenic, and anorectic responses to PrRP can at least be partially uncoupled. While it is tempting to conjecture that the involvement of CRH in PrRP’s intake inhibition pinpoints stress as the causative agent, multiple studies have remarked that PrRP administration does not induce avoidance responses including conditioned taste avoidance (Seal et al. 2001; Lawrence et al. 2002). Thus, while evidence clearly demonstrate an interaction between PrRP and stress responses (Dodd and Luckman 2013), the directionality of their involvement cannot presently be determined.
We showed that NTS/AP PrRP administration is sufficient to decrease food intake and trigger hyperthermia. Future studies will be needed to address related questions. For example, as all published experiments on PrRP’s effects on feeding were assessed in male rats, the present work was also limited to males in order to minimize variability and avoid being confounded by potential sex effects. Whether PrRP influences behavior differentially based on sex remains unknown. Also, as PrRP is produced by neurons locally in the NTS but also in the VLM and DMH, it will be important to identify the source[s] of endogenous PrRP ligand available to hindbrain GPR10 and of the physiologic antecedents of increased PrRP gene expression require targeted investigation. On a different theme, to the best of our knowledge, this is the first investigation of whether PrRP influences the intake of palatable food as well as motivational and appetitive aspects of feeding behavior. A key question that remains is whether lateral ventricular or forebrain parenchymal PrRP delivery affects hedonic feeding. Finally, there is evidence that at least some effects of PrRP may be mediated through the neuropeptide FF receptor (NPFF2) rather than GPR10. NPFF2 binds PrRP with high affinity (Engström et al. 2003) and i.c.v. PrRP’s ability to decrease food intake remains intact in rats possessing a naturally occurring GPR10 polymorphism that makes it functionally unable to bind radiolabeled PrRP (Ellacott et al. 2005). Additionally, the cardiovascular effects of PrRP administration can be blocked with a NPFF2 antagonist (Ma et al. 2009), although whether PrRP’s feeding effects are similarly abolished is unknown. However, knockout data as well as the hyperphagic and obese phenotype of the GPR10-lacking OLETF rat strongly and specifically implicate GPR10 in PrRP’s effects on feeding (Gu et al. 2004; Watanabe et al. 2005; Bechtold and Luckman 2006; Bjursell et al. 2007). It is worth noting that OLETF rats have preserved cardiovascular responses to PrRP (Ma et al. 2009), supporting a dissociation between PrRP’s sympathetic and anorectic effects and suggesting that this difference may be due to its action at NPFF2 vs. GPR10 receptors. Other work shows that anti-opioid effects of NPFF are mediated through the PrRP system, suggesting that while the two systems are interconnected, they are not redundant (Laurent et al. 2005); a proposition supported by the fact that while NPFF2 binds PrRP, GPR10 does not bind NPFF (Engström et al. 2003). Regardless, a possible contribution of NPFF2 to 4V PrRP’s intake inhibitory effect cannot be ruled out, especially as NPFF2 are also expressed in the NTS (Panula et al. 1996).
In summary, results show that 4V-delivered PrRP induced hyperthermia as well as reduced chow intake, meal size, and body weight under ad libitum conditions. Other experiments examining the effects of direct NTS PrRP delivery but at lower doses confirmed that GPR10 in NTS, and possibly also the adjacent AP, is a site of PrRP’s energy balance action. 4V PrRP did not reduce motivation to seek, work for, or consume palatable food in energy-replete rats. Together, these findings expand our understanding of the PrRP-GPR10 system in the CNS by identifying new sites of action and by determining its effects on different food types as they interact with energy status and body weight.
Acknowledgements
We thank Zhi Yi Ong, Hallie Wald, and Amber Alhadeff for their assistance with experiments.
Funding This study was funded by NIH R01 DK21397 (HJG) and T32 DK007314 (XSD).
Footnotes
Compliance with ethical standards
All procedures conformed to and received approval from the institutional standards of the University of Pennsylvania Animal Care and Use Committee.
Conflict of interest The authors declare that they have no conflict of interest.
References
- Alhadeff AL, Grill HJ (2014) Hindbrain nucleus tractus solitarius glucagon-like peptide-1 receptor signaling reduces appetitive and motivational aspects of feeding. Am J Physiol Regul Integr Comp Physiol 19104:465–470. 10.1152/ajpregu.00179.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadeff AL, Mergler BD, Zimmer DJ, Turner CA, Reiner DJ, Schmidt HD, Grill HJ, Hayes MR (2017) Endogenous glucagon-like peptide-1 receptor signaling in the nucleus tractus solitarius is required for food intake control. Neuropsychopharmacology 42:1471–1479. 10.1038/npp.2016.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold DA, Luckman SM (2006) Prolactin-releasing peptide mediates cholecystokinin-induced satiety in mice. Endocrinology 147:4723–4729. 10.1210/en.2006-0753 [DOI] [PubMed] [Google Scholar]
- Bjursell M, Lennerås M, Göoransson M, Elmgren A, Bohlooly-Y M (2007) GPR10 deficiency in mice results in altered energy expenditure and obesity. Biochem Biophys Res Commun 363:633–638. 10.1016/j.bbrc.2007.09.016 [DOI] [PubMed] [Google Scholar]
- Chen CT, Dun SL, Dun NJ, Chang JK (1999) Prolactin-releasing peptide-immunoreactivity in A1 and A2 noradrenergic neurons of the rat medulla. Brain Res 822:276–279. 10.1016/S0006-8993(99)01153-1 [DOI] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Magrisso IJ, Fitzgerald ME, Lipton JW, Benoit SC (2012) Orexin signaling in the paraventricular thalamic nucleus modulates mesolimbic dopamine and hedonic feeding in the rat. Neuroscience 210:243–248. 10.1016/j.neuroscience.2012.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings DE, Overduin J (2007) Gastrointestinal regulation of food intake. J Clin Invest 117:13–23. 10.1172/JCI30227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JF, Choi DL, Schurdak JD, Fitzgerald MF, Clegg DJ, Lipton JW, Figlewicz DP, Benoit SC (2011) Leptin regulates energy balance and motivation through action at distinct neural circuits. BPS 69:668–674. 10.1016/j.biopsych.2010.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd GT, Luckman SM (2013) Physiological roles of GPR10 and PrRP signaling. Front Endocrinol (Lausanne) 4:1–9. 10.3389/fendo.2013.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd GT, Worth AA, Nunn N, Korpal AK, Bechtold DA, Allison MB, Myers MG Jr, Statnick MA, Luckman SM (2014) The Thermogenic effect of Leptin is dependent on a distinct population of prolactin-releasing peptide neurons in the dorsomedial hypothalamus. Cell Metab 20:639–649. 10.1016/j.cmet.2014.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellacott KLJ, Donald EL, Clarkson P, Morten J, Masters D, Brennand J, Luckman SM (2005) Characterization of a naturally-occurring polymorphism in the UHR-1 gene encoding the putative rat prolactin-releasing peptide receptor. Peptides 26:675–681. 10.1016/j.peptides.2004.11.020 [DOI] [PubMed] [Google Scholar]
- Ellacott KLJ, Lawrence CB, Pritchard LE, Luckman SM (2003) Repeated administration of the anorectic factor prolactin-releasing peptide leads to tolerance to its effects on energy homeostasis. Am J Physiol Regul Integr Comp Physiol 285:R1005–R1010. 10.1152/ajpregu.00237.2003 [DOI] [PubMed] [Google Scholar]
- Ellacott KLJ, Lawrence CB, Rothwell NJ, Luckman SM (2002) PRL-releasing peptide interacts with leptin to reduce food intake and body weight. Endocrinology 143:368–374. 10.1210/en.143.2.368 [DOI] [PubMed] [Google Scholar]
- Engström M, Brandt A, Wurster S, Savola JM, Panula P (2003) Prolactin releasing peptide has high affinity and efficacy at neuropeptide FF2 receptors. J Pharmacol Exp Ther 305:825–832. 10.1124/jpet.102.047118 [DOI] [PubMed] [Google Scholar]
- Fujii R, Fukusumi S, Hosoya M, Kawamata Y, Habata Y, Hinuma S, Sekiguchi M, Kitada C, Kurokawa T, Nishimura O, Onda H, Sumino Y, Fujino M (1999) Tissue distribution of prolactin-releasing peptide (PrRP) and its receptor. Regul Pept 83:1–10. 10.1016/S0167-0115(99)00028-2 [DOI] [PubMed] [Google Scholar]
- Grill HJ (2006) Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity (Silver Spring) 14(Suppl 5):216S–221S. 10.1038/oby.2006.312 [DOI] [PubMed] [Google Scholar]
- Grill HJ, Hayes MR (2012) Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab 16:296–309. 10.1016/j.cmet.2012.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Geddes BJ, Zhang C, Foley KP, Stricker-Krongrad A (2004) The prolactin-releasing peptide receptor (GPR10) regulates body weight homeostasis in mice. J Mol Neurosci 22:93–103. 10.1385/JMN:22:1-2:93 [DOI] [PubMed] [Google Scholar]
- Hayes MR, Bradley L, Grill HJ (2009) Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 150: 2654–2659. 10.1210/en.2008-1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, de Jonghe BC, Kanoski SE, Grill HJ, Bence KK (2011) Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab 13:320–330. 10.1016/j.cmet.2011.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinuma S, Habata Y, Fujii R, Kawamata Y, Hosoya M, Fukusumi S, Kitada C, Masuo Y, Asano T, Matsumoto H, Sekiguchi M, Kurokawa T, Nishimura O, Onda H, Fujino M (1998) A prolactin-releasing peptide in the brain. Nature 393:272–276. 10.1038/30515 [DOI] [PubMed] [Google Scholar]
- Horiuchi J, Saigusa T, Sugiyama N, Kanba S, Nishida Y, Sato Y, Hinuma S, Arita J (2002) Effects of prolactin-releasing peptide microinjection into the ventrolateral medulla on arterial pressure and sympathetic activity in rats. Brain Res 958:201–209. 10.1016/S0006-8993(02)03718-6 [DOI] [PubMed] [Google Scholar]
- Huo L, Gamber KM, Grill HJ, Bjørbæk C (2008) Divergent leptin signaling in proglucagon neurons of the nucleus of the solitary tract in mice and rats. Endocrinology 149:492–497. 10.1210/en.2007-0633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibata Y, Iijima N, Kataoka Y, Kakihara K (2000) Morphological survey of prolactin-releasing peptide and its receptor with special reference to their functional roles in the brain. Neurosci Res 38(3):223–230. 10.1016/S0168-0102(00)00182-6 [DOI] [PubMed] [Google Scholar]
- Iijima N, Kataoka Y, Kakihara K, Bamba H, Tamada Y, Hayashi S, Matsuda T, Tanaka M, Honjyo H, Hosoya M, Hinuma S, Ibata Y (1999) Cytochemical study of prolactin-releasing peptide (PrRP) in the rat brain. Neuroreport 10:1713–1716. 10.1097/00001756-199906030-00016 [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Alhadeff AL, Fortin SM, Gilbert JR, Grill HJ (2014) Leptin signaling in the medial nucleus tractus solitarius reduces food seeking and willingness to work for food. Neuropsychopharmacology 39:605–613. 10.1038/npp.2013.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Fortin SM, Ricks KM, Grill HJ (2013) Ghrelin signaling in the ventral hippocampus stimulates learned and motivational aspects of feeding via PI3K-Akt signaling. Biol Psychiatry 73:915–923. 10.1016/j.biopsych.2012.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Zhao S, Guarnieri DJ, DiLeone RJ, Yan J, de Jonghe BC, Bence KK, Hayes MR, Grill HJ (2012) Endogenous leptin receptor signaling in the medial nucleus tractus solitarius affects meal size and potentiates intestinal satiation signals. AJP Endocrinol Metab 303:E496–E503. 10.1152/ajpendo.00205.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka Y, Iijima N, Yano T, Kakihara K, Hayashi S, Hinuma S, Honjo H, Hayashi S, Tanaka M, Ibata Y (2001) Gonadal regulation of PrRP mRNA expression in the nucleus tractus solitarius and ventral and lateral reticular nuclei of the rat. Brain Res Mol Brain Res 87:42–47. 10.1016/S0169-328X(00)00280-1 [DOI] [PubMed] [Google Scholar]
- Kreisler AD, Davis EA, Rinaman L (2014) Differential activation of chemically identified neurons in the caudal nucleus of the solitary tract in non-entrained rats after intake of satiating vs. non-satiating meals. Physiol Behav 136:47–54. 10.1016/j.physbeh.2014.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent P, Becker JAJ, Valverde O, Ledent C, de Kerchove d’Exaerde A, Schiffmann SN, Maldonado R, Vassart G, Parmentier M (2005) The prolactin-releasing peptide antagonizes the opioid system through its receptor GPR10. Nat Neurosci 8:1735–1741. 10.1038/nn1585 [DOI] [PubMed] [Google Scholar]
- Lawrence CB, Celsi F, Brennand J, Luckman SM (2000) Alternative role for prolactin-releasing peptide in the regulation of food intake. Nat Neurosci 3:645–646. 10.1038/76597 [DOI] [PubMed] [Google Scholar]
- Lawrence CB, Ellacott KLJ, Luckman SM (2002) PRL-releasing peptide reduces food intake and may mediate satiety signaling. Endocrinology 143:360–367. 10.1210/en.143.2.360 [DOI] [PubMed] [Google Scholar]
- Lawrence CB, Liu Y-L, Stock MJ, Luckman SM (2004) Anorectic actions of prolactin-releasing peptide are mediated by corticotropin-releasing hormone receptors. Am J Physiol Regul Integr Comp Physiol 286:R101–R107. 10.1152/ajpregu.00402.2003 [DOI] [PubMed] [Google Scholar]
- Lee Y, Yang SP, Soares MJ, Voogt JL (2000) Distribution of prolactin-releasing peptide mRNA in the rat brain. Brain Res Bull 51:171–176. 10.1016/S0361-9230(99)00212-9 [DOI] [PubMed] [Google Scholar]
- Loewy AD, Wallach JH, McKellar S (1981) Efferent connections of the ventral medulla oblongata in the rat. Brain Res Rev 3:63–80. 10.1016/0165-0173(81)90012-6 [DOI] [PubMed] [Google Scholar]
- Ma L, MacTavish D, Simonin F, Bourguignon JJ, Watanabe T, Jhamandas JH (2009) Prolactin-releasing peptide effects in the rat brain are mediated through the neuropeptide FF receptor. Eur J Neurosci 30:1585–1593. 10.1111/j.1460-9568.2009.06956.x [DOI] [PubMed] [Google Scholar]
- Maletínská L, Nagelová V, Tichá A et al. (2015) Novel lipidized analogs of prolactin-releasing peptide have prolonged half-lives and exert anti-obesity effects after peripheral administration. Int J Obes:986–993. 10.1038/ijo.2015.28 [DOI] [PubMed] [Google Scholar]
- Maniscalco JW, Kreisler AD, Rinaman L (2012) Satiation and stress-induced hypophagia: examining the role of hindbrain neurons expressing prolactin-releasing peptide or glucagon-like peptide 1. Front Neurosci 6:1–17. 10.3389/fnins.2012.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniscalco JW, Zheng H, Gordon PJ, Rinaman L (2015) Negative energy balance blocks neural and behavioral responses to acute stress by “silencing” central glucagon-like peptide 1 signaling in rats. J Neurosci 35:10701–10714. 10.1523/JNEUROSCI.3464-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama M, Matsumoto H, Fujiwara K, Kitada C, Hinuma S, Onda H, Fujino M, Inoue K (1999) Immunocytochemical localization of prolactin-releasing peptide in the rat brain. Endocrinology 140: 2326–2333. 10.1210/endo.140.5.6685 [DOI] [PubMed] [Google Scholar]
- Maruyama M, Matsumoto H, Fujiwara K, Noguchi J, Kitada C, Fujino M, Inoue K (2001) Prolactin-releasing peptide as a novel stress mediator in the central nervous system. Endocrinology 142:2032–2038. 10.1210/en.142.5.2032 [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Maruyama M, Noguchi J, Horikoshi Y, Fujiwara K, Kitada C, Hinuma S, Onda H, Nishimura O, Inoue K, Fujino M (2000) Stimulation of corticotropin-releasing hormone-mediated adrenocorticotropin secretion by central administration of prolactin-releasing peptide in rats. Neurosci Lett 285:234–238. 10.1016/S0304-3940(00)01077-6 [DOI] [PubMed] [Google Scholar]
- Mera T, Fujihara H, Kawasaki M, Hashimoto H, Saito T, Shibata M, Saito J, Oka T, Tsuji S, Onaka T, Ueta Y (2006) Prolactin-releasing peptide is a potent mediator of stress responses through the hypothalamic paraventricular nucleus. Neuroscience 141:1069–1086. 10.1016/j.neuroscience.2006.04.023 [DOI] [PubMed] [Google Scholar]
- Mikulášková B, Zemenová J, Pirník Z, Pražienková V, Bednárová L, Železná B, Maletínská L, Kuneš J (2015) Effect of palmitoylated prolactin-releasing peptide on food intake and neural activation after different routes of peripheral administration in rats. Peptides 75:109–117. 10.1016/j.peptides.2015.11.005 [DOI] [PubMed] [Google Scholar]
- Moran TH, Dailey MJ (2011) Intestinal feedback signaling and satiety. Physiol Behav 105:77–81. 10.1016/j.physbeh.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski PK, Klockars A, Levine AS (2016) Oxytocin: a conditional anorexigen whose effects on appetite depend on the physiological, behavioural and social contexts. J Neuroendocrinol 28(4). 10.1111/jne.12376 [DOI] [PubMed] [Google Scholar]
- Panula P, Aarnisalo AA, Wasowicz K (1996) Neuropeptide FF, a mammalian neuropeptide with multiple functions. Prog Neurobiol 48:461–487. 10.1016/0301-0082(96)00001-9 [DOI] [PubMed] [Google Scholar]
- Rinaman L (2011) Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol 300:R222–R235. 10.1152/ajpregu.00556.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter RC, Slusser PG, Stone S (1981) Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science 213:451–452. 10.1126/science.6264602 [DOI] [PubMed] [Google Scholar]
- Roland BL, Sutton SW, Wilson SJ, Luo L, Pyati J, Huvar R, Erlander MG, Lovenberg TW (1999) Anatomical distribution of prolactin-releasing peptide and its receptor suggests additional functions in the central nervous system and periphery. Endocrinology 140:5736–5745. 10.1210/endo.140.12.7211 [DOI] [PubMed] [Google Scholar]
- Samson WK, Keown C, Samson CK, Samson HW, Lane B, Baker JR, Taylor MM (2003) Prolactin-releasing peptide and its homolog RFRP-1 act in hypothalamus but not in anterior pituitary gland to stimulate stress hormone secretion. Endocrine 20:59–66. 10.1385/ENDO:20:1-2:59 [DOI] [PubMed] [Google Scholar]
- Samson WK, Resch ZT, Murphy TC (2000) A novel action of the newly described prolactin-releasing peptides: cardiovascular regulation. Brain Res 858:19–25. 10.1016/S0006-8993(99)02451-8 [DOI] [PubMed] [Google Scholar]
- Seal LJ, Small CJ, Dhillo WS, Stanley SA, Abbott CR, Ghatei MA, Bloom SR (2001) PRL-releasing peptide inhibits food intake in male rats via the dorsomedial hypothalamic nucleus and not the paraventricular hypothalamic nucleus. Endocrinology 142:4236–4243. 10.1210/en.142.10.4236 [DOI] [PubMed] [Google Scholar]
- Skibicka KP, Grill HJ (2008) Energetic responses are triggered by caudal brainstem melanocortin receptor stimulation and mediated by local sympathetic effector circuits. Endocrinology 149:3605–3616. 10.1210/en.2007-1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GP (2000) The controls of eating: a shift from nutritional homeostasis to behavioral neuroscience. Nutrition 16(10):814–820. 10.1016/S0899-9007(00)00457-3 [DOI] [PubMed] [Google Scholar]
- Stice E, Figlewicz DP, Gosnell BA, Levine AS, Pratt WE (2013) The contribution of brain reward circuits to the obesity epidemic. Neurosci Biobehav Rev 37:2047–2058. 10.1016/j.neubiorev.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, Matsumoto H, Nakata M, Mera T, Fukusumi S, Hinuma S, Ueta Y, Yada T, Leng G, Onaka T (2008) Endogenous prolactin-releasing peptide regulates food intake in rodents. J Clin Invest 118:4014–4024. 10.1172/JCI34682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, Onaka T (2010) Roles of prolactin-releasing peptide and RFamide related peptides in the control of stress and food intake. FEBS J 277:4998–5005. 10.1111/j.1742-4658.2010.07932.x [DOI] [PubMed] [Google Scholar]
- Thompson RH, Canteras NS, Swanson LW (1996) Organization of projections from the dorsomedial nucleus of the hypothalamus: a PHA-L study in the rat. J Comp Neurol 376:143–173. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Mikio S, Yuki Y, et al. (2005) Mutated G-protein-coupled receptor GPR10 is responsible for the hyperphagia/dyslipidaemia/ obesity locus of Dmo1 in the OLETF rat. Clin Exp Pharmacol Physiol 32(5-6):355–366. 10.1111/j.1440-1681.2005.04196.x [DOI] [PubMed] [Google Scholar]
- Yamada T, Mochiduki A, Sugimoto Y et al. (2009) Prolactin-releasing peptide regulates the cardiovascular system via corticotrophin-releasing hormone. J Neuroendocrinol 21:586–593. 10.1111/j.1365-2826.2009.01875.x [DOI] [PubMed] [Google Scholar]
- Zheng H (2005) Brain stem melanocortinergic modulation of meal size and identification of hypothalamic POMC projections. AJP Regul Integr Comp Physiol 289:R247–R258. 10.1152/ajpregu.00869.2004 [DOI] [PubMed] [Google Scholar]