Abstract
This study elucidated the function role of dietary selenium-enriched yeast (SeY) supplementation on growth performance, immune function, and antioxidant capacity in weaned pigs exposure to oxidative stress. Thirty-two similarity weight pigs were randomly divided into four treatments: (1) nonchallenged control, (2) control+SeY, (3) control+diquat, and (4) control+SeY+diquat. The period of experiment was 21 days; on day 16, pigs were injected with diquat or sterile saline. Results revealed that oxidative stress was notably detrimental to the growth performance of piglets, but SeY supplementation ameliorated this phenomenon, which might be regarding the increasing of body antioxidant capacity and immune functions. In details, SeY supplementation improved the digestibility of crude protein (CP), ash, and gross energy (GE). Moreover, the serum concentrations of proinflammatory cytokines (TNF-α, IL-1β, and IL-6), glutamic-pyruvic transaminase(GPT), and glutamic-oxaloacetic transaminase (GOT) were reduced via SeY supplemented, and serum concentrations of immunoglobulins A (IgA), IgG, and activities of antioxidant enzymes such as the superoxide dismutase (SOD), catalase (CAT) ,and glutathione peroxidase (GSH-Px) were improved in the diquat-challenged pigs (P < 0.05). In addition, SeY supplementation acutely enhanced the activities of these antioxidant enzymes in the liver and thymus upon diquat challenge, which involved with the upregulation of the critical genes related antioxidant signaling such as the nuclear factor erythroid-derived 2-related factor 2 (Nrf-2) and heme oxygenase-1 (HO-1) (P < 0.05). Importantly, we also found that SeY supplementation apparently reduced the malondialdehyde (MDA) concentrations in the liver, thymus, and serum (P < 0.05). Specifically, the expression levels of TNF-α, IL-6, IL-1β, Toll-like receptor 4 (TLR-4), and nuclear factor-κB (NF-κB) in the liver and thymus were downregulated by SeY upon diquat challenge. These results suggested that SeY can attenuate oxidative stress-induced growth retardation, which was associated with elevating body antioxidant capacity, immune functions, and suppressed inflammatory response.
1. Introduction
Recent studies have shown that neonatal animals such as the weaning pigs (approximately 3-5 weeks of age) are more prone to gastrointestinal diseases, infections, and diarrhea because of incomplete development of immune and digestive systems [1]. In addition, weaning can cause a variety of oxidative stress-induced injuries, including growth retardation, disease, and even death, resulting in considerable economic losses [2]. Under normal circumstances, ROS produced in the body participates a variety of biological events including signal transduction, gene expression, and activation of receptors [3]. Excessive production of oxidative radicals is generally neutralized or eliminated by the antioxidant system including nonenzymatic components, such as glutathione, selenium, vitamin E, and vitamin C, and a series of antioxidant enzymes, such as SOD, CAT, and GSH-Px [4, 5]. Previous study indicated that overproduction of ROS can bring the damage to DNA, proteins, and lipids and further provoke the production of reactive species such as MDA and 4-hydroxynonenal [6, 7]. As a result, oxidative stress is harmful and causes irreversible damage to the cell structure and function, leading to cell death through the process of necrosis and apoptosis (Hetz & Claudio, 2007).
Selenium is an essential trace element in the animal body [8], which exerts various functions in improving antioxidant capacity, immunity, growth, and meat quality [9]. Selenium plays an important role in pig nutrition via participating in selenoprotein synthesis, which is central for the antioxidant system regulation in the body [10]. As compared to inorganic selenium (i.e., sodium selenite and sodium selenate), organic selenium (i.e., selenium methionine and selenium-enriched yeast) has higher absorption and utilization rates [11]. Previous study indicated that selenium-enriched yeast can relieve various oxidative stress-induced damages through improving the body antioxidant enzymes activities [12]. However, our understanding of the precise growth-promoting function of SeY remains indistinct, especially weaned pigs in the oxidative stress. Thus, to clarify the role of SeY, we investigated the effect of dietary SeY supplementation on growth performance, immune function, and antioxidant capacity in weaned pigs upon oxidative stress. Meanwhile, the mechanisms for the SeY-modulated immune and antioxidative functions have also been explored. This study could also provide novel insights into the application of SeY for the livestock industry.
2. Materials and Methods
2.1. Animal Trial
Thirty-two similarity weight (7.30 ± 0.14 kg) pigs were purchased from a commercial farm. The weaned pigs with the same breed (DLY) and gender and nearly the same initial body weight (age) were used in this study and randomly divided into four treatments consisting of nonchallenged pigs (CON, fed with basal diet), diquat-challenged pigs (DT, fed with basal diet), and SeY-treated pigs (fed with basal diet containing 250 mg/kg SeY) challenged by sterile saline (SSY) or diquat (DSY). Diquat came from Shanghai Yuanye Biotechnology CO., Ltd., average molecular weight 362.06. and purity of 99%, then prepared into 10 mg/kg diquat solution with sterilized physiological saline and stored at 4-8°C (Shanghai, China). The SeY was purchased from Sichuan Junzheng Biofeed Co., Ltd. (Organic Selenium ≥95%). We used the recommendations of the National Research Council in 2012 [13] to formulate the nutritional requirements of basal diet (Table 1). Pigs were kept in metabolism cages individually and fresh water and feed available. The trial conducted for 21 d. The oxidative stress model was established by using diquat. On 16 d, pigs received intraperitoneally injection of sterile saline (0.9%) or diquat (10 mg/kg BW).
Table 1.
Composition and nutrient levels of experimental diets.
| Ingredients | % | Nutrient level | Contents |
|---|---|---|---|
| Corn | 28.31 | Digestible energy (calculated, MJ/kg) | 14.78 |
| Extruded corn | 24.87 | Crude protein(%) | 19.68 |
| Soybean meal | 8.50 | Calcium(%) | 0.81 |
| Extruded full-fat soybean | 10.30 | Available phosphorus (%) | 0.55 |
| Fish meal | 4.20 | Lysine | 1.35 |
| Whey powder | 7.00 | Methionine | 0.42 |
| Soybean protein concentrate | 8.00 | Methionine+cysteine | 0.60 |
| Soybean oil | 2.00 | Threonine | 0.79 |
| Sucrose | 4.00 | Tryptophan | 0.22 |
| Limestone | 0.90 | ||
| Dicalcium phosphate | 0.50 | ||
| NaCl | 0.30 | ||
| L-Lysine$HCl (78%) | 0.47 | ||
| DL-Methionine | 0.15 | ||
| L-Threonine (98.5%) | 0.13 | ||
| Tryptophan (98%) | 0.03 | ||
| Chloride choline | 0.10 | ||
| Vitamin premixa | 0.04 | ||
| Mineral premixb | 0.20 | ||
| Total | 100 |
aThe vitamin premix provided the following per kg of diet: 9000 IU of VA, 3000 IU of VD 3, 20 IU of VE, 3 mg of VK 3, 1.50 mg of VB1, 4 mg of VB 2, 3 mg of VB6, 0.02 mg of VB12, 30 mg of niacin, 15 mg of pantothenic acid, 0.75 mg of folic acid, and 0.10 mg of biotin. The premix provided the following per kg of diets: 75 mg of Fe, 150 mg of Cu, 75 mg of Zn, 60 mg of Mn, and 0.35 mg of I.
2.2. Sample Collection and Preparation
To begin with the trial, about 150 g of each treated diet samples was collected by the quartering method, the sterile plastic bags containing feed samples were vacuum-extracted, and the source information was marked and stored at -20°C for chemical analysis. The digestion test was carried out for all piglets on days 12-15 of the experiment, and 10 mL of a 10% H2SO4 solution and 0.5 mL of toluene were added to 100 g fresh feces for preservative and nitrogen fixation. An appropriate amount of fully mixed feces was taken and placed in the sample plate, dried at 60-65°C to a constant weight, dried and crushed, screened for 40 mesh, and stored at -20°C for subsequent nutrient analysis. At the end of the experiment (morning of day 22), all pigs were weighed on an empty stomach, and blood was collected from the anterior vena cava. The samples were placed at room temperature for 30 min and centrifuged at 3000 r/min for 15 min. The supernatant was collected and stored at -20°C for the detection of various physiological and biochemical indexes in the serum. After blood collection, the sodium pentobarbital was used to euthanize all the pigs through intravenous injection and slaughtered by exsanguination protocols. The liver, spleen, kidney, and thymus were weighted, collected, and snap frozen in liquid N2 and then stored at −80°C until analysis.
2.3. Measurement of Growth Performance
Daily feed intake of each pig was accurately recorded throughout the experiment. Fasting weightings were performed on the morning of days 1 and 22, respectively. Average daily gain (ADG), average daily feed intake (ADFI), and feed/gain (F/G) of each pig from stages 1 to 21 were recorded.
2.4. Determination of the Apparent Total Tract Digestibility
Determination of apparent total gastrointestinal digestibility (ATTD) calculated by acid insoluble ash (AIA) [14]. Chinese National Standard (GB/T23742) was applied to measure the AIA in diets and feces samples. DM, CP, EE, and ash were determined in the samples and fecal samples. Also, adiabatic bomb calorimeter (LECO, St. Joseph, Michigan, USA) was utilized to calculate the GE content of diets and fecal samples. Finally, the ATTD was carried out by (100 − A1F2/A2F1 × 100), where A1 serves as the AIA content of the diet, A2 acts as the AIA content of feces, F1 regards as the nutrient content of the diet, and F2 represents the nutrient content of feces.
2.5. Measurement of the Viscera Indexes
Liver, spleen, kidney, and thymus were separated and weighed. The relative organ weight was calculated according to the ratio of the organ weight to the body weight [15].
2.6. Measurements of Serum Immunoglobulin and Cytokine Concentrations
Serum contents of TNF-α, IL-1β, IL-6, TP, and immunoglobulin (Ig) consisting of IgA and IgG were determined referred to the procedures described by the corresponding kit manufacturer using commercially available swine enzyme-linked immunosorbent assay (ELISA) kits (Jiangsu Jingmei Biotechnology Co., Ltd., Yancheng, China).
2.7. Antioxidant Enzymes and MDA Concentration in the Serum, Thymus, and Liver
The MDA content and the activities of several enzymatic as well as nonenzymatic antioxidants consists of GSH-PX, CAT, SOD, and T-AOC were tested in the serum, thymus, and liver. All antioxidant-related indices were detected using the corresponding diagnostic kits obtained from Nanjing Jian Cheng Bioengineering Institute (Nanjing, China) that were used according to the manufacturer's instructions.
2.8. Measurement of Serum GOT and GPT
The activities of GOT and GPT were measured using commercial kits (Nanjing Jiancheng Biological Product, Nanjing, China) according to the manufacturer's recommendations.
2.9. Gene mRNA Expression Analysis by Real-Time PCR
TRIzol Reagent (TaKaTa, Dalian, China) was used to extract the total RNA from the liver and thymus on the basis of the manufacturer's instructions. Spectrophotometer (Beckman Coulter DU800; Beckman Coulter Inc.) was applied to measure the concentration and purity of RNA at 260 and 280 nm to ensure the ratios of absorption (OD260/OD280 nm) various between 1.8 and 2.0 for all samples. The integrity of RNA was verified by formaldehyde gel electrophoresis. Reverse transcription of each sample was conducted by PrimeScript RT reagent kit with gDNA Eraser (TaKaRa, Dalian, China) following the manufacturer's instructions. The primers were synthesized commercially by Life Technologies Limited and exhibited in Table 2.
Table 2.
Primers sequences used for quantitative RT-PCR.
| Gene | Primer sequences (5′-3′) | Product length, bp |
|---|---|---|
| Nrf2 | F: GCCCCTGGAAGCGTTAAAC | 67 |
| R: GGACTGTATCCCCAGAAGGTTGT | ||
| HO-1 | F: CGCTCCCGAATGAACAC | 112 |
| R: GCTCCTGCACCTCCTC | ||
| TNF-α | F: TCTCATGCACCACCATCAAGGACT | 178 |
| R: ACCACTCTCCCTTTGCAGAACTCA | ||
| IL-1β | F: ACCTGTGTCTTTCCCGTGG | 92 |
| R: TCATCTCGGAGCCTGTAGTG | ||
| IL-6 | F: ATCCAGTTGCCTTCTTGGGACTGA | 162 |
| R: TAAGCCTCCGACTTGTGAAGTGGT | ||
| β-Actin | F: TGGAA CGGTG AAGGT GACAGC | 177 |
| R:GCTTTTGGGAA GGCAG GGACT | ||
| NF-κB | F: CACTGTCACCTGGAAGCAGAG | 139 |
| R: CACACATCTCCTTTCTCATTGC | ||
| TLR-4 | F: TTACAGAAGCTGGTTGCCGT | 152 |
| R: TCCAGGTTGGGCAGGTTAGA |
TLR4: Toll-like receptor 4; NF-κB: nuclear factor-κB; Nrf2: nuclear factor erythroid-derived 2-related factor 2; HO-1: heme oxygenase-1; interleukin-1β: IL-1β; IL-6: interleukin-6; TNF-α: tumor necrosis factor-α.
The analysis of the expression abundance of TNF-α, IL-6, IL-1β, TLR4, NF-κB, Nrf-2, and HO-1 was calculated by quantitative real-time PCR in the liver and thymus using SYBR Premix Ex Taq II (Tli RnaseH Plus) reagents (TaKaRa, Dalian, China) and the CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Richmond, CA). The β-actin gene was chosen as the reference gene to normalize the mRNA expression of target genes. The relative expression ratio of target genes relative to the reference gene was calculated using the 2−ΔΔCT method [16]. Each sample was simultaneously performed on the same PCR plate.
2.10. Statistical Analysis
Data were carried out as a 2 × 2 factorial arrangement by ANOVA through the general linear model procedure of SPSS 24.0 (SPSS Inc., Chicago). The statistical model contains the effects of diquat (challenged or unchallenged), SeY (supplemented or not supplemented), and their interaction. Data were exhibited as means and stand errors. Differences were regarded as significant when P < 0.05. Meanwhile, P < 0.10 is discussed as trends. Variable means for treatments showing significant differences in the ANOVA were separated by Tukey's multiple range test (P < 0.05).
3. Results
3.1. Growth Performance
As showed in Table 3, the ADG and ADFI were cut down in the DT group than the CON group (P < 0.05). Dietary SeY supplementation significantly promoted ADG, ADFI, and the feed efficiency in the diquat-challenged pigs (P < 0.05).
Table 3.
Effect of dietary Se Y on the growth performance in weaned pigs.
| Items | Treatments | P value | ||||||
|---|---|---|---|---|---|---|---|---|
| CON | SSY | DT | DSY | SEM | SeY | Diquat | SeY × diquat | |
| 1-21 | ||||||||
| ADG (g/day) | 390.86a | 335.05ac | 221.71b | 271.62bc | 88.07 | 0.65 | 0.01 | 0.10 |
| ADFI (g/day) | 499.42a | 428.37ac | 338.86bc | 377.34bc | 93.40 | 0.96 | 0.02 | 0.22 |
| F:G (g/g) | 1.27b | 1.27b | 1.60a | 1.40ab | 0.20 | 0.02 | <0.01 | 0.02 |
Values are means ± SEM, (n = 8), nonchallenged pigs (CON, fed with basal diet), diquat-challenged pigs (DT, fed with basal diet), and SeY-treated pigs (fed with basal diet containing 250 mg/kg SeY) challenged by sterile saline (SSY) or diquat (DSY). a,b,cMean values within a row with unlike superscript letters were significantly different (P < 0.05). ADFI: average daily feed intake; ADG: average daily gain; G/F: the ratio of gain to feed intake.
3.2. Effect of SeY on Nutrient Digestibility in Weaned Pigs
SeY supplementation had no effect on the apparent digestibility of DM and EE (Table 4). The digestibility of CP and ash was significant higher in SSY group than the CON group (P < 0.05). Interestingly, SeY supplementation significantly elevated the digestibility of GE and ash in the DSY group than the DT group (P < 0.05).
Table 4.
Effect of SeY on ATTD of nutrients in weaned pigs.
| Items (%) | Treatments | SEM | P value | |||
|---|---|---|---|---|---|---|
| CON | SSY | DT | DSY | |||
| DM | 83.12 | 83.67 | 83.65 | 83.20 | 0.67 | 0.89 |
| CP | 81.69b | 86.33a | 82.14b | 85.31ab | 2.57 | 0.03 |
| EE | 73.53 | 75.27 | 71.26 | 81.91 | 3.47 | 0.65 |
| Ash | 62.18b | 69.42a | 63.5b | 65.76a | 1.13 | 0.02 |
| GE | 80.98ab | 86.61a | 76.96b | 84.23a | 1.75 | 0.04 |
Values are means ± SEM, (n = 8), nonchallenged pigs (CON, fed with basal diet), diquat-challenged pigs (DT, fed with basal diet), and SeY-treated pigs (fed with basal diet containing 250 mg/kg SeY) challenged by sterile saline (SSY) or diquat (DSY). a,b,cMean values within a row with unlike superscript letters were significantly different (P < 0.05). DM: dry matter; CP: crude protein; EE: ether extract; GE: gross energy.
3.3. Effect of SeY on the Viscera Indexes in Weaned Pigs upon Oxidative Stress
As showed in Table 5, dietary SeY supplementation had no effect on the liver, spleen, kidney, and thymus index under normal condition (P > 0.05). However, the indexes of the liver and kidney in the DSY group were significantly higher than those in the DT group (P < 0.05). There was no effect on the spleen index under both conditions (P > 0.05).
Table 5.
Effect of dietary SeY on the viscera index in weaned pigs.
| Items | Treatments | P value | ||||||
|---|---|---|---|---|---|---|---|---|
| CON | SSY | DT | DSY | SEM | SeY | Diquat | SeY × diquat | |
| Liver index | 20.35ab | 20.94ab | 19.47bc | 24.90a | 0.85 | 0.06 | 0.32 | 0.15 |
| Spleen index | 5.13 | 4.75 | 4.74 | 5.60 | 0.74 | 0.42 | 0.41 | 0.10 |
| Kidney index | 1.65ab | 1.81ac | 1.20bc | 2.03a | 0.65 | 0.09 | 0.97 | 0.48 |
| Thymus index | 1.04ab | 1.50a | 0.74b | 1.13ab | 0.10 | 0.02 | 0.17 | 0.47 |
Values are means ± SEM, (n = 8), nonchallenged pigs (CON, fed with basal diet), diquat-challenged pigs (DT, fed with basal diet), and SeY-treated pigs (fed with basal diet containing 250 mg/kg SeY) challenged by sterile saline (SSY) or diquat (DSY). a,b,cMean values within a row with unlike superscript letters were significantly different (P < 0.05).
3.4. Effect of SeY on Serum GPT and GOT in Weaned Pigs upon Oxidative Stress
As shown in Figure 1, compared to the CON group, the GPT content in the serum was significantly lowered in the SSY group (P < 0.05). However, the GOT and GPT levels were significantly reduced in the DSY group compared to the DT group (P < 0.05).
Figure 1.
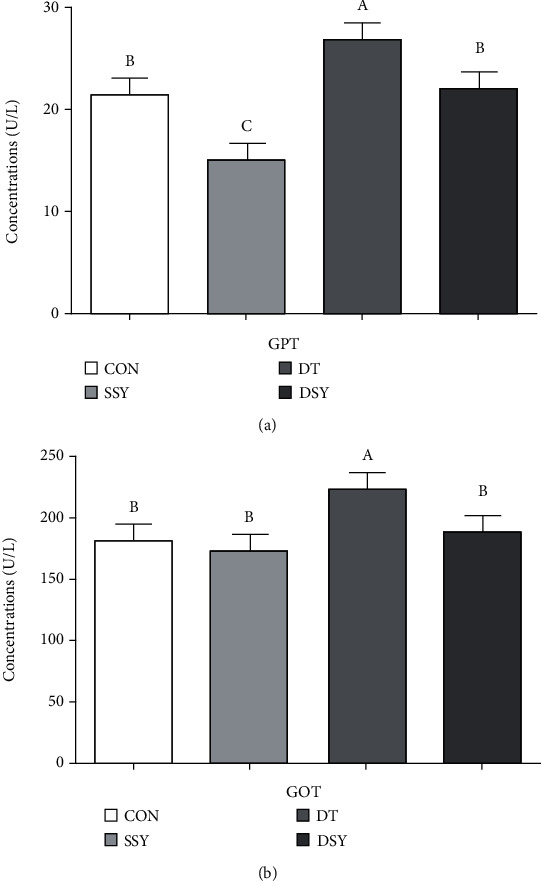
Effect of SeY on serum GPT and GOT in weaned pigs upon oxidative stress. (a)–(c) Mean values with different letters on vertical bars indicate significant differences (P < 0.05). CON, pigs were fed with basal diet and challenged by sterile saline, SSY: pigs were fed with SeY-containing diet and challenged by sterile saline, DT: pigs were fed with basal diet and challenged by diquat, and DSY: pigs were fed with SeY-containing diet and challenged by diquat. GPT: glutamic-pyruvic transaminase; GOT: glutamic-oxaloacetic transaminase.
3.5. Effect of SeY on Serum Immunoglobulin and Cytokine Concentrations in Weaned Pigs upon Oxidative Stress
Dietary SeY supplementation significantly amplified the serum TP, IgG, and IgA levels and inhibited the serum IL-1β concentration in weaned piglets without diquat administration (P < 0.05, Table 6); however, dietary SeY supplementation also reduced the TNF-α, IL-1β, and IL-6 (P < 0.05) content in piglets when piglets were subjected to the diquat injection. In addition, the contents of TP, IgG, and IgA in the DSY group were higher than those in the DT group.
Table 6.
Effect of SeY on serum immunoglobulin and cytokine concentrations in weaned pigs upon oxidative stress.
| Items | Treatments | P value | ||||||
|---|---|---|---|---|---|---|---|---|
| CON | SSY | DT | DSY | SEM | SeY | Diquat | SeY × diquat | |
| TNF-α (pg/mL) | 466.99a | 427.6b | 464.92 a | 370.63b | 15.51 | 0.03 | 0.30 | 0.33 |
| IL-1β (ng/L) | 42.8a | 23.12c | 45.73a | 34.35b | 2.43 | <0.01 | 0.03 | 0.18 |
| IL-6 (ng/mL) | 2.46ac | 1.74bc | 3.04a | 1.91bc | 0.21 | 0.02 | 0.33 | 0.59 |
| TP (gprot/L) | 26.31b | 32.09a | 24.68b | 30.38a | 1.01 | <0.01 | 0.79 | 0.31 |
| IgG (μg/mL) | 306.35c | 588.03a | 323.27c | 497.36b | 28.74 | <0.01 | 0.09 | 0.02 |
| IgA (μg/mL) | 93.08b | 166.94a | 114.28b | 159a | 7.90 | <0.01 | 0.37 | 0.06 |
Values are means ± SEM, (n = 8), nonchallenged pigs (CON, fed with basal diet), diquat-challenged pigs (DT, fed with basal diet), and SeY-treated pigs (fed with basal diet containing 250 mg/kg SeY) challenged by sterile saline (SSY) or diquat (DSY). a,b,cMean values within a row with unlike superscript letters were significantly different (P < 0.05). TP: total protein; IgG: immunoglobulin G; IgA: immunoglobulin A; TNF-α: tumor necrosis factor-α; IL-6: interleukin-6; IL-1β: interleukin-1β.
3.6. Effect of SeY on Antioxidant Capacity and MDA Content in the Serum
The data for antioxidant capacity parameters in serum are presented in Table 7. Compared to the CON group, higher serum content of the MDA was observed in the DT group (P < 0.05). However, SeY supplementation not only brought down the MDA content but also enhanced the GSH-Px, CAT, and SOD activities in the serum (P < 0.05). Compared to the DT group, the T-AOC was extremely raised in the DSY group (P < 0.05).
Table 7.
Effect of SeY on antioxidant capacity of the serum in weaned pig upon oxidative stress.
| Items | Treatments | P value | ||||||
|---|---|---|---|---|---|---|---|---|
| CON | SSY | DT | DSY | SEM | SeY | Diquat | SeY × diquat | |
| GSH-Px, mg/mL | 747.19b | 878.67ac | 793.48bc | 837.24a | 99.60 | <0.01 | 0.12 | 0.94 |
| T-AOC, U/mL | 0.61b | 0.57b | 0.42b | 1.47a | 0.13 | 0.02 | 0.07 | 0.02 |
| MDA, nmol/mL | 2.49b | 1.71c | 3.73a | 2.06b | 0.21 | <0.01 | 0.08 | 0.11 |
| CAT U/mL | 2.85b | 5.85a | 2.92b | 5.21a | 0.47 | <0.01 | 0.71 | 0.65 |
| SOD U/mL | 109.82b | 129.81a | 103.29b | 133.65a | 3.72 | <0.01 | 0.79 | 0.31 |
Values are means ± SEM, (n = 8), nonchallenged pigs (CON, fed with basal diet), diquat-challenged pigs (DT, fed with basal diet), and SeY-treated pigs (fed with basal diet containing 250 mg/kg SeY) challenged by sterile saline (SSY) or diquat (DSY). a,b,cMean values within a row with unlike superscript letters were significantly different (P < 0.05). GSH-Px: glutathione peroxidase; T-AOC: total antioxidant capacity, MDA: malondialdehyde; SOD: superoxide dismutase; CAT: catalase.
3.7. Effect of SeY on Antioxidant Capacity and MDA Content in the Liver and Thymus
As shown in Table 8, compared to the CON group, the MDA content in the liver and thymus was significantly reduced in the SSY group (P < 0.05), and the CAT and SOD activities were significantly reduced in the DT group (P < 0.05). However, the T-AOC, CAT, and SOD activities in the liver and the GSH-Px, SOD, and CAT activities and T-AOC in the thymus were significantly elevated in the DSY group compared with the DT group (P < 0.05).
Table 8.
Effect of SeY on antioxidant capacity of the liver in weaned pig upon oxidative stress.
| Items | Treatments | P value | ||||||
|---|---|---|---|---|---|---|---|---|
| CON | SSY | DT | DSY | SEM | SeY | Diquat | SeY × diquat | |
| Liver | ||||||||
| T-AOC, U/mgprot | 1.50ac | 1.30a | 0.80bc | 1.33a | 0.39 | 0.23 | 0.02 | 0.02 |
| MDA, nmol/mL | 1.87a | 1.14b | 1.78a | 1.43ac | 0.47 | 0.03 | 0.83 | 0.40 |
| CAT, U/gprot | 72.10a | 64.80a | 51.86b | 72.00a | 2.87 | 0.13 | 0.34 | 0.03 |
| SOD, U/mL | 97.27a | 93.92a | 68.63b | 117.02a | 5.88 | 0.02 | 0.85 | 0.02 |
| GSH-Px U/mgprot | 47.19c | 58.67ab | 43.28bc | 67.24a | 10.05 | 0.03 | 0.05 | 0.03 |
| Thymus | ||||||||
| T-AOC, U/mgprot | 7.05a | 6.16a | 4.04b | 8.71a | 0.58 | <0.01 | 0.81 | 0.06 |
| CAT U/mgprot | 62.10a | 64.80a | 51.86b | 65.60a | 2.87 | 0.03 | 0.34 | 0.03 |
| MDA nmol/mL | 2.17b | 1.64c | 3.78a | 1.93bc | 0.57 | 0.03 | 0.56 | 0.30 |
| SOD U/mgprot | 97.27a | 93.92a | 68.63b | 107.02a | 5.88 | 0.07 | 0.85 | 0.02 |
| GSH-Px U/mgprot | 80.43b | 90.56a | 78.22b | 98.76a | 6.44 | <0.01 | 0.67 | 0.04 |
Values are means ± SEM, (n = 8), nonchallenged pigs (CON, fed with basal diet), diquat-challenged pigs (DT, fed with basal diet), and SeY-treated pigs (fed with basal diet containing 250 mg/kg SeY) challenged by sterile saline (SSY) or diquat (DSY). a,b,cMean values within a row with unlike superscript letters were significantly different (P < 0.05). T-AOC: total antioxidant capacity; CAT: catalase; MDA: malondialdehyde; SOD: superoxide dismutase; GSH-Px: glutathione peroxidase.
3.8. Effect of SeY on Expressions of Critical Genes Related to the Inflammatory Response
As shown in Figure 2, compared to the CON group, the expression abundance of IL-6 and IL-1β was upregulated in the DT group of the liver and thymus (P < 0.05); however, their expressions were declined in the DSY group. Lower expression levels of TNF-α in the liver and thymus were found in the DSY group compared with the DT group (P < 0.05). Meanwhile, the expression levels TLR-4 and NF-κB in the liver were upregulated in the DT group compared with the CON group (Figure 3); on the contrary, their expressions were downregulated in the DSY group. The expression abundance of Nrf-2 and HO-1 in the liver and thymus was upper in the DSY group than the DT group (P < 0.05). However, no effect was occurred to the expression of TNF-α, IL-6, and IL-1β, TLR4, NF-κB, Nrf-2, and HO-1 in the SSY group compared with the CON group (P > 0.05).
Figure 2.
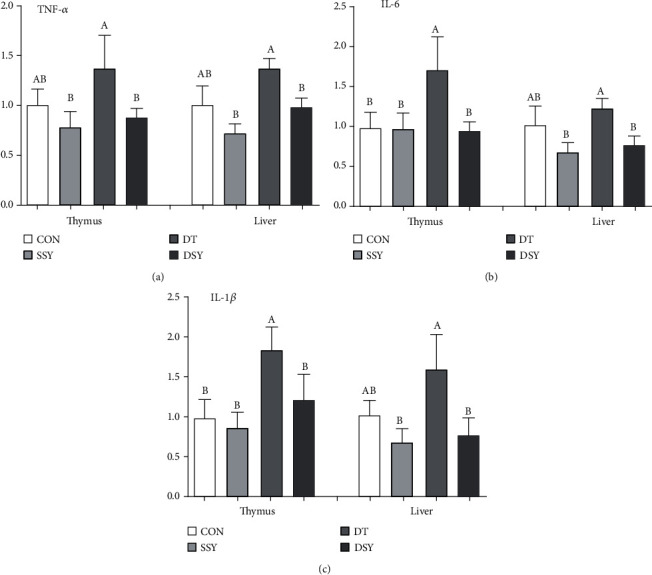
Relative expression levels of critical genes involved in the inflammatory response. (a)–(c) Mean values with different letters on vertical bars indicate significant differences (P < 0.05). CON, pigs were fed with basal diet and challenged by sterile saline, SSY: pigs were fed with SeY-containing diet and challenged by sterile saline, DT: pigs were fed with basal diet and challenged by diquat, and DSY: pigs were fed with SeY-containing diet and challenged by diquat. TNF-α :tumor necrosis factor-α; IL-6 interleukin-6; IL-1β: interleukin-1β.
Figure 3.
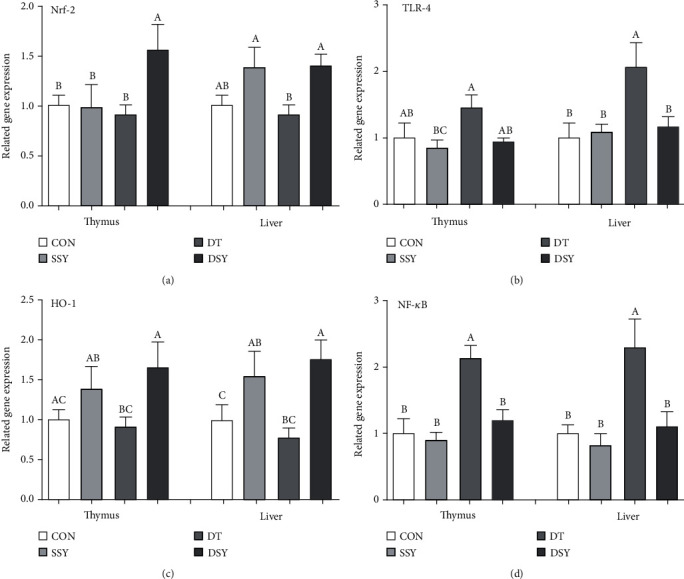
Relative expression levels of critical genes involved in the inflammatory response. (a)–(c) Mean values with different letters on vertical bars indicate significant differences (P < 0.05). CON: pigs were fed with basal diet and challenged by sterile saline, SSY: pigs were fed with SeY-containing diet and challenged by sterile saline, DT: pigs were fed with basal diet and challenged by diquat, and DSY: pigs were fed with SeY-containing diet and challenged by diquat. TLR-4: Toll-like receptor 4; NF-κB: nuclear factor-κB; Nrf-2: nuclear factor erythroid-derived 2-related factor 2; HO-1: heme oxygenase-1.
4. Discussion
To evaluate whether SeY supplementation could alleviate oxidative stress injury through improving body antioxidative capacity and immune functions roles in weaned pigs, we established a model for inducing oxidative stress in pigs by using diquat injection [17]. Multiple lines of evidence show that diquat-induced oxidative stress has been used commonly as an experimental animal model in which to analyze the mechanism of chemical agent-induced acute oxidative injury [18]. Vomiting and anorexia were initially occurred to all piglets with diquat challenge. In parallel with our study, diquat injected was adverse to the growth performance indicating conspicuously dropped down the ADG and ADFI, which are consistent with previous observations showing that the performance of weaned pigs are negatively affected by diquat challenge [19]. Meanwhile, dietary SeY supplementation significantly promoted ADG, ADFI, feed efficiency, and apparent nutrient digestibility in pigs. It is suggested that SeY exerted a potentially crucial role in inhibiting adverse reactions in weaned piglets exposure to the oxidative stress. This result also is in agreement with former outcomes that SeY supplementation can boost the growth performance and antioxidant capacity of animals under oxidative stress [20].
Nutrition and management at the weaning phase are crucial to the growth and development of piglets. Additionally, in this phase, piglets are more susceptible to multifarious stresses (i.e., oxidative stress), and oxidative stress exploits full advantages to the pathophysiology of numerous cardiovascular diseases (i.e., endothelial dysfunction, atherosclerosis, and ischemia–reperfusion injury). Exceeding accumulation levels of ROS in the animal body—such as H2O2, O2−, and OH−—seriously undermine the function of proteins, DNA, and lipids, which lead to tissue injury and organ dysfunction, causing considerable economic losses to the pig industry [21]. The homeostasis of the ROS is maintained in vivo associating with signal transduction, gene expression, and receptor activation [22]. Nevertheless, excessive accumulation of ROS is usually removed promptly by the antioxidant system, including nonenzymatic components, such as vitamins E and C, and a range of antioxidant enzymes consisting of SOD, CAT, and GSH-Px. They are considered to be the principal line of the antioxidant enzyme system to boycott ROS production subjected to oxidative stress. SOD is distributed in plenty of tissues and organisms, which exert the protective function role of cells injury caused by O2–. CAT and GSH-Px can serve as catalysts to convert H2O2 into nontoxic H2O. Furthermore, T-AOC is a participant in the nonenzymatic antioxidant defense systems [23]. The SeY is reported to be a particularly attractive antioxidant due to its stability in the presence of strong function in reducing the production of lipid peroxidation, eliminating ROS, relieving DNA oxidative injury, and preventing cell apoptosis. Our experiment found that dietary SeY supplementation significantly increased the activities of T-AOC, SOD, CAT, and GSH-Px in the serum, liver, and thymus, suggesting that dietary SeY supplementation played protective role on diquat-induced oxidative stress of weaned piglets by enhancing the antioxidant function.
Cellular biomembranes are one of the main targets subjected to the attack of ROS, which in turn induces lipid peroxidation, resulting in MDA production. MDA can interact with biomolecules result in cytotoxicity and genotoxicity. Collectively, the detection of the MDA content is usually used as a key label for oxidative stress in organisms [24]. We found that dietary supplementation of SeY significantly reduced the content of the MDA in the serum, thymus, and liver, declaring that SeY supplementation suppressed lipid peroxidation. Our results are in line with the former studies that SeY supplementation could reduce oxidative stress and has the potential effects on ameliorating the deleterious impacts of oxidative stress injury. Organ index reflects the development and functional status of the organ. In the present study, the liver and kidney index was significantly improved in the DSY group compared with the DT group, indicating that SeY had beneficial effects on attenuating the tissue injury of weaned pigs undergoing oxidative stress.
GOT and GPT are hepatic intracellular enzymes, which principally work in the synthesis and metabolism of nonessential amino acids. Excessive serum elevation of these two enzymes revealed leakage of damaged liver cells and is regarded as sensitive indexes of liver injury [25]. In the present study, the serum activities of GOT and GPT were apparently reduced in the DSY group than the DT group, indicating that SeY had favorable effect in improving the liver function upon diquat injection. These results also got agreement with former findings [26].
Linkages between oxidative stress and inflammation are closely correlated [27]. In addition, oxidative stress disrupts the body's redox balance, which also leads to systemic inflammation. Systemic responses may be triggered by the overexpression of proinflammatory (TNF-ɑ, IL-6, and IL-1β) in the body, resulting in cell damage [28]. Both IgA and IgG are crucial markers of the body's immune capacity and figure a profoundly essential role in the body's response to a series of pathogens [29, 30]. Total serum protein is a complex mixture of proteins, consisting of ALB and globulin, which on behalf of the nutritional levels of dietary protein available to the animal as well as the degree of digestion and absorption [31]. Previous studies demonstrated that dietary SeY supplementation enhances the immune response in early weaned piglets and mice as well as the human by modulating the production of cytokines and antibodies through enhancing B lymphocytes and their functional activity [32, 33]. In this study, we observed that SeY prominently elevated the contents of serum TP, IgA, and IgG and downregulated the content of the IL-1β, IL-6, and TNF-α in piglets exposed to oxidative stress. These findings were in accordance with the reports. On the basis of these results, we can come to a conclusion that dietary supplementation of SeY showed great advantages over alleviating body injury of diquat-challenged pigs. It is speculated that the protective effect of SeY may be related to the decrease of serum proinflammatory cytokines and the advancement immunoglobulin level.
To elucidate the molecular mechanisms by which SeY supplementation might attenuate thymus and liver antioxidative function and inflammatory response under oxidative stress, we determined several vital genes associating with the antioxidative function and inflammatory response. TLR4 is a typical member in the TLR family and widely distributed on various types of cells [27, 34]. Activation of TLR4 accompanies with initiating the activation of NF-κB signal pathway and then expands the expression levels of omnifarious inflammatory cytokines' genes, including IL-6, TNF-α, and IL-1β [35]. In the present study, we have observed that the expression levels of TLR4, NF-κB, TNF-α, IL-6, and IL-1β in the liver and thymus were lower in the DSY group than the DT group. Therefore, the protective effect of SeY on oxidative stress injury in the liver and thymus may be related to the downregulation mRNA expression of TLR4 signaling-related genes. Together speaking, our results are also consistent with reports that SeY improves hepatic protection by regulating proinflammatory cytokines and antioxidant capacity in broilers under heat stress conditions [36].
Nrf-2 is an essential redox-sensitive transcription component that has been involved in cellular responses to oxidative stress, which coordinates the expression of multiple genes such as antioxidant and detoxification via a motion-promoting sequence named antioxidant response element (ARE), which synergically forms a pleiotropic cellular defense that eliminates ROS, detoxifies electrophiles and xenobiotics, and maintains the reductive potential in the cell [37]. HO-1 is an important antioxidant enzyme that plays a key role in endogenous and exogenous cell protection against harmful stimuli. On the one hand, its antioxidant function is related to the prevention of free heme from participating in the oxidative reaction; on the other hand, HO-1 and its enzymolysis products including bilirubin and CO exert a key role in antioxidant, anti-inflammatory and inhibiting cell apoptosis. Accumulating evidence suggesting that the Nrf-2/HO-1 pathway is one of the most important endogenous protective systems, which is widely involved in the oxidative stress injury of the heart, brain, liver, kidney, and nervous system [38]. In the present study, we observed that the expression abundance of the Nrf-2 and HO-1 was elevated in the DSY group compared with the DT group in the liver and thymus subjected to oxidative stress. The result is accompanied with the measurements of the antioxidative enzymes in the liver and thymus. Both results indicated that the SeY could act as a potential therapeutic antioxidant agent to relieve the tissue injury induced by diquat injection.
5. Conclusions
SeY plays a key role in antioxidation and anti-inflammation. In conclusion, dietary SeY supplementation alleviates the negative effects of diquat injection on growth performance, inflammatory response, and organ injury in piglets via improving antioxidative activity. These results would provide scientific evidences for the development of antioxidant therapies and anti-inflammatory drugs in the future as well as the application of SeY in piglets.
Acknowledgments
Herein, thank Wang Quyuan and Wang Huifen for their help during the animal trial and sample collections. This study was supported by the Key Research and Development Program of Sichuan Province (no. 2018NZDZX0005) and the National Natural Science Foundation of China (no. 31972599).
Data Availability
The datasets used to support the findings of this study are available from the corresponding author upon request.
Additional Points
Animal Welfare Statement. All animal care and experimental procedures were performed following the Guide for the Care and Use of Laboratory Animals prepared by the Institutional Animal Care and Use Committee, Sichuan Agricultural University, China.
Conflicts of Interest
The authors have declared no conflict of interest.
Authors' Contributions
LL and JH conceived the study, performed the experiment, performed data analysis, and contributed to drafting the manuscript. LL carried out the animal experiment. DWC, BY, ZQH, YHL, PZ,,XBM, JY, and JQL conceived the experiment and proof read the manuscript. All authors read and approved the final manuscript.
References
- 1.Touchette K. J., Carroll J. A., Allee G. L., et al. Effect of spray-dried plasma and lipopolysaccharide exposure on weaned pigs: I. Effects on the immune axis of weaned pigs. Journal of Animal Science. 2002;80(2):494–501. doi: 10.2527/2002.802494x. [DOI] [PubMed] [Google Scholar]
- 2.Smith F., Clark J. E., Overman B. L., et al. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2010;298(3):G352–G363. doi: 10.1152/ajpgi.00081.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gul M. Z., Kalam S., Singh R., Ankati S. Free radicals: implications in etiology of chronic diseases and their amelioration through nutraceuticals. Pharmacologia. 2015;6(1):11–20. doi: 10.5567/pharmacologia.2015.11.20. [DOI] [Google Scholar]
- 4.Lightfoot T. J., Skibola C. F., Smith A. G., et al. Polymorphisms in the oxidative stress genes, superoxide dismutase, glutathione peroxidase and catalase and risk of non-Hodgkin's lymphoma. Haematologica. 2006;91(9):1222–1227. [PubMed] [Google Scholar]
- 5.Bolzán A. D., Bianchi M. S., Bianchi N. O. Superoxide dismutase, catalase and glutathione peroxidase activities in human blood: influence of sex, age and cigarette smoking. Clinical Biochemistry. 1997;30(6):449–454. doi: 10.1016/S0009-9120(97)00047-7. [DOI] [PubMed] [Google Scholar]
- 6.Ďuračková Z. Some current insights into oxidative stress. Physiological Research. 2010;59(4):459–469. doi: 10.33549/physiolres.931844. [DOI] [PubMed] [Google Scholar]
- 7.Hussain S. P., Hofseth L. J., Harris C. C. Radical causes of cancer. Nature Reviews Cancer. 2003;3(4):276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 8.Zachara B. A. Mammalian selenoproteins. Journal of Trace Elements & Electrolytes in Health & Disease. 1992;6(3):137–151. [PubMed] [Google Scholar]
- 9.Mahan D. C., Cline T. R., Richert B. Effects of dietary levels of selenium-enriched yeast and sodium selenite as selenium sources fed to growing-finishing pigs on performance, tissue selenium, serum glutathione peroxidase activity, carcass characteristics, and loin quality. Journal of Animal Science. 1999;77(8):2172–2179. doi: 10.2527/1999.7782172x. [DOI] [PubMed] [Google Scholar]
- 10.Gopalakrishna R., Gundimeda U., Zhou S., Zung K., Forell K., Holmgren A. Imbalance in protein thiol redox regulation and cancer-preventive efficacy of selenium. Reactive Oxygen Species. 2016;2(4) doi: 10.20455/ros.2016.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrauzer G. N. Nutritional selenium supplements: product types, quality, and safety. Journal of the American College of Nutrition. 2001;20(1):1–4. doi: 10.1080/07315724.2001.10719007. [DOI] [PubMed] [Google Scholar]
- 12.Waters D. J., Shen S., Cooley D. M., et al. Effects of dietary selenium supplementation on DNA damage and apoptosis in canine prostate. Jnci Journal of the National Cancer Institute. 2003;95(3):237–241. doi: 10.1093/jnci/95.3.237. [DOI] [PubMed] [Google Scholar]
- 13.Usa N. C. Nutrient requirements of swine. Nutrient Requirements of Swine. 2012;44(3) [Google Scholar]
- 14.Lee M. H. Official methods of analysis of AOAC International (16th edn) Trends in Food Science & Technology. 1995;6(11):382–382. doi: 10.1016/0924-2244(95)90022-5. [DOI] [Google Scholar]
- 15.Wu L., Wang W., Yao K., et al. Effects of dietary arginine and glutamine on alleviating the impairment induced by deoxynivalenol stress and immune relevant cytokines in growing pigs. PLoS ONE. 2013;8(7) doi: 10.1371/journal.pone.0069502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.dos Santos Costa C., Rohden F., Hammes T. O., et al. Resveratrol upregulated SIRT1, FOXO1, and adiponectin and downregulated PPARγ1–3 mRNA expression in human visceral adipocytes. Obesity Surgery. 2011;21(3):356–361. doi: 10.1007/s11695-010-0251-7. [DOI] [PubMed] [Google Scholar]
- 17.Yuan S., Chen D. W., Zhang K. Y., Yu B. Effects of oxidative stress on growth performance, nutrient digestibilities and activities of antioxidative enzymes of weanling pigs. Asian-Australasian Journal of Animal Sciences. 2007;20(10):1600–1605. doi: 10.5713/ajas.2007.1600. [DOI] [Google Scholar]
- 18.Zheng P., Yu B., Lv M., Chen D. Effects of oxidative stress induced by diquat on arginine metabolism of postweaning pigs. Asian Australasian Journal of Animal Sciences. 2010;23(1):98–105. doi: 10.5713/ajas.2010.90270. [DOI] [Google Scholar]
- 19.Yin J., Liu M., Ren W., et al. Effects of dietary supplementation with glutamate and aspartate on diquat-induced oxidative stress in piglets. PLoS One. 2015;10(4, article e0122893) doi: 10.1371/journal.pone.0122893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He J., Zhang K. Y., Chen D. W., Ding X. M., Feng G. D., Ao X. Effects of vitamin E and selenium yeast on growth performance and immune function in ducks fed maize naturally contaminated with aflatoxin B1. Livestock Science. 2013;152(2-3):200–207. doi: 10.1016/j.livsci.2012.12.018. [DOI] [Google Scholar]
- 21.Pohl C. S., Medland J. E., Mackey E., et al. Early weaning stress induces chronic functional diarrhea, intestinal barrier defects, and increased mast cell activity in a porcine model of early life adversity. Neurogastroenterology & Motility. 2017;29(11) doi: 10.1111/nmo.13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y., Han J., Huang J., Wang X., Wang F., Wang J. Dietary L-arginine supplementation improves intestinal function in weaned pigs after an Escherichia coli lipopolysaccharide challenge. Asian Australasian Journal of Animal Sciences. 2009;22(12):1667–1675. doi: 10.5713/ajas.2009.90100. [DOI] [Google Scholar]
- 23.Cabiscol E., Tamarit J., Ros J. Oxidative stress in bacteria and protein damage by reactive oxygen species. International Microbiology the Official Journal of the Spanish Society for Microbiology. 2000;3(1):3–8. [PubMed] [Google Scholar]
- 24.Halliwell B., Gutteridge J. M. C. The importance of free radicals and catalytic metal ions in human diseases. Molecular Aspects of Medicine. 1985;8(2):89–193. doi: 10.1016/0098-2997(85)90001-9. [DOI] [PubMed] [Google Scholar]
- 25.Miyake S. The mechanism of release of hepatic enzymes in various liver diseases. II. Altered activity ratios of GOT to GPT in serum and liver of patients with liver diseases. Acta Medica Okayama. 1979;33(5) [PubMed] [Google Scholar]
- 26.El-Bayoumy K., Richie J. P., Jr., Boyiri T., et al. Influence of selenium-enriched yeast supplementation on biomarkers of oxidative damage and hormone status in healthy adult males a clinical pilot study. Cancer Epidemiology, Biomarkers & Prevention. 2002;11(11):1459–1465. [PubMed] [Google Scholar]
- 27.Becker C. E., O’Neill L. A. J. Inflammasomes in inflammatory disorders: the role of TLRs and their interactions with NLRs. Seminars in Immunopathology. 2007;29(3):239–248. doi: 10.1007/s00281-007-0081-4. [DOI] [PubMed] [Google Scholar]
- 28.Gabay C., Kushner I. Acute-phase proteins and other systemic responses to inflammation. New England Journal of Medicine. 1999;340(6):448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 29.Woof J. M., Mestecky J. Mucosal immunoglobulins. Mucosal Immunology. 2005;1:287–324. doi: 10.1111/j.0105-2896.2005.00290.x. [DOI] [Google Scholar]
- 30.Rath T., Baker K., Pyzik M., Blumberg R. S. Regulation of immune responses by the neonatal fc receptor and its therapeutic implications. Frontiers in Immunology. 2015;5(3) doi: 10.3389/fimmu.2014.00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vieira A. T., Silveira K. D., Arruda M. C. C., et al. Treatment with Selemax, a selenium-enriched yeast, ameliorates experimental arthritis in rats and mice. British Journal of Nutrition. 2012;108(10):1829–1838. doi: 10.1017/S0007114512000013. [DOI] [PubMed] [Google Scholar]
- 32.Stabnikova O., Ivanov V., Larionova I., Stabnikov V., Bryszewska M. A., Lewis J. Ukrainian dietary bakery product with selenium-enriched yeast. LWT-Food Science and Technology. 2008;41(5):890–895. doi: 10.1016/j.lwt.2007.05.021. [DOI] [Google Scholar]
- 33.Wang D., Pu L., Wei G. Improved antioxidant capacity and immune function of broiler chickens fed with selenium-enriched Candida utilis. Revista Brasileira De Ciência Avícola. 2020;22(2) doi: 10.1590/1806-9061-2019-1047. [DOI] [Google Scholar]
- 34.Sabroe I., Parker L. C., Dower S. K., Whyte M. K. B. The role of TLR activation in inflammation. Journal of Pathology. 2008;214(2):126–135. doi: 10.1002/path.2264. [DOI] [PubMed] [Google Scholar]
- 35.Li Q., Verma I. M. NF-κB regulation in the immune system. Nature Reviews Immunology. 2002;2(10):725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 36.Khan A., Khan I., Khan S., et al. Selenium-enriched probiotics improve hepatic protection by regulating pro-inflammatory cytokines and antioxidant capacity in broilers under heat stress conditions. Journal of Advanced Veterinary & Animal Research. 2019;6(3):355–361. doi: 10.5455/javar.2019.f354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen L., Wang L., Zhang X., et al. The protection by Octreotide against experimental ischemic stroke: Up- regulated transcription factor Nrf2, HO-1 and down-regulated NF-κB expression. Brain Research. 2012;1475(1):80–87. doi: 10.1016/j.brainres.2012.07.052. [DOI] [PubMed] [Google Scholar]
- 38.Kim Y.-M., Pae H.-O., Park J. E., et al. Heme oxygenase in the regulation of vascular biology: from molecular mechanisms to therapeutic opportunities. Antioxidants & Redox Signalling. 2011;14(1):137–167. doi: 10.1089/ars.2010.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used to support the findings of this study are available from the corresponding author upon request.


