Abstract
Mastocytosis is a neoplasm characterized by an accumulation of mast cells in various organs and increased risk for severe anaphylaxis in patients with concomitant allergies. Coronavirus disease 2019 (COVID-19) is a pandemic that is associated with a relatively high rate of severe lung disease and mortality. The mortality is particularly high in those with certain comorbidities and increases with age. Recently, several companies have developed an effective vaccination against COVID-19. Although the reported frequency of severe side effects is low, there is an emerging discussion about the safety of COVID-19 vaccination in patients with severe allergies and mastocytosis. However, even in these patients, severe adverse reactions are rare. We therefore recommend the broad use of COVID-19 vaccination in patients with mastocytosis on a global basis. The only well-established exception is a known or suspected allergy against a constituent of the vaccine. Safety measures, including premedication and postvaccination observation, should be considered in all patients with mastocytosis, depending on the individual personal risk and overall situation in each case. The current article provides a summary of published data, observations, and expert opinion that form the basis of these recommendations.
Key words: Vaccine, COVID-19, Mastocytosis, Mast cell diseases, Adverse reaction to medications, Hypersensitivity reactions
Abbreviations used: CM, Cutaneous mastocytosis; COVID-19, Coronavirus disease 2019; MC, Mast cell; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SM, Systemic mastocytosis
Introduction
Mastocytosis is a disease that is characterized by abnormal expansion and accumulation of mast cells (MCs) in (1) the skin, (2) internal organs, or (3) both the skin and internal organs. As a result, mastocytosis is diagnosed on the basis of a histologically confirmed increase and accumulation of MCs in these organs.1 , 2 In general, mastocytosis can be divided into cutaneous mastocytosis (CM) and systemic mastocytosis (SM). In patients with CM, MC accumulation is only or primarily found in the skin. To diagnose CM, a biopsy of affected skin is often performed. The pure cutaneous form of mastocytosis is usually diagnosed in (early) childhood. In contrast, most patients in adulthood are diagnosed to have SM.1 , 3 , 4
Symptoms of mastocytosis can result from biologically active mediators that are released from MCs (mediator-related symptoms) or from a malignant local infiltration and expansion of neoplastic MCs in various organ systems. MCs produce and release a large number of biologically active mediators, including vasoactive mediators, coaguloactive substances, and immunoregulatory molecules, such as histamine, heparin, tryptases, prostaglandins, cytokines, or chemokines.2 , 5 , 6 These mediators can cause various symptoms, including headache, fatigue, bone pain, nausea, itching, urticaria, flushing, diarrhea, abdominal discomfort and cramping, hypotension, up to severe anaphylaxis with shock. Symptoms can occur in any variant of mastocytosis and at any time during the course of disease.2 , 7 , 8 Patients with mastocytosis are prone to recurrent severe anaphylactic reactions especially to hymenoptera venom, with an incidence being much higher than in the general population.9, 10, 11 Fatal anaphylaxis has also been described in these patients, especially after discontinuing allergen-specific immunotherapy.12 Severe anaphylactic reactions to other triggers such as foods (3%-16% of cases) and drugs (5%-9%) have also been reported, although allergy testing may show negative results in these cases.13, 14, 15, 16, 17, 18, 19, 20
The coronavirus disease 2019 (COVID-19) pandemic infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 2019 was described first in Wuhan, China, and developed into a global pandemic in 2020, with a case-fatality rate of up to 3.5% in Europe and 1.7% in the United States.21 The fact that certain comorbidities confer a higher risk for a severe course have raised fears and concerns also in patients with MC disorders and their physicians. Representatives of the European Competence Network on Mastocytosis and the American Initiative in Mast Cell Diseases have recently issued an expert opinion on the risk and management of patients with mastocytosis in the COVID pandemic.22 Although solid data on which to base recommendations are not yet available, on the basis of their experience with CM/SM and other hematologic neoplasms, and the biology of SARS-CoV-2, the experts confirmed that there is yet no reason to believe that patients with mastocytosis or mast cell activation syndrome (MCAS) have a higher risk to acquire a SARS-CoV-2 infection. Some of these patients may even live in more or less social isolation and avoid public meetings and crowded places, reducing their risk of infection compared with the general population. This is supported by a study on the impact of SARS-CoV-2 infection on mastocytosis, in which 28 patients did neither have increased COVID-19 mortality nor clinical symptoms of MC activation.23 Therefore, in those with CM and indolent SM, the risk may be comparable to that in those without mastocytosis, and may be more dependent on other coexisting risk factors such as age, obesity, and cardiovascular disease.22 However, in patients with advanced SM (aggressive SM, SM with an associated hematologic neoplasm, or MC leukemia), the risk may be increased because these patients may have an impaired immune system that is further suppressed by some antineoplastic drugs such as cladribine or glucocorticoids, whereas no (further) immunosuppression is introduced by the KIT-targeting drugs used in advanced SM. There is no evidence to suggest that antiviral drugs and drugs used to treat SARS-CoV-2 infection induce or aggravate MC activation in patients with allergies and patients with mastocytosis.24
The cooperation between the scientific community and the pharmaceutical industry has resulted in the fast development of many vaccines with different mechanisms of action, which stimulate the immune system and protect from the potentially deadly COVID-19 infection.25, 26, 27, 28 The first months of the vaccination with currently approved vaccines (primarily using mRNA-based technologies) resulted in several reports of severe allergic reactions including anaphylaxis.26 , 29 These reports led to a degree of concern among patients with mastocytosis and their physicians whether vaccination to COVID 19 can be safely administered in this population and whether there is an increased risk for reactions due to vaccination in patient with MC disorders. The aim of the current article was to help medical staff managing the COVID-19 immunization program on how to deal with patients suffering from mastocytosis needing vaccination. Recommendations given in this article are based on expert opinion. Although evidence to provide recommendations is still limited at this time, the authors see the need to evaluate the potential risk of vaccination in patients with mastocytosis.
A recent work report of the American Academy of Allergy, Asthma, and Immunology on adverse reactions to drugs and biologics in patients with clonal MC disorders19 reviewed adverse reactions to vaccines in patients with mastocytosis. The literature search and personal experience of the working group found no evidence for an increased number of reactions to vaccines in adults with mastocytosis.30 , 31 Based on these observations and although large and controlled studies are not available, there is a consensus among the authors that mastocytosis alone is not a contraindication to vaccines in adults with mastocytosis.
Severe allergic reactions to the previous dose of mRNA COVID-19 vaccines, to any of its components, including polyethylene glycol and potentially cross-reacting polysorbate, are contraindications for vaccination.24 Recent data on 2 health care workers with cutaneous and systemic mastocytosis who had a history of prior MC mediator–related symptoms has not shown any problems after mRNA vaccination using BioNtech/Pfizer vaccine under anti-H1, anti-H2, and leukotriene receptor antagonist premedication.32
Recommendations
General recommendations
On the basis of risk related to the SARS-CoV-2 infection and the data reviewed above, members of the European Competence Network on Mastocytosis and American Initiative in Mast Cell Diseases analyzed the risk-benefit ratio, concluding that there is presently no evidence that the incidence and severity of reactions to available vaccines is higher in patients with mastocytosis and against believing that patients suffering from mastocytosis are at comparable risk to the general population from COVID-19. Thus, they should be offered vaccination to SARS-CoV-2 COVID-19. COVID-19 vaccination is effective in reducing the risk of a potentially fatal infection, and available data so far suggest an overall low risk of anaphylaxis in the general population, even though the risk is higher as compared with other vaccines. However, because these subjects have a higher risk of nonspecific release of mediators, it is suggested to proceed with caution both not to exclude them from vaccination and to reduce the risk of any adverse reactions.
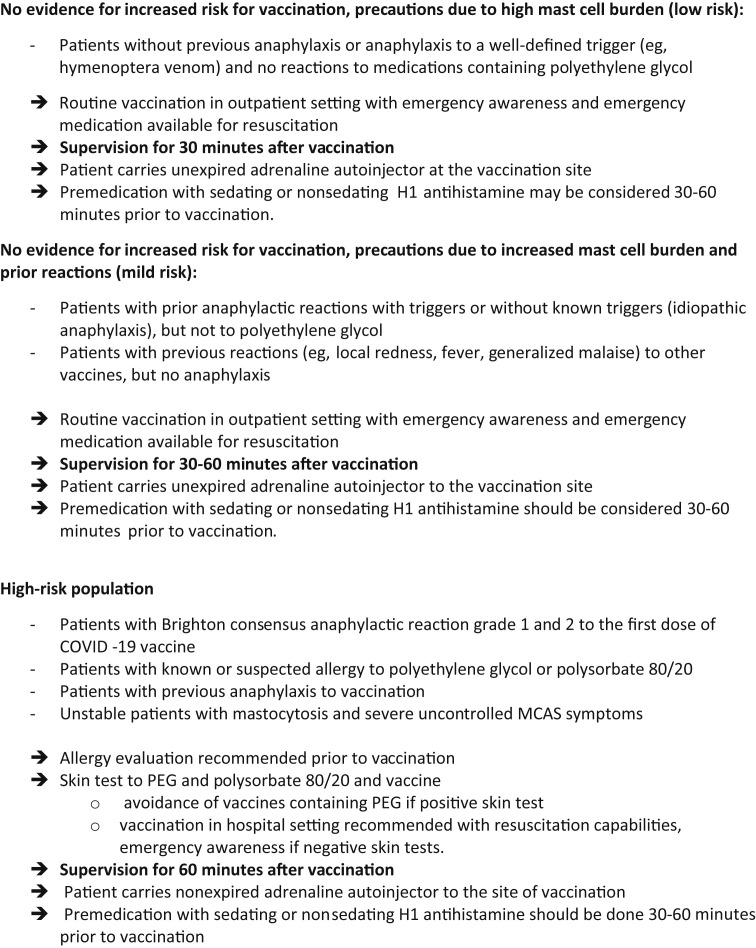
For this reason, to ensure vaccination in as many individuals as quickly as possible, our guidance results in a stratification of risk in patients with mastocytosis and it identifies 3 categories of patients at low, mild, and high risk of vaccination (Table I ). The rapid identification of high-risk individuals needing allergist assessment do not preclude the large groups of individuals with lower risk allergy histories from receiving the COVID-19 vaccine per usual protocol. In patients with uncontrolled MC-mediator–induced symptoms (intense flushing, episodes of hypotension, other uncontrolled symptoms from cardiovascular, respiratory, gastrointestinal, neurological systems) at the time of intended vaccination, the symptoms should be treated accordingly and vaccination should be delayed until proper control of the symptoms is achieved.19 , 33
Table I.
Recommended equipment for the management of anaphylactic events
| External defibrillator or an automated external defibrillator | Supplies for intubation—laryngoscope, tracheal tubes where appropriate |
|---|---|
| Medical monitor analyzing electrocardiogram, saturation, NIBP (if not integrated with defibrillator) where appropriate | Oxygen and nebulizer set with oxygen mask |
| Sphygmomanometer with blood pressure cuffs | Suction device |
| Stethoscope | Oropharyngeal airway (6, 7, 8, 9, 10 cm) |
| Pulse oximeter | Medical latex-free gloves, alcohol swabs |
| Oxygen—it should be possible to give flow of 6-8 mL/min 100% using a non-rebreather mask | Needles or catheters with a wide-bore cannula (14-16 gauge for adults), syringes |
| Bag valve masks with HEPA filters |
HEPA, high-efficiency particulate air; NIBP, non-invasive blood pressure.
The only well-established exception to vaccination is a known or suspected allergy against a constituent of the vaccine.33 A potential risk for having such an allergy to vaccine constituents can be assessed in those with (other) severe allergies and concomitant MCAS by comparing the constituents of the triggers of anaphylaxis with the lipids present in the vaccine. Those with a known allergy to polyethylene glycol, polysorbate, or another vaccine ingredient should be vaccinated with an alternative product not having these ingredients, if possible. Potential cross-reactivity between polyethylene glycol and polysorbate has to be considered. Patients with previous anaphylaxis to vaccination should also undergo allergy workup to look for common ingredients and elicitors.34 Skin testing may be of value in shared decision making around future COVID-19 vaccination.34
Unstable patients with mastocytosis and severe uncontrolled MCAS symptoms should first be treated until the symptoms are well controlled before receiving vaccination. It is important to inform the vaccination team about the histories of anaphylaxis in patients with mastocytosis.
Premedication and anti–mediator-based treatment
The role of premedication to avoid adverse reactions to medications, such as radiocontrast media, hymenoptera immunotherapy, or anesthetics, has not been evaluated in controlled studies but is currently best clinical practice. Earlier studies on the use of premedications for patients with severe contrast media reactions were successful at 10-fold lowering the rate of reactions with reexposures and paved the way for the current premedication protocols.35, 36, 37, 38
Whether premedications can protect from anaphylaxis in IgE-mediated reactions has not been evaluated, and no controlled studies are available to provide evidence of protection from reactions to vaccines. There is a general consensus among experts about the use of premedications in patients with mastocytosis at high risk for anaphylaxis, and H1-antihistamines taken 30 to 60 minutes before vaccination are the preferred drugs. Corticosteroids, H2-antihistamines, and montelukast are also considered; however, there is concern that corticosteroids may influence vaccination efficacy. The benefit of premedication with H2-antihistamines and montelukast has been even less well documented. Currently, premedication with an H1-antihistamine is recommended in patients with mastocytosis at high risk for anaphylaxis, whereas the addition of other prophylactic drugs such as H2-antihistamines or montelukast can be considered on a case-by-case basis, once risk stratification is done.
Many patients with mastocytosis are treated with anti–mediator-type drugs, including antihistamines, antileukotrienes, cromones, and omalizumab (anti-IgE).39 There is no reason to suggest that these drugs exert immunosuppressive effects or would reduce the efficacy of the vaccination, even when used over several years. Therefore, patients on anti–mediator-based treatment should continue their therapy during vaccination. Omalizumab is effective in preventing anaphylaxis and improving chronic MC mediator–related symptoms in patients with anaphylaxis insufficiently controlled by conventional therapy and should not be discontinued.39 Patients on omalizumab may receive a dose 1 week before vaccination for sufficient omalizumab levels during the procedure.
Emergency awareness and equipment for anaphylaxis treatment in the vaccination center
Because hypersensitivity reactions can occur to any vaccine in any patient and the reaction severity to known triggers for anaphylaxis is often more severe in patients with mastocytosis, they should be vaccinated in a health care facility equipped and experienced with the treatment of anaphylaxis, with resuscitation equipment readily available (see Figure 1 and Table I).
Figure 1.
European Competence Network on Mastocytosis and American Initiative in Mast Cell Diseases Consensus Guidelines for COVID-19 Vaccine Risk Stratification in Mastocytosis.∗ MCAS, mast cell activation syndrome; PEG, polyethylene glycol. ∗These recommendations are based on expert opinion and have not been evaluated in regard to effectiveness.
It is prudent and not demanding to extend the general observation period in patients with mastocytosis to at least 30 minutes, and potentially even longer in those with an increased risk to develop anaphylaxis with recognition of logistic limitations in larger vaccination centers.
In the vaccination center, the equipment and drugs for the management of anaphylactic events should be available40 (Tables I and II ), and an emergency protocol should be in effect for acute treatment performed by the local medical staff. Emergency contact details are crucial for the intervention of anesthesiologists and possible transportation to intensive care unit. Because of the COVID-19 pandemic, safety of the staff should be emphasized; thus, medical latex-free gloves should be used, as well as ventilation using bag valve masks with high-efficiency particulate air filters, and cardiac compressors if available. The availability of adrenaline at vaccination centers could speed urgent therapeutic procedures. In addition, all patients with mastocytosis should bring their own autoinjector with them when going to the vaccination center.
Table II.
Drugs for the management of anaphylactic events
| Adrenaline autoinjector (0.15 mg, 0.3 mg, 0.5 mg or 2 × 0.3 mg according to weight) |
| Adrenaline 1 mg/mL |
| Glucagon |
| 0.9% isotonic saline |
| Glucocorticoid for intravenous infusion (dexamethasone, methylprednisolone) |
| H1-antihistamine for intravenous infusion (ie, chlorpheniramine, diphenhydramine) |
| Short-acting beta-2 adrenoreceptor agonists |
Considerations in patients with advanced mastocytosis
Patients with cancer are endangered by COVID-19 and should receive vaccination. Likewise, all patients with mastocytosis, including CM, indolent SM, and advanced SM, such as aggressive SM, or SM with an associated hematologic neoplasm should be vaccinated against COVID-19.
With regard to the efficacy of vaccination, common therapies used in patients with mastocytosis have not been shown to interfere with antibody production. Patients with advanced SM who receive polychemotherapy, cladribine (2CdA), or high-dose corticosteroids are at risk for severe COVID-19 infection and should be vaccinated, but may have a reduced vaccination response. Therefore, patients in whom intensive and/or immunosuppressive therapy is planned should preferentially be vaccinated before such treatment. For patients under immunosuppressive therapy, shared decision making should be applied, if waiting for a therapy-free interval is appropriate or proceeding with vaccination is preferable. Patients treated with low-dose corticosteroids (<10 mg/d), vitamin D, bisphosphonates, or antihistamines should have a normal antibody response to vaccination and should not discontinue therapies.
Conclusions
Together, on the basis of the risk related to the COVID-19 infection and the above considerations, the European Competence Network on Mastocytosis and American Initiative in Mast Cell Diseases experts analyzed the risk-benefit ratio, and have concluded that patients suffering from mastocytosis should be vaccinated to COVID-19 safely with available vaccines and mentioned precautions. This allows for clinical phenotyping, risk stratification, and reassurance to patients deemed at lower risk to safely receive the vaccine. The recommendations given in this article are based on expert opinion and have not yet been evaluated. Finally, in the present document, we have not considered the management of COVID-19 vaccination in children with mastocytosis because so far, none of the vaccines have been tested in clinical trials in children younger than 16 years.
Footnotes
M.C.C. and D.D.M. are supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases/National Institutes of Health. P.V. is supported by the Austrian Science Fund (FWF) (grant no. P32470-B).
Conflicts of interest: The authors declare that they have no relevant conflicts of interest.
References
- 1.Valent P., Horny H.P., Escribano L., Longley B.J., Li C.Y., Schwartz L.B., et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25:603–625. doi: 10.1016/s0145-2126(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 2.Metcalfe D.D. Mast cells and mastocytosis. Blood. 2008;112:946–956. doi: 10.1182/blood-2007-11-078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valent P., Sperr W.R., Schwartz L.B., Horny H.-P. Diagnosis and classification of mast cell proliferative disorders: delineation from immunologic diseases and non-mast cell hematopoietic neoplasms. J Allergy Clin Immunol. 2004;114:3–11. doi: 10.1016/j.jaci.2004.02.045. quiz 12. [DOI] [PubMed] [Google Scholar]
- 4.Valent P., Akin C., Metcalfe D.D., Mastocytosis 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017;129:1420–1427. doi: 10.1182/blood-2016-09-731893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valent P., Akin C., Arock M., Brockow K., Butterfield J.H., Carter M.C., et al. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int Arch Allergy Immunol. 2012;157:215–225. doi: 10.1159/000328760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brockow K., Plata-Nazar K., Lange M., Nedoszytko B., Niedoszytko M., Valent P. Mediator-related symptoms and anaphylaxis in children with mastocytosis. Int J Mol Sci. 2021;22:2684. doi: 10.3390/ijms22052684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valent P. Mast cells, masters, and mastocytosis: development of research since the times of Paul Ehrlich. Wien Klin Wochenschr. 2004;116:645–646. doi: 10.1007/s00508-004-0254-2. [DOI] [PubMed] [Google Scholar]
- 8.Theoharides T.C., Valent P., Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med. 2015;373:163–172. doi: 10.1056/NEJMra1409760. [DOI] [PubMed] [Google Scholar]
- 9.Bonadonna P., Perbellini O., Passalacqua G., Caruso B., Colarossi S., Dal Fior D., et al. Clonal mast cell disorders in patients with systemic reactions to Hymenoptera stings and increased serum tryptase levels. J Allergy Clin Immunol. 2009;123:680–686. doi: 10.1016/j.jaci.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 10.Niedoszytko M., Bonadonna P., Elberink J.N.G.O., Golden D.B.K. Epidemiology, diagnosis, and treatment of Hymenoptera venom allergy in mastocytosis patients. Immunol Allergy Clin North Am. 2014;34:365–381. doi: 10.1016/j.iac.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Vos B.J.P.R., van Anrooij B., van Doormaal J.J., Dubois A.E.J., Oude Elberink J.N.G. Fatal anaphylaxis to yellow jacket stings in mastocytosis: options for identification and treatment of at-risk patients. J Allergy Clin Immunol Pract. 2017;5:1264–1271. doi: 10.1016/j.jaip.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Oude Elberink J.N.G., de Monchy J.G.R., Kors J.W., van Doormaal J.J., Dubois A.E.J. Fatal anaphylaxis after a yellow jacket sting, despite venom immunotherapy, in two patients with mastocytosis. J Allergy Clin Immunol. 1997;99:153–154. doi: 10.1016/s0091-6749(97)70314-2. [DOI] [PubMed] [Google Scholar]
- 13.González de Olano D., de la Hoz Caballer B., Núñez López R., Sánchez Muñoz L., Cuevas Agustín M., Diéguez M.C., et al. Prevalence of allergy and anaphylactic symptoms in 210 adult and pediatric patients with mastocytosis in Spain: a study of the Spanish network on mastocytosis (REMA) Clin Exp Allergy. 2007;37:1547–1555. doi: 10.1111/j.1365-2222.2007.02804.x. [DOI] [PubMed] [Google Scholar]
- 14.Brockow K., Jofer C., Behrendt H., Ring J. Anaphylaxis in patients with mastocytosis: a study on history, clinical features and risk factors in 120 patients. Allergy. 2008;63:226–232. doi: 10.1111/j.1398-9995.2007.01569.x. [DOI] [PubMed] [Google Scholar]
- 15.Brockow K., Bonadonna P. Drug allergy in mast cell disease. Curr Opin Allergy Clin Immunol. 2012;12:354–360. doi: 10.1097/ACI.0b013e328355b7cb. [DOI] [PubMed] [Google Scholar]
- 16.Gülen T., Hägglund H., Dahlén B., Nilsson G. High prevalence of anaphylaxis in patients with systemic mastocytosis—a single-centre experience. Clin Exp Allergy. 2014;44:121–129. doi: 10.1111/cea.12225. [DOI] [PubMed] [Google Scholar]
- 17.Bonadonna P., Zanotti R., Pagani M., Caruso B., Perbellini O., Colarossi S., et al. How much specific is the association between hymenoptera venom allergy and mastocytosis? Allergy. 2009;64:1379–1382. doi: 10.1111/j.1398-9995.2009.02108.x. [DOI] [PubMed] [Google Scholar]
- 18.Pieri L., Bonadonna P., Elena C., Papayannidis C., Grifoni F.I., Rondoni M., et al. Clinical presentation and management practice of systemic mastocytosis. A survey on 460 Italian patients. Am J Hematol. 2016;91:692–699. doi: 10.1002/ajh.24382. [DOI] [PubMed] [Google Scholar]
- 19.Carter M.C., Metcalfe D.D., Matito A., Escribano L., Butterfield J.H., Schwartz L.B., et al. Adverse reactions to drugs and biologics in patients with clonal mast cell disorders: a Work Group Report of the Mast Cells Disorder Committee, American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2019;143:880–893. doi: 10.1016/j.jaci.2018.10.063. [DOI] [PubMed] [Google Scholar]
- 20.Jarkvist J., Brockow K., Gülen T. Low frequency of IgE-mediated food hypersensitivity in mastocytosis. J Allergy Clin Immunol Pract. 2020;8:3093–3101. doi: 10.1016/j.jaip.2020.05.044. [DOI] [PubMed] [Google Scholar]
- 21.Johns Hopkins Coronavirus Resource Center Mortality analyses. https://coronavirus.jhu.edu/data/mortality Available from:
- 22.Valent P., Akin C., Bonadonna P., Brockow K., Niedoszytko M., Nedoszytko B., et al. Risk and management of patients with mastocytosis and MCAS in the SARS-CoV-2 (COVID-19) pandemic: expert opinions. J Allergy Clin Immunol. 2020;146:300–306. doi: 10.1016/j.jaci.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giannetti M.P., Weller E., Alvarez-Twose I., Torrado I., Bonadonna P., Zanotti R., et al. COVID-19 infection in patients with mast cell disorders including mastocytosis does not impact mast cell activation symptoms. J Allergy Clin Immunol Pract. 2021;9:2083–2086. doi: 10.1016/j.jaip.2021.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castells M.C., Phillips E.J. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384:643–649. doi: 10.1056/NEJMra2035343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.U.S. Food and Drug Administration . U.S. Food and Drug Administration, Vaccines and Related Biological Products Advisory Committee; Silver Spring, MD: 2020. Moderna COVID-19 vaccine [FDA briefing document] [Google Scholar]
- 27.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Widge A.T., Rouphael N.G., Jackson L.A., Anderson E.J., Roberts P.C., Makhene M., et al. mRNA-1273 Study Group. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384:80–82. doi: 10.1056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimabukuro T.T., Cole M., Su J.R. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US—December 14, 2020-January 18, 2021. JAMA. 2021;325:1101. doi: 10.1001/jama.2021.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zanoni G., Zanotti R., Schena D., Sabbadini C., Opri R., Bonadonna P. Vaccination management in children and adults with mastocytosis. Clin Exp Allergy. 2017;47:593–596. doi: 10.1111/cea.12882. [DOI] [PubMed] [Google Scholar]
- 31.Nilsson L., Brockow K., Alm J., Cardona V., Caubet J.-C., Gomes E., et al. Vaccination and allergy: EAACI position paper, practical aspects. Pediatr Allergy Immunol. 2017;28:628–640. doi: 10.1111/pai.12762. [DOI] [PubMed] [Google Scholar]
- 32.Rama T.A., Moreira A., Castells M. mRNA COVID-19 vaccine is well tolerated in patients with cutaneous and systemic mastocytosis with mast cell activation symptoms and anaphylaxis. J Allergy Clin Immunol. 2021;147:877–878. doi: 10.1016/j.jaci.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parente R., Pucino V., Magliacane D., Petraroli A., Loffredo S., Marone G., et al. Evaluation of vaccination safety in children with mastocytosis. Pediatr Allergy Immunol. 2017;28:93–95. doi: 10.1111/pai.12647. [DOI] [PubMed] [Google Scholar]
- 34.Banerji A., Wickner P.G., Saff R., Stone C.A., Robinson L.B., Long A.A., et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9:1423–1437. doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brockow K., Kiehn M., Riethmüller C., Vieluf D., Berger J., Ring J. Efficacy of antihistamine pretreatment in the prevention of adverse reactions to Hymenoptera immunotherapy: a prospective, randomized, placebo-controlled trial. J Allergy Clin Immunol. 1997;100:458–463. doi: 10.1016/s0091-6749(97)70135-0. [DOI] [PubMed] [Google Scholar]
- 36.Torres M., Trautmann A., Böhm I., Scherer K., Barbaud A., Bavbek S., et al. Practice parameters for diagnosing and managing iodinated contrast media hypersensitivity. https://doi.org/10.1111/all.14656 [published online ahead of print November 10, 2020]. Allergy. [DOI] [PubMed]
- 37.Greenberger P.A., Patterson R. The prevention of immediate generalized reactions to radiocontrast media in high-risk patients. J Allergy Clin Immunol. 1991;87:867–872. doi: 10.1016/0091-6749(91)90135-b. [DOI] [PubMed] [Google Scholar]
- 38.Lee S.-Y., Yang M.S., Choi Y.-H., Park C.M., Park H.-W., Cho S.H., et al. Stratified premedication strategy for the prevention of contrast media hypersensitivity in high-risk patients. Ann Allergy Asthma Immunol. 2017;118:339–344.e1. doi: 10.1016/j.anai.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 39.Broesby-Olsen S., Vestergaard H., Mortz C.G., Jensen B., Havelund T., Hermann A.P., et al. Omalizumab prevents anaphylaxis and improves symptoms in systemic mastocytosis: efficacy and safety observations. Allergy. 2018;73:230–238. doi: 10.1111/all.13237. [DOI] [PubMed] [Google Scholar]
- 40.Lieberman P., Nicklas R.A., Randolph C., Oppenheimer J., Bernstein D., Bernstein J., et al. Anaphylaxis—a practice parameter update 2015. Ann Allergy Asthma Immunol. 2015;115:341–384. doi: 10.1016/j.anai.2015.07.019. [DOI] [PubMed] [Google Scholar]