Abstract
The life cycle of Toxoplasma gondii is characterized by active replication alternating with periods of rest. Encysted dormant sporozoites and bradyzoites initiate active replication as tachyzoites and merozoites. Here we explore the role of the cell cycle with a focus on the canonical G1 RESTRICTION checkpoint (R-point) as the integrator governing developmental decisions in T. gondii. This surveillance mechanism which licenses replication, creates a window of opportunity in G1 for cellular reorganization in the execution of developmental transitions. We also explore the unique status of the bradyzoite, the only life cycle stage executing both a forward (entry into the sexual cycle) and reverse (recrudescence) developmental transitions as a multipotent cell. These opposing decisions are executed through the common machinery of the RESTRICTION checkpoint.
Keywords: Toxoplasma gondii, growth, development, cell cycle, checkpoint, metabolism
INTRODUCTION
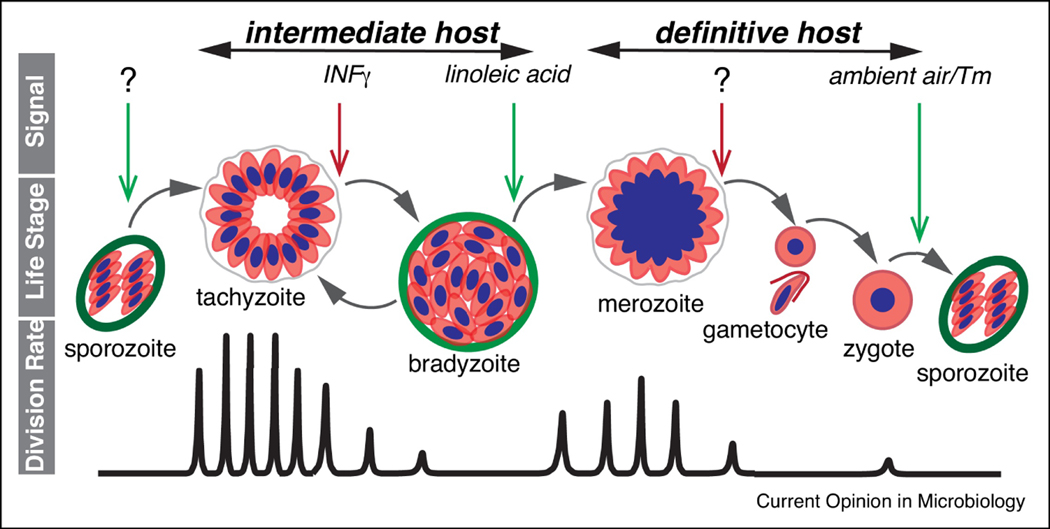
The life cycle of the apicomplexan parasite Toxoplasma gondii is comprised of alternating periods of replication and rest, with developmental decisions governing the transitions (Fig. 1). Upon infection of an intermediate host, resting sporozoites convert into rapidly dividing tachyzoites [1,2]. The rapid expansion of progeny triggers a potent immune response and changes in the nutritional environment [3]. This causes tachyzoites to slow their division converting into largely dormant bradyzoites [3]. After transmission to the definitive feline host, bradyzoites initiate the replicative stage of merogony which is followed by morphological transitions to generate gametocytes [4,5]. Gametocytes fuse and form unsporulated oocysts [6]. Meiosis and limited rounds of replication convert each zygote into a mature oocyst with eight non-replicative sporozoites [5]. Bradyzoite infection of intermediate hosts results in conversion to tachyzoites which can also occur during tissue cyst reactivation within a host [3]. Rapid division of tachyzoites is responsible for the symptomatic stages of infection, while resting bradyzoites associate with the silent chronic infection [3]. Of note, only encysted resting stages (bradyzoites and sporozoites) are capable of transmission between intermediate and definitive hosts [1,7], which involves re-initiation of the active growth.
Figure 1. Major growth-rest transitions in the T. gondii life cycle.
Diagram highlights correlation between extrinsic signals from the host, parasite life stages and replication rates. Transitions between parasite life stages are driven by the host-specific signals, many of which are still unknown. Green arrows indicate signals to enhance parasite division, and the red arrows specify signals to repress division. Alternating T. gondii life stages are indicated in the middle cartoon. Bottom graph visualizes the frequency of the cell division as parasite progresses through the life cycle. First infection of intermediate and definitive host is caused by the resting T. gondii forms, sporozoite and bradyzoite respectively. Each host supports at least one replicative and one resting form of the parasite. Replication in different hosts follow different mechanisms. Tachyzoite employs binary division, endodyogeny to amplify in intermediate hosts, while merozoites divide by multinuclear mechanism, endopolygeny, in definitive feline host. Note that besides the self-renewal, the bradyzoite is the only T. gondii stage capable of forward (merozoite) and reverse (tachyzoite) development.
Our understanding of regulation of the stage conversions has been largely limited to studies of the unidirectional tachyzoite to bradyzoite development [8]. Most of the insights emerge from in vitro systems using diverse triggers [8]. The role of extrinsic stressors is supported by in vivo observations which strongly implicate infection-induced inflammation as a major trigger of differentiation [9]. Accumulation of such extrinsic factors as IFN and other cytokines [10,11] creates an unfavorable host environment marked by toxic reactive oxygen and nitrogen species [12,13]. Furthermore, the inflammatory state is associated with metabolic changes impacting key building blocks and affecting cell energy metabolism [14]. Due to T. gondii auxotrophies, limitation in arginine and tryptophan arrests parasite biosynthetic activity [15]. A new cellular environment in the host represses parasite replication promoting a resting stage that had long been considered as bradyzoite dormancy [16]. The microenvironment created by immune effectors is central to the maintenance of the parasite rest in vivo, as the loss of this control significantly increases reactivation and a return to active growth [3].
The bradyzoite is the only life cycle stage that can execute both a forward step to merozoites and the reverse step to tachyzoites (Fig. 1). The contribution of lipid metabolism for the forward step has been elegantly demonstrated by the Knoll laboratory as involving the sensing of accumulated unsaturated fatty acids (linoleic acid) [17]. This anomaly specific to feline intestinal physiology explains the highly restricted T. gondii host range for the sexual cycle. Nevertheless, despite of the clinical importance of the rest-growth transitions, we know very little about the signals and how the commands are executed.
How are diverse extrinsic stimuli governing parasite developmental transitions are processed within the parasite? The machinery to promote these changes is likely integrated into the basic physiology of the parasite that governs the decision to grow or not. At the heart of this decision is the canonical cell cycle RESTRICTION (or R-point) checkpoint. Here we present arguments pointing to the crucial role of the R-point surveillance mechanism in determining T. gondii developmental fate. In addition, we propose that the bradyzoite functionally resembles a multipotent cell whose fate is determined by the specific factors/signals processed at the RESTRICTION checkpoint.
RESTRICTION checkpoint balances replication and differentiation in T. gondii
Cell division is regulated by the set of surveillance mechanisms called checkpoints to ensure orderly progression through the cell cycle [18]. Located in the G1 phase the RESTRICTION checkpoint is a molecular switch that controls the binary decision to enter or not enter the DNA replication phase [19], consequently, to divide or delay division. This universal mechanism mediates cell adaptation to the changing environment by balancing replication and rest. Originally viewed as a G1 stop, the R-point was later re-evaluated as a cell cycle period or “window of opportunity” that is critically needed to introduce intracellular changes [20]. Undoubtedly, the R-point functions in apicomplexan parasites, including T. gondii. It has been shown that encysted T. gondii stages, sporozoites and bradyzoites, are primarily a G1 form of the parasite, suggesting retention within the window of opportunity [21].
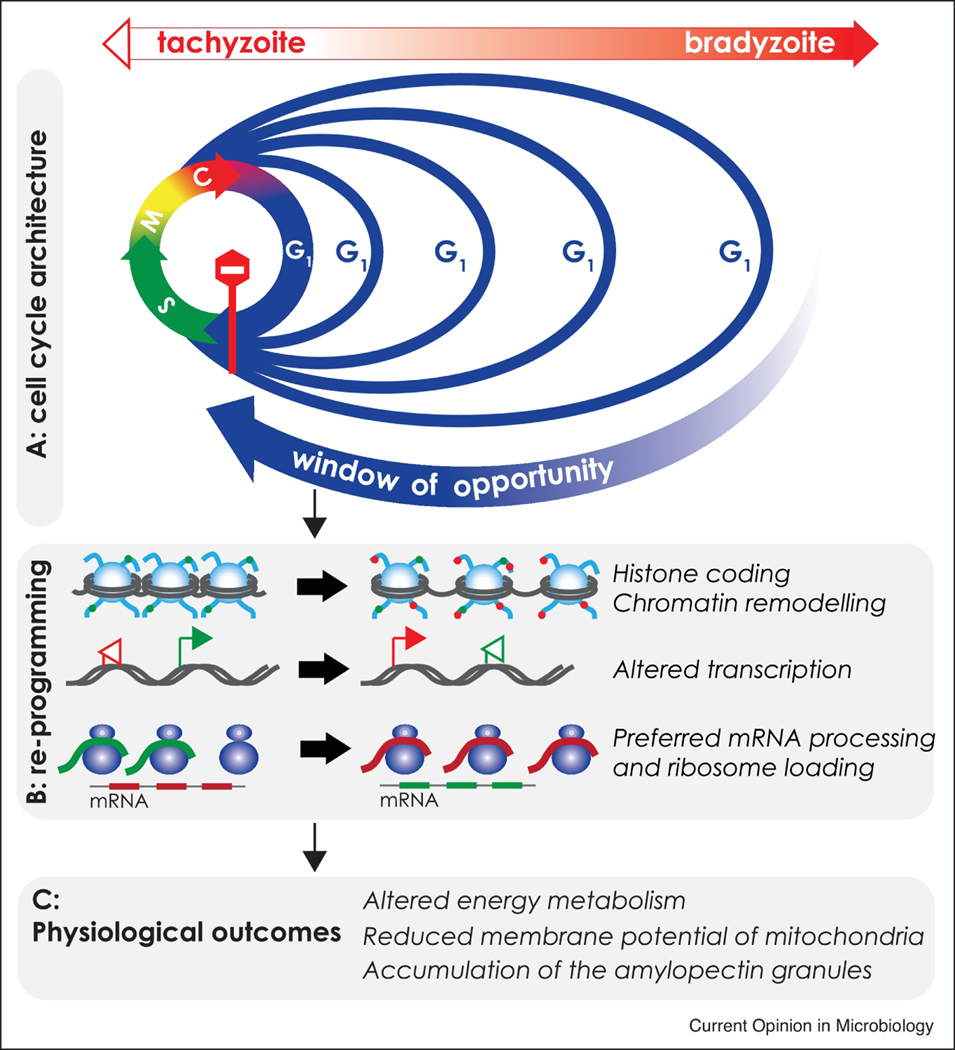
Depending on the conditions, R-point passage may be swift or prolonged, which translates into a growth or rest, respectively. We propose that the R-point mechanism supports the gradual change of the T. gondii tachyzoite into the bradyzoite (Fig. 2A). Rapidly dividing tachyzoites have a short G1 phase, indicative of the uninterrupted R-point passage. Upon bradyzoite differentiation the proportion of the G1 parasites increases reaching nearly 100% G1 parasites in the late stages [21–23]. This phenomenon can be explained by dramatic temporal reorganization of the parasite cell cycle recently verified by single-cell RNA sequencing approach [23]. At each division cycle, the G1 phase of differentiating bradyzoites disproportionally extends with little or no change in the S-phase, mitosis or budding (Fig. 2A). In contrast to a common view, persistent differentiation signal does not trigger the exit from the cell cycle (G0), but rather intervenes with the R-point passage making it increasingly difficult to enter the S-phase, thereby blocking commitment to replication. This mechanism explains why in the late stages, larger proportions of bradyzoites are detained within the widening window of opportunity that progressively extends the cell cycle duration (Fig. 2A). We recently demonstrated that even at the late stage of development, bradyzoites do divide within the tissue cysts in vivo [22]. Therefore, despite increasing difficulty to progress through the G1 phase, once all the checkpoint conditions are met, licensed bradyzoites pass the R-point and complete a division cycle.
Figure 2. The RESTRICTION checkpoint controls developmental transitions in T. gondii.
Diagram illustrates a tachyzoite-to-bradyzoite conversion. A. Reorganization of the cell cycle upon bradyzoite development. Color arrows circle represents cell cycle phases. Blue – Gap 1 (G1) phase, devoted to cell growth and preparation for cell division. Green – a Synthesis (S)-phase, devoted to DNA replication. Yellow – Mitosis (M) phase, during which the sister chromatids are segregated on bipolar spindle. Red – cytokinesis (C), is the last stage of mitosis where the mother cell is resolved into two daughter cells. Stop sign indicates the threshold of the RESTRICTION checkpoint where external signals govern the entry into division cycle. Rapidly dividing tachyzoite has a short G1 period (blue arrow). Unfavorable bradyzoite differentiation conditions repress cell division by disproportional increase of the G1 phase (blue line). This change creates a window of opportunity much needed for cell reprogramming. B. Major reprogramming events taking place in G1 phase of the developing bradyzoites. Window of opportunity permits regional relaxation of the chromatin and changes in histone coding, which in turn activates transcription of the previously silent genes. Transcripts of the bradyzoite-specific genes receive preferential processing. Green colored modifications are tachyzoite specific and red colored are bradyzoite specific. C. Parasite reprogramming during window of opportunity culminates in indicated metabolic changes. New metabolic status of the parasite becomes an internal signal to maintain the current developmental pathway.
Although the R-point is a binary switch, the length of the window of opportunity determines the degree of changes introduced at each division cycle [24]. For example, bradyzoite maturation can be modeled as sequential, increasingly difficult passages through the R-point (Fig 2A). The tachyzoite’s window of opportunity is too short to introduce developmental changes. Differentiation signals initiate re-programming in G1 executed at the epigenetic, transcriptional and translational levels (Fig. 2B) [23,25–28]. Implementation of the developmental reprogramming has been recently demonstrated in the in vitro studies of transcriptional regulators. Among these are TgBDF1, several AP2 factors and MORC1 complex which either promote or inhibit the developmental transitions, or dysregulate these processes [25,28–32]. Active reprogramming establishes a new normal that promotes further epigenetic changes to assure the new state is sustained. Gene expression changes coupled with post-translational modifications culminate in adaptive changes of parasite growth and physiology most clearly seen with regard to energy metabolism (Fig. 2C) [15] [33–35]. The in vitro and more recently in vivo bradyzoite (Patwardhan and Sinai, unpublished) studies reveal altered mitochondrial activity/morphology [36], and changes in the glucose storage, amylopectin granules [37,38]. Additionally, changes in amino acid transport/synthesis are also observed as is likely the case for other classes of metabolites [15]. Cumulative external signals from the host and altered metabolism of the developing bradyzoite continue to apply pressure and make the R-point passage more challenging. Consequently, lengthening the window of opportunity allows extensive reprogramming and further changes in metabolism. Under such conditions, the rounds of the parasite division create a feedback loop building a deeper stage of the bradyzoite differentiation. Evidence from diverse experimental systems show that developmental transitions are probabilistically driven by accumulation of small changes, until a threshold is reached [39]. As more parasites reach the threshold, the population shifts, an event we recognize as the specific stage conversion.
RESTRICTION checkpoint regulation in T. gondii
The RESTRICTION checkpoint is operated by a set of factors that connect environmental signals to the biosynthetic pathways [40]. In conventional eukaryotes, the core R-point network is composed of the CDK4/6 family kinases, D-type cyclins and the specific CDK effector, Retinoblastoma protein, that coordinate gene expression by activation of the e2F transcription factor [40]. None of these canonical R-point regulators were found in apicomplexans, including T. gondii, suggesting that apicomplexan R-point is regulated by alternative network [41]. We recently demonstrated that T. gondii tachyzoites deficient in either CDK-related kinase TgCrk2 or P-type cyclin struggle to complete G1 [42]. Stable interaction of TgCrk2 kinase with TgPHO80 cyclin makes this complex the most likely candidate for the R-point regulator in T. gondii. A few studies indicate that the TgCrk2/P-cyclins network may function as a universal regulator of the RESTRICTION checkpoint in apicomplexan parasites and other protozoa. Orthologs of TgCrk2 and P-cyclins are encoded in all Apicomplexa and were also found in kinetoplastid genomes [42–45]. The TgCrk2 related kinase CRK1 and P-cyclins regulate the G1/S transition and cytoskeletal morphogenesis in Trypanosoma brucei [43,45,46], while studies of Plasmodium berghei development demonstrated a critical role for P-cyclin CYC3 in oocyst sporulation in mosquito stages [44]. Interestingly, TgCrk2 and P-cyclins are distantly related the PHO (phosphate) network found in yeast and plants [43,46–49], but are absent in animals [41,42,44]. In yeast, PHO network regulates various G1 functions, including ion-sensing pathways, nutrient uptake and carbon utilization [50,51]. Moreover, PHO complexes control the passage through the yeast START checkpoint analogous to the R-point in higher eukaryotes [52]. Similar function of the yeast PHO and T. gondii G1 network suggests origination from the same inherited requirements to regulate replication in response environmental changes.
Cyst heterogeneity and developmental fate of the Toxoplasma bradyzoite
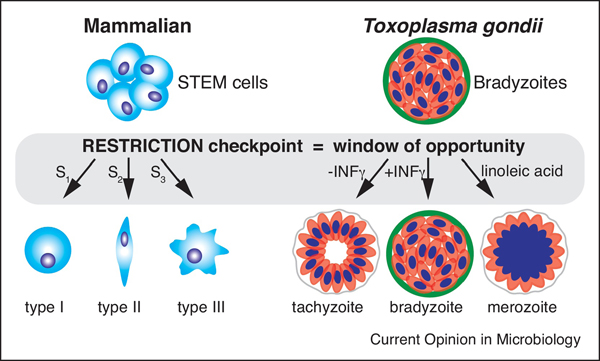
In mammals, the RESTRICTION checkpoint plays a key role in maintenance and fate commitment of the pluripotent cells, suggesting ancestral functions of the R-point in control population diversity [24,53]. There are clear parallels between differentiation of the mammalian stem cells and differentiation in T. gondii, particularly as relates to bradyzoites (Fig. 3). The multipotency of the bradyzoite allows the parasite to remain in its resting state, convert to a tachyzoite or initiate merogony (Fig. 1 and 3). Developmental plasticity of the bradyzoite responding to its extrinsic environment by integrating intrinsic signals into decisions that alter the developmental fate of its immediate progeny, mirrors mammalian stem cell behavior. In our view these decisions are intimately tied to the G1 function. At the RESTRICTION checkpoint, bradyzoites integrate extrinsic (immune environment and metabolites) and intrinsic cues (parasite metabolic state) and execute the developmental programs in a stepwise fashion to reach thresholds associated with the transition. Specificity of the signals drives specific gene expression leading a functional shift in the life cycle stage.
Figure 3. Bradyzoite is the only multipotent T. gondii stage.
Schematics on the left (blue colored cells) represents ability of the mammalian STEM cells to convert into several type of cells. Different signals (S) trigger different developmental pathways (cell type). Similarly, alternative external stimuli direct development of the T. gondii bradyzoites (red colored cells) toward different fates. Bradyzoites recrudesce into tachyzoites when IFNγ is depleted, while excess of the linoleic acid drives forward development into merozoites. In both models, signals are converted into a specific developmental program during window of opportunity in the G1 phase (grey area).
One of the outstanding characteristics of the bradyzoite stage is a remarkable diversity of the intracyst population [22,23]. It is tempting to suggest that cyst heterogeneity facilitates the developmental choice of individual bradyzoites. Early bradyzoites may have higher chance to reinitiate tachyzoite cycle while mature bradyzoites may efficiently converting into merozoite stage. The maintenance of heterogeneity is likely under the cell cycle control. Bradyzoite diversity is probably the result of the cell cycle changes defined by the duration of the window of opportunity creating both cell cycle and physiological diversity. Although the average cyst consists of primarily the G1 bradyzoites, metabolic state of the parasites passing thought the window of opportunity of the first division cycles will be very different than the state of the parasites progressing through the G1 phase of the later division cycles. Indeed, our recent work revealed that individual bradyzoites within tissue cysts, while being genetically clonal are remarkably heterogenous with regard to their physiology [22,23]. This is seen most clearly in the loss of replicative synchrony, diversity in mitochondrial activity/morphology (Patwardhan and Sinai, unpublished) as well as in the levels, distribution and utilization of the amylopectin granules ([38,54], Murphy, Gentry and Sinai, unpublished). As we model transitions in the T. gondii life cycle, quantification of the physiological parameters coupled with detailed “omic” studies at the single cell level are going to be crucial to dissecting how the window of opportunity is harnessed to execute developmental transitions.
In conclusion, confirmation of the R-point’s role in T. gondii developmental will require elucidation of how the stage-specific stimuli are integrated. While the immune and metabolic environment for each transition is different, the corresponding signals converge at and recognized by the R-point machinery. Identification of stimuli, pathways and factors regulating the clinically relevant bradyzoite reactivation will be of a particular importance. Given the central role of the R-point in the growth-rest decision, the checkpoint dissection is likely to reveal targets for pharmacological intervention that may raise R-point barrier thereby block reactivation. This and fundamental discoveries about how environmental and metabolic cues are integrated in the basic decisions governing the Toxoplasma growth offers a new perspective for ongoing investigations into a fascinating parasite.
HIGHLIGHTS.
The developmental fate of Toxoplasma gondii is decided at the G1 RESTRICTION checkpoint (R-point).
The G1 elongation creates a window of opportunity to integrate cues into developmental changes.
Specific host signals direct development of the multipotent bradyzoites at the R-point.
The inherent heterogeneity of individual bradyzoites is a direct consequence of cell cycle reconstruction.
AKNOWLEDGMENTS
The work was supported by grants awarded to ESS R01AI141467 and APS R01AI145335.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Dubey JP, Speer CA, Shen SK, Kwok OC, Blixt JA: Oocyst-induced murine toxoplasmosis: life cycle, pathogenicity, and stage conversion in mice fed Toxoplasma gondii oocysts. J Parasitol 1997, 83:870–882. [PubMed] [Google Scholar]
- [2].Jerome ME, Radke JR, Bohne W, Roos DS, White MW: Toxoplasma gondii bradyzoites form spontaneously during sporozoite-initiated development. Infect Immun 1998, 66:4838–4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Khan IA, Ouellette C, Chen K, Moretto M: Toxoplasma: Immunity and Pathogenesis. Curr Clin Microbiol Rep 2019, 6:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hehl AB, Basso WU, Lippuner C, Ramakrishnan C, Okoniewski M, Walker RA, Grigg ME, Smith NC, Deplazes P: Asexual expansion of Toxoplasma gondii merozoites is distinct from tachyzoites and entails expression of non-overlapping gene families to attach, invade, and replicate within feline enterocytes. BMC Genomics 2015, 16:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Speer CA, Dubey JP: Ultrastructural differentiation of Toxoplasma gondii schizonts (types B to E) and gamonts in the intestines of cats fed bradyzoites. Int J Parasitol 2005, 35:193–206. [DOI] [PubMed] [Google Scholar]
- [6].Dubey JP: Advances in the life cycle of Toxoplasma gondii. Int J Parasitol 1998, 28:1019–1024. [DOI] [PubMed] [Google Scholar]
- [7].Dubey JP: Bradyzoite-induced murine toxoplasmosis: stage conversion, pathogenesis, and tissue cyst formation in mice fed bradyzoites of different strains of Toxoplasma gondii [published erratum appears in J Eukaryot Microbiol 1998 May-Jun;45(3):367]. J Eukaryot Microbiol 1997, 44:592–602. [DOI] [PubMed] [Google Scholar]
- [8].Jeffers V, Tampaki Z, Kim K, Sullivan WJ, Jr.: A latent ability to persist: differentiation in Toxoplasma gondii. Cell Mol Life Sci 2018, 75:2355–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sasai M, Yamamoto M: Innate, adaptive, and cell-autonomous immunity against Toxoplasma gondii infection. Exp Mol Med 2019, 51:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Aviles H, Stiles J, O’Donnell P, Orshal J, Leid J, Sonnenfeld G, Monroy F: Kinetics of systemic cytokine and brain chemokine gene expression in murine toxoplasma infection. J Parasitol 2008, 94:1282–1288. [DOI] [PubMed] [Google Scholar]
- [11].He JJ, Ma J, Song HQ, Zhou DH, Wang JL, Huang SY, Zhu XQ: Transcriptomic analysis of global changes in cytokine expression in mouse spleens following acute Toxoplasma gondii infection. Parasitol Res 2016, 115:703–712. [DOI] [PubMed] [Google Scholar]
- [12].Bohne W, Heesemann J, Gross U: Induction of bradyzoite-specific Toxoplasma gondii antigens in gamma interferon-treated mouse macrophages. Infect Immun 1993, 61:1141–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bohne W, Heesemann J, Gross U: Reduced replication of Toxoplasma gondii is necessary for induction of bradyzoite-specific antigens: a possible role for nitric oxide in triggering stage conversion. Infect Immun 1994, 62:1761–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Neagu M, Constantin C, Popescu ID, Zipeto D, Tzanakakis G, Nikitovic D, Fenga C, Stratakis CA, Spandidos DA, Tsatsakis AM: Inflammation and Metabolism in Cancer Cell-Mitochondria Key Player. Front Oncol 2019, 9:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Krishnan A, Kloehn J, Lunghi M, Chiappino-Pepe A, Waldman BS, Nicolas D, Varesio E, Hehl A, Lourido S, Hatzimanikatis V, et al. : Functional and Computational Genomics Reveal Unprecedented Flexibility in Stage-Specific Toxoplasma Metabolism. Cell Host Microbe 2020, 27:290–306 e211.** By integrating experimentally derived fitness scores identifying essential genes and synthetic lethal pairs with metabolic outputs the plasticity of Toxoplasma metabolism is revealed to be defined in both common and stage specific reactions. These metabolic outputs define the capacity of the parasite to replicate and as such define the thresholds for crossing the R-point barrier or not.
- [16].Sinai AP, Watts EA, Dhara A, Murphy RD, Gentry MS, Patwardhan A: Reexamining Chronic Toxoplasma gondii Infection: Surprising Activity for a “Dormant” Parasite. Current Clinical Microbiology Reports 2016, 3:175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Olson WJ, Martorelli Di Genova B, Gallego-Lopez G, Dawson AR, Stevenson D, Amador-Noguez D, Knoll LJ: Dual metabolomic profiling uncovers Toxoplasma manipulation of the host metabolome and the discovery of a novel parasite metabolic capability. PLoS Pathog 2020, 16:e1008432.This work highlights the complexity of the dynamic dialogue between the host and parasite plays out in the context of metabolism. It exposes how changes influence both the extrinsic (host) and intrinsic (parasite) metabolomes by linking metabolite levels to transcriptional changes. This integration is a central player in defining the decision to replicate or not that defines the R-point.
- [18].Nasmyth K: A prize for proliferation. Cell 2001, 107:689–701. [DOI] [PubMed] [Google Scholar]
- [19].Blagosklonny MV, Pardee AB: The restriction point of the cell cycle. Cell Cycle 2002, 1:103–110. [PubMed] [Google Scholar]
- [20].Schwarz C, Johnson A, Koivomagi M, Zatulovskiy E, Kravitz CJ, Doncic A, Skotheim JM: A Precise Cdk Activity Threshold Determines Passage through the Restriction Point. Mol Cell 2018, 69:253–264 e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Radke JR, Guerini MN, Jerome M, White MW: A change in the premitotic period of the cell cycle is associated with bradyzoite differentiation in Toxoplasma gondii. Mol Biochem Parasitol 2003, 131:119–127. [DOI] [PubMed] [Google Scholar]
- [22].Watts E, Zhao Y, Dhara A, Eller B, Patwardhan A, Sinai AP: Novel Approaches Reveal that Toxoplasma gondii Bradyzoites within Tissue Cysts Are Dynamic and Replicating Entities In Vivo. MBio 2015, 6:e01155–01115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Xue Y, Theisen TC, Rastogi S, Ferrel A, Quake SR, Boothroyd JC: A single-parasite transcriptional atlas of Toxoplasma Gondii reveals novel control of antigen expression. Elife 2020, 9.** Pioneering study of the bradyzoite development in vitro using single-cell RNA sequencing technology. This novel approach not only confirmed extraordinary heterogeneity of the bradyzoite population but for the first time expremintally demonstrated the altered cell cycle architecture of the developing bradyzoite. Elegant experiments reveal extention of the window of opportunity in the G1 phase.
- [24].Dalton S: Linking the Cell Cycle to Cell Fate Decisions. Trends Cell Biol 2015, 25:592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Farhat DC, Swale C, Dard C, Cannella D, Ortet P, Barakat M, Sindikubwabo F, Belmudes L, De Bock PJ, Coute Y, et al. : A MORC-driven transcriptional switch controls Toxoplasma developmental trajectories and sexual commitment. Nat Microbiol 2020, 5:570–583.** This study highlighted critical role for epigenetic machinary in T. gondii development and provided a captivating evidence that under special conditions tachyzoite can skip bradyzoite developmental stage and initiate sexual development. Identified MORC complex likely operates within the window of opportunity prior the R-point threshold.
- [26].Sindikubwabo F, Ding S, Hussain T, Ortet P, Barakat M, Baumgarten S, Cannella D, Palencia A, Bougdour A, Belmudes L, et al. : Modifications at K31 on the lateral surface of histone H4 contribute to genome structure and expression in apicomplexan parasites. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sullivan WJ Jr., Narasimhan J, Bhatti MM, Wek RC: Parasite-specific eIF2 (eukaryotic initiation factor-2) kinase required for stress-induced translation control. Biochem J 2004, 380:523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Waldman BS, Schwarz D, Wadsworth MH 2nd, Saeij JP, Shalek AK, Lourido S: Identification of a Master Regulator of Differentiation in Toxoplasma. Cell 2020, 180:359–372 e316.* The study examined the novel class of the gene expression regulators and their role in T. gondii bradyzoite development. Authors demonstrated a remarkable global shutdown of the bradyzoite developmental program upon knockdown of a single zinc-finger protein TgBDF1 that may represent one of the key connections to the R-point control.
- [29].Huang S, Holmes MJ, Radke JB, Hong DP, Liu TK, White MW, Sullivan WJ Jr., Toxoplasma gondii AP2IX-4 Regulates Gene Expression during Bradyzoite Development. mSphere 2017, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kim K: The Epigenome, Cell Cycle, and Development in Toxoplasma. Annu Rev Microbiol 2018, 72:479–499. [DOI] [PubMed] [Google Scholar]
- [31].Radke JB, Lucas O, De Silva EK, Ma Y, Sullivan WJ, Jr., Weiss LM, Llinas M, White MW: ApiAP2 transcription factor restricts development of the Toxoplasma tissue cyst. Proc Natl Acad Sci U S A 2013, 110:6871–6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Radke JB, Worth D, Hong D, Huang S, Sullivan WJ Jr., Wilson EH, White MW: Transcriptional repression by ApiAP2 factors is central to chronic toxoplasmosis. PLoS Pathog 2018, 14:e1007035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Denton H, Roberts CW, Alexander J, Thong KW, Coombs GH: Enzymes of energy metabolism in the bradyzoites and tachyzoites of Toxoplasma gondii. FEMS Microbiol Lett 1996, 137:103–108. [DOI] [PubMed] [Google Scholar]
- [34].Manger ID, Hehl A, Parmley S, Sibley LD, Marra M, Hillier L, Waterston R, Boothroyd JC: Expressed sequence tag analysis of the bradyzoite stage of Toxoplasma gondii: identification of developmentally regulated genes. Infect Immun 1998, 66:1632–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yahiaoui B, Dzierszinski F, Bernigaud A, Slomianny C, Camus D, Tomavo S: Isolation and characterization of a subtractive library enriched for developmentally regulated transcripts expressed during encystation of Toxoplasma gondii. Mol Biochem Parasitol 1999, 99:223–235. [DOI] [PubMed] [Google Scholar]
- [36].Lin SS, Gross U, Bohne W: Type II NADH dehydrogenase inhibitor 1-hydroxy-2-dodecyl-4(1H)quinolone leads to collapse of mitochondrial inner-membrane potential and ATP depletion in Toxoplasma gondii. Eukaryot Cell 2009, 8:877–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Coppin A, Dzierszinski F, Legrand S, Mortuaire M, Ferguson D, Tomavo S: Developmentally regulated biosynthesis of carbohydrate and storage polysaccharide during differentiation and tissue cyst formation in Toxoplasma gondii. Biochimie 2003, 85:353–361. [DOI] [PubMed] [Google Scholar]
- [38].Guerardel Y, Leleu D, Coppin A, Lienard L, Slomianny C, Strecker G, Ball S, Tomavo S: Amylopectin biogenesis and characterization in the protozoan parasite Toxoplasma gondii, the intracellular development of which is restricted in the HepG2 cell line. Microbes Infect 2005, 7:41–48. [DOI] [PubMed] [Google Scholar]
- [39].Julian LM, Carpenedo RL, Rothberg JL, Stanford WL: Formula G1: Cell cycle in the driver’s seat of stem cell fate determination. Bioessays 2016, 38:325–332. [DOI] [PubMed] [Google Scholar]
- [40].Fisher RP: Getting to S: CDK functions and targets on the path to cell-cycle commitment. F1000Res 2016, 5:2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].White MW, Suvorova ES: Apicomplexa Cell Cycles: Something Old, Borrowed, Lost, and New. Trends Parasitol 2018, 34:759–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Alvarez CA, Suvorova ES: Checkpoints of apicomplexan cell division identified in Toxoplasma gondii. PLoS Pathog 2017, 13:e1006483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Liu Y, Hu H, Li Z: The cooperative roles of PHO80-like cyclins in regulating the G1/S transition and posterior cytoskeletal morphogenesis in Trypanosoma brucei. Mol Microbiol 2013, 90:130–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Roques M, Wall RJ, Douglass AP, Ramaprasad A, Ferguson DJ, Kaindama ML, Brusini L, Joshi N, Rchiad Z, Brady D, et al. : Plasmodium P-Type Cyclin CYC3 Modulates Endomitotic Growth during Oocyst Development in Mosquitoes. PLoS Pathog 2015, 11:e1005273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tu X, Wang CC: Pairwise knockdowns of cdc2-related kinases (CRKs) in Trypanosoma brucei identified the CRKs for G1/S and G2/M transitions and demonstrated distinctive cytokinetic regulations between two developmental stages of the organism. Eukaryot Cell 2005, 4:755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hammarton TC, Engstler M, Mottram JC: The Trypanosoma brucei cyclin, CYC2, is required for cell cycle progression through G1 phase and for maintenance of procyclic form cell morphology. J Biol Chem 2004, 279:24757–24764. [DOI] [PubMed] [Google Scholar]
- [47].Li Z, Wang CC: A PHO80-like cyclin and a B-type cyclin control the cell cycle of the procyclic form of Trypanosoma brucei. J Biol Chem 2003, 278:20652–20658. [DOI] [PubMed] [Google Scholar]
- [48].Torres Acosta JA, de Almeida Engler J, Raes J, Magyar Z, De Groodt R, Inze D, De Veylder L: Molecular characterization of Arabidopsis PHO80-like proteins, a novel class of CDKA;1-interacting cyclins. Cell Mol Life Sci 2004, 61:1485–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Waters NC, Knight JP, Creasy CL, Bergman LW: The yeast Pho80-Pho85 cyclin-CDK complex has multiple substrates. Curr Genet 2004, 46:1–9. [DOI] [PubMed] [Google Scholar]
- [50].Huang D, Friesen H, Andrews B: Pho85, a multifunctional cyclin-dependent protein kinase in budding yeast. Mol Microbiol 2007, 66:303–314. [DOI] [PubMed] [Google Scholar]
- [51].Wang Z, Wilson WA, Fujino MA, Roach PJ: The yeast cyclins Pc16p and Pc17p are involved in the control of glycogen storage by the cyclin-dependent protein kinase Pho85p. FEBS Lett 2001, 506:277–280. [DOI] [PubMed] [Google Scholar]
- [52].Wysocki R, Javaheri A, Kristjansdottir K, Sha F, Kron SJ: CDK Pho85 targets CDK inhibitor Sic1 to relieve yeast G1 checkpoint arrest after DNA damage. Nat Struct Mol Biol 2006, 13:908–914. [DOI] [PubMed] [Google Scholar]
- [53].Soufi A, Dalton S: Cycling through developmental decisions: how cell cycle dynamics control pluripotency, differentiation and reprogramming. Development 2016, 143:4301–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Coppin A, Varre JS, Lienard L, Dauvillee D, Guerardel Y, Soyer-Gobillard MO, Buleon A, Ball S, Tomavo S: Evolution of plant-like crystalline storage polysaccharide in the protozoan parasite Toxoplasma gondii argues for a red alga ancestry. J Mol Evol 2005, 60:257–267. [DOI] [PubMed] [Google Scholar]