Abstract
The genus Entamoeba includes a variety of widely distributed species adapted to live in the digestive tracts of humans and a large variety of animals of different classes. The objective of this study was to investigate the prevalence, distribution, and molecular epidemiology of Entamoeba spp. in different classes of hosts in Brazil. Studies that analyzed hosts from several classes, including humans and domestic, wild, or captive animals, were considered. The pooled prevalence of Entamoeba spp. was calculated using the random-effects model. A total of 166 studies on humans and 16 on animals were included. The prevalence of Entamoeba spp. in the Brazilian population was 22% (95% CI: 21–24). The state with the highest prevalence was Paraiba with 72%, followed by Federal District with 53%, and Rondonia with 50%. In immunocompromized patients, the prevalence was 18%, and cancer (36%) was the most prevalent cause of immunosuppression. The prevalence of Entamoeba spp. in animal hosts was 12% (95% CI: 7–17). Captive wild animals and domestic farm animals showed the highest prevalence, with 16% and 15%, respectively. The species found more often were E. coli (86.5%), E. dispar (7.9%), and E. histolytica (3.1%). In conclusion, a high prevalence (22%) of Entamoeba spp. was found in the Brazilian population, with a prevalence of up to 50% mainly in the northern, northeastern, and central-western regions. The pathogenic species E. histolytica is distributed in most Brazilian regions, with significant prevalence percentages. Among animals, unidentified Entamoeba species were most prevalent in mammals.
Keywords: Parasitic disease, Amebiasis, Diarrhea, Zoonoses, Protozoan
Abstract
Le genre Entamoeba comprend une variété d’espèces largement distribuées, adaptées à vivre dans le tube digestif des humains et une grande variété d’animaux de différentes classes. L’objectif de cette étude était d’étudier la prévalence, la distribution et l’épidémiologie moléculaire d’Entamoeba spp. dans différentes classes d’hôtes au Brésil. Les études qui ont analysé les hôtes de plusieurs classes, y compris les humains et les animaux domestiques, sauvages ou captifs, ont été prises en compte. La prévalence combinée d’Entamoeba spp. a été calculée à l’aide du modèle à effets aléatoires. Au total, 166 études sur l’homme et 16 sur les animaux ont été incluses. La prévalence d’Entamoeba spp. dans la population brésilienne était de 22 % (IC à 95 % : 21–24). L’état avec la prévalence la plus élevée était Paraiba avec 72 %, suivi du District fédéral avec 53 % et Rondonia avec 50 %. Chez les patients immunodéprimés, la prévalence était de 18 % et le cancer (36 %) était la cause la plus fréquente d’immunosuppression. La prévalence d’Entamoeba spp. chez les hôtes animaux était de 12 % (IC à 95 % : 7–17). Les animaux sauvages en captivité et les animaux domestiques d’élevage ont affiché la prévalence la plus élevée, avec respectivement 16 % et 15 %. Les espèces trouvées le plus souvent étaient E. coli (86,5 %), E. dispar (7,9 %) et E. histolytica (3,1 %). En conclusion, une prévalence élevée (22 %) d’Entamoeba spp. a été trouvée dans la population brésilienne, allant jusqu’à 50 % principalement dans les régions du nord, du nord-est et du centre-ouest. L’espèce pathogène E. histolytica est répartie dans la plupart des régions du Brésil, avec des pourcentages de prévalence importants. Parmi les animaux, les espèces d’Entamoeba non identifiées étaient les plus répandues chez les mammifères.
Introduction
The genus Entamoeba includes a variety of anaerobic, unicellular, and monoxenic protozoan species adapted to live as parasites or commensals in the digestive tracts of humans and a large variety of animals of different classes [5, 7, 64, 110, 112, 205, 206].
The main species of this genus that parasitize humans are E. histolytica, E. dispar, E. moshkovskii, E. coli, E. polecki, E. bangladeshi, and E. hartmanni [84, 124, 151, 174]. Morphologically, the species E. histolytica, E. dispar, and E. moshkovskii are considered identical, but only E. histolytica is the causative agent of amebiasis, a gastrointestinal disease that commonly occurs worldwide; amebiasis is considered endemic in tropical regions and is associated with inadequate socioeconomic and sanitary conditions [8, 166, 216]. Entamoeba histolytica shows several degrees of virulence and is capable of invading a wide variety of tissues in the host, including those of the colon and liver, and more rarely the lung, skin, urogenital tract, brain, and spleen. This invasive feature separates it from the other species [70]. It is estimated that amebiasis accounts for 55 500 all-age deaths and causes disability-adjusted life years at 2.237 million [211].
In contrast, E. dispar can cause focal intestinal lesions in laboratory animals [133]. However, in humans, it is considered a stable commensal with no virulent characteristics, producing an asymptomatic carrier state and being generally much more prevalent worldwide than E. histolytica [64, 124]. On the other hand, the idea that E. dispar is a simple commensal parasite is under discussion, and some authors discuss the importance of this species in damage of the intestine and liver [73].
Globally, the overall prevalence of Entamoeba spp. in humans is 3.5%. Entamoeba histolytica and E. dispar account for 81.7% of this global prevalence in documented infections. The comparison of prevalence by regions showed differences in prevalence between Australia (1.7%) and North America (21.6%) [64].
Regarding zoonotic potential, research on E. histolytica, E. dispar, E. hartmanni, E. coli, E. moshkovskii, and E. polecki is remarkably important because of previous reports on these species in both humans and different species of animals worldwide [76, 110, 152, 165, 206]. Furthermore, regarding pathogenic potential, some of these species can cause diarrhea and other symptomatic presentations in non-human primates [165].
The Entamoeba spp. have a variety of vertebrate hosts: E. moshkovskii is found in cattle, elephants, and reptiles [94, 110]; E. coli and E. hartmanni are found in non-human primates [26, 57, 113, 220]; and finally, some studies suggest that different subtypes of E. polecki, infect human, non-human primates, pigs and ostriches [41, 59, 76, 84, 112].
In Brazil, several studies based on microscopic examination have investigated the prevalence of amebiasis in different population groups, but discriminatory studies between species (using molecular methods) are relatively scarce and mainly address different animal hosts. Although there are data on the prevalence of Entamoeba spp. in some regions, there is no aggregate analysis of the prevalence and distribution of species of this protozoan by geographic area, sex, age group, and host type in Brazil. Therefore, the objective of this systematic review and meta-analysis was to determine the prevalence and distribution of different species of Entamoeba in several host classes in Brazil.
Materials and methods
The protocol of this systematic review was registered in the International Prospective Register of Systematic Reviews (PROSPERO 2019: CRD42020167222) before its implementation. The protocol and final report were developed according to the Cochrane Handbook for Systematic Reviews of Interventions [105].
The review question
What is the prevalence and geographical distribution of Entamoeba spp. in different host species in Brazil?
Inclusion and exclusion criteria
This review included studies on various hosts (humans and domestic, wild, or captive animals) of different classes to determine the prevalence and genetic identification of Entamoeba spp. in Brazil through coprological analyses and molecular techniques.
Studies analyzing fecal samples of humans and domestic, wild, or captive animals that did not report percentages of samples positive for Entamoeba spp. were excluded.
Types of studies
This review included cross-sectional epidemiological studies assessing the prevalence of Entamoeba spp. in humans and wild, captive, and domestic animals.
Search strategy
An initial search limited to MEDLINE was conducted using MeSH index terms and related keywords. Subsequently, the words contained in the title, abstract, and index terms used to describe the articles were analyzed. A second search using all identified keywords and index terms was performed using all included databases. As a source of gray literature, a search was conducted in the reference lists of dissertations and theses that evaluated the prevalence of protozoan intestinal parasites. Because this search was limited to Brazil, it was limited to studies in the English, Spanish, and Portuguese languages. This search had no start date limitation but was completed in November 2020.
The studies were searched in the following databases: Spanish Bibliographic Index of Health Sciences (IBECS), Latin American and Caribbean Literature in Health Sciences (LILACS), Virtual Health Library (BVS), US National Library of Medicine bibliographic database (Medline), Elsevier database EMBASE, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Web of Science, Cochrane Library, and National Institute of Health and Clinical Excellence (NICE). The MeSH index terms searched were Entamoeba and Brazil. The keywords Brasil and Endamoeba were also included in the search. The MeSH terms and keywords were combined via the boolean operators “AND” and/or “OR” to compose the search strings.
Assessment of methodological quality
The articles selected for data retrieval were analyzed by two independent reviewers to evaluate the methodological validity of each text before inclusion in this review. The quality of the publications included was evaluated based on the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria. Studies received one point for not presenting a study design or execution limitations (risk of bias), inconsistency of results, indirectness of evidence, imprecision, and publication bias. A score of 4–5 points was considered high quality, 3 as moderate quality, and 0–2 as low quality.
Data extraction
The selected texts were evaluated by two independent reviewers for validity before inclusion; discrepancies were resolved by an independent reviewer. The data were entered into the Review Manager (RevMan 5.3) [168] software for analysis. A data extraction table was used to evaluate the quality of demographic data, study location, sample size, number of cases, number of positive cases, and diagnostic test.
Data summary
The random-effects meta-analysis model was used to analyze the overall combined prevalence of Entamoeba spp. in humans and animals. The heterogeneity among studies was evaluated using I2-statistic, which shows the percentage of variation among studies. These analyses were performed using the Stata software, version 12.
Results
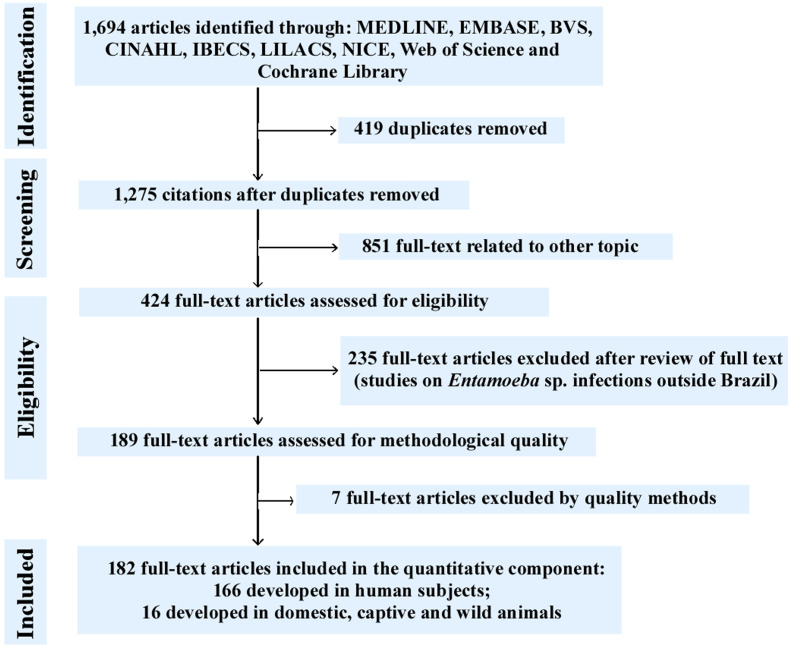
Our systematic literature search yielded 1694 manuscripts using the established search strategies. As per the eligibility criteria (after exclusion of duplicate texts and articles related to other topics and exclusion of text based on review criteria or owing to method quality), 182 studies were selected for analysis (Table 1) [2–4, 6, 7, 9–25, 27–37, 39, 40, 42–45, 47–56, 58, 60–63, 65–69, 71, 72, 74, 75, 77–83, 85–93, 95–104, 106–109, 111, 114–123, 125–130, 132, 135, 136, 138–140, 142–146, 148–150, 153–164, 167, 169–173, 175–192, 194–204, 207–210, 212–215, 217–219]. Of these studies, 166 evaluated the prevalence of Entamoeba spp. in human fecal samples from different Brazilian states during different periods; the remaining 16 studies analyzed the prevalence of Entamoeba spp. parasites in different wild, captive, and domestic animals. Of the 182 studies included, 9 identified the species of the genus Entamoeba by molecular characterization, 17 by serology, and 2 by isoenzyme analysis. The results of this search strategy are presented in a Preferred Reporting Items for Systematic Reviews and Meta-Analyzes (PRISMA) flowchart (Fig. 1). Data were extracted according to the PRISMA Statement [141].
Table 1.
A summary of the included studies.
| No. | Region | City – State | Total N | Prevalence (%) | Diagnostic method | Author/year |
|---|---|---|---|---|---|---|
| Human host | ||||||
| 1 | Midwest | Caceres – MT | 53 | 9.4 | C | Alencar et al. [7] |
| 2 | Midwest | Campo Novo do Parecis – MT | 43 | 37.2 | C | Zenazokenae et al. [219] |
| 3 | Midwest | Caceres – MT | 183 | 36.6 | C | Silva et al. [196] |
| 4 | Midwest | Rondonopolis – MT | 215 | 11.5 | C | Luz et al. [125] |
| 5 | Midwest | Parque do Xingu – MT | 304 | 52.9 | C | Escobar-Pardo et al. [77] |
| 6 | Midwest | MT | 173 | 16.8 | C | Coimbra Jr and Santos [60] |
| 7 | Midwest | Parque Xingu – MT | 62 | 75.8 | C | Ferreira et al. [87] |
| 8 | Midwest | Mirassol D’Oeste – MT | 149 | 38.2 | C | Latorraca et al. [118] |
| 9 | Midwest | Corumba – MS | 200 | 52.0 | C | Silva et al. [198] |
| 10 | Midwest | Corumba – MS | 196 | 55.1 | C | Silva et al. [197] |
| 11 | Midwest | Campo Grande – MS | 510 | 4.6 | C | Curval et al. [65] |
| 12 | Midwest | Campo Grande – MS | 66 | 25.7 | C | Higa Júnior et al. [104] |
| 13 | Midwest | MS | 103 | 43.7 | C | Neres-Norberg et al. [150] |
| 14 | Midwest | Bonito – MS | 115 | 23.5 | C | Gomes et al. [97] |
| 15 | Midwest | Sidrolandia – MS | 313 | 64.8 | C | Aguiar et al. [4] |
| 16 | Midwest | DF | 75 | 53.3 | C | Pereira et al. [157] |
| 17 | Midwest | Cumari – GO | 1029 | 2.7 | C | Borges et al. [33] |
| 18 | South | Moreira Sales – PR | 42 | 4.8 | C | Barbosa and Pavanelli [20] |
| 19 | South | Maringa – PR | 150 | 16.0 | C | Colli et al. [61] |
| 20 | South | Campo Mourao – PR | 5219 | 7.2 | C | Mortean et al. [144] |
| 21 | South | Maria Helena – PR | 431 | 6.5 | C | Santos and Merlini [177] |
| 22 | South | Cascavel – PR | 343 | 17.8 | C | Takizawa et al. [207] |
| 23 | South | Ubirata – PR | 86 | 4.6 | C | Falavigna et al. [79] |
| 24 | South | Campo Mourao – PR | 86 | 4.6 | C | Kulik et al. [117] |
| 25 | South | Jataizinho – PR | 264 | 26.9 | C | Lopes et al. [122] |
| 26 | South | Pitanga – PR | 181 | 20.9 | C | Nascimento and Moitinho [149] |
| 27 | South | Maringa – PR | 369 | 5.9 | C | Guilherme et al. [101] |
| 28 | South | Porto Alegre – PR | 17,951 | 15.1 | C | De Carli et al. [69] |
| 29 | South | Pelotas – RS | 73 | 35.6 | C | Jeske et al. [111] |
| 30 | South | Ipe – RS | 124 | 4.0 | C | Zanotto et al. [218] |
| 31 | South | Palmeiras das Missoes – RS | 209 | 20.6 | C | Nagel et al. [148] |
| 32 | South | Caxias do Sul – RS | 257 | 1.5 | C | Camello et al. [44] |
| 33 | South | Caxias do Sul – RS | 331 | 3.3 | C | Porto et al. [162] |
| 34 | South | Flores da Cunha – RS | 341 | 3.2 | C | Cavagnolli et al. [53] |
| 35 | South | Rio Grande – RS | 144 | 28.5 | C | Mata-Santos et al. [136] |
| 36 | South | Porto Alegre – RS | 146 | 10.3 | C | Silva et al. [192] |
| 37 | South | Caxias do Sul – RS | 9787 | 14.6 | C | Basso et al. [22] |
| 38 | South | Porto Alegre – RS | 181 | 14.9 | C | Bencke et al. [24] |
| 39 | South | Campos Novos – SC | 109 | 13.7 | C | Biolchi et al. [28] |
| 40 | South | Florianopolis – SC | 3126 | 3.5 | C | Bueno et al. [40] |
| 41 | South | Florianopolis – SC | 57 | 31.6 | C | Santos et al. [180] |
| 42 | South | Blumenau – SC | 53 | 18.9 | C | Andrade et al. [11] |
| 43 | South | Criciuma – SC | 94 | 56.4 | E | Schnack et al. [185] |
| 44 | South | Florianopolis – SC | 43 | 4.6 | C | Korzeniowski et al. [116] |
| 45 | Northeast | Teresina – PI | 39,539 | 8.4 | C | Ibiapina et al. [108] |
| 46 | Northeast | Burti dos Lopes – PI | 511 | 8.4 | C | Sousa et al. [201] |
| 47 | Northeast | Parnaiba – PI | 251 | 29.9 | C | Fernandes et al. [85] |
| 48 | Northeast | Sao Raimundo Nonato – PI | 265 | 42.6 | C | Alves et al. [10] |
| 49 | Northeast | Santa Cruz – RN | 3480 | 2.3 | C | Lima et al. [121] |
| 50 | Northeast | Aracaju – SE | 476 | 31.3 | C | Oliveira et al. [155] |
| 51 | Northeast | Aracaju – SE | 500 | 32.6 | C | Rollemberg et al. [172] |
| 52 | Northeast | Aracaju – SE | 298 | 14.1 | C and E | Lawson et al. [119] |
| 53 | Northeast | Santo Antonio de Jesus – BA | 144 | 45.8 | C | Reis et al. [167] |
| 54 | Northeast | Salvador – BA | 48,028 | 0.5 | C and M | Soares et al. [200] |
| 55 | Northeast | Santo Antonio de Jesus – BA | 144 | 45.8 | C | Andrade et al. [12] |
| 56 | Northeast | Aiquara – BA | 236 | 15.7 | C | Santos et al. [183] |
| 57 | Northeast | Feira de Santana – BA | 349 | 50.1 | C | Almeida et al. [9] |
| 58 | Northeast | Ilheus – BA | 97 | 49.5 | C and E | Santos et al. [181] |
| 59 | Northeast | Salvador – BA | 200 | 65.0 | C | Seixas et al. [186] |
| 60 | Northeast | Salvador – BA | 52,704 | 3.4 | C and M | Santos et al. [178] |
| 61 | Northeast | Salvador – BA | 5624 | 15.6 | C | Santos et al. [176] |
| 62 | Northeast | Ipira – BA | 410 | 12.2 | C | Santos-Junior et al. [184] |
| 63 | Northeast | Cuite – PB | 45 | 40.0 | C | Bezerra et al. [27] |
| 64 | Northeast | Joao Pessoa – PB | 150 | 18.6 | C | Monteiro et al. [143] |
| 65 | Northeast | Campina Grande – PB | 1195 | 69.0 | C and E | Silva et al. [195] |
| 66 | Northeast | Joao Pessoa – PB | 67 | 28.3 | C | Magalhães et al. [129] |
| 67 | Northeast | Campina Grande – PB | 742 | 93.1 | C | Silva et al. [188] |
| 68 | Northeast | Russas – CE | 213 | 21.6 | C and M | Calegar et al. [43] |
| 69 | Northeast | Fortaleza – CE | 582 | 29.4 | C | Bachur et al. [15] |
| 70 | Northeast | Fortaleza – CE | 735 | 38.3 | C and E | Braga et al. [36] |
| 71 | Northeast | Fortaleza – CE | 161 | 20.5 | E | Braga et al. [35] |
| 72 | Northeast | Fortaleza – CE | 564 | 36.2 | C and E | Braga et al. [34] |
| 73 | Northeast | Maceio – AL | 1003 | 6.4 | C and M | Santos et al. [182] |
| 74 | Northeast | Maceio – AL | 1798 | 3.8 | C and E | Duarte et al. [74] |
| 75 | Northeast | Recife – PE | 213 | 4.7 | C and E | Dourado et al. [72] |
| 76 | Northeast | Recife e Macaparana – PE | 1783 | 5.8 | C and M | Pinheiro et al. [159] |
| 77 | Northeast | Macaparana – PE | 1437 | 2.6 | C and M | Pinheiro et al. [158] |
| 78 | Northeast | Recife, Palmares e Bodoco – PE | 633 | 28.3 | C, Z and E | Aca et al. [3] |
| 79 | Northeast | Sao Lourenço da Mata – PE | 485 | 41.2 | C and E | Gonçalves et al. [98] |
| 80 | Northeast | Recife – PE | 459 | 50.9 | E | Okazaki et al. [153] |
| 81 | Northeast | Chapadinha – MA | 3933 | 26.9 | C | Silva et al. [190] |
| 82 | Northeast, North | Timo – MA, Macapa – AP | 10,260 | 3.8 | C | Ferraz et al. [86] |
| 83 | North | Belem – PA | 320 | 3.7 | C | Carvalho et al. [50] |
| 84 | North | Santarem – PA | 367 | 34.3 | C | Banhos et al. [16] |
| 85 | North | Belem – PA | 334 | 28.4 | C and E | Silva et al. [187] |
| 86 | North | Belem – PA | 438 | 28.9 | E | Póvoa et al. [163] |
| 87 | North | PA | 300 | 57.6 | C | Miranda et al. [140] |
| 88 | North | Presidente Figueiredo – AM | 143 | 4.2 | C | Gonçalves et al. [99] |
| 89 | North | Coari – AM | 65 | 9.2 | C | Silva et al. [194] |
| 90 | North | Santa Izabel do Rio Negro – AM | 463 | 25.3 | C | Valverde et al. [215] |
| 91 | North | Manaus – AM | 400 | 40.5 | C | Oliveira et al. [154] |
| 92 | North | Iauarete – AM | 333 | 31.2 | C | Boia et al. [32] |
| 93 | North | Manaus – AM | 451 | 23.9 | C | Maia et al. [130] |
| 94 | North | Coari – AM | 211 | 29.4 | C | Monteiro et al. [142] |
| 95 | North | Coari – AM | 123 | 21.1 | C | Silva et al. [189] |
| 96 | North | Sao Gabriel da Cachoeira – AM | 895 | 29.9 | C | Rios et al. [170] |
| 97 | North | Santa Izabel do Rio Negro – AM | 308 | 71.7 | C | Boia et al. [31] |
| 98 | North | Eirunepe – AM | 413 | 38.2 | C | Araújo and Fernandez [13] |
| 99 | North | Manaus – AM | 1585 | 37.3 | C and E | Benetton et al. [25] |
| 100 | North | Nova Olinda do Norte – AM | 81 | 23.4 | C | Hurtado-Guerrero et al. [106] |
| 101 | North | Novo Airao – AM | 316 | 29.1 | C | Boia et al. [30] |
| 102 | North | Manaus – AM | 110 | 9.1 | C | Giugliano et al. [96] |
| 103 | North | Ariquemes e Monte Negro – RO | 216 | 50.4 | C and E | Santos et al. [179] |
| 104 | North | Acrelandia – AC | 429 | 25.6 | C | Souza et al. [202] |
| 105 | Southeast | Diamantina – MG | 66 | 18.2 | C | Eustachio et al. [78] |
| 106 | Southeast | Belo Horizonte – MG | 6289 | 6.5 | C and M | Costa et al. [62] |
| 107 | Southeast | Viçosa – MG | 419 | 32.9 | C | Iasbik et al. [107] |
| 108 | Southeast | Alfenas – MG | 277 | 2.5 | C | Felizardo et al. [83] |
| 109 | Southeast | Ituiutaba – MG | 140 | 22.1 | C | Moura et al. [146] |
| 110 | Southeast | Sete Lagoas – MG | 26 | 30.8 | C | Pires et al. [160] |
| 111 | Southeast | Uberaba – MG | 1323 | 6.4 | C | Cabrine-Santos et al. [42] |
| 112 | Southeast | Caldas – MG | 60 | 66.6 | … | Simões et al. [199] |
| 113 | Southeast | Divinopolis – MG | 1403 | 5.7 | C and E | Pereira et al. [156] |
| 114 | Southeast | MG | 409 | 89.7 | C | Assis et al. [14] |
| 115 | Southeast | Uberaba – MG | 82 | 63.4 | M | Cembranelli et al. [54] |
| 116 | Southeast | Ouro verde de minas – MG | 315 | 28.2 | C | Carvalho et al. [49] |
| 117 | Southeast | Uberlandia – MG | 110 | 17.3 | C | Ferreira-Filho et al. [89] |
| 118 | Southeast | Viçosa – MG | 246 | 4.1 | C | Einloft et al. [75] |
| 119 | Southeast | Pato de Minas – MG | 161 | 16.1 | C | Silva and Silva [191] |
| 120 | Southeast | Berilo – MG | 149 | 24.8 | C | Martins et al. [135] |
| 121 | Southeast | Vespasiano – MG | 176 | 16.5 | C | Barçante et al. [21] |
| 122 | Southeast | Uberlandia – MG | 160 | 23.1 | C | Machado et al. [127] |
| 123 | Southeast | Abadia dos Dourados – MG | 376 | 20.5 | C | Machado et al. [128] |
| 124 | Southeast | Belo Horizonte – MG | 472 | 14.6 | C | Menezes et al. [138] |
| 125 | Southeast | Vespasiano – MG | 537 | 6.3 | C | Santos et al. [175] |
| 126 | Southeast | Bambui – MG | 2811 | 7.4 | C | Rocha et al. [171] |
| 127 | Southeast | Uberlandia – MG | 264 | 1.5 | C | Rezende et al. [169] |
| 128 | Southeast | Uberlandia – MG | 104 | 24.0 | C | Costa-Cruz et al. [63] |
| 129 | Southeast | Uberlandia – MG | 100 | 62.0 | C | Favoreto Jr and Machado [82] |
| 130 | Southeast | Sao Mateus – ES | 50 | 36.0 | C | Albuquerque and Souza [6] |
| 131 | Southeast | Sao Matheus – ES | 42 | 19.0 | C | Brauer et al. [39] |
| 132 | Southeast | Sao Mateus – ES | 221 | 31.2 | C | Damázio et al. [67] |
| 133 | Southeast | Sao Mateus – ES | 82 | 31.7 | C | Damázio et al. [66] |
| 134 | Southeast | Sumidouro – RJ | 294 | 12.9 | C | Barbosa et al. [19] |
| 135 | Southeast | Rio de Janeiro – RJ | 3245 | 6.8 | C | Faria et al. [81] |
| 136 | Southeast | Rio de Janeiro – RJ | 595 | 12.2 | C | Ignácio et al. [109] |
| 137 | Southeast | Rio de Janeiro – RJ | 180 | 10.5 | … | Valença-Barbosa et al. [214] |
| 138 | Southeast | Niteroi – RJ | 68 | 17.6 | C | Leite et al. [120] |
| 139 | Southeast | Niteroi – RJ | 1749 | 5.4 | C | Macedo et al. [126] |
| 140 | Southeast | Niteroi – RJ | 429 | 11.6 | C | Uchôa et al. [213] |
| 141 | Southeast | Rio de Janeiro – RJ | 218 | 1.4 | C | Carvalho-Costa et al. [51] |
| 142 | Southeast | Niteroi – RJ | 140 | 15.7 | C | Port-Lourenço et al. [161] |
| 143 | Southeast | Niteroi – RJ | 261 | 21.8 | C | Uchôa et al. [212] |
| 144 | Southeast | RJ | 99 | 31.3 | C | Moura et al. [145] |
| 145 | Southeast | Ribeirao Preto – SP | 233 | 13.3 | C | Fonseca et al. [91] |
| 146 | Southeast | Sao Jose do Rio Preto – SP | 100 | 7.0 | C | Castro et al. [52] |
| 147 | Southeast | Campos do Jordao – SP | 185 | 22.2 | C | Branco et al. [37] |
| 148 | Southeast | Mirassol – SP | 310 | 15.1 | C | Belloto et al. [23] |
| 149 | Southeast | Sao Jose do Rio Preto – SP | 500 | 0.8 | C | Cardoso et al. [48] |
| 150 | Southeast | Sao Paulo – SP | 66 | 40.9 | C | Lopes et al. [123] |
| 151 | Southeast | Catanduva – SP | 133 | 9.7 | C | Biscegli et al. [29] |
| 152 | Southeast | Presidente Bernardes – SP | 101 | 8.9 | C | Tashima et al. [209] |
| 153 | Southeast | Ribeirao Preto – SP | 429 | 9.3 | C | Capuano et al. [47] |
| 154 | Southeast | Araraquara – SP | 503 | 14.5 | C | Miné and Rosa [139] |
| 155 | Southeast | Sao Paulo – SP | 120 | 16.6 | C | Korkes et al. [115] |
| 156 | Southeast | Catanduva – SP | 250 | 34.4 | C | Faleiros et al. [80] |
| 157 | Southeast | Presidente Prudente – SP | 1000 | 7.1 | C | Tashima and Simões [208] |
| 158 | Southeast | Sao Paulo – SP | 200 | 13.0 | C | Cimerman et al. [58] |
| 159 | Southeast | Sao Jose da Bela Vista – SP | 1032 | 0.2 | C | Tavares-Dias and Grandini [210] |
| 160 | Southeast | Botucatu – SP | 147 | 22.4 | C | Guimarães and Sogayar [102] |
| 161 | Southeast | Holambra – SP | 222 | 15.7 | C | Kobayashi et al. [114] |
| 162 | Southeast | Sao Paulo – SP | 407 | 1.5 | C | Ferreira et al. [88] |
| 163 | Southeast | Osasco – SP | 155 | 21.3 | Z | Aca et al. [2] |
| 164 | Southeast | Sao Paulo – SP | 395 | 25.8 | C | Guerra et al. [100] |
| 165 | Southeast | Guarulhos – SP | 913 | 21.9 | C | Chieffi et al. [56] |
| 166 | Southeast | Ribeirao Preto – SP | 1351 | 23.1 | C | Ferriolli-Filho [90] |
| Animal host | ||||||
| 167 | Southeast | Rio de Janeiro – RJ | 13 (bird – emu) | 23.1 | C and M | Gallo et al. [93] |
| 168 | Southeast | Rio de Janeiro – RJ | 1190 (non-human primate) | 33.4 | C | Barbosa et al. [18] |
| 169 | Southeast | Petropolis – RJ | 790 (pig) | 21.5 | C | Barbosa et al. [17] |
| 170 | Southeast | Sao Paulo – SP | 21 (rodent – mouse) | 9.5 | C | Chagas et al. [55] |
| 171 | Southeast | Bauru – SP | 47 (non-human primate) | 23.4 | C | David et al. [68] |
| 172 | Southeast | Botucatu – SP | 207 (bird) | 1.9 | C | Marietto-Gonçalves et al. [132] |
| 173 | Southeast | Sao Paulo – SP | 31 (canid – guara wolf) | 22.6 | C | Gilioli and Silva [95] |
| 174 | Southeast | Sao Paulo – SP | 103 (edentate – anteater) | 4.8 | C | Diniz et al. [71] |
| 175 | Northeast | CE – MA – PI – PE – BA | 340 (dog) | 3.8 | C | Zanetti et al. [217] |
| 176 | Northeast | Aracaju – SE | 44 (rodent – mouse) | 2.3 | C | Guimarães et al. [103] |
| 177 | Northeast | Lajes – RN | 64 (sheep) | 17.2 | C | Souza et al. [203] |
| 178 | Northeast | Itabuna – BA | 119 (dog) | 0.8 | C | Campos-Filho et al. [45] |
| 179 | Northeast | Recife – PE | 685 (bird) | 5.7 | C | Freitas et al. [92] |
| 180 | North | Sena Madureira – AC | 18 (bird) | 22.2 | C | Souza et al. [204] |
| 181 | Midwest | Caceres – MT | 120 (dog) | 15.8 | C | Rosales and Malheiros [173] |
| 182 | South | SC | 217 (goat) | 1.8 | C | Radavelli et al. [164] |
Abbreviations: MT – Mato Grosso; PR – Parana; PI – Piaui; RN – Rio Grande do Norte; PA – Para; MG – Minas Gerais; SE – Sergipe; BA – Bahia; MS – Mato Grosso do Sul; ES – Espirito Santo; RJ – Rio de Janeiro; PB – Paraiba; RS – Rio Grande do Sul; SP – Sao Paulo; CE – Ceara; AL – Alagoas; SC – Santa Catarina; DF – Federal District (capital of Brazil); MA – Maranhao; AP – Amapa; AM – Amazonas; RO – Rondonia; GO – Goias; AC – Acre; PE – Pernambuco. C – conventional method, based on detection by optical microscopy; M – molecular method, based on DNA detection; E – Elisa method, serology-based; Z – zymodema method, based on isoenzyme analysis.
Figure 1.
A flowchart of the steps performed in the systematic review.
Regarding the methodological quality, according to the GRADE criteria used, all 166 studies evaluating the prevalence of Entamoeba spp. in different Brazilian populations as well as the 16 studies evaluating its prevalence in different animal host species presented a high methodological quality, all with a score of 5.
Entamoeba spp. in the Brazilian population
Overall, the 166 studies on human samples included 268,465 coprological tests and 114 from the oral cavity, including samples from 24 Brazilian states and the Federal District. The only states not analyzed were Roraima and Tocantins, both in the northern region. Test distribution by state showed that 10 studies were performed in Bahia (representing 40.2% of the analyzed samples), 4 in Piaui (15.1%), 11 in Rio Grande do Sul (11.0%), 25 in Minas Gerais (6.1%), 10 in Parana (4.0%), 22 in Sao Paulo (3.3%), 11 in Rio de Janeiro (2.7%), 15 in Amazonas (2.2%), 6 in Pernambuco (1.9%), 6 in Santa Catarina (1.3%), 5 in Ceara (0.8%), 5 in Paraiba (0.8%), 5 in Para (0.6%), 7 in Mato Grosso do Sul (0.6%), 3 in Sergipe (0.5%), 8 in Mato Grosso (0.4%), and 4 in Espirito Santo (0.2%). Two studies were conducted in the states of Maranhao (1.6% of the included samples) and Alagoas (1.0%). Only one study was conducted in Amapa (3.7%), Rio Grande do Norte (1.3%), Goias (0.4%), Acre (0.2%), Rondonia (0.1%), and the Federal District (0.03%).
Of the 166 studies analyzed, only 19 distributed patient samples by sex, totaling 56,442 samples, of which 65% were female and 35% male, with 1992 (3.5%) positive samples. Of the positive samples, 1082 (54.3%) were from females and 910 (45.7%) from males.
Fifty-six studies distributed the samples by age group, totaling 35,411 samples. Of these samples, 26,143 (73.8%) were from children aged 0–9 years; 5971 (16.8%) from aged 10–19 years, and 3297 (9.4%) from adults aged over 19 years. Of these samples, 5684 (16.1%) were positive for Entamoeba spp., with 4133 (72.7%) from children aged 0–9 years, 609 (10.8%) from 10–19 years, and 942 (16.5%) from adults over 19 years.
Regarding the status of the immune system, 266,794 (99.3%) of the samples were from patients with no previously reported compromized immune system, whereas 1785 (0.7%) samples were from immunocompromized patients. Regarding the causes of immunosuppression, it was found that 1463 (82%) samples were from human immunodeficiency virus (HIV) carriers, 249 (14%) from patients undergoing hemodialysis, and 73 (4%) from patients with cancer. Of the samples from immunosuppressed patients, 338 (19%) were positive for Entamoeba spp.; 284 (84%) of these patients had HIV, 28 (8.3%) were undergoing hemodialysis, and 26 (7.7%) had cancer.
Pooled prevalence of Entamoeba spp.
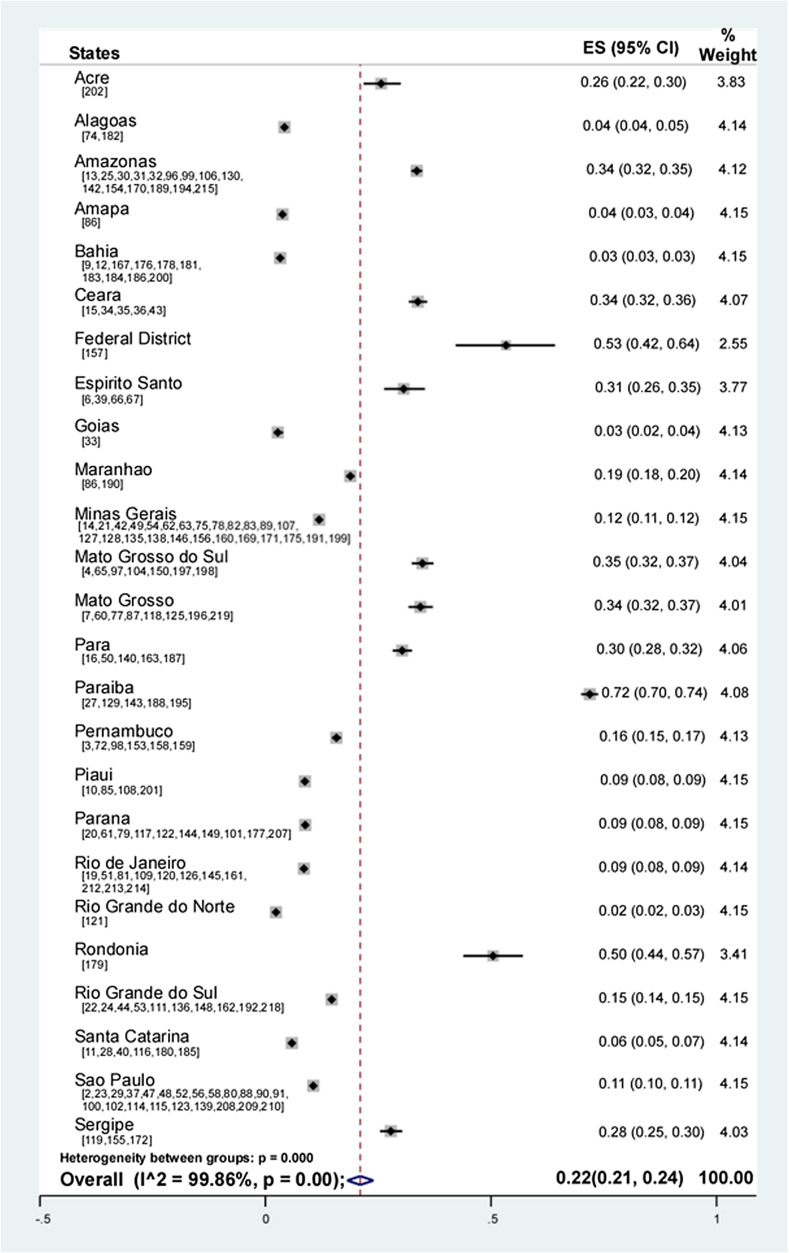
The prevalence of Entamoeba spp. reported in the analyzed studies was between 0.2% and 93.1%. Random-effects meta-analysis showed a pooled prevalence of 22% (95% CI: 21–24; weight 100%) of Entamoeba spp. in the Brazilian population (Fig. 2).
Figure 2.
Forest plot for a random-effect meta-analysis of the pooled prevalence of Entamoeba spp. in the Brazilian population by state. In parentheses the studies used for each state.
The analysis of pooled prevalence by state showed that it was 72% in Paraiba, 53% in the Federal District, 50% in Rondonia, 35% in Mato Grosso do Sul, 34% in Mato Grosso and Amazonas and Ceara, 31% in Espirito Santo, 30% in Para, 28% in Sergipe, 26% in Acre, 19% in Maranhao, 16% in Pernambuco, 15% in Rio Grande do Sul, 12% in Minas Gerais, 11% in Sao Paulo, 9% in Parana, Piaui and Rio de Janeiro, 6% in Santa Catarina, 4% in Alagoas and Amapa, 3% in Bahia and Goias, and 2% in Rio Grande do Norte (Fig. 2). The pooled prevalence with complete 95% CI values for each state is shown in Table 2.
Table 2.
Distribution of the pooled prevalence of Entamoeba spp. according to state and age.
| Overall |
≤9 |
10–19 |
>20 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| State | Overall prevalence | 95% CI | Weight (%) | Prevalence | 95% CI | Weight (%) | Prevalence | 95% CI | Weight (%) | Prevalence | 95% CI | Weight (%) |
| PR | 13 | 1–25 | 4.30 | 13 | 1–25 | 7.16 | – | – | – | – | – | – |
| SE | 31 | 27–36 | 1.44 | 31 | 27–36 | 2.39 | – | – | – | – | – | – |
| RS | 20 | 7–33 | 5.63 | 15 | 2–29 | 7.13 | – | – | – | 36 | 26–47 | 5.19 |
| PA | 34 | 30–39 | 1.43 | 34 | 30–39 | 2.38 | – | – | – | – | – | – |
| MG | 33 | 22–45 | 24.58 | 23 | 9–36 | 22.36 | 45 | 24–67 | 41.1 | 47 | 7–100 | 21.17 |
| SP | 19 | 13–26 | 12.89 | 17 | 10–24 | 14.31 | 34 | 28–41 | 10.49 | 21 | 19–23 | 10.72 |
| MT | 28 | 6–50 | 5.66 | 34 | 6–62 | 7.10 | – | – | – | 9 | 4–20 | 5.28 |
| MA | 4 | 3–6 | 1.45 | 4 | 3–6 | 2.41 | – | – | – | – | – | – |
| AP | 4 | 3–4 | 1.45 | 4 | 3–4 | 2.42 | – | – | – | – | – | – |
| SC | 36 | 13–58 | 4.06 | 36 | 13–58 | 6.79 | – | – | – | – | – | – |
| PB | 85 | 84–87 | 2.9 | 85 | 84–87 | 4.82 | – | – | – | – | – | – |
| BA | 30 | 17–42 | 6.3 | 13 | 9–16 | 4.18 | 50 | 28–72 | 6.99 | 20 | 16–25 | 10.50 |
| AM | 20 | 14–26 | 9.88 | 16 | 8–24 | 9.49 | 30 | 22–39 | 10.18 | 26 | 21–32 | 10.56 |
| MS | 56 | 36–76 | 5.50 | 55 | 45–64 | 2.29 | 75 | 65–83 | 10.11 | 51 | 44–57 | 10.48 |
| RJ | 22 | 17–27 | 2.74 | 21 | 16–27 | 2.38 | – | – | – | 26 | 15–40 | 5.12 |
| PE | 23 | 8–39 | 5.7 | 25 | 20–30 | 2.39 | 6 | 5–7 | 21.13 | 35 | 28–41 | 5.31 |
| ES | 19 | 10–33 | 1.33 | – | – | – | – | – | – | 19 | 10–33 | 5.16 |
| FD | 53 | 42–64 | 1.34 | – | – | – | – | – | – | 53 | 42–64 | 5.18 |
| PI | 30 | 25–36 | 1.42 | – | – | – | – | – | – | 30 | 25–36 | 5.33 |
| Overall Prevalence | 29 | 24–34 | 100 | 25 | 18–31 | 100 | 40 | 29–50 | 100 | 34 | 20–47 | 100 |
Abbreviations: 95% CI, 95% confidence interval. PR – Parana, SE – Sergipe, RS – Rio Grande do Sul, PA – Para, MG – Minas Gerais, SP – Sao Paulo, MT – Mato Grosso, MA – Maranhao, AP – Amapa, SC – Santa Catarina, PB – Paraiba, BA – Bahia, AM – Amazonas, MS, Mato Grosso do Sul, RJ – Rio de Janeiro, PE – Pernambuco, ES – Espirito Santo, DF – Federal District, PI – Piaui.
Pooled prevalence by age group showed that the age group between 10 and 19 years had the highest prevalence (40%; 95% CI: 29–50; weight 100%). The state with the highest prevalence in this age group was Mato Grosso do Sul (75%), followed by Bahia (50%), Minas Gerais (45%), Sao Paulo (34%), Amazonas (30%), and Pernambuco (6%). In the group over 19 years of age, the pooled prevalence was 34% (95% CI: 20–47; weight 100%). The state with the highest prevalence in this age group was the Federal District (53%), followed by Mato Grosso do Sul (51%), Minas Gerais (47%), Rio Grande do Sul (36%), Pernambuco (35%), Piaui (30%), Rio de Janeiro and Amazonas (26%), Sao Paulo (21%), Bahia (20%), Espirito Santo (19%), and Mato Grosso (9%). Children below 9 years of age had a pooled prevalence of 25% (95% CI: 18–31; weight 100%). The state with the highest prevalence for this age group was Paraiba (85%), followed by Mato Grosso do Sul (55%), Santa Catarina (36%), Mato Grosso and Para (34%), Sergipe (31%), Pernambuco (25%), Minas Gerais (23%), Rio de Janeiro (21%), Sao Paulo (17%), Amazonas (16%), Rio Grande do Sul (15%), Parana and Bahia (13%), and Maranhao and Amapa (4%) (Table 2).
The pooled prevalence in the 19,721 male samples was 26% (95% CI: 20–31; weight 100%). The state with the highest prevalence was Para (57%), followed by Pernambuco (33%), Amazonas (28%), Parana (20%), Espirito Santo (19%), Sao Paulo (18%), Mato Grosso and Rio de Janeiro (15%), Minas Gerais (8%), Mato Grosso do Sul (7%), and Bahia (1%). In contrast, the pooled prevalence in the 36,721 female samples was 29% (95% CI: 14–43; weight 100%). The state with the highest prevalence of Entamoeba spp. in female samples was Mato Grosso do Sul (62%), followed by Para (59%), Amazonas (33%), Espirito Santo (31%), Pernambuco (25%), Parana (21%), Sao Paulo (19%), Rio de Janeiro (11%), Minas Gerais (7%), and Mato Grosso (4%).
The pooled prevalence in immunosuppressed patients was 18% (95% CI: 7–30; weight 100%). The most prevalent cause of immunosuppression with Entamoeba spp. was cancer (36%), followed by HIV infection (27%), and hemodialysis (10%) (Table 3).
Table 3.
Distribution of the pooled prevalence of Entamoeba spp. according to the type of immunosuppression.
| Immunosuppression | Overall subtotal | 95% CI | Weight (%) |
|---|---|---|---|
| Cancer | 36 | 26–47 | 10.45 |
| HIV infection | 27 | 9–45 | 55.96 |
| Hemodialysis | 10 | 2–18 | 33.59 |
| Overall prevalence | 18 | 7–30 | 100 |
Abbreviations: 95% CI, 95% confidence interval.
Entamoeba spp. in animals in Brazil
The 16 studies that analyzed the prevalence of Entamoeba spp. in animals included 3805 coprological tests in different species (79.1% mammals and 20.9% birds). The classification by direct interaction with humans showed that 54% were wild animals in captivity, 2.3% were free-living wild animals, 15.2% were pets, and 28.5% were farm animals.
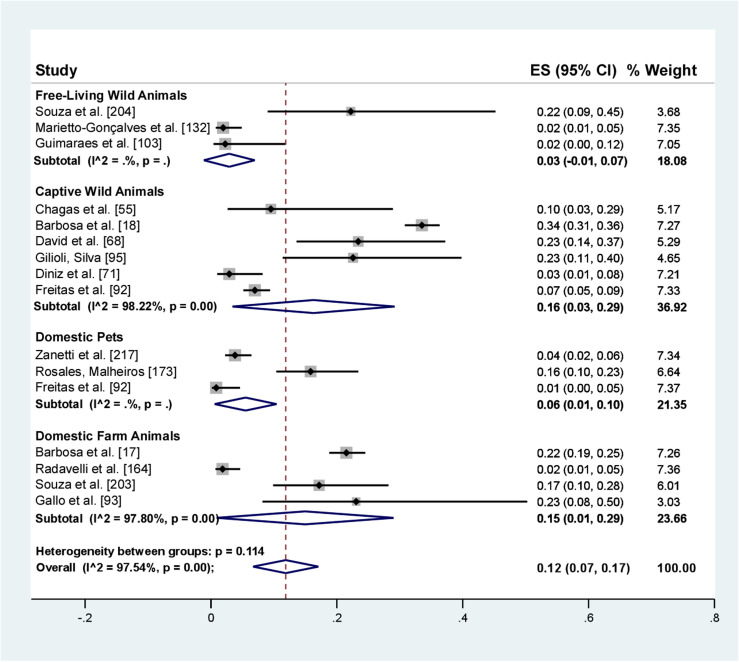
The analysis of prevalence of Entamoeba spp. in Brazilian animals from different orders and with different types of human interaction showed a pooled prevalence of 12% (95% CI: 7–17). Wild animals in captivity had a prevalence of 16% (95% CI: 3–29), free-living wild animals 3% (95% CI: 1–7), farm animals 15% (CI95%: 1–29.00), and pets 6% (95% CI: 1–10) (Fig. 3).
Figure 3.
Forest plot for a random-effect meta-analysis of the pooled prevalence of Entamoeba spp. in different animals in Brazil, according to the type of interaction with humans.
The prevalence of Entamoeba spp. by taxonomic class showed a prevalence of 12% (95% CI: 6–19) in mammals and 6% (95% CI: 1–12) in birds (Table 4).
Table 4.
Distribution of the pooled prevalence of Entamoeba spp. according to taxonomic class and interaction with humans.
| Study | Taxonomic class | Overall prevalence (%) | 95% CI | Weight (%) |
|---|---|---|---|---|
| Mammals | 12 | 6–19 | 78.60 | |
| Guimarães et al. [103] | Rodents | 2 | 0–12 | 7.05 |
| Chagas et al. [55] | Rodents | 10 | 3–29 | 5.17 |
| Barbosa et al. [18] | Non-human primates | 34 | 31–36 | 7.27 |
| David et al. [68] | Non-human primates | 23 | 14–37 | 5.29 |
| Gilioli and Silva [95] | Guara wolf | 23 | 11–40 | 4.65 |
| Diniz et al. [71] | Anteaters | 3 | 1–8 | 7.21 |
| Zanetti et al. [217] | Dogs | 4 | 2–6 | 7.34 |
| Rosales and Malheiros [173] | Dogs | 16 | 10–23 | 6.64 |
| Campos-Filho et al. [45] | Dogs | 1 | 0–5 | 7.37 |
| Barbosa et al. [17] | Pigs | 22 | 19–25 | 7.26 |
| Radavelli et al. [164] | Goat | 2 | 1–5 | 7.36 |
| Souza et al. [203] | Sheep | 17 | 10–28 | 6.01 |
| Birds | 6 | 1–12 | 21.40 | |
| Souza et al. [204] | Birds | 22 | 9–45 | 3.68 |
| Marietto-Gonçalves et al. [132] | Birds | 2 | 1–5 | 7.35 |
| Freitas et al. [92] | Birds | 7 | 5–9 | 7.33 |
| Gallo et al. [93] | Emus | 23 | 8–50 | 3.03 |
| Interaction with humans | ||||
| Free-living wild animals | 3 | 1–7 | 18.08 | |
| Captive wild animals | 16 | 3–29 | 36.92 | |
| Domestic pets | 6 | 1–10 | 21.35 | |
| Domestic farm animals | 15 | 1–29 | 23.66 |
Abbreviations: 95% CI, 95% confidence interval
Of the captive wild mammals, non-human primates were the most studied, with prevalence percentages of 34% and 23%. In contrast, of the farm mammals, pigs had a prevalence of 22%. Notably, the only animal considered a pet in the studies analyzed was the dog, representing 16% (Table 4). Of the domestic farm birds, emus had a prevalence of 23% and free-living wild birds had a prevalence of 22% (Table 4).
Entamoeba spp. diversity in different host species in Brazil
Conventional microscopy analysis, molecular characterization, serology, and isoenzyme analysis were used to identify Entamoeba spp. in 150 studies, totaling 17,651 human samples. In contrast, only six studies on host animals characterized 51 positive samples at the species level.
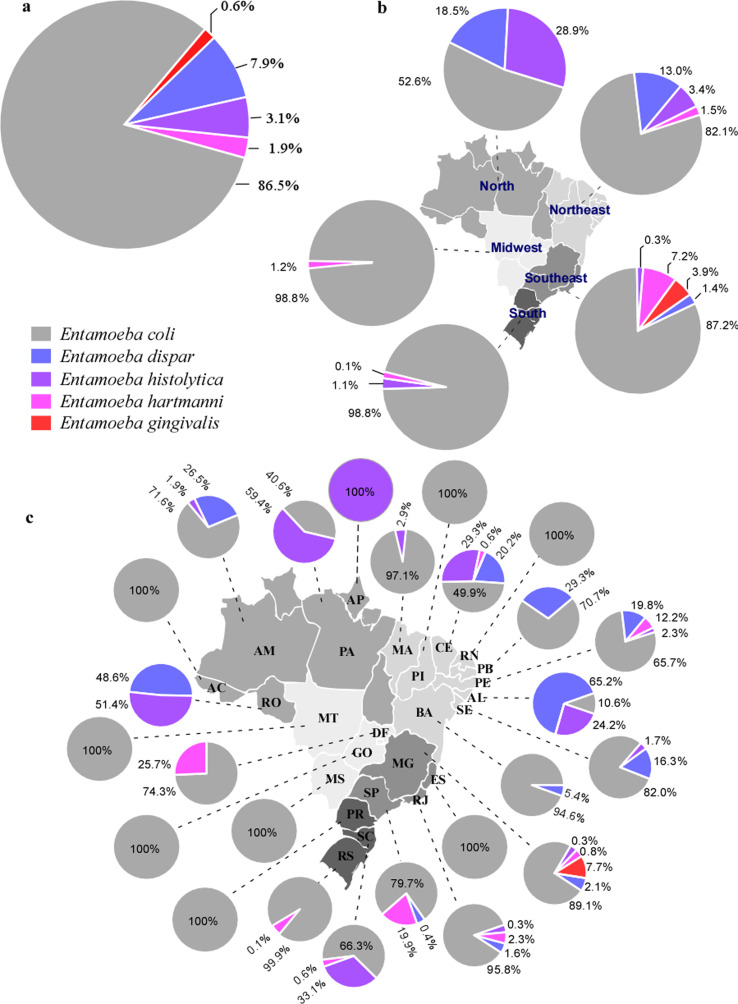
To calculate the prevalence of the reported species, only the samples that performed this procedure were used. For this purpose, 17,651 samples (fecal and oral cavity) with identification of Entamoeba species, were used. In these samples, the most prevalent species identified in human hosts were E. coli (86.5%), followed by E. dispar (7.9%), E. histolytica (3.1%), E. hartmanni (1.9%), and E. gingivalis 0.6% (Fig. 4). The species identified as non-pathogenic E. histolytica, through zymodeme [2, 3], were considered as E. dispar. On the other hand, E. coli was the only species with a taxonomic classification, identified in animal hosts. In addition, unidentified Entamoeba species were reported in animal hosts.
Figure 4.
Geographical distribution of Entamoeba spp. detected in Brazil. (a) Species detected in 17,651 human samples. (b) Species distribution in human and animal hosts according to Brazilian regions. (c) Species distribution in human and animal hosts in Brazilian states. Abbreviations: AC – Acre; AM – Amazonas; RO – Rondonia; PA – Para; MA – Maranhao; PI – Piaui; CE – Ceara; RN – Rio Grande do Norte; PB – Paraiba; PE – Pernambuco; AL – Alagoas; SE – Sergipe; BA – Bahia; MG – Minas Gerais; ES – Espirito Santo; RJ – Rio de Janeiro; SP – Sao Paulo; PR – Parana; SC – Santa Catarina, RS – Rio Grande do Sul; MS – Mato Grosso do Sul; GO – Goias; MT – Mato Grosso; DF – Federal District (Capital of Brazil).
The prevalence of species by geographical regions showed that E. coli was the most prevalent species in the five regions, with high percentages. Entamoeba histolytica was identified in the north (28.9%), northeast (3.4%), south (1.1%), and southeast (0.3%) regions. The southeast region presented the greatest species diversity, with the identification of the five Entamoeba spp. registered in Brazil, followed by the northeast region with four species, north and south with three, and center-west with two different species (Fig. 4).
The detailed distribution of protozoan species by the Brazilian state is shown in Figure 4.
Discussion
Data on the prevalence of Entamoeba spp. were documented in 24 of 26 Brazilian states and in the Federal District. In this meta-analysis, a pooled prevalence of 22% of Entamoeba spp. was found in the Brazilian population. The pooled prevalence was calculated with samples of studies published between 1962 to 2020, so this percentage represents an overall prevalence of Entamoeba spp. in different hosts during this period of time, in Brazil. These results reflect a sampling of the five Brazilian regions, but the northeastern, southern, and southeastern regions are better characterized since these regions present higher scientific production. The northeastern region contributed 38 articles, representing 63.3% of the samples analyzed in this meta-analysis, the southern region 27 studies (16.3%), the southeastern region 62 (12.3%), the northern region 23 (6.7%), and the central-western region 17 studies (1.4%).
The analysis of the prevalence of Entamoeba spp. by region showed contrasting realities within the states of each region. The northeastern region showed high pooled prevalence percentages in the states of Paraiba (72%), Ceara (34%), Sergipe (28%), Pernambuco (16%), Piaui (9%) and Bahia (3%). Alagoas and the Rio Grande do Norte showed another reality, with a prevalence of 4% and 2%, respectively. The central-western region showed high pooled prevalence in the Federal District (53%) and the states of Mato Grosso do Sul (35%) and Mato Grosso (34%), but the state of Goias presented a pooled prevalence of 3%. In the northern region, the states of Rondonia (50%), Para (30%), Acre (26%), Amazonas (30%) and Maranhao (19%) showed high percentages of prevalence, while and Amapa showed a prevalence of 4%. In the southeastern region, the states of Espirito Santo, Minas Gerais and Sao Paulo showed a pooled prevalences of 31%, 12% and 11% respectively, while Rio Janeiro presented a moderate prevalence of 9%. The same data were found for the southern region, where the state of Rio Grande do Sul had a high pooled prevalence of 15% and the states Parana and Santa Catarina had a moderate prevalence of 9% and 6%, respectively.
The differences in the prevalence of intestinal parasites among the Brazilian regions were recently documented in a previous study [81]. However, in addition to the differences among the regions, this present study showed great prevalence differences within the same region. This epidemiological data can be used as a tool to identify areas of social vulnerability as intestinal parasitosis is strongly associated with the socioeconomic level of the population. In contrast, Brazil is an extensive country and presents many regional and intraregional socioeconomic and health development differences. Only 39% of the cities collect and treat 100% of the sewage [38], with the lack of adequate basic sanitation system increasing the continuous dissemination of neglected diseases linked to sanitary problems, such as intestinal parasitosis, including those caused by Entamoeba spp.
Regarding sex, both showed a similar pooled prevalence of Entamoeba spp., with 29% for women and 26% for men, suggesting that sex may not be a determinant for protozoan contamination. Regarding age, there was a high prevalence in the three groups, 40% in the 10–19 years group, 34% in adults aged over 19 years, and 25% in children aged below 9 years.
Age is an important risk factor for intestinal parasitic infections. Children are often more susceptible to intestinal infectious diseases than adults owing to inadequate hygiene habits. Children aged below 9 years were the group that presented the highest number of samples analyzed in this meta-analysis, and even though it is the least prevalent for Entamoeba spp., 25% is a percentage of great importance within this population. In contrast, this study showed that the most prevalent group for Entamoeba spp. were the people aged 10–19 years. Therefore, school age represents a higher risk for amebiasis than the age of the general population. A previous study in Indonesia showed a high rate of Entamoeba spp. (52.8%) in the school-age (7–15 years) group [137]. The age group between 10 and 19 years was the most heterogeneous, including pre-adolescents, adolescents, and young adults. However, this group provides a possible panorama for the prevalence of intestinal parasitosis in high school students in Brazil.
The pooled prevalence of Entamoeba spp. infection in immunocompromized patients was 18%. This parasitic infection was most prevalent in cancer patients, with 36%, although they presented fewer samples for analysis, followed by HIV and hemodialysis patients, with a prevalence of 27% and 10%, respectively. Some studies indicate that this parasite frequently causes opportunistic infections in immunosuppressed patients [46, 111]; it was one of the most common causes of morbidity in this group. This study recorded high prevalence percentages in immunosuppressed patients, especially with cancer. Cancer, HIV, and hemodialysis patients become immunocompromized as a result of the disease itself or due to therapeutic procedures that cause immunosuppression [134, 193]. Although intestinal parasitic infections are a great risk with persistent diarrhea and severe clinical symptoms in immunocompromized patients, the routine diagnosis of these infections is often ignored during chemotherapy or disease [1, 131]. For this reason, it is extremely important to diagnose and treat parasitic infections to decrease morbidity in this group.
The overall pooled prevalence of Entamoeba spp. in animal hosts was 12%. Of these animals, Entamoeba spp. was most prevalent in mammals (12%), followed by birds (6%). Regarding human interaction, Entamoeba spp. was most prevalent in captive wild animals, which are not easily accessible to the general population, followed by domestic farm animals. Farm animal breeding is a possible risk factor for Entamoeba spp. transmission. Therefore, it is necessary to establish control measures to minimize the transmission of these parasites among different animal hosts and humans.
For Entamoeba spp. diversity, this study showed little variability in human hosts, with differentiation into five different species. Studies on animal hosts characterized only E. coli. Of the species identified in humans, E. coli was the most prevalent (86.5%), followed by E. dispar (7.9%), E. histolytica (3.1%), E. hartmanni (1.9%), and E. gingivalis (0.6%). The prevalence of these species in Brazil determined in this meta-analysis differed from the world scenario, which presented E. dispar with the highest prevalence (49.4%), followed by E. histolytica (32.3%), E. coli (1.9%), and E. hartmanni (0.9%) [64]. The Brazilian situation could be different if the 89 studies that used conventional identification methods also used molecular analysis in the 5234 samples to separate the species E. dispar from E. histolytica, which are morphologically indistinguishable and were not included in the general percentage.
Although this study presents the commensal parasite E. coli as the most prevalent in Brazil, it is important to highlight that this species has the same transmission route as that of other pathogenic species, such as E. histolytica, E. dispar, and even Giardia lamblia as well as helminths. The prevalence of this parasite can be used as an indicator of fecal/oral transmission, suggesting intestinal parasite transmission through water supply for human consumption or through contaminated food.
Entamoeba histolytica causes severe intestinal and extraintestinal amebiasis, representing a health risk in countries with inadequate sanitary barriers. This study identified significant prevalence and distribution percentages of E. histolytica in Brazil, with 28.9% prevalence in the north, 3.4% in the northeast, 1.1% in the south, and 0.3% in the southeast. In the central-western region, no study distinguished E. histolytica from E. dispar. It is important to note that more studies need to be developed in this region to resolve this sampling bias.
This study has some limitations. First, in human studies, some authors did not distribute the positive sample results by sex and/or age, decreasing the number of classified samples to better evaluate the prevalence by these variables. Second, many samples were not identified at the protozoan species level, which could improve data on the species distribution and prevalence in Brazil, especially those of the pathogenic E. histolytica. Finally, it is recommended that publication biases be evaluated using statistical methods in meta-analyses. However, the currently available methods, such as funnel graphs and the Egger regression test, are not considered useful in proportion studies [147].
In conclusion, this study showed a high prevalence of Entamoeba spp. in the Brazilian population (22%), with a prevalence of up to 50% in the northern, northeastern, and central-western regions. Although there were contrasting prevalence percentages among the regions, there is a wide distribution of Entamoeba spp. in Brazil. There was no difference between males and females, and the age group of 10–19 years had the highest prevalence, broadly indicating the prevalence of intestinal parasitosis in high-school students in Brazil. The most diagnosed species was E. coli, which may suggest the transmission of intestinal parasites through water supply for human consumption or through contaminated food. This may lead to the possibility of infection due to other protozoan pathogenic species. The pathogenic species E. histolytica is distributed in most Brazilian regions, with significant prevalence percentages. The prevalence in mammals was the highest among animals, with interactions among humans and captive, wild, or domestic farm animals presenting the higher protozoan prevalence.
The implementation of molecular methods to detect Entamoeba spp. in scientific productions is extremely important to reduce possible false-negatives using coprological methods and to differentiate protozoan species. Patients with any type of immunosuppression should undergo routine intestinal protozoa screening and early treatment to avoid future complications because a significant prevalence was identified in this population.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgments
The authors thank the National Council for Scientific and Technological Development (CNPq – Brazil), Universal Project 423391/2018-6 for funding. A.S.Z. received a fellowship from Mato Grosso State University.
Cite this article as: Zanetti AS, Malheiros AF, de Matos TA, dos Santos C, Battaglini PF, Moreira LM, Lemos LMS, Castrillon SKI, Cortela DCB, Ignotti E & Espinosa OA. 2021. Diversity, geographical distribution, and prevalence of Entamoeba spp. in Brazil: a systematic review and meta-analysis. Parasite 28, 17.
References
- 1.Abdel-Hafeez EH, Ahmad AK, Ali BA, Moslam FA. 2012. Opportunistic parasites among immunosuppressed children in Minia District, Egypt. Korean Journal of Parasitology, 50, 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aca IS, França E Jr, Nozaki T, Freitas GB, Tateno S. 1993. Entamoeba histolytica zymodemes in children of Osasco, São Paulo. Revista do Instituto de Medicina Tropical de São Paulo, 35, 581–582. [DOI] [PubMed] [Google Scholar]
- 3.Aca IS, Kobayashi S, Carvalho LZ Jr, Tateno S, Takeuchi T. 1994. Prevalence and pathogenicity of Entamoeba histolytica in three different regions of Pernambuco, northeast Brazil. Revista do Instituto Medicina Tropical de São Paulo, 36, 519–524. [DOI] [PubMed] [Google Scholar]
- 4.Aguiar J, Goncalves A, Sodre F, Pereira SR, Boia M, Lemos E, Daher R. 2007. Intestinal protozoa and helminths among Terena Indians in the State of Mato Grosso do Sul: high prevalence of Blastocystis hominis. Revista da Sociedade Brasileira de Medicina Tropical, 40, 631–634. [DOI] [PubMed] [Google Scholar]
- 5.Al-Habsi K, Yang R, Ryan U, Jacobson C, Miller DW. 2017. Morphological and molecular characterization of an uninucleated cyst-producing Entamoeba spp. in captured Rangeland goats in Western Australia. Veterinary Parasitology, 235, 41–46. [DOI] [PubMed] [Google Scholar]
- 6.Albuquerque NO, Souza MAA. 2018. Análise parasitológica em estudantes com deficiência intelectual e/ou múltipla (o múltiple). Sociedad Iberoamericana de Información Científica, 2018, 1–7. [Google Scholar]
- 7.Alencar BT, Zanetti AS, Vilella SH, Araújo MSM, Silva LNL, Alencar RT, Espinosa AO, Malheiros AF. 2020. Fatores socioambientais e prevalência de enteroparasitas em pacientes em hemodiálise no pantanal mato-grossense, Brasil. Research, Society and Development, 9, e5109108738. [Google Scholar]
- 8.Ali IKM. 2015. Intestinal Amebae. Clinics in Laboratory Medicine, 35, 393–422. [DOI] [PubMed] [Google Scholar]
- 9.Almeida PHA, Santana PCS, Silva AV. 2012. Prevalência de protozoários e helmintos entéricos em residentes de São Cristóvão, Feira de Santana, Bahia, Brasil. Arquivos de Ciências da Saúde UNIPAR, 16, 61–66. [Google Scholar]
- 10.Alves JR, Macedo HW, Ramos AN Jr, Ferreira LF, Gonҫalves MLC, Araújo A. 2003. Parasitoses intestinais em região semi-árida do Nordeste do Brasil: resultados preliminares distintos das prevalências esperadas. Caderno de Saúde Pública, 19, 667–670. [DOI] [PubMed] [Google Scholar]
- 11.Andrade F, Rode G, Silva Filho HH, Greinert-Goulart JA. 2008. Parasitoses intestinais em um centro de educação infantil público do municipio de Blumenau (SC), Brasil, com ênfase em Cryptosporidium spp. e outros protozoários. Revista de Patologia Tropical, 37, 332–340. [Google Scholar]
- 12.Andrade RS, Albuquerque WA, Miranda FS, Marques BC, Mota LHS, Santos RS, Silva IMM, Amor AM. 2018. Presence of enteroparasites in the environment and the resident population in a rural community in Santo Antonio de Jesus in the reconcavo da Bahia, Brazil. Revista de Patologia Tropical, 47, 31–45. [Google Scholar]
- 13.Araújo CF, Fernández CL. 2005. Prevalência de parasitoses intestinais na cidade de Eirunepé, Amazonas. Revista da Sociedade Brasileira de Medicina Tropical, 38, 69. [DOI] [PubMed] [Google Scholar]
- 14.Assis EM, Oliveira RC, Moreira LE, Pena JL, Rodrigues LC, Machado-Coelho GLL. 2013. Prevalência de parasitos intestinais na comunidade indígena Maxakali, Minas Gerais, Brasil, 2009. Cadernos de Saúde Pública, 29, 681–690. [DOI] [PubMed] [Google Scholar]
- 15.Bachur TPR, Vale JM, Coêlho ICB, Queiroz TRBS, Chaves CS. 2008. Enteric parasitic infections in HIV/AIDS patients before and after the highly active antiretroviral therapy. Brazilian Journal of Infectious Diseases, 12, 115–122. [DOI] [PubMed] [Google Scholar]
- 16.Banhos EF, Rocha JAM, Pimentel ML, Batista ETM, Silva LM. 2017. Prevalence and risk factors for intestinal parasite infections in schoolchildren, in the city of Santarém, Pará state, Brazil. Arquivos Brasileiros de Ciências da Saúde, 42, 137–142. [Google Scholar]
- 17.Barbosa AS, Bastos OMP, Dib LV, Siqueira MP, Cardozo ML, Ferreira LC, Chaves WT, Fonseca ABM, Uchôa CMA, Amendoeira MRR. 1995. Gastrointestinal parasites of swine raised in diferente management systems in the state of Rio de Janeiro, Brazil. Prequisa Veterinária Brasileira, 35, 941–946. [Google Scholar]
- 18.Barbosa AS, Pissinatti A, Dib LV, Siqueira MP, Cardozo ML, Fonseca ABM, Oliveira AB, Silva FA, Uchôa CMA, Bastos OMP, Amendoeira MRR. 2015. Balantidium coli and other gastrointestinal parasites in captives non-human primates of the Rio de Janeiro, Brazil. Journal of Medical Primatology, 44, 18–26. [DOI] [PubMed] [Google Scholar]
- 19.Barbosa CV, Barreto MM, Andrade RJ, Sodré F, d’Avila-Levy CM, Peralta JM, Igreja RP, Macedo HW, Santos HLC. 2018. Intestinal parasite infections in a rural community of Rio de Janeiro (Brazil): prevalence and genetic diversity of Blastocystis subtypes. PLoS One, 13, e0193860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbosa IA, Pavanelli MF. 2019. Alta prevalencia de Balantidium coli em crianças de uma escola municipal de Moreira Salas – PR. Arquivos de Ciências da Saúde UNIPAR, 23, 41–45. [Google Scholar]
- 21.Barçante TA, Cavalcanti DV, Silva GAV, Lopoes PB, Barros RF, Ribeiro GP, Neubert LF, Barçante JMP. 2008. Enteroparasitos em crianças matriculadas em creches públicas do município de Vespasiano, Minas Gerais. Revista de Patologia Tropical, 37, 33–42. [Google Scholar]
- 22.Basso RMC, Silva-Ribeiro RT, Soligo DS, Ribacki SI, Callegari-Jacques SM, Zoppas BCA. 2008. Evolução da prevalência de parasitoses intestinais em escolares em Caxias do Sul, RS. Revista da Sociedade Brasileira de Medicina Tropical, 41, 263–268. [DOI] [PubMed] [Google Scholar]
- 23.Belloto MVT, Junior JES, Macedo EA, Ponce A, Galisteu KJ, Castro E, Tauyr LV, Rossit ARB, Machado RLD. 2011. Enteroparasitoses numa população de escolares da rede pública de ensino do município de Mirassol, São Paulo, Brasil. Revista Pan-Amazonica de Saúde, 2, 37–44. [Google Scholar]
- 24.Bencke A, Artuso GL, Reis RS, Barbieri NL, Rott MB. 2006. Enteroparasitoses em escolares residentes na periferia de Porto Alegre, RS, Brasil. Revista de Patologia Tropical, 35, 31–36. [Google Scholar]
- 25.Benetton MLFN, Gonçalves AV, Meneghini MEF, Silva EF, Carneiro M. 2005. Risk factors for infection by the Entamoeba histolytica/E. dispar complex: na epidemiological study conducted in outpatient clinics in the city of Manaus, Amazon Region, Brazil. Transactions of the Royal Society of Tropical Medicine and Hygiene, 99, 532–540. [DOI] [PubMed] [Google Scholar]
- 26.Berrilli F, Prisco C, Friedrich KG, Cerbo PD, Cave DD, Liberato CD. 2011. Giardia duodenalis assemblages and Entamoeba species infecting non-human primates in an Italian zoological garden: zoonotic potential and management traits. Parasites & Vectors, 4, 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bezerra AS, Cardoso VVBP, Barbosa VSA. 2018. Estado nutricional, anemia e parasitoses intestinais em gestantes de um municipio do Curimataú Paraibano. Revista de Atenção Primária à Saúde, 21, 399–407. [Google Scholar]
- 28.Biolchi LC, Collet ML, Dallanora FJ, D’Agostini FM, Nardi GM, Muller GA, Wagner G. 2015. Enteroparaistes and commensals in students aged 7 to 14 years in rural and urban áreas of Campos Novos, west of Santa Catarina, Brazil. Revista de Patologia Tropical, 44, 337–342. [Google Scholar]
- 29.Biscegli TS, Romera J, Candido AB, Santos JM, Candido ECA, Binotto AL. 2009. Estado nutricional e prevalência de enteroparsitoses em crianças matriculadas em creche. Revista Paulista de Pediatria, 27, 289–295. [Google Scholar]
- 30.Boia MN, Motta LP, Salazar MSP, Mutis MPS, Coutinho RBA, Coura JR. 1999. Estudo das parasitoses intestinais e da infecção chagásica no município de Novo Airão, estado do Amazonas, Brasil. Caderno de Saúde Pública, 15, 497–504. [DOI] [PubMed] [Google Scholar]
- 31.Boia MN, Carvalho-Costa FA, Sodré FC, Eyer-Silva WA, Lamas CC, Lyra MR, Pinto-Junior VL, Cantalice-Filho JP, Oliveira ALL, Carvalho LMA, Gross JB, Souza ALS, Moraes TI, Bermudez-Aza EH, Martins EB, Coura JR. 2006. Mass treatment for intestinal helminthiasis control in na amazonian endemic área in Brazil. Revista do Intituto de Medicina Tropical de São Paulo, 48, 189–195. [DOI] [PubMed] [Google Scholar]
- 32.Boia MN, Carvalho-Costa FA, Sodré FC, Porras-Pedroza BE, Faria EC, Magalhães GAP, Silva IM. 2009. Tuberculose e parasitismo intestinal em população indígena na Amazônia brasileira. Revista de Saúde Pública, 43, 176–178. [DOI] [PubMed] [Google Scholar]
- 33.Borges WF, Marciano FM, Oliveira HB. 2011. Parasitos intestinais: elevada prevalencia de Giardia lamblia em pacientes atendidos pelo serviço público de saúde da região sudeste de Goiás, Brasil. Revista de Patologia Tropical, 40, 149–157. [Google Scholar]
- 34.Braga LL, Mendonça Y, Paiva CA, Sales A, Cavalcante ALM, Mann BJ. 1998. Seropositivity for and Intestinal Colonization with Entamoeba histolytica and Entamoeba dispar in Individuals in Northeastern Brazil. Journal of Clinical Microbiology, 36, 3044–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braga LLBC, Gomes ML, Silva MW, Façanha FE Jr, Fiuza L, Mann BJ. 2001. Household epidemiology of Entamoeba histolytica infection in an urban community in northeastern Brazil. American Journal of Tropical Medicine and Hygiene, 65, 268–271. [DOI] [PubMed] [Google Scholar]
- 36.Braga LLBC, Gomes ML, Silva MW, Paiva C, Sales A, Mann BJ. 2001. Entamoeba histolytica and Entamoeba dispar infections as detected by monoclonal antibody in an urban slum in Fortaleza, Northeastern Brazil. Revista da Sociedade Brasileira de Medicina Tropical, 34, 467–471. [DOI] [PubMed] [Google Scholar]
- 37.Branco N, Leal DAG, Franco RMB. 2012. A parasitological survey of natural water springs and inhabitants of a tourist city in southeastern Brazil. Vector-borne and Zoonotic Diseases, 12, 410–417. [DOI] [PubMed] [Google Scholar]
- 38.Brasil. 2016. Ministério das Cidades; Secretaria Nacional de Saneamento Ambiental – SNSA. Sistema Nacional de Informações sobre Saneamento: diagnóstico dos serviços de água e esgotos – 2014. Brasília: : SNSA/MCIDADES. [Google Scholar]
- 39.Brauer AMNW, Silva JC, Souza AA, Souoza MAA. 2017. Intestinal parasites among employees of restaurants and cafeterias in a city of Brazil. Revista de Salud Publica, 19, 691–696. [DOI] [PubMed] [Google Scholar]
- 40.Bueno GCL, Reis M, Dantas-Correa EB, Schiavon LL, Narciso-Schiavon J. 2015. The prevalence of intestinal parasitosis according to gender in a university hospital in southern Brazil. Revista de Patologia Tropical, 44, 441–452. [Google Scholar]
- 41.Burrows RB. 1959. Morphological differentiation of Entamoeba hartmanni and E. polecki from E. histolytica. American Journal of Tropical Medicine and Hygiene, 8, 583–589. [DOI] [PubMed] [Google Scholar]
- 42.Cabrine-Santos M, Cintra EN, Carmo RA, Nascentes GAN, Pedrosa AL, Correia D, Oliveira-Silva MB. 2015. Occurrence of Blastocystis spp. in Uberaba, Minas Gerais, Brazil. Revista do Instituto de Medicina Tropical de São Paulo, 57, 211–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calegar DA, Nunes BC, Monteiro KJL, Santos JP, Toma HK, Gomes TF, Lima MM, Boia MN, Carvalho-Costa FA. 2016. Frequency and molecuar characterization of Entamoeba histolytica, Entamoeba dispar, Entamoeba moshkovskii, and Entamoeba hartmanni in the context of wáter scarcity in northeastern Brazil. Memórias do Instituto Oswaldo Cruz, 111, 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Camello JT, Cavagnolli NI, Spada PKWDS, Poeta J, Rodrigues AD. 2016. Prevalência de parasitoses intestinais e condições de saneamento básico das moradias em escolares da zona urbana de Caxias do Sul, Rio Grande do Sul. Scientia Medica, 26, ID 21716. [Google Scholar]
- 45.Campos-Filho PC, Barros LM, Campos JO, Braga VB, Cazorla IM, Albuquerque GR, Carvalho SMS. 2008. Parasitas zoonóticos em fezes de cães em praças públicas do município de Itabuna, Bahia, Brasil. Revista Brasileira de Parasitologia Veterinária, 17, 206–209. [PubMed] [Google Scholar]
- 46.Caner A, Zorbozan O, Tunali V, Kantar M, Aydogdu S, Aksoylar S, Guruz Y, Turgay N. 2019. Intestinal protozoan parasitc infections in immunocompromised child patients with diarrhea. Japanese Journal of Infectious Diseases, 73, 187–192. [DOI] [PubMed] [Google Scholar]
- 47.Capuano DM, Lazzrini MPT, Junior EG, Takayanagui OM. 2008. Enteroparasitoses em manipuladores de alimentos do município de Ribeirão Preto – SP, Brasil, 2000. Revista Brasileira de Epidemiologia, 11, 687–695. [Google Scholar]
- 48.Cardoso LV, Galisteu KJ, Junior AS, Chahla LAOA, Canille RMS, Belloto MVT, Franco C, Maia IL, Rossit ARB, Machado RLD. 2011. Enteric parasites in HIV-1/AIDS – infected patients from a northwestern São Paulo reference unit in the highly active antirretroviral therapy era. Revista da Sociedade Brasileira de Medicina Tropical, 44, 665–669. [DOI] [PubMed] [Google Scholar]
- 49.Carvalho GLX, Moreira LE, Pena JL, Marinho CC, Bahia MT, Machado-Coelho GLL. 2012. A comoparative study of the TF-test, Kato-Katz, Hoffman-Pons-Janer, Willis and Baermann-Moraes coprologic methods for the detection of human parasitosis. Memórias do Instituto Oswaldo Cruz, 107, 80–84. [DOI] [PubMed] [Google Scholar]
- 50.Carvalho APGC, Santos MCNP, Morais ROA, Pinto LC. 2019. Detection of intestinal parasites in public transport buses in Belém, Pará state, Northern Brazil. Revista de Patologia Tropical, 48, 170–178. [Google Scholar]
- 51.Carvalho-Costa FA, Gonçalves AQ, Lassance SL, Albuquerque CP, Leite JPG, Boia MN. 2007. Detection of Cryptosporidium spp. and other intestinal parasites in children with acute diarrhea and severe dehydration in Rio de Janeiro. Revista da Sociedade Brasileira de Medicina Tropical, 40, 346–348. [DOI] [PubMed] [Google Scholar]
- 52.Castro EDR, Germini MCBY, Mascarenhas JDP, Gabbay YB, Lima ICG, Lobo PS, Fraga VD, Conceição LM, Machado RLD, Rossit ARB. 2015. Enteropathogens detected in a daycare center, southeastern Brazil: bacteria, vírus, and parasite research. Revista do Instituto de Medicina Tropical de São Paulo, 57, 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cavagnolli NI, Camello JT, Tesser S, Poeta J, Rodrigues AD. 2015. Prevalência de enteroparasitoses e análise socioeconómica de escolares em Flores da Cunha – RS. Revista de Patologia Tropical, 44, 312–322. [Google Scholar]
- 54.Cembranelli SBS, Souto FO, Ferreira-Paim K, Richinho TT, Nunes PL, Nascentes GAN, Ferreira TB, Correia D, Lages-Silva E. 2013. First evidence of genetic intraspecific variability and occurrence of Entamoeba gingivalis in HIV +/ AIDS. PLoS One, 8, e82864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chagas CRF, Gonzalez IHL, Favoretto SM, Ramos PL. 2017. Parasitological surveillance in a rat (Rattus norvegicus) colony in São Paulo Zoo animal house. Annals of Parasitology, 63, 291–297. [DOI] [PubMed] [Google Scholar]
- 56.Chieffi PP, Waldman EA, Dias RMDS, Torres DMAGV, Chimara R, Mizumoto LC, Silva AMA, Uehara M. 1988. Enteroparasitoses no município de Guarulhos, SP, Brasil: prevalencia de infecção entre escolares residentes no bairro de Taboão, em junho de 1984. Revista do Instituto Adolfo Lutz, 48, 75–80. [Google Scholar]
- 57.Chihi A, Stensvold CR, Ben-Abda I, Ben-Romdhane R, Aoun K, Siala E, Bouratbine A. 2019. Development and evaluation of molecular tools for detecting and differentiating intestinal amoebae in healthy individuals. Parasitology, 146, 821–827. [DOI] [PubMed] [Google Scholar]
- 58.Cimerman S, Cimerman B, Lewi DS. 1999. Revalence of intestinal parasitic infections in patients with acquired immunodeficiency syndrome in Brazil. International Journal of Infectious Diseases, 3, 203–206. [DOI] [PubMed] [Google Scholar]
- 59.Clark CG, Kaffashian F, Tawari B, Windsor JJ, Twigg-Flesner A, Davies-Morel MCG, Blessmann J, Ebert F, Peschel B, Van AL, Jackson CJ, Macfarlane L, Tannich E. 2006. New insights into the phylogeny of Entamoeba species provided by analysis of four new small-subunit rRNA genes. International Journal of Systematic and Evolutionary Microbiology, 56, 2235–2239. [DOI] [PubMed] [Google Scholar]
- 60.Coimbra CEA Jr, Santos RV. 1991. Parasitismo intestinal entre o grupo indígena Zoró, Estado de Mato Grosso (Brasil). Cadernos de Saúde Pública, 7, 100–103. [Google Scholar]
- 61.Colli CM, Mizutani AS, Martins VA, Ferreira ÉC, Gomes ML. 2014. Prevalence and risk factors for intestinal parasites in food handlers, southern Brazil. International Journal of Environmental Health Research, 24, 450–458. [DOI] [PubMed] [Google Scholar]
- 62.Costa JO, Resende JÁ, Gil FF, Santos JFG, Gomes MA. 2018. Prevalence of Entamoeba histolytica and other enteral parasitic disease in the metropolitan region of Belo Horizonte, Brazil. A cross-sectional study. São Paulo Medical Journal, 136, 319–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Costa-Cruz JM, Cardoso MLG, Marques DE. 1995. Intestinal parasites in school food handlers in the city of Uberlândia, Minas Gerais, Brazil. Revista do Instituto de Medicina Tropical de São Paulo, 37, 191–196. [DOI] [PubMed] [Google Scholar]
- 64.Cui Z, Li J, Chen Y, Zhang L. 2019. Molecular epidemiology, evolution, and phylogeny of Entamoeba spp. Infection, Genetics and Evolution, 75, e104018. [DOI] [PubMed] [Google Scholar]
- 65.Curval LG, França AdO, Fernandes HJ, Mendes RP, de Carvalho LR, Higa MG, Ferreira EC, Dorval MEC. 2017. Prevalence of intestinal parasites among inmates in Midwest Brazil. PLoS One, 12, e0182248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Damázio SM, Lima MS, Soares AR, Souza MAA. 2013. Intestinal parasites in a quilombola community of the northern state of Espírito Santo, Brazil. Revista do Instituto de Medicina Tropical de São Paulo, 55, 179–183. [DOI] [PubMed] [Google Scholar]
- 67.Damázio SM, Soares AR, Souza MAA. 2016. Perfil parasitológico de escolares da localidade de Santa Maria, zona rural do município de São Mateus/ES, Brasil. Revista de Atenção Primária a Saúde, 19, 261–267. [Google Scholar]
- 68.David EB, Patti M, Coradi ST, Oliveira-Sequeira TCG, Ribolla PEM, Guimarães S. 2014. Molecular typing of Giardia duodenalis isolates from nonhuman primates housed in a Brazilian zoo. Revista do Instituto de Medicina Tropical de São Paulo, 56, 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Carli GA, Mentz M, Rott MB, Silva ACA, Wendorff A, Tasca T, Castilhos DS, Hypolito L, Mylius L, Montenegro R, De Carli G. 1997. Prevalência das enteroparasitoses nas vilas periféricas da grande Porto Alegre, nos assentamentos de trabalhadores rurais e na cidade de arroio dos ratos no estado do Rio Grande do Sul. Revista Brasileira de Análises Clínicas, 29, 185–189. [Google Scholar]
- 70.Diamond LS, Clark CG. 1993. A redescription of Entamoeba histolytica Schaudinn, 1903 (Emended Walker, 1911) separating it from Entamoeba dispar Brumpt, 1925. Journal of Eukaryotic Microbiology, 40, 340–344. [DOI] [PubMed] [Google Scholar]
- 71.Diniz LSM, Costa EO, Oliveira PMA. 1995. Clinical disorders observed in anteaters (Myrmecophagidae, Edentata) in captivity. Veterinary Research Communications, 19, 409–415. [DOI] [PubMed] [Google Scholar]
- 72.Dourado A, Maciel A, Aca IS. 2006. Ocorrência de Entamoeba histolytica/Entamoeba dispar em pacientes ambulatoriais de Recife, PE. Revista da Sociedade Brasileira de Medicina Tropical, 39, 388–389. [DOI] [PubMed] [Google Scholar]
- 73.Dolabella SS, Serrano-Luna J, Navarro-García F, Cerritos R, Ximénez C, Glaván-Moroyoqui JM, Silva FF, Tsutsumi V, Shibayama M. 2012. Amoebic liver abscess production by Entamoeba dispar. Annals of Hepatology, 11, 107–117. [PubMed] [Google Scholar]
- 74.Duarte IAC, Santos RV, Fontes G, Galindo LF, Ximenes RAA, Maciel MAV, Aca IS, Rocha EMM. 2013. Revalencia e infección por Entamoeba histolytica en escuelas publicas de la ciudad de Maceió, Alagoas, Brasil. Revista Cubana de Medicina Tropical, 65, 4–12. [Google Scholar]
- 75.Einloft ABN, Vitor CFH, Sant’Ana LFR, Priore SE, Franceschini SCC. 2010. Efeito das infecções parasitárias e da anemia materna sobre o peso ao nascer de crianas no município de Viçosa, MG. Revista Médica de Minas Gerais, 20, 317–322. [Google Scholar]
- 76.Elsheikha HM, Regan CS, Clark CG. 2018. Novel Entamoeba findings in nonhuman primates. Trends in Parasitology, 1, 12–25. [DOI] [PubMed] [Google Scholar]
- 77.Escobar-Pardo ML, Godoy APO, Machado RS, Rodrigues D, Neto UF, Kawakami E. 2010. Prevalência de parasitoses intestinais em crianças do Parque Indígena do Xingu. Jornal de Pediatria, 86, 493–496. [DOI] [PubMed] [Google Scholar]
- 78.Eustachio PFP, Avelar LA, Dias JVL, Queiroz DRM, Murta NMG, Oliveira GHB, Cambraia RP, Pires HHR, Martins HR. 2019. Parasitismo intestinal y contaminación ambiental con helmintos y protozoários en una comunidade quilombola del sudeste de Brasil. Revista Cubana de Medicina Tropical, 71, 1–13. [Google Scholar]
- 79.Falavigna DLM, Almeida AA, Iwazaki RS, Araújo SM. 2008. Intestinal parasites in ecotourism región of the state of Paraná, Brazil. Brazilian Archives of Biology and Technology, 51, 693–699. [Google Scholar]
- 80.Faleiros JMM, Gallo G, Silva MMK, Raful R, Nasorri AR, Pipino LFR, Junqueira RB, Pinto PLS. 2004. Ocorrência de enteroparsitoses em alunos da escola pública de ensino fundamental do município de Catanduva (São Paulo, Brasil). Revista do Instituto Adolfo Lutz, 63, 243–247. [Google Scholar]
- 81.Faria CP, Zanini GM, Dias GS, Silva S, Freitas MB, Almendra R, Santana P, Sousa MC. 2017. Geospatial distribution of intestinal parasitic infections in Rio de Janeiro (Brazil) and its association with social determinats. PLoS Neglected Tropical Diseases, 11, e0005445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Favoreto-Junior S, Machado MI. 1995. Estudos de frequência, morfologia e diagnóstico de Entamoeba gingivalis, Gross, 1849. Revista da Sociedade Brasileira de Medicina Tropical, 28, 379–387. [DOI] [PubMed] [Google Scholar]
- 83.Felizardo AA, Souza LM, Siqueira RV, Kanamura HY. 2017. Prevalence and risk factors for intestinal parasitic infections in children attending daycare centers in Alfenas, Southern Minas Gerais, Brazil. Revista de Patologia Tropical, 46, 263–275. [Google Scholar]
- 84.Feng M, Pandey K, Yanagi T, Wang T, Putaporntip C, Jongwutiwes S, Cheng X, Sherchand JB, Pandey BD, Tachibana H. 2018. Prevalence and genotypic diversity of Entamoeba species in inhabitants in Kathmandu, Nepal. Parasitology Research, 117, 2467–2472. [DOI] [PubMed] [Google Scholar]
- 85.Fernandes NS, Guimarães HR, Amorim ACS, Brito VM, Borges EP, Reis MB, Trindade RA, Melo ACFL. 2014. Ocorrência de enteroparasitoses em manipuladores de alimentos de restaurantes em Parnaíba, Piauí-Brasil. Revista de Patologia Tropical, 43, 459–469. [Google Scholar]
- 86.Ferraz RRN, Barnabé AS, Porcy C, D’Eça Junior A, Feitosa T, Figueiredo PM. 2014. Parasitoses intestinais e baixos índices de Gini em Macapá (AP) e Timon (MA), Brasil. Cadernos de Saúde Coletiva, 22, 173–176. [Google Scholar]
- 87.Ferreira CS, Camargo LMA, Moitinho MLR, Azevedo RA. 1991. Intestinal parasites in Iaualapiti Indians from Xingu Park, Mato Grosso, Brazil. Memórias do Instituto Oswaldo Cruz, 86, 441–442. [DOI] [PubMed] [Google Scholar]
- 88.Ferreira CS, Ferreira MU, Nogueira MR. 1994. The prevalence of infection by intestinal parasites in an urban slum in São Paulo, Brazil. Journal of Tropical Medicine and Hygiene, 97, 121–127. [PubMed] [Google Scholar]
- 89.Ferreira-Filho SR, Braga FCC, Sa DM, Nunes EB, Soares JSP, Padovese SM, Oliveira AC, Oliveira GMF, Passos G, Lemes HP. 2011. Entamoeba histolytica/Entamoeba dispar infection in chronic hemodialysis patients. Saudi Journal of Kidney Diseases and Transplantation, 22, 237–244. [PubMed] [Google Scholar]
- 90.Ferriolli-Filho F. 1962. Prevalência da Entamoeba histolytica e da Entamoeba hartmanni no município de Ribeirão Preto, São Paulo (Brasil). Revista do Instituto de Medicina Tropical de São Paulo, 4, 305–310. [PubMed] [Google Scholar]
- 91.Fonseca REP, Barbosa MCR, Ferreira BR. 2017. High prevalence of enteroparasites in children from Ribeirão Preto, São Paulo, Brazil. Revista Brasileira de Enfermagem, 70, 566–571. [DOI] [PubMed] [Google Scholar]
- 92.Freitas MFL, Oliveira JB, Cavalcanti MB, Leite AS, Magalhaes VS, Oliveira RA, Sobrino AE. 2002. Parásitos gastrointetinales de aves silvestres en cautiverio en el estado de Pernambuco, Brasil. Parasitologia Latinoamericana, 57, 50–54. [Google Scholar]
- 93.Gallo SSM, Teixeira CS, Ederli NB, Oliveira FCR. 2019. Gastrointestinal parasites of a population of emus (Dromaius novaehollandiae) in Brazil. Brazilian Journal of Biology, 2019, 1–7. [DOI] [PubMed] [Google Scholar]
- 94.Garcia G, Ramos F, Pérez RG, Yañez J, Estrada MS, Mendoza LH, Martinez-Hernandez F, Gaytán P. 2014. Molecular epidemiology and genetic diversity of Entamoeba species in a chelonian collection. Journal of Medical Microbiology, 63, 271–283. [DOI] [PubMed] [Google Scholar]
- 95.Gilioli R, Silva FA. 2000. Frequência de parasitas e infecção de Salmonella em lobo-guará, Chrysocyon brachyurus, mantido em zoológicos do estado de São Paulo, Brasil. Arquivos Brasileiros de Medicina Veterinária e Zootecnia, 52, 15–19. [Google Scholar]
- 96.Giugliano LG, Bernardi MGP, Vasconcelos JC, Costa CA, Giugliano R. 1986. Longitudinal study of diarrhoeal disease in a periurban community in Manaus, Amazon-Brazil. Annals of Tropical Medicine e Parasitology, 80, 443–450. [DOI] [PubMed] [Google Scholar]
- 97.Gomes PDMF, Nunes VLB, Knechtel DS, Brilhante AF. 2010. Enteroparasitos em escolares do distrito Águas do Miranda, município de Bonito, Mato Grosso do Sul. Revista de Patologia Tropical, 39, 299–307. [Google Scholar]
- 98.Gonçalves JF, Tanabe M, Medeiros FPM, Gonçalves FJ, Aca IS, Motta SRN, Tateno S, Takeuchi T. 1990. Parasitological and serological studies on amoebiasis and other intestinal parasitic infections in the rural sector arond recife, northeast Brazil. Revista do Instituto de Medicina Tropical de São Paulo, 32, 428–435. [DOI] [PubMed] [Google Scholar]
- 99.Gonçalves AQ, Abellana R, Pereira-da-Silva HD, Santos I, Serra PT, Julião GR, Orlandi PP, Ascaso C. 2014. Comparison of the performance of two spontaneous sedimentation techniques for the diagnosis of human intestinal parasites in the absence of a gold standard. Acta Tropica, 131, 63–70. [DOI] [PubMed] [Google Scholar]
- 100.Guerra EM, Vaz AJ, Toledo LAS, Ianoni AS, Quadros CMS, Dias RMDS, Barretto OCO. 1991. Infecções por helmintos e protozoários intestinais em gestantes de primeira consulta antendidas em centros de saúde da rede estadual no subdistrito do Butantã, município de São Paulo. Revista do Instituto de Medicina Tropical de São Paulo, 33, 303–308. [PubMed] [Google Scholar]
- 101.Guilherme ALF, Araújo SM, Pupulim ART, Lima Júnior JE, Falavigna DLM. 2004. Parasitas intestinais e comensais em individuos de três vilas rurais do estado do Paraná, Brasil. Acta Scientiarum Health Sciences, 26, 331–336. [Google Scholar]
- 102.Guimarães S, Sogayar MIL. 1995. Occurrence of Giardia lamblia in children of municipal day-care centers from Botucatu, São Paulo state, Brazil. Revista do Instituto de Medicina Tropical de São Paulo, 37, 501–506. [DOI] [PubMed] [Google Scholar]
- 103.Guimarães AO, Valença FM, Sousa JBS, Souza SA, Madi RR, Melo CM. 2014. Parasitic and fungal infections in synanthropic rodents in na área of urban expansion, Aracaju, Sergipe state, Brazil. Acta Scientiarum, 36, 113–120. [Google Scholar]
- 104.Higa-Júnior MG, Cardoso WM, Weis SMS, Franҫa AO, Pontes ERJC, Silva PV, Oliveira MP, Dorval MEMC. 2017. Intestinal parasitism among waste pickers in Mato Grosso do Sul, Midwest Brazil. Revista do Instituto de Medicina Tropical de São Paulo, 59, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Higgins JPT, Green S. 2011. Cochrane handbook for systematic reviews of interventions [Internet]. London: The Cochrane Collaboration. Version 5.1.0. [cited 2020 Dez 20]. Available from: http://handbook-5-1.cochrane.org/. [Google Scholar]
- 106.Hurtado-Guerrero AF, Alencar FH, Hurtado-Guerrero JC. 2005. Ocorrência de enteroparasitas na população geronte de Nova Olinda do Norte – Amazonas, Brasil. Acta Amazonica, 35, 487–490. [Google Scholar]
- 107.Iasbik AF, Pinto PSA, Guimarães-Peixoto RPM, Santos TO, Fernandes FM, Silva LF, Silva AR, Vieira SE, Araújo JV. 2018. Prevalence and transmission of intestinal parasitosis in human beings from zona da mata, Minas Gerais, Brazil. Bioscience Journal, 34, 802–809. [Google Scholar]
- 108.Ibiapina AB, Leal JS, Santana PRA, Mesquita MR, Lopes TLC, Braz DC. 2020. Enteroparasitosis in patients attended by the health public service: epidemiology and spatial distribution. Scientia Medica, 30, 1–10. [Google Scholar]
- 109.Ignácio CF, Silva MECD, Handam NB, Alencar MFL, Sotero-Martins A, Barata MML, Neto AHAM. 2017. Socioenvironmental conditions and intestinal parasitic infections in Brazilian urban slums: a cross-sectional study. Revista do Instituto de Medicina Tropical de São Paulo, 59, e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jacob AS, Busby EJ, Levy AD, Komm N, Clark CG. 2016. Expanding the Entamoeba universe: new hosts yield novel ribosomal lineages. Journal of Eukaryotic Microbiology, 63, 69–78. [DOI] [PubMed] [Google Scholar]
- 111.Jeske S, Bianchi TF, Moura MQ, Baccega B, Pinto NB, Berne MEA, Villela MM. 2018. Intestinal parasites in cancer patients in the South of Brazil. Brazilian Journal of Biology, 78, 574–578. [DOI] [PubMed] [Google Scholar]
- 112.Ji T, Cao HX, Wu R, Cui LL, Su GM, Niu C, Zhang N, Wang SK, Zhou DH. 2019. Prevalence and genetic identification of three Entamoeba species in pigs in Southeastern China. Hindawi BioMed Research International, 2019, e2824017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jirků-Pomajbíková K, Čepička I, Kalousová B, Jirků M, Stewart F, Levecke B, Modrý D, Piel AK, Petrželková KJ. 2016. Molecular identification of Entamoeba species in savanna woodland chimpanzees (Pan troglodytes schweinfurthii). Parasitology, 143, 741–748. [DOI] [PubMed] [Google Scholar]
- 114.Kobayashi J, Hasegawa H, Forli AA, Nishimura NF, Yamanaka A, Shimabururo T, Sato Y. 1995. Prevalence of intestinal parasitic infection in five farms in Holambra São Paulo, Brazil. Revista do Instituto de Medicina Tropical de São Paulo, 37, 13–18. [DOI] [PubMed] [Google Scholar]
- 115.Korkes F, Kumagai FU, Belfort RN, Szjnfeld D, Abud TG, Kleinman A, Florez GM, Szejnfeld T, Chieffi PP. 2007. Relationship between intestinal parasitic infection in children and soil contamination in na urban slum. Journal of Tropical Pediatrics, 55, 42–45. [DOI] [PubMed] [Google Scholar]
- 116.Korzeniowski OM, Dantas W, Trabulsi LR, Guerrant RL. 1984. A controlled study of endemic sporadic diarrhoea among adult residents of southern Brazil. Transaction of the Royal Society of Tropical Medicine and Hygiene, 8, 363–373. [DOI] [PubMed] [Google Scholar]
- 117.Kulik RA, Falavigna DL, Nishi L, Araujo SM. 2008. Blastocystis sp. and other intestinal parasites in hemodialysis patients. Brazilian Journal Infect Diseases, 12, 338–341. [DOI] [PubMed] [Google Scholar]
- 118.Latorraca MQ, Meirelles SMP, Marchini JS. 1988. Indicadores das condições nutricionais na região polonoroeste versos desnutrição protéico-energética e parasitoses intestinais em um grupo de crianças de 3 a 72 meses de idade da cidade de Mirassol D’Oeste, Mato Grosso, Brasil. Revista do Instituto de Medicina Tropical de São Paulo, 30, 192–196. [DOI] [PubMed] [Google Scholar]
- 119.Lawson LLO, Bailey JW, Beeching NJ, Gurgel RG, Cuevas LE. 2004. The stool examination reports amoeba cysts: should you treat in the face of over diagnosis and lack of specificity of light microscopy? Tropical Doctor, 34, 28–30. [DOI] [PubMed] [Google Scholar]
- 120.Leite RO, Toma HK, Adani YL. 2014. Diagnóstico parasitológico e molecular de enteroparsitos entre crianças residentes e funcionários de uma instituição beneficente para menores no município de Niterói-RJ, Brasil. Revista de Patologia Tropical, 43, 446–458. [Google Scholar]
- 121.Lima ECS, Leon CMP, Oliveira HMBF, Barbosa VSA. 2020. Prevalência de parasitoses intestinais em usuários de um hospital universitário, Santa Cruz-RN, Brasil. Revista de Atenção a Saúde, 18, 21–30. [Google Scholar]
- 122.Lopes FMR, Gonçalves DD, Reis CR, Breganó RM, Anaruma Filho F, Murad VA, Menezes MCND, Freire RL, Freitas JC, Santana MAZ, Navarro IT. 2006. Occurrence of enteroparasitosis in schoolchildren of the municipal district of Jataizinho, State of Paraná Brazil. Acta Scientiarum Health Sciences, 28, 107–111. [Google Scholar]
- 123.Lopes LM, Santos ES, Savegnago TL, Salvador FA, Ribeiro-Barbosa ER. 2010. Ocorrência de parasitas e comensais intestinais em crianças da comunidade de Vila Inglesa em São Paulo, SP, Brasil. Revista do Instituto Adolfo Lutz, 69, 252–254. [Google Scholar]
- 124.López MC, León CM, Fonseca J, Reyes P, Moncada L, Olivera MJ, Ramírez JD. 2015. Molecular epidemiology of Entamoeba: first description of Entamoeba moshkovskii in a rural area from Central Colombia. PLoS One, 10, e0140302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Luz JGG, Carvalho AG, Marques AP, Marcondes AA, Roma JHF, Castro LS, Castro LS, Dias JVL, Pavoni JHC. 2017. Intestinal parasitic infections and associated risk factors in preschoolers from different urban settings in Central-Western Brazil. Asian Pacific Journal of Tropical Disease, 7, 405–410. [Google Scholar]
- 126.Macedo HW, Gonçalves AMH, Almeida CB, Dias LVB, Muniz MF. 2010. Infecção por Blastocystis hominis e Entamoeba histolytica/ Entamoeba dispar em pacientes atendidos em um hospital localizado em Niterói, Rio de Janeiro. Revista de Patologia Tropical, 39, 56–62. [Google Scholar]
- 127.Machado ER, Santos DS, Costa-Cruz JM. 2008. Enteroparasites and commensals among children in four peripheral districts of Uberlândia, satate of Minas Gerais. Revista da Sociedade Brasileira de Medicina Tropical, 41, 581–585. [DOI] [PubMed] [Google Scholar]
- 128.Machado ER, Souza TS, Costa JM, Costa-Cruz JM. 2008. Enteroparasites and commensals among individuals living in rural and urban áreas in Adadia dos Dourados, Minas Gerais state, Brazil. Parasitología Latinoamericana, 63, 34–39. [Google Scholar]
- 129.Magalhães VM, Carvalho AG, Freitas FIS. 2010. Inquérito parasitológico em manipuladores de alimentos em João Pessoa, PB, Brasil. Revista de Patologia Tropical, 39, 335–342. [Google Scholar]
- 130.Maia MMM, Fausto MA, Vieira ELM, Benetton MLFN, Carneiro M. 2009. Intestinal parasitic infection and associated risk factors, among children presenting at outpatient clinics in Manaus, Amazonas state, Brazil. Annals of Tropical Medicine & Parasitology, 103, 583–591. [DOI] [PubMed] [Google Scholar]
- 131.Marcos LA, Gotuzzo E. 2013. Intestinal protozoan infections in the immunocompromised host. Current Opinion in Infectious Diseases, 26, 295–301. [DOI] [PubMed] [Google Scholar]
- 132.Marietto-Gonçalves GA, Fernandes TM, Silva RJ, Lopes RS, Andreatti Filho RL. 2008. Intestinal protozoan parasites with zoonotic potential in birds. Parasitology Research, 103, 1237–1240. [DOI] [PubMed] [Google Scholar]
- 133.Markell EK, John DT, Krotoski WA. 2003. Protozoários que habitam a luz, in Parasitologia médica, Markell EK, Editor. Guanabara-Koogan: Rio de Janeiro. p. 265–283. [Google Scholar]
- 134.Marques AB, Pereira DC, Ribeiro RC. 2005. Motivos e frequência de internação dos pacientes com IRC em tratamento hemodialítico. Arquivos de Ciências da Saúde, 12, 67–72. [Google Scholar]
- 135.Martins LPA, Serapião AATB, Valenciano RF, Oliveira GT, Santos KJA, Castanho REP. 2009. Avaliação inicial da prevalência de algumas enteroparasitoses na comunidade de Palmital, município de Berilo-MG. Revista Médica de Minas Gerais, 19, 26–31. [Google Scholar]
- 136.Mata-Santos T, Gatti FA, Mascarenhas CS, Martins LH, Mata-Santos HA, Fenalti JM, Netto ICO, Mendoza-Sassi RA, Scaini CJ. 2013. Prevalence of enteroparasites in children atended at basic health unities in a Brazilian southern city. Revista do Instituto Adolfo Lutz, 72, 175–178. [Google Scholar]
- 137.Matsumura T, Hendarto J, Mizuno T, Syafruddin D, Yoshikawa H, Matsubayashi M, Nishimura T, Tokoro M. 2019. Possible Pathogenicity of commensal Entamoeba hartmanni revealed by molecular screening of healthy school children in Indonesia. Tropical Medicine and Health, 47, 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Menezes AL, Lima VMP, Freitas MTS, Rocha MO, Silva EF, Dolabella SS. 2008. Prevalence of intestinal parasites in children from public daycare center in the city of Belo Horizonte, Minas Gerais, Brazil. Revista do Instituto de Medicina Tropical de São Paulo, 50, 57–59. [DOI] [PubMed] [Google Scholar]
- 139.Miné JC, Rosa JA. 2008. Frequency of Blastocystis hominis and other intestinal parasites in stool samples examined at the parasitology laboratory of the School of Pharmaceutical Sciences at the São Paulo State University, Araraquara. Revista da Sociedade Brasileira de Medicina Tropical, 41, 565–569. [DOI] [PubMed] [Google Scholar]
- 140.Miranda RA, Xavier FB, Menezes RC. 1998. Parasitismo intestinal em uma aldeia indígena Parakanã, sudeste do estado do Pará, Brasil. Caderno de Saúde Pública, 14, 507–511. [DOI] [PubMed] [Google Scholar]
- 141.Moher D, Liberati A, Tetzlaff J, Altman DG. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine, 6, e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Monteiro AMC, Silva EF, Almeida KS, Sousa JJN, Mathias LA, Baptista F, Freitas FLC. 2009. Parasitoses intestinais em crianças de creches públicas localizadas em bairros periféricos do município de Coari, Amazonas, Brasil. Revista de Patologia Tropical, 38, 284–290. [Google Scholar]
- 143.Monteiro ACS, Soares DA, Diniz SCPOR, Cavalcante UMB, Silva AB, Vianna RPT, Freitas FIS, Souza TC, Lima CMBL. 2018. Intestinal parasitismo and related risk factors for primary school students in the municipality of João Pessoa, Northeast Brazil. Bioscientia Journal, 34, 1062–1072. [Google Scholar]
- 144.Mortean ECM, Falavigna DLM, Janeiro V, Falavigna-Guilherme AL, Gomes ML. 2012. Low intestinal parasites as an health indicator in a municipality of southern Brazil with intesnive agricultural mechanization. Revista de Saúde e Biologia, 7, 23–29. [Google Scholar]
- 145.Moura H, Fernandes O, Viola JPB, Silva SP, Passos RH, Lima DB. 1989. Enteric parasites and HIV infection: occurrence in AIDS patients in Rio de Janeiro, Brazil. Memórias do Instituto Oswaldo Cruz, 84, 527–533. [DOI] [PubMed] [Google Scholar]
- 146.Moura RGF, Ramos ELP, Colombo MS, Aidar FLM, Hernández CG, Silva MBO, Oliveira KR. 2017. Prevalence of intestinal parasites in child daycare centers: epidemiological significance. Revista de Patologia Tropical, 46, 75–84. [Google Scholar]
- 147.Murad MH, Chu H, Lin L, Wang Z. 2018. The effect of publication bias magnitude and direction on the certainty in evidence. BMJ Evidence Based Medicine, 23, 84–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nagel AS, Baccega B, Hernandes JC, Santos CV, Gallo MC, Quevedo PS, Villela MM. 2017. Intestinal parasite prevalence in shoolchildren from northwestern Rio Grande do Sul state, Brazil. Revista de Patologia Tropical, 46, 277–286. [Google Scholar]
- 149.Nascimento SA, Moitinho MLR. 2005. Blastocystis hominis and other intestinal parasites in a community of Pitanga city, Paraná state, Brazil. Revista do Instituto de Medicina Tropical de São Paulo, 47, 213–217. [DOI] [PubMed] [Google Scholar]
- 150.Neres-Norberg A, Guerra-Sanches F, Moreira-Norberg PRB, Madeira-Oliveira JT, Santa-Helena AA, Serra-Freire NM. 2014. Enteroparasitismo en indígenas Terena em el Estado de Mato Grosso do Sul, Brasil. Revista de Salud Publica, 16, 859–870. [PubMed] [Google Scholar]
- 151.Ngobeni R, Samie A, Moonah S, Watanabe K, Petri WA, Gilchrist C. 2017. Entamoeba species in South Africa: correlations with the host microbiome, parasite burdens, and first description of Entamoeba bangladeshi outside of Asia. Journal of Infectious Diseases, 216, 1592–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nolan MJ, Unger YYT, Rogers E, Millet I, Harman K, Fox M, Kalema-Zikusoka G, Blake DP. 2017. Molecular characterisation of protest parasites in human-habituated mountain gorillas (Gorilla beringei beringei), humans and livestock, from Bwindi impenetrable National Park, Uganda. Parasites & Vectors, 10, 340–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Okazaki M, Okazaki M, Miranda P, Neto J, Diegues V, Alves J, Cauas M, Tanabe M, Kobayashi S, Kaneko N, Nagakura K, Kobayashi M, Motta S, Tateno S, Takeuchi T. 1988. Parasitological and serological studies on amoebiasis and other intestinal parasitic infections in recife and its suburban area, northeast Brazil. Revista do Instituto de Medicina Tropical de São Paulo, 30, 313–321. [DOI] [PubMed] [Google Scholar]
- 154.Oliveira CLM, Ferreira WA, Vasquez FG, Barbosa MGV. 2010. Parasitoses intestinais e fatores socioambientais de uma população da área periurbana de Manaus – AM. Revista Brasileira em Promoção da Saúde, 23, 307–315. [Google Scholar]
- 155.Oliveira YLDC, Oliveira LM, Oliveira YLM, Nascimento AMD, Corte RL, Geraldini RM, Barbosa L, Gazzinelli-Guimarães PH, Fujiwara RT, Bueno LL, Dolabella SS. 2020. Changes in the epidemiological profile of intestinal parasites after a school-based large-scale treatment for soil-transmitted helminths in a community in northeastern Brazil. Acta Tropica, 202, ID 105279. [DOI] [PubMed] [Google Scholar]
- 156.Pereira VV, Conceição AS, Maximiano LHS, Belligoli LQG, Silva ES. 2014. Laboratory diagnosis of amebiasis in a sample of students from southeastern Brazil and a comparison of microscopy with enzyme-linked immunosorbent assay for screening of infections with Entamoeba sp. Revista da Sociedade Brasileira de Medicina Tropical, 47, 52–56. [DOI] [PubMed] [Google Scholar]
- 157.Pereira IGS, Rodrigues CS, Gurgel-Gonçalves R, Machado ER. 2015. Frequency of intestinal parsites and commensals in street waste pickers from two cooperatives in the Brazilian Federal district. Revista de Patologia Tropical, 44, 432–440. [Google Scholar]
- 158.Pinheiro SMB, Carneiro RM, Aca IS, Irmão JI, Morais JRMA, Coimbra MRM, Carvalho JRLB. 2004. Determination of the prevalence of Entamoeba histolytica and E. dispar in the Pernambuco state of northeastern Brazil by a polymerase chain reaction. American Journal of Tropical Medicine and Hygiene, 70, 221–224. [PubMed] [Google Scholar]
- 159.Pinheiro SMB, Maciel RF, Morais JRMA, Aca IS, Carvalho JRLB, Coimbra MRM. 2005. Genetic characterization of Entamoeba dispar isolates in Northeast Brazil. Acta Tropica, 94, 35–40. [DOI] [PubMed] [Google Scholar]
- 160.Pires ECR, Guimarães FP, Diniz JC, Froeseler MVG, Mata LCC. 2016. Abordagem interdisciplinar das parasitoses intestinais em escolares da microrregião de Sete Lagoas-MG. Arquivos de Ciências da Saúde UNIPAR, 20, 111–116. [Google Scholar]
- 161.Port-Lourenço AE, Uchoa ACM, Bastos MPO. 2004. Hospital food handlers in Niterói, RJ, Brazil: intestinal parasitism. Archivos Latinoamericanos de Nutrición, 54, 395–401. [PubMed] [Google Scholar]
- 162.Porto LP, Cavagnolli NI, Reis DS, Spada PKWDS, Rodrigues AD. 2016. Prevalência de parasitoses em trabalhadores de restaurantes de Caxias do Sul – RS. Revista de Patologia Tropical, 45, 115–120. [Google Scholar]
- 163.Póvoa MM, Arruda JEG, Silva MCM, Bichara CNC, Esteves P, Gabbay YB, Machado RLD. 2000. Diagnóstico de amebíase intestinal utilizando métodos coproscópicos e imunológicos em amostras da população da área metropolitana de Belém, Pará, Brasil. Cadernos de Saúde Pública, 16, 843–846. [DOI] [PubMed] [Google Scholar]
- 164.Radavelli WM, Pazinato R, Klauck V, Volpato A, Balzan A, Rossett J, Cazorotto CJ, Lopes LS, Kessler JD, Cucco DC, Tonin AA, Da Silva AS. 2014. Occurrence of gastrointestinal parasites in goats from the western Santa Catarina, Brazil. Brazilian Journal of Veterinary Parasitology, 23, 101–104. [DOI] [PubMed] [Google Scholar]
- 165.Ragazzo LJ, Zohdy S, Velonabison M, Herrera J, Wright PC, Gillespie TR. 2018. Entamoeba histolytica infection in wild lemurs associated with proximity to humans. Parasitology Research, 249, 98–101. [DOI] [PubMed] [Google Scholar]
- 166.Raza A, Iqbal Z, Muhammad G, Ahmad M, Hanif K. 2013. Amebiasis as a major risk to human health: a review. International Journal of Molecular Medical Science, 3, 13–24. [Google Scholar]
- 167.Reis LB, Santos RS, Mota LHS, Jesus JSA, Oliveira JMO, Andrade RS, Santos GA, Amor ALM. 2019. Enteroparasites, demographic profile socioeconomic status and education level in the rural popuolation of the recôncavo of Bahia, Brazil. Revista de Patologia Tropical, 48, 197–210. [Google Scholar]
- 168.Review Manager (Computer program). 2020. Version 5 The Cochrane Collaboration. Available at: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/revman-5-download. [Google Scholar]
- 169.Rezende CHA, Costa-Cruz JM, Gennari-Cardoso ML. 1997. Enteroparasitoses em manipuladores de alimentos de escolas públicas em Uberlândia, Minas Gerais, Brasil. Revista Panamericana de Salud Publica, 2, 392–397. [PubMed] [Google Scholar]
- 170.Rios L, Cutolo AS, Giatti LL, Castro M, Rocha AA, Toledo RF, Pelicioni MCF, Barreira LP, Santos JG. 2007. Prevalência de parasitos intestinais e aspectos socioambientais em comunidade indígena no distrito de Iauaretê, município de São Gabriel da Cachoeira-AM, Brasil. Revista Saúde e Sociedade, 16, 76–86. [Google Scholar]
- 171.Rocha RS, Silva JG, Peixoto SV, Caldeira RL, Firmo JOA, Carvalho OS, Katz N. 2000. Avaliação da esquistossomose e de outras parasitoses intestinais, em escolares do município de Bambuí, Minas Gerais, Brasil. Revista da Sociedade Brasileira de Medicina Tropical, 33, 431–436. [PubMed] [Google Scholar]
- 172.Rollemberg CVV, Silva MMBL, Rollemberg KC, Amorim FR, Lessa NMN, Santos MDS, Souza AMB, Melo EV, Almeida RP, Silva AM, Werneck GL, Santos MA, Almeida JAP, Jesus AR. 2015. Predicting frequency distribution and influence of sociodemographic and behavioral risk factors of Schistosoma mansoni infection and analysis of co-infection with intestinal parasites. Geospatial Health, 10, 13–19. [DOI] [PubMed] [Google Scholar]
- 173.Rosales TFL, Malheiros AF. 2017. Contaminação ambiental por enteroparasitas presentes em fezes de cães em uma região do Pantanal. O Mundo da Saúde, 41, 368–377. [Google Scholar]
- 174.Royer TL, Gilchrist C, Kabir M, Arju T, Ralston KS, Haque R, Clark CG, Petri-Junior WA. 2012. Entamoeba bangladeshi nov. sp., Bangladesh. Emerging Infectious Diseases, 18, 1543–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Santos MES, Ogando T, Fonseca BPV, Junior CEG, Barçante JMP. 2006. Ocorrência de enteroparasitos em crianças atendidas no programa de saúde da família de uma área de abrangência do município de Vespasiano, Minas Gerais, Brasil. Revista Eletrônica de Enfermagem, 8, 25–29. [Google Scholar]
- 176.Santos LP, Santos FLN, Soares NM. 2007. Prevalência de parasitoses intestinais em pacientes atendidos no hospital universitário Professor Edgar Santos, Salvador, Bahia. Revista de Patologia Tropical, 36, 237–246. [Google Scholar]
- 177.Santos SA, Merlini LS. 2010. Prevalência de enteroparasitoses na população do município de Maria Helena, Paraná. Ciência & Saúde Coletiva, 15, 899–905. [DOI] [PubMed] [Google Scholar]
- 178.Santos FLN, Gonçalves MS, Soares NM. 2011. Validation and utilization of PCR for differential diagnosis and prevalence determination of Entamoeba histolytica/Entamoeba dispar in Salvador city, Brazil. Brazilian Journal of Infectious Diseases, 15, 119–125. [PubMed] [Google Scholar]
- 179.Santos RV, Nunes JS, Camargo JASA, Rocha EMM, Fontes G, Camargo LMA. 2013. High occurrence of Entamoeba histolytica in the municipalities of Ariquemes and Monte Negro, State of Rondônia, Western Amazonia, Brazil. Revista do Intituto de Medicina Tropical de São Paulo, 55, 193–196. [DOI] [PubMed] [Google Scholar]
- 180.Santos J, Duarte ARM, Gadotti G, Lima LM. 2014. Parasitoses intestinais em crianças de creche comunitária em Florianópolis, SC, Brasil. Revista de Patologia Tropical, 43, 332–340. [Google Scholar]
- 181.Santos HLC, Martins LAF, Peralta RHS, Peralta JM, Macedo HW. 2014. Frequency of amoebiasis and other intestinal parasitosis in a settlement in Ilhéus city, state of Bahia, Brazil. Revista da Sociedade Brasileira de Medicina Tropical, 47, 11–14. [DOI] [PubMed] [Google Scholar]
- 182.Santos RV, Fontes G, Duarte IAC, Santos-Júnior JA, Rocha EMM. 2016. Identification of Entamoeba histolytica and E. dispar infection in Maceió, Alagoas State, northeast Brazil. Journal of Infection in Developing Countries, 10, 1146–1150. [DOI] [PubMed] [Google Scholar]
- 183.Santos PHS, Barros RCS, Gomes KVG, Nery AA, Casotti CA. 2017. Prevalência de parasitoses intestinais e fatores associados em idosos. Revista Brasileira de Geriatria e Gerontologia, 20, 244–254. [Google Scholar]
- 184.Santos-Júnior GO, Silva MM, Santos FLN. 2006. Prevalência de enteroparasitoses em crianças do sertão baiano pelo método de sedimentação espontânea. Revista de Patologia Tropical, 35, 233–240. [Google Scholar]
- 185.Schnack FJ, Fontana LM, Barbosa PR, Silva LSM, Baillargeon CMM, Barichello T, Póvoa MM, Cavasini CE, Machado RLD. 2003. Enteropatógenos associados com diarreia infantil (< 5 anos de idade) em amostras da população da área metropolitana de Criciúma, Santa Catarina, Brasil. Caderno de Saúde Pública, 19, 1205–1208. [DOI] [PubMed] [Google Scholar]
- 186.Seixas MTL, Souza JN, Souza RP, Teixeira MCA, Soares NM. 2011. Avaliação da frequência de parasitos intestinais e do estado nutricional em escolares de uma área periurbana de salvador, Bahia, Brasil. Revista de Patologia Tropical, 40, 304–314. [Google Scholar]
- 187.Silva MCM, Monteiro CSP, Araújo BAV, Silva JV, Póvoa MM. 2005. Determinação da infeção por Entamoeba histolytica em residentes da área metropolitana de Belém, Pará, Brasil, utilizando o ensaio imunoenzimático (ELISA) para detecção de antígenos. Cadernos de Saúde Pública, 21, 969–973. [DOI] [PubMed] [Google Scholar]
- 188.Silva MTN, Pontes A, Aragão P, Andrade J, Tavares-Neto J. 2005. Prevalência de parasitas intestinais em crianças, com baixos indicadores socio-econômicos, de Campina Grande (Paraíba). Revista Baiana de Saúde Pública, 29, 121–125. [Google Scholar]
- 189.Silva EF, Silva EB, Almeida KS, Sousa JJN, Freitas FL. 2009. Enteroparasitoses em crianças de áreas rurais do município de Coari, Amazonas, Brasil. Revista de Patologia Tropical, 38, 35–43. [Google Scholar]
- 190.Silva FS, Paulo ADC, Braga CMM, Almeida RJ, Galvão VP. 2010. Frequência de parasitos intestinais no municipio de Chapadinha, Maranhão, Brasil. Revista de Patologia Tropical, 39, 63–68. [Google Scholar]
- 191.Silva LP, Silva RMG. 2010. Ocorrência de enteroparasitos em centros de educação infantil no município de Patos de Minas, MG, Brasil. Biocience Journal, 26, 147–151. [Google Scholar]
- 192.Silva SRP, Arrosi N, Jesus RS, Reis RS, Rott MB. 2010. Enteroparasitoses em portadores de necessidades especiais – prevalência em individuos atendidos em instituições do municipio de Porto Alegre-RS. Revista de Patologia Tropical, 39, 123–129. [Google Scholar]
- 193.Silva LP, Silva RMG, Fernandes NA, Oliveira JAA. 2011. Parasitos e/ou comensais em pacientes neoplásicos submetidos à quimioterapia. Bioscience Journal, 27, 170–177. [Google Scholar]
- 194.Silva EF, Silva VBC, Freitas FLC. 2012. Parasitoses intestinais em crianças residentes na comunidade ribeirinha São Francisco do Laranjal, município de Coari, estado do Amazonas, Brasil. Revista de Patologia Tropical, 41, 97–101. [Google Scholar]
- 195.Silva MTN, Santana JV, Bragagnoli G, Marinho AMN, Malagueño E. 2014. Prevalence of Entamoeba histolytica/Entamoeba dispar in the city of Campina Grande, in northeastern Brazil. Revista do Instituto de Medicina Tropical de São Paulo, 56, 451–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Silva JSH, Silva DJ, Shaw JJ, Malheiros AF. 2018. Prevalência de enteroparasitos em moradores da cidade de Cáceres/MT. Revista Ibero Americana de Ciências Ambientais, 9, 154–164. [Google Scholar]
- 197.Silva PV, Maciel LS, Castro LS, Murat PG, Higa-Jr MG, Zerlotti PH, Motta-Castro ARC, Pontes ERJC, Dorval MEC. 2018. Enteroparasites in riverside settlements in the Pantanal wetlands ecosystem. Journal of Parasitology Research, 2018, e6839745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Silva RSB, Malheiros AF, Santos DP, Shaw JJ, Araújo MSM, Moraes MFA, Campos WNL. 2019. Estudo de parasitoses intestinais em moradores de Corumbá, Mato Grosso do Sul, Brasil. Revista Ibero Americana de Ciências Ambientais, 10, 109–128. [Google Scholar]
- 199.Simões BS, Machado-Coelho GLL, Pena JL, Freitas SN. 2015. Condições ambientais e prevalência de infecção parasitária em indígenas Xukuru-Kariri, Caldas, Brasil. Revista Panamericana de Salud Publica, 38, 42–48. [PubMed] [Google Scholar]
- 200.Soares NM, Azevedo HC, Pacheco FTF, Souza JN, Del-Rei RP, Teixeira MCA, Santos FLN. 2019. A cross-sectional study of Entamoeba histolytica/dispar/moshkovskii complex in Salvador, Bahia, Brazil. Hindawi BioMed Research International, 2019, ID 7523670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sousa ACP, Costa LNG, Vieira JMS. 2018. Prevalência de enteroparasitas em individuos atendidos no Laboratório Municipal de Buriti dos Lopes, Piauí, Brasil. Revista Brasileira de Análises Clínicas, 50, 184–188. [Google Scholar]
- 202.Souza EA, Silva-Nunes M, Malafronte RS, Muniz PT, Cardoso MA, Ferreira MU. 2007. Prevalence and spatial distribution of intestinal parasitic infections in a rural Amazonian settlement, Acre state, Brazil. Caderno de Saúde Pública, 23, 427–434. [DOI] [PubMed] [Google Scholar]
- 203.Souza MF, Pimentel-Neto M, Silva RM, Farias ACB, Guimarães MP. 2012. Gastrointestinal parasites of sheep, municipality of Lajes Rio Grande do Norte, Brazil. Revista Brasileira de Parasitologia Veterinária, 21, 71–73. [DOI] [PubMed] [Google Scholar]
- 204.Souza LS, Guilherme E, Andrade AMF, Santos FGA. 2019. Occurrence o fendo and hemoparasites in Sporophila caerulescens captured in the eastern region of the state of Acre, Brazil. Ciência Rural, 49, e20180811. [Google Scholar]
- 205.Stensvold CR, Lebbad M, Clark CG. 2010. Genetic characterisation of uninucleated cyst-producing Entamoeba spp. from ruminants. International Journal for Parasitology, 40, 775–778. [DOI] [PubMed] [Google Scholar]
- 206.Stensvold CR, Lebbad M, Victory EL, Verweij JJ, Tannich E, Alfellani M, Legarraga P, Clark CG. 2011. Increased sampling reveals novel lineages of Entamoeba: consequences of genetic diversity and host specificity for taxonomy and molecular detection. Protist, 162, 525–541. [DOI] [PubMed] [Google Scholar]
- 207.Takizawa MGMH, Falavigna DLM, Gomes ML. 2009. Enteroparasitosis and their ethnographic relationship to food handlers in a tourist and economic center in Paraná, southern Brazil. Revista Instituto de Medicina Tropical de São Paulo, 51, 31–35. [DOI] [PubMed] [Google Scholar]
- 208.Tashima NT, Simões MJS. 2004. Enteroparasitic occurrence in fecal samples analyzed at the University of western São Paulo-UNOESTE clinical laboratory, Presidente Prudente São Paulo state, Brazil. Revista do Instituto de Medicina Tropical de São Paulo, 46, 243–248. [DOI] [PubMed] [Google Scholar]
- 209.Tashima NT, Simões MJS, Leite CQF, Fluminhan A, Nogueira MA, Malaspina AC. 2009. Classic and molecular study of Giardia duodenalis in children from a daycare center in the region of Presidente Prudente, São Paulo, Brazil. Revista do Instituto de Medicina Tropical de São Paulo, 51, 19–24. [DOI] [PubMed] [Google Scholar]
- 210.Tavares-Dias M, Grandini AA. 1999. Prevalencia e aspectos epidemiológicos de enteroparasitoses na população de São José da Bela Vista, São Paulo. Revista da Sociedade Brasileira de Medicina Tropical, 32, 63–65. [DOI] [PubMed] [Google Scholar]
- 211.Turkeltaub JA, McCarty TR, Hotez PJ. 2015. The intestinal protozoa: emerging impacto n global health and development. Current Opinion in Gastroenterology, 31, 38–44. [DOI] [PubMed] [Google Scholar]
- 212.Uchoa CMA, Lobo AGB, Bastos OMP, Matos AD. 2001. Parasitoses intestinais: prevalência em creches comunitárias da cidade de Niterói, Rio de Janeiro-Brasil. Revista do Instituto Adolfo Lutz, 60, 97–101. [Google Scholar]
- 213.Uchoa CMA, Albuquerque MC, Carvalho FM, Falcão AO, Silva P, Bastos OMP. 2009. Parasitismo intestinal em crianças e funcionários de creches comunitárias na cidade de Niterói-RJ, Brasil. Revista de Patologia Tropical, 38, 267–278. [Google Scholar]
- 214.Valença-Barbosa C, Batista RJ, Igreja RP, d’Avila-Levy CM, Macedo HW, Santos HLC. 2017. Distribution of Blastocystis subtypes isolated from humans from an urban community in Rio de Janeiro, Brazil. Parasites & Vectors, 10, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Valverde JG, Gomes-Slva A, Moreira CJC, Souza DL, Jaeger LH, Martins PP, Meneses VF, Boia MN, Carvalho-Costa FA. 2011. Prevalence and epidemiology of intestinal parasitism, as revealed by three distinct techniques in an endemic area in the Brazilian Amazon. Annals of Tropical Medicine and Parasitology, 105, 413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ximénez C, Morán P, Rojas L, Valadez A, Gómez A. 2009. Reassessment of the epidemiology of amebiasis: state of the art. Infection, Genetics and Evolution, 9, 1023–1032. [DOI] [PubMed] [Google Scholar]
- 217.Zanetti AS, Silva Junior IC, Barros LF, Domínguez OAE, Lima GS, Silva AS, Danelichen OS, Silva SL, Moreira LM, Shaw JJ, Malheiros AF. 2019. Parasitas intestinais em cães provenientes dos biomas do nordeste brasileiro: aspecto zoonótico e ambiental. Revista Ibero-Americana de Ciências Ambientais, 10, 42–51. [Google Scholar]
- 218.Zanotto M, Cavagnolli NI, Breda JC, Spada PKWDS, Bortolini GV, Rodrigues AD. 2018. Prevalence of intestinal parasites and socioeconomic evaluation of a country town in the Serra Gaucha región, Rio Grande do Sul, Brazil. Revista de Patologia Tropical, 47, 19–30. [Google Scholar]
- 219.Zenazokenae LE, Terças-Trettel ACP, Nascimento VF, Hattori TY, Atanaka M, Lemos ERS, Boia MN. 2019. Prevalence of enteroparasitosis in the indigenous communit y of Mato Grosso, Brazil: a look into the sanitation and ethno-development. Saúde e Pesquisa, 12, 253–264. [Google Scholar]
- 220.Zhang Q, Liu K, Wang C, Luo J, Lu J, He H. 2019. Molecular characterization of Entamoeba spp. in wild Taihangshan macaques (Macaca mulatta tcheliensis) in China. Acta Parasitologica, 64, 228–231. [DOI] [PubMed] [Google Scholar]