Abstract
Adenosine deaminases that act on RNA (ADARs) are present in all animals and function to both bind double-stranded RNA (dsRNA) and catalyze the deamination of adenosine (A) to inosine (I). As inosine is a biological mimic of guanosine, deamination by ADARs changes the genetic information in the RNA sequence and is commonly referred to as RNA editing. Millions of A-to-I editing events have been reported for metazoan transcriptomes, indicating that RNA editing is a widespread mechanism used to generate molecular and phenotypic diversity. Loss of ADARs results in lethality in mice and behavioral phenotypes in worm and fly model systems. Furthermore, alterations in RNA editing occur in over 35 human pathologies, including several neurological disorders, metabolic diseases and cancers. In this review, a basic introduction to ADAR structure and target recognition will be provided before summarizing how ADARs affect the fate of cellular RNAs and how researchers are using this knowledge to engineer ADARs for personalized medicine. In addition, we will highlight the important roles of ADARs and RNA editing in innate immunity and cancer biology.
Keywords: double-stranded RNA (dsRNA), ADAR, RNA editing, inosine, dsRBP, cancer, innate immunity, RNA modification
Graphical Abstract

Adenosine deamination by ADARs. ADARs search the cellular milieu for dsRNA targets. Upon recognizing a substrate, ADARs can bind and exert effects on gene expression and a number of cellular pathways (shown by white shading of boxes). ADARs also convert adenosines within cellular dsRNAs into inosines, through a process called “RNA editing”. RNA editing by ADARs play critical roles in specific cellular pathways (shaded in orange) and are being harnessed to generate novel therapeutics to treat disease. Please note a color version of this figure is available online.
Introduction
RNA sequence and structure serves as a fundamental code for directing specific RNA-protein interactions. RNA secondary and tertiary structures are recognized by different classes of RNA-binding proteins (RBPs). Specifically, the recognition of double-stranded RNA (dsRNA) by dsRNA-binding proteins (dsRBPs) is crucial for proper regulation of gene expression and recognition of foreign nucleic acids (Hur, 2019; Saunders and Barber, 2003). A family of dsRBPs that is central to both of these molecular processes is the adenosine deaminase acting on RNA (ADAR) family (Savva, Rieder, and Reenan, 2012). ADARs affect these processes through both dsRNA binding and/or converting adenosine (A) to inosine (I) within cellular dsRNAs (Walkley and Li, 2017; Wang, Zheng, and Beal, 2017). These molecular functions play critical roles in neuronal function, development and innate immunity (Jain, Jantsch, and Licht, 2019; Rosenthal, 2015).
What makes a protein an ADAR?
The basic domain structure of ADARs consists of a varying number of amino-terminal dsRNA binding domains (dsRBDs) as well as a highly conserved carboxy-terminal deaminase domain. While ADARs are present across metazoans, the number of ADAR proteins in each organism differs. These differences arise either from separate genetic loci or from a single locus that makes use of multiple promoters, splice sites or translation initiation sites (George and Samuel, 1999; Gerber, O’Connell, and Keller, 1997; Palladino et al., 2000). In addition to the variable combinations of conserved domains, the ADAR proteins produced can also include additional domains that impart unique functions (Figure 1).
Figure 1. Domain structure of ADARs from various organisms.
Overall domain structure of human (Homo sapiens, hs), fly (Drosophila melanogaster, dm), squid (Doryteuthis opalescens, sq), and nematode (Caenorhabditis elegans, ce) ADAR proteins. Structures highlighted include dsRNA binding domains (purple), C-terminal catalytic (deaminase) domains (blue), Z-DNA binding motifs (red, hsADAR1 only), and the arginine-rich R-domain (yellow, hsADAR3 only). Domain boundaries and protein length data was obtained from the UniProt database, accession numbers are as follows: hsADAR1 (P55265), hsADAR2 (P78563), hsADAR3 (Q9NS39), dmADAR (Q9NII1), sqADAR2a (C1JAR3), sqADAR2b (C1JAR4), ceADR-1 (Q9U3D6), ceADR-2 (Q22618).
The number as well as the distance between dsRBDs confers differing enzymatic activity and substrate specificity to each ADAR (see details in “Target Recognition by the ADAR family”). Human ADAR1 differs from the canonical ADAR structure by containing one or two Z-DNA binding domains (Berger et al., 1998; Herbert et al., 1997). This may allow ADAR1 to bind Z-DNA that trails a moving RNA polymerase, facilitating the targeting of nascent RNA immediately after transcription. Additionally, stabilization of promoter Z-DNA may enhance transcription, suggesting a potential gene regulatory role for ADAR1 (Oh, Kim, and Rich, 2002). Human ADAR1 is produced as two different isoforms generated via the use of two different promoters (George and Samuel, 1999). One promoter is constitutively active and produces the shorter p110 isoform, while the other promoter is interferon-inducible and produces the longer p150 isoform (Figure 1). The p110 isoform contains one Z-DNA binding domain and localizes to the nucleus, while the p150 isoform contains two Z-DNA binding domains and a nuclear export signal (NES), which leads to shuttling between the nucleus and cytoplasm (Poulsen et al., 2001).
Human ADAR3 contains an amino-terminal, arginine (R)-rich domain (referred to as the R-domain), which allows ADAR3 to bind single-stranded RNA (ssRNA) in addition to the dsRNA binding capabilities afforded by its dsRBDs (Chen et al., 2000). However, ssRNA binding by ADAR3 has only been demonstrated in vitro, and the biological importance of the R-domain for in vivo RNA binding and ADAR3 function remains unknown. One study observed an interaction between the R-domain and KPNA2, a nuclear localization signal (NLS) receptor, suggesting the R-domain acts as an NLS (Maas and Gommans, 2009). In addition to the canonical domain structure (Figure 1), a tissue-specific ADAR2 splice variant includes a motif closely related to the R-domain of ADAR3 (Maas and Gommans, 2009). Whether or not this splice variant results in the ability of ADAR2 to bind ssRNA is unclear. However, in vitro studies indicate a similar general editing activity of the extended and canonical ADAR2 variants (Maas and Gommans, 2009).
Despite containing a deaminase domain, not all ADARs catalyze A-to-I editing. In particular, human ADAR3 and C. elegans ADR-1 both lack editing activity. While the deaminase domain of C. elegans ADR-1 lacks essential catalytic residues (Tonkin et al., 2002), human ADAR3 has the essential amino acids to carry out adenosine deamination but is editing deficient (Chen et al., 2000). It has been suggested that the lack of ADAR3 editing activity is due to lack of conservation in residues of the newly identified RNA binding loop present in the deaminase domain (Matthews et al., 2016). Importantly, a recent computational and screening approach generated an ADAR3 protein with five point mutations that resulted in functional deaminase activity in vitro and in cells (Wang et al., 2019). To provide biological relevance to the observed ADAR3 editing activity, it will be interesting to see if any of these gain-of-function mutations, or others, occur in human disease. Despite the lack of naturally occurring editing activity, both C. elegans ADR-1 and human ADAR3 have been implicated in regulating the activity of catalytically active ADARs through dimerization and/or competition for dsRNA binding (Chen et al., 2000; Oakes et al., 2017; Rajendren et al., 2018; Washburn and Hundley, 2016; Washburn et al., 2014). Going forward, whether inhibition of RNA editing is a critical biological function of human ADAR3 and C. elegans ADR-1 and/or whether these proteins perform other important gene regulatory functions should be determined.
Although some organisms have more ADAR genes than others, this does not always correlate with more editing activity, which leads to the question of the biological function of multiple ADARs. While the exact purpose remains unclear, reconstruction of ADAR gene evolution revealed that the metazoan last common ancestor likely had a set of multiple ADARs similar to those of modern humans and higher metazoans, and that the differences in ADAR number and structure between different organisms likely resulted from gene loss or domain loss along the lineage (Grice and Degnan, 2015). This would be consistent with the idea that RNA editing is an essential metazoan regulatory function, and that lineages that have lost ADAR genes/domains have evolved other, complementary regulatory mechanisms.
Target Recognition by the ADAR Family
The modular domain structure of ADARs allows for separation of the dsRNA binding and deaminase functions. In substrate recognition by ADARs, the dsRBDs directly interact with dsRNA and are required for substrate binding in vivo (Nishikura, 2010). After the dsRBDs bind, the deaminase domain provides additional contacts around the target adenosine and catalyzes deamination (Thomas and Beal, 2017). Herein, we will discuss what is known about how these two domains contribute to ADAR function with an emphasis on knowledge gained from in vitro studies and whether these same conclusions apply to in vivo substrate binding and deamination.
How do dsRBDs contribute to the affinity and specificity of ADAR binding to dsRNA?
The general dogma for dsRBPs is that specificity for dsRNA is based on the overall A-form helical structure. This is largely based on early crystal structures of the dsRBD of Xenopus laevis RNA-binding protein A (Xlbpa) bound to dsRNA and NMR structures of dsRBD3 of Staufen bound to an RNA stem-loop (Ramos et al., 2000; Ryter and Schultz, 1998). Both structures indicated the majority of interactions between the dsRBD and dsRNA involve the phosphodiester backbone across the major groove and 2’-hydroxyl groups of riboses. As these contacts would occur with dsRNA of any sequence, these early studies suggested that dsRBDs bind dsRNA in a sequence-independent, but structure-dependent manner. While dsRBDs may largely recognize overall dsRNA structure, the solution structure of ADAR2 dsRBDs bound to dsRNA suggests that dsRNA binding may also be specific to RNA sequence (Stefl et al., 2010). This observed sequence specificity could have arisen due to the use of a substrate related to the native GRIA2 R/G editing site, capturing the structural context that occurs in vivo, while the previous Xlbpa and Staufen structures were solved with a synthetic dsRNA.
In large part, details of how dsRBDs contribute to in vivo recognition of specific sequences by ADARs are lacking. Immunofluorescence studies of Xenopus ADAR1 with lampbrush chromosomes revealed that individual dsRBDs can bind different subtrates in vivo (Doyle and Jantsch, 2003). The specificity of dsRBDs for different targets was also observed in experiments comparing human ADARs and PKR. In domain swapping experiments, replacement of dsRBD1 and dsRBD2 in ADAR1 with those from PKR significantly reduced in vitro editing of native substrates (Liu, Lei, and Samuel, 2000). Using a hydroxy radical footprinting approach, the dsRBDs of ADAR2 and PKR interacted with different regions of a duplex RNA (Stephens, Haudenschild, and Beal, 2004). These studies also suggest dsRBD specificity can be studied both in vitro and in vivo for human ADARs. In contrast, RNA immunoprecipitation (RIP) studies indicated that dsRBD1, but not dsRBD2 of C. elegans ADR-1 contributed to substrate binding in vivo (Rajendren et al., 2018), while both dsRBDs of ADR-1 were capable of dsRNA binding in vitro. These differences indicate that further in vivo studies are needed for a better understanding of substrate specificity of the dsRBDs of ADARs.
Transcriptome-wide identification of human ADAR1 and ADAR2 targets, as well as C. elegans ADARs have been obtained from RIP studies (Bahn et al., 2015; Galipon et al., 2017; Ganem et al., 2019; Ohlson et al., 2005; Rajendren et al., 2018). However, the in vivo binding sites on these targets and whether a given dsRBD occupies a specific binding site is largely unknown. Crosslinking immunoprecipitation followed by high-throughput sequencing (CLIP-Seq) has identified human ADAR1 bound regions on endogenous RNAs (Bahn et al., 2015), which includes both the expected highly edited Alu repeats as well as non-Alu regions (15% of ADAR1-bound targets). This latter discovery was critical to uncovering a role for ADAR1 in regulating 3’ untranslated regions (3’ UTRs) length and altering miRNA biogenesis. However, ADAR1 contains multiple dsRBDs and the role of individual domains in these molecular processes and whether specific domains contribute to recognition of specific targets in vivo was not examined. It is also important to note a major caveat of analyzing binding sites of dsRBPs, like ADARs, using CLIP-seq is that the bound region identified is limited to one strand of the dsRNA. As the other half of the ADAR target dsRNA can be hundreds to even thousands of nucleotides downstream in terms of linear transcriptome distance, information obtained from CLIP-seq is limited in contributing to understanding specific ADAR binding sites in vivo.
A number of methods, such as CLASH (Helwak and Tollervey, 2014) and hiCLIP (Sugimoto et al., 2015), were developed to capture RNA-RNA interactions/secondary structures, the latter of which was applied to a transcriptome-wide study of the bound targets of Staufen. A recent advancement to the CLASH methodology, termed irCLASH, adds an infrared-dye conjugated and biotinylated adapter to allow for more rapid and non-isotopic analysis of bound dsRNAs (Song et al., 2020). The bound targets of exogenously expressed human ADAR1, ADAR2 and ADAR3 were examined using irCLASH. This approach identified a significant number of non-Alu targets, as ~60% of human ADAR1, ADAR2 and ADAR3 bound regions were of non-Alu sequences. Taken together, future irCLASH experiments examining endogenous ADARs and CRISPR engineered dsRBD mutants of ADARs will be critical for understanding how the dsRBDs of ADARs contact substrates in vivo.
In addition to playing a role in recognition of specific substrates, in vitro studies have indicated that the number of dsRBDs contributes to the affinity of ADARs for dsRNA. This is most clearly evidenced by biochemical studies of squid ADARs. Squids express two isoforms of ADAR2; sqADAR2a, with three dsRBDs, as well as an alternatively spliced isoform from the same gene, sqADAR2b, with only two dsRBDs (Figure 1) (Palavicini, O’Connell, and Rosenthal, 2009). Comparison of the binding dissociation constant (KD) of the sqADAR2 isoforms indicates that the extra dsRBD strengthens the dsRNA binding affinity of sqADAR2 by 30–100 fold in vitro (Palavicini, Correa-Rojas, and Rosenthal, 2012). Furthermore, sqADAR2a edits more sites than sqADAR2b in vitro, suggesting that an additional dsRBD confers not only tighter dsRNA binding but also more editing (Palavicini, O’Connell, and Rosenthal, 2009). In contrast to the squid editing enzymes, ADR-2, the sole ADAR enzyme in C. elegans, has the weakest dsRNA binding affinity of all ADARs studied to date (Rajendren et al., 2018). Interestingly, C. elegans ADR-2 and two as yet uncharacterized ADAR1 proteins from Octopus bimaculoides and Acropora millepora are the only ADARs which possess a single dsRBD (Albertin et al., 2015; Hough, Lingam, and Bass, 1999; Porath et al., 2017). Future studies aimed at addressing whether these unusual ADARs with one dsRBD bind dsRNA directly in vivo may reveal important insights into the mechanisms ADAR enzymes use for substrate recognition.
Diving into the details of the dsRBDs of ADARs
While multiple dsRBDs may contribute to both substrate specificity and binding affinity of ADARs, it is largely unknown which amino acids within dsRBDs contribute to these functions in vivo. The dsRBD was first identified based on amino acid sequence similarity amongst dsRBPs that perform diverse cellular functions (St Johnston et al., 1992). As mentioned above, structural studies have indicated that dsRBDs interact with the major groove and two consecutive minor grooves, which in total spans ~16 base pairs (bps) of dsRNA (Ryter and Schultz, 1998). The dsRBD is comprised of 65–70 amino acids that fold into a distinctive αβββα secondary structure (Bycroft et al., 1995; Nanduri et al., 1998). The helix α2 includes a highly conserved KKxxK motif (K is lysine, x is any amino acid) that is crucial for the dsRBD to interact with dsRNA (Figure 2). This is supported by many studies of ADARs and other dsRBPs, which perform mutagenesis of the lysines in the KKxxK motif and demonstrate loss of dsRNA binding in vitro and/or in vivo. Moreover, early structural studies of dsRBPs indicate that these lysines are located in a surface-exposed loop which recognizes the major groove of dsRNA, suggesting the importance of electrostatic interactions by the basic residues (Ramos et al., 2000; Ryter and Schultz, 1998). These studies also suggest that the first and last lysines of this motif stabilize the overall dsRBD structure (Ramos et al., 2000; Stefl et al., 2006). Taken together, this implies each lysine in this motif may have a different function, and in vivo binding studies of ADARs should examine the contribution of each of these residues.
Figure 2. Multiple sequence alignment of dsRNA binding domains of ADARs.
Sequences of the dsRBDs for the indicated proteins were obtained from UniProt database using the accession numbers described for Figure 1. Domain boundaries were adjusted based on structural data. The final figure was produced using ESPript 3 (Robert and Gouet, 2014). Sequences that are identical are shown in a red-filled box, while those meet consensus (> 70%, <100%) are boxed with consensus residues in red font. The residues important for dsRNA binding (Masliah, Barraud, and Allain, 2013) and the secondary structure elements (conserved αβββα fold) are shown below. The conserved GPxH motif and di-alanine residues (AA) as well as the aromatic residues that reside within the hydrophobic core (HC) of the dsRBD are indicated above the alignment.
Mutagenesis and structural studies of the dsRBP family have identified other residues that are important for in vitro dsRNA binding, such as the glutamate (E) within helix α1 and the GPxH motif in the β1– β2 loop (Bycroft et al., 1995; Ramos et al., 2000). However, in ADARs, only the G of the GPxH motif is highly conserved (87% amongst ADARs shown in Figure 2). Interestingly, C. elegans ADR-2 is the only ADAR that does not contain a glycine or a small, hydrophobic amino acid at the start of the GPxH motif (Figure 2) and, as described above, has the weakest dsRNA affinity observed in vitro (Rajendren et al., 2018). From studies of Staufen, it is known that the two aromatic side chains, tyrosine in β1 loop and phenylalanine in β2 loop, reside within the hydrophobic core of the dsRBD (the homologous residues in ADARs are indicated by HC above the alignments in Figure 2). Specifically, the tyrosine is in the center of the hydrophobic core, suggesting a structural role in maintaining other key surface residues in an optimal orientation for dsRNA binding. In contrast, the phenylalanine is at the edge of the hydrophobic core/partially surface exposed. While mutation of this residue to alanine completely abolishes Staufen binding to dsRNA in vitro (Bycroft et al., 1995), this may be due to a structural change and thus, the phenylalanine residue only indirectly contributes to dsRNA binding. As would be expected for residues important in stabilizing the dsRBD fold, these aromatic groups are conserved across all ADARs, with the exception of C. elegans ADR-1 dsRBD1 having a glutamic acid in place of the aromatic residue in the β1 loop (Figure 2). The impact of these variances on in vivo dsRNA binding by ADARs should be explored.
Overall, human ADAR2 dsRBD2 and squid ADAR2 dsRBD3 exhibit complete sequence identity to the twenty-one defined consensus (>70%) residues present amongst human, fly, squid and nematode ADARs (Figure 2). In contrast, the dsRBDs of C. elegans ADARs are most diverged, with ADR-1 dsRBD1 and ADR-2 dsRBD containing only 13 and 14 of the 21 consensus residues, respectively (Figure 2). One striking difference is that these two domains harbor a pair of adjacent sulfur containing amino acids while all other ADARs have two alanines in the middle of helix α2 (Figure 2, marked with AA above alignment). As C. elegans ADARs have a unique partnership and mechanism for editing in vivo (Rajendren et al., 2018), the importance of these residues should be explored in future studies.
Not all ADAR dsRBDs function in dsRNA binding
It is important to note that some dsRBDs have a strong affinity to dsRNA while others display a weak affinity (Mohibi, Chen, and Zhang, 2019). Based on these differences, there have been attempts to classify dsRBDs into two groups (Doyle and Jantsch, 2002; Gleghorn and Maquat, 2014). The former dsRBDs are defined as type A and have strong sequence homology to other dsRBPs along the entire length of the dsRBD. The latter are known as type B dsRBDs and are proposed to have poor sequence similarity in the left half of the dsRBD including the N-terminus, which contributes to the observed weaker dsRNA binding affinity in vitro (Krovat and Jantsch, 1996; St Johnston et al., 1992). The type B dsRBDs often serve a function besides dsRNA binding, including mediating protein-protein interactions.
The protein-protein interactions of ADARs has been most extensively studied for mammalian ADARs. Mammalian ADAR1 has three dsRBDs (Figure 1) and mutation of any individual dsRBD reduces dsRNA binding in vitro (Liu and Samuel, 1996). However, human ADAR1 also uses dsRBD2 and dsRBD3 for dimerization with different dsRBPs (Ota et al., 2013). Specifically, ADAR1 is proposed to homodimerize via dsRBD3, as ADAR1 dsRBD3 deletion mutants lacked a physical interaction with wildtype ADAR1 (Ota et al., 2013). Similar experiments revealed that dsRBD2 of human ADAR1 is critical for heterodimerization with Dicer, a key dsRBP in the RNA interference (RNAi) pathway (Ota et al., 2013). These data suggest an individual dsRBD is capable of binding both dsRNA and protein and raises the idea that ADAR dsRBDs cannot be strictly classified as type A or B dsRBDs. Furthermore, the ability of some dsRBDs to interact with both dsRNA and proteins can lead to RNA-binding dependent regulation of the interaction of ADARs with specific proteins. A striking example is the interaction of human ADAR1 dsRBD3 with the nuclear import factor Transportin-1 (Trn-1), which recognizes a bimodal nuclear localization signal (NLS) formed by the C-terminus region of the dsRBD and an extended α-helix at the N-terminal region of the dsRBD (Barraud et al., 2014; Fritz et al., 2009). Binding of dsRNA to the dsRBD disrupts the NLS and thus, inhibits the interaction of Trn-1 and ADAR1. As ADAR1 is known to shuttle between the nucleus and cytoplasm (Strehblow, Hallegger, and Jantsch, 2002), the regulated interaction with Trn-1 would prevent dsRNA-bound ADAR1 from entering the nucleus, thus ensuring dsRNAs exported from the nucleus bound to ADAR1 remain in the cytoplasm.
The entanglement of dsRBDs used for dsRNA binding as well as protein-protein interactions has complicated our understanding of whether ADAR dimerization is RNA-dependent or independent. In studies using human ADARs, mutagenesis of the KKxxK motif within all dsRBDs of ADAR1 or ADAR2 resulted in lack of dsRNA binding but did not effect homodimerization, suggesting both human ADAR1 and ADAR2 homodimerize independent of dsRNA binding (Valente and Nishikura, 2007). In contrast, studies of Drosophila ADAR (dADAR) have indicated dsRNA-dependent homodimerization (Gallo et al., 2003). Alanine mutations in the dADAR dsRBD1, which correspond to an alanine in the second variable position of the KKxxK motif and the first amino acid of the di-alanines present in helix α2 (Figure 2), lack dsRNA binding and fail to interact with wildtype dADAR. Furthermore, these mutants do not edit, suggesting dsRBD1 is required for dsRNA-dependent dimerization and dADAR editing activity. A similar mutational analysis of the first amino acid of the di-alanines present in helix α2 within rat ADAR2 indicated that dsRBD1, but not dsRBD2, is important for homodimerization (Poulsen et al., 2006). In contrast, a recent crystal structure of an ADAR2 homodimer revealed that ADAR2 forms an asymmetric dimer on dsRNA and provided the first evidence of critical contacts between the deaminase domains of each ADAR2 monomer (Thuy-Boun et al., 2020). In particular a highly conserved TWDG motif was identified, and mutation of these residues reduced deaminase activity in vitro. It is important to note that the structures were obtained with truncated ADAR2 proteins lacking dsRBD1, and gel filtration studies indicated the ADAR2 dsRBD2-deaminase domain homodimers were not readily formed in the absence of dsRNA. Therefore, it may be possible that, dsRNA binding and dimerization happen simultaneously, and this cooperative mechanism could lead to enhanced editing at specific sites in vivo. This possibility is intruiging and could be addressed either with transcriptome-wide studies that determine the impacts of dimerization and RNA binding mutants on all editing sites and/or saturation mutagenesis studies of dsRNA subjected to in vitro editing assays with dimerization and RNA binding mutant ADAR proteins.
The recent ADAR2 dsRBD2-deaminase domain crystal structure also revealed important insights into substrate recognition by ADAR2 as the deaminase domain of one of the monomers was in contact with dsRNA as well as dsRBD2 of the other monomer (Thuy-Boun et al., 2020). The contributions from each monomer are consistent with earlier studies demonstrating that disruption of either dsRNA binding by dsRBDs or catalytic residues within the deaminase domain of one monomer led to decreased in vitro editing activity of ADAR2 (Valente and Nishikura, 2007). Interestingly, overexpression of both human ADAR1 and ADAR2 TWDG motif mutants in human cells led to reduced editing of some substrates, but not others (Thuy-Boun et al., 2020). These data suggest human ADAR homodimerization positions the dsRBD nearest the deaminase domain onto a specific region of dsRNA and provides a means to recognize and efficiently edit specific substrates and is consistent with several earlier studies of ADAR activity in vitro (Liu, Emeson, and Samuel, 1999; Liu and Samuel, 1999; Maas et al., 1996; Poulsen et al., 2006; Stephens, Haudenschild, and Beal, 2004). Expanding these studies in multiple organisms and over the transcriptome will be important for furthering our understanding of the complex role of the ADAR dsRBDs in dimerization, substrate recognition and editing.
How ADARs Determine which Adenosine to Edit
ADARs are selective in that specific adenosines within dsRNA are edited, while others are not edited. In part, ADAR selectivity is dependent on the length and structure of the dsRNA (Wahlstedt and Ohman, 2011). Incubating HeLa cell extracts with dsRNA led to the observations that shorter dsRNA (<50 bps) exhibited low levels of editing while longer dsRNA (>50 bps) were extensively edited (Nishikura et al., 1991). From in vitro mapping of editing sites within artificial dsRNA substrates less than 50 bps, it was observed that Xenopus ADAR1 modified a small number of adenosines, indicating high selectivity in short substrates (Polson and Bass, 1994). Together, these studies suggest ADARs edit dsRNA both selectively and promiscuously and the length of the dsRNA influences the extent of modification. However, it is important to note these early studies used dsRNA that is perfectly base-paired, which rarely occurs in cellular transcripts. In contrast to the in vitro studies, the extent of editing in vivo does not directly correlate to dsRNA length and editing within long double-stranded regions, such as UTRs, occurs at specific sites (Greenberger et al., 2010; Wheeler et al., 2015). Understanding how the selectivity and extent of editing occurs in vivo is critical for ADAR based therapeutics for personalized medicine (discussed in detail in “Harnessing ADAR editing for personalized medicine”).
Selective deamination of adenosines is facilitated by loops, bulges and mismatches in the dsRNA. The importance of imperfect base-pairing for selectivity was observed with in vitro editing assays using the dsRNA of GRIA2 and mutations that disrupted the natural structure (Ohman, Kallman, and Bass, 2000). The mutations did not affect the affinity of rat ADAR2 for dsRNA but allowed specific binding of ADAR2 to a region surrounding the editing site. Consistent with this, the presence of internal loops limits the extent of RNA editing by ADAR1 in vitro (Lehmann and Bass, 1999). Together, these studies suggest the dsRBDs are important for determining the location of ADAR binding on dsRNA, which is critical for selectivity.
Upon binding of ADAR dsRBDs to a specific dsRNA region, the deaminase domain catalyzes the conversion of adenosine to inosine. For deamination to occur, the target adenosine must flip out of the RNA helix (Stephens, Yi-Brunozzi, and Beal, 2000). A crystal structure of the human ADAR2 deaminase domain bound to dsRNA revealed three major contacts between the deaminase domain and dsRNA, including the minor groove containing the edited adenosine, a major groove site located 5’ to the edited adenosine and a major groove site 3’ to the edited adenosine (Matthews et al., 2016). The portion of the deaminase domain that contacts the dsRNA is referred to as the RNA binding loop, which is composed of seven residues that are highly conserved in ADAR2 homologs. In addition to the RNA binding loop, this structure identified several residues that interact with the nucleotides surrounding the target adenosine, including the orphaned base opposite of the flipped-out adenosine (Matthews et al., 2016). In particular, the ADAR2 E488 residue was observed to directly contact the orphaned base and stabilize the distorted RNA structure during deamination, providing additional evidence for how mutation of this residue results in hyperactive ADAR2 editing activity (Kuttan and Bass, 2012). The RNA binding loop also plays an important role in substrate selectivity. From in vitro deamination assays performed with ADAR1 mutants harboring portions of the ADAR2 deaminase domain, it was observed that a human ADAR1 protein with the 5’ portion of the ADAR2 RNA binding loop displayed the selectivity of ADAR2 (Wang, Park, and Beal, 2018). These findings suggest that how ADARs bind and edit specific targets are regulated by a number of factors both intrinsic to the deaminase domain and specific to the RNA target.
ADAR binding and editing influences the fate of cellular RNAs
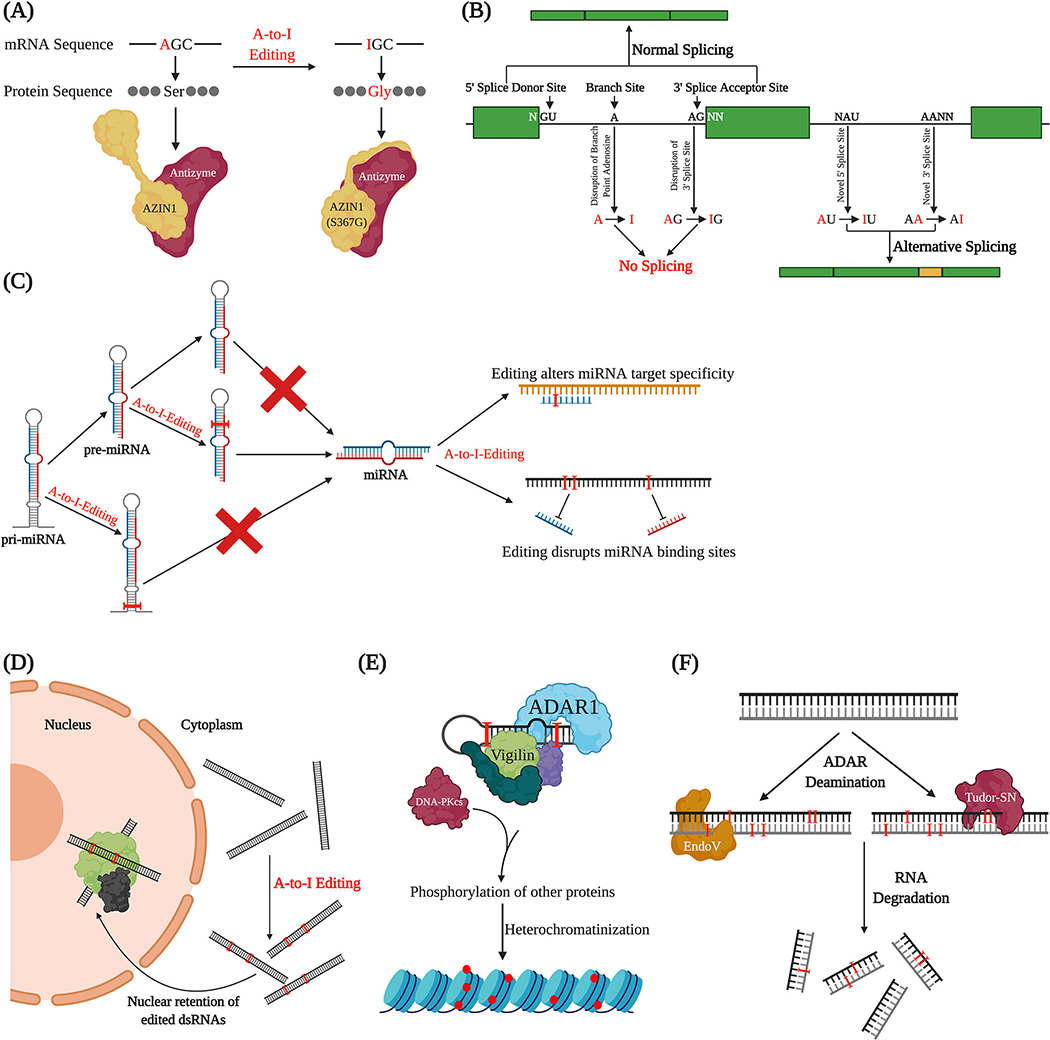
Both ADAR binding and editing can affect cellular dsRNA fate and, thereby, gene expression. A number of regulatory processes involving ADARs have been described, including recoding genetic information within the coding region of mRNAs, altering splice sites and exon inclusion, disrupting or creating miRNA binding sites, directing both transcriptional and translational silencing, and possibly marking transcripts for nuclear retention or degradation (Figure 3).
Figure 3. Influence of ADARs and editing on cellular fate of RNAs.
A) A-to-I editing within coding regions can lead to codon changes, resulting in altered protein sequence and, potentially, changes in protein structure and function. B) Editing can affect splicing by disrupting the 3’ splice site or the branch point adenosine, or by creating novel 5’ or 3’ splice sites. C) A-to-I editing can interfere with miRNA biogenesis and processing. Editing can also alter miRNA specificity and binding to the 3’ UTRs of target genes. D) ADARs may cause nuclear retention of target transcripts, either by binding transcripts and competing with RNA shuttling factors or by editing transcripts, allowing them to be targeted and bound by the nuclear p54nrb complex. E) Inosine-containing RNA can recruit the Vigilin complex, which promotes heterochromatic gene silencing. F) Certain endonucleases specifically target and cleave inosine-containing RNA, suggesting that editing may mark certain transcripts for degradation.
Nonsynonymous editing in coding regions
Because the translational machinery reads inosine as guanosine, A-to-I editing events within coding regions of mRNA can result in codon changes that ultimately affect the structure and function of the protein produced (Figure 3A). These editing changes, referred to as recoding editing events, allow for diversification of the proteome without requiring additional genes. Furthermore, since editing activity by ADARs is regulated (Deffit and Hundley, 2016), recoding can increase diversity with more control than gene mutation (Nishikura, 2010). While coding editing is abundant in cephalopods (Alon et al., 2015) and in Drosophila neural transcripts (Graveley et al., 2011; St Laurent et al., 2013), the majority of editing sites in mammals, flies and nematodes occur in non-coding regions of the transcriptome. However, while rare, human recoding events have been identified in neurotransmitter receptors and ion channels, and modification of at least one of these targets, the Q/R site in GRIA2, is essential (Bhalla et al., 2004; Burns et al., 1997; Higuchi et al., 2000; Hoopengardner et al., 2003; Ingleby et al., 2009). Interestingly, a recent study using an in vitro translation assay found that inosines in certain contexts are recognized as adenosine or, rarely, uracil by the translational machinery (Licht et al., 2019). This suggests that A-to-I recoding events and their consequences at the protein level may be more complex than originally thought.
ADAR-mediated reprogramming of alternative splicing and exonization
RNA editing is thought to occur co-transcriptionally with nearly 100,000 editing sites detected on nascent mouse transcripts (Licht et al., 2019) and over 95% of human mRNA editing sites occurring on chromatin-associated RNA prior to polyadenylation (Hsiao et al., 2018). As splicing and editing occur at a similar time in the lifetime of an RNA, there can be considerable crosstalk between these two processes (reviewed in (Shevchenko and Morris, 2018; Tajaddod, Jantsch, and Licht, 2016). Editing has the potential to create splice donors (GU) and destroy branch point adenosines, as well as both create and destroy splice acceptors (AG) (Figure 3B). One important example of how RNA editing affects splicing is with mammalian ADAR2 regulating its own activity by generating splice sites within ADAR2 transcripts to produce different isoforms (Rueter, Dawson, and Emeson, 1999). Another study identified an Alu-element-derived, primate-specific exon of human nuclear prelamin A recognition factor (NARF) which is exonized via RNA editing, by both the creation of a splice site and the alteration of splicing enhancers (Lev-Maor et al., 2007). Exonization levels of NARF in different tissues follow the pattern of RNA editing in those tissues, suggesting that RNA editing can regulate exon inclusion in a tissue-specific manner. This finding demonstrates a role for A-to-I editing not only in alternative splicing and gene expression, but also in the creation of new exons in the human genome.
The impacts of ADARs and RNA editing on splicing are not limited to these few examples. Transcriptome-wide sequencing analyses have identified abundant intronic editing in worms, flies, mice and humans (Deffit et al., 2017; Graveley et al., 2011; Licht et al., 2019; Picardi et al., 2015) and strong correlations between editing and alternative splicing (Kapoor et al., 2020; St Laurent et al., 2013). However, it is also important to note that in addition to editing of a nascent RNA directly influencing splicing, binding of ADARs to nascent RNA as well as ADAR regulation of splicing factor expression also both contribute to regulation of alternative splicing (Kapoor et al., 2020; Solomon et al., 2013).
Regulation of miRNA expression and binding
In addition to regulating processing of mRNA, RNA editing can also alter the biogenesis, target specificity and/or binding affinity of microRNAs (miRNAs) (Figure 3C). miRNAs are short, ~22 nucleotide long, endogenous small RNAs that post-transcriptionally regulate gene expression (Dexheimer and Cochella, 2020). Since miRNAs regulate gene expression by base-pairing to the 3’ UTRs of target genes and a large number of A-to-I editing sites occur in 3’ UTRs, it has long been speculated that editing could disrupt or even create miRNA binding sites. Additionally, miRNAs arise from dsRNA precursors, which could be edited to block processing and/or alter 3’ UTR pairing. While very few editing sites overlap with miRNA binding sites in human transcripts (Liang and Landweber, 2007), a small fraction of miRNAs are edited in at least one tissue in humans (Blow et al., 2006). In C. elegans, editing of miRNAs is also rare; however, about 40% of miRNAs exhibit altered levels in ADAR mutants (Warf et al., 2012). These differences also correspond to alterations in levels of mRNA targets of the aberrantly expressed miRNAs, suggesting that C. elegans ADARs impact gene regulation via miRNA abundance.
Although relatively uncommon, miRNA editing has been shown to have functional significance (also discussed in “Role of oncogenic A-to-I editing events on miRNA biogenesis and specificity”). Editing of one miRNA precursor, pri-miR-142, blocks processing by Drosha and results in degradation by Tudor-SN (Yang et al., 2006). Another miRNA precursor, pri-miR-151, requires editing to avoid cleavage by Dicer (Kawahara et al., 2007). Edited human miR-376 was found to target and silence a different set of genes than unedited miR-376 (Kawahara et al., 2007). Additionally, editing of miRNAs in the brain increases through mammalian development (Ekdahl et al., 2012). These examples implicate editing in the regulation of the miRNA-mediated gene silencing. However, how this regulation changes in development and in specific cells to influence gene expression remains largely unexplored.
Regulation of dsRNA localization
To prevent the translation of viral or otherwise unwanted dsRNA in the cytoplasm, cells use a variety of responses such as interferon activation or RNAi. Additionally, dsRNA can be retained in the nucleus, and some evidence suggests that ADARs may act in this retention. Recent reports suggest a role for mammalian ADAR1 in regulating nuclear transport by competing with the RNA shuttling factor STAU1 for binding to transcripts (Yang et al., 2017). Additionally, from HeLa cell nuclear extracts, Zhang and Carmichael isolated p54nrb, an abundant nuclear protein that specifically binds inosine-containing dsRNA (Zhang and Carmichael, 2001). A complex of p54nrb, PSF (a splicing factor), and matrin3 (a nuclear matrix structural protein) binds and attaches edited dsRNA to the inner nuclear matrix, effectively retaining the RNA in the nucleus (Figure 3D). Further study of this complex revealed its association with paraspeckles and the requirement of a long noncoding RNA, hNEAT1, for paraspeckle formation and retention of edited dsRNAs (Chen and Carmichael, 2009). Confirming the function of the p54nrb complex, Prasanth et al. identified CTN-RNA, a nuclear retained dsRNA in mice, which is edited and interacts with the p54nrb complex (Prasanth et al., 2005). CTN-RNA regulates expression of its protein-coding partner, mCAT2, again demonstrating that editing in noncoding regions can affect gene expression. Newer evidence, however, suggests that the edited mRNAs structure, rather than editing status, is responsible for nuclear retention (reviewed in (Chen and Yang, 2017).
While nuclear retention may occur in some edited transcripts or under certain conditions, it is not an absolute phenomenon, as many essential mRNAs are edited and complete retention would prevent translation of these critical proteins. Additionally, mRNAs with edited 3’ UTRs are found on translating ribosomes, stress granules and bound to other dsRBPs in the cytoplasm, confirming that nuclear retention is not a general mechanism for dealing with edited mRNAs (Capshew, Dusenbury, and Hundley, 2012; Elbarbary et al., 2013; Fitzpatrick and Huang, 2012; Hundley, Krauchuk, and Bass, 2008).
Transcriptional and translational silencing of gene expression
In both Drosophila and human cell lines, members of the Vigilin class of proteins bind tightly to inosine-containing RNA in vitro (Wang et al., 2005). Vigilin (DDP1 in Drosophila) proteins localize to heterochromatin and interact with ADAR1 as well as RNA helicase A and Ku86/70. The Vigilin complex when assembled on RNA, recruits DNA-PKcs, which phosphorylates a number of targets, including several proteins that promote gene silencing. In this way, edited dsRNA may direct heterochromatin formation and transcriptionally silence gene expression (Figure 3E). However, it is unknown what specific regions of DNA and in response to what cellular signals ADARs would be important for directing transcriptional gene silencing.
Editing by ADARs may also be involved in translational silencing. Under stress conditions, cells reprogram to synthesize proteins necessary for survival. Cytoplasmic Stress Granules (SGs) sequester and block translation of unnecessary mRNAs. SG formation is believed to be triggered by failed translation initiation (Wolozin and Ivanov, 2019). One study demonstrated that transfecting cells with inosine-containing dsRNA (I-dsRNA) can inhibit initiation of translation, and I-dsRNA is bound by a complex containing SG components (Scadden, 2007). This suggests that edited transcripts may trigger SG formation and thereby downregulate gene expression via translational silencing. However, to date, no endogenous RNAs that trigger translational silencing are known. A recent study has shown that inosines in transcripts cause ribosomal stalling, which may represent a previously unknown effect of editing on translation (Licht et al., 2019).
Targeted Cleavage of Edited Transcripts
Studies of CTN-RNA (described above) and others have suggested that edited transcripts may undergo targeted cleavage upon entry to the cytoplasm (Figure 3F). One study showed that hyper-edited dsRNA containing sites with alternating IU and UI base pairs are specifically cleaved by an endoribonuclease activity found in many cell extracts (Scadden and Smith, 2001). A following study showed that ADAR1, ADAR2, and dADAR preferentially generate this alternating IU and UI pattern when editing long dsRNAs (Scadden and O’Connell, 2005). Tudor staphylococcal nuclease (Tudor-SN), a subunit of the RNAi induced silencing complex (RISC), specifically cleaves synthetic RNAs containing IU and UI base pairs (Scadden, 2005). Another nuclease that targets edited RNAs is Endonuclease V (EndoV), which specifically binds and cleaves inosines in RNAs, including endogenous edited transcripts in human cells (Vik et al., 2013). However, a recent study using mice lacking EndoV showed little change in inosine levels compared to wildtype, suggesting that EndoV causes little, if any, degradation of transcripts in vivo (Kong et al., 2020). Rather, its RNA binding activity may be more functionally important, especially relating to the reduced tumorigenesis phenotype observed in EndoV−/− mice. In sum, while both the Tudor-SN and EndoV nucleases can target and degrade edited RNAs, it remains unclear whether these proteins significantly affect cellular editomes.
Of note, the examples above would suggest that editing by ADARs has an overall effect of downregulating gene expression. However, this is not the case. A study which analyzed the expression of ADARs compared with their editing targets in the human brain found a bimodal distribution between upregulated and downregulated genes (Liscovitch et al., 2014). The upregulated genes were enriched for genes involved in RNA processing and regulation of gene expression. While several connections between A-to-I editing and gene expression have been made, this study makes it clear that this regulation is complex and may involve several mechanisms working in concert to fine-tune expression of critical genes.
Harnessing ADAR editing for personalized medicine
The ability of ADARs to bind RNA and deaminate adenosines makes ADARs an attractive therapeutic means to correct specific genetic mutations (where a G has been mutated to an A in the DNA) at the RNA level (Montiel-Gonzalez, Diaz Quiroz, and Rosenthal, 2019). Targeting mRNA is beneficial to restore proper gene expression without modifying the genome, which can often have additional off-target mutations permanently installed in patient DNA. Herein, we will discuss the multiple approaches developed to use ADARs for personalized medicine. While we are focusing on mechanisms that promote RNA editing, it is also important to note that antisense oligoribonucleotides have been used to inhibit editing in cell culture (Mizrahi, Schirle, and Beal, 2013; Penn, Balik, and Greger, 2013) and could be of potential therapeutic use in diseases caused by hyperediting of a given transcript.
One method to promote RNA editing relies on an antisense RNA oligonucleotide to bind to the mRNA of interest and generate a dsRNA structure at the target site, which will be recognized and edited by endogenous ADARs. Studies performed over two decades ago used nuclear extracts containing ADARs to act on the dystrophin RNA, where an adenosine within a premature stop codon (UAG) gave rise to a shortened, non-functioning protein. Upon editing, the stop codon was converted into a tryptophan (UIG), resulting in translation of the full-length Dystrophin protein in vitro (Woolf, Chase, and Stinchcomb, 1995). A more recent approach used an antisense guide RNA that has an complementary region to bind the target mRNA as well as a stem-loop structure that mimicked part of the highly edited GRIA2 mRNA, the latter of which recruits ADAR2 (Wettengel et al., 2017). Interestingly, the dsRNA structure had three mismatches including the target adenosine, supporting the idea that mismatches promote specific editing by ADARs. This approach was also tested on a premature stop codon, but in an effort to repair the loss-of-function mutation in PINK1 (W437X), which is one of the causes of early Parkinson’s disease (Wettengel et al., 2017). This approach was further optimized and developed into RESTORE (recruiting endogenous ADAR to specific transcripts for oligonucleotide-mediated RNA editing), where the complementary region (also referred to as a specificity domain) was designed and tested on multiple transcripts. One of uses of RESTORE was for mutations within PiZZ (E342K) that lead to α1-antitrypsin deficiency, which were efficiently corrected by the recruited ADARs. In addition, the RESTORE method was used to edit phosphotyrosine 701 within the STAT1 transcript in primary and HeLa cells (Merkle et al., 2019).
A second major approach to perform site-directed RNA editing uses antisense RNA oligonucleotides as guide RNAs to recruit engineered ADARs to the target transcript. Here, the ADAR catalytic domain is engineered to specifically interact with a guide RNA that binds an endogenous target RNA and generates a dsRNA structure (Montiel-Gonzalez, Diaz Quiroz, and Rosenthal, 2019). One method fuses ADAR to a SNAP tag, which is an engineered O6- alkylguanine-DNA-alkyl transferase. The SNAP tagged protein selectively binds to 5’-O-benzylguanine (BG) and undergoes a covalent labeling reaction (Keppler et al., 2003). The SNAP-tag is fused to the N- or C-terminus of the ADAR1 deaminase domain, which allows a BG-attached guide RNA to selectively bind to the deaminase domain and form a covalent guide RNA-deaminase conjugate (Stafforst and Schneider, 2012). However, redirecting SNAP-tag ADARs has limited editing activity at some codon contexts such as 5’-XAG (X is U, A, C, G). An attempt to optimize the guide RNA showed a codon-specific effect to enhance editing when using 5′-mismatched neighboring base-pairs or a matching base-pair (Schneider et al., 2014).
A similar strategy to direct engineered ADARs uses the λN peptide that specifically binds BoxB sequences (~17 nt stem-loops) in RNA. The λN peptide is fused to the deaminase domain to direct ADAR2 to the BoxB sites, which are bound by a complementary guide RNA. This engineered enzyme and guide RNA can be genetically encoded from plasmids delivered by transfection in vivo (Montiel-Gonzalez et al., 2013). In an attempt to enhance efficiency, the E488Q mutation was introduced within the deaminase domain. Quantification of editing in vitro revealed that the λN-BoxB strategy in combination with the catalytic mutant increased the editing efficiency by approximately 50% (Montiel-Gonzalez, Vallecillo-Viejo, and Rosenthal, 2016). However, the E488Q mutation also increased off-target editing, and further refinement has been needed to reduce these unwanted effects. One way is nuclear localization of the editing enzyme by insertion of a nuclear localization sequence (NLS) to the enzyme (Vallecillo-Viejo et al., 2018). In another, an AAV (Adeno-associated virus) vector, which is commonly used for gene therapies, was engineered to express the human ADAR2 deaminase domain harboring an NLS and the hyperactive E488Q mutation. This approach targeted a G to A mutation that leads to a missense mutation in the Mecp2 transcription factor, which occurs in the neurological disorder, Rett syndrome. This strategy resulted in 72% editing of Mecp2 mRNA and partially restored the MECP2 protein in neurons from a Rett Syndrome mouse model (Sinnamon et al., 2017). Recently, the in vivo applicability of this approach was tested in a Rett Syndrome mouse model. The correction was mediated by hippocampal injection of an AAV vector expressing the ADAR2 deaminase domain with the E488Q mutation and a guide RNA to target the Mecp2 mRNA. This resulted in 50% editing across several neuronal populations as well as 50% restoration in MECP2 protein levels (Sinnamon et al., 2020). Another study using an AAV vector harboring ADAR2 and a GRIA2 guide RNA or a guide RNA with MS2 hairpins resulted ~40% of editing activity in human embryonic kidney 293T (HEK293T) cells. Excitingly, use of this approach in two mouse models of human disease demonstrated in vivo therapeutic applications of ADARs (Katrekar et al., 2019).
Overall, by using these site-directed RNA-editing in therapeutic approaches it is possible to alter the mRNA sequence of aberrantly encoded transcripts to improve gene expression. It is also important to note, as described above, improved gene expression can occur regardless of complete restoration of genomic sequence. Of utmost importance is determining the extent to which these alterations and improved gene expression positively impact disease physiology. Furthermore, these approaches should be verified for high specificity and efficiency before therapeutic use can occur in patients (Aquino-Jarquin, 2020). In addition, the effects of these therapies on endogenous ADAR function should be analyzed due to the well-known biological consequences caused by loss of ADARs, that we will discuss below.
Biological Consequences of Loss of ADARs
Loss of ADARs can cause defects ranging from mild behavioral phenotypes to severe, even lethal, effects in different organisms. ADAR mutant animals often have developmental and neural defects (Nishikura, 2010). In Drosophila melanogaster, loss of the single ADAR protein (dADAR) causes progressive neurodegeneration with aging but does not limit lifespan. The neurodegeneration in dADAR null flies causes behavioral defects such as uncoordination, tremors and excessive grooming, as well as male mating defects (Palladino et al., 2000). Recent evidence suggests the neurodegeneration and behavioral defects result from inadequate autophagy rather than from cell death, suggesting a role for dADAR in regulating the degradation and recycling of cellular components (Khan et al., 2020).
In the nematode Caenorhabditis elegans, mutants lacking both ADARs display defects in chemotaxis (Tonkin et al., 2002). The chemotaxis defects seen in adr-1(−);adr-2(−) worms are also seen in both adr-1(−) and adr-2(−) single mutants, though weaker in the adr-1(−) mutants (Ganem et al., 2019; Tonkin et al., 2002). Since both adr-2(−) and adr-1(−);adr-2(−) mutants lack editing, defects in both animals suggest the chemotaxis defects are editing dependent. While adr-1(−) mutants do not lack editing, the worms have altered editing, which could explain the milder defects. Recently, the chemotaxis defects of adr-2(−) worms were rescued by overexpressing one specific gene, clec-41, in neural cells (Deffit et al., 2017). In addition, the differential neural expression of clec-41 was shown to be dependent on ADR-1-regulated deamination.
ADAR mutant worms also have altered lifespans, with adr-1 single mutant and adr-1;adr-2 double mutant worms having a shortened lifespan compared to controls, and adr-2 mutant worms having an extended lifespan compared to wildtype worms (Ganem et al., 2019; Sebastiani et al., 2009). The shortened lifespan in adr-1;adr-2 double mutants can be rescued by inactivating rde-4, a dsRNA binding protein that is required for the RNAi pathway, suggesting antagonism between RNA editing and RNAi (Sebastiani et al., 2009). An interesting feature of C. elegans ADAR double mutants is the ability of transgenic sequences to trigger gene silencing via RNAi. This suggests that ADARs may protect dsRNA arising from convergent transcription of repetitive arrays from cleavage by Dicer and subsequent entry into the RNAi pathway (Knight and Bass, 2002). Triple mutant worms with knockouts of both ADARs as well as eri-6/7 or rrf-3 (genes involved in endogenous RNAi pathways in C. elegans) have a synthetic phenotype of lower brood size and adult bursting (Fischer and Ruvkun, 2020; Reich, Tyc, and Bass, 2018). Interactions between ADARs and the RNAi pathway will be discussed further in “Invertebrates are not immune to protection by ADARs.”
In mice, loss of ADARs results in more severe defects. Mice homozygous for an ADAR1 null allele are embryonic lethal, with disintegration of liver tissue and severe defects in hematopoiesis caused by death of hematopoietic stem cells (HSCs) (Hartner et al., 2004; Hartner et al., 2009; Wang et al., 2004). ADAR1 null mice showed an upregulation of interferon-inducible transcripts along with apoptosis in HSCs, suggesting that ADAR1 may regulate HSCs by suppressing aberrant interferon activation that can lead to cell death (Hartner et al., 2009) (see details in “A role for mammalian ADAR1 in regulating dsRNA triggered innate immunity”). It is important to note that these essential ADAR1 phenotypes also occur in mice specifically lacking the ADAR1 p150 isoform (Ward et al., 2011), suggesting that suppression of interferon activation is a function of cytoplasmic ADAR1. Mice homozygous for an ADAR2 null allele are prone to seizures and die almost immediately after birth (Higuchi et al., 2000). Reflecting the high expression of ADAR3 in the hippocampus, ADAR3-deficient mice show altered hippocampus-dependent behavior, including increased anxiety and decreased fear conditioning response (Mladenova et al., 2018). While the brain morphology appears normal, differential gene expression analysis of these ADAR3-deficient mice revealed a change in expression of genes involved in synaptic function in the hippocampus compared to controls, further suggesting an important role for ADAR3 in hippocampal function (Mladenova et al., 2018).
Since ADARs have separable RNA binding and catalysis functions, loss of ADARs can result in phenotypes either from lack of editing or from lack of some editing-independent function. By assessing these separately, we can better understand the function of editing and reveal the nature of the editing-independent functions of ADARs. In dADAR mutant flies, overexpression of an editing-deficient dADAR rescues neurodegeneration, suggesting this phenotype is caused by a lack of editing-independent functions of dADAR (Deng et al., 2020).
The separation of editing and RNA binding functions can also be easily delineated in C. elegans, as one ADAR protein, ADR-1, lacks deaminase activity. Two phenotypes, a low penetrance protruding vulva morphology and a synthetic “bag of worms” phenotype, occur in adr-1(−), but not adr-2(−) animals (Ganem et al., 2019; Tonkin et al., 2002). While the exact molecular cause underlying these phenotypes is unknown, the “bag of worms” phenotype was observed in adr-1(−) animals after additional loss of unc-22 expression (Ganem et al., 2019). As both adr-1 and unc-22 are expressed the vulva, and the bag of worms defect results from inability to lay eggs, it has been suggested that ADR-1 may play an important role in vulva function and/or development (Ganem et al., 2019; Tonkin et al., 2002). Furthermore, as ADR-1 binds to the unc-22 transcript but unc-22 is not edited, the associated bag of worms phenotype appears to result from the lack of a non-editing-related function of adr-1 (Ganem et al., 2019).
Mice expressing an editing-deficient ADAR1 exhibited embryonic lethality similar to ADAR1 null mice, albeit the lethality occurred one day later than ADAR1 or ADAR1 p150 null mice (Liddicoat et al., 2015). This data taken together indicate that there is an essential editing-dependent function of ADAR1 p150, which several studies have recently determined to be attenuation of the immunogenic potential of endogenous dsRNAs (discussed below in “A role for mammalian ADAR1 in regulating dsRNA triggered innate immunity”). However, it is also important to note that in addition to the essential editing-dependent function of ADAR1 p150, there are essential editing-independent functions of ADAR1 p110 in postnatal mammalian development, including organ development (Bajad et al., 2020; Pestal et al., 2015).
In mice, ADAR2 edits the Q/R site of GRIA2, an AMPA receptor gene. When ADAR2−/− mice are provided with exonically introduced “pre-edited” alleles of the GRIA2 transcript, the seizure and lethal phenotypes are rescued, implicating the Q/R site of GRIA2 as an essential ADAR2 substrate and editing site (Higuchi et al., 2000). Together, these studies indicate that RNA editing is essential for mammalian development. However, as the viability defects of both ADAR1 and ADAR2 mice can be rescued, a recent study sought to address the physiological effects of complete loss of a A-to-I editing activity in mammals (Chalk et al., 2019). Consistent with the essential editing dependent functions described above, when the editing-deficient ADAR1 mice rescued with loss of the MDA5 dsRNA sensing pathway were crossed to the GRIA2 rescued ADAR2-deficient mice, the resulting mice were viable (Chalk et al., 2019). These data indicate that the essential editing-dependent functions of ADAR1 and ADAR2 are non-redundant. In addition, though an extensive phenotypic analysis was not performed, the mice expressing editing-deficient ADAR1 (rescued with loss of the MDA5/MAVS dsRNA sensing pathway) and lacking ADAR2 (rescued by edited GRIA2) were reported to be phenotypically normal (Chalk et al., 2019). While these data suggest that editing of substrates (outside the rescued ones) are not important for mammalian homeostasis, it is possible that more subtle phenotypes may have gone unnoticed and/or that challenging mice to perform complex behaviors or with viral infection will reveal additional roles for RNA editing in mammals.
“Self Care” by ADARs
Recently, dysregulation of the innate immune response is emerging as a common phenotype observed in ADAR mutant animals. This is thought to be due to the fact that along with serving as a substrate for ADARs, dsRNA is a trigger of immune responses. Some RNA virus genomes are double-stranded, and the presence of these foreign molecules is sensed to fight infection. Similarly, during single-stranded RNA virus replication, dsRNA intermediates can form and trigger an immune response (Weber et al., 2006). Even in some DNA viruses, dsRNAs may accumulate as a result of overlapping convergent transcription (Jacobs and Langland, 1996). The immunity pathways that respond to dsRNA are interferon based in mammals and RNAi based in insects, nematodes, plants and fungi (Gammon and Mello, 2015; Gantier and Williams, 2007). Initiation of the responses to dsRNA occur in the cytoplasm of cells and involve various host proteins that sense viral infection (Akira, Uematsu, and Takeuchi, 2006). There are many different classes of sensor proteins. One such example is the retinoic acid-inducible gene I-like receptors (RLRs). RLRs are cytoplasmic sensors of the pathogen associated molecular patterns present within viral dsRNA. These RLRs are thus responsible for eliciting an intracellular immune response to control viral infection (Loo and Gale, 2011).
Although cytoplasmic dsRNA is a signature of viral infection, expression of endogenous genetic elements such as retrotransposons and even portions of cellular mRNAs, rRNAs and tRNAs can form dsRNA structures in cells. These endogenous dsRNA structures could also engage dsRNA sensors and activate an immune response, even in the absence of viral infection (Lamers, van den Hoogen, and Haagmans, 2019). Hence, it is crucial for the immune system to distinguish between self and non-self dsRNAs to prevent aberrant immune responses and the development of autoimmune disorders. Recent studies suggest that ADARs play an important role in regulating these responses, including ensuring that self dsRNAs are protected from initiating aberrant immune activity.
A role for mammalian ADAR1 in regulating dsRNA triggered innate immunity
Stimuli such as viral infections lead to interferon (IFN) signaling in mammals. Mammalian IFN signaling is critical for innate immune responses, and hence, the initial discovery of ADAR1 p150 as an IFN-stimulated gene (ISG) suggested that ADAR1 could play an important role in innate immunity (Patterson and Samuel, 1995). Recent studies of mice lacking ADAR1 further support the idea of ADAR1 having an innate immune function. When compared to control mice, a global upregulation of ISGs is observed in hematopoietic stem cells from the livers of ADAR1 knockout mice (Hartner et al., 2009). To determine if the ISG upregulation is caused by lack of ADAR1 editing, mice with an editing-deficient ADAR1 mutation (ADAR1E861A) were generated and analyzed (Liddicoat, Chalk, and Walkley, 2016). The mouse ADAR1E861A mutation is homologous to the human ADAR1E912A mutant allele and is located in the HAE motif of the ADAR1 deaminase domain (Lai, Drakas, and Nishikura, 1995). The glutamate (E) residue in the HAE motif is responsible for accepting a proton from nucleophilic water, to form a reactive hydroxide ion which attacks the sixth carbon of adenosine residues (Goodman, Macbeth, and Beal, 2012). The proton loss from the water molecule is a crucial step in the hydrolytic deamination reaction catalyzed by ADARs, and hence, the ADAR1E861A mutation lacks deaminase activity (Lai, Drakas, and Nishikura, 1995). While ADAR1 knockout mice are embryonic lethal, the ADAR1E861A mutant mice exhibit a delay in embryonic lethality, such that death occurs 1–1.5 days later than ADAR1 knockout mice (Liddicoat, Chalk, and Walkley, 2016). The ADAR1E861A mice are developmentally delayed as compared to wildtype mice and have a drastic upregulation in ISG expression. The ISG upregulation in both ADAR1 null and ADAR1E861A mice indicates that RNA editing by ADAR1 indeed plays a role in innate immune regulation.
The ADAR1 mouse studies indicate that ADAR1 is an essential suppressor of IFN signaling, and that the editing activity of ADAR1 is critical for this suppressor function. Additional genetic rescue experiments have also been performed to dissect the IFN suppressor function of ADAR1. ADAR1 knockout mice are rescued to live birth by additionally deleting a gene encoding the mitochondrial antiviral signaling protein (MAVS) (Mannion et al., 2014). MAVS is activated upon viral infection when sensor proteins, RIG-I and MDA5, recognize foreign molecules, including dsRNA (Seth et al., 2005). Upon MAVS activation, transcription factors such as IRF3 and NFkB promote transcription of cytokines and ISGs. The loss of MAVS rescuing the ADAR1 embryonic lethality suggests that ADAR1 prevents aberrant signaling through the MAVS axis (Figure 4). Consistent with this, deletion of MDA5 (encoded by the IFIH1 gene) also rescues the ADAR1 embryonic lethality and the ISG hyperactivation phenotype of ADAR1 knockout mice as well as ADAR1E861A mutants (Liddicoat et al., 2015). However, it is important to note that mice lacking ADAR1 and MDA5 only survive for a few days after birth (Pestal et al., 2015), while the mice that lack MDA5 and express ADAR1E861A exhibit a normal lifespan (Heraud-Farlow et al., 2017). These data suggest that the essential function of ADAR1 during mammalian development is suppression of aberrant MDA5/MAVS signaling. Furthermore, as mice specifically lacking ADAR1 p150 and MDA5 live until weaning (approximately one postnatal week) (Pestal et al., 2015), the essential immunosuppressive function of ADAR1 is presumably due to the action of cytoplasmic ADAR1 p150.
Figure 4. ADAR binding and editing of endogenous dsRNA prevents aberrant sensing of self dsRNA in nematodes, flies and mammals.
ADARs can have both binding and editing effects that prevent the sensing of endogenous dsRNA structures by the indicated sensor proteins. Loss of ADAR leads to upregulation of antiviral, innate immune and interferon stimulated genes.
From the observations above, it is apparent that ADAR1 acts antagonistically to the MAVS pathway. Studies with human embryonic kidney 293 (HEK293) cells also have shown ADAR1 as a specific negative regulator of the MDA5-MAVS pathway (Pestal et al., 2015). The MDA5-activated interferon response is enhanced in ADAR1-null HEK293T cells as compared to control cells (Pestal et al., 2015). This is consistent with the mouse studies and indicates that an ADAR1 deficiency can result in the unnecessary accumulation of dsRNA in human cells, which may lead to autoimmune diseases (discussed in detail below).
During an IFN response, ADAR1 also inhibits hyperactivation of the dsRNA sensor, protein kinase R (PKR) (Chung et al., 2018). Upon IFN treatment, ISG mRNA expression is similar in wildtype and ADAR1 knockout cells, but ISG protein expression is reduced in ADAR1 knockout cells (Chung et al., 2018). This observation suggests that ADAR1 can prevent translational shutdown in response to IFN. To directly address this possibility, global translational efficiency was examined by comparing the polysome profiles of wildtype and ADAR1 knockout cells. IFN treatment of ADAR1 knockout cells resulted in collapse of polysomes to individual ribosomes, which indicates translational shutdown. Following IFN treatment, along with defects in translation, cell growth defects were also observed in ADAR1 deficient cells. Together, these observations indicate that during an IFN response, ADAR1 also has a role in maintaining translation and cell growth. Editing deficient ADAR1 cells are unable to suppress PKR activation after IFN treatment, suggesting that ADAR1 editing is required to fully suppress PKR activation (Chung et al., 2018).
The prevailing model for how ADAR editing alters the innate immune pathways is that editing makes endogenous dsRNA structures less susceptible to recognition by dsRNA sensors (Figure 4). As inosine does not base-pair with uracil, ADAR editing makes completely base-paired dsRNA less double-stranded by forming IU mismatches. Consistent with this model, transfection of short dsRNA (~20 bps) containing four IU mismatches causes a decrease in ISG expression, as compared to HeLa cells transfected with a perfect Watson-Crick RNA duplex (Vitali and Scadden, 2010). Furthermore, the level of ISG suppression observed directly correlates with the number of IU mismatches present in the dsRNA. These data indicate that IU mismatches are required for dsRNA to efficiently suppress ISG induction, which suggests that the editing activity of ADARs could be important for suppressing immune responses.
In summary, it is apparent that ADAR1 has an innate immune function and that ADAR editing is important to distinguish between endogenous and non-self dsRNA. However, there are several unanswered questions. A major question is, what are the endogenous dsRNAs that when unedited, aberrantly activate the immune response? Long and perfect duplexes are the ideal candidates to evoke these immune responses. However, it was recently shown that perfect duplexes, such as inverted and tandem duplicated sequences, are depleted in mature mRNAs (Barak et al., 2020). However, inverted repeats of Alu elements can form dsRNA structures, and it is well established that Alu dsRNAs formed in cellular transcripts are ADAR substrates and MDA5 ligands (Ahmad et al., 2018; Athanasiadis, Rich, and Maas, 2004; Kim et al., 2004; Levanon et al., 2004). If Alu containing RNAs are the shared common ligand, how much editing is required to disrupt recognition of the inverted repeat by the dsRNA sensors? Editing levels are usually quite low within Alu inverted repeats, which raises the idea that editing cannot fully protect endogenous dsRNA structures from innate immune sensors (Barak et al., 2020). Finding the elusive endogenous RNAs that engage both ADARs and the innate immune sensors is critical goal for understanding the impact of loss of ADAR function on autoimmune disease.
ADARs and autoimmune disease
A-to-I editing is an RNA modification that requires proper balance. Both insufficient as well as excessive amounts of editing can have detrimental effects on organisms. As mammalian ADAR1 plays a role in regulating innate immunity, it is not surprising that mutations in ADAR1 are present in human autoimmune disorders. Specifically, mutations in ADAR1 have been associated with Aicardi Goutières syndrome (AGS) (Table 1) (reviewed in (Slotkin and Nishikura, 2013). In addition, decreases and increases in RNA editing are implicated in the autoimmune disorders, psoriasis and systemic lupus erythematosus (SLE), respectively (Hung et al., 2015; Shallev et al., 2018). Herein, we will provide details about the molecular function of RNA editing in each of these disorders.
Table 1. Consequences of mutations that occur in AGS patients on ADAR1 functions.
The first column lists the mutations with normal amino acid followed by the amino acid location in the human ADAR1 p150 isoform followed by the mutant amino acid. The domain location as well as the type of inheritance are indicated. Known effects of the mutations on RNA editing and possible ADAR1 functions are provided (Mannion et al., 2014; Rice et al., 2012).
| Identified variants of human ADAR1 that occur in AGS patients | ||||
|---|---|---|---|---|
| Mutation | Location | Disease Description | Effect on editing | Predicted effect on molecular function |
| A870T | – |  |
No significant reduction | Protein destabilization |
| I872T | – | Compound heterozygote with P193A mutation | No significant reduction | Protein destabilization |
| R892H | Deaminase |  |
No significant reduction | Disruption of protein interactions with dsRNA |
| K999N | Deaminase (RNA binding loop) | Autosomal recessive | No significant reduction | – |
| G1007R | Deaminase (RNA binding loop) | Autosomal dominant | Significant reduction | Potential competitive inhibitor of WT ADAR1 protein |
| Y1112H | Deaminase | Autosomal recessive | Not tested | – |
| D1113H | Deaminase | Autosomal recessive | No significant reduction | – |
SLE is a multisystemic autoimmune disease which is characterized by an aberrant loss of tolerance for self-antigens, IFN activation and tissue destruction (Moulton et al., 2017). SLE patients have abnormally increased levels of RNA editing, particularly within Alu repeats, the main ADAR1 substrates (Roth et al., 2018). It has also been proposed that the increased RNA editing in SLE patients can result in editing of self-antigens, thereby leading to the loss of self-tolerance phenotype observed in SLE patients. In addition to hyperediting, expression of ADAR1 is significantly increased in some, but not all, SLE patients (Quinones-Valdez et al., 2019; Roth et al., 2018). Loss of the editing repressor, Ro60 (TROVE2), was recently proposed to provide a secondary mechanism for the increased editing in SLE patients (Quinones-Valdez et al., 2019). Ro60 binds to Alu elements, and loss of Ro60 leads to increased editing within Alu elements (Hung et al., 2015; Quinones-Valdez et al., 2019). It is possible that both loss of Ro60 and increased ADAR1 expression contribute to the aberrant editing observed in SLE patients. However, whether the increased editing events are involved in the pathogenesis of SLE and could be potential therapeutic targets remains to be explored.
In contrast to SLE, decreased global editing is observed in patients suffering from psoriasis. Psoriasis is a chronic autoimmune disease that is characterized by redness of skin and scaly plaques (Di Meglio, Villanova, and Nestle, 2014). There is evidence which suggests that type I IFNs play a role in the initiation of psoriasis (Shallev et al., 2018). Psoriatic lesions have decreased global editing, and presumed, increased cellular dsRNA structures. From what has been discussed about the effects of global editing so far, one can expect an increase in innate immune responses in the lesions. Consistent with this expectation, psoriatic lesions have an overactivation of MDA5 pathway as well as elevated ISG expression.
Another inherited skin disease, dyschromatosis symmetrica hereditaria (DSH) is known to be caused by mutations in ADAR1. DSH is a rare autosomal dominant disorder, characterized by skin pigmentation defects on the hands and face (Hayashi and Suzuki, 2013). Most DSH cases have been reported in East Asian countries, and studies of these patients have revealed a broad spectrum of ADAR1 mutations, including single nucleotide deletions, insertions and mutations (Kono et al., 2014; Lai et al., 2012; Liu et al., 2006; Liu et al., 2014; Tang et al., 2018; Wang et al., 2019; Xing et al., 2005; Zhang et al., 2004). Of the over 200 identified ADAR1 mutations that occur in DSH patients, most are localized to the deaminase domain, but some mutations are also found in the dsRBDs (Wang et al., 2019) To date, RNA editing levels in DSH patients have not been examined. While the exact defect in RNA editing and/or RNA binding by ADAR1 in DSH patients is unknown, the severity of skin pigmentation defects in DSH1 patients has been linked to in utero viral infection and IFN induction (Kondo et al., 2008). Interestingly, a recent study demonstrated that mice lacking ADAR1 in neural crest cells exhibited depigmentation due to high levels of melanocyte apoptosis (Gacem et al., 2020). Loss of MDA5 rescued these defects, suggesting that ADAR1 functions to suppress ISGs and promote melanocyte cell survival. It will be interesting to examine whether the ADAR1 mutations in DSH also have similar effects on melanocyte and ISG levels.
Another ADAR1-linked genetically determined inflammatory disorder that affects the brain and skin is Aicardi Goutières syndrome (AGS). As a neurodevelopmental autoimmune disorder, AGS is characterized by severities such as cerebral atrophy, intracranial calcifications and chronic cerebrospinal fluid lymphocytosis (Rice et al., 2012). AGS symptoms mimic those of in-utero acquired infections, including an increased production of interferons. Genes such as TREX1, RNASEH2A/B/C, SAMHDI, IFIH1 (MDA5) and ADAR1 are all involved in nucleic acid metabolism/signaling, and mutations in these genes are associated with AGS (Livingston and Crow, 2016). Individuals with an ADAR1 deficiency are also prone to developing other severe conditions such as bilateral striatal necrosis, childhood-onset multivalvular stenosis or even fatal cardiac failure due to increased interferon signaling (Crow et al., 2019). In whole blood samples from AGS patients with ADAR1 mutations, the mRNA levels of ISGs were upregulated compared to control samples (Livingston et al., 2014; Rice et al., 2012). The ISG upregulation suggests a crucial role of ADAR1 in preventing the development of AGS.
Even though ADAR1 deficiency is implicated in AGS, the exact molecular function of ADAR1 required to prevent the AGS development is still unknown. Out of the eight AGS missense mutations identified in the human ADAR1 gene (Table 1), five map to the deaminase domain. The high number of AGS mutations in the ADAR1 deaminase domain suggests that editing activity is important to prevent the development of AGS. To understand the molecular defects of ADAR1 in AGS patients, editing assays were performed in HEK293 cells transfected with wildtype ADAR1 or the individual AGS mutations. The ADAR1 proteins were expressed as ADAR1 p110 variants and co-transfected with a plasmid expressing miR376-a2, a known ADAR1 substrate (Rice et al., 2012). As HEK293 cells express endogenous ADAR1, effects on editing were compared to transfection of the known ADAR1 deaminase-deficient mutant, ADAR1E912A. From this assay, only ADAR1G1007R showed a significant reduction in editing activity (Rice et al., 2012). Interestingly, this mutant is the only AGS mutant that appears in patients as a heterozygote. Furthermore, it is possible that the effect of ADAR1G1007R on editing observed in this assay could be due to inhibition of the wildtype ADAR1 present. Consistent with this, a competition assay where both wildtype ADAR1 and ADAR1G1007R were co-transfected revealed a dominant negative effect of ADAR1G1007R on editing (Rice et al., 2012).
The lack of observed effects of AGS mutations on RNA editing was surprising. However, a follow-up study analyzed these same mutations expressed as ADAR1 p150 variants and found significant effects on editing (Mannion et al., 2014). Specifically, the authors found that the A870T, R892H and K999N mutations had significantly reduced editing when expressed as ADAR1 p150 proteins. Interestingly, two of these mutations, A870T and R892H, were only observed in AGS patients that also exhibited a P193A mutation, which is specific to the ADAR1 p150 isoform (Rice et al., 2012). Despite being in the Z-α domain of ADAR1, the P193A mutation reduces editing by ADAR1 p150 (Mannion et al., 2014). As noted by others, clinical observations suggest that AGS symptoms develop after a viral infection, which should result in the expression of these ADAR1 p150 variants. However, it is important to note that not all AGS mutations in ADAR1 significantly affect editing, and some AGS mutants have not yet been examined for effects on editing (Table 1). Therefore, it is possible that editing-independent functions of ADAR1 are also altered in AGS patients. Consistent with this idea, at least one group has demonstrated that RNA binding by ADAR1 is also important for suppressing IFN signaling (Yang et al., 2014). Future studies aimed at identifying RNAs specifically bound and/or edited by ADAR1 p150 will be critical for understanding the molecular defects underlying AGS symptoms in patients with ADAR1 mutations.
As discussed above, editing of endogenous dsRNAs is suggested to prevent the aberrant binding of self dsRNA to sensors such as MDA5. It is possible that a reduction in editing leads to the accumulation of endogenous dsRNA, which is sensed by MDA5 (Liddicoat et al., 2015). Interestingly, AGS patients can also have mutations in MDA5; however, mutations in ADAR1 and MDA5 occur in AGS patients in a mutually exclusive manner (Rice et al., 2014). AGS mutations in MDA5 result in a stronger affinity for dsRNA, which can result in the aberrant detection of self dsRNA. In addition, unlike wildtype MDA5, the AGS mutations in MDA5 are gain-of-function, in that the mutant MDA5 proteins tolerate editing in Alu repeats as evidenced by similar binding to modified as well as unmodified dsRNA (Ahmad et al., 2018). Together, these data suggest that unmodified dsRNAs can aberrantly activate wildtype MDA5 in the absence of ADAR1, leading to the development of AGS.
It is evident from the observations so far that altered ADAR1 activity occurs in autoimmune disease. Even though an upregulation of ISGs is a recurring phenotype observed, it is unclear if ADAR1 edits specific transcripts that might be crucial in IFN regulation. Future studies need to focus on identifying these transcripts. Furthermore, the sustained immune activation that occurs in autoimmune disease and promotes ADAR1 expression may led to aberrant hyperediting of ADAR1 targets not involved in IFN regulation. It is also possible that hypoediting of certain transcripts could occur in these conditions if the excessive ADAR1 expression competitively inhibited ADAR2, as has been observed in glioblastoma (Cenci et al., 2008). Understanding the mechanism of ADAR1 function in innate immunity will help deduce the cause of the aberrant phenotypes in the above-mentioned diseases, thereby opening doors for therapeutic interventions.
Invertebrates are not immune to protection by ADARs
Instead of an IFN based immune system, invertebrates use an RNAi based antiviral response against dsRNA. Viral dsRNA is cleaved into small interfering RNAs (siRNAs) by the endonuclease, Dicer (Gammon and Mello, 2015). After cleavage, siRNAs are loaded onto RISC, which uses the siRNA as a template to bind and degrade viral RNAs.
In C. elegans, the dsRBPs RDE-1 and RDE-4, as well as the nuclease, Dicer, play critical roles in the RNAi pathway (Parrish and Fire, 2001; Tabara et al., 2002). In addition, to silencing exogenous dsRNA, the RNAi pathway can also affect expression of transgenes in C. elegans (Knight and Bass, 2002). Transgene silencing is thought to occur when dsRNA arises from convergent transcription of genes that are repeated in an extrachromosomal array, which is formed when recombinant DNA is artificially introduced into C. elegans. An increase in transgene silencing is observed in adr-1(−);adr-2(−) mutant worms as well as in adr-1(−) and adr-2(−) single mutant animals (Knight and Bass, 2002). To understand the intersection of ADARs and RNAi in dsRNA silencing, mutations in rde-1 were introduced into the adr mutant worms and reduced levels of dsRNA silencing were observed (Knight and Bass, 2002). Together, these data suggest that dsRNA generated in vivo is used by the RNAi pathway to silence gene expression but editing of the dsRNA can prevent silencing.
By analogy to vertebrates, the above study suggests that ADARs function to mark transgenically expressed dsRNA as self. Importantly, recent studies suggests this function extends to endogenous dsRNA (Fischer and Ruvkun, 2020; Reich, Tyc, and Bass, 2018). In both of these studies, loss of genes involved in endogenous siRNA (endo-siRNA) pathways in animals that also lack adr-1 and adr-2 resulted in a synthetic phenotype of frequent adult bursting and decreased brood sizes. The triple mutant synthetic phenotype is rescued by deletions in RNAi factors, including rde-1 and rde-4, as well as loss of drh-1, which is the C. elegans ortholog of the sensor protein RIG-1 (Figure 4). Together, these studies indicate that ADARs and endo-siRNA-mediated gene silencing pathways function in parallel to prevent a DRH-1/RIG-I based response triggered against genomically encoded dsRNA.
Interestingly, the triple mutant worms lacking endo-siRNA pathways and adrs also exhibit elevated expression of antiviral genes (Fischer and Ruvkun, 2020; Reich, Tyc, and Bass, 2018). In addition, transcriptome analysis of the triple mutants that lack adr-1(−), adr-2(−) and eri-6/7(−) revealed an increased expression of LTR retrotransposons, suggesting the ERI-6/7 helicase and ADARs work together to silence these recently integrated viral genes (Fischer and Ruvkun, 2020). However, as the loss of drh-1 does not rescue the elevated retrotransposon expression and retrotransposons lack dsRNA structure, the exact molecular mechanism of how ADARs and ERI-6/7 silence these genes is unknown, but RNAi independent. Interestingly, the increased retrotransposon expression is accompanied by an elevated unfolded protein response, which is a common antiviral response (Fischer and Ruvkun, 2020). It will be important in future studies to dissect the gene regulatory pathway leading to increased retrotransposon expression in C. elegans as well whether this pathway functions in specific tissues to affect organismal health.
ADARs are suspected to have a function in innate immunity in Drosophila as well. The Drosophila Adar gene (dADAR) was originally shown to be homologous to mammalian ADAR2 (Keegan et al., 2011). However, recent studies suggest dADAR may also perform functions similar to mammalian ADAR1 (Deng et al., 2020). Animals expressing a dAdarE374A mutant (homologous to the ADAR1E861A mice mentioned previously) were generated using genome engineering. Isolation of RNA from the heads of these mutant flies, wildtype flies and flies lacking dADAR expression (dADAR(−)) was subjected to high-throughput sequencing. Compared to wildtype, both dADAR(−) and dAdarE374A mutants exhibit increased expression of immune-related genes. These data suggest that RNA editing is important for suppressing expression of innate immune genes in the head tissues of Drosophila. However, it is important to note that the level of induction in heads of dAdarE374A mutant flies was only 20–50% of the level of induction in the dAdar(−) flies. Interestingly, the level of immune gene induction is similar in dAdar(−) and dAdarE374A animals when examining RNA isolated from whole flies, suggesting RNA editing is less important in non-head tissues. Consistent with this, ubiquitous overexpression of dAdarE374A is as efficient as wildtype dADAR at suppressing immune gene induction in whole flies.
The ability of dADAR to regulate innate immune genes in both neural and non-neural tissue is regulated by the Dicer-2 pathway (Figure 4). In Drosophila, distinct immune gene transcripts are induced by different pathogens through defined signaling pathways. In particular Dicer-2 mediated signaling, is analogous to the MDA-5 pathway in mammals (Figure 4). Specific loss of Dicer-2 from cholinergic neurons reduced the increased immune gene expression of both dAdar(−) and dAdarE374A animals (Deng et al., 2020). A synthetic induction of immune gene expression occurred when animals lacked dADAR and expressed a Dicer-2 mutant (Dicer-2R416X) that cannot cleave dsRNA but can still bind dsRNA and signal. Interestingly, this synthetic induction does not occur in dAdarE374A flies, further suggesting that the immune induction in whole flies is dependent on dADAR RNA binding and not RNA editing. Together, these results are intriguing and suggest that proper RNA editing in Drosophila brain tissues is critical for controlling innate immunity, which correlates with the fact that mutations in human ADAR1 are prevalent in the neurodevelopmental autoimmune disease, AGS. Future work studying gene expression and signaling pathways in specific tissues of multicellular organisms will be important in providing key molecular insights into not only the role of ADARs in regulating innate immunity, but also the tissue-specific responses to dsRNA.
Overall, it is clear that ADARs have an immune function, which includes both binding and editing of self dsRNA to help distinguish these molecules from foreign dsRNA. It is also established that ADARs contribute to the regulation of balance between self-tolerance and immune activity, which is necessary to prevent development of autoimmune diseases in humans. To date, there are eight mutations in human ADAR1 observed in the autoimmune disease, AGS (Table 1). As the vast majority of mutations occur within the deaminase domain, focus has been on understanding the impact of these mutations on RNA editing. However, the deaminase domain also contains residues that directly contact dsRNA (see previous discussion of RNA binding loop), and there are two ADAR1 mutations that occur in AGS and are located within this loop (Table 1). Of the seven human ADAR1 deaminase domain mutations that occur in AGS, two residues are identical to those found in C. elegans and Drosophila ADAR enzymes and three additional residues are similar across all three ADARs (Figure 5). It will be important in future studies to determine the impact of these conserved residues on both RNA editing and ADAR binding to target RNA in vivo in both mammalian and invertebrate systems.
Figure 5. Conservation of the deaminase domain sequences between human ADAR1, Drosophila ADAR and C. elegans ADR-2.
Sequences of the deaminase domains for the indicated proteins were obtained from UniProt database using the accession numbers described for Figure 1. Sequences that are identical are shown in a red-filled box, while those meet consensus (> 70%, <100%) are boxed with consensus residues in red font. Variants in human ADAR1 p150 deaminase domain known to cause AGS (Rice et al., 2012) are indicated using symbols: A870T (#), I872T (*), R892H (!), K999N (^), G1007R(&), Y1112H (+) and D1113H ($). Please refer to Table 1 for detailed information about the consequences of these mutations on ADAR1 function.
Malignancy and dysregulated A-to-I editing
Just as an imbalance in RNA editing leads to autoimmune disorders, altered ADAR expression and activity also participates in tumor progression. For decades, cancer research has been geared towards identifying and targeting somatic mutations and epigenetic alterations that drive tumor progression. However, the notion that RNA modifications and, more specifically, A-to-I editing, can influence carcinogenesis has recently been brought to light (Fritzell et al., 2018; Ganem, Ben-Asher, and Lamm, 2017; Jain, Jantsch, and Licht, 2019). As A-to-I editing can rewire genetically encoded information, cancer-specific editing events provide malignant cells with an advantage over their normal counterparts. Moreover, owing to the ability of ADARs to respond to environmental cues, this kind of dynamic signaling is ideal for the ever-changing tumor microenvironment. Several transcriptomic analyses have revealed that RNA editing is dysregulated in a wide variety of cancers (Chan et al., 2014; Han et al., 2015; Paz et al., 2007; Paz-Yaacov et al., 2015). Breast, bladder, colon, head & neck and thyroid cancers are associated with hyperedited RNA (Han et al., 2015; Paz-Yaacov et al., 2015) whereas hypoediting is observed in brain, prostate and testicular tumors (Paz et al., 2007). A-to-I editing levels can also vary amongst different subtypes of a particular cancer. A classic example includes kidney cancer, wherein RNA from kidney chromophobe (KICH) and kidney renal papillary cell carcinoma (KIRP) subtypes are hypoedited and RNA from kidney renal clear cell carcinoma (KIRC) is hyperedited (Han et al., 2015) compared to normal kidney tissue.
Even though most conclusions from these independent studies are in agreement, contradictory results regarding global editing trends have been reported for lung, liver and kidney cancer (Chan et al., 2014; Han et al., 2015; Paz et al., 2007; Paz-Yaacov et al., 2015). The observed contradictions might be attributed to differences in tumor type or stage, patient gender or ethnicity, sample size or preparation and read coverage between different datasets. Ergo, if the underlying variables are taken into consideration, systemic studies are beneficial in providing a bird’s eye-view to observe tumor-associated alterations in editomes.
Specific editing events that regulate carcinogenesis
Depending on where an edited adenosine lies within the RNA, editing events have the ability to affect gene and/or protein expression (Figure 3). Recoding editing events are known to promote tumorigenesis by inactivating tumor suppressor genes, activating oncogenes and generating novel oncoproteins (Chen et al., 2013; Chen et al., 2017; Fu et al., 2017; Galeano et al., 2013; Gumireddy et al., 2016; Han et al., 2015; Peng et al., 2018). Similarly, intronic editing can affect alternative splicing and introduce novel splice sites that generate tumor-specific isoforms or disrupt efficient splicing of tumor suppressors (Penn, Balik, and Greger, 2013; Schoft, Schopoff, and Jantsch, 2007). A-to-I changes within 3’ UTRs of transcripts can lead to differential expression of oncogenes or tumor suppressors owing to altered miRNA recognition and binding (Pinto et al., 2018; Wang et al., 2013; Xu et al., 2019). This section focuses on different types of editing events that have a role in regulating tumor progression. Keeping brevity in mind, a few representative examples of each category have been discussed. For a more complete list of editing events that lead to cancer-specific protein recoding and miRNA biogenesis, tables that summarize these events are provided (Tables 2 and 3, respectively).
Table 2. Summary of recoding editing events previously shown to regulate tumor progression in different tissues.
Recoding events are classified into tumor promoting or inhibiting. Additional provided details include cancer type, ADAR enzyme responsible for editing and oncogenic mechanism (where known).
| Editing promotes cancer growth and progression | ||||||
|---|---|---|---|---|---|---|
| Gene | Protein | Editing Site | ADAR | Mechanism | Cancer | Reference |
| AZIN1 | Antizyme Inhibitor 1 | S367G | ADAR1 | –Edited AZIN1 binds to Antizyme 1 with a stronger affinity and inhibits degradation of oncoproteins –Editing of AZIN1 is probably also associated with cytoplasmic-to-nuclear translocation |
HCC, CRC, ESCC, NSCLC | (Chen et al. 2013; Qin et al. 2014; Hu et al. 2017; Shigeyasu et al. 2018) |
| BLCAP | Bladder Cancer Associated Protein | Y2C | ADAR1 | –Edited BLCAP increases phosphorylation and activation of Akt/MDM2/mTOR and inhibits p53 phosphorylation | HCC | (Galeano et al. 2010; Hu et al. 2015) |
| Y2C; Q5R; K15R | ADAR1 & ADAR2 | –Edited BLCAP prevents inhibition of STAT phosphorylation, promoting IL6-mediated JAK-STAT signaling | CC | (Galeano et al. 2010; Chen W et al. 2017) | ||
| COG3 | Component of Oligomeric Golgi Complex 3 | I635V | ADAR2 | N.D. | GBM, LGG, BRCA, HNSC, KIRC, KIRP, LUAD, LUSC, STAD, THCA, SARC, SKCM | (Han L et al. 2015; Peng et al. 2018) |
| COPA* | COPI Coat Complex Subunit Alpha | I164V | ADAR2 | –In HCC, unedited COPA promotes tumor cell proliferation and clonogenicity. –Upon editing, COPA serves as tumor suppressor and downregulates CAV1-mediated PI3K/AKT/mTOR pathway |
SARC, SKCM, LGG, BRCA, GBM, HNSC, KIRC, LUAD, LUSC, STAD, ESCA, HCC | (Chan et al. 2014; Han L et al. 2015; Peng et al. 2018; Song et al. 2020) |
| FLNB | Filamin B | M2269V | ADAR1 & ADAR2 | N.D. | HCC, ESCC | (Chan et al. 2014; Qin et al. 2014) |
| Q2327R | ADAR2 | PCPG, KIRC, LGG, SKCM, SARC | (Peng et al. 2018) | |||
| GLI1 | Glioma-Associated Oncogene 1 | R701G | ADAR1 | –Editing disrupts SUFU (Suppressor of Fused Homolog)-mediated inhibition of GLI1 activity/hedgehog signaling | MM | (Lazzari et al. 2017) |
| NEIL1 | Nei Like DNA Glycosylase 1 | K242R | ADAR1 | –Edited NEIL1 has impaired oxidative damage and single-strand break (SSB) DNA repair abilities, which predisposes cells to double-strand breaks (DSBs) and increased sensitivity towards DSB inducing agents | MM | (Teoh et al. 2018) |
| RhoQ | Ras homologue family member Q | N136S | N.D. | –Editing increases RhoQ activity –Editing promotes actin cytoskeletal remodeling and tumor invasion |
CRC | (Han SW et al. 2014) |
| SLC22A3 | Solute Carrier Family 22 Member 3 | N72D | ADAR2 | –SLC22A3 inhibits cytoskeletal remodeling by sequestering ACTN4 to the cell membrane Edited SLC22A3 relieves ACTN4 suppression and promotes tumor metastasis |
ESCC | (Fu et al. 2017) |
| Editing promotes cancer growth and progression | ||||||
| Gene | Protein | Editing Site | ADAR | Function | Cancer | Reference |
| CCN1 | Cyclin I | R75G | ADAR1 | –Peptide derived from edited CCN1 is presented by MHC molecules on tumor cell surfaces and elicits antitumorigenic immune responses | Melanoma | (Zhang et al. 2018) |
| GABRA3 | Gamma-Aminobutyric Acid Type A Receptor Subunit Alpha 3 | I342M | ADAR1 | –Edited GABRA3 promotes tumor suppression by acting in a dominant negative manner to reduce cell surface expression of unedited GABRA3 and inhibition of Akt phosphorylation | BC | (Gumireddy et al. 2016) |
| GLI1 | Glioma-Associated Oncogene 1 | R701G | ADAR1 & ADAR2 | –Editing prevents SUFU-mediated inhibition of GLI1 and reduces GLI1 activation by Dyrk1a kinase | MB, BCC | (Shimokawa et al. 2013) |
| GRIA2 | Glutamate Ionotropic Receptor AMPA Type Subunit 2 | Q607R | ADAR2 | –Edited GRIA2 inhibits Ca2+ influx mediated Akt Phosphorylation | Gliomas | (Maas et al. 2001; Cenci et al. 2008) |
| IGFBP7 | Insulin Like Growth Factor Binding Protein 7 | K95R | ADAR2 | –Editing protects IGFBP7 from matriptase-mediated proteolysis thereby promoting apoptosis | ESCC | (Chen YB et al. 2017) |
| PODXL** | Podocalyxin Like | H241R | ADAR2 | N.D. | GC | (Chan et al. 2016) |
COPA under-edited in Hepatocellular and Gastric Carcinoma (Chan et al., 2014; Chan et al., 2016)
ADAR1 edits PODXL resulting in a synonymous T238T mutation. However, both ADAR1 and ADAR2 cannot edit PODXL simultaneously. As a result, this synonymous editing event is capable of inhibiting ADAR2-mediated non-synonymous PODXL editing.
Abbreviations Used: Not determined (N.D.), Breast Cancer (BC), Basal Cell Carcinoma (BCC), Breast Invasive Carcinoma (BRCA), Cervical Cancer (CC), Colorectal Cancer (CRC), Esophageal Carcinoma (ESCA), Esophageal Squamous Cell Carcinoma (ESCC), Gastric Cancer (GC), Glioblastoma (GBM), Hepatocellular Cancer (HCC), Head And Neck Squamous Cell Carcinoma (HNSC), Kidney Renal Clear Cell Carcinoma (KIRC), Kidney Renal Papillary Cell Carcinoma (KIRP), Lower Grade Glioma (LGG), Lung Adenocarcinoma (LUAD), Lung Squamous Cell Carcinoma (LUSC), Medulloblastoma (MB), Multiple Myeloma (MM), Non-Small Cell Lung Carcinoma (NSCLC), Pheochromocytoma and Paraganglioma (PCPG), Sarcoma (SARC), Skin Cutaneous Melanoma (SKCM), Stomach Adenocarcinoma (STAD), Thyroid Carcinoma (THCA)
Table 3. Summary of cancer-associated editing events regulating miRNA biogenesis and activity.
Editing events have been grouped into three categories based on whether they affect miRNA target specificity, binding or biogenesis and processing. Additional details including the type of cancer the editing event is found in, ADAR enzyme responsible, targets identified and associated mechanism (where known) has been provided along with respective references.
| Editing alters miRNA target specificity | ||||||
|---|---|---|---|---|---|---|
| miRNA | ADAR | Target(s) | Function | Cancer | Reference | |
| Unedited | Edited | |||||
| miR-200b | ADAR1 & ADAR2 | ZEB1 ZEB2 |
LIFR | –Wild type miR-200b inhibits cell migration and invasion –Edited miR-200b acts upon Leukemia Inhibitory Factor Receptor (LIFR), a gene known to suppress Epithelial-to-Mesenchymal transition (EMT) and aids tumor metastasis |
BRCA, HNSC, COAD, KICH, KIRC, LUAD, STAD, THCA, UCEC | (Ramirez-Moya et al., 2020; Wang et al., 2017) |
| miR-379–5p | ADAR2 | PTK2 | CD97 | –Unlike wild type, edited miR-379–5p inhibits cellular proliferation and promotes apoptosis | CRC, HNSC, LUAD, LUSC, SC, THCA, UC | (Xu et al., 2019) |
| miR-455–5p | ADAR1 | CPEB1 | RhoC MDM4 Integrin ⍺2 |
–Through regulation of tumor suppressor CPEB1, wild type miR-455–5p promotes tumor growth and metastasis –ADAR1-mediated editing of miR-455–5p inhibits expression of mature miRNA and suppresses melanoma progression |
Melanoma | (Shoshan et al., 2015) |
| miR-378a-3p | ADAR1 | – | PARVA | –PARVA promotes tumor growth and invasion through increased expression of MMP-2 and c-Jun | Melanoma | (Velazquez-Torres et al., 2018) |
| miR-376a* | ADAR2 | RAP2A | AMFR | –Wild type miR-376a* promotes tumor cell proliferation, migration and invasion through suppression of RAP2A –Edited miR-376a* inhibits tumor progression through regulation of AMFR |
GBM | (Choudhury et al., 2012) |
| miR-589–3p | Primarily ADAR2 | PCDH9 | ADAM12 | –In contrast to wild type miRNA, edited miR-589–3p suppresses tumor cell proliferation and motility –Edited miR-589–3p also suppresses MMP9 activity |
GBM | (Cesarini et al., 2018) |
| Editing alters miRNA binding | ||||||
| miRNA | ADAR | Target(s) | Function | Cancer | Reference | |
|
miR-25–3p miR-125a-3p |
ADAR1 | DHFR | –Editing of the DHFR 3’ UTR disrupts miR-25–3p and miR-125a-3p binding sites DHFR promotes cell viability |
BRCA | (Nakano et al., 2017) | |
|
miR-30b-3p miR-573 |
ADAR1 | ARHGAP26 | –Editing of the ARHGAP26 3’ UTR disrupts miR-30b-3p and miR-573 binding sites –ARHGAP26 inactivates oncogenic proteins RhoA and Cdc42 |
BRCA, GBM | (Wang et al., 2013) | |
| Editing alters miRNA biogenesis and processing | ||||||
| miRNA | ADAR | Target(s) | Function | Cancer | Reference | |
| miR-21 | ADAR2 | PDCD4 | –Mature miR-21 promotes tumor migration –Editing inhibits mature miR-21 expression |
GBM | (Tomaselli et al., 2015) | |
| miR-221/222 | ADAR2 | p27Kip1 | –Mature miR-221/-222 promotes tumor cell proliferation –Editing inhibits mature mi221/222 expression |
GBM | (Tomaselli et al., 2015) | |
| miR-214 | ADAR2 | Rab15 | –Editing of the transcript antisense to pri-miR-214 decreases expression of mature miR-214 –miR-214 inhibition is associated with an increase in metastatic phenotype through regulation of Twist and E-Cadherin expression |
HCC | (Li et al., 2012; Liu et al., 2013) | |
| miR-222 | ADAR1 | ICAM1 | –Editing decreases miR-222 expression and inhibits suppression of ICAM1 expression | Melanoma | (Galore-Haskel et al., 2015) | |
# represents bioinformatically predicted targets.
Abbreviations Used: (see Table 2 legend as well) Colon Adenocarcinoma (COAD), Kidney Chromophobe (KICH), Stomach Cancer (SC), Uterus Cancer (UC), Uterine Corpus Endometrial Carcinoma (UCEC)
Aberrant recoding editing events in cancer
Bioinformatic analyses have revealed that aberrant levels of editing within coding regions give rise to cancer-specific proteomic diversity (Han et al., 2015; Paz-Yaacov et al., 2015; Peng et al., 2018) which can promote oncogenic cellular phenotypes. To illustrate the versatility of A-to-I editing in mediating both oncogenic and tumor suppressive roles, a few recoding editing events are highlighted below and a more complete summary of cancer-associated recoding editing events and associated mechanisms is provided in Table 2.
In liver cancer, editing of Antizyme Inhibitor 1 (AZIN1) is associated with tumor progression (Chan et al., 2014). Wildtype AZIN1 can bind to Antizyme 1 and prevent Antizyme 1 from degrading several oncoproteins like Ornithine Decarboxylase (ODC) and Cyclin D1 (CCND1). When edited by ADAR1, a serine (S) to glycine (G) substitution at position 367 of AZIN1 induces a conformational change, which increases the affinity of AZIN1 towards Antizyme 1. Consequently, edited AZIN1 sequesters Antizyme 1, preventing degradation of ODC and CCND1 and thus, promoting cell proliferation. The oncogenic potential of edited AZIN1 is not limited to liver cancer. In esophageal squamous cell carcinoma and colorectal cancer, edited AZIN1 promotes cell proliferation, invasion, migration and stemness (Qin et al., 2014; Shigeyasu et al., 2018). Recoding editing by ADAR2 also exhibits tumor promoting capabilities. In esophageal cancer, ADAR2-mediated editing of Solute Carrier Family 22 Member 3 (SLC22A3) promotes cancer metastasis (Fu et al., 2017). SLC22A3 is an organic cation transporter that inhibits α-Actinin 4 (ACTN4) mediated actin crosslinking. SLCA22A3 inhibits ACTN4 by sequestering ACTN4 to the cell membrane. When edited, a single amino acid substitution (N72E) in SLC22A3 relieves this ACTN4 suppression and aids cancer metastasis through cytoskeletal remodeling.
ADARs can also act upon specific targets in normal cells to suppress tumor progression. ADAR1-mediated editing of Gamma-Aminobutyric Acid Type A Receptor Subunit Alpha 3 (GABRA3) serves as a perfect example. Although GABRA3 is primarily expressed in brain, breast cancers express high levels of unedited GABRA3, which activates Akt and leads to increased stem cell populations. Upon ADAR1p110 editing at the I/M site (Isoleucine to Methionine at position 342), edited GABRA3 suppresses tumor invasion and metastasis. This suppression occurs through inhibition of Akt phosphorylation (Gumireddy et al., 2016). Consistent with this important role, expression of edited GABRA3 in a breast cancer cell significantly reduces tumor metastasis in a mouse model.
In tumors, ADAR activity can also be regulated by cofactors leading to modulation of cancer-specific recoding editing events. Death Associated Protein 3 (DAP3) is a mitochondrial protein overexpressed in a number of cancers. One function of DAP3 is to bind ADAR1 and ADAR2 via the dsRBD and deaminase domains, respectively. As a result, DAP3 prevents ADARs from binding to and editing target RNAs. One example in which DAP3-mediated editing repression promotes tumorigenesis is the alteration of PDZ Domain Containing 7 (PDZD7). Unedited PDZD7 is oncogenic. However, ADAR1- or ADAR2-mediated editing of PDZD7 at position 518 converts a STOP codon into tryptophan (W) leading to a gain of 18 amino acids and reduced PDZD7 oncogenicity (Han et al., 2020). Thus, depending on substrate and cellular context, ADARs can function to promote or suppress tumor progression.
Role of oncogenic A-to-I editing events on miRNA biogenesis and specificity
In addition to dysregulation in mRNA editing, cancers are also associated with aberrant editing of microRNAs (miRNAs) (Pinto et al., 2018; Wang et al., 2017). In a growing tumor, malignant cells exploit ADAR-mediated editing of miRNAs to gain an oncogenic advantage over non-cancerous competitors. Of the many miRNA editing events that thus far have been identified to affect tumor progression (Table 3), a few characteristic examples have been highlighted below.
It is well established that the seed sequence (2–8 nucleotides) of a mature miRNA is crucial in recognizing targets and a single nucleotide substitution within the seed sequence can confer altered miRNA target recognition (Kawahara et al., 2007). Thus, one way in which ADARs influence miRNA activity is by editing the seed sequence (Figure 3C). In case of miR-200b, a single editing event at the 5th position of the mature miRNA allows edited miR-200b to recognize an alternate set of targets and aid tumor progression. Amongst these, edited miR-200b recognizes and silences the metastasis suppressor leukemia inhibitory factor receptor (LIFR). LIFR suppression thereby promotes cancer cell migration, invasion and metastasis (Wang et al., 2017). In contrast, unedited miR-200b acts as a tumor suppressor and represses epithelial-to-mesenchymal transition (EMT) through the inhibition of oncogenic targets ZEB1 and ZEB2.
A-to-I editing can also regulate mature miRNA expression by interfering with miRNA biogenesis and processing (Figure 3C). For instance, editing-dependent regulation of onco-miRNAs: miR-21, miR-221 and miR-222 are crucial determinants of glioblastoma progression. miR-21 promotes tumor cell migration whereas miR-221/−222 promotes cell proliferation through downregulation of p27 (Gabriely et al., 2008; Galardi et al., 2007). In glioblastomas, ADAR2-mediated editing leads to reduced expression of mature miRNAs with a concomitant accumulation of respective precursor primary transcripts, pri-miR-21, −221 and −222 (Tomaselli et al., 2015). This function of ADAR2 is associated with decreased cellular proliferation and migration. However, since glioblastomas are associated with decreased ADAR2-mediated editing, expression of these onco-miRNAs is often elevated and aid tumor progression.
ADARs can also affect miRNA function without directly acting upon mature miRNAs or their precursors. Instead, ADARs modulate miRNA activity by disrupting miRNA binding sites on the 3’ UTRs of target genes. A classic example is seen in ADAR1-mediated regulation of Rho GTPase activating protein 26 (ARHGAP26) gene expression (Wang et al., 2013). ARHGAP26 functions to inactivate small oncogenic G-proteins like RhoA and Cdc42. By editing specific sites on the ARPHGAP26 3’ UTR, ADAR1 disrupts binding sites of two miRNAs, miR-30b-3p and miR-573, and promotes ARHGAP26 expression. ARHGAP26 is a tumor suppressor, and reduced editing of ARHGAP26 3’ UTR allows increased miRNA binding and decreases ARHGAP26 protein expression in breast cancer and glioblastomas. Reminiscent of what was also uncovered for DNA modifications in cancer genomes, cancer-associated alterations in individual editing events do not always correlate with respective global trends in editomes. For instance, despite breast cancer exhibiting global hyperediting of RNAs, the ARHGAP26 3’ UTR undergoes hypoediting. Therefore, as is true for tumor-associated recoding events, ADAR-mediated modulation of miRNA processing and activity is highly substrate and/or cancer specific and might not always correlate with global editing profiles.
Cancer-associated effects of ADARs on alternative splicing
Both A-to-I editing and alternative splicing are co-transcriptional and increasing evidence suggests the existence of a crosstalk between both processes (Hsiao et al., 2018; Licht et al., 2019). In the competitive tumor environment, this crosstalk can be exploited to abrogate efficient splicing of tumor suppressors or give rise to novel oncogenic isoforms of existing proteins. As described earlier in this review, by editing an ‘AU’ dinucleotide, ADARs can create novel 5’ splice sites (Figure 3B). Editing can also generate (AA→ AG) or disrupt (AG→ GG) splice acceptor sites (Figure 3B). However, such examples are yet to be identified in the context of a tumor. In contrast, branch point adenosines are affected by A-to-I editing, and this gene regulatory event has been observed in patients suffering from Acute Myeloid Leukemia (AML) (Beghini et al., 2000). An editing event at position 7866 in the Protein Tyrosine Phosphatase Non-Receptor Type 6 (PTPN6) disrupts the branch point adenosine and leads to a variant of PTPN6 that retains intron 3. Although the clinical significance remains unexplored, the alternatively spliced PTPN6 variant has elevated expression at AML diagnosis than remission (Beghini et al., 2000). ADARs can also alter splice isoform levels by either deterring or recruiting splicing modulators to target RNAs. ADAR1-mediated editing of a site within intron 8 of CCDC15 is responsible for recruiting the alternative splicing negative regulator, SRSF7 thereby repressing CCDC15-exon9 inclusion (Tang et al., 2020). Compared to paired normal tissues, almost 60% of esophageal squamous cell carcinoma tumors analyzed have elevated expression of the oncogenic CCDC15-exon9 variant. This alternative splicing event is also regulated by ADARs in an editing-independent manner. Binding of ADAR1 and ADAR2 to intronic double stranded regions of the CCDC15 RNA represses the oncogenic CCDC15-exon9 inclusion event (Tang et al., 2020). In this same study, binding of ADAR2 to RELL2 was shown to impede U2AF65 from binding and exon recognition which leads to repression of the tumor suppressive RELL2-exon3 inclusion. Thus, by modulating alternative splicing of target genes, ADARs can direct cancer-specific gene expression changes and aid tumor progression.
ADAR expression and cancer
Since alterations in tumor editomes are cancer specific, dysregulation could in part be attributed to differential ADAR expression. In fact, when compared to respective normal tissues, bladder, breast, head & neck, liver, lung, prostate and thyroid carcinoma exhibit elevated ADAR1 mRNA expression (Paz-Yaacov et al., 2015). ADAR1 mRNA levels also positively correlate to hyperediting in many cancers (Han et al., 2015). One plausible reason behind elevated ADAR1 expression is that the ADAR1 gene is located in a chromosomal region (Chromosome 1 Arm q) which undergoes amplification in a wide variety of tumors (Knuutila et al., 1998; Qin et al., 2014). Secondly, the ADAR1 p150 isoform is interferon-inducible (Patterson and Samuel, 1995). Thus the elevated ADAR1 expression and associated hyperediting observed in many cancers could simply be a passenger effect of chronic inflammation that occurs in cancer (Ganem, Ben-Asher, and Lamm, 2017). Speculation about the oncogenic potential of ADAR1 is further fueled by independent reports where ectopic ADAR1 expression and/or ADAR1-mediated changes in editing promotes malignancy (Tables 2 and 3) (Chan et al., 2014; Chen et al., 2013; Gumireddy et al., 2016; Qin et al., 2014). On the contrary, ADAR1 has also been shown to inhibit cell proliferation and metastasis in glioblastoma and breast cancer, respectively (Gumireddy et al., 2016; Paz et al., 2007). Thus, ADAR1 plays a highly context-specific role in regulating tumorigenesis.
Unlike ADAR1, no significant correlations between ADAR2 mRNA expression and alterations in global editing profiles have been reported to date. ADAR2 can act as a tumor suppressor, as ADAR2 expression inhibits cell proliferation in glioblastomas and esophageal, gastric and hepatocellular carcinoma (Cenci et al., 2008; Chan et al., 2014; Chan et al., 2016; Chen et al., 2017; Galeano et al., 2013). In 2013, ADAR2 was shown to exert this inhibitory role in an editing-dependent manner by restricting cells in the G1 phase (Galeano et al., 2013). Glioblastomas often have elevated levels of S-Phase Kinase Associated Protein 2 (Skp2), an oncogene responsible for targeting the p21 and p27 cyclin dependent kinase inhibitors for degradation. ADAR2 regulates Skp2 activity indirectly by editing and activating a phosphatase, CDC14B. CDC14B promotes Skp2 degradation and restricts cells in the G1 phase by modulating p21 and p27 activity. However, decreased ADAR2 activity in glioblastomas results in decreased CDC14B activity. The consequent increase in Skp2 expression promotes cell proliferation (Galeano et al., 2013). On the contrary, editing activity of ADAR2 also promotes tumorigenesis in a cancer-specific manner (refer to Tables 2 and 3 for details).
Despite lacking any known deaminase activity, a negative correlation has been reported for ADAR3 mRNA levels and grades of brain tumors (Chen et al., 2000; Paz et al., 2007; Zhang et al., 2018). Kaplan-Meier survival analysis indicates that high ADAR3 mRNA expression parallels better progression-free survival in lower grade gliomas, suggesting that ADAR3 could function as a tumor suppressor (Zhang et al., 2018). However, a major caveat of these previous studies is that it was recently shown that ADAR3 mRNA levels do not directly correlate with protein expression (Wang et al., 2018). Additionally, multiple independent studies have shown that despite having low mRNA, glioblastomas have elevated ADAR3 protein expression when compared to paired normal tissues (Oakes et al., 2017; Wang et al., 2018). However, whether ADAR3 is a driver promoting tumor progression or elevated ADAR3 is simply a consequence of genomic alterations in glioblastomas remains elusive.
ADARs position on tumor immunity
In tumor cells, somatic mutations can lead to generation of novel, tumor-specific peptides that can be processed and presented on tumor cell surfaces by major histocompatibility complexes. These tumor-specific antigens or neoantigens are not expressed in normal cells and recognition of neoantigens by the immune system can lead to destruction of the cancerous cell (Jiang et al., 2019). Thus, neoantigens serve as ideal candidates for cancer immunotherapy.
In addition to increasing proteomic diversity, A-to-I edited RNAs can also contribute to the neoantigen repertoire (Peng et al., 2018). For example, tumor infiltrating lymphocytes (TILs) elicit a strong cytotoxic immune response against melanoma tumor cells presenting edited Cyclin I (CCNI) peptide (Zhang et al., 2018). Triple negative breast cancer tissues having high ADAR1 expression are also associated with increased TIL expression (Song et al., 2017). Moreover, contributions of ADARs towards tumor immunity are not restricted to generating neoantigens. In certain cancers, specific subsets of cells contribute to IFN production per se and are hence associated with elevated IFN-stimulated gene expression (ISG) (Liu et al., 2019). Studies indicate that cells with elevated ISG signature are rendered sensitive to ADAR1 loss in several cancers including hepatocellular carcinoma (HCC) and triple negative breast cancer (TNBC) (Gannon et al., 2018; Kung et al., 2020; Liu et al., 2019). Recently, ADAR1 null mice were also found to evade resistance to immune checkpoint blockade (Ishizuka et al., 2019), a common theme for failure of cancer immunotherapies. In this study, ADAR1 null mice were found to have an inflamed tumor microenvironment, which conferred tumors with increased sensitivity towards immunotherapies promoting IFN production including anti-PD1 treatment. These studies not only highlight how an elevated ISG signature could serve as a prognostic marker but also supports the therapeutic potential of ADAR1.
Thus, despite being nascent in the field, ADARs and associated edited peptides exhibit potential to serve as important targets for cancer immunotherapy. However, one thing to note is that unlike DNA mutations, RNA editing events are often not specific to tumors. Instead, ADAR expression and editing levels are deregulated in tumors. Thus, as true for most available cancer therapeutics, selection of editing-derived neoantigens and/or ADARs as novel therapeutic targets requires factoring in the possibility that healthy cells might also be affected.
Concluding remarks
ADARs impact RNA through both RNA binding and A-to-I editing, thereby regulating gene expression and affecting the cellular dsRNA pools. The importance of these functions is made clear by the neural and developmental defects, autoimmune disorders, and malignancies that arise when ADAR expression and activity are altered. Site-directed RNA editing could be a promising therapeutic strategy to rectify clinical mutations, making the study of ADARs even more beneficial to the field of medicine. Since ADAR activity is dynamic and changes both between tissues and across developmental time, future studies aimed at dissecting how binding and editing by ADARs is spatiotemporally regulated in vivo are critical for understanding not only the fundamentals of these enzymes, but also how best to approach treatment options.
Acknowledgements
The authors thank Suba Rajendren and Reshma Kurup for comments on the initial manuscript and the four anonymous referees for excellent suggestions on improving the review. Some illustrations were created with BioRender.com.
Declaration of Interest
Work in the Hundley lab is supported by the National Science Foundation (Award Number 1917050) and the National Institutes of Health (R01GM130759). Emily Erdmann is supported by the Graduate Training Program in Quantitative and Chemical Biology under Award Number T32 GM131994 and Indiana University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Ahmad S, Mu X, Yang F, Greenwald E, Park JW, Jacob E, Zhang CZ, Hur S. 2018. Breaching Self-Tolerance to Alu Duplex RNA Underlies MDA5-Mediated Inflammation. Cell, 172, 797–810 e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. 2006. Pathogen recognition and innate immunity. Cell, 124, 783–801 [DOI] [PubMed] [Google Scholar]
- Albertin CB, Simakov O, Mitros T, Wang ZY, Pungor JR, Edsinger-Gonzales E, Brenner S, Ragsdale CW, Rokhsar DS. 2015. The octopus genome and the evolution of cephalopod neural and morphological novelties. Nature, 524, 220–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon S, Garrett SC, Levanon EY, Olson S, Graveley BR, Rosenthal JJ, Eisenberg E. 2015. The majority of transcripts in the squid nervous system are extensively recoded by A-to-I RNA editing. Elife, 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquino-Jarquin G 2020. Novel Engineered Programmable Systems for ADAR-Mediated RNA Editing. Mol Ther Nucleic Acids, 19, 1065–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasiadis A, Rich A, Maas S. 2004. Widespread A-to-I RNA editing of Alu-containing mRNAs in the human transcriptome. PLoS Biol, 2, e391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn JH, Ahn J, Lin X, Zhang Q, Lee JH, Civelek M, Xiao X. 2015. Genomic analysis of ADAR1 binding and its involvement in multiple RNA processing pathways. Nat Commun, 6, 6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajad P, Ebner F, Amman F, Szabo B, Kapoor U, Manjali G, Hildebrandt A, Janisiw MP, Jantsch MF. 2020. An internal deletion of ADAR rescued by MAVS deficiency leads to a minute phenotype. Nucleic Acids Res, 48, 3286–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak M, Porath HT, Finkelstein G, Knisbacher BA, Buchumenski I, Roth SH, Levanon EY, Eisenberg E. 2020. Purifying selection of long dsRNA is the first line of defense against false activation of innate immunity. Genome Biol, 21, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraud P, Banerjee S, Mohamed WI, Jantsch MF, Allain FH. 2014. A bimodular nuclear localization signal assembled via an extended double-stranded RNA-binding domain acts as an RNA-sensing signal for transportin 1. Proc Natl Acad Sci U S A, 111, E1852–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beghini A, Ripamonti CB, Peterlongo P, Roversi G, Cairoli R, Morra E, Larizza L. 2000. RNA hyperediting and alternative splicing of hematopoietic cell phosphatase (PTPN6) gene in acute myeloid leukemia. Hum Mol Genet, 9, 2297–304 [DOI] [PubMed] [Google Scholar]
- Berger I, Winston W, Manoharan R, Schwartz T, Alfken J, Kim YG, Lowenhaupt K, Herbert A, Rich A. 1998. Spectroscopic characterization of a DNA-binding domain, Z alpha, from the editing enzyme, dsRNA adenosine deaminase: evidence for left-handed Z-DNA in the Z alpha-DNA complex. Biochemistry, 37, 13313–21 [DOI] [PubMed] [Google Scholar]
- Bhalla T, Rosenthal JJ, Holmgren M, Reenan R. 2004. Control of human potassium channel inactivation by editing of a small mRNA hairpin. Nat Struct Mol Biol, 11, 950–6 [DOI] [PubMed] [Google Scholar]
- Blow MJ, Grocock RJ, van Dongen S, Enright AJ, Dicks E, Futreal PA, Wooster R, Stratton MR. 2006. RNA editing of human microRNAs. Genome Biol, 7, R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns CM, Chu H, Rueter SM, Hutchinson LK, Canton H, Sanders-Bush E, Emeson RB. 1997. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature, 387, 303–8 [DOI] [PubMed] [Google Scholar]
- Bycroft M, Grunert S, Murzin AG, Proctor M, St Johnston D. 1995. NMR solution structure of a dsRNA binding domain from Drosophila staufen protein reveals homology to the N-terminal domain of ribosomal protein S5. EMBO J, 14, 3563–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capshew CR, Dusenbury KL, Hundley HA. 2012. Inverted Alu dsRNA structures do not affect localization but can alter translation efficiency of human mRNAs independent of RNA editing. Nucleic Acids Res, 40, 8637–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci C, Barzotti R, Galeano F, Corbelli S, Rota R, Massimi L, Di Rocco C, O’Connell MA, Gallo A. 2008. Down-regulation of RNA editing in pediatric astrocytomas: ADAR2 editing activity inhibits cell migration and proliferation. J Biol Chem, 283, 7251–60 [DOI] [PubMed] [Google Scholar]
- Chalk AM, Taylor S, Heraud-Farlow JE, Walkley CR. 2019. The majority of A-to-I RNA editing is not required for mammalian homeostasis. Genome Biol, 20, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TH, Lin CH, Qi L, Fei J, Li Y, Yong KJ, Liu M, Song Y, Chow RK, Ng VH, Yuan YF, Tenen DG, Guan XY, Chen L. 2014. A disrupted RNA editing balance mediated by ADARs (Adenosine DeAminases that act on RNA) in human hepatocellular carcinoma. Gut, 63, 832–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TH, Qamra A, Tan KT, Guo J, Yang H, Qi L, Lin JS, Ng VH, Song Y, Hong H, Tay ST, Liu Y, Lee J, Rha SY, Zhu F, So JB, Teh BT, Yeoh KG, Rozen S, Tenen DG, Tan P, Chen L. 2016. ADAR-Mediated RNA Editing Predicts Progression and Prognosis of Gastric Cancer. Gastroenterology, 151, 637–50 e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CX, Cho DS, Wang Q, Lai F, Carter KC, Nishikura K. 2000. A third member of the RNA-specific adenosine deaminase gene family, ADAR3, contains both single- and double-stranded RNA binding domains. RNA, 6, 755–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Li Y, Lin CH, Chan TH, Chow RK, Song Y, Liu M, Yuan YF, Fu L, Kong KL, Qi L, Li Y, Zhang N, Tong AH, Kwong DL, Man K, Lo CM, Lok S, Tenen DG, Guan XY. 2013. Recoding RNA editing of AZIN1 predisposes to hepatocellular carcinoma. Nat Med, 19, 209–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LL, Carmichael GG. 2009. Altered nuclear retention of mRNAs containing inverted repeats in human embryonic stem cells: functional role of a nuclear noncoding RNA. Mol Cell, 35, 467–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LL, Yang L. 2017. ALUternative Regulation for Gene Expression. Trends Cell Biol, 27, 480–90 [DOI] [PubMed] [Google Scholar]
- Chen YB, Liao XY, Zhang JB, Wang F, Qin HD, Zhang L, Shugart YY, Zeng YX, Jia WH. 2017. ADAR2 functions as a tumor suppressor via editing IGFBP7 in esophageal squamous cell carcinoma. Int J Oncol, 50, 622–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H, Calis JJA, Wu X, Sun T, Yu Y, Sarbanes SL, Dao Thi VL, Shilvock AR, Hoffmann HH, Rosenberg BR, Rice CM. 2018. Human ADAR1 Prevents Endogenous RNA from Triggering Translational Shutdown. Cell, 172, 811–24 e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow Y, Keshavan N, Barbet JP, Bercu G, Bondet V, Boussard C, Dedieu N, Duffy D, Hully M, Giardini A, Gitiaux C, Rice GI, Seabra L, Bader-Meunier B, Rahman S. 2019. Cardiac valve involvement in ADAR-related type I interferonopathy. J Med Genet [DOI] [PubMed] [Google Scholar]
- Deffit SN, Hundley HA. 2016. To edit or not to edit: regulation of ADAR editing specificity and efficiency. Wiley Interdiscip Rev RNA, 7, 113–27 [DOI] [PubMed] [Google Scholar]
- Deffit SN, Yee BA, Manning AC, Rajendren S, Vadlamani P, Wheeler EC, Domissy A, Washburn MC, Yeo GW, Hundley HA. 2017. The C. elegans neural editome reveals an ADAR target mRNA required for proper chemotaxis. Elife, 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P, Khan A, Jacobson D, Sambrani N, McGurk L, Li X, Jayasree A, Hejatko J, Shohat-Ophir G, O’Connell MA, Li JB, Keegan LP. 2020. Adar RNA editing-dependent and -independent effects are required for brain and innate immune functions in Drosophila. Nat Commun, 11, 1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexheimer PJ, Cochella L. 2020. MicroRNAs: From Mechanism to Organism. Front Cell Dev Biol, 8, 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Meglio P, Villanova F, Nestle FO. 2014. Psoriasis. Cold Spring Harb Perspect Med, 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M, Jantsch MF. 2002. New and old roles of the double-stranded RNA-binding domain. J Struct Biol, 140, 147–53 [DOI] [PubMed] [Google Scholar]
- Doyle M, Jantsch MF. 2003. Distinct in vivo roles for double-stranded RNA-binding domains of the Xenopus RNA-editing enzyme ADAR1 in chromosomal targeting. J Cell Biol, 161, 309–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl Y, Farahani HS, Behm M, Lagergren J, Öhman M. 2012. A-to-I editing of microRNAs in the mammalian brain increases during development. Genome Res, 22, 1477–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbarbary RA, Li W, Tian B, Maquat LE. 2013. STAU1 binding 3’ UTR IRAlus complements nuclear retention to protect cells from PKR-mediated translational shutdown. Genes Dev, 27, 1495–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer SEJ, Ruvkun G. 2020. Caenorhabditis elegans ADAR editing and the ERI-6/7/MOV10 RNAi pathway silence endogenous viral elements and LTR retrotransposons. Proc Natl Acad Sci U S A, 117, 5987–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick T, Huang S. 2012. 3’-UTR-located inverted Alu repeats facilitate mRNA translational repression and stress granule accumulation. Nucleus, 3, 359–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz J, Strehblow A, Taschner A, Schopoff S, Pasierbek P, Jantsch MF. 2009. RNA-regulated interaction of transportin-1 and exportin-5 with the double-stranded RNA-binding domain regulates nucleocytoplasmic shuttling of ADAR1. Mol Cell Biol, 29, 1487–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzell K, Xu LD, Lagergren J, Ohman M. 2018. ADARs and editing: The role of A-to-I RNA modification in cancer progression. Semin Cell Dev Biol, 79, 123–30 [DOI] [PubMed] [Google Scholar]
- Fu L, Qin YR, Ming XY, Zuo XB, Diao YW, Zhang LY, Ai J, Liu BL, Huang TX, Cao TT, Tan BB, Xiang D, Zeng CM, Gong J, Zhang Q, Dong SS, Chen J, Liu H, Wu JL, Qi RZ, Xie D, Wang LD, Guan XY. 2017. RNA editing of SLC22A3 drives early tumor invasion and metastasis in familial esophageal cancer. Proc Natl Acad Sci U S A, 114, E4631–E40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriely G, Wurdinger T, Kesari S, Esau CC, Burchard J, Linsley PS, Krichevsky AM. 2008. MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol Cell Biol, 28, 5369–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gacem N, Kavo A, Zerad L, Richard L, Mathis S, Kapur RP, Parisot M, Amiel J, Dufour S, de la Grange P, Pingault V, Vallat JM, Bondurand N. 2020. ADAR1 mediated regulation of neural crest derived melanocytes and Schwann cell development. Nat Commun, 11, 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galardi S, Mercatelli N, Giorda E, Massalini S, Frajese GV, Ciafre SA, Farace MG. 2007. miR-221 and miR-222 expression affects the proliferation potential of human prostate carcinoma cell lines by targeting p27Kip1. J Biol Chem, 282, 23716–24 [DOI] [PubMed] [Google Scholar]
- Galeano F, Rossetti C, Tomaselli S, Cifaldi L, Lezzerini M, Pezzullo M, Boldrini R, Massimi L, Di Rocco CM, Locatelli F, Gallo A. 2013. ADAR2-editing activity inhibits glioblastoma growth through the modulation of the CDC14B/Skp2/p21/p27 axis. Oncogene, 32, 998–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galipon J, Ishii R, Suzuki Y, Tomita M, Ui-Tei K. 2017. Differential Binding of Three Major Human ADAR Isoforms to Coding and Long Non-Coding Transcripts. Genes (Basel), 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo A, Keegan LP, Ring GM, O’Connell MA. 2003. An ADAR that edits transcripts encoding ion channel subunits functions as a dimer. EMBO J, 22, 3421–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammon DB, Mello CC. 2015. RNA interference-mediated antiviral defense in insects. Curr Opin Insect Sci, 8, 111–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem NS, Ben-Asher N, Lamm AT. 2017. In cancer, A-to-I RNA editing can be the driver, the passenger, or the mechanic. Drug Resist Updat, 32, 16–22 [DOI] [PubMed] [Google Scholar]
- Ganem NS, Ben-Asher N, Manning AC, Deffit SN, Washburn MC, Wheeler EC, Yeo GW, Zgayer OB, Mantsur E, Hundley HA, Lamm AT. 2019. Disruption in A-to-I Editing Levels Affects C. elegans Development More Than a Complete Lack of Editing. Cell Rep, 27, 1244–53 e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon HS, Zou T, Kiessling MK, Gao GF, Cai D, Choi PS, Ivan AP, Buchumenski I, Berger AC, Goldstein JT, Cherniack AD, Vazquez F, Tsherniak A, Levanon EY, Hahn WC, Meyerson M. 2018. Identification of ADAR1 adenosine deaminase dependency in a subset of cancer cells. Nat Commun, 9, 5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantier MP, Williams BR. 2007. The response of mammalian cells to double-stranded RNA. Cytokine Growth Factor Rev, 18, 363–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George CX, Samuel CE. 1999. Human RNA-specific adenosine deaminase ADAR1 transcripts possess alternative exon 1 structures that initiate from different promoters, one constitutively active and the other interferon inducible. Proc Natl Acad Sci U S A, 96, 4621–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber A, O’Connell MA, Keller W. 1997. Two forms of human double-stranded RNA-specific editase 1 (hRED1) generated by the insertion of an Alu cassette. RNA, 3, 453–63 [PMC free article] [PubMed] [Google Scholar]
- Gleghorn ML, Maquat LE. 2014. ‘Black sheep’ that don’t leave the double-stranded RNA-binding domain fold. Trends Biochem Sci, 39, 328–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RA, Macbeth MR, Beal PA. 2012. ADAR proteins: structure and catalytic mechanism. Curr Top Microbiol Immunol, 353, 1–33 [DOI] [PubMed] [Google Scholar]
- Graveley BR, Brooks AN, Carlson JW, Duff MO, Landolin JM, Yang L, Artieri CG, van Baren MJ, Boley N, Booth BW, Brown JB, Cherbas L, Davis CA, Dobin A, Li R, Lin W, Malone JH, Mattiuzzo NR, Miller D, Sturgill D, Tuch BB, Zaleski C, Zhang D, Blanchette M, Dudoit S, Eads B, Green RE, Hammonds A, Jiang L, Kapranov P, Langton L, Perrimon N, Sandler JE, Wan KH, Willingham A, Zhang Y, Zou Y, Andrews J, Bickel PJ, Brenner SE, Brent MR, Cherbas P, Gingeras TR, Hoskins RA, Kaufman TC, Oliver B, Celniker SE. 2011. The developmental transcriptome of Drosophila melanogaster. Nature, 471, 473–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberger S, Levanon EY, Paz-Yaacov N, Barzilai A, Safran M, Osenberg S, Amariglio N, Rechavi G, Eisenberg E. 2010. Consistent levels of A-to-I RNA editing across individuals in coding sequences and non-conserved Alu repeats. BMC Genomics, 11, 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice LF, Degnan BM. 2015. The origin of the ADAR gene family and animal RNA editing. BMC Evol Biol, 15, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumireddy K, Li A, Kossenkov AV, Sakurai M, Yan J, Li Y, Xu H, Wang J, Zhang PJ, Zhang L, Showe LC, Nishikura K, Huang Q. 2016. The mRNA-edited form of GABRA3 suppresses GABRA3-mediated Akt activation and breast cancer metastasis. Nat Commun, 7, 10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, An O, Hong H, Chan THM, Song Y, Shen H, Tang SJ, Lin JS, Ng VHE, Tay DJT, Molias FB, Pitcheshwar P, Tan HQ, Yang H, Chen L. 2020. Suppression of adenosine-to-inosine (A-to-I) RNA editome by death associated protein 3 (DAP3) promotes cancer progression. Sci Adv, 6, eaba5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Diao L, Yu S, Xu X, Li J, Zhang R, Yang Y, Werner HMJ, Eterovic AK, Yuan Y, Li J, Nair N, Minelli R, Tsang YH, Cheung LWT, Jeong KJ, Roszik J, Ju Z, Woodman SE, Lu Y, Scott KL, Li JB, Mills GB, Liang H. 2015. The Genomic Landscape and Clinical Relevance of A-to-I RNA Editing in Human Cancers. Cancer Cell, 28, 515–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartner JC, Schmittwolf C, Kispert A, Müller AM, Higuchi M, Seeburg PH. 2004. Liver disintegration in the mouse embryo caused by deficiency in the RNA-editing enzyme ADAR1. J Biol Chem, 279, 4894–902 [DOI] [PubMed] [Google Scholar]
- Hartner JC, Walkley CR, Lu J, Orkin SH. 2009. ADAR1 is essential for the maintenance of hematopoiesis and suppression of interferon signaling. Nat Immunol, 10, 109–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Suzuki T. 2013. Dyschromatosis symmetrica hereditaria. J Dermatol, 40, 336–43 [DOI] [PubMed] [Google Scholar]
- Helwak A, Tollervey D. 2014. Mapping the miRNA interactome by cross-linking ligation and sequencing of hybrids (CLASH). Nat Protoc, 9, 711–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heraud-Farlow JE, Chalk AM, Linder SE, Li Q, Taylor S, White JM, Pang L, Liddicoat BJ, Gupte A, Li JB, Walkley CR. 2017. Protein recoding by ADAR1-mediated RNA editing is not essential for normal development and homeostasis. Genome Biol, 18, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert A, Alfken J, Kim YG, Mian IS, Nishikura K, Rich A. 1997. A Z-DNA binding domain present in the human editing enzyme, double-stranded RNA adenosine deaminase. Proc Natl Acad Sci U S A, 94, 8421–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M, Maas S, Single FN, Hartner J, Rozov A, Burnashev N, Feldmeyer D, Sprengel R, Seeburg PH. 2000. Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature, 406, 78–81 [DOI] [PubMed] [Google Scholar]
- Hoopengardner B, Bhalla T, Staber C, Reenan R. 2003. Nervous system targets of RNA editing identified by comparative genomics. Science, 301, 832–6 [DOI] [PubMed] [Google Scholar]
- Hough RF, Lingam AT, Bass BL. 1999. Caenorhabditis elegans mRNAs that encode a protein similar to ADARs derive from an operon containing six genes. Nucleic Acids Res, 27, 3424–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao YE, Bahn JH, Yang Y, Lin X, Tran S, Yang EW, Quinones-Valdez G, Xiao X. 2018. RNA editing in nascent RNA affects pre-mRNA splicing. Genome Res, 28, 812–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundley HA, Krauchuk AA, Bass BL. 2008. C. elegans and H. sapiens mRNAs with edited 3’ UTRs are present on polysomes. RNA, 14, 2050–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung T, Pratt GA, Sundararaman B, Townsend MJ, Chaivorapol C, Bhangale T, Graham RR, Ortmann W, Criswell LA, Yeo GW, Behrens TW. 2015. The Ro60 autoantigen binds endogenous retroelements and regulates inflammatory gene expression. Science, 350, 455–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur S 2019. Double-Stranded RNA Sensors and Modulators in Innate Immunity. Annu Rev Immunol, 37, 349–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingleby L, Maloney R, Jepson J, Horn R, Reenan R. 2009. Regulated RNA editing and functional epistasis in Shaker potassium channels. J Gen Physiol, 133, 17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka JJ, Manguso RT, Cheruiyot CK, Bi K, Panda A, Iracheta-Vellve A, Miller BC, Du PP, Yates KB, Dubrot J, Buchumenski I, Comstock DE, Brown FD, Ayer A, Kohnle IC, Pope HW, Zimmer MD, Sen DR, Lane-Reticker SK, Robitschek EJ, Griffin GK, Collins NB, Long AH, Doench JG, Kozono D, Levanon EY, Haining WN. 2019. Loss of ADAR1 in tumours overcomes resistance to immune checkpoint blockade. Nature, 565, 43–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Langland JO. 1996. When two strands are better than one: the mediators and modulators of the cellular responses to double-stranded RNA. Virology, 219, 339–49 [DOI] [PubMed] [Google Scholar]
- Jain M, Jantsch MF, Licht K. 2019. The Editor’s I on Disease Development. Trends Genet, 35, 903–13 [DOI] [PubMed] [Google Scholar]
- Jiang T, Shi T, Zhang H, Hu J, Song Y, Wei J, Ren S, Zhou C. 2019. Tumor neoantigens: from basic research to clinical applications. J Hematol Oncol, 12, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor U, Licht K, Amman F, Jakobi T, Martin D, Dieterich C, Jantsch MF. 2020. ADAR-deficiency perturbs the global splicing landscape in mouse tissues. Genome Res, 30, 1107–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katrekar D, Chen G, Meluzzi D, Ganesh A, Worlikar A, Shih YR, Varghese S, Mali P. 2019. In vivo RNA editing of point mutations via RNA-guided adenosine deaminases. Nat Methods, 16, 239–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara Y, Zinshteyn B, Chendrimada TP, Shiekhattar R, Nishikura K. 2007. RNA editing of the microRNA-151 precursor blocks cleavage by the Dicer-TRBP complex. EMBO Rep, 8, 763–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara Y, Zinshteyn B, Sethupathy P, Iizasa H, Hatzigeorgiou AG, Nishikura K. 2007. Redirection of silencing targets by adenosine-to-inosine editing of miRNAs. Science, 315, 1137–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan LP, McGurk L, Palavicini JP, Brindle J, Paro S, Li X, Rosenthal JJ, O’Connell MA. 2011. Functional conservation in human and Drosophila of Metazoan ADAR2 involved in RNA editing: loss of ADAR1 in insects. Nucleic Acids Res, 39, 7249–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppler A, Gendreizig S, Gronemeyer T, Pick H, Vogel H, Johnsson K. 2003. A general method for the covalent labeling of fusion proteins with small molecules in vivo. Nat Biotechnol, 21, 86–9 [DOI] [PubMed] [Google Scholar]
- Khan A, Paro S, McGurk L, Sambrani N, Hogg MC, Brindle J, Pennetta G, Keegan LP, O’Connell MA. 2020. Membrane and synaptic defects leading to neurodegeneration in Adar mutant Drosophila are rescued by increased autophagy. BMC Biol, 18, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DD, Kim TT, Walsh T, Kobayashi Y, Matise TC, Buyske S, Gabriel A. 2004. Widespread RNA editing of embedded alu elements in the human transcriptome. Genome Res, 14, 1719–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight SW, Bass BL. 2002. The role of RNA editing by ADARs in RNAi. Mol Cell, 10, 809–17 [DOI] [PubMed] [Google Scholar]
- Knuutila S, Bjorkqvist AM, Autio K, Tarkkanen M, Wolf M, Monni O, Szymanska J, Larramendy ML, Tapper J, Pere H, El-Rifai W, Hemmer S, Wasenius VM, Vidgren V, Zhu Y. 1998. DNA copy number amplifications in human neoplasms: review of comparative genomic hybridization studies. Am J Pathol, 152, 1107–23 [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Suzuki T, Mitsuhashi Y, Ito S, Kono M, Komine M, Akita H, Tomita Y. 2008. Six novel mutations of the ADAR1 gene in patients with dyschromatosis symmetrica hereditaria: histological observation and comparison of genotypes and clinical phenotypes. J Dermatol, 35, 395–406 [DOI] [PubMed] [Google Scholar]
- Kong XY, Vik ES, Nawaz MS, Berges N, Dahl TB, Vågbø C, Suganthan R, Segers F, Holm S, Quiles-Jiménez A, Gregersen I, Fladeby C, Aukrust P, Bjørås M, Klungland A, Halvorsen B, Alseth I. 2020. Deletion of Endonuclease V suppresses chemically induced hepatocellular carcinoma. Nucleic Acids Res, 48, 4463–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono M, Suganuma M, Akiyama M, Ito Y, Ujiie H, Morimoto K. 2014. Novel ADAR1 mutations including a single amino acid deletion in the deaminase domain underlie dyschromatosis symmetrica hereditaria in Japanese families. Int J Dermatol, 53, e194–6 [DOI] [PubMed] [Google Scholar]
- Krovat BC, Jantsch MF. 1996. Comparative mutational analysis of the double-stranded RNA binding domains of Xenopus laevis RNA-binding protein A. J Biol Chem, 271, 28112–9 [DOI] [PubMed] [Google Scholar]
- Kung CP, Cottrell KA, Ryu S, Bramel ER, Kladney RD, Bao EA, Freeman EC, Sabloak T, Maggi L Jr., Weber JD. 2020. Evaluating the therapeutic potential of ADAR1 inhibition for triple-negative breast cancer. Oncogene [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuttan A, Bass BL. 2012. Mechanistic insights into editing-site specificity of ADARs. Proc Natl Acad Sci U S A, 109, E3295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F, Drakas R, Nishikura K. 1995. Mutagenic analysis of double-stranded RNA adenosine deaminase, a candidate enzyme for RNA editing of glutamate-gated ion channel transcripts. J Biol Chem, 270, 17098–105 [DOI] [PubMed] [Google Scholar]
- Lai ML, Yang LJ, Zhu XH, Li M. 2012. A novel mutation of the DSRAD gene in a Chinese family with dyschromatosis symmetrica hereditaria. Genet Mol Res, 11, 1731–7 [DOI] [PubMed] [Google Scholar]
- Lamers MM, van den Hoogen BG, Haagmans BL. 2019. ADAR1: “Editor-in-Chief” of Cytoplasmic Innate Immunity. Front Immunol, 10, 1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann KA, Bass BL. 1999. The importance of internal loops within RNA substrates of ADAR1. J Mol Biol, 291, 1–13 [DOI] [PubMed] [Google Scholar]
- Lev-Maor G, Sorek R, Levanon EY, Paz N, Eisenberg E, Ast G. 2007. RNA-editing-mediated exon evolution. Genome Biol, 8, R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levanon EY, Eisenberg E, Yelin R, Nemzer S, Hallegger M, Shemesh R, Fligelman ZY, Shoshan A, Pollock SR, Sztybel D, Olshansky M, Rechavi G, Jantsch MF. 2004. Systematic identification of abundant A-to-I editing sites in the human transcriptome. Nat Biotechnol, 22, 1001–5 [DOI] [PubMed] [Google Scholar]
- Liang H, Landweber LF. 2007. Hypothesis: RNA editing of microRNA target sites in humans? RNA, 13, 463–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht K, Hartl M, Amman F, Anrather D, Janisiw MP, Jantsch MF. 2019. Inosine induces context-dependent recoding and translational stalling. Nucleic Acids Res, 47, 3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht K, Kapoor U, Amman F, Picardi E, Martin D, Bajad P, Jantsch MF. 2019. A high resolution A-to-I editing map in the mouse identifies editing events controlled by pre-mRNA splicing. Genome Res, 29, 1453–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddicoat BJ, Chalk AM, Walkley CR. 2016. ADAR1, inosine and the immune sensing system: distinguishing self from non-self. Wiley Interdiscip Rev RNA, 7, 157–72 [DOI] [PubMed] [Google Scholar]
- Liddicoat BJ, Piskol R, Chalk AM, Ramaswami G, Higuchi M, Hartner JC, Li JB, Seeburg PH, Walkley CR. 2015. RNA editing by ADAR1 prevents MDA5 sensing of endogenous dsRNA as nonself. Science, 349, 1115–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscovitch N, Bazak L, Levanon EY, Chechik G. 2014. Positive correlation between ADAR expression and its targets suggests a complex regulation mediated by RNA editing in the human brain. RNA Biol, 11, 1447–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Golji J, Brodeur LK, Chung FS, Chen JT, deBeaumont RS, Bullock CP, Jones MD, Kerr G, Li L, Rakiec DP, Schlabach MR, Sovath S, Growney JD, Pagliarini RA, Ruddy DA, MacIsaac KD, Korn JM, McDonald ER 3rd. 2019. Tumor-derived IFN triggers chronic pathway agonism and sensitivity to ADAR loss. Nat Med, 25, 95–102 [DOI] [PubMed] [Google Scholar]
- Liu Q, Jiang L, Liu WL, Kang XJ, Ao Y, Sun M, Luo Y, Song Y, Lo WH, Zhang X. 2006. Two novel mutations and evidence for haploinsufficiency of the ADAR gene in dyschromatosis symmetrica hereditaria. Br J Dermatol, 154, 636–42 [DOI] [PubMed] [Google Scholar]
- Liu Q, Wang Z, Wu Y, Cao L, Tang Q, Xing X, Ma H, Zhang S, Luo Y. 2014. Five novel mutations in the ADAR1 gene associated with dyschromatosis symmetrica hereditaria. BMC Med Genet, 15, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Emeson RB, Samuel CE. 1999. Serotonin-2C receptor pre-mRNA editing in rat brain and in vitro by splice site variants of the interferon-inducible double-stranded RNA-specific adenosine deaminase ADAR1. J Biol Chem, 274, 18351–8 [DOI] [PubMed] [Google Scholar]
- Liu Y, Lei M, Samuel CE. 2000. Chimeric double-stranded RNA-specific adenosine deaminase ADAR1 proteins reveal functional selectivity of double-stranded RNA-binding domains from ADAR1 and protein kinase PKR. Proc Natl Acad Sci U S A, 97, 12541–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Samuel CE. 1996. Mechanism of interferon action: functionally distinct RNA-binding and catalytic domains in the interferon-inducible, double-stranded RNA-specific adenosine deaminase. J Virol, 70, 1961–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Samuel CE. 1999. Editing of glutamate receptor subunit B pre-mRNA by splice-site variants of interferon-inducible double-stranded RNA-specific adenosine deaminase ADAR1. J Biol Chem, 274, 5070–7 [DOI] [PubMed] [Google Scholar]
- Livingston JH, Crow YJ. 2016. Neurologic Phenotypes Associated with Mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR1, and IFIH1: Aicardi-Goutieres Syndrome and Beyond. Neuropediatrics, 47, 355–60 [DOI] [PubMed] [Google Scholar]
- Livingston JH, Lin JP, Dale RC, Gill D, Brogan P, Munnich A, Kurian MA, Gonzalez-Martinez V, De Goede CG, Falconer A, Forte G, Jenkinson EM, Kasher PR, Szynkiewicz M, Rice GI, Crow YJ. 2014. A type I interferon signature identifies bilateral striatal necrosis due to mutations in ADAR1. J Med Genet, 51, 76–82 [DOI] [PubMed] [Google Scholar]
- Loo YM, Gale M, Jr. 2011. Immune signaling by RIG-I-like receptors. Immunity, 34, 680–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas S, Gommans WM. 2009. Identification of a selective nuclear import signal in adenosine deaminases acting on RNA. Nucleic Acids Res, 37, 5822–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas S, Gommans WM. 2009. Novel exon of mammalian ADAR2 extends open reading frame. PLoS One, 4, e4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas S, Melcher T, Herb A, Seeburg PH, Keller W, Krause S, Higuchi M, O’Connell MA. 1996. Structural requirements for RNA editing in glutamate receptor pre-mRNAs by recombinant double-stranded RNA adenosine deaminase. J Biol Chem, 271, 12221–6 [DOI] [PubMed] [Google Scholar]
- Mannion NM, Greenwood SM, Young R, Cox S, Brindle J, Read D, Nellaker C, Vesely C, Ponting CP, McLaughlin PJ, Jantsch MF, Dorin J, Adams IR, Scadden AD, Ohman M, Keegan LP, O’Connell MA. 2014. The RNA-editing enzyme ADAR1 controls innate immune responses to RNA. Cell Rep, 9, 1482–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah G, Barraud P, Allain FH. 2013. RNA recognition by double-stranded RNA binding domains: a matter of shape and sequence. Cell Mol Life Sci, 70, 1875–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews MM, Thomas JM, Zheng Y, Tran K, Phelps KJ, Scott AI, Havel J, Fisher AJ, Beal PA. 2016. Structures of human ADAR2 bound to dsRNA reveal base-flipping mechanism and basis for site selectivity. Nat Struct Mol Biol, 23, 426–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle T, Merz S, Reautschnig P, Blaha A, Li Q, Vogel P, Wettengel J, Li JB, Stafforst T. 2019. Precise RNA editing by recruiting endogenous ADARs with antisense oligonucleotides. Nat Biotechnol, 37, 133–38 [DOI] [PubMed] [Google Scholar]
- Mizrahi RA, Schirle NT, Beal PA. 2013. Potent and selective inhibition of A-to-I RNA editing with 2’-O-methyl/locked nucleic acid-containing antisense oligoribonucleotides. ACS Chem Biol, 8, 832–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mladenova D, Barry G, Konen LM, Pineda SS, Guennewig B, Avesson L, Zinn R, Schonrock N, Bitar M, Jonkhout N, Crumlish L, Kaczorowski DC, Gong A, Pinese M, Franco GR, Walkley CR, Vissel B, Mattick JS. 2018. Adar3 Is Involved in Learning and Memory in Mice. Front Neurosci, 12, 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohibi S, Chen X, Zhang J. 2019. Cancer the’RBP’eutics-RNA-binding proteins as therapeutic targets for cancer. Pharmacol Ther, 203, 107390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montiel-Gonzalez MF, Diaz Quiroz JF, Rosenthal JJC. 2019. Current strategies for Site-Directed RNA Editing using ADARs. Methods, 156, 16–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montiel-Gonzalez MF, Vallecillo-Viejo I, Yudowski GA, Rosenthal JJ. 2013. Correction of mutations within the cystic fibrosis transmembrane conductance regulator by site-directed RNA editing. Proc Natl Acad Sci U S A, 110, 18285–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montiel-Gonzalez MF, Vallecillo-Viejo IC, Rosenthal JJ. 2016. An efficient system for selectively altering genetic information within mRNAs. Nucleic Acids Res, 44, e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton VR, Suarez-Fueyo A, Meidan E, Li H, Mizui M, Tsokos GC. 2017. Pathogenesis of Human Systemic Lupus Erythematosus: A Cellular Perspective. Trends Mol Med, 23, 615–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanduri S, Carpick BW, Yang Y, Williams BR, Qin J. 1998. Structure of the double-stranded RNA-binding domain of the protein kinase PKR reveals the molecular basis of its dsRNA-mediated activation. EMBO J, 17, 5458–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikura K 2010. Functions and regulation of RNA editing by ADAR deaminases. Annu Rev Biochem, 79, 321–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikura K, Yoo C, Kim U, Murray JM, Estes PA, Cash FE, Liebhaber SA. 1991. Substrate specificity of the dsRNA unwinding/modifying activity. EMBO J, 10, 3523–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes E, Anderson A, Cohen-Gadol A, Hundley HA. 2017. Adenosine Deaminase That Acts on RNA 3 (ADAR3) Binding to Glutamate Receptor Subunit B Pre-mRNA Inhibits RNA Editing in Glioblastoma. J Biol Chem, 292, 4326–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DB, Kim YG, Rich A. 2002. Z-DNA-binding proteins can act as potent effectors of gene expression in vivo. Proc Natl Acad Sci U S A, 99, 16666–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlson J, Enstero M, Sjoberg BM, Ohman M. 2005. A method to find tissue-specific novel sites of selective adenosine deamination. Nucleic Acids Res, 33, e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman M, Kallman AM, Bass BL. 2000. In vitro analysis of the binding of ADAR2 to the pre-mRNA encoding the GluR-B R/G site. RNA, 6, 687–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota H, Sakurai M, Gupta R, Valente L, Wulff BE, Ariyoshi K, Iizasa H, Davuluri RV, Nishikura K. 2013. ADAR1 forms a complex with Dicer to promote microRNA processing and RNA-induced gene silencing. Cell, 153, 575–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palavicini JP, Correa-Rojas RA, Rosenthal JJ. 2012. Extra double-stranded RNA binding domain (dsRBD) in a squid RNA editing enzyme confers resistance to high salt environment. J Biol Chem, 287, 17754–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palavicini JP, O’Connell MA, Rosenthal JJ. 2009. An extra double-stranded RNA binding domain confers high activity to a squid RNA editing enzyme. RNA, 15, 1208–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino MJ, Keegan LP, O’Connell MA, Reenan RA. 2000. dADAR, a Drosophila double-stranded RNA-specific adenosine deaminase is highly developmentally regulated and is itself a target for RNA editing. RNA, 6, 1004–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino MJ, Keegan LP, O’Connell MA, Reenan RA. 2000. A-to-I pre-mRNA editing in Drosophila is primarily involved in adult nervous system function and integrity. Cell, 102, 437–49 [DOI] [PubMed] [Google Scholar]
- Parrish S, Fire A. 2001. Distinct roles for RDE-1 and RDE-4 during RNA interference in Caenorhabditis elegans. RNA, 7, 1397–402 [PMC free article] [PubMed] [Google Scholar]
- Patterson JB, Samuel CE. 1995. Expression and regulation by interferon of a double-stranded-RNA-specific adenosine deaminase from human cells: evidence for two forms of the deaminase. Mol Cell Biol, 15, 5376–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz N, Levanon EY, Amariglio N, Heimberger AB, Ram Z, Constantini S, Barbash ZS, Adamsky K, Safran M, Hirschberg A, Krupsky M, Ben-Dov I, Cazacu S, Mikkelsen T, Brodie C, Eisenberg E, Rechavi G. 2007. Altered adenosine-to-inosine RNA editing in human cancer. Genome Res, 17, 1586–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Yaacov N, Bazak L, Buchumenski I, Porath HT, Danan-Gotthold M, Knisbacher BA, Eisenberg E, Levanon EY. 2015. Elevated RNA Editing Activity Is a Major Contributor to Transcriptomic Diversity in Tumors. Cell Rep, 13, 267–76 [DOI] [PubMed] [Google Scholar]
- Peng X, Xu X, Wang Y, Hawke DH, Yu S, Han L, Zhou Z, Mojumdar K, Jeong KJ, Labrie M, Tsang YH, Zhang M, Lu Y, Hwu P, Scott KL, Liang H, Mills GB. 2018. A-to-I RNA Editing Contributes to Proteomic Diversity in Cancer. Cancer Cell, 33, 817–28 e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn AC, Balik A, Greger IH. 2013. Steric antisense inhibition of AMPA receptor Q/R editing reveals tight coupling to intronic editing sites and splicing. Nucleic Acids Res, 41, 1113–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestal K, Funk CC, Snyder JM, Price ND, Treuting PM, Stetson DB. 2015. Isoforms of RNA-Editing Enzyme ADAR1 Independently Control Nucleic Acid Sensor MDA5-Driven Autoimmunity and Multi-organ Development. Immunity, 43, 933–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picardi E, Manzari C, Mastropasqua F, Aiello I, D’Erchia AM, Pesole G. 2015. Profiling RNA editing in human tissues: towards the inosinome Atlas. Sci Rep, 5, 14941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto Y, Buchumenski I, Levanon EY, Eisenberg E. 2018. Human cancer tissues exhibit reduced A-to-I editing of miRNAs coupled with elevated editing of their targets. Nucleic Acids Res, 46, 71–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polson AG, Bass BL. 1994. Preferential selection of adenosines for modification by double-stranded RNA adenosine deaminase. EMBO J, 13, 5701–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porath HT, Schaffer AA, Kaniewska P, Alon S, Eisenberg E, Rosenthal J, Levanon EY, Levy O. 2017. A-to-I RNA Editing in the Earliest-Diverging Eumetazoan Phyla. Mol Biol Evol, 34, 1890–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen H, Jorgensen R, Heding A, Nielsen FC, Bonven B, Egebjerg J. 2006. Dimerization of ADAR2 is mediated by the double-stranded RNA binding domain. RNA, 12, 1350–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen H, Nilsson J, Damgaard CK, Egebjerg J, Kjems J. 2001. CRM1 mediates the export of ADAR1 through a nuclear export signal within the Z-DNA binding domain. Mol Cell Biol, 21, 7862–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasanth KV, Prasanth SG, Xuan Z, Hearn S, Freier SM, Bennett CF, Zhang MQ, Spector DL. 2005. Regulating gene expression through RNA nuclear retention. Cell, 123, 249–63 [DOI] [PubMed] [Google Scholar]
- Qin YR, Qiao JJ, Chan TH, Zhu YH, Li FF, Liu H, Fei J, Li Y, Guan XY, Chen L. 2014. Adenosine-to-inosine RNA editing mediated by ADARs in esophageal squamous cell carcinoma. Cancer Res, 74, 840–51 [DOI] [PubMed] [Google Scholar]
- Quinones-Valdez G, Tran SS, Jun HI, Bahn JH, Yang EW, Zhan L, Brummer A, Wei X, Van Nostrand EL, Pratt GA, Yeo GW, Graveley BR, Xiao X. 2019. Regulation of RNA editing by RNA-binding proteins in human cells. Commun Biol, 2, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendren S, Manning AC, Al-Awadi H, Yamada K, Takagi Y, Hundley HA. 2018. A protein-protein interaction underlies the molecular basis for substrate recognition by an adenosine-to-inosine RNA-editing enzyme. Nucleic Acids Res, 46, 9647–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos A, Grunert S, Adams J, Micklem DR, Proctor MR, Freund S, Bycroft M, St Johnston D, Varani G. 2000. RNA recognition by a Staufen double-stranded RNA-binding domain. EMBO J, 19, 997–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich DP, Tyc KM, Bass BL. 2018. C. elegans ADARs antagonize silencing of cellular dsRNAs by the antiviral RNAi pathway. Genes Dev, 32, 271–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice GI, Del Toro Duany Y, Jenkinson EM, Forte GM, Anderson BH, Ariaudo G, Bader-Meunier B, Baildam EM, Battini R, Beresford MW, Casarano M, Chouchane M, Cimaz R, Collins AE, Cordeiro NJ, Dale RC, Davidson JE, De Waele L, Desguerre I, Faivre L, Fazzi E, Isidor B, Lagae L, Latchman AR, Lebon P, Li C, Livingston JH, Lourenco CM, Mancardi MM, Masurel-Paulet A, McInnes IB, Menezes MP, Mignot C, O’Sullivan J, Orcesi S, Picco PP, Riva E, Robinson RA, Rodriguez D, Salvatici E, Scott C, Szybowska M, Tolmie JL, Vanderver A, Vanhulle C, Vieira JP, Webb K, Whitney RN, Williams SG, Wolfe LA, Zuberi SM, Hur S, Crow YJ. 2014. Gain-of-function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I interferon signaling. Nat Genet, 46, 503–09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice GI, Kasher PR, Forte GM, Mannion NM, Greenwood SM, Szynkiewicz M, Dickerson JE, Bhaskar SS, Zampini M, Briggs TA, Jenkinson EM, Bacino CA, Battini R, Bertini E, Brogan PA, Brueton LA, Carpanelli M, De Laet C, de Lonlay P, del Toro M, Desguerre I, Fazzi E, Garcia-Cazorla A, Heiberg A, Kawaguchi M, Kumar R, Lin JP, Lourenco CM, Male AM, Marques W Jr., Mignot C, Olivieri I, Orcesi S, Prabhakar P, Rasmussen M, Robinson RA, Rozenberg F, Schmidt JL, Steindl K, Tan TY, van der Merwe WG, Vanderver A, Vassallo G, Wakeling EL, Wassmer E, Whittaker E, Livingston JH, Lebon P, Suzuki T, McLaughlin PJ, Keegan LP, O’Connell MA, Lovell SC, Crow YJ. 2012. Mutations in ADAR1 cause Aicardi-Goutieres syndrome associated with a type I interferon signature. Nat Genet, 44, 1243–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert X, Gouet P. 2014. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res, 42, W320–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal JJ. 2015. The emerging role of RNA editing in plasticity. J Exp Biol, 218, 1812–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth SH, Danan-Gotthold M, Ben-Izhak M, Rechavi G, Cohen CJ, Louzoun Y, Levanon EY. 2018. Increased RNA Editing May Provide a Source for Autoantigens in Systemic Lupus Erythematosus. Cell Rep, 23, 50–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueter SM, Dawson TR, Emeson RB. 1999. Regulation of alternative splicing by RNA editing. Nature, 399, 75–80 [DOI] [PubMed] [Google Scholar]
- Ryter JM, Schultz SC. 1998. Molecular basis of double-stranded RNA-protein interactions: structure of a dsRNA-binding domain complexed with dsRNA. EMBO J, 17, 7505–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders LR, Barber GN. 2003. The dsRNA binding protein family: critical roles, diverse cellular functions. FASEB J, 17, 961–83 [DOI] [PubMed] [Google Scholar]
- Savva YA, Rieder LE, Reenan RA. 2012. The ADAR protein family. Genome Biol, 13, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scadden AD. 2005. The RISC subunit Tudor-SN binds to hyper-edited double-stranded RNA and promotes its cleavage. Nat Struct Mol Biol, 12, 489–96 [DOI] [PubMed] [Google Scholar]
- Scadden AD. 2007. Inosine-containing dsRNA binds a stress-granule-like complex and downregulates gene expression in trans. Mol Cell, 28, 491–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scadden AD, O’Connell MA. 2005. Cleavage of dsRNAs hyper-edited by ADARs occurs at preferred editing sites. Nucleic Acids Res, 33, 5954–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scadden AD, Smith CW. 2001. Specific cleavage of hyper-edited dsRNAs. EMBO J, 20, 4243–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MF, Wettengel J, Hoffmann PC, Stafforst T. 2014. Optimal guideRNAs for redirecting deaminase activity of hADAR1 and hADAR2 in trans. Nucleic Acids Res, 42, e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoft VK, Schopoff S, Jantsch MF. 2007. Regulation of glutamate receptor B pre-mRNA splicing by RNA editing. Nucleic Acids Res, 35, 3723–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastiani P, Montano M, Puca A, Solovieff N, Kojima T, Wang MC, Melista E, Meltzer M, Fischer SE, Andersen S, Hartley SH, Sedgewick A, Arai Y, Bergman A, Barzilai N, Terry DF, Riva A, Anselmi CV, Malovini A, Kitamoto A, Sawabe M, Arai T, Gondo Y, Steinberg MH, Hirose N, Atzmon G, Ruvkun G, Baldwin CT, Perls TT. 2009. RNA editing genes associated with extreme old age in humans and with lifespan in C. elegans. PLoS One, 4, e8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth RB, Sun L, Ea CK, Chen ZJ. 2005. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell, 122, 669–82 [DOI] [PubMed] [Google Scholar]
- Shallev L, Kopel E, Feiglin A, Leichner GS, Avni D, Sidi Y, Eisenberg E, Barzilai A, Levanon EY, Greenberger S. 2018. Decreased A-to-I RNA editing as a source of keratinocytes’ dsRNA in psoriasis. RNA, 24, 828–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko G, Morris KV. 2018. All I’s on the RADAR: role of ADAR in gene regulation. FEBS Lett, 592, 2860–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeyasu K, Okugawa Y, Toden S, Miyoshi J, Toiyama Y, Nagasaka T, Takahashi N, Kusunoki M, Takayama T, Yamada Y, Fujiwara T, Chen L, Goel A. 2018. AZIN1 RNA editing confers cancer stemness and enhances oncogenic potential in colorectal cancer. JCI Insight, 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnamon JR, Kim SY, Corson GM, Song Z, Nakai H, Adelman JP, Mandel G. 2017. Site-directed RNA repair of endogenous Mecp2 RNA in neurons. Proc Natl Acad Sci U S A, 114, E9395–E402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnamon JR, Kim SY, Fisk JR, Song Z, Nakai H, Jeng S, McWeeney SK, Mandel G. 2020. In Vivo Repair of a Protein Underlying a Neurological Disorder by Programmable RNA Editing. Cell Rep, 32, 107878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin W, Nishikura K. 2013. Adenosine-to-inosine RNA editing and human disease. Genome Med, 5, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon O, Oren S, Safran M, Deshet-Unger N, Akiva P, Jacob-Hirsch J, Cesarkas K, Kabesa R, Amariglio N, Unger R, Rechavi G, Eyal E. 2013. Global regulation of alternative splicing by adenosine deaminase acting on RNA (ADAR). RNA, 19, 591–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song IH, Kim YA, Heo SH, Park IA, Lee M, Bang WS, Park HS, Gong G, Lee HJ. 2017. ADAR1 expression is associated with tumour-infiltrating lymphocytes in triple-negative breast cancer. Tumour Biol, 39, 1010428317734816 [DOI] [PubMed] [Google Scholar]
- Song Y, Yang W, Fu Q, Wu L, Zhao X, Zhang Y, Zhang R. 2020. irCLASH reveals RNA substrates recognized by human ADARs. Nat Struct Mol Biol [DOI] [PubMed] [Google Scholar]
- St Johnston D, Brown NH, Gall JG, Jantsch M. 1992. A conserved double-stranded RNA-binding domain. Proc Natl Acad Sci U S A, 89, 10979–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Laurent G, Tackett MR, Nechkin S, Shtokalo D, Antonets D, Savva YA, Maloney R, Kapranov P, Lawrence CE, Reenan RA. 2013. Genome-wide analysis of A-to-I RNA editing by single-molecule sequencing in Drosophila. Nat Struct Mol Biol, 20, 1333–9 [DOI] [PubMed] [Google Scholar]
- Stafforst T, Schneider MF. 2012. An RNA-deaminase conjugate selectively repairs point mutations. Angew Chem Int Ed Engl, 51, 11166–9 [DOI] [PubMed] [Google Scholar]
- Stefl R, Oberstrass FC, Hood JL, Jourdan M, Zimmermann M, Skrisovska L, Maris C, Peng L, Hofr C, Emeson RB, Allain FH. 2010. The solution structure of the ADAR2 dsRBM-RNA complex reveals a sequence-specific readout of the minor groove. Cell, 143, 225–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefl R, Xu M, Skrisovska L, Emeson RB, Allain FH. 2006. Structure and specific RNA binding of ADAR2 double-stranded RNA binding motifs. Structure, 14, 345–55 [DOI] [PubMed] [Google Scholar]
- Stephens OM, Haudenschild BL, Beal PA. 2004. The binding selectivity of ADAR2’s dsRBMs contributes to RNA-editing selectivity. Chem Biol, 11, 1239–50 [DOI] [PubMed] [Google Scholar]
- Stephens OM, Yi-Brunozzi HY, Beal PA. 2000. Analysis of the RNA-editing reaction of ADAR2 with structural and fluorescent analogues of the GluR-B R/G editing site. Biochemistry, 39, 12243–51 [DOI] [PubMed] [Google Scholar]
- Strehblow A, Hallegger M, Jantsch MF. 2002. Nucleocytoplasmic distribution of human RNA-editing enzyme ADAR1 is modulated by double-stranded RNA-binding domains, a leucine-rich export signal, and a putative dimerization domain. Mol Biol Cell, 13, 3822–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y, Vigilante A, Darbo E, Zirra A, Militti C, D’Ambrogio A, Luscombe NM, Ule J. 2015. hiCLIP reveals the in vivo atlas of mRNA secondary structures recognized by Staufen 1. Nature, 519, 491–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabara H, Yigit E, Siomi H, Mello CC. 2002. The dsRNA binding protein RDE-4 interacts with RDE-1, DCR-1, and a DExH-box helicase to direct RNAi in C. elegans. Cell, 109, 861–71 [DOI] [PubMed] [Google Scholar]
- Tajaddod M, Jantsch MF, Licht K. 2016. The dynamic epitranscriptome: A to I editing modulates genetic information. Chromosoma, 125, 51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SJ, Shen H, An O, Hong H, Li J, Song Y, Han J, Tay DJT, Ng VHE, Bellido Molias F, Leong KW, Pitcheshwar P, Yang H, Chen L. 2020. Cis- and trans-regulations of pre-mRNA splicing by RNA editing enzymes influence cancer development. Nat Commun, 11, 799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang ZL, Wang S, Tu C, Wang T, Ma CW, Liu Y, Xiao SX, Wang XP. 2018. Eight Novel Mutations of the ADAR1 Gene in Chinese Patients with Dyschromatosis Symmetrica Hereditaria. Genet Test Mol Biomarkers, 22, 104–08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JM, Beal PA. 2017. How do ADARs bind RNA? New protein-RNA structures illuminate substrate recognition by the RNA editing ADARs. Bioessays, 39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuy-Boun AS, Thomas JM, Grajo HL, Palumbo CM, Park S, Nguyen LT, Fisher AJ, Beal PA. 2020. Asymmetric dimerization of adenosine deaminase acting on RNA facilitates substrate recognition. Nucleic Acids Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli S, Galeano F, Alon S, Raho S, Galardi S, Polito VA, Presutti C, Vincenti S, Eisenberg E, Locatelli F, Gallo A. 2015. Modulation of microRNA editing, expression and processing by ADAR2 deaminase in glioblastoma. Genome Biol, 16, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkin LA, Saccomanno L, Morse DP, Brodigan T, Krause M, Bass BL. 2002. RNA editing by ADARs is important for normal behavior in Caenorhabditis elegans. EMBO J, 21, 6025–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente L, Nishikura K. 2007. RNA binding-independent dimerization of adenosine deaminases acting on RNA and dominant negative effects of nonfunctional subunits on dimer functions. J Biol Chem, 282, 16054–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallecillo-Viejo IC, Liscovitch-Brauer N, Montiel-Gonzalez MF, Eisenberg E, Rosenthal JJC. 2018. Abundant off-target edits from site-directed RNA editing can be reduced by nuclear localization of the editing enzyme. RNA Biol, 15, 104–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vik ES, Nawaz MS, Strøm Andersen P, Fladeby C, Bjørås M, Dalhus B, Alseth I. 2013. Endonuclease V cleaves at inosines in RNA. Nat Commun, 4, 2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitali P, Scadden AD. 2010. Double-stranded RNAs containing multiple IU pairs are sufficient to suppress interferon induction and apoptosis. Nat Struct Mol Biol, 17, 1043–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstedt H, Ohman M. 2011. Site-selective versus promiscuous A-to-I editing. Wiley Interdiscip Rev RNA, 2, 761–71 [DOI] [PubMed] [Google Scholar]
- Walkley CR, Li JB. 2017. Rewriting the transcriptome: adenosine-to-inosine RNA editing by ADARs. Genome Biol, 18, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Yu S, Liu J, Zhang D, Kang X. 2019. Seven novel mutations of ADAR in multi-ethnic pedigrees with dyschromatosis symmetrica hereditaria in China. Mol Genet Genomic Med, 7, e00905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Hui H, Guo Z, Zhang W, Hu Y, He T, Tai Y, Peng P, Wang L. 2013. ADAR1 regulates ARHGAP26 gene expression through RNA editing by disrupting miR-30b-3p and miR-573 binding. RNA, 19, 1525–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Miyakoda M, Yang W, Khillan J, Stachura DL, Weiss MJ, Nishikura K. 2004. Stress-induced apoptosis associated with null mutation of ADAR1 RNA editing deaminase gene. J Biol Chem, 279, 4952–61 [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhang Z, Blackwell K, Carmichael GG. 2005. Vigilins bind to promiscuously A-to-I-edited RNAs and are involved in the formation of heterochromatin. Curr Biol, 15, 384–91 [DOI] [PubMed] [Google Scholar]
- Wang R, Zhang S, Chen X, Li N, Li J, Jia R, Pan Y, Liang H. 2018. CircNT5E Acts as a Sponge of miR-422a to Promote Glioblastoma Tumorigenesis. Cancer Res, 78, 4812–25 [DOI] [PubMed] [Google Scholar]
- Wang Y, Chung DH, Monteleone LR, Li J, Chiang Y, Toney MD, Beal PA. 2019. RNA binding candidates for human ADAR3 from substrates of a gain of function mutant expressed in neuronal cells. Nucleic Acids Res, 47, 10801–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Park S, Beal PA. 2018. Selective Recognition of RNA Substrates by ADAR Deaminase Domains. Biochemistry, 57, 1640–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xu X, Yu S, Jeong KJ, Zhou Z, Han L, Tsang YH, Li J, Chen H, Mangala LS, Yuan Y, Eterovic AK, Lu Y, Sood AK, Scott KL, Mills GB, Liang H. 2017. Systematic characterization of A-to-I RNA editing hotspots in microRNAs across human cancers. Genome Res, 27, 1112–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zheng Y, Beal PA. 2017. Adenosine Deaminases That Act on RNA (ADARs). Enzymes, 41, 215–68 [DOI] [PubMed] [Google Scholar]
- Ward SV, George CX, Welch MJ, Liou LY, Hahm B, Lewicki H, de la Torre JC, Samuel CE, Oldstone MB. 2011. RNA editing enzyme adenosine deaminase is a restriction factor for controlling measles virus replication that also is required for embryogenesis. Proc Natl Acad Sci U S A, 108, 331–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warf MB, Shepherd BA, Johnson WE, Bass BL. 2012. Effects of ADARs on small RNA processing pathways in C. elegans. Genome Res, 22, 1488–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn MC, Hundley HA. 2016. Trans and cis factors affecting A-to-I RNA editing efficiency of a noncoding editing target in C. elegans. RNA, 22, 722–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn MC, Kakaradov B, Sundararaman B, Wheeler E, Hoon S, Yeo GW, Hundley HA. 2014. The dsRBP and inactive editor ADR-1 utilizes dsRNA binding to regulate A-to-I RNA editing across the C. elegans transcriptome. Cell Rep, 6, 599–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F, Wagner V, Rasmussen SB, Hartmann R, Paludan SR. 2006. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J Virol, 80, 5059–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettengel J, Reautschnig P, Geisler S, Kahle PJ, Stafforst T. 2017. Harnessing human ADAR2 for RNA repair - Recoding a PINK1 mutation rescues mitophagy. Nucleic Acids Res, 45, 2797–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler EC, Washburn MC, Major F, Rusch DB, Hundley HA. 2015. Noncoding regions of C. elegans mRNA undergo selective adenosine to inosine deamination and contain a small number of editing sites per transcript. RNA Biol, 12, 162–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolozin B, Ivanov P. 2019. Stress granules and neurodegeneration. Nat Rev Neurosci, 20, 649–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf TM, Chase JM, Stinchcomb DT. 1995. Toward the therapeutic editing of mutated RNA sequences. Proc Natl Acad Sci U S A, 92, 8298–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Q, Wang M, Chen X, Qian X, Qin W, Gao J, Wu S, Gao R, Feng G, He L. 2005. Identification of a novel ADAR mutation in a Chinese family with dyschromatosis symmetrica hereditaria (DSH). Arch Dermatol Res, 297, 139–42 [DOI] [PubMed] [Google Scholar]
- Xu X, Wang Y, Mojumdar K, Zhou Z, Jeong KJ, Mangala LS, Yu S, Tsang YH, Rodriguez-Aguayo C, Lu Y, Lopez-Berestein G, Sood AK, Mills GB, Liang H. 2019. A-to-I-edited miRNA-379–5p inhibits cancer cell proliferation through CD97-induced apoptosis. J Clin Invest, 129, 5343–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CC, Chen YT, Chang YF, Liu H, Kuo YP, Shih CT, Liao WC, Chen HW, Tsai WS, Tan BC. 2017. ADAR1-mediated 3’ UTR editing and expression control of antiapoptosis genes fine-tunes cellular apoptosis response. Cell Death Dis, 8, e2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Deng P, Zhu Z, Zhu J, Wang G, Zhang L, Chen AF, Wang T, Sarkar SN, Billiar TR, Wang Q. 2014. Adenosine deaminase acting on RNA 1 limits RIG-I RNA detection and suppresses IFN production responding to viral and endogenous RNAs. J Immunol, 193, 3436–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Chendrimada TP, Wang Q, Higuchi M, Seeburg PH, Shiekhattar R, Nishikura K. 2006. Modulation of microRNA processing and expression through RNA editing by ADAR deaminases. Nat Struct Mol Biol, 13, 13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Fritsche J, Roszik J, Williams LJ, Peng X, Chiu Y, Tsou CC, Hoffgaard F, Goldfinger V, Schoor O, Talukder A, Forget MA, Haymaker C, Bernatchez C, Han L, Tsang YH, Kong K, Xu X, Scott KL, Singh-Jasuja H, Lizee G, Liang H, Weinschenk T, Mills GB, Hwu P. 2018. RNA editing derived epitopes function as cancer antigens to elicit immune responses. Nat Commun, 9, 3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XJ, He PP, Li M, He CD, Yan KL, Cui Y, Yang S, Zhang KY, Gao M, Chen JJ, Li CR, Jin L, Chen HD, Xu SJ, Huang W. 2004. Seven novel mutations of the ADAR gene in Chinese families and sporadic patients with dyschromatosis symmetrica hereditaria (DSH). Hum Mutat, 23, 629–30 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang K, Zhao Z, Sun S, Zhang K, Huang R, Zeng F, Hu H. 2018. ADAR3 expression is an independent prognostic factor in lower-grade diffuse gliomas and positively correlated with the editing level of GRIA2(Q607R). Cancer Cell Int, 18, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Carmichael GG. 2001. The fate of dsRNA in the nucleus: a p54(nrb)-containing complex mediates the nuclear retention of promiscuously A-to-I edited RNAs. Cell, 106, 465–75 [DOI] [PubMed] [Google Scholar]