Abstract
Purpose
Long-term macrolide treatment is recommended for patients with chronic obstructive pulmonary disease (COPD) with frequent exacerbations. Bronchiectasis is a common comorbid condition in patients with COPD, for which long-term azithromycin is effective in preventing exacerbation. This study aimed to compare the effect of long-term azithromycin between bronchiectasis patients with chronic airflow obstruction (CAO) and COPD patients without bronchiectasis.
Patients and Methods
Patients with CAO who received azithromycin for more than 12 weeks were retrospectively identified at a single referral hospital. CAO was defined as a post-bronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) <0.7, and bronchiectasis was determined using computed tomography. The development of exacerbation and symptom improvement were compared between bronchiectasis patients with CAO and COPD patients without bronchiectasis.
Results
A total of 59 patients (43 in bronchiectasis with CAO group vs 16 in COPD without bronchiectasis group) were included in this study. Compared to COPD patients without bronchiectasis, those in bronchiectasis with CAO group were younger, more likely to be female, and never smokers. There was no difference in the previous exacerbation history or FEV1 between the two groups. The median duration of azithromycin treatment was 15 months (interquartile range, 8–25 months). At the 12-month follow-up, the development of ≥2 moderate or ≥1 severe exacerbations was significantly lower in bronchiectasis with CAO group than in COPD without bronchiectasis group (46.5% vs 87.5%, P = 0.005). The proportion of patients with symptom improvement determined by the COPD assessment test score was also significantly higher in bronchiectasis with CAO group than COPD without bronchiectasis group at the 12-month follow-up (68.2% vs 16.7%, P = 0.004).
Conclusion
Bronchiectasis patients with CAO could benefit more from long-term azithromycin treatment than COPD patients without bronchiectasis.
Keywords: COPD, bronchiectasis, azithromycin, exacerbation
Introduction
Frequent exacerbations of chronic obstructive pulmonary disease (COPD) are a major cause of morbidity and mortality, leading to a substantial healthcare burden.1–4 To reduce exacerbations, the current COPD guidelines recommend pharmacological treatment with anti-inflammatory agents, including inhaled corticosteroids (ICS), roflumilast, and long-term macrolides.5 Among macrolides, long-term azithromycin added to the usual treatment reduced the frequency of exacerbations and improved quality of life,6,7 particularly in those who are not current smokers.8 As this long-term macrolide therapy significantly reduces the incidence of non-cystic fibrosis bronchiectasis (NCFB) exacerbations,9–11 NCFB management guidelines also recommend long-term macrolide therapy for bronchiectasis patients with frequent exacerbations,12,13 regardless of the presence of chronic airflow obstruction (CAO).
With the wide availability of chest computed tomography (CT), previously unrecognized bronchiectasis is increasingly identified in patients with COPD. Indeed, bronchiectasis has been reported in 29–57% of patients with COPD, and the presence of bronchiectasis is associated with frequent exacerbations, COPD severity, and even increased mortality.14–17 COPD is also a common comorbidity in patients with bronchiectasis, reporting up to 58% of prevalence of COPD diagnosis in patients with bronchiectasis using health-insurance claim data.18,19 Another data from US bronchiectasis research registry also showed that airflow limitation was present in more than half patients of bronchiectasis patients.20
Despite this frequent coexistence of COPD and bronchiectasis, previous studies on the benefits of long-term macrolides in patients with COPD did not assess the presence of bronchiectasis; thus, there is no available study to show the treatment efficacy according to the presence of bronchiectasis in COPD.6–8 Similarly, it remains unclear whether coexisting CAO in bronchiectasis patients are associated with a greater benefit of long-term macrolide treatment than COPD without bronchiectasis.9–11 Therefore, this study aimed to compare the effect of long-term azithromycin between bronchiectasis patients with CAO and COPD patients without bronchiectasis.
Methods
Study Population
Between July 2014 and October 2018, 82 patients who 1) received azithromycin for more than 12 weeks, and 2) were free of nontuberculous mycobacterial (NTM) pulmonary disease were retrospectively identified at a single referral hospital in South Korea. After excluding 21 patients with post-bronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ≥ 0.7 and two patients without post-bronchodilator spirometry, 59 patients with CAO, defined as post-bronchodilator FEV1/FVC < 0.7 were included in this study. The diagnosis of bronchiectasis was based on the CT (bronchial dilatation, lack of bronchial tapering, or identification of bronchi within 1 cm of the pleura)21 and compatible symptoms. In this study, the group with bronchiectasis and CAO was named as bronchiectasis with CAO rather than COPD with bronchiectasis. Up to 39% of COPD patients are never smokers22 and they are diagnosed based on CAO. Nevertheless, so far, as there is no solid definition of COPD and bronchiectasis overlap, bronchiectasis with CAO would be more appropriate nomenclature.
This study was approved by the Institutional Review Board of the Samsung Medical Center (IRB No. SMC 2019–10-096), and the requirement for informed consent was waived due to the retrospective nature of the study. All patients’ data were anonymized and deidentified before the analysis, and this study was conducted according to the Declaration of Helsinki.
Data Collection and Clinical Assessment
Clinical characteristics, including demographics, symptoms, history of exacerbations, medications before initiating azithromycin, pulmonary function, laboratory findings, sputum culture, and adverse events of azithromycin therapy, were obtained from the medical records. Symptomatic burden and quality of life were assessed using the modified Medical Research Council (mMRC) and COPD Assessment Test (CAT).23 The severity of bronchiectasis was evaluated using the Bronchiectasis Severity Index (BSI).24
Spirometry was performed using Vmax 22 (SensorMedics, Yorba Linda, CA, USA) according to the ATS/European Respiratory Society criteria.25 Absolute values of FVC and FEV1 were obtained, and the percentage predicted values (%pred) were calculated using data obtained from a representative Korean sample.26 A positive bronchodilator response was defined as a post-bronchodilator increase in FEV1 of at least 12% and 200 mL from the baseline value.27
The primary outcome of this study was the development of exacerbations. Moderate exacerbation was defined as an outpatient clinic visit, while severe exacerbation was defined as hospitalization or an emergency room visit owing to one or more of the following: worsening of dyspnea, increased sputum volume, and purulent sputum. Exacerbations were treated with short-acting bronchodilators and antibiotics with or without systemic corticosteroids. The secondary outcome was symptom improvement during azithromycin treatment. An asymptomatic responder was defined as a patient who achieved a minimum clinically important difference (MCID) for CAT, which is a ≥2 point decrement from the initial CAT score.28 We used the CAT score measured at 3, 6, and 12 months after azithromycin initiation.
Statistical Analysis
Categorical variables were analyzed using Pearson’s χ2 test or Fisher’s exact test. Continuous variables were analyzed using the Mann–Whitney U-test. Univariable and multivariable analyses with logistic regression models were performed to identify factors associated with the development of exacerbations during azithromycin treatment. In multivariable analyses, variables with P < 0.20 on univariate analyses and clinically relevant variables were included. With 95% confidence intervals (CIs), P values of less than 0.05 were considered significant. All analyses were performed using IBM SPSS 25.0 (IBM Corp., Armonk, NY).
Results
Baseline Characteristics
Fifty-nine patients with CAO were categorized into two groups: bronchiectasis with CAO group (N = 43) vs COPD without bronchiectasis group (N = 16). The median age was 64 years, and patients in bronchiectasis with CAO group were younger (P = 0.023), more likely to be female (P = 0.017), and never smokers (P < 0.001) than those in COPD without bronchiectasis group (Table 1). While no patients had allergic bronchopulmonary mycosis, six patients (14.0%) and a patient (6.3%) had co-existing bronchial asthma in bronchiectasis with CAO group and COPD without bronchiectasis group, respectively (P = 0.661). Most patients reported dyspnea with mMRC grade ≥ 2 (83.1%) and CAT score ≥ 10 points (98.0%), which did not differ between the two groups. About 90% of patients had a history of moderate or severe exacerbations in the previous year, which were similar between the two groups.
Table 1.
Baseline Characteristics at the Time of Azithromycin Treatment Initiation
| Total (N = 59) | Bronchiectasis with CAO (n = 43) | COPD without Bronchiectasis (n = 16) | P-value | |
|---|---|---|---|---|
| Age, years | 64.0 (58.0–72.0) | 62.0 (57.0–70.0) | 70.0 (62.5–76.3) | 0.023 |
| Sex, male | 33 (55.9) | 20 (46.5) | 13 (81.3) | 0.017 |
| BMI, kg/m2 | 21.0 (18.2–23.1) | 21.4 (18.5–23.1) | 20.8 (16.8–22.8) | 0.549 |
| Smoking history | ||||
| Never | 31 (52.5) | 29 (67.4) | 2 (12.5) | <0.001 |
| Former | 26 (44.1) | 13 (30.2) | 13 (81.3) | |
| Current | 2 (3.4) | 1 (2.3) | 1 (6.3) | |
| History of PTB | 20 (33.9) | 16 (37.2) | 4 (25.0) | 0.378 |
| Bronchial asthma | 7 (11.9) | 6 (14.0) | 1 (6.3) | 0.661 |
| mMRC | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) | 0.348 |
| ≥2 | 49 (83.1) | 34 (79.1) | 15 (93.8) | 0.259 |
| CAT score (n = 50) | 27.5 (22.8–31.0) | 26.5 (20.8–31.5) | 28.0 (23.5–30.5) | 0.471 |
| ≥10 | 49 (98.0) | 33 (97.1) | 16 (100.0) | 1.000 |
| Exacerbations in the previous year | ||||
| Any exacerbations | 54 (91.5) | 39 (90.7) | 15 (93.8) | 1.000 |
| ≥2 moderate or ≥1 severe | 51 (86.4) | 36 (83.7) | 15 (93.8) | 0.427 |
| Post-bronchodilator spirometry | ||||
| FVC, L | 2.47 (1.90–3.15) | 2.26 (1.82–2.86) | 2.83 (2.65–3.48) | 0.010 |
| FVC, % pred | 69.0 (56.0–82.0) | 66.0 (54.0–79.0) | 72.5 (64.5–86.5) | 0.039 |
| FEV1, L | 1.11 (0.88–1.40) | 1.11 (0.91–1.40) | 1.13 (0.77–1.47) | 0.580 |
| FEV1, % pred | 41.0 (54.0–33.0) | 41.0 (33.0–57.0) | 40.0 (34.0–48.8) | 0.406 |
| ≥50% pred | 19 (32.2) | 16 (37.2) | 3 (18.8) | 0.177 |
| <50% pred | 40 (67.8) | 27 (62.8) | 13 (81.3) | |
| FEV1/FVC, % pred | 46.0 (38.0–63.0) | 55.0 (40.0–63.0) | 39.0 (33.0–44.5) | 0.002 |
| Bronchodilator response | 9 (15.3) | 7 (16.3) | 2 (12.5) | 1.000 |
| Laboratory finding | ||||
| WBC, × 103 /uL | 8.2 (6.2–10.6) | 8.3 (6.2–11.1) | 7.9 (5.6–9.9) | 0.422 |
| CRP, mg/dL (n = 58) | 0.4 (0.1–2.1) | 0.4 (0.2–2.7) | 0.1 (0.0–1.7) | 0.059 |
| Eosinophils, /uL | 130.0 (39.4–245.7) | 145.6 (86.6–250.0) | 44.0 (10.0–144.8) | 0.019 |
| Sputum culture (n = 50) | ||||
| Pseudomonas | 6/50 (12.0) | 6/40 (15.0) | 0/10 (0.0) | 0.327 |
| Other organismsa | 4/50 (8.0) | 4/40 (10.0) | 0/10 (0.0) | 0.571 |
Notes: Data are presented as number (%) or median (interquartile range). aTwo patients had Haemophilus influenzae, and the others had M. catarrhalis and Enterobacteriaceae coli, respectively.
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; CAO, chronic airflow obstruction; CAT, COPD Assessment Test; CRP, C-reactive protein; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; mMRC, modified Medical Research Council dyspnea questionnaire; PTB, pulmonary tuberculosis; WBC, white blood cell.
Regarding pulmonary function, FVC of patients in bronchiectasis with CAO group was lower than those in COPD without bronchiectasis group, whereas there were no significant differences in FEV1. Compared to patients in COPD without bronchiectasis group, those in bronchiectasis with CAO group had higher eosinophil counts (P = 0.019), which was persistent even after excluding 7 patients with asthma. Although it did not reach statistical significance, patients with bronchiectasis had a higher rate of positive sputum culture (10/40, 25.0%) than those without bronchiectasis (0/10, 0.0%). In addition, among patients with bronchiectasis, the median number of lobes involved in bronchiectasis was 4, and 26 patients (60.5%) had BSI ≥9 points (data not shown).
Outcomes of Long-Term Azithromycin Treatment
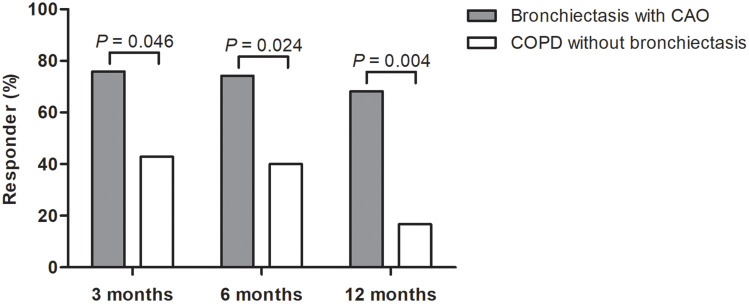
The median dose of azithromycin was 1750 mg per week, and the median duration of treatment was 15 (IQR 8.0–25.0) months. Most of the patients in COPD without bronchiectasis group (93.8%) were treated with ICS before initiating azithromycin, while only 58.1% of bronchiectasis with CAO patients did so (P = 0.010) (Table 2). During the 12-month follow-up, the development of ≥2 moderate or ≥1 severe exacerbation was significantly lower in bronchiectasis with CAO group than in COPD without bronchiectasis group (46.5% vs 87.5%, P = 0.005). However, there was no difference in the development of severe exacerbations between the two groups (Table 3). The proportion of patients who achieved MCID for CAT score was significantly higher in bronchiectasis with CAO group than in COPD without bronchiectasis group at 3 months (75.9% vs 42.9%), 6 months (74.2% vs 40.0%), and 12 months (68.2% vs 16.7%) of azithromycin treatment (Figure 1).
Table 2.
Baseline Characteristics of Given Treatment
| Total (N = 59) | Bronchiectasis with CAO (n = 43) |
COPD without Bronchiectasis (n = 16) | P-value | |
|---|---|---|---|---|
| Treatment regimen | ||||
| Weekly dose, mg | 1750 (1736–1750) | 1750 (1478–1750) | 1750 (1750–1750) | 0.171 |
| 250 mg once daily | 50 (84.7) | 35 (81.4) | 15 (93.8) | 0.421 |
| 500 mg once, three days a week | 1 (1.7) | 0 (0.0) | 1 (6.3) | 0.271 |
| Othersa | 8 (13.6) | 8 (18.6) | 0 (0.0) | 0.093 |
| Duration, months | 15.0 (8.0–25.0) | 14.8 (7.1–24.6) | 16.5 (10.2–29.1) | 0.569 |
| Inhaler medication | 0.010 | |||
| None | 7 (11.9) | 7 (16.3) | 0 (0.0) | |
| LAMA | 3 (5.1) | 2 (4.7) | 1 (6.3) | |
| LAMA/LABA | 9 (15.3) | 9 (20.9) | 0 (0.0) | |
| ICS/LABA | 9 (15.3) | 8 (18.6) | 1 (6.3) | |
| ICS/LAMA/LABA | 31 (52.5) | 17 (39.5) | 14 (87.5) | |
| Roflumilast | 9 (15.3) | 5 (11.6) | 4 (25.0) | 0.236 |
Notes: Data are presented as number (%) or median (interquartile range). aThree patients received 250 mg three times a week; two patients received 500 mg daily; one patient received 250 mg for five days per month; one patient received 250 mg for ten days per month; and the other received 500 mg for 7 days per month.
Abbreviations: CAO, chronic airflow obstruction; COPD, chronic obstructive pulmonary disease; ICS, inhaled corticosteroid; LABA, long-acting beta2-agonist; LAMA, long-acting muscarinic antagonist.
Table 3.
Outcomes of Long-Term Azithromycin Treatment at 12 Months Follow-Up
| Total (N = 59) | Bronchiectasis with CAO (n = 43) | COPD without Bronchiectasis (n = 16) | P-value | |
|---|---|---|---|---|
| Exacerbation | ||||
| Moderate | 27 (45.8) | 15 (34.9) | 12 (75.0) | 0.006 |
| Severe | 17 (28.8) | 12 (27.9) | 5 (31.3) | 1.000 |
| ≥2 moderate or ≥1 severe | 34 (57.6) | 20 (46.5) | 14 (87.5) | 0.005 |
| Adverse event | 3 (5.1) | 1 (2.3)a | 2 (12.5)b | 0.176 |
Notes: Data are presented as number (%). aA patient reported lip swelling. bA patient reported abdominal discomfort, and the other presented general weakness.
Abbreviations: CAO, chronic airflow obstruction; COPD, chronic obstructive pulmonary disease.
Figure 1.
The proportion of symptomatic responders* after long-term azithromycin treatment. *Defined as patients with ≥2 points decrement from the initial COPD Assessment Test (CAT) score. CAT was measured in 43, 47, and 35 patients at 3, 6, and 12 months after initiation of azithromycin treatment.
Abbreviations: CAO, chronic airflow obstruction; COPD, chronic obstructive pulmonary disease.
Adverse events were reported in three patients: lip swelling, abdominal discomfort, and general weakness. In terms of hearing loss, audiometry was performed in 25 patients at baseline and 14 patients repeated audiometry following azithromycin treatment. No hearing impairment was observed in these patients, and there was no subjective hearing loss following azithromycin treatment in all patients.
Risk Factors Associated with Acute Exacerbation During Azithromycin Treatment
Factors associated with the development of ≥2 moderate or ≥1 severe exacerbation during the 12-month follow-up were evaluated using logistic regression analysis. In univariable analysis, male sex (unadjusted odds ratio [OR] = 3.14, 95% CI = 1.07–9.19), smoking history (unadjusted OR = 3.04, 95% CI = 1.03–8.97) were associated with a higher risk of exacerbation, while the bronchiectasis with CAO group had a lower risk of exacerbation compared with COPD without bronchiectasis group (unadjusted OR = 0.12, 95% CI = 0.03–0.61). In multivariable analysis, patients with bronchiectasis with CAO had a significantly lower risk for exacerbation after long-term azithromycin therapy, compared with COPD patients without bronchiectasis (adjusted OR = 0.15, 95% CI = 0.03–0.87) (Table 4).
Table 4.
Risk Factors for Acute Exacerbationsa During 12 Months of Follow-Up
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value | |
| Age | 1.03 (0.99–1.08) | 0.137 | 1.01 (0.96–1.06) | 0.693 |
| Sex, male | 3.14 (1.07–9.19) | 0.037 | 2.52 (0.37–17.03) | 0.343 |
| Smoking, ever | 3.04 (1.03–8.97) | 0.044 | 0.74 (0.11–4.96) | 0.760 |
| FEV1 < 50% pred | 1.85 (0.61–5.59) | 0.274 | 1.04 (0.26–4.14) | 0.959 |
| Bronchiectasis with CAO (vs COPD without bronchiectasis) | 0.12 (0.03–0.61) | 0.011 | 0.15 (0.03–0.87) | 0.035 |
| ICS use | 1.85 (0.61–5.59) | 0.274 | 0.92 (0.25–3.40) | 0.904 |
Notes: aDefined as ≥2 moderate (hospital visit) or ≥1 severe (emergency department or hospitalization) acute exacerbations.
Abbreviations: CAO, chronic airflow obstruction; CI, confidence interval; FEV1, forced expiratory volume in one second; ICS, inhaled corticosteroid; OR, odds ratio.
Discussion
In the present study, bronchiectasis patients with CAO had a significantly lower risk of exacerbation than COPD patients without bronchiectasis during azithromycin therapy with a median follow-up of 15 months. It is noteworthy that there was no difference in the previous exacerbation history or the degree of airflow limitation between the two groups. In addition, the proportion of CAT responders indicating symptomatic improvement was markedly higher in bronchiectasis with CAO group than in COPD without bronchiectasis group. Given that the diagnosis of COPD is based on CAO and the delineation between bronchiectasis with CAO and COPD-bronchiectasis overlap is not always clear in practice,29 our study would provide important therapeutic implication of coexisting bronchiectasis among COPD patients with frequent exacerbations.
In addition to their antimicrobial action, macrolides are well known for their immunomodulatory effects by mitigating neutrophil-mediated lung damage and promoting mucociliary clearance, for which the effect occurs below the minimum inhibitory concentration for bacterial infection.30,31 Several studies have proven the effect of long-term macrolide treatment for exacerbation prevention in COPD patients.6,7,32–34 A recent meta-analysis including 2151 patients showed that long-term macrolide treatment reduced COPD exacerbation compared with the control (OR = 0.40; 95% CI = 0.24–0.65).35 Long-term macrolide treatment also improved the quality of life measured with the St George’s Respiratory Questionnaire score, although it did not exceed the MCID. However, there are notable adverse effects associated with macrolide therapy, including gastrointestinal disturbance, hearing impairment, and risk of antibiotic resistance, which were more evident with azithromycin, among other macrolides.35 Therefore, it is important to identify patients who are most likely to benefit from long-term azithromycin therapy. Landmark trials reported that daily azithromycin treatment for 12 months decreased the frequency of COPD exacerbation,6 and the post hoc analysis found that older (age > 65) patients, those with milder airflow limitation (FEV1 > 50% pred), and ex-smokers were subgroups with greater efficacy of azithromycin therapy.8 However, the presence of bronchiectasis was not assessed, although it was not an exclusion criterion of the trial. In another study of azithromycin therapy, patients with bronchiectasis were excluded from the beginning.7 In the aspect of bronchiectasis, the reported efficacy of long-term azithromycin use in bronchiectasis is approximately halving the exacerbation rate (adjusted incidence rate ratio 0.49, 95% CI = 0.36–0.66) in a recent individual participant data meta-analysis, which was consistently observed in all subgroups.36 However, no study has addressed the question regarding the different efficacy of long-term azithromycin between these two frequently overlapping diseases since most studies on azithromycin treatment in bronchiectasis did not exclude patients with coexisting COPD or spirometric CAO.9–11 Correspondingly, 65% of bronchiectasis patients in azithromycin trials were using ICS,36 similar to our study (approximately 60%). Thus, our study compared the efficacy of azithromycin treatment between bronchiectasis with CAO and COPD patients without bronchiectasis, and showed the lower exacerbation risk and better symptomatic improvement in bronchiectasis with CAO patients than in COPD patients without bronchiectasis during long-term azithromycin treatment, even using much less ICS. Our study suggests that azithromycin is a more effective anti-inflammatory treatment in bronchiectasis with CAO and this is also aligned with the fact that bronchiectasis patients taking macrolide monotherapy were less likely to be hospitalized for a respiratory infection or exacerbated compared with ICS.37
The microbiological burden of coexisting bronchiectasis likely has a beneficial effect on azithromycin treatment. Several studies consistently demonstrated that coexisting bronchiectasis in COPD is associated with chronic bronchial infection (CBI) by potentially pathogenic microorganisms and greater bacterial load,38–40 with more than 50% of sputum samples yielding a positive result.40 Our study also showed that patients with bronchiectasis were more likely to have a positive sputum culture than those without bronchiectasis. This CBI perpetuates inflammation and contributes to COPD progression and frequent exacerbations.41,42 Indeed, some previous trials in patients with COPD showed a nonsignificant reduction in airway bacterial load with three months of azithromycin treatment.43,44 Taken together, the presence of bronchiectasis might indicate the CBI phenotype of COPD, which is associated with a better response to long-term azithromycin treatment for preventing exacerbation.
We acknowledge several limitations of this study. First, it was a retrospective study at a single referral hospital in Korea, including a small number of patients, limiting its generalizability. Notably, long-term azithromycin as an anti-inflammatory agent for bronchiectasis or COPD has not yet been covered by national health insurance in South Korea. Second, comprehensive microbiological data were not available due to the retrospective nature of this study. Although we speculate that CBI might have played a major role in the favorable outcomes of azithromycin treatment, we did not obtain data regarding the change in bacterial load or the emergence of antibiotic resistance following azithromycin treatment.45 We excluded patients with NTM pulmonary disease, in which concerns about inducing macrolide resistance are critical to macrolide monotherapy. Further studies with a larger number of patients and prospectively collected microbiologic data are warranted to prove that coexisting bronchiectasis in patients with COPD is a predictive factor for the treatment response to long-term azithromycin. Finally, this study reported a markedly lower occurrence of adverse events than the previous trials,6,7,9 of which the prevalence ranged from 36–42%, probably owing to its retrospective nature or partly because we only selected those who had already tolerated azithromycin for more than 12 weeks. It is noteworthy that subjective hearing loss was not reported in all patients, while audiometry was performed in 42% and showed no hearing impairment. A further prospective study with sufficiently long follow-up is required to clarify the optimal duration of treatment to balance the benefits and risks.
Conclusion
In conclusion, this study generated a hypothesis suggesting that bronchiectasis patients with CAO could benefit more from long-term azithromycin treatment in terms of exacerbation prevention and quality of life improvement than COPD patients without bronchiectasis. Further prospective studies are necessary to confirm our findings.
Abbreviations
BSI, bronchiectasis severity index; CAO, chronic airflow obstruction; CAT, chronic obstructive pulmonary disease assessment test; CBI, chronic bronchial infection; CI, confidence intervals; COPD, chronic obstructive pulmonary disease; CT, computed tomography; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; ICS, inhaled corticosteroids; MCID, minimum clinically important difference; mMRC, modified Medical Research Council; NCFB, non-cystic fibrosis bronchiectasis; NTM, nontuberculous mycobacterial; OR, odds ratio.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript. The authors report no conflicts of interest in this work.
References
- 1.Mullerova H, Maselli DJ, Locantore N, et al. Hospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohort. Chest. 2015;147(4):999–1007. doi: 10.1378/chest.14-0655 [DOI] [PubMed] [Google Scholar]
- 2.Spencer S, Calverley PM, Burge PS, Jones PW. Impact of preventing exacerbations on deterioration of health status in COPD. Eur Respir J. 2004;23(5):698–702. doi: 10.1183/09031936.04.00121404 [DOI] [PubMed] [Google Scholar]
- 3.Hoogendoorn M, Hoogenveen RT, Rutten-van Molken MP, Vestbo J, Feenstra TL. Case fatality of COPD exacerbations: a meta-analysis and statistical modelling approach. Eur Respir J. 2011;37(3):508–515. doi: 10.1183/09031936.00043710 [DOI] [PubMed] [Google Scholar]
- 4.López-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology. 2016;21(1):14–23. doi: 10.1111/resp.12660 [DOI] [PubMed] [Google Scholar]
- 5.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. Report; 2020. Available from: www.goldcopd.org. Accessed April6, 2020.
- 6.Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365(8):689–698. doi: 10.1056/NEJMoa1104623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uzun S, Djamin RS, Kluytmans JA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2(5):361–368. doi: 10.1016/S2213-2600(14)70019-0 [DOI] [PubMed] [Google Scholar]
- 8.Han MK, Tayob N, Murray S, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Respir Crit Care Med. 2014;189(12):1503–1508. doi: 10.1164/rccm.201402-0207OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altenburg J, de Graaff CS, Stienstra Y, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA. 2013;309(12):1251–1259. doi: 10.1001/jama.2013.1937 [DOI] [PubMed] [Google Scholar]
- 10.Serisier DJ, Martin ML, McGuckin MA, et al. Effect of long-term, low-dose erythromycin on pulmonary exacerbations among patients with non-cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. JAMA. 2013;309(12):1260–1267. doi: 10.1001/jama.2013.2290 [DOI] [PubMed] [Google Scholar]
- 11.Wong C, Jayaram L, Karalus N, et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):660–667. doi: 10.1016/S0140-6736(12)60953-2 [DOI] [PubMed] [Google Scholar]
- 12.Polverino E, Goeminne PC, McDonnell MJ, et al. European respiratory society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50(3):3. doi: 10.1183/13993003.00629-2017 [DOI] [PubMed] [Google Scholar]
- 13.Hill AT, Sullivan AL, Chalmers JD, et al. British Thoracic Society Guideline for bronchiectasis in adults. Thorax. 2019;74(Suppl 1):1–69. [DOI] [PubMed] [Google Scholar]
- 14.O’Brien C, Guest PJ, Hill SL, Stockley RA. Physiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary care. Thorax. 2000;55(8):635–642. doi: 10.1136/thorax.55.8.635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martínez-García MA, de la Rosa Carrillo D, Soler-Cataluña JJ, et al. Prognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(8):823–831. doi: 10.1164/rccm.201208-1518OC [DOI] [PubMed] [Google Scholar]
- 16.Mao B, Lu H-W, Li M-H, et al. The existence of bronchiectasis predicts worse prognosis in patients with COPD. Sci Rep. 2015;5(1):10961. doi: 10.1038/srep10961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du Q, Jin J, Liu X, Sun Y, Sethi S. Bronchiectasis as a comorbidity of chronic obstructive pulmonary disease: a systematic review and meta-analysis. PLoS One. 2016;11(3):e0150532. doi: 10.1371/journal.pone.0150532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi H, Yang B, Nam H, et al. Population-based prevalence of bronchiectasis and associated comorbidities in South Korea. Eur Respir J. 2019;54(2):1900194. doi: 10.1183/13993003.00194-2019 [DOI] [PubMed] [Google Scholar]
- 19.Ringshausen FC, de Roux A, Diel R, Hohmann D, Welte T, Rademacher J. Bronchiectasis in Germany: a population-based estimation of disease prevalence. Eur Respir J. 2015;46(6):1805–1807. doi: 10.1183/13993003.00954-2015 [DOI] [PubMed] [Google Scholar]
- 20.Aksamit TR, O’Donnell AE, Barker A, et al. Adult patients with bronchiectasis: a first look at the US bronchiectasis research registry. Chest. 2017;151(5):982–992. doi: 10.1016/j.chest.2016.10.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Muller NL, Remy J. Fleischner society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi: 10.1148/radiol.2462070712 [DOI] [PubMed] [Google Scholar]
- 22.Zhou Y, Wang C, Yao W, et al. COPD in Chinese nonsmokers. Eur Respir J. 2009;33(3):509–518. doi: 10.1183/09031936.00084408 [DOI] [PubMed] [Google Scholar]
- 23.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–654. doi: 10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 24.Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med. 2014;189(5):576–585. doi: 10.1164/rccm.201309-1575OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller M. ATS/ERS task force: standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 26.Choi JK, Paek D, Lee JO. Normal predictive values of spirometry in Korean population. Tuberc Respir Dis. 2005;58(3):230–242. doi: 10.4046/trd.2005.58.3.230 [DOI] [Google Scholar]
- 27.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205 [DOI] [PubMed] [Google Scholar]
- 28.Kon SS, Canavan JL, Jones SE, et al. Minimum clinically important difference for the COPD assessment test: a prospective analysis. Lancet Respir Med. 2014;2(3):195–203. doi: 10.1016/S2213-2600(14)70001-3 [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Garcia MA, Polverino E, Aksamit T. Bronchiectasis and chronic airway disease: it is not just about asthma and COPD. Chest. 2018;154(4):737–739. doi: 10.1016/j.chest.2018.02.024 [DOI] [PubMed] [Google Scholar]
- 30.Spagnolo P, Fabbri LM, Bush A. Long-term macrolide treatment for chronic respiratory disease. Eur Respir J. 2013;42(1):239–251. doi: 10.1183/09031936.00136712 [DOI] [PubMed] [Google Scholar]
- 31.Martinez FJ, Curtis JL, Albert R. Role of macrolide therapy in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2008;3(3):331–350. doi: 10.2147/COPD.S681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki T, Yanai M, Yamaya M, et al. Erythromycin and common cold in COPD. Chest. 2001;120(3):730–733. doi: 10.1378/chest.120.3.730 [DOI] [PubMed] [Google Scholar]
- 33.Seemungal TAR, Wilkinson TMA, Hurst JR, Perera WR, Sapsford RJ, Wedzicha JA. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178(11):1139–1147. doi: 10.1164/rccm.200801-145OC [DOI] [PubMed] [Google Scholar]
- 34.Naderi N, Assayag D, Mostafavi-Pour-Manshadi SM, et al. Long-term azithromycin therapy to reduce acute exacerbations in patients with severe chronic obstructive pulmonary disease. Respir Med. 2018;138:129–136. doi: 10.1016/j.rmed.2018.03.035 [DOI] [PubMed] [Google Scholar]
- 35.Cui Y, Luo L, Li C, Chen P, Chen Y. Long-term macrolide treatment for the prevention of acute exacerbations in COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2018;13:3813–3829. doi: 10.2147/COPD.S181246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chalmers JD, Boersma W, Lonergan M, et al. Long-term macrolide antibiotics for the treatment of bronchiectasis in adults: an individual participant data meta-analysis. Lancet Respir Med. 2019;7(10):845–854. doi: 10.1016/S2213-2600(19)30191-2 [DOI] [PubMed] [Google Scholar]
- 37.Henkle E, Curtis JR, Chen L, et al. Comparative risks of chronic inhaled corticosteroids and macrolides for bronchiectasis. Eur Respir J. 2019;54(1):1. doi: 10.1183/13993003.01896-2018 [DOI] [PubMed] [Google Scholar]
- 38.Gatheral T, Kumar N, Sansom B, et al. COPD-related bronchiectasis; independent impact on disease course and outcomes. Copd. 2014;11(6):605–614. doi: 10.3109/15412555.2014.922174 [DOI] [PubMed] [Google Scholar]
- 39.Martínez-García M, Soler-Cataluña JJ, Donat Sanz Y, et al. Factors associated with bronchiectasis in patients with COPD. Chest. 2011;140(5):1130–1137. doi: 10.1378/chest.10-1758 [DOI] [PubMed] [Google Scholar]
- 40.Patel IS, Vlahos I, Wilkinson TM, et al. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170(4):400–407. doi: 10.1164/rccm.200305-648OC [DOI] [PubMed] [Google Scholar]
- 41.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359(22):2355–2365. doi: 10.1056/NEJMra0800353 [DOI] [PubMed] [Google Scholar]
- 42.Patel IS, Seemungal TA, Wilks M, Lloyd-Owen SJ, Donaldson GC, Wedzicha JA. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax. 2002;57(9):759–764. doi: 10.1136/thorax.57.9.759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simpson JL, Powell H, Baines KJ, et al. The effect of azithromycin in adults with stable neutrophilic COPD: a double blind randomised, placebo controlled trial. PLoS One. 2014;9(8):e105609. doi: 10.1371/journal.pone.0105609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brill SE, Law M, El-Emir E, et al. Effects of different antibiotic classes on airway bacteria in stable COPD using culture and molecular techniques: a randomised controlled trial. Thorax. 2015;70(10):930–938. doi: 10.1136/thoraxjnl-2015-207194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogers GB, Bruce KD, Martin ML, Burr LD, Serisier DJ. The effect of long-term macrolide treatment on respiratory microbiota composition in non-cystic fibrosis bronchiectasis: an analysis from the randomised, double-blind, placebo-controlled BLESS trial. Lancet Respir Med. 2014;2(12):988–996. doi: 10.1016/S2213-2600(14)70213-9 [DOI] [PubMed] [Google Scholar]