Abstract
Introduction:
Absence epilepsy is a brief non-convulsive seizure associated with sudden abruptness in consciousness. Because of the unpredictable occurrence of absence seizures and the ethical issues of human investigation on the pathogenesis and drug assessment, researchers tend to study animal models. This paper aims to review the advantages and disadvantages of several animal models of nonconvulsive induced seizure.
Methods:
The articles that were published since 1990 were assessed. The publications that used genetic animals were analyzed, too. Besides, we reviewed possible application methods of each model, clinical types of seizures induced, purposed mechanism of epileptogenesis, their validity, and relevance to the absence epileptic patients.
Results:
The number of studies that used genetic models of absence epilepsy from years of 2000 was noticeably more than pharmacological models. Genetic animal models have a close correlation of electroencephalogram features and epileptic behaviors to the human condition.
Conclusion:
The validity of genetic models of absence epilepsy would motivate the researchers to focus on genetic modes in their studies. As there are some differences in the pathophysiology of absence epilepsy between animal models and humans, the development of new animal models is necessary to understand better the epileptogenic process and, or discover novel therapies for this disorder.
Keywords: Epilepsy, Absence, Animal models, Seizures, Genetic models
Highlights
There are several animal models of absence epilepsy.
It seems that genetic models are more valid than chemical models.
WAG/Rij rats as one of genetic model of absence epilepsy have most similarity to human absence epilepsy.
Plain Language Summary
We studied arious laboratory models of absent epilepsy. Typical absence seizures are defined behaviorally by a paroxysmal loss of consciousness without aura or postictal mood and accompanied with bilateral synchronous Spike and Wave Discharges (SWDs). This study helps young students and researchers to select a suitable model for their research by reviewing and validating the models that we have discussed in this article.
1. Introduction
Absence epilepsy, as a childhood disease, is characterized by generalized epileptic activities in both hemispheres of the brain and accompanied by unconsciousness. Absence epilepsy is a nonconvulsive seizure and can be classified into typical and atypical forms ( Berg et al., 2010; Manning, Richards, & Bowery, 2003; Snead, 1995 ). Typical absence seizures are defined behaviorally by a paroxysmal loss of consciousness without aura or postictal mood and accompanied with bilateral synchronous spike and wave discharges (SWDs) about 3 Hz in the electroencephalogram (EEG). Usually, the duration of the seizure persists for 3–10 seconds (Figure 1). Misinterpretation with day-dreaming happens in such episodes because they are associated with a fixed and gaze, especially in children ( Manning et al., 2003).
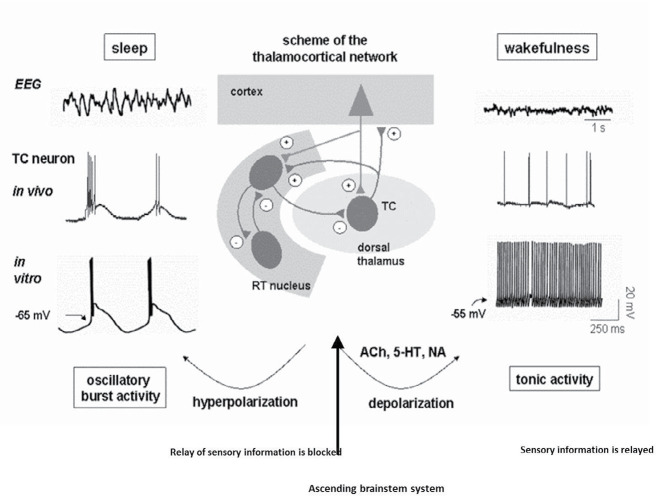
Figure 1.
The cellular and network activity of thalamocortical circuit during an absence seizure
During periods of absence seizure activity (like sleep or drowsiness), the neurons are hyperpolarized and show rhythmic burst firing, which spreads towards the reticular nucleus and the cortex, then the system starts to oscillate in synchrony, which is visible on a large scale EEG as high amplitude and low frequency (3–4 Hz). Under these conditions, the system is incapable of receiving information from the periphery. In wakefulness state, relay neurons receive sensory inputs from the environment and the brainstem (locus seroluos, raphe magnus, the reticular nucleus of the pons and hypothalamus nuclei), then project them into 3–5 layers of the somatosensory cortex. After processing, it sets a series of nerve branches from layer 6 neurons of the cortex to relay neurons. Thalamocortical and corticothalamic neurons via excitatory axon collaterals evoke the reticular nucleus of the thalamus. Activation of these neurons leads to thalamic neurons depolarization and showing tonic single-spike activity. In this state, the conscious perception of our environment appears ( Crunelli & Leresche, 2002; Sejnowski, McCormick, & Steriade, 1998; Steriade, 2005)
Atypical absence seizures are described with complex orofacial automatisms, abnormal neurodevelopmental outcomes, and cognitive impairment in patients. Such atypical episodes are more frequent and lasted more than typical seizure duration. SWDs frequency is less than 3 Hz. Seizure onset and offset are gradual, and there is a minor correlation between the EEG and behavioral changes. For instance, during seizure episodes children might be able to walk or talk ( Manning et al., 2003).
The incidence of absence epilepsy in children up to the age of sixteen years is about 2 to 8 out of every 100000 children and with a prevalence of 10% of children with any form of epilepsy ( Crunelli & Leresche, 2002). Since the precise etiology of absence epilepsy is unknown and multiple mechanisms are involved in the pathophysiology ( Karimzadeh et al., 2017), hopefully, most of our knowledge about mechanisms and treatment of human absence epilepsy arise from the use of appropriate chemical and genetic animal models. This study aimed to review the pathophysiology of absence epilepsy as well as different kinds of experimental models. Besides, the validity and similarity of different absence epilepsy models have been illustrated.
1.1. Search strategy
We searched for the relevant publications in PubMed, Wiley, and Google scholar. The keywords of “absence epilepsy”, “absence seizure”, “animal model”, “pharmacological animal models”, “chemical models”, and “genetic models” were used in the title and abstract of articles.
The articles were searched from 1990 to 2018. To explore most researchers’ focus on the genetic models, the number of publications that used genetic animals were analyzed, too.
1.2. Statistical analysis
The number of publications was analyzed by 1-way Analysis of Variance (ANOVA) followed by Tukey post hoc test in SPSS V. 20. The significance level was established by P≤0.05.
1.3. Pathogenesis of absence epilepsy
Studies reveal that absence epilepsy arises from an aberration of the interplay between some areas of the cortex, especially the somatosensory cortex and the thalamus. Investigations in human and animal models identified hyper-synchronization in the thalamocortical circuit that generates SWDs in absence seizure ( Avoli, Gloor, Kostopoulos, & Gotman, 1983). In typical absence seizures, the main components of the thalamocortical circuit contribute to the creating SWDs involving the reticular and ventrobasal (medial and lateral) nuclei of the thalamus and somatosensory perioral region of the somatosensory cortex ( Meeren, van Luijtelaar, da Silva, & Coenen, 2005). Besides, the channelopathy and imbalance of excitatory and inhibitory receptors in the other thalamocortical neurons such as intrathalamic and dorsal thalamic nuclei are involved in SWDs generation ( Karimzadeh et al., 2017). The atypical absence seizure involves both the thalamocortical circuit and the limbic system ( Cortez, McKerlie, & Snead, 2001).
Absence epilepsy may be related to several malfunctions in the nervous system. Hyperexcitability of the cortex and hyperfunction of the GABAergic tonus in the thalamus trigger hyperactivity of low-threshold T-type calcium currents activated by hyperpolarization in the thalamus. Naturally, there are reciprocal excitatory glutamatergic and inhibitory GABAergic projections between the cortex and thalamus. These neurons can produce both tonics and burst firings in different conditions which are specialized for the flow of information and sleep spindles at the network, but hyperactivation of the GABAergic neurons in the reticular nucleus of the thalamus leads to a burst of synchronized firing and causing inhibitory postsynaptic potentiation in thalamocortical neurons (Figure 1). Gamma-aminobutyric acid (GABA) induces hyperpolarization, especially through GABAB receptors in the specific relay nuclei and the reticular nuclei of the thalamus and then hyperactivation of the low-voltage-activated T-type calcium currents that create a rebound burst of fast spikes leading to the next cycle of the oscillation ( Budde, Pape, Kumar, & Huguenard, 2006; Meeren, Pijn, Van Luijtelaar, Coenen, & da Silva, 2002; Polack, Guillemain, Deransart, Depaulis, & Charpier, 2007).
1.4. Animal models of absence epilepsy
The validity of animal models in the experimental studies is identified by three characters of reproducibility, predictability, and quantifiability. There are several animal models for absence epilepsy such as electrical stimulation, chemical injection, and genetic models of Drosophila, mice, rats, baboons, and cats. They should be comparable behaviorally, electroencephalographic, ontogenetic, etiologic, and pharmacologically with human absence epileptic disease. These models must indicate SWDs in the EEG as the hallmark of absence epilepsy in humans, but there are some differences in some clinical criteria in the animal models that are presented in Table 1. Therefore, researchers should select the models based on the aim of the study considering the limitation of each model ( Fisher, 1989; Manning et al., 2003; Pitkänen, Schwartzkroin, & Moshé, 2005 ).
Table 1.
Characteristics of experimental animal models for absence seizures
| Most Important Feature | Pharmacologic Animal Models | Genetic Animal Models |
|---|---|---|
| EEG and behavior similar to the human condition | LD-PTZ, GHB, THIP, AY-9944, MAM-AY | GAERS, WAG/Rij, Lethargic, slow-wave-epilepsy mouse, tottering mouse |
| Reproducibility and predictability | LD-PTZ, GHB, THIP, AY-9944, MAM-AY | GAERS, WAG/Rij, lethargic |
| Quantifiable | LD-PTZ, GHB, THIP, AY-9944, MAM-AY | GAERS, WAG/Rij, lethargic |
| Appropriate pharmacology | LD-PTZ, GHB, PLC, AY9944 | GAERS, WAG/Rij, Lethargic, slow-wave epilepsy, tottering |
| Unique developmental profile | LD-PTZ, GHB, AY9944 | GAERS, WAG/Rij, lethargic |
| Exacerbated by GABAergic drugs | LD-PTZ, GHB, AY9944, MAM-AY | GAERS, WAG/Rij, lethargic |
| Involvement of thalamocortical mechanisms | LD-PTZ, GHB, PLC, AY9944, MAM-AY | GAERS, WAG/Rij, lethargic |
| Blocked by GABAB receptor antagonists | LD-PTZ, GHB, AY9944, MAM-AY | GAERS, WAG/Rij, |
1.5. Pharmacological animal models of absence epilepsy
Several pharmacological compounds induce acute or chronic absence seizures (Table 2).
Table 2.
Special features of each model
| Models | Human Seizure Type | Pharmacologic Response | Appropriate Study | |||
|---|---|---|---|---|---|---|
| Ethosuximide | Valproic Acid | |||||
| Acute | PCL | TAS | + | + | Pathogenesis | |
| LD-PTZ | + | + | ||||
| GHB | + | + | ||||
| THIP | + | - | ||||
| Chronic acquire | AM9944 | CAAS | + | + | Pathogenesis & treatment | |
| MAM-AY | CRAAS | + | - | |||
| Genetic animal models (Chronic models) | Mutant mice | Leaner | TAS | + | + | Pathogenesis |
| Lethargic | + | ND | ||||
| Stargazer | + | ND | ||||
| Mocha | + | ND | ||||
| Slow-wave epilepsy | + | ND | ||||
| Ducky | + | ND | ||||
| Inbreed rats | + | ND | Pathogenesis & treatment | |||
| WAG/Rij | + | + | ||||
| GAERS | + | + | ||||
ND: Not significant; CAAS: Chronic Atypical Absence Seizure; CRAAS: Chronic Refractory Atypical Absence Seizures; TAS: Typical Absence Seizures.
Penicillin, low-dose pentylenetetrazole (L-PTZ), 4,5,6,7 tetrahydroisoxazolo (4,5,-c) pyridine-3-ol (THIP), and gamma-hydroxybutyrate (GHB) are the most common compounds for the induction of typical absence epilepsy. Also, AY-9944 (trans-1, 4-bis[2-chloro-benzylamino-ethyl] cyclohexane dihydrochloride) and methylazoxymethanol acetate (MAM)-AY-9944 are administered for the induction of atypical absence seizures (Tang, & Loke, 2011).
1.6. Chemical models of typical absence epilepsy
1.6.1. Penicillin
Local administration of some antibiotics such as cephalosporin and penicillin onto the cortex or systemically (250000–6000000 U/kg) at high doses can induce a seizure in cats and rodents. Penicillin acts as a GABA agonist (Deyn, Hooge, Marescau, & Pei, 1992; Tang & Loke, 2011) and has limited effects in rodents rather than cats. Because of the unstable penetration of penicillin through the blood-brain barrier, it produces multifocal spikes with only occasional bursts of bilaterally synchronous SWDs associated with the attention deficit. In other words, seizures initiated through parenteral penicillin have minor similarities to clinical absence seizures in rats.
1.6.2. Low-dose pentylenetetrazole
PTZ, a tetrazole derivative, is the most commonly used epileptic induced agent for systemic convulsive epilepsy. PTZ is a GABAA receptor antagonist and interacts with GABAA receptors at the picrotoxin binding site. The extensive use of PTZ in different types of epilepsy has made this model an identifier of anticonvulsants and proconvulsants. The low dose of PTZ (20–30 mg/kg) injected intraperitoneally (IP) or subcutaneously produces freezing and is used to induce absence-like seizures from 3 weeks of age. L-PTZ produces twitching of vibrissae at the frequency of 6–7 Hz SWD on the EEG in rodents. Generally, seizure initiates 2–10 min after injection and depends on the dose and species. SWDs duration is about 2–3 s in rodents (Deyn et al., 1992; MacDonald & Barker, 1977; Tang & Loke, 2011).
1.6.3. GHB and g-butyrolactone (GBL) models
GHB is a GABA metabolic substance that is produced naturally in the mammalian brain. Endogenous GHB exists in low concentration in different areas of the nervous system. Auto-radiographic studies have shown the presence of GHB receptors in the cortex, thalamus, striatum, hypothalamus, substantia nigra, and hippocampus. The affinity of GHB for GABAB receptors has been shown, especially in the thalamocortical network. Following the IP administration of GHB, electrographic and behavioral events occur similar to generalized absence seizures in cats, rats, and monkeys (Deyn et al., 1992; Pitkänen et al., 2005). The intravenous administration of GHB (200 mg/kg) induces the absence epileptic attacks in the same frequency of human SWDs (2.5 Hz) and clinical symptoms such as head drops, behavioral immobility, pupillary dilation, staring, rhythmic eye movements, and stereotypical automatisms. This model is considered a valid model because it is predictable, reproducible, and produces electrographic and behavioral symptoms similar to the human absence epilepsy condition. Besides, as the kinetics of the GHB is detected well then it is a good model for the study of some drugs with unknown pharmacokinetic features ( Cortez, & Snead, 2006).
Furthermore, GBL as a pro-drug of GHB increases the reproducibility and predictability of the GHB model of absence seizures. GBL is used more because of the consistency and rapidity of onset of its effects (Bearden, Snead, Healy, & Pegram, 1980) and has been shown to produce the same EEG and behavioral effect as that of GHB. A single dose application of GBL generates absence seizures through the activation of the GHB-related neuromodulation or interaction with GABAergic inhibitory and excitatory neurotransmission in the cortico-thalamo-cortical circuit (Bearden et al., 1980; Pitkänen et al., 2005).
Administration of GHB (240 mM) changes the rhythmicity of EEG in the GHB models. The peak components of GBL rise 1 minute after GBL injection and fell rapidly to undetectable levels within 5 minutes. GBL-induced SWDs can be quantitated as cumulative duration (in seconds) per 20-min cycle or as a percentage of control SWDs duration. This pharmacological rat model is an appropriate experimental model for the study of the mechanisms of bilaterally synchronous SWDs production and screen for the anti-seizure activity of antiepileptic drugs in generalized absence seizures ( Depaulis, Snead, Marescaux, & Vergnes, 1989; Karamahmutoğlu et al., 2013; Tang & Loke, 2011).
1.7. 4,5,6,7 Tetrahydroisoxazolo (4,5,-c) pyridine-3-ol (THIP)
THIP, as a GABA agonist, induces bilaterally synchronous SWDs in rats. THIP is a selective GABA agonist for the extrasynaptic delta-subunits of GABAA receptors. Delta-subunits of GABAA receptors were abundantly found in the thalamus and neocortex. The optimum dose of THIP to induce bilaterally synchronous SWDs is 5–10 mg/kg and lasted 7 to 9 s. The dose of THIP to elicit SWDs is 7.5 mg/kg (IP). The model is quantified similarly to the GHB model ( Depaulis et al., 1989; Fariello & Golden, 1987; Fisher, 1989; Pitkänen et al., 2005).
1.8. Chemical Models of atypical absence epilepsy
1.8.1. AY-9944
AY-9944 is a common compound for induction of atypical absence seizure. Subcutaneous administration of AY-9944 (7.5 mg/kg) inhibits the reduction of 7-dehydrocholesterol to cholesterol and leads to an abnormal cognitive outcome. It causes seizure onset in the prepubescent and maximum peak in the adult periods. Anti-absence drugs decrease these seizures and phenytoin and GABA agonist drugs exacerbate them. The AY-treated rat can be used to investigate the pathogenesis and treatment of this malignant disorder ( Chan et al., 2004; Cortez, Cunnane, & Snead, 2002; Cortez et al., 2001).
In some atypical absence epilepsy studies, antimitotic agent MAM with AY-9944 is used to produce seizures. IN the MAM-AY model (double-hit model), rats display bilaterally synchronous SWDs with a frequency of 4 to 6 Hz. The MM-AY-induced atypical absence seizures; however, ethosuximide, as well as sodium valproate, suppress the induced seizures. The histopathologic assessment showed the MAM-treated rat brains have hippocampal heterotopias, atrophy, and abnormalities of cortical lamination. The MAM-AY-treated rat is a reproducible model for studying refractory atypical absence seizures in children with brain maldevelopment (Pitkänen et al., 2005; Serbanescu, Cortez, McKerlie, & Snead, 2004).
1.8.2. Genetic models of absence epilepsy
During recent decades, it has been revealed that genetic factors play a critical role in idiopathic generalized epilepsies, including absence epilepsy. Some evidence emphasizes the role of genes in the pathogenesis of Childhood Absence Epilepsy (CAE). It has been reported that monozygotic twins compared with dizygotic twins suffered more from CAE ( Berkovic, Howell, Hay, & Hopper, 1994; Marini et al., 2003). Although there are several types of chemical animal models for CAE, genetic models are more valid for research. Spontaneous absence seizures and reproduced symptoms in the genetic models could be considered as the most important advantages compared to chemical models and they are reliable to clarify the pathophysiology of the human condition.
Some genetic models of rats and mutant mice are currently used in most experiments. Six mutant mice are suitable models for absence epilepsy, including lethargic, tottering, mocha, stargazer, slow-wave-epilepsy, and ducky.
1.8.3. Genetic mutant mouse models
Spontaneous mutations in mice associated with other epilepsy models and generated by genetic engineering approaches are critical models to comprehend the pathogenesis of human epilepsies. They were considered as a reproducible biological model for experimental strategies to prevent or reverse the onset of seizures. Spontaneous SWDs have also been observed in mutant mousses, which is usually accompanied by other neurological disorders (ataxia). A Mendelian basis underlies epileptic phenotype in most of the mice models. Since epileptic mutant mice is a favored model in neurobiology and neuropharmacology for the study of abnormal brain synchronization, it has a pioneering role in the neurogenic analysis of seizure disorders. These models were a key role to figure out the significance of single genes in the hereditary transmission of epilepsy in the mammalian nervous system. Yet there are several absence epileptic mutants mouse (tottering, lethargic, stargazer, mocha, slow-wave-epilepsy, and ducky), but their number are restricted by the rate of mutations and feasible dysfunctions ( Crunelli & Leresche, 2002; Pitkänen et al., 2005).
1.8.4. Tottering mouse
The phenotype of tottering mice includes motor seizures and ataxia ( Tokuda et al., 2007). There were no abnormalities in their EEG recordings associated with motor seizures ( Tokuda et al., 2007). The mutation in these mice is the tottering locus on chromosome 8 ( Campbell & Hess, 1996). This chromosomal mutation creates an abnormal α1A subunit of voltage-gated Ca2+ channel. This subunit forms the core of P/Q Ca2+ channels and this malformation decreases the Ca2+ current ( Bourinet et al., 1999). It has been believed that a decrease in the Ca2+ current in Purkinje cells has been involved in the pathophysiology of ataxia ( Rhyu, Abbott, Walker, & Sotelo, 1999). Tottering mice are the best model for ataxia.
The manifestation of tottering seizures (behavioral arrest, assuming a fixed staring posture) is the same as CAE in humans. The frequency of SWDs in these mice is higher (6–7 Hz) than humans and absence seizures are suppressed by anti-absence drugs ( Heller, Dichter, & Sidman, 1983; Kaplan, Seyfried, & Glaser, 1979).
1.8.5. Lethargic mouse
The lethargic mouse has a spontaneous mutation on chromosome 2 in the gene encoding of the β4 subunit of voltage-gated calcium channels ( Frankel, 1999). This subunit is an auxiliary part of voltage-gated calcium channels that regulate Ca2+ influx ( Burgess, Jones, Meisler, & Noebels, 1997). The phenotype of a lethargic mouse appears after 15 days of age by ataxia and lethargic behavior accompanied by focal motor seizures. Generalized cortical SWDs are the second seizure type which is recorded in their EEG ( Pinault et al., 1998). The characteristic of their absence seizures is the same as human CAE and tottering absence seizures ( Pinault et al., 1998).
There are no structural and pathological changes in the brain and spinal cord but some immunological discrepancies such as splenic and thymic degeneration and lymphocytopenia are seen ( Dung, 1977). Interestingly, after two months of age, their immune function and loss of body weight would be recovered but their fertility is reduced ( Devanagondi, Egami, LeDoux, Hess, & Jinnah, 2007).
1.8.6. Stargazer mouse
This mutant was named stargazer because of mutation in stargazing protein (γ2 subunit of T-type or Cav3.1 calcium channel). A single mutation occurs in the gene encoding stargazing on mouse chromosome 15 ( Letts et al., 1997; Noebels, Qiao, Bronson, Spencer, & Davisson, 1990). This protein has a modulatory role in voltage-gated calcium channels ( Burgess & Noebels, 1999). Also, γ2 subunit is necessary for AMPA (a-amino-3-hydroxy5-methyl-4-isoxazole propionic acid) receptors for synaptic targeting in the ionotropic glutamate receptors ( Chen et al., 2000).
An increase in calcium currents in thalamic relay neurons may have a crucial role in the absence seizure initiation in stargazer mice (Snead, 1995). The phenotype of stargazer is similar to the other mutant mice that are ataxia, paroxysmal dyskinesia, absence seizure, and head tossing ( Sharp et al., 2001).
1.8.7. Mocha mouse
The characteristic of absence seizures in mocha is the same as a stargazer mouse. The frequency of SWDs is 6–7 Hz and phenotype is accompanied by ataxia, tonic-clonic seizures, pigment dilution, and increased bleeding time ( Sarkisian, 2001). The gene encoding the AP-3δ protein on chromosome 10 has been mutated in these mice ( Noebels, 2003). The AP-3 complex is a heterodimer that regulates lysosome trafficking and other related organelles ( Di Pietro et al., 2006; Kantheti et al., 1998).
1.8.8. Slow-wave epilepsy mouse
SWDs in these mutants appear with a frequency of 3 to 4.5 Hz during absence seizures ( Cox et al., 1997). It has been considered as a valid model for absence epilepsy because of seizure phenotype and anti-absence-drug responses ( Blumenfeld, 2005). The main mutation underlying SWDs generation in the spike-wave epilepsy mice has been occurred in the gene encoding the ubiquitous sodium hydrogen exchanger on chromosome 4 ( Papale et al., 2009). This exchanger regulates the hemostasis, cell volume, and mitogenic responses to growth factors.
These mutants have some extra phenotype as the same as the other mice models for absence epilepsy. Ataxia, tonic-clonic seizures, and cerebellum degeneration are some phenotypes that appear in these mutants in addition to absence seizures ( Qiao & Noebels, 1993).
1.8.9. Ducky mouse
The frequency of SWDs in ducky mice like most of the mice model of absence epilepsy is 6 Hz ( Barclay et al., 2001). Ataxia, limb dyskinesia, and developmental dysgenesis of some brain areas like the cerebellum, medulla, and spinal cord are some additional phenotypes that exist in these mice. The gene encoding α2 and a δ2 subunit of high-voltage activated Ca2+ channels on chromosome 9 have been mutated ( Porter et al., 1993). These channels are extremely permeable to Ca2+ in excitable and non-excitable cells and have a critical role in the absence seizures genesis ( Zhang, Mori, Burgess, & Noebels, 2002).
1.8.10. Genetic rat models of absence epilepsy
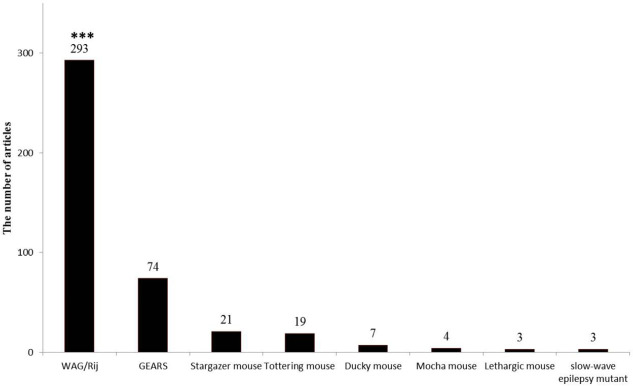
In the mutant mice models, generally, a single gene underlies the epileptic phenotype (SWDs); however, in humans, a polygenetic mode of inheritance occurs. So it seems that polygenic rat models are more valid in comparison to other models ( Sarkisova & van Luijtelaar, 2011). Genetic absence epileptic rats from Strasbourg (GAERS) and WAG/Rij rats represented spontaneous absence seizure attacks for the first time about 25 years ago. It is believed that a single gene might be responsible for causing absence seizure attacks ( Rudolf et al., 2004) but different genes are involved in the characteristics of absence seizures and SWDs ( Rudolf et al., 2004) . Different studies with various approaches revealed that these two rats have a similar feature to human absence epilepsy. Statistical data showed fabulous information about absence epilepsy that was published with the use of these two rats (more than 500 articles; Figure 2).
Figure 2.
The number of related articles to each genetic models of absence epilepsy since 2000
The bar graphs show the high number of articles that were focused on the WAG/Rij rats to study absence epilepsy; *** Indicates P<0.001.
1.8.11. Genetic Absence Epilepsy Rat From Strasbourg (GAERS) rats
In France (Strasbourg), a fully inbred strain of rats was derived from an outbred Wistar colony. It is notable that, 100% of animals show the EEG and behavioral characteristics of absence seizures in three-month-old rats. However, the first detectable SWDs appear around 30 days of age. In the first seizures, the SWDs appear rarely and in short duration (1–3 s). During a lifetime, the severity of seizures (duration and frequency) increases gradually. The most SWDs occur at around 4 to 6 months old. There are not any differences in the characteristics of SWDs between males and females, that represent autosomal genetic transmission ( Coenen & Van Luijtelaar, 2003; Crunelli & Leresche, 2002).
The frequency of SWDs in these rats is 7–10 Hz ( Seidenbecher & Pape, 2001). The behavioral manifestation is the same as a typical absence seizure such as loss of cautiousness and some chewing motion during the seizure. No pathological and structural abnormalities have been reported in these rats ( Avanzini, Vergnes, Spreafico, & Marescaux, 1993). Anti-absence drugs can suppress seizure attacks. The most important difference between absence seizures in GEARS and human is the higher frequency of SWDs ( Avanzini et al., 1993).
It is believed that polygenetic mutation is involved in the absence seizures genesis in this rat model ( Rudolf et al., 2004). A polygenic mutation on the chromosomes of 4, 7, and 8 seems that regulate the SWDs initiation in the GAERS ( Rudolf et al., 2004). Single nucleotide mutation in the gene encoding T-type Ca2+ channel has occurred in GAERS. Future studies are necessary to determine the exact chromosomal mutations ( Powell et al., 2009).
1.8.12. WAG/Rij rats
The WAG/Rij strain is a subline of the WAG strain that was derived from Wistar supply by A. L. Bacharach at the Glaxo Laboratories in London (UK) in 1924. In other words, WAG/Rij is an inbred strain in which brother-sister mating has been carried for more than 130 generations. The behavioral study showed that WAG/Rij rats have a short latency to move out from the home cage into familiar and new environments ( Coenen & Van Luijtelaar, 2003; Sarkisova & van Luijtelaar, 2011). Many similarities in the cognitive deficits, as well as behavioral disturbances, have been shown in the WAG/Rij rats and CAE patients ( Karson, Utkan, Balcı, Arıcıoğlu, & Ateş, 2012).
SWDs are emerged at around 60 to 80 days age in WAG/Rij ( Seidenbecher & Pape, 2001). At the age of 3 months, half of the WAG/Rij display fully developed SWDs, and at 6 months of age all of the animals show SWDs and it occurs alternatively until the death of the animals (Pitkänen et al., 2005). SWDs in the WAG/Rij rats are very age-dependent. In 6-month-old rats, the number of SWDs is about 16–18 discharges per hour.
Sex differences are very little ( Coenen & Van Luijtelaar, 1987). Generally, burst activities last 1–30 s with a spike-wave frequency of 7–11 Hz in the cortical EEG of adult WAG/Rij rats ( Sarkisova & van Luijtelaar, 2011). The behavioral manifestation of seizures (facial myoclonic jerks, eye twitching, and head tilting) is the same as GAERS rats ( Sitnikova & Van Luijtelaar, 2007). It is noticeable that besides the high frequency of SWDs, the age of SWDs initiation is different from humans. The pharmacological responses to anti-absence drugs in these rats are the same as humans ( Karimzadeh et al., 2013). A polygenetic mutation (chromosome 5 and 9) control the SWDs in these rats ( Gauguier et al., 2004).
It seems that channelopathy of slow and fast Ca2+ channels has a critical role in the SWDs generation of WAG/Rij rats as well as the other genetic animal models ( Gorji, Mittag, Shahabi, Seidenbecher, & Pape, 2011). Besides, some cognitive deficits, as well as pathological changes (neuronal injury and cell death) in several brain areas (hippocampus and neocortex) have been reported in these rats ( Jafarian et al., 2015).
1.8.13. The quantitative comparison of genetic animal models of absence epilepsy
The articles which used genetic models to study absence epilepsy were counted from 2000 to 2018. The articles included 293 WAG/Rij, 74 GEARS, 21 stargazers, 19 tottering mice, 7 ducky mice, 3 lethargic, and 3 slow-wave epilepsy. The number of articles that used WAG/Rij rats was significantly more than the others (P<0.001).
2. Conclusion
Our findings of many diseases have been found from valid animal models. Although there are several models for the screening, quantification, and evaluation of absence epilepsy, the important issue is still the reproducibility of the full clinical syndrome and pathogenesis as well as different etiology of the absence seizures. In this regard, it should be noted that how much the existing models are useful for discovering new drugs for 30% of medically refractory epilepsy. It seems that current animal models are designed based on the underlying mechanism of seizure, not epileptogenesis. Besides, epilepsy is classified as a chronic disorder and most animal models are not appropriate for long-term chronic induction of diseases ( Havasimehr, Saffarzadeh, Divanbeigi, & Karimzadeh, 2018). As regards, childhood absence epilepsy has multifactorial genetic etiology, so it seems that genetic animal models are more suitable than chemical models, because of the close correlation of their EEG features and clinical appearances to the human condition. Among genetic models of mice and rats, the WAG/Rij and GAERS strains of Wistar rats have asserted to be valid and predictive models of human absence epilepsy. Although there are some differences in seizure characteristics such as frequency, ontogeny, and the time of seizure developing, the pharmacologic re-activities and the pathogenesis explained in both strains are very similar to human absence epilepsy. Differences between the two strains suggest the involvement of several genetic mechanisms in the SWDs pathogenesis. Multidisciplinary studies of these two strains lead to finding trustable information about the role of the cortex and the thalamus, and other subcortical circuits. As statistics show, the most published papers in the absence epilepsy was designed based on the WAG/Rij rats. It should be mentioned that pharmacological models such as the GHB model are used to assess the mechanisms of receptors in the pathogenesis and new anti-seizure compounds.
As all of these models have some shortcomings and limitations, so researchers should be open-minded and enthusiastic to more valid animal models for a better understanding of the epileptogenic process and or discovery of novel therapies for this heterogeneous disorder.
Footnotes
Conflict of interest
The authors declared no conflicts of interest.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of the Iran University of Medical Sciences.
Funding
This work was supported by Iran University of Medical Sciences and Shefa Neuroscience Research Center (Grant No.: 95-01-117-27884).
Authors’ contributions
Writing – original draft: Maryam Jafarian; Data collection: Mohammad Esmaeil Alipour; Data analysis: Fariba Karimzadeh.
References
- Avanzini G., Vergnes M., Spreafico R., Marescaux C. (1993). Calcium-dependent regulation of genetically determined spike and waves by the reticular thalamic nucleus of rats. Epilepsia, 34(1), 1–7. [DOI: 10.1111/j.1528-1157.1993.tb02369.x] [PMID https://www.ncbi.nlm.nih.gov/pubmed/8422841] [DOI] [PubMed] [Google Scholar]
- Avoli M., Gloor P., Kostopoulos G., Gotman J. (1983). An analysis of penicillin-induced generalized spike and wave discharges using simultaneous recordings of cortical and thalamic single neurons. Journal of Neurophysiology, 50(4), 819–37. [DOI: 10.1152/jn.1983.50.4.819] [PMID https://www.ncbi.nlm.nih.gov/pubmed/6631465] [DOI] [PubMed] [Google Scholar]
- Barclay J., Balaguero N., Mione M., Ackerman S. L., Letts V. A., Brodbeck J., et al. (2001). Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. The Journal of Neuroscience, 21(16), 6095–104. [DOI: 10.1523/JNEUROSCI.21-16-06095.2001] [PMID https://www.ncbi.nlm.nih.gov/pubmed/11487633] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC6763162] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden L. J., Snead O. C., Healy C. T., Pegram G. V. (1980). Laboratory note: Antagonism of gamma-hydroxybutyric acid-induced frequency shifts in the cortical EEG of rats by dipropylacetate. Electroencephalography and Clinical Neurophysiology, 49(1–2), 181–3. [DOI: 10.1016/0013-4694(80)90365-X] [PMID https://pubmed.ncbi.nlm.nih.gov/6159161/] [DOI] [PubMed] [Google Scholar]
- Berg A. T., Berkovic S. F., Brodie M. J., Buchhalter J., Cross J. H., Van Emde Boas W., et al. (2010). Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE commission on classification and terminology, 2005–2009. Epilepsia, 51(4), 676–85. [DOI: 10.1111/j.1528-1167.2010.02522.x] [PMID https://www.ncbi.nlm.nih.gov/pubmed/20196795] [DOI] [PubMed] [Google Scholar]
- Berkovic S. F., Howell R. A., Hay D. A., Hopper J. L. (1994). Epilepsies in twins. In Wolf P. (Ed.), Epileptic Seizures and Syndromes: With Some of Their Theoretical Implications (pp. 157–164). London: John Libbey Eurotext. https://books.google.com/books?id=3FILwNf3kVUC&vq [Google Scholar]
- Blumenfeld H. (2005). Cellular and network mechanisms of spike-wave seizures. Epilepsia, 46(s9), 21–33. [DOI: 10.1111/j.1528-1167.2005.00311.x] [PMID https://www.ncbi.nlm.nih.gov/pubmed/16302873] [DOI] [PubMed] [Google Scholar]
- Bourinet E., Soong T. W., Sutton K., Slaymaker S., Mathews E., Monteil A., et al. (1999). Splicing of α1A subunit gene generates phenotypic variants of P-and Q-type calcium channels. Nature Neuroscience, 2(5), 407–15. [DOI: 10.1038/8070] [PMID https://www.ncbi.nlm.nih.gov/pubmed/10321243] [DOI] [PubMed] [Google Scholar]
- Budde T., Pape H. C., Kumar S. S., Huguenard J. R. (2006). Thalamic, thalamocortical, and corticocortical models of epilepsy with an emphasis on absence seizures. In Pitkänen A., Schwartzkroin P. A., Moshé S. L. (Eds.), Models of Seizures And Epilepsy (pp. 73–88). Cambridge, MA: Academic Press. [DOI: 10.1016/B978-012088554-1/50009-8] [DOI] [Google Scholar]
- Burgess D. L., Noebels J. L. (1999). Single gene defects in mice: The role of voltage-dependent calcium channels in absence models. Epilepsy Research, 36(2–3), 111–22. [DOI: 10.1016/S0920-1211(99)00045-5] [DOI] [PubMed] [Google Scholar]
- Burgess D. L., Jones J. M., Meisler M. H., Noebels J. L. (1997). Mutation of the Ca2+ channel β subunit gene Cchb4 is associated with ataxia and seizures in the lethargic (lh) mouse. Cell, 88(3), 385–92. [DOI: 10.1016/S0092-8674(00)81877-2] [PMID https://pubmed.ncbi.nlm.nih.gov/9039265/] [DOI] [PubMed] [Google Scholar]
- Campbell D. B., Hess E. J. (1996). Chromosomal localization of the neurological mouse mutations tottering (tg), Purkinje cell degeneration (pcd), and nervous (nr). Molecular Brain Research, 37(1–2), 79–84. [DOI: 10.1016/0169-328X(95)00275-W] [DOI] [PubMed] [Google Scholar]
- Chan K. F. Y., Jia Z., Murphy P. A., Burnham W. M., Cortez M. A., Snead O. C. (2004). Learning and memory impairment in rats with chronic atypical absence seizures. Experimental Neurology, 190(2), 328–36. [DOI: 10.1016/j.expneurol.2004.08.001] [PMID https://www.ncbi.nlm.nih.gov/pubmed/15530872] [DOI] [PubMed] [Google Scholar]
- Chen L., Chetkovich D. M., Petralia R. S., Sweeney N. T., Kawasaki Y., Wenthold R. J., et al. (2000). Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature, 408(6815), 936–43. [DOI: 10.1038/35050030] [PMID https://www.ncbi.nlm.nih.gov/pubmed/11140673] [DOI] [PubMed] [Google Scholar]
- Coenen A. M. L., Van Luijtelaar E. L. J. M. (1987). The WAG/Rij rat model for absence epilepsy: Age and sex factors. Epilepsy Research, 1(5), 297–301. [DOI: 10.1016/0920-1211(87)90005-2] [DOI] [PubMed] [Google Scholar]
- Coenen A. M. L., Van Luijtelaar E. L. J. M. (2003). Genetic animal models for absence epilepsy: A review of the WAG/Rij strain of rats. Behavior Genetics, 33(6), 635–55. [DOI: 10.1023/A:1026179013847] [PMID https://www.ncbi.nlm.nih.gov/pubmed/14574120] [DOI] [PubMed] [Google Scholar]
- Cortez M. A., Snead III O. C. (2006). Pharmacologic models of generalized absence seizures in rodents. In Pitkänen A., Schwartzkroin P. A., Moshé S. L. (Eds.), Models of Seizures And Epilepsy (pp. 111–126). Cambridge, MA: Academic Press. [DOI: 10.1016/B978-012088554-1/50012-8] [DOI] [Google Scholar]
- Cortez M. A., Cunnane S. C., Snead O. C., III (2002). Brain sterols in the AY-9944 rat model of atypical absence seizures. Epilepsia, 43(1), 3–8. [DOI: 10.1046/j.1528-1157.2002.22401.x] [PMID https://www.ncbi.nlm.nih.gov/pubmed/11879380] [DOI] [PubMed] [Google Scholar]
- Cortez M. A., McKerlie C., Snead O. C., III (2001). A model of atypical absence seizures: EEG, pharmacology, and developmental characterization. Neurology, 56(3), 341–9. [DOI: 10.1212/WNL.56.3.341] [PMID https://www.ncbi.nlm.nih.gov/pubmed/11171899] [DOI] [PubMed] [Google Scholar]
- Cox G. A., Lutz C. M., Yang C. L., Biemesderfer D., Bronson R. T., Fu A., et al. (1997). Sodium/hydrogen exchanger gene defect in slow-wave epilepsy mutant mice. Cell, 91(1), 139–48. [DOI: 10.1016/S0092-8674(01)80016-7] [DOI] [PubMed] [Google Scholar]
- Crunelli V., Leresche N. (2002). Childhood absence epilepsy: Genes, channels, neurons and networks. Nature Reviews Neuroscience, 3(5), 371–82. [DOI: 10.1038/nrn811] [PMID https://www.ncbi.nlm.nih.gov/pubmed/11988776] [DOI] [PubMed] [Google Scholar]
- De Deyn P. P., D’Hooge R., Marescau B., Pei Y. Q. (1992). Chemical models of epilepsy with some reference to their applicability in the development of anticonvulsants. Epilepsy Research, 12(2), 87–110. [DOI: 10.1016/0920-1211(92)90030-W] [DOI] [PubMed] [Google Scholar]
- Depaulis A., Snead III O. C., Marescaux C., Vergnes M. (1989). Suppressive effects of intranigral injection of muscimol in three models of generalized non-convulsive epilepsy induced by chemical agents. Brain Research, 498(1), 64–72. [DOI: 10.1016/0006-8993(89)90399-5] [DOI] [PubMed] [Google Scholar]
- Devanagondi R., Egami K., LeDoux M. S., Hess E. J., Jinnah H. A. (2007). Neuroanatomical substrates for paroxysmal dyskinesia in lethargic mice. Neurobiology of Disease, 27(3), 249–57. [DOI: 10.1016/j.nbd.2007.05.001] [PMID https://www.ncbi.nlm.nih.gov/pubmed/17561408] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pietro S. M., Falcón-Pérez J. M., Tenza D., Setty S. R. G., Marks M. S., Raposo G., et al. (2006). BLOC-1 interacts with BLOC-2 and the AP-3 complex to facilitate protein trafficking on endosomes. Molecular Biology of the Cell, 17(9), 4027–38. [DOI: 10.1091/mbc.e06-05-0379] [PMID https://www.ncbi.nlm.nih.gov/pubmed/16837549] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1593172] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dung H. C. (1977). Deficiency in the thymus-dependent immunity in “lethargic” mutant mice1. Transplantation, 23(1), 39–43. [DOI: 10.1097/00007890-197701000-00007] [PMID https://www.ncbi.nlm.nih.gov/pubmed/13525] [DOI] [PubMed] [Google Scholar]
- Fariello R. G., Golden G. T. (1987). The THIP-induced model of bilateral synchronous spike and wave in rodents. Neuropharmacology, 26(2–3), 161–5. [DOI: 10.1016/0028-3908(87)90204-8] [DOI] [PubMed] [Google Scholar]
- Fisher R. S. (1989). Animal models of the epilepsies. Brain Research Reviews, 14(3), 245–78. [DOI: 10.1016/0165-0173(89)90003-9] [DOI] [PubMed] [Google Scholar]
- Frankel W. N. (1999). Detecting genes in new and old mouse models for epilepsy: A prospectus through the magnifying glass. Epilepsy Research, 36(2–3), 97–110. [DOI: 10.1016/S0920-1211(99)00044-3] [DOI] [PubMed] [Google Scholar]
- Gauguier D., van Luijtelaar G., Bihoreau M. T., Wilder S. P., Godfrey R. F., Vossen J., et al. (2004). Chromosomal mapping of genetic loci controlling absence epilepsy phenotypes in the WAG/Rij rat. Epilepsia, 45(8), 908–15. [DOI: 10.1111/j.0013-9580.2004.13104.x] [PMID https://www.ncbi.nlm.nih.gov/pubmed/15270755] [DOI] [PubMed] [Google Scholar]
- Gorji A., Mittag C., Shahabi P., Seidenbecher T., Pape H. C. (2011). Seizure-related activity of intralaminar thalamic neurons in a genetic model of absence epilepsy. Neurobiology of Disease, 43(1), 266–74. [DOI: 10.1016/j.nbd.2011.03.019] [PMID https://www.ncbi.nlm.nih.gov/pubmed/21458572] [DOI] [PubMed] [Google Scholar]
- Havasimehr M., Saffarzadeh F., Divanbeigi A., Karimzadeh F. (2018). [A review on the animal models of seizure: Review article (Persian)]. Tehran University Medical Journal, 76(2), 79–89. http://tumj.tums.ac.ir/article-1-8767-en.html [Google Scholar]
- Heller A. H., Dichter M. A., Sidman R. L. (1983). Anticonvulsant sensitivity of absence seizures in the tottering mutant mouse. Epilepsia, 24(1), 25–34. [DOI: 10.1111/j.1528-1157.1983.tb04862.x] [PMID https://www.ncbi.nlm.nih.gov/pubmed/6401628] [DOI] [PubMed] [Google Scholar]
- Jafarian M., Karimzadeh F., Alipour F., Attari F., Lotfinia A. A., Speckmann E. J., et al. (2015). Cognitive impairments and neuronal injury in different brain regions of a genetic rat model of absence epilepsy. Neuroscience, 298, 161–70. [DOI: 10.1016/j.neuroscience.2015.04.033] [PMID https://www.ncbi.nlm.nih.gov/pubmed/25907443] [DOI] [PubMed] [Google Scholar]
- Kantheti P., Qiao X., Diaz M. E., Peden A. A., Meyer G. E., Carskadon S. L., et al. (1998). Mutation in AP-3 δ in the mocha mouse links endosomal transport to storage deficiency in platelets, melanosomes, and synaptic vesicles. Neuron, 21(1), 111–22. [DOI: 10.1016/S0896-6273(00)80519-X] [DOI] [PubMed] [Google Scholar]
- Kaplan B. J., Seyfried T. N., Glaser G. H. (1979). Spontaneous polyspike discharges in an epileptic mutant mouse (tottering). Experimental Neurology, 66(3), 577–86. [DOI: 10.1016/0014-4886(79)90203-6] [DOI] [PubMed] [Google Scholar]
- Karamahmutoğlu T. E., Çarçak N., şahiner M., Akman Ö., Snead O. C., Eşkazan E., et al. (2013). The gamma-butyrolactone model of absence epilepsy: Acute and chronic effects in Wistar rats. Türk Epilepsi İle Savas Dernegi, 19(2), 48–52. http://acikerisim.istanbulbilim.edu.tr:8080/xmlui/handle/11446/410 [Google Scholar]
- Karimzadeh F., Modarres Mousavi S. M., Alipour F., Hosseini Ravandi H., Kovac S., Gorji A. (2017). Developmental changes in Notch1 and NLE1 expression in a genetic model of absence epilepsy. Brain Structure and Function, 222(6), 2773–85. [DOI: 10.1007/s00429-017-1371-9] [PMID https://www.ncbi.nlm.nih.gov/pubmed/28210849] [DOI] [PubMed] [Google Scholar]
- Karimzadeh F., Modarres Mousavi S. M., Ghadiri T., Jafarian M., Soleimani M., Mohammad Sadeghi Sh., et al. (2017). The modulatory effect of metabotropic glutamate receptor type-1α on spike-wave discharges in WAG/Rij Rats. Molecular Neurobiology, 54(2), 846–54. [DOI: 10.1007/s12035-016-9692-x] [PMID https://www.ncbi.nlm.nih.gov/pubmed/26780454] [DOI] [PubMed] [Google Scholar]
- Karimzadeh F., Soleimani M., Mehdizadeh M., Jafarian M., Mohamadpour M., Kazemi H., et al. (2013). Diminution of the NMDA receptor NR2B subunit in cortical and subcortical areas of WAG/Rij rats. Synapse, 67(12), 839–46. [DOI: 10.1002/syn.21687] [PMID https://www.ncbi.nlm.nih.gov/pubmed/23754322] [DOI] [PubMed] [Google Scholar]
- Karson A., Utkan T., Balcı F., Arıcıoğlu F., Ateş N. (2012). Age-dependent decline in learning and memory performances of WAG/Rij rat model of absence epilepsy. Behavioral and Brain Functions, 8, 51. [DOI: 10.1186/1744-9081-8-51] [PMID https://www.ncbi.nlm.nih.gov/pubmed/22998946] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3514399] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letts V. A., Valenzuela A., Kirley J. P., Sweet H. O., Davisson M. T., Frankel W. N. (1997). Genetic and physical maps of the stargazer locus on mouse chromosome 15. Genomics, 43(1), 62–8. [DOI: 10.1006/geno.1997.4780] [PMID https://www.ncbi.nlm.nih.gov/pubmed/9226373] [DOI] [PubMed] [Google Scholar]
- MacDonald R. L., Barker J. L. (1977). Pentylenetetrazol and penicillin are selective antagonists of GABA-mediated post-synaptic inhibition in cultured mammalian neurones. Nature, 267(5613), 720–1. [DOI: 10.1038/267720a0] [PMID https://www.ncbi.nlm.nih.gov/pubmed/195224] [DOI] [PubMed] [Google Scholar]
- Manning J. P. A., Richards D. A., Bowery N. G. (2003). Pharmacology of absence epilepsy. Trends in Pharmacological Sciences, 24(10), 542–9. [DOI: 10.1016/j.tips.2003.08.006] [PMID https://www.ncbi.nlm.nih.gov/pubmed/14559407] [DOI] [PubMed] [Google Scholar]
- Marini C., Harkin L. A., Wallace R. H., Mulley J. C., Scheffer I. E., Berkovic S. F. (2003). Childhood absence epilepsy and febrile seizures: A family with a GABAA receptor mutation. Brain, 126(1), 230–40. [DOI: 10.1093/brain/awg018] [PMID https://www.ncbi.nlm.nih.gov/pubmed/12477709] [DOI] [PubMed] [Google Scholar]
- Meeren H. K. M., Pijn J. P. M., Van Luijtelaar E. L. J. M., Coenen A. M. L., da Silva F. H. L. (2002). Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. The Journal of Neuroscience, 22(4), 1480–95. [DOI: 10.1523/JNEUROSCI.22-04-01480.2002] [PMID https://www.ncbi.nlm.nih.gov/pubmed/11850474] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC6757554] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeren H., van Luijtelaar G., da Silva F. L., Coenen A. (2005). Evolving concepts on the pathophysiology of absence seizures: The cortical focus theory. Archives of Neurology, 62(3), 371–6. [DOI: 10.1001/archneur.62.3.371] [PMID https://www.ncbi.nlm.nih.gov/pubmed/15767501] [DOI] [PubMed] [Google Scholar]
- Noebels J. L. (2003). The biology of epilepsy genes. Annual Review of Neuroscience, 26, 599–625. [DOI: 10.1146/annurev.neuro.26.010302.081210] [PMID https://www.ncbi.nlm.nih.gov/pubmed/14527270] [DOI] [PubMed] [Google Scholar]
- Noebels J. L., Qiao X., Bronson R. T., Spencer C., Davisson M. T. (1990). Stargazer: A new neurological mutant on chromosome 15 in the mouse with prolonged cortical seizures. Epilepsy Research, 7(2), 129–35. [DOI: 10.1016/0920-1211(90)90098-G] [DOI] [PubMed] [Google Scholar]
- Papale L. A., Beyer B., Jones J. M., Sharkey L. M., Tufik S., Epstein M., et al. (2009). Heterozygous mutations of the voltage-gated sodium channel SCN8A are associated with spike-wave discharges and absence epilepsy in mice. Human Molecular Genetics, 18(9), 1633–41. [DOI: 10.1093/hmg/ddp081] [PMID https://www.ncbi.nlm.nih.gov/pubmed/19254928] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2667290] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault D., Leresche N., Charpier S., Deniau J. M., Marescaux C., Vergnes M., et al. (1998). Intracellular recordings in thalamic neurones during spontaneous spike and wave discharges in rats with absence epilepsy. The Journal of Physiology, 509(Pt 2), 449–56. [DOI: 10.1111/j.1469-7793.1998.449bn.x] [PMID https://www.ncbi.nlm.nih.gov/pubmed/9575294] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2230966] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen A., Schwartzkroin P. A., Moshé S. L., Eds. (2005). Models of seizures and epilepsy. Burlington, MA: Academic Press. https://books.google.com/books?id=Qw6KqLjwtZQC&dq [Google Scholar]
- Polack P. O., Guillemain I., Hu E., Deransart C., Depaulis A., Charpier S. (2007). Deep layer somatosensory cortical neurons initiate spike-and-wave discharges in a genetic model of absence seizures. The Journal of Neuroscience, 27(24), 6590–9. [DOI: 10.1523/JNEUROSCI.0753-07.2007] [PMID https://www.ncbi.nlm.nih.gov/pubmed/17567820] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC6672429] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter B. E., Justice M. J., Copeland N. G., Jenkins N. A., Hunter D. D., Merlie J. P., et al. (1993). S-Laminin: Mapping to mouse chromosome 9 and expression in the linked Mutants tippy and ducky. Genomics, 16(1), 278–81. [DOI: 10.1006/geno.1993.1178] [PMID https://www.ncbi.nlm.nih.gov/pubmed/8486374] [DOI] [PubMed] [Google Scholar]
- Powell K. L., Cain S. M., Ng C., Sirdesai Sh., David L. S., Kyi M., et al. (2009). A Cav3.2 T-type calcium channel point mutation has splice-variant-specific effects on function and segregates with seizure expression in a polygenic rat model of absence epilepsy. The Journal of Neuroscience, 29(2), 371–80. [DOI: 10.1523/JNEUROSCI.5295-08.2009] [PMID https://www.ncbi.nlm.nih.gov/pubmed/19144837] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC6664949] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao X., Noebels J. L. (1993). Developmental analysis of hippocampal mossy fiber outgrowth in a mutant mouse with inherited spike-wave seizures. The Journal of Neuroscience, 13(11), 4622–35. [DOI: 10.1523/JNEUROSCI.13-11-04622.1993] [PMID https://www.ncbi.nlm.nih.gov/pubmed/8229188] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC6576361] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhyu I. J., Abbott L. C., Walker D. B., Sotelo C. (1999). An ultrastructural study of granule cell/Purkinje cell synapses in tottering (tg/tg), leaner (tgla/tgla) and compound heterozygous tottering/leaner (tg/tgla) mice. Neuroscience, 90(3), 717–28. [DOI: 10.1016/S0306-4522(98)00518-1] [DOI] [PubMed] [Google Scholar]
- Rudolf G., Bihoreau M. T., Godfrey R. F., Wilder S. P., Cox R. D., Lathrop M., et al. (2004). Polygenic control of idiopathic generalized epilepsy phenotypes in the genetic absence rats from Strasbourg (GAERS). Epilepsia, 45(4), 301–8. [DOI: 10.1111/j.0013-9580.2004.50303.x] [PMID https://www.ncbi.nlm.nih.gov/pubmed/15030491] [DOI] [PubMed] [Google Scholar]
- Sarkisian M. R. (2001). Overview of the current animal models for human seizure and epileptic disorders. Epilepsy & Behavior, 2(3), 201–16. [DOI: 10.1006/ebeh.2001.0193] [PMID https://www.ncbi.nlm.nih.gov/pubmed/12609365] [DOI] [PubMed] [Google Scholar]
- Sarkisova K., van Luijtelaar G. (2011). The WAG/Rij strain: A genetic animal model of absence epilepsy with comorbidity of depressiony. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 35(4), 854–76. [DOI: 10.1016/j.pnpbp.2010.11.010] [PMID https://www.ncbi.nlm.nih.gov/pubmed/21093520] [DOI] [PubMed] [Google Scholar]
- Seidenbecher T., Pape H. C. (2001). Contribution of intralaminar thalamic nuclei to spike-and-wave-discharges during spontaneous seizures in a genetic rat model of absence epilepsy. European Journal of Neuroscience, 13(8), 1537–46. 10.1046/j.0953-816x.2001.01537.x [DOI] [PubMed] [Google Scholar]
- Serbanescu I., Cortez M. A., McKerlie C., Snead O. C., III (2004). Refractory atypical absence seizures in rat: A two hit model. Epilepsy Research, 62(1), 53–63. [DOI: 10.1016/j.eplepsyres.2004.08.003] [PMID https://www.ncbi.nlm.nih.gov/pubmed/15519132] [DOI] [PubMed] [Google Scholar]
- Sharp A. H., Black J. L., Dubel S. J., Sundarraj S., Shen J. P., Yunker A. M. R., et al. (2001). Biochemical and anatomical evidence for specialized voltage-dependent calcium channel γ isoform expression in the epileptic and ataxic mouse, stargazer. Neuroscience, 105(3), 599–617. [DOI: 10.1016/S0306-4522(01)00220-2] [DOI] [PubMed] [Google Scholar]
- Sitnikova E., Van Luijtelaar G. (2007). Electroencephalographic characterization of spike-wave discharges in cortex and thalamus in WAG/Rij rats. Epilepsia, 48(12), 2296–311. [DOI: 10.1111/j.1528-1167.2007.01250.x] [PMID https://www.ncbi.nlm.nih.gov/pubmed/18196621] [DOI] [PubMed] [Google Scholar]
- Snead O. C., III (1995). Basic mechanisms of generalized absence seizures. Annals of Neurology, 37(2), 146–57. [DOI: 10.1002/ana.410370204] [PMID https://www.ncbi.nlm.nih.gov/pubmed/7847856] [DOI] [PubMed] [Google Scholar]
- Steriade M. (2005). Sleep, epilepsy and thalamic reticular inhibitory neurons. Trends in Neurosciences, 28(6), 317–24. [DOI: 10.1016/j.tins.2005.03.007] [PMID https://www.ncbi.nlm.nih.gov/pubmed/15927688] [DOI] [PubMed] [Google Scholar]
- Tang F. R., Loke W. K., Eds. (2011). Chemical-induced seizures: Mechanisms, consequences and treatment. Sharjah: Bentham Science Publishers. https://books.google.com/books?id=9H4m7T9nG1YC&pg=PA3&dq [Google Scholar]
- Tokuda S., Kuramoto T., Tanaka K., Kaneko Sh., Takeuchi I. K., Sasa M., et al. (2007). The ataxic groggy rat has a mis-sense mutation in the P/Q-type voltage-gated Ca2+ channel α1A subunit gene and exhibits absence seizures. Brain Research, 1133, 168–77. [DOI: 10.1016/j.brainres.2006.10.086] [PMID https://www.ncbi.nlm.nih.gov/pubmed/17196942] [DOI] [PubMed] [Google Scholar]
- Zhang Y., Mori M., Burgess D. L., Noebels J. L. (2002). Mutations in high-voltage-activated calcium channel genes stimulate low-voltage-activated currents in mouse thalamic relay neurons. The Journal of Neuroscience, 22(15), 6362–71. [DOI: 10.1523/JNEUROSCI.22-15-06362.2002] [PMID https://pubmed.ncbi.nlm.nih.gov/12151514/] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC6758149] [DOI] [PMC free article] [PubMed] [Google Scholar]