Abstract
Nitric oxide (NO) is a ubiquitous signaling molecule that is critical for supporting a plethora of processes in biological organisms. Among these, its role in the innate immune system as a first line of defense against pathogens has received less attention. In asthma, levels of exhaled NO have been utilized as a window into airway inflammation caused by allergic processes. However, respiratory infections count among the most important triggers of disease exacerbations. Among the multitude of factors that affect NO levels are psychological processes. In particular, longer lasting states of psychological stress and depression have been shown to attenuate NO production. The novel SARS-CoV-2 virus, which has caused a pandemic, and with that, sustained levels of psychological stress globally, also adversely affects NO signaling. We review evidence on the role of NO in respiratory infection, including COVID-19, and stress, and argue that boosting NO bioavailability may be beneficial in protection from infections, thus benefitting individuals who suffer from stress in asthma or SARS-CoV-2 infection.
Keywords: nitric oxide, Respiratory infection, Psychological stress, Asthma, SARS-CoV-2, Nitric oxide donor, Dietary nitrate
Highlights
-
•
Asthma, respiratory infections, and COVID-19, pose a global health care burden.
-
•
Airway nitric oxide provides a major innate defense against respiratory infection.
-
•
Stress, depression, and COVID-19 compromise nitric oxide production.
-
•
Dietary nitrate, including beetroot juice, boost nitric oxide production.
-
•
Beetroot juice supplementation protects against respiratory infection.
1. Introduction
Asthma and respiratory infections are diseases that cause an enormous health care, economical, societal, and individual burden. The COVID-19 pandemic is the most recent dramatic example for the global scale of the threat from respiratory disease. Psychological factors are known to affect asthma and respiratory infections and their burden in turn can affect psychological and behavioral functioning. Airway nitric oxide (NO) has been identified as a protective factor against respiratory pathogens. Recent research has shown that NO production is linked to psychological factor. In the following, we review the role of NO and stress in asthma and respiratory infection, including COVID-19. We propose that boosting airway NO may benefit the innate immune defense and elevate resistance against respiratory infections in psychological stress and other conditions of heightened vulnerability.
2. The burden of asthma and respiratory infections
Asthma is a common chronic respiratory disease affecting an estimated 339 million people worldwide (Global Asthma Network, 2018), including 25.7 million individuals in the US alone. Among children and adolescents, asthma is the most common chronic illness and has continued to increase in prevalence in the past several decades. Asthma extolls a substantial burden on the patients’ well-being and can be life threatening if not controlled adequately. Asthma-related morbidity and mortality highlight the need for better control and exacerbation prevention. With approximately $56 billion each year in direct health care costs and lost productivity (Barnett and Nurmagambetov, 2011), the economic costs of asthma are significant, as are the costs to the sufferer’s well-being. Although medical treatment has improved, overall asthma control remains unsatisfactory.
Respiratory tract infections are the leading cause of physician visits in developed countries (Shann et al., 1999), accounting for an estimated 10 million outpatient visits a year (Thomas et al., 2020). Their adverse effects on personal well-being, mood, cognition, and performance are well-known (Smith, 2016). The economic costs of respiratory tract infections are high with an estimated $22 billion annual and accounting for more than 20 million missed days of school and work in the US (Fendrick et al., 2003; Adams et al., 1999). Respiratory infections are difficult to treat (Musher and Thorner, 2014). Most recently, COVID-19 has increased the economic, health care, and psychological burden of respiratory virus infections worldwide dramatically (Subbaro and Mahanty, 2020).
3. Asthma and respiratory infections
Asthma is a chronic inflammatory airway disease characterized by episodic exacerbations, which are associated with distressing symptoms of shortness of breath, chest tightness, cough, and wheezing (National Heart Lung and Blood Institute (NHLBI), 2007). Symptom worsening, loss of asthma control, or exacerbations can be precipitated by various allergens, air pollution or irritants, weather/climate changes, respiratory infections, exercise, or psychological stress, as suggested by both observation and challenge tests and patient self-report (Ritz et al., 2006, 2008, 2016). Upper respiratory infections and common colds are particularly potent triggers of exacerbations and are estimated to be a factor in up to 80–90% of patients (Busse et al., 2010; Jartti and Gern, 2017). Compared to allergic triggers, which can be more salient and often can be avoided more easily, infections are less easily evaded (especially in critical seasons). Allergic inflammation of the airways interacts with respiratory viruses (Edwards et al., 2017), resulting in more severe and longer illness periods in asthma and increased asthma exacerbations.
4. Psychological stress and respiratory infections
People commonly report that they contract colds during stressful periods of their lives. Research has indeed demonstrated that psychosocial stress is associated with cold symptoms or upper respiratory tract infections (Falagas et al., 2010; Pedersen et al., 2010). Self-reported stress is associated with common cold symptoms in both children and adults (Cobb and Steptoe, 1996; Evans and Edgerton, 1991; Smolderen et al., 2007; Turner-Cobb and Steptoe, 1998) and experimental infection studies have shown associations between the development of cold symptoms and perceived stress, lack of social support, or loneliness. (Cohen et al., 1991; LeRoy et al., 2017; Stone et al., 1992). Virtually all major constructs of negative affect, including anxiety, depression, neuroticism, perceived stress, chronic stress, negative life-events, or daily hassles have been associated with susceptibility to respiratory infections or outcomes such as cold symptom severity or duration (Falagas et al., 2010). Importantly, associations with stress do not vary whether studies monitor biological indicators of respiratory infection or cold symptoms (Pedersen et al., 2010).
Biological factors underlying the stress-infection association have also been explored, with studies suggesting a role for systemic proinflammatory cytokine production (Cohen et al., 1999), natural killer cell cytotoxicity (Cohen et al., 2002), catecholamine levels (Cohen et al., 1997), leukocyte telomere length (Cohen et al., 2013), cardiovascular stress reactivity (Boyce et al., 1995), and cortisol levels or cortisol stress reactivity (Cohen et al., 2002; Janicki-Deverts et al., 2016). Comparably fewer studies have explored local airway processes, in particular airway mucosal immune responses, to stress (Trueba and Ritz, 2013). Cohen et al. (1997) found that social support increased mucociliary clearance of infection. A number of studies have examined immunoglobulin A (IgA) (Bosch et al., 2004) and other molecules relevant to adaptive and innate immunity extracted from saliva (Bosch et al., 2003), but a clear relationship with cold symptoms has not been established and factors more directly linked to airway mucosal immunity have not been studied in detail.
5. Asthma, psychological stress, and respiratory infections
Asthma has long been associated with emotions and psychological stress (Lehrer et al., 2002; Ritz et al., 2013). Anecdotal and clinical reports have detailed psychologically induced asthma symptoms or exacerbations. Longitudinal epidemiological research has also made progress in demonstrating the role of psychopathology and stress as a precursor of asthmatic pathology. Additionally, laboratory studies have demonstrated the susceptibility of the airway to stress induction (Ritz, 2012) and observational studies have detailed associations between life stress factors, airway inflammation, and asthma management outcomes (Chen and Schreier, 2008; Kullowatz et al., 2008; Liu et al., 2002; Rosenkranz et al., 2016). Consequently, asthma treatment and management guidelines have listed stress or psychosocial influences among both host factors that favor the development of asthma and as antecedent factors triggering exacerbations of existing asthma (NHLBI, 2007). Research with a psychometrically validated perceived asthma trigger questionnaire has shown that patients who experience psychological triggers suffer from low perceived control of asthma, high anxiety and depression, and a reduced quality of life (Ritz et al., 2006, 2008, 2016). In addition, among all perceived triggers, only psychological triggers are consistently associated with a lower asthma control, explaining up to 10% of the variance in symptoms, daily life function, nighttime sleep, and bronchodilator use (Ritz et al., 2014a, Ritz et al., 2014b). Psychological triggers are associated with physician-recorded asthma exacerbations and emergency treatments over and above other trigger factors, anxiety, and depression (Ritz et al., 2016). Elevated susceptibility for respiratory infections with stress is one pathway for the effects of stress on asthma (Trueba and Ritz, 2013; Wright et al., 1998). Perceived psychological asthma triggers usually correlate with respiratory infection triggers (Ritz et al., 2006, 2008). However, there is a lack of longitudinal studies that monitor stress, respiratory infections or common cold symptoms, and asthma control across periods of critical life stress.
6. The COVID-19 pandemic: effects on psychological well-being
The recent COVID-19 pandemic has disrupted or destroyed regular daily life, social networks, livelihoods, and lives of billions of people around the globe. Whereas early into this crisis, detrimental mental health effects on health care personnel, who works on the frontlines of patient care, have been recognized (Lai et al., 2020), increasingly the broader impact on global mental health is being documented (Szcześniak et al., 2021; Torales et al., 2020). The threat of infection and potential long-term health consequences or death, combined with stay-at-home orders or lockdowns, loss of occupation, and separation from families and friends, have created multiple sources of challenges that are prototypes of intense and sustained stress, which can result in helplessness, hopelessness, and depression. Although challenges vary and subpopulations can mobilize different resources and resiliency factors (Nikolaidis et al., 2021), even young and healthy populations such as college students can show mood effects that predispose to depression (Elmer et al., 2020; Hasratian, under review). Given the evidence on stress and respiratory infections, it can be expected psychological factors can worsen the course of this illness.
7. Airway NO in asthma
NO is a ubiquitous signaling molecule employed in multiple organismic processes, including vasodilation and bronchodilation, impulse transmission in peripheral and central nervous system, and inflammation (Förstermann and Sessa, 2012). At the molecular level, NO has an unpaired electron in the ground state and exists as a free radical under biological conditions (Fukuto et al., 2012). As a free radical, reactions are generally fast with other radical species. NO reacts with oxygen (O2) in a termolecular process to produce two equivalents of nitrogen dioxide (NO2•), which can subsequently react with another equivalent of NO to form dinitrogen trioxide (N2O3) (Wink et al., 1992). N2O3 is a reactive nitrosating species that can react with thiols, amines, and other biomolecules to form nitroso compounds that have important roles in cellular signaling, but also potentially deleterious effects (Hess et al., 2005). N2O3 will ultimately decompose to form nitrite (NO2–), which is often used as a marker of NO production. The oxidation of NO by oxygen is second order in NO, which means the rate the reaction of NO with oxygen is slow at low concentrations of NO and faster at higher concentrations of NO. This is an important consideration when comparing the signaling of low levels of NO to pharmacological or even pathological effects at higher levels of NO, due to the production of highly reactive oxygen species. NO can also react with superoxide (O2•–) to produce peroxynitrite (ONOO–), which can further react to form the indiscriminately damaging hydroxyl radical (HO•) and nitrogen dioxide (NO2•) (Pryor and Squadrito, 1995). Additionally, NO can react with reductants like hydrogen sulfide (H2S) or other cellular reductants to produce azanone (nitroxyl, HNO), a molecule that can quickly react with ubiquitous biological nucleophiles by two-electron mechanisms (An et al., 2019). Although N2O3, ONOO–, HNO, and other reactive species do have proposed roles for cellular signaling, they can cause deleterious cell damage, and are more likely to be produced when levels of NO exceed healthy physiological concentrations. These considerations highlight the fine details that must be considered when using NO or NO donors in a therapeutic approach (Daiber and Münzel, 2015).
NO has been measured in exhaled air as the fraction of exhaled NO (FeNO) ("ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005," 2005; Dweik et al., 2011). In asthma, FeNO has found support as an indicator of airway inflammatory processes, because large amounts of it are produced by immune cells involved in allergic inflammation (Dweik et al., 2011; Forsythe et al., 2001; Ricciardolo et al., 2004; Silkoff et al., 2006). A major source of elevated levels FeNO levels in atopic asthmatics are eosinophils (Ricciardolo et al., 2004), which contribute to asthmatic inflammation and tissue damage (Pijnenburg and De Jongste, 2008; Yates, 2001). FeNO has become a widely accepted marker of Type 2 airway inflammatory status in asthma (Barnes et al., 2010; Dweik et al., 2011). Anti-inflammatory treatment with inhaled corticosteroids reduces FeNO levels (Pijnenburg and De Jongste, 2008; Yates, 2001).
However, the function of elevated NO levels in asthma is far from clear and both detrimental as well as beneficial effects of airway NO have been demonstrated (Ricciardolo et al., 1996; Sanders, 1999). NO is formed from L-arginine by NO synthase (NOS) for which three isoforms have been identified: two constitutive forms NOS1 (or nNOS) and NOS3 (or eNOS), which are mostly found in postsynaptic terminals of neurons or endothelial cells, respectively, and an inducible isoform NOS2 (or iNOS) in immune cells such as eosinophils or macrophages (Ricciardolo et al., 2004). In the lung, depletion of neuronal NO can interfere with bronchodilation, reduce bronchoprotection from constricting agents, and induce airway hyperreactivity (Belvisi et al., 1992; Persson et al., 1995; Ricciardolo et al., 1996). Reduction of endothelial NO is associated with excessive vascular smooth muscle contraction and pulmonary hypertension (Klinger and Kadowitz, 2017). In healthy individuals, FeNO is almost exclusively dominated by iNOS activity in epithelial cells (Lane et al., 2004), whereas in asthma, iNOS activity from an additional variety of immune cells linked to allergic inflammation contribute to high levels of FeNO. Given the short half-life of NO, a number of factors that can limit its diffusion from cell compartments (Villanueva and Giulivi, 2011), and potential activity of NOS inhibitors or NO competitors, it is unlikely that other NOS isoforms contribute substantially to FeNO.
8. Airway NO as a major factor in pathogen defense
Beyond its role as an indicator of allergic airway inflammation, NO is a major player in the mucosal defense of the airways (Proud, 2005; Vareille et al., 2011; Xu et al., 2006). NO produced by constitutive NOS2 activity in airway epithelial cells is part of the innate immune response. It is secreted upon contact with pathogens and unfolds cytostatic and cytotoxic properties (Xu et al., 2006). NO levels are substantially increased in airway infection (Kharitonov et al., 1995) and higher NO levels in experimental human rhinovirus infection have been linked to more effective viral clearance, fewer symptoms (Sanders et al., 2004), and reductions in the chemokine infection marker CXCL10/IP-10 (Koetzler et al., 2009). The role of NO appears to be linked to early stages of infection, limiting viral replication, while adaptive immune processes seem to be more important in later stages of pathogen clearance from the airways and the resolution of infections (Proud, 2005).
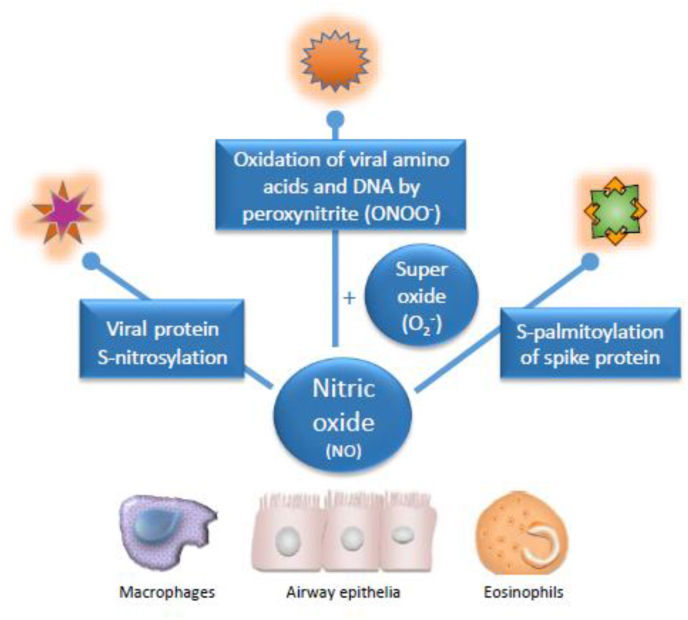
NO is active against a range of DNA and RNA viruses, including respiratory virus strains such as influenza virus, respiratory syncytial virus, rhinovirus, and coronavirus, by unfolding a range of suppressive actions against these pathogens. Among the possible antiviral mechanisms are S-nitrosylation of cysteine residues of viral proteins that are essential for replication (Colasanti et al., 1999), deamination of DNA (Wink et al., 1991), and generation of peroxynitrite by reaction of NO with superoxide anions, which can interfere with viral entry into the host cell (Padalko et al., 2004) and oxidize amino acids of viral capsid proteins (Bastin et al., 2020). In the case of the coronavirus family, NO inhibits viral replication by reducing the palmitoylation of the spike protein on the virus envelope, thereby interfering with binding to the target receptor on the host cell (Akerström et al., 2009) (Fig. 1). Whereas these mechanisms have been studied mostly in airway epithelial cells, other immune cells, such as eosinophils and macrophages, have also been found to contribute to the antiviral NO response (Benencia and Courreges, 1999; Drake et al., 2016).
Fig. 1.
Mechanisms involved in antiviral effects of NO. A range of viral enzymes and proteins that are critical for viral replication can be inactivated by S-nitrosylation of their cysteine residues. NO can also react with superoxide anions to form the highly reactive peroxynitrite, which oxidizes DNA and the amino acids of capsids that form the envelope of the virus, and thus interfere with entry of the virus into the host cell by cross-linking the capsids. In coronavirus strains, NO reduces the addition of palmitate, a saturated fatty acid, to the spike proteins on the envelope of the virus and thereby interferes with binding to the host cell’s angiotensin-converting enzyme (ACE)-2 receptor. Airway epithelial cells are the first to contact respiratory viruses and play the main role in NO defenses, but other cells can also participate in antiviral NO activity.
Endogenous NO also enhances ciliary beat frequency of the nasal respiratory epithelium facilitating mucociliary clearance of pathogens (Alberty et al., 2004, 2006). Therefore, NO would pose as an intriguing target for respiratory infection prevention efforts. In support of this idea, the role of FeNO relative to cold symptoms was prospectively studied in healthy and asthmatic students (Ritz et al., 2018). Cold symptoms were measured with the Wisconsin Upper Respiratory Symptom Survey (Barrett et al., 2005) (WURSS), a reliable measure validated against viral infection markers. Higher basal FeNO was associated with lower cold symptoms 5–10 days after academic finals. Additionally, baseline perceived stress, cortisol levels during the finals, and vascular endothelial growth factor measured in saliva and exhaled breath condensate predicted cold symptoms. Overall, these factors explained 60% of the variance in reported cold symptoms.
9. Stress and depression compromise airway NO
Emerging research has shown that FeNO varies with psychosocial factors in particular stress and mood states (Ritz and Trueba, 2014). Across a number of studies we and others have found FeNO is elevated following acute laboratory challenges, such as public speech paradigms, stressful interviews, or unpleasant odor exposure, and that higher FeNO levels are associated with stronger acute negative affect (Chen et al., 2010; Jaén and Dalton, 2014; Kullowatz et al., 2008; Ritz et al., 2011; Ritz et al., 2014). On the other hand, FeNO increases following acute laboratory stressors are reduced (Ritz et al., 2014), or overall FeNO levels are lower, with higher levels of depressive symptoms (Cepeda et al., 2016; Ritz et al., 2015). Reports of more daily hassles in the past month have also been associated with lower FeNO levels (Kullowatz et al., 2008). Moreover, compared to low stress in mid-semester, FeNO is reduced during a week-long academic stress period in healthy students (Trueba et al., 2013). Another study observed a gradual decline in FeNO levels during final examinations, which was particularly prominent for students with asthma and those with depressive symptoms showed an accelerated decline of FeNO (Ritz et al., 2015). Others have observed a FeNO decrease in healthy students but not asthma during an academic stress period (Höglund et al., 2006). In contrast, higher levels of social support in healthy students were also associated with higher basal FeNO (Trueba et al., 2014). Taken together, sustained acute, longer-lasting, or chronic stress appear to reduce FeNO. Reduced mobilization of NO could compromise mucosal immunity and make individuals more susceptible to respiratory infections.
A number of mechanistic pathways have been proposed for enhanced NO release due to stress, including sympathetic stimulation of epithelial or mast cells, or enhanced interferon-γ production by T-helper cells stimulating iNOS production (Ritz and Trueba, 2014). NO reduction due to longer lasting stress could be due to action on NO inhibitors or competitors, such as oxidative stress processes. Additionally, stress-related hypothalamic-pituitary-adrenal axis activation has been suggested as a factor, with higher salivary cortisol levels linked to attenuated FeNO increases to acute stress (Ritz et al., 2011) and reduced FeNO levels in sustained stress of the academic finals (Ritz et al., 2015).
10. NO in SARS-CoV-2 infection
SARS-CoV-2 infection has also been shown to disrupt signaling that is critical for endothelial NO production. The angiotensin-converting enzyme 2 (ACE2) receptor that is used by the virus as a main gateway into cells, in health supports a signaling cascade that counteracts the ACE – angiotensin II - angiotensin receptor-1 pathway, which is traditionally known to lead to sympathetic activation, vasoconstriction, and inflammation, and is responsible for vascular damage and disease outcomes including hypertension and heart failure (Patel and Schultz, 2013). Conversion of angiotensin II by the ACE2 receptor to angiotensin (1–7) supports an alternative metabolic pathway that, among other effects, enhances NO production and is vasoprotective. This protective system is also active in lung epithelial cells (Adusumilli et al., 2020; Samavati and Uhal, 2020). Viral activity inactivates this pathway and leads to widespread cardiovascular and pulmonary damage, including low levels of NO. NO production is also reduced in populations that are at particular risk from SARS-CoV-2 infection, including those of older age or suffering from underlying conditions such as type 2 diabetes, metabolic syndrome, chronic obstructive pulmonary disease (COPD), obesity, or autoimmune disorders (Adusumilli et al., 2020; Ozdemir and Yazici, 2020). Sufficiently high levels of NO can offset at least some of the drastic adverse consequences of the infection, including vascular inflammation, hypercoagulation, impaired microvascular blood flow and oxygen delivery with accumulation of toxic byproducts, and pulmonary hypertension (Adusumilli et al., 2020; Zhang et al., 2020).
11. Vaccinations and supplements for prevention of respiratory infections
The prevalence and burden of respiratory infections has resulted in numerous research strategies to aid in prevention, including vaccines, predominately live attenuated or inactivated viruses (Papadopoulos et al., 2017). However, a recent Cochrane systematic review showed that there was no significant difference between vaccine and placebo in preventing the common cold (Simancas-Racines et al., 2017). Respiratory syncytial virus, which is known to exacerbate symptoms of asthma (Falsey et al., 2005; Simpson et al., 2003), has no effective vaccine (Jorquera and Tripp, 2017). Supplements have commonly been used as prevention strategies for respiratory infections, but findings have remained equivocal. Studies of vitamin C (Hemilia and Chalker, 2013) and D (Martineau et al., 2017) have not supported initial enthusiasm (Li-Ng et al., 2009; Murdoch et al., 2012). Probiotics have shown some promise, but they do not reduce length of respiratory infection (Hao et al., 2011). Thus, although a magnitude of research tested possible prevention strategies for respiratory infections, development of impactful preventative treatments continues to remain a challenge (Papadopoulos et al., 2017).
12. NO donors and their potential in asthma and SARS-CoV-2 infection
An understudied prevention strategy for respiratory infections is boosting airway NO. In cell-based models, NO has been shown to inhibit replication of the human rhinovirus and virus-induced epithelial cytokine and chemokine production (Koetzler et al., 2009; Sanders et al., 1998) and higher NO levels have shown benefits in viral clearance and symptom reduction after experimental infection (Sanders et al., 2004).
Increasing circulating NO levels is possible through provision of exogenous NO in the form of inhaled gas, dietary supplementation, or administration of direct or indirect donors. However, the complex physiological pathways involved in NO production and regulation result in varying levels of efficacy and safety depending on the method used, and thus multiple variables must be considered before administration. For example, the potent vasodilatory and anti-inflammatory properties of inhaled gaseous NO have been effective in treating acute respiratory disease, but its administration and storage challenges could make it less ideal for outpatient use (Zhou et al., 2020). High costs and the production of toxic byproducts during NO delivery, such as NO2 and O3, have limited its use (Yu et al., 2015), with severe hypoxic conditions and pulmonary hypertension in neonatology as the main application (Gentile, 2011).
Regarding pharmaceuticals targeting the NO pathway, drugs that enhance production of NO by increasing cGMP levels or inhibiting phosphodiesterase type 5 (PDE5) show particular promise (Kovamees et al., 2016; Vasquez et al., 2016). Alternatively, soluble organic nitrates are indirect NO donors that have been used in cardiovascular medicine for decades, but evidence for the deleterious effects of such compounds has mounted over time (Münzel et al., 2011). Inorganic nitrate and nitrites found in leafy vegetables improve vasomotor function and balance endogenous NO in humans, and murine model studies have indicated potential anti-inflammatory effects and improvements in age-related endothelial dysfunction (Sindler et al., 2011). Further, vegetable products containing high levels of inorganic nitrite/nitrate have shown increased effectiveness over nitrate salts in some studies – a benefit potentially attributed to the presence of phytochemicals, such as polyphenols, that also utilize a NO-dependent pathway to produce vasodilatory effects (Clifford et al., 2019).
The strength of inhaled NO therapy appears to lie in vascular and immunological benefits. Inhaled NO has been successfully used in the 2003 severe acute respiratory syndrome (SARS) epidemic (Chen et al., 2004) caused by a coronavirus that is genetically similar to the novel coronavirus SARS-CoV-2. Consequently, inhaled NO has been recommended as a treatment strategy in the SARS-CoV-2 virus pandemic (Hedenstierna et al., 2020; Ignarro, 2020) and is currently being tested for affected patients (Alvarez et al., 2020, Lei et al., 2020a, Lei et al., 2020b, Parikh et al., 2020), capitalizing on both NO’s vasodilatory effects improving oxygenation and its strong antiviral properties (Ignarro, 2020). PDE5 inhibitors that improve NO availability have also been proposed for protection from widespread cytokine dysregulation and vascular and pulmonary damage inflicted by the SARS-CoV-2 virus (Isidori et al., 2020). Developing convenient ways to elevate airway NO could establish the biological strategy of early prevention of respiratory infections, including protection against SARS-CoV-2.
13. Beetroot juice as a source of dietary nitrate and nitric oxide
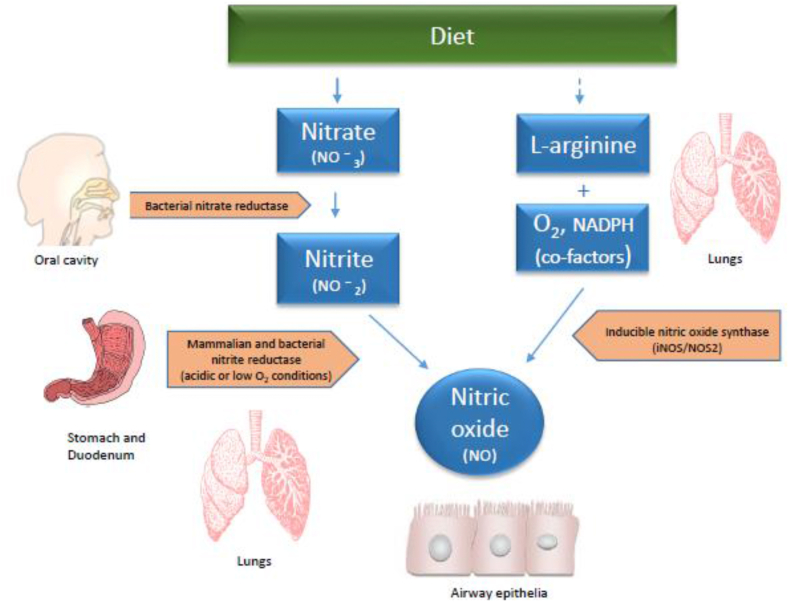
An additional dietary pathway has recently been identified that relies on dietary nitrate as an alternative source of NO. After ingestion, bacterial nitrate reductases convert nitrate to nitrite, which is then further reduced to NO by a range of enzymatic and non-enzymatic pathways in the hypoxic environment of the stomach, gut, and other tissues (Hezel and Weitzberg, 2015; Lundberg et al., 2008; Marteus et al., 2005) (Fig. 2). Nitrite, an end product of NO, can also be reduced in the body to NO and/or NO metabolites in a controlled way to produce physiologically relevant NO levels and mediate beneficial NO signaling (Kim-Shapiro and Gladwin, 2014). When considering the risks of generating deleterious reactive products with high NO concentrations, using natural supplements high in nitrite, like beetroot juice, poses an attractive clinical strategy for NO therapy (Calvert and Lefer, 2010). Dietary nitrate supplementation may help offset some of the adverse effects of low NO across organ systems and tissues, including the cardiovascular system, the airways, and mucosal pathogen defense. Clinical trials have utilized beetroot juice as a source of dietary nitrate, to improve cardiovascular health (Coles and Clifton, 2012; Hobbs et al., 2012; Jajja et al., 2014; Kapil et al., 2010; Webb et al., 2008). A meta-analysis examining 22 studies suggests that beetroot juice reduces blood pressure, with the greatest impacts being shown for systolic blood pressure (Bahadoran et al., 2017). Beetroot supplementation is a cost-effective method to improve cardiovascular health in both healthy and hypertensive populations (Bonilla Ocampo et al., 2018; Cicero and Colletti, 2015). Additionally, it improves cardiovascular functioning during exercise (Bahadoran et al., 2017; Bailey et al., 2009; Domínguez et al., 2017; Kerksick et al., 2018).
Fig. 2.
Alternative pathways of NO production: The regular physiological pathways is through breakdown of the conditionally essential amino acid L-arginine by nitric oxide synthase (NOS) under participation of cofactors such as oxygen and nicotinamide adenine dinucleotide phosphate. In the epithelial cells, this happens through inducible NOS (NOS2, one of three types of NOS). The alternative dietary pathway is through intake of dietary nitrate (e.g., in vegetables), which is converted to nitrite in the oral cavity by bacterial nitrate reductase. The nitrite is then further converted to NO in the stomach and various tissues by bacterial and mammalian reductases under acidic or low oxygen conditions.
Clinical trials vary in duration and dosage of beetroot juice administration (for examples, see Table 1). A recent meta-analysis of beetroot juice interventions using only organic NO3- and in non-exercise settings, reported varying duration of the supplementation period (1–21 days supplementation), volumes ranging from 70 to 500 mL, and the amount of NO3- ranging from 5 to 8 mmol per dose (Bonilla Ocampo et al., 2018). Supplementation over multiple days (Eggebeen et al., 2016; Webb et al., 2008) or higher doses of beetroot juice, have also shown positive benefits (Kerley et al., 2019). While most studies reported no side effects, some with higher concentrations of beetroot juice noted beeturia (pink or red coloration of urine) as a minor side effect (Rasica et al., 2018).
Table 1.
Examples of variations in dosages, supplementation periods, and outcomes for clinical trials examining the impact of beetroot juice.
| Authors (year) | Beetroot Juice Dosage | NO3−Concentration | Supplementation Period | Outcome for Beetroot Juice |
|---|---|---|---|---|
| Kukadia et al. (2019) | 70 mL | ≈6.5–7.3 mmol | 1 day | SBP decreased with BRJ at 30 and 60 min, but was not sustained over 24 h |
| Volino-Souza et al. (2018) | 140 mL | Not listed | 1 day | Improved macrovascular endothelial function, but not muscle oxygen saturation parameters |
| Rasica et al. (2018) | 70 mL | 5 mmol | 6 days | Plasma NO3− higher with BRJ; O2 cost of moderate-intensity exercise was not different in BRJ vs. PLA; reduced amplitude of O2 uptake slow component with BRJ and longer time to exhaustion with BRJ |
| Eggebeen et al. (2016) | 70 mL | 6.1 mmol | 7 days | Single dose and one week of daily BRJ increased plasma NO3− submaximal aerobic endurance improved 24% after 1 week of daily BRJ; SBP decreased with single dose and 1 week of daily BRJ |
| Whitfield et al. (2016) | 280 mL | 6.5 mmol | 7 days | Reduced submaximal exercise oxygen consumption with BRJ; measures of mitochondrial coupling and respiratory efficiency not altered in muscle; rates of mitochondrial H2O2 emission were increased in the absence of markers of lipid or protein oxidative damage |
| Ashor et al. (2015) | 70 mL | ≈4.8–6.4 mmol | 21 days | SBP and DBP decreased 3 weeks after BRJ |
14. Beetroot juice for boosting airway NO
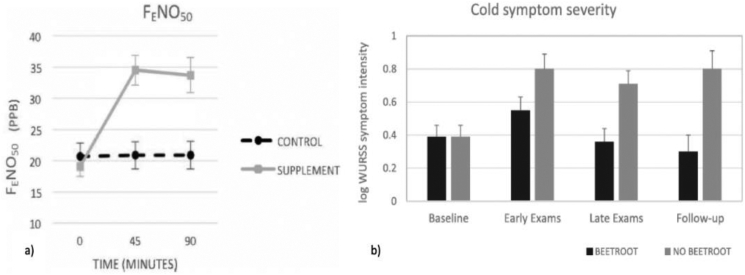
Given the tight association of cold symptoms with low FeNO levels, beetroot juice was explored as a convenient and well-tolerable intervention to raise airway NO levels, testing the influence of a beetroot juice supplement on FeNO in healthy participants (Kroll et al., 2018). FeNO was measured at baseline, 45 min, and 90 min following ingestion of 70 mL beetroot juice (6.5 mmol nitrate) (Fig. 3a). Forty-five minutes after beetroot consumption, FeNO values increased by 21.3% (d = 1.54) and were still elevated by 20.3% (d = 1.45) after 90 min. Less than 1% increase in FeNO was observed after consumption of water as control on a separate day. Elevated FeNO levels persisted over 3 h when tested in a small subset of participants.
Fig. 3.
a) FeNO increases after acute ingestion of 70 mL of beetroot juice (6.5 mmol of dietary nitrate) (reproduced from Kroll et al., 2018), b) Cold symptom severity across baseline, 7 days of academic finals stress with or without beetroot juice supplementation, and follow-up 7 days after finals in students (reproduced with permission from Ritz et al., 2019).
The potential of beetroot juice to protect against illness and cold symptoms was recently tested with N = 76 students during an academic finals period (Ritz et al., 2019). Participants were randomly assigned to a 7-day trial of one daily dose of the supplement or no supplementation control during their final exams. Findings showed that the supplement was associated with reduced symptoms of cold and illness during and 7 days after finals (Fig. 3b). Students with asthma showed the greatest symptom reduction. Higher exhaled NO was prospectively associated with reduced symptoms. Thus, beetroot juice intake during periods of psychological stress protects against cold symptoms. The benefits in asthma could extend to protection against exacerbations due to respiratory infections.
15. Open questions
15.1. NO produced by allergic immune processes, epithelial cells, and NO donors
It may appear counter-intuitive to raise airway NO in asthma, because FeNO has been interpreted as a marker of airway inflammation and targeted by anti-inflammatory therapy. However, the function of elevated NO in asthma has been debated and benefits of NO have also been emphasized (Sanders, 1999). Because FeNO is not specific to the source of NOS activity or dietary supplementation, NO elevations through allergic pathways coexist with NO elevations through epithelial production and conversion of dietary nitrate. It should be noted that while inflammatory and epithelial cells use the same NOS isoform (NOS2), its activation is calcium-independent in the former (classically labeled as “inducible”), but calcium-dependent in the latter (“constitutional”) (Mattila and Thomas, 2014). Thus, more research is needed to estimate the contribution of different cell populations, tissue compartments and NO-generation through dietary and NOS2-dependent pathways to FeNO and to delineate their functions.
There is also a need to better understand the dynamics of NO production through beetroot juice in various tissues and potential inhibitors and competitors of NO. The mechanisms underlying NO deficits in stress and depression and the impact of beetroot juice on these are unclear. Asymmetric dimethylarginine (ADMA) is a potent inhibitor of NOS activity (Mangoni et al., 2019) and the ratio of l-arginine to ADMA as an indicator of NO availability has been shown to be reduced with depressive mood in heart failure patients (Mommersteeg et al., 2015). In addition, the enzyme arginase competes for L-arginine, thus reducing its availability for NO production. Increased airway arginase activity interferes with beneficial effects of NO, compromising bronchodilation and increasing airway hyperresponsiveness (van den Berg et al., 2018). Both arginase and ADMA levels have been shown to be elevated in depression (Baranyi et al., 2015; Elgün and Kumbasar, 2000) and asthma (Morris et al., 2004; Scott et al., 2011).
15.2. The role of the oral and intestinal microbiome
The oral cavity has been shown to substantially contribute to exhaled NO (Törnberg et al., 2002). An increasingly large body of evidence has pointed to microbiota in the mouth and gut as essential in the nitrate-nitrite-NO pathway, with species of Actinomyces, Haemophilus, Neisseria and Veillonella most readily implicated (Grant and Jönsson, 2019). It has been estimated that up to 25% of dietary nitrate is reduced by oral bacteria, and circulatory and metabolic benefits of dietary nitrate can be negated by antimicrobial mouthwash administration (Hezel and Weitzberg, 2015; Moretti et al., 2019). Similarly, the ability to regulate blood pressure is compromised in individuals with lower levels of nitrite-reducing bacteria on the tongue (Tribble et al., 2019).
In the gut, bacterial species also play a crucial role in NO production through the metabolism of nitrate and polyphenols, and changes to intestinal bacterial communities can affect systemic inflammatory pathways (Rocha et al., 2016). There is up to 45% species overlap of bacteria found in the large intestine and oral cavity, and disruptions in one bacterial community within the body often elucidate changes in another (Segata et al., 2012). Such body-wide microbiome alterations, possibly mediated by lymphoid migration (Mestecky, 1987), have been implicated in chronic illness. In eosinophilic respiratory disease, alterations are present in the oral, respiratory, or gut microbiomes (Hiremath et al., 2019; Huang and Boushey, 2015; Sverrild et al., 2017) and can occur through external (e.g., diet, environment) or endogenous influences (e.g., psychological stress, systemic inflammation) (Duran-Pinedo et al., 2018; Huang and Boushey, 2015). However, the relationship between NO production, microbiome disruptions, stress, and chronic disease is complex and further research is required to elucidate underlying connections.
15.3. Potential use of dietary nitrate in respiratory disease an infection
The potential of NO donors as prophylaxis against upper respiratory tract infections requires further exploration. Infection peaks in children coincide with school reopening and the fall season (Olenec et al., 2010; Perry Markovich et al., 2015), which in turn coincide with high rates of exacerbations and emergency room treatments in asthma (Sears and Johnston, 2007). Elevated exposure to pathogens and psychosocial stress in return to school and work could be critical factors. Individuals with other vulnerabilities could equally profit from NO donors in this context. In critical daily life situations during a viral pandemic, such as crowding or close human contact in professional or leisure time settings, strategic use of NO supplementation could be a promising avenue for intervention research. Because aging is associated with a reduced production of NO (Ozdemir and Yazici, 2020), supplementation with NO donors may hold promise for boosting resistance to respiratory infection, including COVID-19, in older individuals, beyond its benefits for cardiovascular health (Ignarro, 2020). Other chronic respiratory illnesses, such as COPD or cystic fibrosis, which are at elevated risk of exacerbation by respiratory infections (Flight and Jones, 2017; Viniol and Vogelmeier, 2018), could profit from an investigation of NO-based prophylaxis and therapies. In cystic fibrosis, airway NO is low due to a lack of sufficient iNOS production, a risk factor for a range of bacterial and viral infections (Moeller et al., 2006; Nichols et al., 2008). NO donors have been proposed as a promising strategy for cystic fibrosis (Barnes et al., 2010; Deppisch et al., 2016; Lundberg et al., 2008; Proud, 2005), but benefits of dietary nitrate supplements has not yet been explored systematically.
15.4. Limits and risks of dietary nitrate use
NO’s endogenous role as both a pro- and anti-inflammatory agent, and its relationship with circulating radical oxygen species (ROS), requires that levels be modulated and/or monitored in tissue to avoid negative effects during administration (Förstermann et al., 2017). For example, circulating NO may promote or inhibit tumor growth and metastasis under different conditions depending on its concentration (Xu et al., 2002). Of particular concern is the finding that generation of N-nitroso compounds (NOCs), a potential carcinogen, can be stimulated by acute nitrate ingestion (Zamani et al., 2020). One study has examined effects of beetroot juice on NOC excretion, and results indicate that acute consumption did result in increased levels of NOCs in urine (Berends et al., 2019). However, more recent epidemiological studies have not found associations between nitrite and gastric cancer or esophageal cancer (Ma et al., 2018). There is also no evidence that a diet high in nitrate-rich vegetables increases the risk of cancer – on the contrary, incorporation of vegetables like beetroot and spinach may have protective effects against cancer. While reason for these contrary findings is unknown, high levels of vitamin C in these vegetables could counteract the formation of NOCs and the resulting increase in circulating NO could actually facilitate optimal uptake of tumor-inhibiting phytochemicals (van Breda and de Kok, 2018; Zamani et al., 2020). Effects of ingested nitrate seem to be context-dependent, in that it is potentially carcinogenic only on the background of intake with dietary amines or amides as found in red and cured meats (Koch et al., 2017). Thus, although increasing NO levels through exogenous donors such as inhaled NO gas, inorganic nitrite/nitrate consumption, or targeted NO-pathway treatments show therapeutic promise, more human research is required on the effects of such treatments before dosage and treatment course recommendations can be made (Gori, 2020).
16. Conclusion
Preventing and combatting respiratory tract infections has remained a formidable scientific challenge despite decades of investigative efforts. While individuals with respiratory disease or compromised immune function are most vulnerable to viral infections, the current COVID-19 crises has highlighted the need for developing new strategies that preserve airway and cardiovascular health in the general population. Here we have proposed that boosting NO, which is depleted by psychological stress and viral assault, could provide protection against viral proliferation and its many adverse pulmonary and vascular consequences. A focus on dietary means of elevating nitrate levels, such as intake of particular types of vegetables, could also be more cost-effective and unfold additional effects on overall health. However, before wider application of such strategies, further exploration of NO donor effects on respiratory infection are needed.
Declaration of competing interest
A.R.L. declares a financial stake in BioLum Sciences, LLC, Houston, TX, USA. The other authors have no conflicts to declare.
Acknowledgements
Work on this manuscript was partially funded by the National Heart, Lung, and Blood Institute (NHLBI, R01 HL142775). The authors thank David Proud for helpful comments and advice.
References
- Adams P.F., Hendershot G.E., Marano M.A. Current estimates from the national health interview Survey. Vital Health Stat. 1999;10:1–260. [PubMed] [Google Scholar]
- Adusumilli N.C., Zhang D., Friedman J.M., Friedman A.J. Harnessing nitric oxide for preventing, limiting and treating the severe pulmonary consequences of COVID-19. Nitric Oxide: Biol. Chem. 2020;103:4–8. doi: 10.1016/j.niox.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerström S., Gunalan V., Keng C.T., Tan Y.J., Mirazimi A. Dual effect of nitric oxide on SARS-CoV replication: viral RNA production and palmitoylation of the S protein are affected. Virology. 2009;395(1):1–9. doi: 10.1016/j.virol.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberty J., August C., Stoll W., Rudack C. The effect of endogenous nitric oxide on cholinergic ciliary stimulation of human nasal mucosa. Laryngoscope. 2004;114(9):1642–1647. doi: 10.1097/00005537-200409000-00026. [DOI] [PubMed] [Google Scholar]
- Alberty J., Stoll W., Rudack C. The effect of endogenous nitric oxide on mechanical ciliostimulation of human nasal mucosa. Clin. Exp. Allergy. 2006;36(10):1254–1259. doi: 10.1111/j.1365-2222.2006.02563.x. [DOI] [PubMed] [Google Scholar]
- Ali-Sisto T., Tolmunen T., Viinamäki H., Mäntyselkä P., Valkonen-Korhonen M., Koivumaa-Honkanen H., Honkalampi K., Ruusunen A., Nandania J., Velagapudi V., Lehto S.M. Mar 15). Global arginine bioavailability ratio is decreased in patients with major depressive disorder. J. Affect. Disord. 2018;229:145–151. doi: 10.1016/j.jad.2017.12.030. [DOI] [PubMed] [Google Scholar]
- Alvarez R.A., Berra L., Gladwin M.T. Home nitric oxide therapy for COVID-19. Am. J. Respir. Crit. Care Med. 2020;202(1):16–20. doi: 10.1164/rccm.202005-1906ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashor A.W., Jajja A., Sutyarjoko A., Brandt K., Qadir O., Lara J., Siervo M. Effects of beetroot juice supplementation on microvascular blood flow in older overweight and obese subjects: A pilot randomised controlled study. Journal of Human Hypertension. 2015;29(8):511–513. doi: 10.1038/jhh.2014.114. [DOI] [PubMed] [Google Scholar]
- ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005 Am. J. Respir. Crit. Care Med. 2005;171(8):912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- Bahadoran Z., Mirmiran P., Kabir A., Azizi F., Ghasemi A. The nitrate-independent blood pressure-lowering effect of beetroot juice: a systematic review and meta-analysis. Adv. Nutr. 2017;8(6):830–838. doi: 10.3945/an.117.016717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey S.J., Winyard P., Vanhatalo A., Blackwell J.R., Dimenna F.J., Wilkerson D.P., Tarr J., Benjamin N., Jones A.M. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J. Appl. Physiol. 2009;107(4):1144–1155. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- Baranyi A., Amouzadeh-Ghadikolai O., Rothenhäusler H.B., Theokas S., Robier C., Baranyi M., Koppitz M., Reicht G., Hlade P., Meinitzer A. Nitric oxide-related biological pathways in patients with major depression. PloS One. 2015;10(11) doi: 10.1371/journal.pone.0143397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P.J., Dweik R.A., Gelb A.F., Gibson P.G., George S.C., Grasemann H., Pavord I.D., Ratjen F., Silkoff P.E., Taylor D.R., Zamel N. Exhaled nitric oxide in pulmonary diseases: a comprehensive review. Chest. 2010;138(3):682–692. doi: 10.1378/chest.09-2090. [DOI] [PubMed] [Google Scholar]
- Barnett S.B., Nurmagambetov T.A. Costs of asthma in the United States: 2002-2007. J. Allergy Clin. Immunol. 2011;127(1):145–152. doi: 10.1016/j.jaci.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Barrett B., Brown R., Mundt M., Safdar N., Dye L., Maberry R., Alt J. The Wisconsin Upper Respiratory Symptom Survey is responsive, reliable, and valid. J. Clin. Epidemiol. 2005;58(6):609–617. doi: 10.1016/j.jclinepi.2004.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin G., Loison P., Vernex-Loset L., Dupire F., Challant J., Majou D., Boudaud N., Krier G., Gantzer C. Structural organizations of Qβ and MS2 phages affect capsid protein modifications by oxidants hypochlorous acid and peroxynitrite. Front. Microbiol. 2020;11:1157. doi: 10.3389/fmicb.2020.01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belvisi M.G., Stretton C.D., Yacoub M., Barnes P.J. Nitric oxide is the endogenous neurotransmitter of bronchodilator nerves in humans. Eur. J. Pharmacol. 1992;210(2):221–222. doi: 10.1016/0014-2999(92)90676-u. [DOI] [PubMed] [Google Scholar]
- Benencia F., Courreges M.C. Nitric oxide and macrophage antiviral extrinsic activity. Immunology. 1999;98(3):363–370. doi: 10.1046/j.1365-2567.1999.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berends J.E., van den Berg L.M.M., Guggeis M.A., Henckens N.F.T., Hossein I.J., de Joode M.E.J.R., Zamani H., van Pelt K.A.A.J., Beelen N.A., Kuhnle G.G., de Kok T.M.C.M., Van Breda S.G.J. Consumption of nitrate-rich beetroot juice with or without vitamin C supplementation increases the excretion of urinary nitrate, nitrite, and N-nitroso compounds in humans. Int. J. Mol. Sci. 2019;20(9):2277. doi: 10.3390/ijms20092277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla Ocampo D.A., Paipilla A.F., Marín E., Vargas-Molina S., Petro J.L., Pérez-Idárraga A. Dietary nitrate from beetroot juice for hypertension: a systematic review. Biomolecules. 2018;8(4):134. doi: 10.3390/biom8040134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch J.A., de Geus E.E., Ring C., Nieuw Amerongen A.V., Stowell J.R. Academic examinations and immunity: academic stress or examination stress? Psychosom. Med. 2004;66(4):625–626. doi: 10.1097/01.psy.0000133254.46947.ac. author reply 626-627. [DOI] [PubMed] [Google Scholar]
- Bosch J.A., de Geus E.J., Veerman E.C., Hoogstraten J., Nieuw Amerongen A.V. Innate secretory immunity in response to laboratory stressors that evoke distinct patterns of cardiac autonomic activity. Psychosom. Med. 2003;65(2):245–258. doi: 10.1097/01.psy.0000058376.50240.2d. [DOI] [PubMed] [Google Scholar]
- Boyce W.T., Chesney M., Alkon A., Tschann J.M., Adams S., Chesterman B., Cohen F., Kaiser P., Folkman S., Wara D. Psychobiologic reactivity to stress and childhood respiratory illnesses: results of two prospective studies. Psychosom. Med. 1995;57(5):411–422. doi: 10.1097/00006842-199509000-00001. [DOI] [PubMed] [Google Scholar]
- Busse W.W., Lemanske R.F., Jr., Gern J.E. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376(9743):826–834. doi: 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert J.W., Lefer D.J. Clinical translation of nitrite therapy for cardiovascular diseases. Nitric Oxide: Biol. Chem. 2010;22(2):91–97. doi: 10.1016/j.niox.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda M.S., Stang P., Makadia R. Depression is associated with high levels of C-reactive protein and low levels of fractional exhaled nitric oxide: results from the 2007-2012 national health and nutrition examination surveys. J. Clin. Psychiatr. 2016;77(12):1666–1671. doi: 10.4088/JCP.15m10267. [DOI] [PubMed] [Google Scholar]
- Chen E., Schreier H.M. Does the social environment contribute to asthma? Immunol. Allergy Clin. North Am. 2008;28(3):649–664. doi: 10.1016/j.iac.2008.03.007. x. [DOI] [PubMed] [Google Scholar]
- Chen E., Strunk R.C., Bacharier L.B., Chan M., Miller G.E. Socioeconomic status associated with exhaled nitric oxide responses to acute stress in children with asthma. Brain Behav. Immun. 2010;24(3):444–450. doi: 10.1016/j.bbi.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Liu P., Gao H., Sun B., Chao D., Wang F., Zhu Y., Hedenstierna G., Wang C.G. Inhalation of nitric oxide in the treatment of severe acute respiratory syndrome: a rescue trial in Beijing. Clin. Infect. Dis. : Off. Publ. Infect. Dis. Soc. Am. 2004;39(10):1531–1535. doi: 10.1086/425357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero A.F., Colletti A. Nutraceuticals and blood pressure control: results from clinical trials and meta-analyses. High Blood Pres. Cardiovasc. Prev. 2015;22(3):203–213. doi: 10.1007/s40292-015-0081-8. [DOI] [PubMed] [Google Scholar]
- Clifford T., Babateen A., Shannon O.M., Capper T., Ashor A., Stephan B., Robinson L., O’Hara J.P., Mathers J.C., Stevenson E., Siervo M. Effects of inorganic nitrate and nitrite consumption on cognitive function and cerebral blood flow: a systematic review and meta-analysis of randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2019;59(15):2400–2410. doi: 10.1080/10408398.2018.1453779. [DOI] [PubMed] [Google Scholar]
- Cobb J.M., Steptoe A. Psychosocial stress and susceptibility to upper respiratory tract illness in an adult population sample. Psychosom. Med. 1996;58(5):404–412. doi: 10.1097/00006842-199609000-00003. [DOI] [PubMed] [Google Scholar]
- Cohen S., Doyle W.J., Skoner D.P. Psychological stress, cytokine production, and severity of upper respiratory illness. Psychosom. Med. 1999;61(2):175–180. doi: 10.1097/00006842-199903000-00009. [DOI] [PubMed] [Google Scholar]
- Cohen S., Doyle W.J., Skoner D.P., Rabin B.S., Gwaltney J.M., Jr. Social ties and susceptibility to the common cold. J. Am. Med. Assoc. 1997;277(24):1940–1944. [PubMed] [Google Scholar]
- Cohen S., Hamrick N., Rodriguez M.S., Feldman P.J., Rabin B.S., Manuck S.B. Reactivity and vulnerability to stress-associated risk for upper respiratory illness. Psychosom. Med. 2002;64(2):302–310. doi: 10.1097/00006842-200203000-00014. [DOI] [PubMed] [Google Scholar]
- Cohen S., Janicki-Deverts D., Turner R.B., Casselbrant M.L., Li-Korotky H.S., Epel E.S., Doyle W.J. Association between telomere length and experimentally induced upper respiratory viral infection in healthy adults. J. Am. Med. Assoc. 2013;309(7):699–705. doi: 10.1001/jama.2013.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Tyrrell D.A., Smith A.P. Psychological stress and susceptibility to the common cold. N. Engl. J. Med. 1991;325(9):606–612. doi: 10.1056/nejm199108293250903. [DOI] [PubMed] [Google Scholar]
- Colasanti M., Persichini T., Venturini G., Ascenzi P. S-nitrosylation of viral proteins: molecular bases for antiviral effect of nitric oxide. IUBMB Life. 1999;48(1):25–31. doi: 10.1080/713803459. [DOI] [PubMed] [Google Scholar]
- Coles L.T., Clifton P.M. Effect of beetroot juice on lowering blood pressure in free-living, disease-free adults: a randomized, placebo-controlled trial. Nutr. J. 2012;11 doi: 10.1186/1475-2891-11-106. 106-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiber A., Münzel T. Organic nitrate therapy, nitrate tolerance, and nitrate-induced endothelial dysfunction: emphasis on redox biology and oxidative stress. Antioxidants Redox Signal. 2015;23(11):899–942. doi: 10.1089/ars.2015.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppisch C., Herrmann G., Graepler-Mainka U., Wirtz H., Heyder S., Engel C., Marschal M., Miller C.C., Riethmüller J. Gaseous nitric oxide to treat antibiotic resistant bacterial and fungal lung infections in patients with cystic fibrosis: a phase I clinical study. Infection. 2016;44(4):513–520. doi: 10.1007/s15010-016-0879-x. [DOI] [PubMed] [Google Scholar]
- Domínguez R., Cuenca E., Maté-Muñoz J.L., García-Fernández P., Serra-Paya N., Estevan M.C.L., Herreros P.V., Garnacho-Castaño M.V. Effects of beetroot juice supplementation on cardiorespiratory endurance in athletes. A systematic review. Nutrients. 2017;9(1):43. doi: 10.3390/nu9010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake M.G., Bivins-Smith E.R., Proskocil B.J., Nie Z., Scott G.D., Lee J.J., Lee N.A., Fryer A.D., Jacoby D.B. Human and mouse eosinophils have antiviral activity against parainfluenza virus. Am. J. Respir. Cell Mol. Biol. 2016;55(3):387–394. doi: 10.1165/rcmb.2015-0405OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Pinedo A.E., Solbiati J., Frias-Lopez J. The effect of the stress hormone cortisol on the metatranscriptome of the oral microbiome. NPJ Biofilms Microbiomes. 2018;4:25. doi: 10.1038/s41522-018-0068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dweik R.A., Boggs P.B., Erzurum S.C., Irvin C.G., Leigh M.W., Lundberg J.O., Olin A.-C., Plummer A.L., Taylor D.R., American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels for Clinical, A An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am. J. Respir. Crit. Care Med. 2011;184(5):602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M.R., Strong K., Cameron A., Walton R.P., Jackson D.J., Johnston S.L. Viral infections in allergy and immunology: how allergic inflammation influences viral infections and illness. J. Allergy Clin. Immunol. 2017;140(4):909–920. doi: 10.1016/j.jaci.2017.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggebeen J., Kim-Shapiro D.B., Haykowsky M., Morgan T.M., Basu S., Brubaker P., Rejeski J., Kitzman D.W. One week of daily dosing with beetroot juice improves submaximal endurance and blood pressure in older patients with heart failure and preserved ejection fraction. J. Am. Coll. Cardiol. Heart Failure. 2016;4(6):428–437. doi: 10.1016/j.jchf.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgün S., Kumbasar H. Increased serum arginase activity in depressed patients. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2000;24(2):227–232. doi: 10.1016/s0278-5846(99)00100-1. [DOI] [PubMed] [Google Scholar]
- Elmer T., Mepham K., Stadtfeld C. Students under lockdown: comparisons of students’ social networks and mental health before and during the COVID-19 crisis in Switzerland. PloS One. 2020;15(7) doi: 10.1371/journal.pone.0236337. e0236337-e0236337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P.D., Edgerton N. Life-events and mood as predictors of the common cold. Br. J. Med. Psychol. 1991;64(Pt 1):35–44. doi: 10.1111/j.2044-8341.1991.tb01640.x. [DOI] [PubMed] [Google Scholar]
- Falagas M.E., Karamanidou C., Kastoris A.C., Karlis G., Rafailidis P.I. Psychosocial factors and susceptibility to or outcome of acute respiratory tract infections. Int. J. Tubercul. Lung Dis. 2010;14(2):141–148. [PubMed] [Google Scholar]
- Falsey A.R., Hennessey P.A., Formica M.A., Cox C., Walsh E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005;352(17):1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- Fendrick A.M., Monto A.s., Nightengale B., Sarnes M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch. Intern. Med. 2003;163(4):487–494. doi: 10.1001/achinte.163.4.487. [DOI] [PubMed] [Google Scholar]
- Flight W., Jones A. The diagnosis and management of respiratory viral infections in cystic fibrosis. Expet Rev. Respir. Med. 2017;11(3):221–227. doi: 10.1080/17476348.2017.1288102. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Sessa W.C. Nitric oxide synthases: regulation and function. Eur. Heart J. 2012;33(7):829–837d. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förstermann U., Xia N., Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 2017;120(4):713–735. doi: 10.1161/circresaha.116.309326. [DOI] [PubMed] [Google Scholar]
- Forsythe P., Gilchrist M., Kulka M., Befus A.D. Mast cells and nitric oxide: control of production, mechanisms of response. Int. Immunopharm. 2001;1(8):1525–1541. doi: 10.1016/s1567-5769(01)00096-0. [DOI] [PubMed] [Google Scholar]
- Fukuto J.M., Carrington S.J., Tantillo D.J., Harrison J.G., Ignarro L.J., Freeman B.A., Chen A., Wink D.A. Small molecule signaling agents: the integrated chemistry and biochemistry of nitrogen oxides, oxides of carbon, dioxygen, hydrogen sulfide, and their derived species. Chem. Res. Toxicol. 2012;25(4):769–793. doi: 10.1021/tx2005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile M.A. Inhaled medical gases: more to breathe than oxygen. Respir. Care. 2011;56(9):1341–1357. doi: 10.4187/respcare.01442. discussion 1357-1349. [DOI] [PubMed] [Google Scholar]
- Global Asthma Network Global asthma report. 2018. http://www.globalasthmareport.org/ Downloaded at. (09/11/2019)
- Gori T. Exogenous NO therapy for the treatment and prevention of atherosclerosis. Int. J. Mol. Sci. 2020;21(8):2703. doi: 10.3390/ijms21082703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant M.M., Jönsson D. Next generation sequencing discoveries of the nitrate-responsive oral microbiome and its effect on vascular responses. J. Clin. Med. 2019;8(8):1110. doi: 10.3390/jcm8081110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Q., Lu Z., Dong B.R., Huang C.Q., Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst. Rev. 2011;(9) doi: 10.1002/14651858.CD006895.pub2. [DOI] [PubMed] [Google Scholar]
- Hasratian, A. M., Nordberg, H.O., Meuret, A.E., Ritz, t. . (under review). Fear and Coping in Students during the COVID-19 Pandeimc: A Combined Cross-Sectional and Longitudinal Study. [DOI] [PMC free article] [PubMed]
- Hedenstierna G., Chen L., Hedenstierna M., Lieberman R., Fine D. Nitric Oxide dosed in short bursts at high concentrations may protect against Covid 19. Nitric Oxide. 2020;103 doi: 10.1016/j.niox.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemilia H., Chalker E. Vitamin C for preventing and treating common cold. Cochrane Database Syst. Rev. 2013;1 doi: 10.1002/14651858.CD000980.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess D.T., Matsumoto A., Kim S.O., Marshall H.E., Stamler J.S. Protein S- nitrosylation: purview and parameters. Nat. Rev. Mol. Cell Biol. 2005;6(2):150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- Hezel M.P., Weitzberg E. The oral microbiome and nitric oxide homoeostasis. Oral Dis. 2015;21(1):7–16. doi: 10.1111/odi.12157. [DOI] [PubMed] [Google Scholar]
- Hiremath G., Shilts M.H., Boone H.H., Correa H., Acra S., Tovchigrechko A., Rajagopala S.V., Das S.R. The salivary microbiome is altered in children with eosinophilic esophagitis and correlates with disease activity. Clin. Transl. Gastroenterol. 2019;10(6) doi: 10.14309/ctg.0000000000000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs D.A., Kaffa N., George T.W., Methven L., Lovegrove J.A. Blood pressure-lowering effects of beetroot juice and novel beetroot-enriched bread products in normotensive male subjects. Br. J. Nutr. 2012;108(11):2066–2074. doi: 10.1017/s0007114512000190. [DOI] [PubMed] [Google Scholar]
- Höglund C.O., Axén J., Kemi C., Jernelöv S., Grunewald J., Müller-Suur C., Smith Y., Grönneberg R., Eklund A., Stierna P., Lekander M. Changes in immune regulation in response to examination stress in atopic and healthy individuals. Clin. Exp. Allergy. 2006;36(8):982–992. doi: 10.1111/j.1365-2222.2006.02529.x. [DOI] [PubMed] [Google Scholar]
- Huang Y.J., Boushey H.A. The microbiome in asthma. J. Allergy Clin. Immunol. 2015;135(1):25–30. doi: 10.1016/j.jaci.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L.J. Inhaled NO and COVID-19. Br. J. Pharmacol. 2020;177(16):3848–3849. doi: 10.1111/bph.15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isidori A.M., Giannetta E., Pofi R., Venneri M.A., Gianfrilli D., Campolo F., Mastroianni C.M., Lenzi A., d’Ettorre G. Targeting the NO-cGMP-PDE5 pathway in COVID-19 infection. DEDALO Project. 2020;9(1):33–38. doi: 10.1111/andr.12837. Andrology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaén C., Dalton P. Asthma and odors: the role of risk perception in asthma exacerbation. J. Psychosom. Res. 2014;77(4):302–308. doi: 10.1016/j.jpsychores.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jajja A., Sutyarjoko A., Lara J., Rennie K., Brandt K., Qadir O., Siervo M. Beetroot supplementation lowers daily systolic blood pressure in older, overweight subjects. Nutr. Res. 2014;34(10):868–875. doi: 10.1016/j.nutres.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Janicki-Deverts D., Cohen S., Turner R.B., Doyle W.J. Basal salivary cortisol secretion and susceptibility to upper respiratory infection. Brain Behav. Immun. 2016;53:255–261. doi: 10.1016/j.bbi.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jartti T., Gern J.E. Role of viral infections in the development and exacerbation of asthma in children. J. Allergy Clin. Immunol. 2017;140(4):895–906. doi: 10.1016/j.jaci.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorquera P.A., Tripp R.A. Respiratory syncytial virus: prospects for new and emerging therapeutics. Expet Rev. Respir. Med. 2017;11(8):609–615. doi: 10.1080/17476348.2017.1338567. [DOI] [PubMed] [Google Scholar]
- Kapil V., Webb A.J., Ahluwalia A. Inorganic nitrate and the cardiovascular system. Heart. 2010;96(21):1703–1709. doi: 10.1136/hrt.2009.180372. [DOI] [PubMed] [Google Scholar]
- Kerksick C.M., Wilborn C.D., Roberts M.D., Smith-Ryan A., Kleiner S.M., Jäger R., Collins R., Cooke M., Davis J.N., Galvan E., Greenwood M., Lowery L.M., Wildman R., Antonio J., Kreider R.B. ISSN exercise & sports nutrition review update: research & recommendations. Sports Nutr. Rev. J. 2018;15(1) doi: 10.1186/s12970-018-0242-y. 38-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerley C.P., James P.E., McGowan A., Faul J., Cormican L. Dietary nitrate improved exercise capacity in COPD but not blood pressure or pulmonary function: a 2 week, double-blind randomised, placebo-controlled crossover trial. Int. J. Food Sci. Nutr. 2019;70(2):222–231. doi: 10.1080/09637486.2018.1492521. [DOI] [PubMed] [Google Scholar]
- Kharitonov S.A., Yates D., Barnes P.J. Increased nitric oxide in exhaled air of normal human subjects with upper respiratory tract infections. Eur. Respir. J. 1995;8(2):295–297. doi: 10.1183/09031936.95.08020295. [DOI] [PubMed] [Google Scholar]
- Kim-Shapiro D.B., Gladwin M.T. Mechanisms of nitrite bioactivation. Nitric Oxide: Biol. Chem. 2014;38:58–68. doi: 10.1016/j.niox.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C.D., Gladwin M.T., Freeman B.A., Lundberg J.O., Weitzberg E., Morris A. Enterosalivary nitrate metabolism and the microbiome: intersection of microbial metabolism, nitric oxide and diet in cardiac and pulmonary vascular health. Free Radic. Biol. Med. 2017;105:48–67. doi: 10.1016/j.freeradbiomed.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koetzler R., Zaheer R.S., Wiehler S., Holden N.S., Giembycz M.A., Proud D. Nitric oxide inhibits human rhinovirus-induced transcriptional activation of CXCL10 in airway epithelial cells. J. Allergy Clin. Immunol. 2009;123(1):201–208. doi: 10.1016/j.jaci.2008.09.041. e209. [DOI] [PubMed] [Google Scholar]
- Kovamees O., Shemyakin A., Eriksson M., Angelin B., Pernow J. Arginase inhibition improves endothelial function in patients with familial hypercholesterolaemia irrespective of their cholesterol levels. J. Intern. Med. 2016;279(5):477–484. doi: 10.1111/joim.12461. [DOI] [PubMed] [Google Scholar]
- Kroll J.L., Werchan C.A., Rosenfield D., Ritz T. Acute ingestion of beetroot juice increases exhaled nitric oxide in healthy individuals. PloS One. 2018;13(1) doi: 10.1371/journal.pone.0191030. e0191030-e0191030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukadia S., Dehbi H.M., Tillin T., Coady E., Chaturvedi N., Hughes A.D. A double-blind placebo-controlled crossover study of the effect of beetroot juice containing dietary nitrate on aortic and brachial blood pressure over 24 h. Frontiers in Physiology. 2019;10(47):1–6. doi: 10.3389/fphys.2019.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullowatz A., Rosenfield D., Dahme B., Magnussen H., Kanniess F., Ritz T. Stress effects on lung function in asthma are mediated by changes in airway inflammation. Psychosom. Med. 2008;70(4):468–475. doi: 10.1097/PSY.0b013e31816f9c2f. [DOI] [PubMed] [Google Scholar]
- Lei, C., Su, B., Dong, H., Bellavia, A., Di Fenza, R., Safaee Fakhr, B., Gianni, S., Grassi, L.G., Kacmarek, R., Araujo Morais, C.C., Pinciroli, R., Vassena, E., & Berra, L. (2020a). Protocol of a randomized controlled trial testing inhaled Nitric Oxide in mechanically ventilated patients with severe acute respiratory syndrome in COVID-19 (SARS-CoV-2). medRxiv [Preprint]. 2020.03.09.20033530. doi: 10.1101/2020.03.09.20033530.
- Lai J., Ma S., Wang Y., Cai Z., Hu J., Wei N., Wu J., Du H., Chen T., Li R., Tan H., Kang L., Yao L., Huang M., Wang H., Wang G., Liu Z., Hu S. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. J. Am. Med. Assoc. Netw. Open. 2020;3(3) doi: 10.1001/jamanetworkopen.2020.3976. e203976-e203976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane C., Knight D., Burgess S., Franklin P., Horak F., Legg J., Moeller A., Stick S. Epithelial inducible nitric oxide synthase activity is the major determinant of nitric oxide concentration in exhaled breath. Thorax. 2004;59(9):757–760. doi: 10.1136/thx.2003.014894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer P., Feldman J., Giardino N., Song H.S., Schmaling K. Psychological aspects of asthma. J. Consult. Clin. Psychol. 2002;70(3):691–711. doi: 10.1037//0022-006x.70.3.691. [DOI] [PubMed] [Google Scholar]
- Lei C., Su B., Dong H., Fakhr B.S., Grassi L.G., Di Fenza R., Gianni S., Pinciroli R., Vassena E., Morais C.C.A., Bellavia A., Spina S., Kacmarek R., Berra L. Protocol for a randomized controlled trial testing inhaled nitric oxide therapy in spontaneously breathing patients with COVID-19. medRxiv. 2020 doi: 10.1101/2020.03.10.20033522. [DOI] [Google Scholar]
- LeRoy A.S., Murdock K.W., Jaremka L.M., Loya A., Fagundes C.P. Loneliness predicts self-reported cold symptoms after a viral challenge. Health Psychol. 2017;36(5):512–520. doi: 10.1037/hea0000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Ng M., Aloia J.F., Pollack S., Cunha B.A., Mikhail M., Yeh J., Berbari N. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol. Infect. 2009;137(10):1396–1404. doi: 10.1017/s0950268809002404. [DOI] [PubMed] [Google Scholar]
- Liu L.Y., Coe C.L., Swenson C.A., Kelly E.A., Kita H., Busse W.W. School examinations enhance airway inflammation to antigen challenge. Am. J. Respir. Crit. Care Med. 2002;165(8):1062–1067. doi: 10.1164/ajrccm.165.8.2109065. [DOI] [PubMed] [Google Scholar]
- Lundberg J.O., Weitzberg E., Gladwin M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008;7(2):156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- Ma L., Hu L., Feng X., Wang S. Nitrate and nitrite in health and disease. Aging Dis. 2018;9(5):938–945. doi: 10.14336/AD.2017.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangoni A.A., Rodionov R.N., McEvoy M., Zinellu A., Carru C., Sotgia S. New horizons in arginine metabolism, ageing and chronic disease states. Age Ageing. 2019;48(6):776–782. doi: 10.1093/ageing/afz083. [DOI] [PubMed] [Google Scholar]
- Marteus H., Törnberg D.C., Weitzberg E., Schedin U., Alving K. Origin of nitrite and nitrate in nasal and exhaled breath condensate and relation to nitric oxide formation. Thorax. 2005;60(3):219–225. doi: 10.1136/thx.2004.030635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau A.R., Jolliffe D.A., Hooper R.L., Greenberg L., Aloia J.F., Bergman P., Dubnov-Raz G., Esposito S., Ganmaa D., Ginde A.A., Goodall E.C., Grant C.C., Griffiths C.J., Janssens W., Laaksi I., Manaseki-Holland S., Mauger D., Murdoch D.R., Neale R., Rees J.R., Simpson S., Jr., Stelmach I., Kumar G.T., Urashima M., Camargo C.A., Jr. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. Br. Med. J. 2017;356 doi: 10.1136/bmj.i6583. i6583-i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila J.T., Thomas A.C. Nitric oxide synthase: non-canonical expression patterns. Front. Immunol. 2014;5 doi: 10.3389/fimmu.2014.00478. 478-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J. Clin. Immunol. 1987;7(4):265–276. doi: 10.1007/bf00915547. [DOI] [PubMed] [Google Scholar]
- Moeller A., Horak F., Jr., Lane C., Knight D., Kicic A., Brennan S., Franklin P., Terpolilli J., Wildhaber J.H., Stick S.M. Inducible NO synthase expression is low in airway epithelium from young children with cystic fibrosis. Thorax. 2006;61(6):514–520. doi: 10.1136/thx.2005.054643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mommersteeg M.T., Yeh M.L., Parnavelas J.G., Andrews W.D. Disrupted Slit-Robo signalling results in membranous ventricular septum defects and bicuspid aortic valves. Cardiovasc. Res. 2015;106(1):55–66. doi: 10.1093/cvr/cvv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti C., Zhuge Z., Zhang G., Haworth S.M., Paulo L.L., Guimarães D.D., Cruz J.C., Montenegro M.F., Cordero-Herrera I., Braga V.A., Weitzberg E., Carlström M., Lundberg J.O. The obligatory role of host microbiota in bioactivation of dietary nitrate. Free Radic. Biol. Med. 2019;145:342–348. doi: 10.1016/j.freeradbiomed.2019.10.003. [DOI] [PubMed] [Google Scholar]
- Morris C.R., Poljakovic M., Lavrisha L., Machado L., Kuypers F.A., Morris S.M., Jr. Decreased arginine bioavailability and increased serum arginase activity in asthma. Am. J. Respir. Crit. Care Med. 2004;170(2):148–153. doi: 10.1164/rccm.200309-1304OC. [DOI] [PubMed] [Google Scholar]
- Münzel T., Daiber A., Gori T. Nitrate therapy: new aspects concerning molecular action and tolerance. Circulation. 2011;123(19):2132–2144. doi: 10.1161/circulationaha.110.981407. [DOI] [PubMed] [Google Scholar]
- Murdoch D.R., Slow S., Chambers S.T., Jennings L.C., Stewart A.W., Priest P.C., Florkowski C.M., Livesey J.H., Camargo C.A., Scragg R. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. J. Am. Med. Assoc. 2012;308(13):1333–1339. doi: 10.1001/jama.2012.12505. [DOI] [PubMed] [Google Scholar]
- Musher, D.M. & Thorner, A.R. Community-acquired pneumonia. N. Engl. J. Med., 371(17), 1619-1628. https:/doi.org/10.1056/NEJMra1312885. [DOI] [PubMed]
- National Heart L. Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma. NIH Publication No. 07-4051; 2007. Blood institute/national asthma education and prevention program (NHLBI/NAEPP) Full report 2007. [Google Scholar]
- Nichols D., Chmiel J., Berger M. Chronic inflammation in the cystic fibrosis lung: alterations in inter- and intracellular signaling. Clin. Rev. Allergy Immunol. 2008;34(2):146–162. doi: 10.1007/s12016-007-8039-9. [DOI] [PubMed] [Google Scholar]
- Nikolaidis A., Paksarian D., Alexander L., DeRosa J., Dunn J., Nielson D.M., Droney I., Kang M., Douka I., Bromet E., Milham M.P., Stringaris A., Merikangas K.R. The Coronavirus Health and Impact Survey (CRISIS) reveals reproducible correlates of pandemic-related mood states across the Atlantic. Sci. Rep. 2021;11(1):8139. doi: 10.1038/s41598-021-87270-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olenec J.P., Kim W.K., Lee W.-M., Vang F., Pappas T.E., Salazar L.E.P., Evans M.D., Bork J., Roberg K., Lemanske R.F., Jr., Gern J.E. Weekly monitoring of children with asthma for infections and illness during common cold seasons. J. Allergy Clin. Immunol. 2010;125(5):1001–1006. doi: 10.1016/j.jaci.2010.01.059. e1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdemir B., Yazici A. Could the decrease in the endothelial nitric oxide (NO) production and NO bioavailability be the crucial cause of COVID-19 related deaths? Med. Hypotheses. 2020;144:109970. doi: 10.1016/j.mehy.2020.109970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padalko E., Ohnishi T., Matsushita K., Sun H., Fox-Talbot K., Bao C., Baldwin W.M., Lowenstein C.J. Peroxynitrite inhibition of Coxsackievirus infection by prevention of viral RNA entry. Proc. Natl. Acad. Sci. U. S. A. 2004;101(32):11731–11736. doi: 10.1073/pnas.0400518101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos N.G., Megremis S., Kitsioulis N.A., Vangelatou O., West P., Xepapadaki P. Promising approaches for the treatment and prevention of viral respiratory illnesses. J. Allergy Clin. Immunol. 2017;140(4):921–932. doi: 10.1016/j.jaci.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh R., Wilson C., Weinberg J., Gavin D., Murphy J., Reardon C.C. Inhaled nitric oxide treatment in spontaneously breathing COVID-19 patients. Ther. Adv. Respir. Dis. 2020;14 doi: 10.1177/1753466620933510. 1753466620933510-1753466620933510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K.P., Schultz H.D. Angiotensin peptides and nitric oxide in cardiovascular disease. Antioxidants Redox Signal. 2013;19(10):1121–1132. doi: 10.1089/ars.2012.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen A., Zachariae R., Bovbjerg D.H. Influence of psychological stress on upper respiratory infection--a meta-analysis of prospective studies. Psychosom. Med. 2010;72(8):823–832. doi: 10.1097/PSY.0b013e3181f1d003. [DOI] [PubMed] [Google Scholar]
- Perry Markovich M., Glatman-Freedman A., Bromberg M., Augarten A., Sefty H., Kaufman Z., Sherbany H., Regev L., Chodick G., Mendelson E., Shohat T., Mandelboim M. Back-to-school upper respiratory infection in preschool and primary school-age children in Israel. Pediatr. Infect. Dis. J. 2015;34(5):476–481. doi: 10.1097/inf.0000000000000627. [DOI] [PubMed] [Google Scholar]
- Persson M.G., Friberg S.G., Gustafsson L.E., Hedqvist P. The promotion of patent airways and inhibition of antigen-induced bronchial obstruction by endogenous nitric oxide. Br. J. Pharmacol. 1995;116(7):2957–2962. doi: 10.1111/j.1476-5381.1995.tb15950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijnenburg M.W., De Jongste J.C. Exhaled nitric oxide in childhood asthma: a review. Clin. Exp. Allergy. 2008;38(2):246–259. doi: 10.1111/j.1365-2222.2007.02897.x. [DOI] [PubMed] [Google Scholar]
- Proud D. Nitric oxide and the common cold. Curr. Opin. Allergy Clin. Immunol. 2005;5(1):37–42. doi: 10.1097/00130832-200502000-00008. [DOI] [PubMed] [Google Scholar]
- Pryor W.A., Squadrito G.L. The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am. J. Physiol. 1995;268(5 Pt 1):L699–L722. doi: 10.1152/ajplung.1995.268.5.L699. [DOI] [PubMed] [Google Scholar]
- Rasica L., Porcelli S., Marzorati M., Salvadego D., Vezzoli A., Agosti F., De Col A., Tringali G., Jones A.M., Sartorio A., Grassi B. Ergogenic effects of beetroot juice supplementation during severe-intensity exercise in obese adolescents. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018;315(3):R453–r460. doi: 10.1152/ajpregu.00017.2018. [DOI] [PubMed] [Google Scholar]
- Ricciardolo F.L., Geppetti P., Mistretta A., Nadel J.A., Sapienza M.A., Bellofiore S., Di Maria G.U. Randomised double-blind placebo-controlled study of the effect of inhibition of nitric oxide synthesis in bradykinin-induced asthma. Lancet. 1996;348(9024):374–377. doi: 10.1016/s0140-6736(96)04450-9. [DOI] [PubMed] [Google Scholar]
- Ricciardolo F.L., Sterk P.J., Gaston B., Folkerts G. Nitric oxide in health and disease of the respiratory system. Physiol. Rev. 2004;84(3):731–765. doi: 10.1152/physrev.00034.2003. [DOI] [PubMed] [Google Scholar]
- Ritz T. Airway responsiveness to psychological processes in asthma and health. Front. Physiol. 2012;3:343. doi: 10.3389/fphys.2012.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz T., Ayala E.S., Trueba A.F., Vance C.D., Auchus R.J. Acute stress-induced increases in exhaled nitric oxide in asthma and their association with endogenous cortisol. Am. J. Respir. Crit. Care Med. 2011;183(1):26–30. doi: 10.1164/rccm.201005-0691OC. [DOI] [PubMed] [Google Scholar]
- Ritz T., Bobb C., Griffiths C. Predicting asthma control: the role of psychological triggers. Allergy Asthma Proc. 2014;35(5):390–397. doi: 10.2500/aap.2014.35.3779. [DOI] [PubMed] [Google Scholar]
- Ritz T., Kullowatz A., Kanniess F., Dahme B., Magnussen H. Perceived triggers of asthma: evaluation of a German version of the Asthma Trigger Inventory. Respir. Med. 2008;102(3):390–398. doi: 10.1016/j.rmed.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Ritz T., Meuret A.E., Trueba A.F., Fritzsche A., von Leupoldt A. Psychosocial factors and behavioral medicine interventions in asthma. J. Consult. Clin. Psychol. 2013;81(2):231–250. doi: 10.1037/a0030187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz T., Steptoe A., Bobb C., Harris A.H., Edwards M. The asthma trigger inventory: validation of a questionnaire for perceived triggers of asthma. Psychosom. Med. 2006;68(6):956–965. doi: 10.1097/01.psy.0000248898.59557.74. [DOI] [PubMed] [Google Scholar]
- Ritz T., Trueba A.F. Airway nitric oxide and psychological processes in asthma and health: a review. Ann. Allergy Asthma Immunol. 2014;112(4):302–308. doi: 10.1016/j.anai.2013.11.022. [DOI] [PubMed] [Google Scholar]
- Ritz T., Trueba A.F., Liu J., Auchus R.J., Rosenfield D. Exhaled nitric oxide decreases during academic examination stress in asthma. Ann. Am. Thorac. Soc. 2015;12(11):1638–1645. doi: 10.1513/AnnalsATS.201504-213OC. [DOI] [PubMed] [Google Scholar]
- Ritz T., Trueba A.F., Simon E., Auchus R.J. Increases in exhaled nitric oxide after acute stress: association with measures of negative affect and depressive mood. Psychosom. Med. 2014;76(9):716–725. doi: 10.1097/psy.0000000000000118. [DOI] [PubMed] [Google Scholar]
- Ritz T., Trueba A.F., Vogel P.D., Auchus R.J., Rosenfield D. Exhaled nitric oxide and vascular endothelial growth factor as predictors of cold symptoms after stress. Biol. Psychol. 2018;132:116–124. doi: 10.1016/j.biopsycho.2017.11.006. [DOI] [PubMed] [Google Scholar]
- Ritz T., Wittchen H.U., Klotsche J., Mühlig S., Riedel O. Asthma trigger reports are associated with low quality of life, exacerbations, and emergency treatments. Ann. Am. Thorac. Soc. 2016;13(2):204–211. doi: 10.1513/AnnalsATS.201506-390OC. [DOI] [PubMed] [Google Scholar]
- Ritz T., Werchan C.A., Kroll J.K., Rosenfield D. Beetroot juice supplementation for the prevention of cold symptoms associated with stress: a proof-of-concept study. Physiol. Behav. 2019;202:45–51. doi: 10.1016/j.physbeh.2019.01.015. [DOI] [PubMed] [Google Scholar]
- Rocha B.S., Nunes C., Laranjinha J. Tuning constitutive and pathological inflammation in the gut via the interaction of dietary nitrate and polyphenols with host microbiome. Int. J. Biochem. Cell Biol. 2016;81:393–402. doi: 10.1016/j.biocel.2016.10.021. [DOI] [PubMed] [Google Scholar]
- Rosenkranz M.A., Esnault S., Christian B.T., Crisafi G., Gresham L.K., Higgins A.T., Moore M.N., Moore S.M., Weng H.Y., Salk R.H., Busse W.W., Davidson R.J. Mind-body interactions in the regulation of airway inflammation in asthma: a PET study of acute and chronic stress. Brain Behav. Immun. 2016;58:18–30. doi: 10.1016/j.bbi.2016.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samavati L., Uhal B.D. ACE2, much more than just a receptor for SARS-COV-2. Front. Cell. Infect. Microbiol. 2020;10 doi: 10.3389/fcimb.2020.00317. 317-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders S.P. Nitric oxide in asthma. Pathogenic, therapeutic, or diagnostic? Am. J. Respir. Cell Mol. Biol. 1999;21(2):147–149. doi: 10.1165/ajrcmb.21.2.f158. [DOI] [PubMed] [Google Scholar]
- Sanders S.P., Proud D., Permutt S., Siekierski E.S., Yachechko R., Liu M.C. Role of nasal nitric oxide in the resolution of experimental rhinovirus infection. J. Allergy Clin. Immunol. 2004;113(4):697–702. doi: 10.1016/j.jaci.2004.01.755. [DOI] [PubMed] [Google Scholar]
- Sanders S.P., Siekierski E.S., Porter J.D., Richards S.M., Proud D. Nitric oxide inhibits rhinovirus-induced cytokine production and viral replication in a human respiratory epithelial cell line. J. Virol. 1998;72(2):934–942. doi: 10.1128/jvi.72.2.934-942.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott L., Morphew T., Bollinger M.E., Samuelson S., Galant S., Clement L., O’Cull K., Jones F., Jones C.A. Achieving and maintaining asthma control in inner-city children. J. Allergy Clin. Immunol. 2011;128(1):56–63. doi: 10.1016/j.jaci.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Sears M.R., Johnston N.W. Understanding the September asthma epidemic. J. Allergy Clin. Immunol. 2007;120(3):526–529. doi: 10.1016/j.jaci.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N., Haake S.K., Mannon P., Lemon K.P., Waldron L., Gevers D., Huttenhower C., Izard J. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol. 2012;13(6):R42. doi: 10.1186/gb-2012-13-6-r42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shann F., Woolcock A., Black R., Cripps A., Foy H., Harris M., D’Souza R. Introduction: acute respiratory tract infections – the forgotten pandemic. Clin. Infect. Dis. 1999;28(2):189–191. doi: 10.1086/515107. [DOI] [PubMed] [Google Scholar]
- Silkoff P.E., Erzurum S.C., Lundberg J.O., George S.C., Marczin N., Hunt J.F., Effros R., Horvath I. ATS workshop proceedings: exhaled nitric oxide and nitric oxide oxidative metabolism in exhaled breath condensate. Proc. Am. Thorac. Soc. 2006;3(2):131–145. doi: 10.1513/pats.200406-710ST. [DOI] [PubMed] [Google Scholar]
- Simancas-Racines D., Franco J.V., Guerra C.V., Felix M.L., Hidalgo R., Martinez-Zapata M.J. Vaccines for the common cold. Cochrane Database Systemat. Rev. 2017;5(5) doi: 10.1002/14651858.CD002190.pub5. CD002190-CD002190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J.L., Moric I., Wark P.A.B., Johnston S.L., Gibson P.G. Use of induced sputum for the diagnosis of influenza and infections in asthma: a comparison of diagnostic techniques. J. Clin. Virol. : Off. Publ. Pan Am. Soc. Clin. Virol. 2003;26(3):339–346. doi: 10.1016/s1386-6532(02)00084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindler A.L., Fleenor B.S., Calvert J.W., Marshall K.D., Zigler M.L., Lefer D.J., Seals D.R. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell. 2011;10(3):429–437. doi: 10.1111/j.1474-9726.2011.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.P. Twenty-five years of research on the behavioural malaise associated with influenza and the common cold. Psychoneuroendocrinology. 2013;38:744–751. doi: 10.1016/j.psyneuen.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolderen K.G.E., Vingerhoets A.J.J.M., Croon M.A., Denollet J. Personality, psychological stress, and self-reported influenza symptomatology. BMC Publ. Health. 2007;7 doi: 10.1186/1471-2458-7-339. 339-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone A.A., Bovbjerg D.H., Neale J.M., Napoli A., Valdimarsdottir H., Cox D., Hayden F.G., Gwaltney J.M., Jr. Development of common cold symptoms following experimental rhinovirus infection is related to prior stressful life events. Behav. Med. 1992;18(3):115–120. doi: 10.1080/08964289.1992.9936961. [DOI] [PubMed] [Google Scholar]
- Subbarao K., Mahanty S. Respiratory virus infections: understanding COVID-19. Immunity. 2020;52(6):905–909. doi: 10.1016/j.immuni.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sverrild A., Kiilerich P., Brejnrod A., Pedersen R., Porsbjerg C., Bergqvist A., Erjefält J.S., Kristiansen K., Backer V. Eosinophilic airway inflammation in asthmatic patients is associated with an altered airway microbiome. J. Allergy Clin. Immunol. 2017;140(2):407–417. doi: 10.1016/j.jaci.2016.10.046. e411. [DOI] [PubMed] [Google Scholar]
- Szcześniak D., Gładka A., Misiak B., Cyran A., Rymaszewska J. The SARS-CoV-2 and mental health: from biological mechanisms to social consequences. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2021;104 doi: 10.1016/j.pnpbp.2020.110046. 110046-110046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., Koutsothanasis G.A., Bomar P.A. StatPearls Publishing; Treasure Island (FL): 2020. Upper Respiratory Tract Infection. StatPearls [Internet] [PubMed] [Google Scholar]
- Tribble G.D., Angelov N., Weltman R., Wang B.Y., Eswaran S.V., Gay I.C., Parthasarathy K., Dao D.V., Richardson K.N., Ismail N.M., Sharina I.G., Hyde E.R., Ajami N.J., Petrosino J.F., Bryan N.S. Frequency of tongue cleaning impacts the human tongue microbiome composition and enterosalivary circulation of nitrate. Front. Cell. Infect. Microbiol. 2019;9:39. doi: 10.3389/fcimb.2019.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Törnberg D.C., Marteus H., Schedin U., Alving K., Lundberg J.O., Weitzberg E. Nasal and oral contribution to inhaled and exhaled nitric oxide: a study in tracheotomized patients. Eur. Respir. J. 2002;19(5):859–864. doi: 10.1183/09031936.02.00273502. [DOI] [PubMed] [Google Scholar]
- Torales J., O’Higgins M., Castaldelli-Maia J.M., Ventriglio A. The outbreak of COVID-19 coronavirus and its impact on global mental health. Int. J. Soc. Psychiatr. 2020;66(4):317–320. doi: 10.1177/0020764020915212. [DOI] [PubMed] [Google Scholar]
- Trueba A.F., Ritz T. Stress, asthma, and respiratory infections: pathways involving airway immunology and microbial endocrinology. Brain Behav. Immun. 2013;29:11–27. doi: 10.1016/j.bbi.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Trueba A.F., Rosenfield D., Smith N.B., Gorena T.L., Ritz T. Social support as a predictor exhaled nitric oxide in healthy individuals across time. Int. J. Psychophysiol. 2014;93(3):356–362. doi: 10.1016/j.ijpsycho.2014.05.011. [DOI] [PubMed] [Google Scholar]
- Trueba A.F., Smith N.B., Auchus R.J., Ritz T. Academic exam stress and depressive mood are associated with reductions in exhaled nitric oxide in healthy individuals. Biol. Psychol. 2013;93(1):206–212. doi: 10.1016/j.biopsycho.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Turner Cobb J.M., Steptoe A. Psychosocial influences on upper respiratory infectious illness in children. J. Psychosom. Res. 1998;45(4):319–330. doi: 10.1016/s0022-3999(97)00311-5. [DOI] [PubMed] [Google Scholar]
- van Breda S.G.J., de Kok T. Smart combinations of bioactive compounds in fruits and vegetables may guide new strategies for personalized prevention of chronic diseases. Mol. Nutr. Food Res. 2018;62(1) doi: 10.1002/mnfr.201700597. [DOI] [PubMed] [Google Scholar]
- van den Berg M.P., Meurs H., Gosens R. Targeting arginase and nitric oxide metabolism in chronic airway diseases and their co-morbidities. Curr. Opin. Pharmacol. 2018;40:126–133. doi: 10.1016/j.coph.2018.04.010. [DOI] [PubMed] [Google Scholar]
- Vareille M., Kieninger E., Edwards M.R., Regamey N. The airway epithelium: soldier in the fight against respiratory viruses. Clin. Microbiol. Rev. 2011;24(1):210–229. doi: 10.1128/CMR.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez E.C., Gava A.L., Graceli J.B., Balarini C.M., Campagnaro B.P., Pereira T.M., Meyrelles S.S. Novel Therapeutic Targets for Phosphodiesterase 5 Inhibitors: current state-of-the-art on systemic arterial hypertension and atherosclerosis. Curr. Pharmaceut. Biotechnol. 2016;17(4):347–364. doi: 10.2174/1389201017666151223123904. [DOI] [PubMed] [Google Scholar]
- Viniol C., Vogelmeier C.F. Exacerbations of COPD. European respiratory review. Off. J. Eur. Respir. Soc. 2018;27(147) doi: 10.1183/16000617.0103-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volino-Souza M., de Oliveira G.V., Alvares T.S. A single dose of beetroot juice improves endothelial function but not tissue oxygenation in pregnant women: A randomised clinical trial. British J. Nutr. 2018;120(9):1006–1013. doi: 10.1017/S0007114518002441. [DOI] [PubMed] [Google Scholar]
- Webb A.J., Patel N., Loukogeorgakis S., Okorie M., Aboud Z., Misra S., Rashid R., Miall P., Deanfield J., Benjamin N., MacAllister R., Hobbs A.J., Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51(3):784–790. doi: 10.1161/hypertensionaha.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield J., Ludzki A., Heigenhauser G.J., Senden J.M., Verdijk L.B., van Loon L.J., Spriet L.L., Holloway G.P. Beetroot juice supplementation reduces whole body oxygen consumption but does not improve indices of mitochondrial efficiency in human skeletal muscle. J. Physiol. 2016;594(2):421–435. doi: 10.1113/JP270844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink D.A., Darbyshire J.F., Nims R.W., Saavedra J.E., Ford P.C. Reactions of the bioregulatory agent nitric oxide in oxygenated aqueous media: determination of the kinetics for oxidation and nitrosation by intermediates generated in the NO/O2 reaction. Chem. Res. Toxicol. 1993;6(1):23–27. doi: 10.1021/tx00031a003. [DOI] [PubMed] [Google Scholar]
- Wink D.A., Kasprzak K.S., Maragos C.M., Elespuru R.K., Misra M., Dunams T.M., Cebula T.A., Koch W.H., Andrews A.W., Allen J.S. DNA deaminating ability and genotoxicity of nitric oxide and its progenitors. Science. 1991;254(5034):1001–1003. doi: 10.1126/science.1948068. [DOI] [PubMed] [Google Scholar]
- Wright R.J., Rodriguez M., Cohen S. Review of psychosocial stress and asthma: an integrated biopsychosocial approach. Thorax. 1998;53(12):1066–1074. doi: 10.1136/thx.53.12.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Liu L.Z., Loizidou M., Ahmed M., Charles I.G. The role of nitric oxide in cancer. Cell Res. 2002;12(5–6):311–320. doi: 10.1038/sj.cr.7290133. [DOI] [PubMed] [Google Scholar]
- Xu W., Zheng S., Dweik R.A., Erzurum S.C. Role of epithelial nitric oxide in airway viral infection. Free Radic. Biol. Med. 2006;41(1):19–28. doi: 10.1016/j.freeradbiomed.2006.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates D.H. Role of exhaled nitric oxide in asthma. Immunol. Cell Biol. 2001;79(2):178–190. doi: 10.1046/j.1440-1711.2001.00990.x. [DOI] [PubMed] [Google Scholar]
- Yu B., Muenster S., Blaesi A.H., Bloch D.B., Zapol W.M. Producing nitric oxide by pulsed electrical discharge in air for portable inhalation therapy. Sci. Transl. Med. 2015;7(294) doi: 10.1126/scitranslmed.aaa3097. 294ra107. [DOI] [PubMed] [Google Scholar]
- Zamani H., de Joode M., Hossein I.J., Henckens N.F.T., Guggeis M.A., Berends J.E., de Kok T., van Breda S.G.J. The benefits and risks of beetroot juice consumption: a systematic review. Crit. Rev. Food Sci. Nutr. 2020:1–17. doi: 10.1080/10408398.2020.1746629. [DOI] [PubMed] [Google Scholar]
- Zhang J., Xie B., Hashimoto K. Current status of potential therapeutic candidates for the COVID-19 crisis. Brain Behav. Immun. 2020;87:59–73. doi: 10.1016/j.bbi.2020.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Gaucher C., Fries I., Hobekkaya M.A., Martin C., Leonard C., Deschamps F., Sapin-Minet A., Parent M. Challenging development of storable particles for oral delivery of a physiological nitric oxide donor. Nitric Oxide. 2020;104–105:1–10. doi: 10.1016/j.niox.2020.08.001. [DOI] [PubMed] [Google Scholar]