Abstract
Alcohol abuse and dependence are world-wide health problems. Most research on alcohol use focuses on the consequences of moderate to high levels of alcohol. However, even at low concentrations, alcohol is capable of producing effects in the brain that can ultimately affect behavior. The current studies seek to understand the effects of low-dose alcohol (blood alcohol levels of ≤10 mM). To do so, these experiments utilize a combination of behavioral and molecular techniques to 1) assess the ability of the interoceptive effects of a low dose of alcohol to gain control over goal-tracking behavior in a Pavlovian discrimination task, 2) determine brain regional differences in cellular activity via expression of immediate early genes (IEGs), and 3) assess the role of the dentate gyrus in modulating sensitivity to the interoceptive effects of a low dose of alcohol. Here, we show that intragastric administration of a dose of 0.8 g/kg alcohol produces blood alcohol levels ≤10 mM in both male and female Long-Evans rats and can readily be trained as a Pavlovian interoceptive drug cue. In rats trained on this procedure, this dose of alcohol also modulates expression of the IEGs c-Fos and Arc in brain regions known to modulate expression of alcohol interoceptive effects. Finally, pharmacological inactivation of the dentate gyrus with GABA agonists baclofen and muscimol disrupted the ability of a low dose of alcohol to serve as an interoceptive cue. Together these findings demonstrate behavioral and molecular consequences of low-dose alcohol.
Keywords: addiction, drug discrimination, ethanol, hippocampus
Introduction
Alcohol use accounts for over 72,000 deaths in the US each year 1. To date, the majority of alcohol research has assessed the effects of moderate to high doses of alcohol. This leaves a considerable lack of research focused on the molecular targets and effects of low doses of alcohol (blood alcohol concentrations of less than 10 mM; 0.046 g%. This is important given that most individuals are not binge drinkers, but instead consume low doses of alcohol. In adults, this constitutes around 1-2 drinks 2. Even at low doses, alcohol has effects on the brain and numerous cognitive functions. For example, human subjects tested on a response inhibition task showed impairments with a blood alcohol concentration (BAC) of 0.043g% 3. Moreover, even at a 0.03g% BAC, a person’s ability to detect acoustic signals and visual information is impaired 4, 5. Given that these low doses are capable of affecting behavior, gaining a better understanding of the underlying neurobiological and molecular effects of low alcohol doses is important for furthering our understanding of the broader consequences of alcohol drinking.
An important aspect of drug taking behavior are the various cues, both external/environmental and internal/interoceptive that drive continued drug use (For review, see 6). In particular, interoceptive drug effects can be used as a valuable marker of whether or not a particular dose of a drug is detectable by an organism. In the laboratory, one well-established method for assessing interoceptive drug effects is to train the interoceptive effect as a discriminative cue. Our lab has demonstrated the ability of alcohol (1.0 g/kg) to serve as a cue predictive of reward using Pavlovian drug discrimination methods 7, 8. In particular, the Pavlovian discrimination model may be a more sensitive tool for detection of low alcohol doses than operant drug discrimination procedures 7, 8, likely related to the different response costs and distinct behavioral outputs (i.e. lever response and goal tracking) of the procedures (see 7). By utilizing this powerful behavioral tool we are able to assess not only the ability of the rat to detect the alcohol but to act selectively based on the specific dose of alcohol they are trained on. This is important given evidence that the ability of an individual to correctly recognize their own interoceptive drug state may be an important contributor the progression of addiction 9.
There is a rich literature assessing neuronal activity across the brain in response to moderate and high doses of alcohol. Cortico-limbic brain circuitry including the nucleus accumbens and prefrontal cortex are common targets, showing increased activity following moderate to high doses of alcohol 10–13. Moreover, cognitive circuitry including the prefrontal cortex and hippocampus are also recognized as playing an important role in alcohol-related behaviors 14. Further, work from our lab and others have demonstrated a functional role of cortico-limbic brain regions (i.e., nucleus accumbens, insular cortex, mPFC, hippocampus) in modulating the interoceptive effects of alcohol 15–19. Given the general lack of studies focused on low doses of alcohol, the current experiments examined immediate early gene expression in some of these known brain regions in rats with an alcohol interoceptive conditioning history.
The dentate gyrus is another key brain region affected by alcohol. There has been much interest in this brain region focused on alcohol-induced reduction of neurogenesis and induction of long-term damage 20. A spectrum of acute alcohol doses have been shown to reduce cell proliferation and enhance cell death in this region ranging from moderate (1.0 g/kg) to high (5.0 g/kg) 21–23. Alcohol doses as low as 3 mM enhance activity in δ subunit containing GABAA receptors which are highly expressed in dentate gyrus granule cells 24, 25, and hippocampal interneuron glutamatergic receptor have been shown to be inhibited by alcohol doses as low as 5 mM 26. Less well-studied is the functional role of the dentate gyrus in modulating sensitivity to drug related cues and behaviors, but given that it is important in conjunctive encoding of multisensory inputs and goal-directed decision making 27, 28 it is likely an important region in neural circuitry related to processing of interoceptive drug effects.
To this end, the current studies utilized a mixture of behavioral and molecular approaches to assess both the ability of a low dose of alcohol to serve as a Pavlovian interoceptive drug cue and to survey of brain regions activated by this cue. As the dentate gyrus was the only region to show a decrease in immediate early gene activity in response to a low dose of alcohol we hypothesized that pharmacological inactivation of this region would result in substitution for the interoceptive effects of alcohol (i.e. inactivation would drive an alcohol-appropriate behavioral response in the absence of alcohol) as we have previously shown in the insular cortex and mPFC 18.
Materials and Methods
Animals
Adult Long-Evans rats (Envigo-Harlan) were used for these experiments. The vivarium was maintained on a 12-h light/dark cycle and experiments were conducted during the light portion of the cycle. Rats had ad libitum access to water in the home cage and were fed daily to maintain body weight at 85% of free-feeding weight. Animals were under continuous care and monitoring by veterinary staff from the Division of Comparative Medicine (DCM) at UNC-Chapel Hill. All animal procedures were approved by the UNC-Chapel Hill Institutional Animal Care and Use Committee (IACUC). All procedures were carried out in accordance with the NIH Guide to Care and Use of Laboratory Animals and institutional guidelines. UNC-Chapel Hill is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC).
Apparatus
Chambers (Med Associates) measuring 31 X 24 x 32 cm were located within sound attenuating cubicles and equipped with an exhaust fan that provided ventilation and masked external sounds. Two cue lights were located on one wall of the chamber adjacent to a liquid dipper receptacle equipped with a photobeam detector that was used to detect head entries into the receptacle. When activated, the dipper arm was raised for 4 sec and presented 0.1 ml of 26% sucrose (w/v). Chambers were also outfitted with infrared photobeams (that divided the chamber into 4 parallel zones) to measure locomotor activity during sessions (number of beam breaks).
Sucrose Access Training
Procedures were similar to those described in detail in 7, 19, 29, 30. Briefly, rats had three 50-min sessions in which sucrose (26% w/v) was randomly presented across the session to train rats to approach the liquid receptacle. The probability of sucrose presentation decreased from the first to the last session and by the last 10 min of the final session rats received approximately 0.75 sucrose presentations/min.
Pavlovian Discrimination Training
A schematic of the training procedure can be found in Figure 1. Training sessions were similar to those described previously by our lab 7, 19, 29, 30. Briefly, training was conducted 5 days per week (M-F) during which alcohol (0.8 g/kg) or water was administered by intragastric gavage (IG) prior to the start of the sessions. Pretreatment was on a double rotation schedule (A,A,W,W) with all animals receiving the same pretreatment on a given day. Immediately following alcohol or water administration the rats were placed in the chambers for a 10 min delay to allow for the blood/brain levels of alcohol to rise. During this time no cue lights were illuminated, no sucrose was presented and head entries into the liquid receptacle were not recorded. The 15-min session began after this 10-min delay. On alcohol sessions (i.e., alcohol administered prior to the start of the session), the offset of each of the 15-sec cue light presentations (both cue lights illuminated) was followed by a 4-sec sucrose presentation (0.1 ml). On water sessions (i.e., water administered prior to the start of the session), no sucrose was delivered following the offset of the cue light presentations. There were 10 cue light presentations (conditioned stimulus, CS) during each session. The onset of the first CS presentation varied from 45-75 s, and the inter-trial intervals (time from CS offset to the next CS onset) ranged from 30-105 s. Under these training conditions, head entries during the light presentations increase on alcohol, but not water sessions 7. Importantly, head entries during the first light presentation (i.e., prior to feedback from sucrose delivery) are the primary index of learning and are calculated as a discrimination score as the primary dependent measure (described in detail in the Data Analysis).
Figure 1.
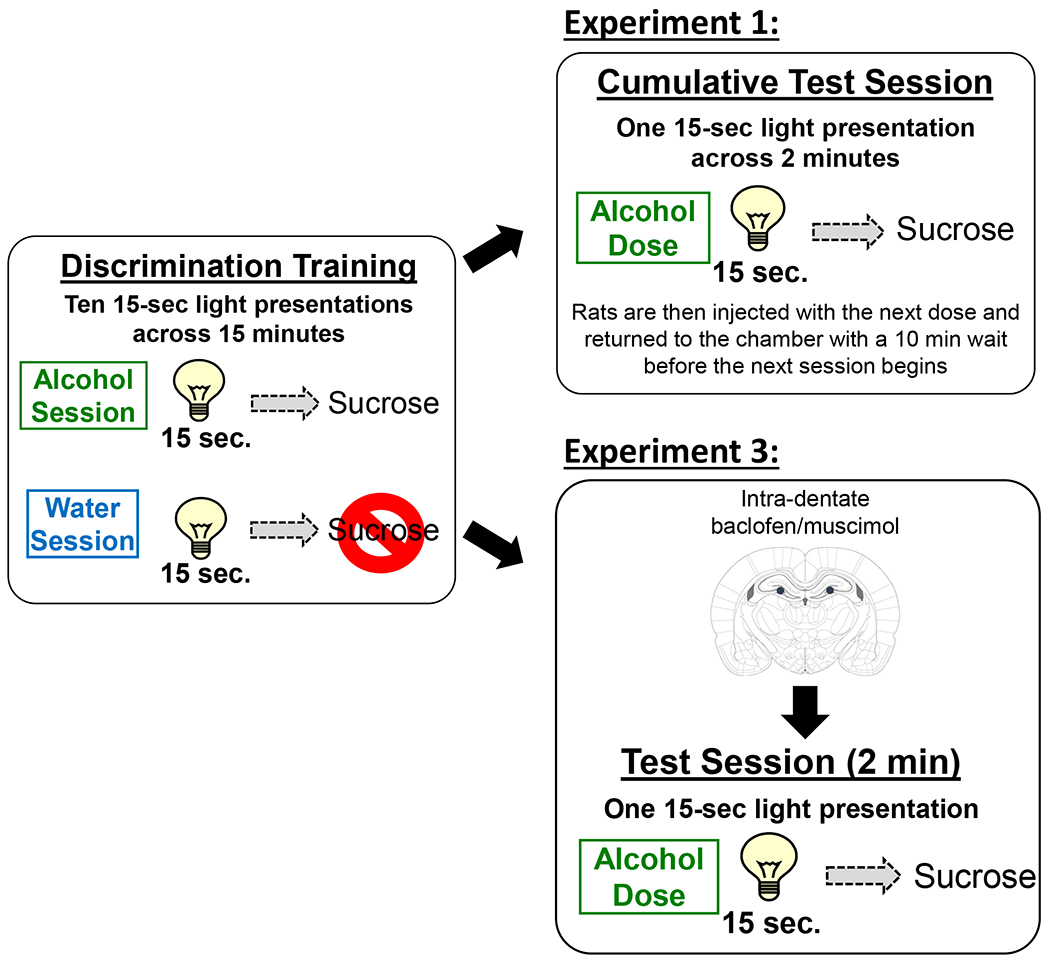
Schematic of the discrimination acquisition and testing procedures. Pretreatment on acquisition sessions was on a double alternation schedule (A,A,W,W) in which only 1 treatment was given per day. Acquisition sessions consisted of a 10 minute pre-treatment time where the chamber was inactive, followed by a 15 minute session in which the stimulus light activated randomly 10 times during the session (15 seconds each). On alcohol sessions, light offset was immediately followed by 4-second access to 0.1ml sucrose. On water sessions, light offset had no outcome. Cumulative test sessions consisted of 4 discrete tests. Each test was preceded by a 10 minute pretreatment off-period, followed by a 2 minute test in which a single, random light activation (15 sec.) occurred that was followed by sucrose access. Rats were then injected with the next dose and placed back in the chamber for the next session.
Drugs
Alcohol (95% v/v, Pharmaco-AAPR) was diluted in tap water to 20% v/v. Alcohol was then administered via intragastric gavage (IG) volume-adjusted to achieve a dose of 0.8 g/kg. Sucrose (Great Value brand granulated sugar) was dissolved in tap water to a concentration of 26% w/v. Muscimol and baclofen (R&D systems, Minneapolis, Minnesota) were dissolved in sterile 0.9% saline to produce a cocktail of 0.1 mm muscimol + 1 mm baclofen, and the doses were chosen based on previous work 18, 31, 32.
Experiment 1: Low-dose alcohol as an interoceptive cue
Rats (n = 12/sex) were trained on the Pavlovian discrimination task described above. After 15 sessions of each (alcohol, water) rats were treated with alcohol (0.8 g/kg, IG) and tail blood was collected at 10, 30, 60, and 120 minutes post-injection to assess blood alcohol concentration (BAC). Blood samples were centrifuged and plasma was pipetted off and analyzed using an Analox alcohol analyzer. Training was withheld on this day. Following this assessment, rats were given a week of training sessions to ensure stable baseline before undergoing a cumulative alcohol test session to verify stimulus control of the training dose of alcohol (0.8 g/kg).
The cumulative alcohol test procedures were used as we describe in 7, 29, 30 and a schematic of the cumulative testing procedure can be found in Figure 1. Briefly, test sessions were identical to training sessions with the exception that 4 separate tests were conducted in immediate succession. Each test was 2 min in duration (following the 10 min delay), with 1 light presentation that was followed by sucrose presentation. Onset of the light presentation was randomized and varied from 45-105 seconds into the 2-minute test period. Dosing for these cumulative tests were scaled to reach each stated dose. For example, to determine the cumulative alcohol dose curve (0.2, 0.4, 0.8, 1.6 g/kg) rats initially received 0.2 g/kg alcohol and were placed in the chamber for a testing session. At the conclusion of the session, the rats received a subsequent alcohol administration of 0.2 g/kg (to reach the 0.4 g/kg cumulative dose) and another test session. This procedure was repeated with two subsequent administrations of 0.4 g/kg alcohol and 0.8 g/kg, to reach the cumulative 0.8 and 1.6 g/kg doses. Thus, testing of the entire dose curve was completed in approximately 48 min.
Experiment 2: c-Fos/Arc immunoreactivity
At the conclusion of Experiment 1, rats continued regular training sessions for 1 additional week. Following this period, on a non-training day, rats were treated with alcohol (0.8 g/kg; n=6/sex) or water (n=6/sex) IG and sacrificed 90 min later. Brain tissue was processed for c-Fos and Arc immunoreactivity as described below to examine alcohol-induced neuronal activation.
Immunohistochemistry and Quantification
Rats were deeply anesthetized with sodium pentobarbital and transcardially perfused with ice cold 0.1M PBS followed by 4% paraformaldehyde (4°; pH=7.4). Brains were extracted and stored in 4% paraformaldehyde overnight at 4°C. Next, brains were moved to a 30% sucrose (w/v) in 0.1M PBS solution and stored at 4°C. 40 micron coronal sections were taken on a freezing sliding microtome. Immunohistochemistry staining and quantification procedures were similar to those we have previously described 18, 33, 34. Free-floating coronal sections were incubated in rabbit anti-c-Fos antibody (1:10,000; Synaptic Systems; #226003; lot #: 3-37) or rabbit anti-Arc antibody (1:4,000; Synaptic Systems; #156003; lot#: 1-62) for 48 h at 4 °C with agitation. Images were acquired utilizing Olympus CX41 light microscope (Olympus America) and analyzed utilizing Image-Pro Premier image analysis software (Media Cybernetics, MD). Immunoreactivity (IR) data (c-Fos positive pixels/mm2 or Arc positive pixels/mm2) were acquired from a minimum of three sections/brain region/animal, and the data were averaged to obtain a single value per subject. The quantification was conducted by an experimenter blind to experimental conditions.
Experiment 3: Inhibition of activity in the dentate on sensitivity to the interoceptive effects of low-dose alcohol
Rats (n = 12/sex) were trained on the Pavlovian discrimination task as described above and reached discrimination criteria by the 16th training session. Rats were anesthetized with 3% isoflurane and 2% oxygen and received implantation of bilateral 26-gauge guide cannulae (Plastics One, Roanoke, Virginia) aimed to terminate 2 mm above the dentate gyrus of the hippocampus (AP −3.5, ML ±2.0, DV −4.0 from bregma; coordinates based on 35. Naproxen (10 mg/kg) was administered immediately after surgery and 24 h postoperatively. Rats were given a week to recover from surgery then training continued. Once rats demonstrated continued successful discrimination, testing began. A schematic of the testing procedure can be found in Figure 1. Tests were 2 min in duration (following the 10 min acclimation period) with 1 light presentation that was followed by sucrose presentation. Test sessions were interspersed with training sessions and testing occurred when a rat reached the criterion (the average of the discrimination score from the preceding two alcohol sessions had to be ≥150% of the average of the discrimination scores from the preceding two water sessions) on four consecutive training days. While uncommon, if an animal did not meet the criteria for testing (i.e., acquisition criteria above), the animal remained in the home cage on that test day. During the test sessions, rats received a microinjection of the 0.1 mM muscimol + 1 mM baclofen cocktail to temporarily inactivate the DG. Baclofen/muscimol injections were delivered through injectors extending 2 mm below the guide cannulae at a volume of 0.5 μl/min for 1 min. The injectors remained in place for an additional 2 min after the infusion to allow for diffusion and then the rats were administered alcohol or water (IG) and placed in the chambers for the test session. This procedure was repeated such that all subjects were tested under all four possible conditions (ethanol/water x muscimol+baclofen/vehicle). At the end of the experiment brains were collected and brain tissue was stained with cresyl violet to verify cannulae placement. Only data from rats with cannulae/injector tracts determined to be in the target brain region were used in the analyses.
Statistical Analyses
BAC was assessed using a two-way repeated measures analysis of variance (RM-ANOVA) with sex as a factor. In addition, area under the curve (AUC) was calculated and compared between males and females. For the discrimination behavior, the number of head entries into the liquid receptacle and corresponding duration (in seconds) was recorded in 15-second intervals throughout the training and testing sessions. The head entry discrimination score was calculated by subtracting the number of head entries that occurred in the 15 seconds before light onset (ie, pre-CS) from the head entries that occurred during the 15-second light CS 7, 8, 29, 30. The first head entry discrimination score (i.e., prior to feedback from sucrose delivery) was used as the primary dependent variable and is represented on all graphs. Similar to the head entry discrimination score, the first duration discrimination score was calculated by subtracting the number of seconds that the rat’s head was in the dipper receptacle in the 15 seconds before light onset from the number of seconds of active head entry that occurred during the 15-second light CS. This measure is important as it assesses the amount of time that rats have their heads in the dipper receptacle, as opposed to raw number of head entries. This accounts for rats that show low head entry counts but wait with their head in the dipper receptacle. Locomotor rate (beam breaks/min) was analyzed for the entire session and served as a measure of nonspecific motor activity. To confirm full substitution for the alcohol training dose (0.8 g/kg), a paired samples t-test was used to compare the discrimination score of the training dose (0.8 g/kg) to the average of the two alcohol sessions prior to testing (i.e., baseline). For all behavioral experiments, RM-ANOVA was used to assess discrimination scores (both head entries and head entry duration in seconds) and locomotor rate across sessions (repeating factor) by sex (between subject factor). Differences between specific doses, time points or sessions were analyzed with post-hoc analysis (Tukey). Immunoreactivity (IR) data (c-Fos positive pixels/mm2 or Arc positive pixels/mm2) were acquired from a minimum of three sections/brain region/animal, and the data were averaged to obtain a single value per subject 18, 30. Positive pixel count values were then converted to percent of control each sex separately and analyzed with t-tests. Raw pixel counts (positive pixels/mm2; mean±SEM) are included in Table 2. Males and females are pooled to ensure sufficient sample size. Patterns were similar for each and sex is represented on the individual data points on the figure. For Experiment 3, discrimination score, discrimination duration, locomotor rate, and head entry rate were analyzed by three-way RM ANOVA with sex as a factor. Given the lack of a main effect of sex on these measures, the sexes were combined in a two-way RM ANOVA. Based on the a priori hypothesis that baclofen/muscimol microinjection would substitute for alcohol in the discrimination task, planned comparisons were conducted to determine whether or not rats could discriminate between water and ethanol under both vehicle and baclofen/muscimol microinjection conditions. Statistical significance was determined at p=0.05.
Table 2:
Immunoreactivity as measured in mean±SEM IR-positive pixels/mm2.
| Prefrontal Cortex | ||||||||
|---|---|---|---|---|---|---|---|---|
| Infralimbic | Prelimbic | |||||||
| Alcohol (g/kg) | 0 | 0.8 | 0 | 0.8 | ||||
| Sex | Male | Female | Male | Female | Male | Female | Male | Female |
| cFos | 28262.4±1416.58 | 23475±1366.16 | 36352.4±2886.16 | 28007.2±1780.32 | 29362.1±1372.61 | 23350.6±3811.09 | 31919±1227.32 | 22705.9±1976.87 |
| Arc | 10649.2±1128.12 | 9591.69±782.813 | 12052.4±1346.9 | 12862.7±1120.69 | 30162±5932.24 | 22258.2±8685.72 | 30346±7887.95 | 31062.8±8589.4 |
| Nucleus Accumbens | ||||||||
|---|---|---|---|---|---|---|---|---|
| Shell | Core | |||||||
| Alcohol (g/kg) | 0 | 0.8 | 0 | 0.8 | ||||
| Sex | Male | Female | Male | Female | Male | Female | Male | Female |
| cFos | 18254.8±980.328 | 12466.4±1093.19 | 22265.5±1935.67 | 14504.8±388.190 | 18190.1±2883.93 | 17194.1±1624.80 | 20006.7±3269.67 | 15301.1±1383.15 |
| Arc | 6928.51±375.227 | 6373.5±978.356 | 8837.67±491.018 | 8015.55±848.907 | 5613.73±421.007 | 5438.99±942.263 | 7404.87±533.954 | 6393.81±711.936 |
| Insular Cortex | ||||
|---|---|---|---|---|
| Alcohol (g/kg) | 0 | 0.8 | ||
| Sex | Male | Female | Male | Female |
| cFos | 2648.59±343.557 | 2552.95±323.820 | 3410.75±459.085 | 2237.05±286.092 |
| Arc | 19411.6±1447.61 | 17986.1±758.147 | 18655.5±2063.73 | 20595.3±778.212 |
| Dentats Gyrus | ||||
|---|---|---|---|---|
| Alcohol (g/kg) | 0 | 0.8 | ||
| Sex | Male | Female | Male | Female |
| cFos | 3295.56±363.111 | 3199.37±516.371 | 2107.62±179.794 | 2194.51±205.701 |
| Arc | 11326.5±1343.71 | 10066.9±699.04 | 8306.29±844.621 | 10312.4±1687.81 |
Results
Experiment 1: Low-dose alcohol serves as a Pavlovian interoceptive drug cue
In male and female rats, a dose of 0.8 g/kg alcohol produced peak BAC at or below 10 mM (Figure 2A). Given that discrimination training sessions occur 10 min post-injection the interoceptive effect of alcohol corresponds to the ascending limb of the blood alcohol curve and is below 10 mM. There was a significant main effect of time (F[3,63] = 9.938, p = 0.001). There was no difference between males or females in alcohol clearance rates. In addition, as shown in Figure 2B, area under the curve (AUC) was calculated and determined there was no difference between males (977.8±107.6) and females (966.6±144.0, t[17] = 0.06, p = 0.95).
Figure 2.
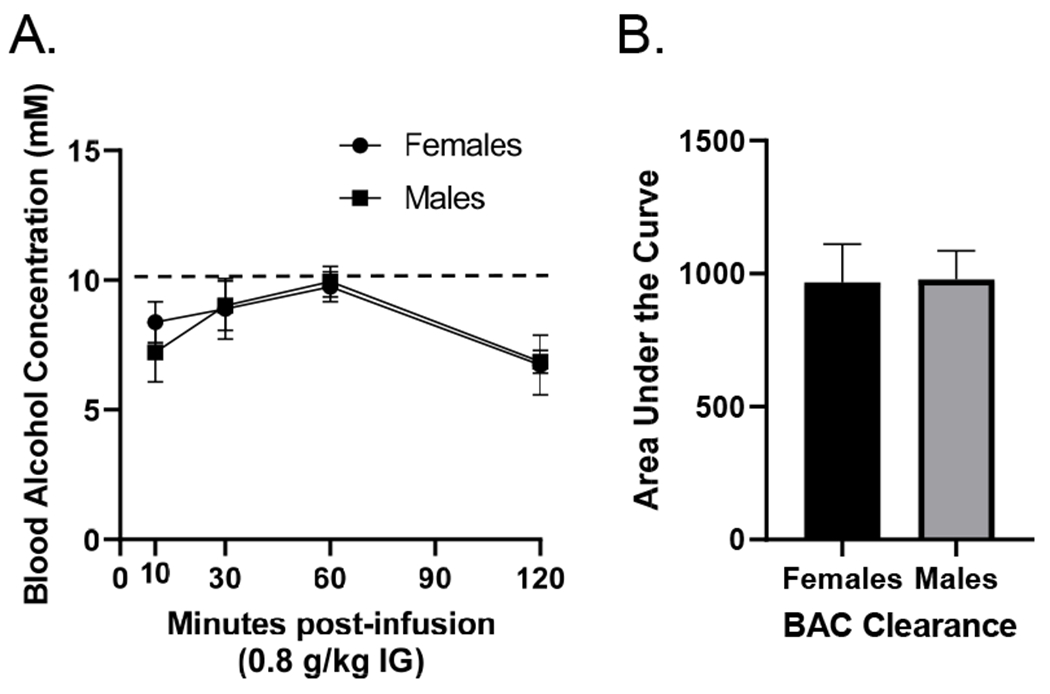
(A) Blood alcohol concentration (mM; mean±SEM) in male and female Long-Evans rats over a 120 minute post-injection period following 0.8 g/kg alcohol. There were no sex differences in BAC. Moreover, there was no sex difference in alcohol clearance as measured by area under the curve (B).
All analyses are reflective of the first light presentation in each session prior to any feedback, avoiding the effects of within-session learning. For a reference of average discrimination performance across each session, average discrimination scores are in Table 1. As shown in Figure 3, both males and females acquired the Pavlovian discrimination. That is, the first discrimination score on alcohol sessions were significantly higher than water sessions (i.e., more head entries while the cue light was on), demonstrating that the alcohol interoceptive cue was modulating goal-tracking behavior during the light CS. This was confirmed by a significant main effect of session (F[14,280] = 4.718, p < 0.0001), treatment (F[1,20] = 14.26, p = 0.0012) and a session by treatment interaction (F[14,280] = 7.987, p < 0.0001), with scores increasing on alcohol days compared to water (Figure 3A). Post hoc analysis showed that after 10 pairings, discrimination scores on alcohol sessions were significantly higher than water sessions (p < 0.05). The first duration discrimination scores showed a similar data pattern (Figure 3B). There was a main effect of session (F[14,280] = 4.817, p = 0.0001) and treatment (F[1,20] = 27.37, p = 0.0001) with scores increasing on alcohol sessions compared to water (Figure 3B). The first duration discrimination scores on alcohol sessions were significantly higher than water sessions after 10 pairings in both males and females (p<0.05). Locomotor rate also increased across training as indicated by a main effect of session (F[14,280] = 5.042, p = 0.0001; Figure 3C). There was no main effect of sex on any of the measures (head entry discrimination score (F[1,20] = 0.053, p = 0.818); duration discrimination score (F[1,20] = 0.979, p = 0.334); locomotor rate (F[1,20] = 0.1566, p = 0.696), indicating similar behavioral performance and acquisition patterns between males and females.
Table 1.
Average discrimination scores (mean+SEM) for acquisition.
| Session | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
| Alcohol | 0.49±0.09 | 0.88±0.11 | 0.82±0.06 | 1.58±0.10 | 2.41±0.11 | 3.30±0.14 | 3.11±0.22 | 3.91±0.21 | 4.34±0.27 | 4.60±0.29 | 4.34±0.48 | 4.45±0.23 | 4.37±0.32 | 4.51±0.19 | 3.88±0.22 |
| Water | 0.74±0.09 | 0.62±0.10 | 1.13±0.08 | 1.01±0.14 | 1.37±0.10 | 1.38±0.11 | 1.23±0.09 | 1.11±0.08 | 1.17±0.09 | 1.20±0.08 | 1.18±0.06 | 1.11±0.07 | 0.74±0.06 | 0.84±0.08 | 0.80±0.10 |
Figure 3.
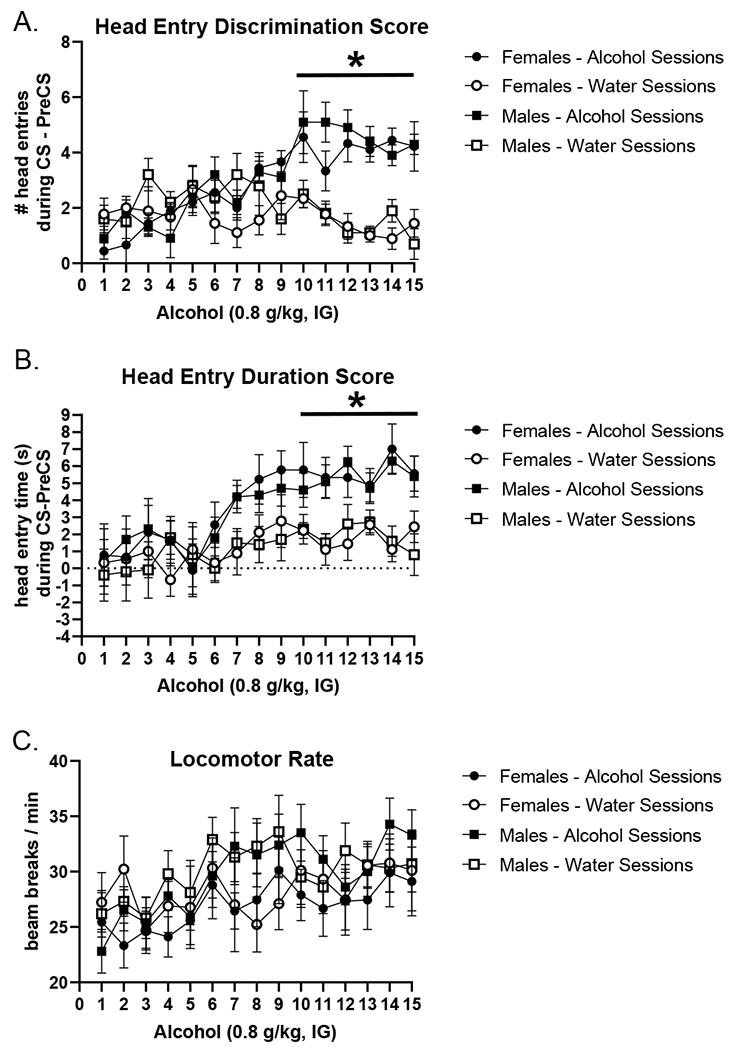
Acquisition of the low-dose alcohol Pavlovian discrimination as measured by the primary dependent variable (mean±SEM) the first head entry discrimination score (i.e., anticipatory head entries prior to any sucrose delivery) (A), in addition to corresponding first head entry duration in seconds (B). First head entry discrimination score and first head entry duration score increased on alcohol sessions compared to water sessions in both male and female rats. There were no sex differences in acquisition. There were no effects on locomotor rate (C). * p<0.05, alcohol different from water sessions.
As shown in the substitution curves in Figure 4A and B, 0.8 g/kg alcohol gained control over goal-tracking behavior. This was supported by a significant main effect of alcohol dose on head entry discrimination score (F[3,63] = 19.551, p = 0.0001; Figure 4A) and duration discrimination score (F[3,53] = 4.645, p = 0.005; Figure 4B) with discrimination scores increasing as the dose approached the 0.8 g/kg training dose. Moreover, post hoc analysis showed that head entry discrimination score and head entry duration following the 0.2 and 0.4 g/kg doses were significantly lower than following the 0.8 g/kg dose in the curve (p < 0.05; Figure 4A/B). There was no effect of sex on head entry discrimination score (F[1,21] = 3.485, p = 0.076) or duration discrimination score (F[1,21] = 1.678, p = 0.209). The alcohol training dose (0.8 g/kg) in the curve fully substituted for the training condition in both head entry discrimination score and duration discrimination scores (discrimination scores at 0.8 g/kg did not differ from baseline). There was a main effect of dose on locomotor rate (F[3,63] = 2.874, p = 0.043; Figure 4C), with decreasing rates as the alcohol dose increased. There was no effect of sex on locomotor rate (F[1,21] = 1.654, p = 0.212).
Figure 4.
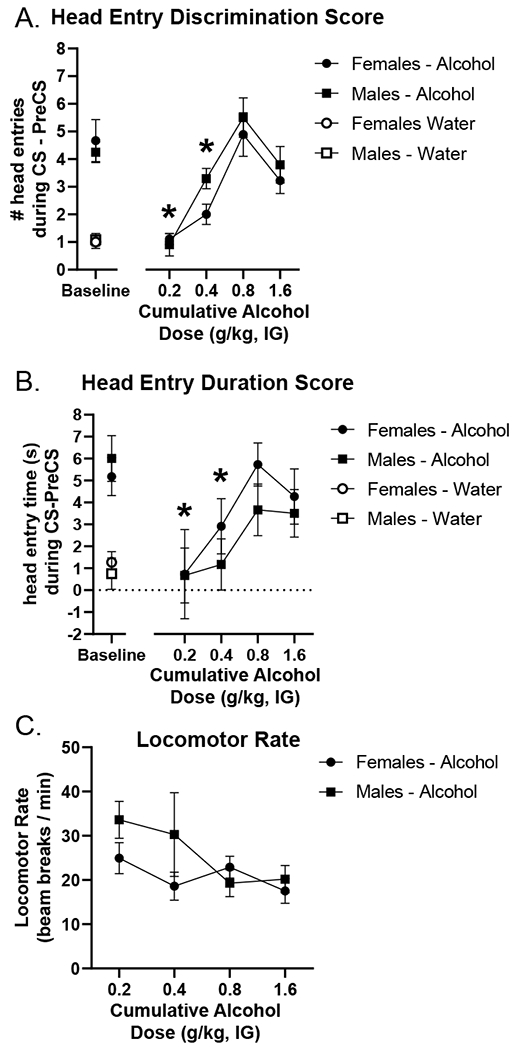
Generalization curve to assess cue specificity as measured by the primary dependent variable (mean±SEM): head entry discrimination score (A) in addition to corresponding head entry duration in seconds (B). Pre-test baseline (average of the previous two water and alcohol sessions) is located on the left. Both head entry discrimination score and duration discrimination scores (mean±SEM) increased as the cumulative dose increased with the 0.8 g/kg dose in the curve not differing from pre-test baseline, showing full substitution for the training dose. Furthermore, head entry discrimination score and head entry duration were significantly lower following 0.2 and 0.4 g/kg doses in the curve compared to 0.8 g/kg in both males and females demonstrating selectivity for the training dose (3A). There were no sex differences on these generalization tests. Locomotor rate was significantly higher at the 0.2 g/kg dose in the curve compared to 0.8 g/kg (C). * p<0.05 different from 0.8 g/kg alcohol.
Experiment 2: Low-dose alcohol alters expression of the immediate early genes c-Fos and Arc in several brain regions.
There were no sex differences in IEG expression patterns and as such, sex was collapsed for these analyses. As shown in Figure 5A, there was an alcohol-induced increase in c-Fos IR in infralimbic PFC (mPFC-IL) (t[20] = 3.377, p = 0.003; Figure 5A) following 0.8 g/kg alcohol compared to water and no change in the prelimbic PFC (Figure 5B). An alcohol-induced increase in c-Fos IR was also observed in the AcbSh (t[20] = 3.520, p = 0.002; Figure 5C), with no change in the AcbC (Figure 5D). There was no change in c-Fos IR in the insular cortex (Figure 5E). In contrast, there was an alcohol-induced decrease in c-Fos IR in the dentate gyrus (t[20] = 5.382, p = 0.001; Figure 5F). The same data patterns were observed with Arc; mPFC-IL (t[20]=2.13, p=0.045; Figure 5G); AcbSh: (t[20] = 2.999, p = 0.007; Figure 5I), dentate: (t[20] = 2.488, p = 0.023; Figure 5L).
Figure 5.
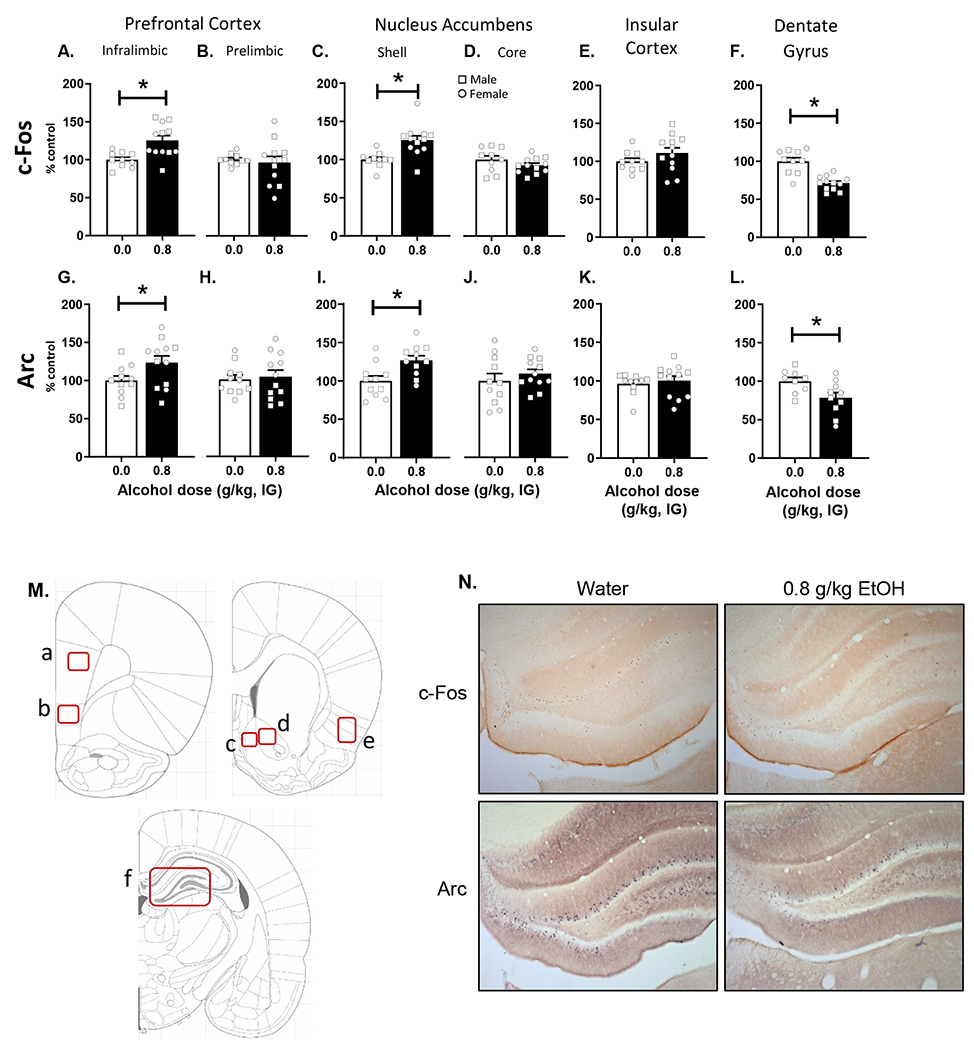
Assessment of immediate early gene immunoreactivity for c-Fos and Arc following alcohol (0.8 g/kg) or water (mean±SEM). c-Fos IR was significantly increased following alcohol in medial prefrontal cortex - infralimbic (A), but not prelimbic (B). c-Fos IR was also increased following alcohol in the accumbens shell (C), but not the core (D) or the insular cortex (E). By contrast, c-Fos IR was significantly decreased following alcohol in dentate gyrus (F). Similar changes were observed in Arc IR with increases in medial prefrontal cortex – infralimbic (G) and accumbens shell (I), with prefrontal cortex – prelimbic (H), accumbens core (J), and insular cortex (K) not showing effects. Furthermore, similar to c-Fos, dentate gyrus showed decreases in Arc expression (L). (M) Atlas images depicting the quantified regions of interest. Letters correspond to the respective figure panel for that region. (N) Photomicrographs of representative DG sections. Symbols: Squares - males, Circles - females * p<0.05 alcohol different from water.
Experiment 3: Infusion of baclofen/muscimol into the dentate gyrus disrupts discrimination
Three male and 6 female rats were excluded due to lost head caps during the experiment and one male was excluded due to poor performance following cannulae implantation. These rats are not included in any analyses or figures. Thus, the data presented are based on 8 males and 6 females. Figure 6A shows the injector tip locations targeting the dentate gyrus. Data were first analyzed via 3-way ANOVA (sex x alcohol dose x microinjection). Sex was not a significant factor in 3-way ANOVAs and therefore, a two-way ANOVA was conducted collapsing across sex. Data are presented with males and females combined into one group with individual data points denoting sex displayed on all graphs (Figure 6).
Figure 6.
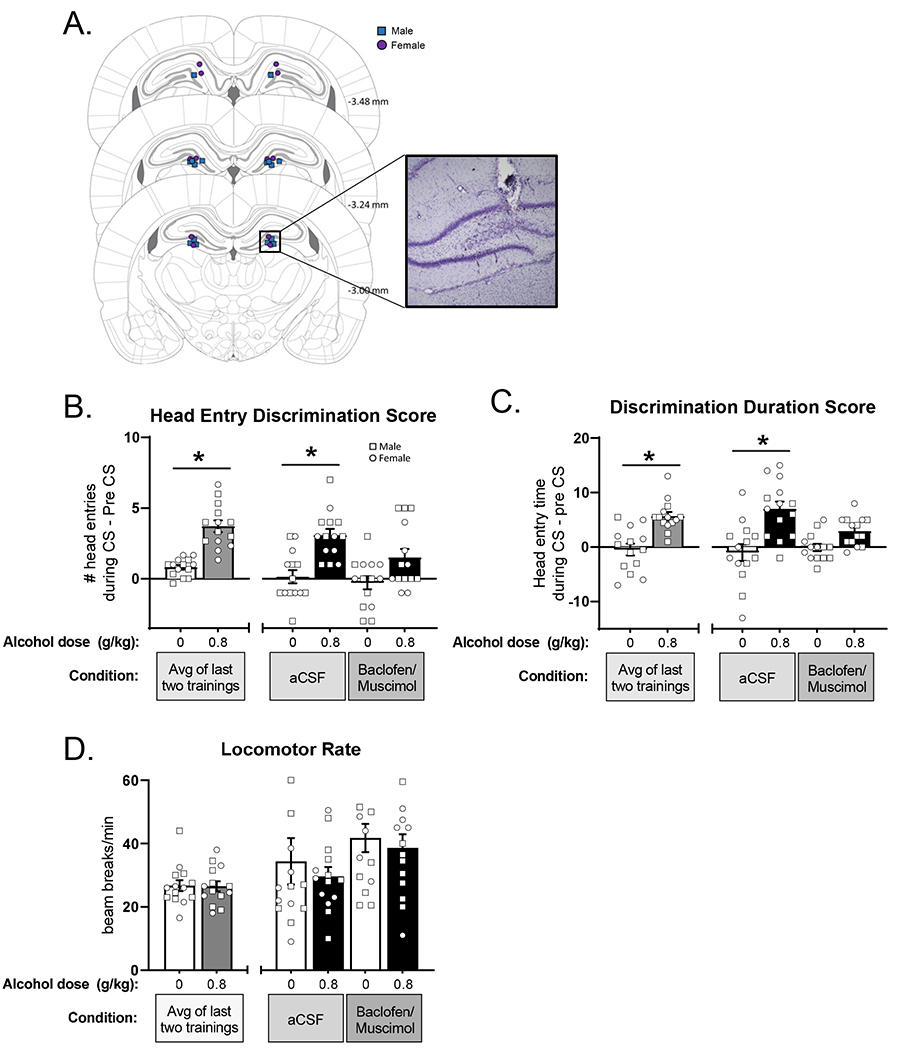
Pharmacological inhibition of the dentate gyrus disrupts sensitivity to the low-dose alcohol cue. (A) Atlas images of confirmed bilateral cannulae placements (squares – males, circles – females), and representative photomicrograph showing injector tract. Behavior was measured by the primary dependent variable (mean±SEM): head entry discrimination score (B) in addition to corresponding duration in seconds (C). Discrimination score following baclofen/muscimol infusion in DG did not differ between water and alcohol pretreatment showing that silencing DG blunted sensitivity to low-dose alcohol. There were no effects on locomotor rate (D). *p<0.05 alcohol different from water.
Figure 6B shows head entry discrimination score following inactivation of the dentate gyrus with baclofen/muscimol. Full substitution for the alcohol training dose following aCSF was confirmed (head entry discrimination score did not differ from baseline), indicating that the microinjection procedures did not affect discrimination performance. Analysis of head entry discrimination score following baclofen+muscimol showed a significant main effect of alcohol (F[1,13]=29.45, p<0.0001) and a trend for a main effect of baclofen/muscimol microinjection (F[1,13]=3.93, p=0.069; Figure 6B). Based on the a priori hypothesis that baclofen+muscimol would substitute for the effects of alcohol, planned comparisons were conducted between ethanol and water following aCSF and baclofen/muscimol. Following aCSF pretreatment, rats accurately discriminated between alcohol vs. water, as head entry discrimination scores were higher following alcohol (p<0.05). Interestingly, baclofen/muscimol pretreatment blunted the discrimination, as head entry discrimination scores did not differ between water and alcohol (p=0.20).
Analysis of discrimination duration score showed a main effect of alcohol (F[1,13]=36.70, p<0.0001) and a significant interaction (F[1,13]=9.13, p<0.01; Figure 6C). Post-hoc tests showed accurate discrimination following aCSF pretreatment, with significantly higher duration scores on alcohol vs. water (p<0.0001). However, following inhibition of the dentate by baclofen+muscimol, rats failed to discriminate between alcohol and water (p=0.104), demonstrating that inactivation of this region disrupted discrimination behavior, consistent with the head entry discrimination score. Additionally, analysis of total head entries (Supplementary Figure 1) found a significant main effect of alcohol with increased head entries on alcohol sessions (F[1,13]=9.04, p=0.01), but no effect of baclofen+muscimol. This finding suggests that the baclofen+muscimol reduction in discrimination was not related to a non-specific reduction in total head entries. Furthermore, no differences in locomotor rate (Figure 6D) were observed, also supporting the lack of nonspecific baclofen+muscimol effects.
Discussion
Understanding the neurobiological effects of low doses of alcohol is necessary in order to better understand the larger scope of alcohol-related effects on both the brain and resulting behavior. The current experiments had several important findings in assessing the behavioral and neurobiological effects of a dose of alcohol that produces a BAC level of <10 mM in male and female rats. First, we demonstrate that a dose of 0.8 g/kg (IG) alcohol is detectable by male and female Long Evans rats as the interoceptive effects of this dose can come to control goal-tracking behavior in a Pavlovian drug discrimination task. Next, we show that in rats trained to discriminate the effects of low-dose alcohol, this dose affects expression of immediate early genes c-Fos and Arc in several brain regions, including the dentate gyrus. Finally, we demonstrated a functional role for the dentate gyrus in modulating sensitivity to the low-dose alcohol interoceptive cue.
Behaviorally, these experiments have several important findings. First, they extend previous work to show that the interoceptive effects of low-dose alcohol can effectively guide reward-seeking behavior. There are other studies showing that rats can detect a dose of 0.5 g/kg in an operant discrimination 36, 37, and one study that utilizes low-dose alcohol as a Pavlovian cue 38 with a dose of 0.75 g/kg, very close to the current work. This is important as learning the two types of discriminations require different approaches. Standard operant drug discrimination requires an instrumental response such as a lever press regardless of the pretreatment (i.e., drug- or vehicle-appropriate lever selection). Pavlovian discrimination on the other hand, requires that the animal learn that only when the correct conditions are present, will they be rewarded and thus only approach the dipper receptacle under reinforced conditions. This distinction between the tasks is important as it demonstrates that a subtle interoceptive cue such as this low dose of alcohol, can gain a learned association with reward. Furthermore, rats training on this task learned the association with a high degree of specificity as shown in the cumulative testing curve (Figure 4) with discrimination score increasing as the dose approached the training dose and slightly decreasing upon reaching a dose higher than the training dose. This suggests that rats could detect the difference between the training dose and the higher dose and adjusted their behavior accordingly. Indeed, we have observed this effect previously when using this task 29, 30. Moreover, this work shows similar acquisition of the low-dose alcohol discrimination in males and females, suggesting similar sensitivity to the interoceptive effects of alcohol in this context. However, future work will need to assess a wider training dose range to determine if this is the case across multiple alcohol doses. Additionally, an advantage of the Pavlovian discrimination is that the opposite association can be trained, such that the drug interoceptive cue signals the absence of reward 29, 30, 39–41. Under these conditions rats decrease their goal-tracking behavior on drug sessions. It will be interesting for future work to determine if there are sex differences under such conditions.
Rats with an extensive conditioning history with low-dose alcohol showed significant increases in c-Fos and Arc IR in mPFC-IL and AcbSH following 0.8 g/kg alcohol. This is consistent with previous studies showing rats treated with 0.5 g/kg alcohol show increased dopamine release in Acb 42 and increased tonic firing rate of dopamine neurons in VTA 43, which have both likely related to alcohol effects on VTA 44. Moreover, endogenous opioid peptide tone in AcbC is also increased by low doses of alcohol 45. Furthermore, the current findings are consistent with human imaging studies showing that 10 mM alcohol alters glucose metabolism in both cortical and subcortical (basal ganglia) regions 46. In contrast, previous work from our lab has implicated mPFC-PL, AcbC, and insular cortex in modulating response to alcohol interoceptive cues 47, three brain regions that did not show increased c-Fos or Arc in the current studies. These differences may be explained by differences in dose (0.8 g/kg vs 1 g/kg). Indeed, a previous study assessing brain metabolic changes in response to low and moderate doses of alcohol found that while 0.25 and 0.5 g/kg doses increased metabolic rates across prefrontal cortex and striatum, at 1 g/kg this transitioned to general decreases in metabolic activity in these regions 48. Taken in the context of the current findings, this suggests that at lower doses of alcohol, different brain regions are recruited in modulating sensitivity to the interoceptive effects of alcohol as compared to higher doses. Indeed, this is consistent with work showing involvement of different receptor systems at low vs. high doses of alcohol 49–51. An important consideration, however, is that dentate gyrus was the only region surveyed that showed a decrease in c-Fos and Arc IR, which suggests that dentate gyrus and potentially hippocampus at large may play a unique role in processing alcohol-related cues. Indeed, Hodge and Cox (1998) showed that local infusion of the NMDA antagonist MK-801 into CA1 region of the hippocampus fully substitutes for alcohol (1 g/kg training dose) in an operant discrimination task. Moreover, Besheer et al (2008) showed that following the training dose of 2 g/kg alcohol, c-Fos IR in the dentate gyrus was significantly decreased in rats trained on an operant alcohol discrimination task relative to a behavior and drug-matched control group. This discrimination training-specific decrease in c-Fos IR in the dentate gyrus is consistent with the reductions in c-Fos in the present work as the present rats were trained on the Pavlovian discrimination.
An important consideration for the IEG findings presented here is that all animals had extensive behavioral training and alcohol administration, so it is not possible to disentangle the effects of training on alcohol-induced c-Fos and Arc response. Moreover, as discussed we have previously shown that an alcohol discrimination training history can impact alcohol-induced c-Fos expression 52. As one of the goals of the present study was to examine IEG expression specifically in animals that had interoceptive training with this alcohol dose, the pattern of IEG expression following this low dose alcohol, may be different in naïve animals or in animals that have not had the extensive training history. Therefore, it will be important for future work to include a drug- and behavior-matched control group in order to determine whether the alcohol-induced c-Fos and Arc IR expression patterns are the result of alcohol exposure, Pavlovian discrimination training, or both.
Finally, in exploring a potential mechanism for modulating sensitivity to the low-dose alcohol interoceptive cue, we demonstrated that inactivation of the dentate gyrus decreased sensitivity to the alcohol cue. We hypothesized that the reduced neuronal activity following low-dose alcohol administration was important for behavioral expression of accurate discrimination and thus that pharmacological inactivation of the dentate gyrus would substitute for the effect of alcohol in the absence of alcohol (i.e., when animals given water). This hypothesis was based on the reduction in c-Fos and Arc IR in the dentate gyrus after alcohol administration in Experiment 2 as well as the previously mentioned training-specific decrease in c-Fos expression in the dentate gyrus 52. Contrary to our hypothesis, pharmacological inactivation of the dentate gyrus disrupted discrimination as evidenced by a lack of difference between water and low-dose alcohol head entry discrimination and discrimination duration scores following baclofen+muscimol microinjection. It is important to consider that the discrimination scores are relative measures of behavior and therefore may not capture overall changes in the number of head entries. However, given that locomotor rate during the session was unaffected by baclofen+muscimol, a non-specific effect on general motor behavior is likely not a tenable explanation for the lack of discrimination behavior. Together, these findings suggest a dissociation between the results of the c-Fos study and the alcohol discrimination behavior. That is, despite reduced immediate early gene activity in the dentate gyrus after alcohol administration, activity in this brain region is important for the expression of the interoceptive effects of alcohol.
The dentate gyrus receives input from many sensory systems including the olfactory, visual, and somatosensory systems 53, 54 and is thought to be involved in conjunctive encoding of visual object and spatial information as well as contributing key information to short-term/working memory 27, 55, 56. For example, lesions of the dorsal dentate gyrus resulted in disruption of short-term spatial memory in a task where rats were required to remember and discriminate between spatial locations based on environmental cues 57. Further, lesions of the dentate gyrus have been shown to disrupt spatial-temporal associations between cues 58 and also disrupt context-dependent pattern separation, a form of discrimination task, based on both the geometry 59 and surface coloration of the environment 60. The dentate gyrus has also been demonstrated to have a critical role in discriminatory contextual fear conditioning where subjects only display fear under the condition of contextual cues related to the fear memory (for review see 61 ). There is some precedence for involvement of the hippocampus in drug discrimination as previous work has found that that microinfusion of nicotine into the dorsal hippocampus partially substitutes for peripherally administered nicotine 62, 63, showing that central nicotinic receptors in the hippocampus are important for the expression of the interoceptive effects of nicotine. In the context of the present work, it is possible that inactivation of the dentate gyrus in the current study prevented working memory from accessing, processing, and/or integrating information about interoceptive, spatial, or environmental cues necessary to perform successful discrimination. Therefore, the dentate gyrus may play an important role in the expression of the learned association that is under the control of the alcohol interoceptive cue and/or that sensitivity to the alcohol interoceptive cue is altered. Alternatively, inactivation of the dentate gyrus may have potentiated sensitivity to the interoceptive effects of low dose alcohol. That is, discrimination scores decrease at alcohol doses higher than the training dose as demonstrated in Figure 4A, B. Therefore, it is possible that following dentate gyrus inactivation behavior is on the descending limb of an alcohol dose response curve. This potential explanation would need to be evaluated directly by testing a complete alcohol dose curve. An additional consideration is that a reduction in c-Fos in this region may not necessarily indicate suppression of dentate gyrus projection neurons. It is possible that the inhibition induced by low dose alcohol is primarily occurring in the region’s large population GABAergic inhibitory interneurons 64, 65 resulting in disinhibition of afferent projections while the baclofen/muscimol microinjection is likely to be suppressing all types of neurons. A more cell-type specific approach would be needed to observe such nuances and determine if this is indeed the case.
Taken together, these findings show that a low dose of alcohol produces measureable effects in the brain and the interoceptive effects of this alcohol dose can be trained to control goal-tracking behavior. Future studies should further expand on these findings by comparing multiple alcohol doses and exploring the role of other implicated brain regions and circuits involved in modulating this behavior. These findings may have important implications for drug taking and seeking behaviors, and identification of critical pathways and brain regions involved in both low and higher doses of alcohol may be of therapeutic benefit in the future.
Supplementary Material
Acknowledgements:
This work was supported in part by the National Institute of Health AA025582 (JB and TLK) and by the Bowles Center for Alcohol Studies. DFL was supported by AA007573.
Footnotes
Data Availability Statement: Data will be made available upon request and following publication of this work.
References
- 1.White AM, Castle IP, Hingson RW, Powell PA. Using Death Certificates to Explore Changes in Alcohol-Related Mortality in the United States, 1999 to 2017. Alcohol Clin Exp Res 2020;44(1): 178–87. [DOI] [PubMed] [Google Scholar]
- 2.Cui C, Koob GF. Titrating Tipsy Targets: The Neurobiology of Low-Dose Alcohol. Trends Pharmacol Sci 2017;38(6): 556–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dry MJ, Burns NR, Nettelbeck T, Farquharson AL, White JM. Dose-related effects of alcohol on cognitive functioning. PLoS One 2012;7(11): e50977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moskowitz H, Murray JT. Decrease of iconic memory after alcohol. J Stud Alcohol 1976;37(3): 278–83. [DOI] [PubMed] [Google Scholar]
- 5.Schneider EW, Carpenter JA. The influence of ethanol on auditory signal detection. Q J Stud Alcohol 1969;30(2): 357–70. [PubMed] [Google Scholar]
- 6.LeCocq MR, Randall PA, Besheer J, Chaudhri N. Considering Drug-Associated Contexts in Substance Use Disorders and Treatment Development. Neurotherapeutics 2020;17(1): 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Besheer J, Fisher KR, Durant B. Assessment of the interoceptive effects of alcohol in rats using short-term training procedures. Alcohol 2012;46(8): 747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaramillo AA, Agan VE, Makhijani VH, Pedroza S, McElligott ZA, Besheer J. Functional role for suppression of the insular-striatal circuit in modulating interoceptive effects of alcohol. Addict Biol 2018;23(5): 1020–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart JL, May AC, Tapert SF, Paulus MP. Hyperactivation to pleasant interoceptive stimuli characterizes the transition to stimulant addiction. Drug Alcohol Depend 2015;154: 264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryabinin AE, Criado JR, Henriksen SJ, Bloom FE, Wilson MC. Differential sensitivity of c-Fos expression in hippocampus and other brain regions to moderate and low doses of alcohol. Mol Psychiatry 1997;2(1): 32–43. [DOI] [PubMed] [Google Scholar]
- 11.Thiele TE, van Dijk G, Bernstein IL. Ethanol-induced c-Fos expression in rat lines selected for low and high alcohol consumption. Brain Res 1997;756(1-2): 278–82. [DOI] [PubMed] [Google Scholar]
- 12.Vilpoux C, Warnault V, Pierrefiche O, Daoust M, Naassila M. Ethanol-sensitive brain regions in rat and mouse: a cartographic review, using immediate early gene expression. Alcohol Clin Exp Res 2009;33(6): 945–69. [DOI] [PubMed] [Google Scholar]
- 13.West RK, Maynard ME, Leasure JL. Binge ethanol effects on prefrontal cortex neurons, spatial working memory and task-induced neuronal activation in male and female rats. Physiol Behav 2018;188: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lukoyanov NV, Madeira MD, Paula-Barbosa MM. Behavioral and neuroanatomical consequences of chronic ethanol intake and withdrawal. Physiol Behav 1999;66(2): 337–46. [DOI] [PubMed] [Google Scholar]
- 15.Besheer J, Cox AA, Hodge CW. Coregulation of ethanol discrimination by the nucleus accumbens and amygdala. Alcohol Clin Exp Res 2003;27(3): 450–6. [DOI] [PubMed] [Google Scholar]
- 16.Besheer J, Grondin JJ, Salling MC, Spanos M, Stevenson RA, Hodge CW. Interoceptive effects of alcohol require mGlu5 receptor activity in the nucleus accumbens. J Neurosci 2009;29(30): 9582–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodge CW, Cox AA. The discriminative stimulus effects of ethanol are mediated by NMDA and GABA(A) receptors in specific limbic brain regions. Psychopharmacology (Berl) 1998;139(1-2): 95–107. [DOI] [PubMed] [Google Scholar]
- 18.Jaramillo AA, Randall PA, Frisbee S, Besheer J. Modulation of sensitivity to alcohol by cortical and thalamic brain regions. Eur J Neurosci 2016;44(8): 2569–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaramillo AA, Randall PA, Stewart S, Fortino B, Van Voorhies K, Besheer J. Functional role for cortical-striatal circuitry in modulating alcohol self-administration. Neuropharmacology 2018;130: 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol 2009;44(2): 115–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience 2006;137(2): 437–45. [DOI] [PubMed] [Google Scholar]
- 22.Jang MH, Shin MC, Jung SB, et al. Alcohol and nicotine reduce cell proliferation and enhance apoptosis in dentate gyrus. Neuroreport 2002;13(12): 1509–13. [DOI] [PubMed] [Google Scholar]
- 23.Morris SA, Eaves DW, Smith AR, Nixon K. Alcohol inhibition of neurogenesis: a mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus 2010;20(5): 596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances alpha 4 beta 3 delta and alpha 6 beta 3 delta gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci U S A 2003;100(25): 15218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABAA receptors in hippocampal neurons. J Neurosci 2004;24(38): 8379–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carta M, Ariwodola OJ, Weiner JL, Valenzuela CF. Alcohol potently inhibits the kainate receptor-dependent excitatory drive of hippocampal interneurons. Proc Natl Acad Sci U S A 2003;100(11): 6813–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kesner RP. An analysis of dentate gyrus function (an update). Behav Brain Res 2018;354: 84–91. [DOI] [PubMed] [Google Scholar]
- 28.Kirk RA, Redmon SN, Kesner RP. The ventral dentate gyrus mediates pattern separation for reward value. Behav Neurosci 2017;131(1): 42–45. [DOI] [PubMed] [Google Scholar]
- 29.Randall PA, Cannady R, Besheer J. The nicotine + alcohol interoceptive drug state: contribution of the components and effects of varenicline in rats. Psychopharmacology (Berl) 2016;233(15-16): 3061–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Randall PA, McElligott ZA, Besheer J. Role of mPFC and nucleus accumbens circuitry in modulation of a nicotine plus alcohol compound drug state. Addict Biol 2019: e12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaudhri N, Woods CA, Sahuque LL, Gill TM, Janak PH. Unilateral inactivation of the basolateral amygdala attenuates context-induced renewal of Pavlovian-conditioned alcohol-seeking. Eur J Neurosci 2013;38(5): 2751–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lasseter HC, Wells AM, Xie X, Fuchs RA. Interaction of the basolateral amygdala and orbitofrontal cortex is critical for drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology 2011;36(3): 711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Besheer J, Fisher KR, Jaramillo AA, Frisbee S, Cannady R. Stress hormone exposure reduces mGluR5 expression in the nucleus accumbens: functional implications for interoceptive sensitivity to alcohol. Neuropsychopharmacology 2014;39(10): 2376–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cannady R, Grondin JJ, Fisher KR, Hodge CW, Besheer J. Activation of group II metabotropic glutamate receptors inhibits the discriminative stimulus effects of alcohol via selective activity within the amygdala. Neuropsychopharmacology 2011;36(11): 2328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. Amsterdam ; Boston: ;: Academic Press/Elsevier; 2007. [Google Scholar]
- 36.McMillan DE, Li M, Shide DJ. Differences between alcohol-preferring and alcohol-nonpreferring rats in ethanol generalization. Pharmacol Biochem Behav 1999;64(2): 415–9. [DOI] [PubMed] [Google Scholar]
- 37.Stefanski R, Bienkowski P, Kostowski W. Studies on the role of 5-HT3 receptors in the mediation of the ethanol interoceptive cue. Eur J Pharmacol 1996;309(2): 141–7. [DOI] [PubMed] [Google Scholar]
- 38.Anderson RI, Spear LP. Age differences in ethanol discrimination: acquisition and ethanol dose generalization curves following multiple training conditions in adolescent and adult rats. Alcohol Clin Exp Res 2014;38(1): 186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bevins RA, Wilkinson JL, Palmatier MI, Siebert HL, Wiltgen SM. Characterization of nicotine’s ability to serve as a negative feature in a Pavlovian appetitive conditioning task in rats. Psychopharmacology (Berl) 2006;184(3-4): 470–81. [DOI] [PubMed] [Google Scholar]
- 40.Murray JE, Walker AW, Li C, Wells NR, Penrod RD, Bevins RA. Nicotine trained as a negative feature passes the retardation-of-acquisition and summation tests of a conditioned inhibitor. Learn Mem 2011;18(7): 452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reichel CM, Wilkinson JL, Bevins RA. Methamphetamine functions as a positive and negative drug feature in a Pavlovian appetitive discrimination task. Behav Pharmacol 2007;18(8): 755–65. [DOI] [PubMed] [Google Scholar]
- 42.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A 1988;85(14): 5274–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res 1985;348(1): 201–3. [DOI] [PubMed] [Google Scholar]
- 44.Morikawa H, Morrisett RA. Ethanol action on dopaminergic neurons in the ventral tegmental area: interaction with intrinsic ion channels and neurotransmitter inputs. Int Rev Neurobiol 2010;91: 235–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitchell JM, O’Neil JP, Janabi M, Marks SM, Jagust WJ, Fields HL. Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Sci Transl Med 2012;4(116): 116ra6. [DOI] [PubMed] [Google Scholar]
- 46.Volkow ND, Wang GJ, Franceschi D, et al. Low doses of alcohol substantially decrease glucose metabolism in the human brain. Neuroimage 2006;29(1): 295–301. [DOI] [PubMed] [Google Scholar]
- 47.Jaramillo AA, Van Voorhies K, Randall PA, Besheer J. Silencing the insular-striatal circuit decreases alcohol self-administration and increases sensitivity to alcohol. Behav Brain Res 2018;348: 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams-Hemby L, Porrino LJ. Low and moderate doses of ethanol produce distinct patterns of cerebral metabolic changes in rats. Alcohol Clin Exp Res 1994;18(4): 982–8. [DOI] [PubMed] [Google Scholar]
- 49.Grant KA, Colombo G. Discriminative stimulus effects of ethanol: effect of training dose on the substitution of N-methyl-D-aspartate antagonists. J Pharmacol Exp Ther 1993;264(3): 1241–7. [PubMed] [Google Scholar]
- 50.Grant KA, Colombo G. Substitution of the 5-HT1 agonist trifluoromethylphenylpiperazine (TFMPP) for the discriminative stimulus effects of ethanol: effect of training dose. Psychopharmacology (Berl) 1993;113(1): 26–30. [DOI] [PubMed] [Google Scholar]
- 51.Stolerman IP, Childs E, Ford MM, Grant KA. Role of training dose in drug discrimination: a review. Behav Pharmacol 2011;22(5–6): 415–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Besheer J, Schroeder JP, Stevenson RA, Hodge CW. Ethanol-induced alterations of c-Fos immunoreactivity in specific limbic brain regions following ethanol discrimination training. Brain Res 2008;1232: 124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature 2005;436(7052): 801–6. [DOI] [PubMed] [Google Scholar]
- 54.Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Prog Neurobiol 2006;79(1): 1–48. [DOI] [PubMed] [Google Scholar]
- 55.Kesner RP. A behavioral analysis of dentate gyrus function. Prog Brain Res 2007;163: 567–76. [DOI] [PubMed] [Google Scholar]
- 56.Kesner RP. An analysis of the dentate gyrus function. Behav Brain Res 2013;254: 1–7. [DOI] [PubMed] [Google Scholar]
- 57.Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus 2001;11(6): 626–36. [DOI] [PubMed] [Google Scholar]
- 58.Morris AM, Curtis BJ, Churchwell JC, Maasberg DW, Kesner RP. Temporal associations for spatial events: the role of the dentate gyrus. Behav Brain Res 2013;256: 250–6. [DOI] [PubMed] [Google Scholar]
- 59.Hunsaker MR, Rosenberg JS, Kesner RP. The role of the dentate gyrus, CA3a,b, and CA3c for detecting spatial and environmental novelty. Hippocampus 2008;18(10): 1064–73. [DOI] [PubMed] [Google Scholar]
- 60.Kesner RP, Kirk RA, Yu Z, Polansky C, Musso ND. Dentate gyrus supports slope recognition memory, shades of grey-context pattern separation and recognition memory, and CA3 supports pattern completion for object memory. Neurobiol Learn Mem 2016;129: 29–37. [DOI] [PubMed] [Google Scholar]
- 61.Hainmueller T, Bartos M. Dentate gyrus circuits for encoding, retrieval and discrimination of episodic memories. Nat Rev Neurosci 2020;21(3): 153–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosecrans JA, Meltzer LT. Central sites and mechanisms of action of nicotine. Neurosci Biobehav Rev 1981;5(4): 497–501. [DOI] [PubMed] [Google Scholar]
- 63.Shoaib M, Stolerman IP. Brain sites mediating the discriminative stimulus effects of nicotine in rats. Behav Brain Res 1996;78(2): 183–8. [DOI] [PubMed] [Google Scholar]
- 64.Buzsaki G, Eidelberg E. Commissural projection to the dentate gyrus of the rat: evidence for feed-forward inhibition. Brain Res 1981;230(1-2): 346–50. [DOI] [PubMed] [Google Scholar]
- 65.Elgueta C, Bartos M. Dendritic inhibition differentially regulates excitability of dentate gyrus parvalbumin-expressing interneurons and granule cells. Nat Commun 2019;10(1): 5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


