Abstract
Cross talking between natural senescence and cell death in response to pathogen attack is an interesting topic; however, its action mechanism is kept open. In this study, 33 OsWRKY genes were obtained by screening with leaf aging procedure through RNA-seq dataset, and 11 of them were confirmed a significant altered expression level in the flag leaves during aging by using the reverse transcript quantitative PCR (RT-qPCR). Among them, the OsWRKY2, OsWRKY14, OsWRKY26, OsWRKY69, and OsWRKY93 members exhibited short-term alteration in transcriptional levels in response to Magnaporthe grisea infection. The CRISPR/Cas9-edited mutants of five genes were developed and confirmed, and a significant sensitivity to M. oryzae infection was observed in CRISPR OsWRKY93-edited lines; on the other hand, a significant resistance to M. oryzae infection was shown in the enhanced expression OsWRKY93 plants compared to mock plants; however, enhanced expression of other four genes have no significant affection. Interestingly, ROS accumulation was also increased in OsWRKY93 enhanced plants after flg22 treatment, compared with the controls, suggesting that OsWRKY93 is involved in PAMP-triggered immune response in rice. It indicated that OsWRKY93 was involved in both flag leaf senescence and in response to fungi attack.
Keywords: OsWRKY93, rice, flag leaf, senescence, biotic stress
Introduction
Rice is the main food crop of the developing world. However, the increase of yield is seriously restricted by flag leaf senescence in rice. The flag leaf, the uppermost leaf in the rice plant, is thought to contribute highly to what is accumulated in grain (Ghosh et al., 1990; Li et al., 1998). Delaying the senescence of rice leaves and prolonging the photosynthesis time are beneficial for increasing the rice yield, and the yield can increase by about 2% after flag leaf senescence is delayed for 1 day (Ma and Lu, 1990). Therefore, studying the mechanism of flag leaf senescence is essential to improving the yield of rice grain.
Leaf senescence is the final stage of leaf development. As an organ level senescence, leaf senescence is a crucial means for plants to reallocate nutrients and valuable substances from senescent leaves to reproducing seeds, eventually maximizing reproductive success (Himelblau and Amasino, 2001). Leaf senescence is a strictly organized process finely governed by developmental age. However, leaf senescence is also influenced by various internal and environmental signals that are integrated with age information (Lim et al., 2007). The internal factors that affect leaf senescence include developmental cues and reproductive development as well as phytohormones (Gan and Amasino, 1995; Pic et al., 2002; Riefler et al., 2006). The environmental cues include various stresses such as extreme temperatures, nutrient deficiency, drought, radiation, and infection from pathogens. Interestingly, the leaf transcriptome varies immensely accompanying the onset and progression of leaf senescence. It was previously reported that 20 different families of transcription factors that are transcriptionally up-regulated in senescent leaves remarkably contain several large groups such as NAC, WRKY, C2H2-type zinc finger, AP2/EREBP, and MYB proteins (Guo and Gan, 2005).
Among these large groups, WRKY proteins are plant specific transcription factors that are especially believed to play central roles in regulating senescence. All WRKY proteins contain at least one WRKY domain that is composed of a zinc finger structure and a 60-amino acid region with WRKYGQK at the N-terminal end. The WRKY domain is a DNA-binding domain that binds directly to various W-box variants (Eulgem et al., 2000; Yu et al., 2001). To date, many WRKY TFs regulating leaf senescence have been characterized in Arabidopsis. WRKY6 is highly induced during leaf senescence (Robatzek and Somssich, 2001). WRKY45 positively regulates age-triggered leaf senescence through interacting with a DELLA protein, RGL1 (Chen L. et al., 2017). Another well-known WRKY member, WRKY53 plays a regulatory role in the early events of leaf senescence (Hinderhofer and Zentgraf, 2001; Miao et al., 2004). Overexpression of WRKY75 accelerates age-dependent leaf senescence (Guo et al., 2017). In rice, WRKY family has over 102 members (Xie et al., 2005). However, relatively few OsWRKY members involved in leaf senescence have been examined. For instance, overexpressing OsWRKY5 promotes leaf senescence under natural and dark-induced senescence conditions (Kim et al., 2019). Heterologous expression of OsWRKY23 promotes dark-induced leaf senescence in Arabidopsis (Jing et al., 2009). OsWRKY42 enhances leaf senescence by repressing the expression of OsMT1d to induce reactive oxygen species (ROS) in rice (Han et al., 2014).
The WRKY family is also known for being the key player in plant biotic stress response. The initial study investigated the expression of WRKY TFs in rice response to M. oryzae and found that 15 OsWRKYs were induced upon pathogen infection (Ryu et al., 2006). Subsequent research revealed more details about the involvement of many OsWRKYs in plant defense. At least nine OsWRKYs have been identified to regulate rice response to M. oryzae positively. For example, overexpression of OsWRKY31, OsWRKY45, OsWRKY47, OsWRKY53, or OsWRKY67 in rice plants enhances resistance to M. oryzae (Chujo et al., 2007; Shimono et al., 2007; Zhang et al., 2008; Wei et al., 2013; Vo et al., 2018). On the contrary, several OsWRKY members function as negative regulators of the rice response to M. oryzae infection. For instance, through suppressing JA signaling-related genes, OsWRKY42 negatively regulate rice response to M. oryzae (Cheng et al., 2015). Overexpression of OsWRKY28 or OsWRKY76 in rice plants resulted in increased susceptibility to M. oryzae (Chujo et al., 2013; Yokotani et al., 2013).
In this study, the transcriptome analysis shows that 33 OsWRKY members in rice flag leaves are differentially expressed during plant aging. Besides, RT-qPCR analysis displayed that the expression of five OsWRKY genes were altered in Guy11-treated rice plants. The Crispr/Cas9-edited mutants of five OsWRKY genes were developed and confirmed. Genetic analysis reveals that enhanced expression of OsWRKY93 resulted in an enhanced resistance to M. oryzae infection in rice. This finding suggests that OsWRKY93 plays a role in the defense response and is also associated with the regulation of flag leaf senescence in rice. All in all, this study provides a new candidate gene for in depth understanding of the regulatory mechanisms of pathogen induced leaf senescence, helping in breeding high yield and disease resistant crops.
Materials and Methods
Plant Materials and Growth Conditions
The rice (Oryza sativa L. subsp. japonica) of the Kitaake accession was used for generating OsWRKY2, OsWRKY14, OsWRKY26, OsWRKY69, and OsWRKY93 transgenic plants with increased OsWRKY2, OsWRKY14, OsWRKY26, OsWRKY69, and OsWRKY93 expression level via a transcriptional activator containing four copies of VP16 (i.e., VP64), and named OsWRKYVP64 (Sadowski et al., 1988; Yaghmai and Cutting, 2002). Rice plants were grown in the growth chamber at 30°C for 12 h (day) and 20°C for 12 h (night) or under outdoor conditions (natural long-day conditions) in Fuzhou Fujian Province, China, from April to September.
Identification of CRISPR/Cas9-Edited Mutants
The OsWRKY2, OsWRKY14, OsWRKY26, OsWRKY69, and OsWRKY93 CRISPR transgenic plants were produced by the Biogle company (Hangzhou, China). Genomic DNA from individual transgenic plants was isolated using Edwards buffer (Edwards et al., 1991) for PCR analysis. The PCR products were amplified with OsWRKY93-specific primers and were sequenced directly. The OsWRKY93-specific primers were designed for amplifying targeted regions of OsWRKY93 (Supplementary Table S2).
Pathogen Inoculation
M. oryzae strain Guy11 was used in this study. At the three-leaf stage, rice seedlings were spray-inoculated with the spore suspension of M. oryzae (1 × 105 spores/ml in water containing 0.02% Tween 20). Subsequently, the inoculated plants were incubated in the dark at high humidity for 24 h and transferred to a growth chamber at 24°C with 12 h of light and 12 h of darkness. The disease lesions in the infected leaves were observed, and were scanned at 0, 1, 3, 4 days post-inoculation (dpi).
Darkness Treatment
Kitaake, NIP, oswrky93-1 mutant and the T2 generation OsWRKY93vp64 plants were cultured in soil for 39 days after germination. The fully expanded part of the sixth leaves were cut into 1–2 cm pieces and pooled, and then the leaf pieces were suspended in 3mM MES (pH5.8) buffer and cultured in the dark at 28°C for 0, 24, 36, 48, 60, 72, 84, and 96 h. The color changes of leaves were observed and photographed. Three biological replicates were used.
Chlorophyll Measurements
The chlorophyll content of flag leaves were measured using a chlorophyll meter (DUALEX SCIENTIFIC). For measurement 3–4 points in the central region of the leaf were picked up.
Reverse Transcription Quantitative PCR
Three-leaf stage rice seedlings were spray-inoculated with Guy11 (1 × 105 spores/ml) and water, and leaf samples were collected at 0, 24, 48, 72, 96, and 108 hrs post-inoculation (hpi). Two biological replicates were tested, and each biological replicate contains leaves from three independent plants. Total RNA was extracted from those leaf samples using TRIzol reagent (Invitrogen), followed by cDNA synthesis with RevertAid Reverse Transcriptase (Thermo Fisher Scientific). Quantitative PCR was performed using TransStart Green qPCR SuperMix Kit (TransGen Biotech, China) and the indicated primers (Supplementary Table S1). The rice actin1 (OsACTIN1) gene was selected as an internal control.
ROS Assay
Oxidative bursts were measured using a luminal-based assay with leaf discs from 5-week-old plants. The leaf discs were incubated in sterile water overnight, and then water was replaced with 20 μM luminal and 2.5 μg/ml peroxidase. To measure ROS, leaf discs were treated with 1 μM flg22 or water (Ctrl). Immediately, the luminescence was measured at 3 min intervals with a Varioskan LUX Multimode Microplate Reader (Thermo Fisher Scientific). Then 3–5 replications were carried out for each sample.
Results
Expression Patterns of OsWRKYs in Rice Flag Leaves During Natural Senescence
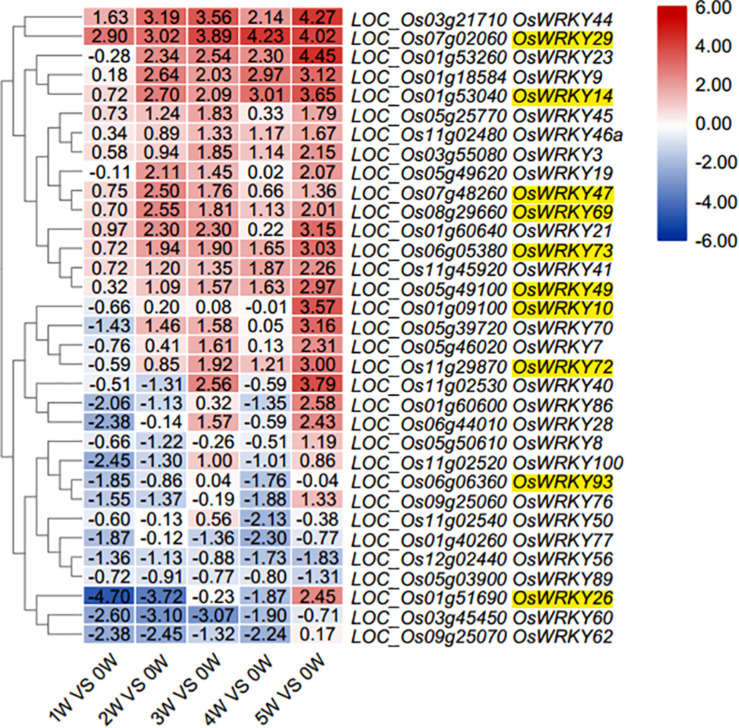
To monitor the transcriptional changes in rice flag leaves during natural senescence, a genome-wide transcriptome analysis was carried out in flag leaf tissue of the Nipponbare through massive RNA sequencing. For generation of RNA-seq libraries, six flag leaf samples were taken. The first sample of the flag leaf was collected at the heading stage when the flag leaf was fully expanded [0 weeks after heading (WAH) and named 0W]; chlorophyll content is higher in 1w than 0w, and then it is gradually decreased from 1w to 5w; the following five flag leaf samples were collected every week (named 1W, 2W, 3W, 4W, and 5W, respectively, 0W used as control). The onset of leaf senescence coincides with the start of Chlorophyll (Chl) degradation, while the initiation of leaf senescence is before Chl degradation. Therefore, the senescence initiation of flag leaves started at the time period between 0W and 2W (Supplementary Figure S1). Through RNA-Seq analysis, the expression patterns of 102 OsWRKY family members in rice flag leaves during aging stages were investigated (Supplementary Dataset S1). EdgeR program was used for differential expression analysis of OsWRKY genes between any of the six samples (Nikolayeva and Robinson, 2014). In comparison with the control (0W), a differential expression profile of a total thirty-three OsWRKY genes were exhibited during natural senescence of flag leaves (Figure 1 and Supplementary Dataset S1).
FIGURE 1.
Heat map diagram of relative gene expression levels of 33 OsWKRYs from total 102 WRKYs (Supplementary Dataset S1) in rice flag leaves at six stages during aging. Developmental stages comprising six stages of flag leaf (0, 1, 2, 3, 4, and 5 weeks after heading). Expression values were scaled by Log2Fold change ≥ 1 and FDR < 0.05 normalized to 0W stage of flag leaf development. 10 OsWRKY candidates are indicated with yellow highlight.
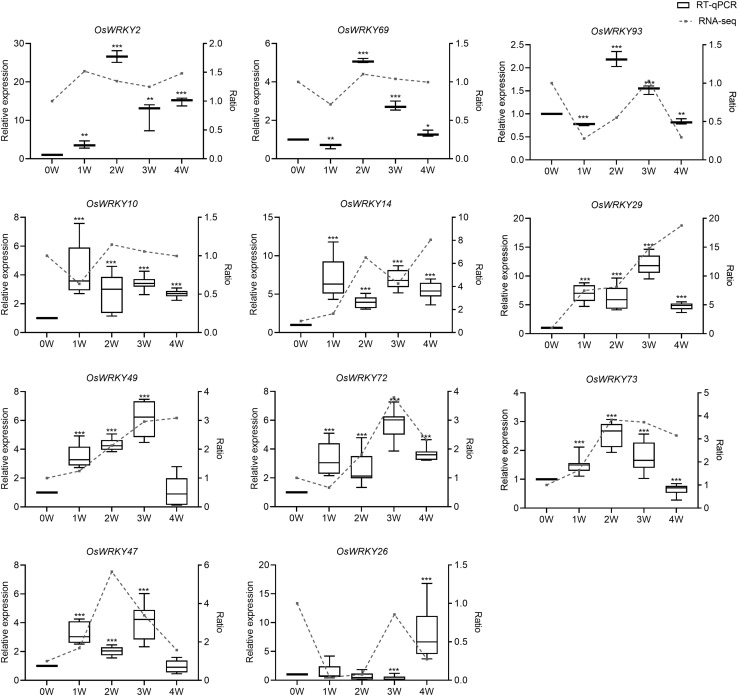
To further confirm the differential expression of thirty-three OsWRKY genes during natural senescence according to transcriptome data (Figure 2 and Supplementary Dataset S1), all of 33 OsWRKY genes were checked by RT-qPCR, the transcript levels of eight OsWRKYs (OsWRKY2, OsWRKY10, OsWRKY14, OsWRKY29, OsWRKY47, OsWRKY49, OsWRKY72, and OsWRKY73) were immediately up-regulated in 1W-vs-0W comparison, while that of three OsWRKYs (OsWRKY69, OsWRKY93, OsWRKY26) were slightly down-regulated in 1W-vs-0W comparison then up-regulated in 2W vs. 0W again (Figure 2), suggesting that they are senescence-related OsWRKY genes. Among the 11 OsWRKY genes, OsWRKY2, OsWRKY69, and OsWRKY93 shared a similar expression pattern in rice flag leaves that the transcript level increased and peaked at the second week after heading (2W) and declined afterward compared with the 0W control. The expression of OsWRKY10 and OsWRKY14 reached the highest level at 1W and remained relatively high afterward. The level of OsWRKY26 mRNA was slightly increased at 1W and then stayed low level at 2W and 3W and suddenly highly increased at 4W. At 3 weeks after heading, the expression of OsWRKY29, OsWRKY47, OsWRKY49, and OsWRKY72 was significantly higher than other controls and began to decrease later (Figure 2). Overall, the results of RT-qPCR were similarly consistent with the RNA-seq data except OsWRKY26 and OsWRKY47 (Figure 2 broken line).
FIGURE 2.
Analyses of several OsWRKYs expression level in rice flag leaves during natural senescence. The expression level was assessed by RT-qPCR. All values were normalized to OsACTIN expression. Box-and-whisker plots show the median value (horizontal lines), interquartile range (boxes), and minimum and maximum values (whiskers). Three biological replicates and three technique replicates were used. The broken-line graphs indicate expression profiles of 11 OsWRKYs from RNA-seq dataset. Asterisks indicate significant differences relative to the 0W controls calculated using the Student t-test: *P < 0.05; **P < 0.01; and ***P < 0.001. The leaf Y_axis denotes relative expression by RT-qPCR. The right y-axis denotes ratio of the fold change of RPKM compared with 0W by RNA-seq. 0W means 0 week after heading.
Expression Profiles of OsWRKYs in Response to Pathogen Infection
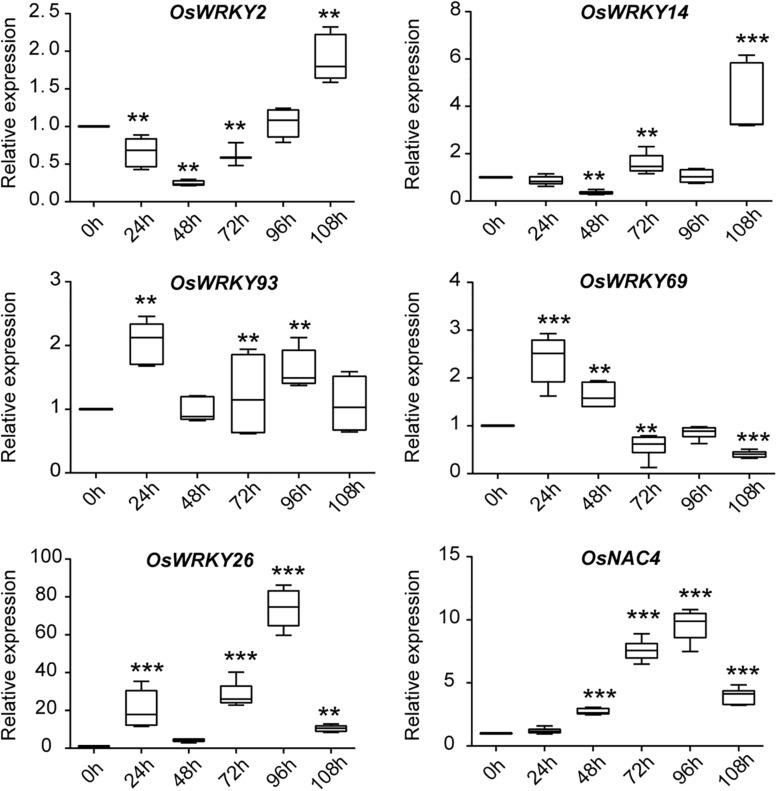
In nature, plants are often attacked by various pathogens, leading to senescence and even death of plants. In this case, plants will initiate a series of immune defense responses to fight back. A number of WRKY family TFs are involved in regulation of both leaf senescence and pathogen defense response, evidently through the ROS and SA pathways, both of which play an important role in leaf senescence and defense responses induced by pathogens (Zhang et al., 2020). To investigate whether these 11 OsWRKYs are induced by infection from pathogens, we performed RT-qPCR (Figure 3). For pathogen treatment, three-leaf-stage rice seedlings were spray-inoculated with Magnaporthe oryzae strain Guy11. The infected leaf samples were collected every 24 h for near 5 days. The defense-related gene, OsNAC4, was used as a positive marker control, showing increased transcript levels in the infected leaves (Kaneda et al., 2009). Among 11 OsWRKYs, OsWRKY2, OsWRKY14, OsWRKY26, OsWRKY69, and OsWRKY93 were induced by M. oryzae infection. For instance, OsWRKY69 and OsWRKY93 had slightly elevated mRNA levels in infected plants, and they were exclusively expressed at the early stage of infection. On the contrary, OsWRKY2 and OsWRKY14 were up-expressed at the late stage after infection. Specifically, the expression of OsWRKY26 was strongly up-regulated at 96 h after inoculation with Guy11. Taken together, the five OsWRKYs appear to play roles in M. oryzae mediated resistance.
FIGURE 3.
Expression analysis of five OsWRKY genes and the defense-related marker gene OsNAC4 in response to M. oryzae infection. qRT-PCR analysis of five OsWRKYs and OsNAC4 in WT at 0, 24, 48, 72, 96, and 108 h after pathogen treatment. The Y-axis represents the relative expression level normalized to OsACTIN. Box-and-whisker plots show median value (line within box), interquartile range (boxes), and minimum and maximum values (whiskers). Three biological replicates and three technique replicates were used. Asterisk indicate significant differences (**P < 0.01, and ***P < 0.001) based on Student t-test compared to 0 h.
We summarized the expression profiles of five OsWRKYs genes both after pathogen infection and during plant aging and showed that OsWRKY2 was down-regulated, which might mean no resistance and no senescence; OsWRKY14 was down-regulated after infection but up-regulated during plant aging, which might imply senescence but no resistance; OsWRKY26 was both up-regulated, which might mean both resistance and senescence. Both OsWRKY69 and OsWRKY93 showed up-resistance after infection but down-regulation during plant aging, which might mean resistance but no senescence (Table 1). Therefore, OsWRKY69 and OsWRKY93 were our favorite candidates for breeding of high yield and disease-resistant rice.
TABLE 1.
Summary of the expression profiles of five OsWRKYs genes after pathogen infection and during plant aging.
| Genes | Expression profile response to M. oryzae | Expression profile during aging |
| OsWRKY2 | Down | Down |
| OsWRKY14 | Down | Up |
| OsWRKY26 | Up | Up |
| OsWRKY69 | Up | Down |
| OsWRKY93 | Up | Down |
Evaluation of Disease Resistance of OsWRKY93 Transgenic Lines to Magnaporthe oryzae Guy11
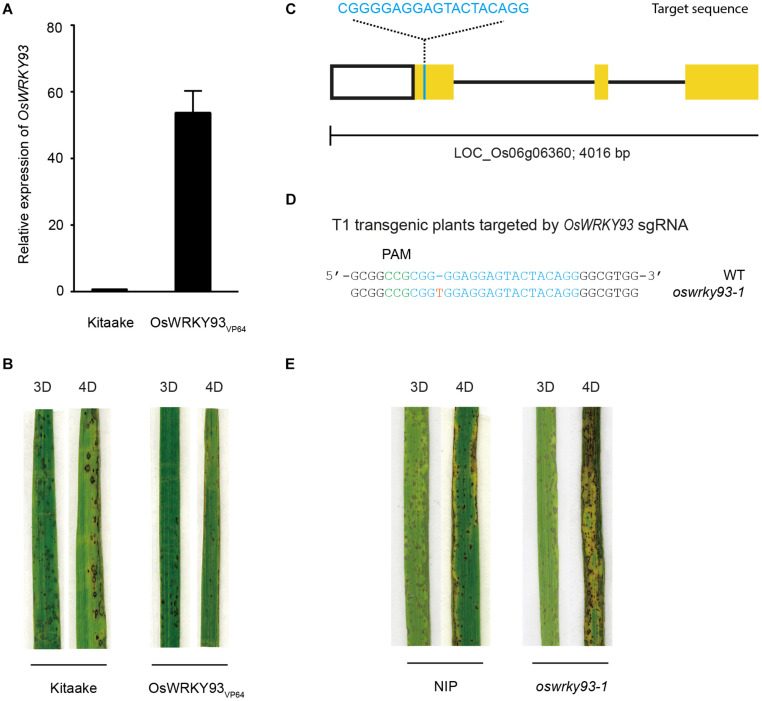
We showed that five OsWRKYs were induced in response to Guy11 treatment. In order to genetically evaluate five OsWRKYs protein functions, five OsWRKYVP64 transgenic lines were generated to explore the potential functions in rice disease resistance (see section “Materials and Methods”). We first detected their transcript levels of five OsWRKY genes by RT-qPCR. The results showed that five OsWRKYs genes all increased their transcript levels in the transgenic lines (OsWRKYs VP64) compared with WT Kitaake (Figure 4A and Supplementary Figure S2). We then inoculated the three-leaf-stage OsWRKYsVP64 plants with Magnaporthe oryzae Guy11 using the spray-inoculation method. Surprisingly, we found that only OsWRKY93VP64 plants showed a significant enhanced resistance to blast disease (Figure 4B). However, the other four of them have no significant alteration of disease resistance to Magnaporthe oryzae Guy11 in the transgenic lines (OsWRKYsVP64) compared with WT Kitaake (Supplementary Figure S3).
FIGURE 4.
Generation and analysis of the OsWRKY93 transgenic lines. (A) Real-time quantitative PCR experiments showing expression changes of OsWRKY93 in Kitaake and the OsWRKY93VP64. (B) Representative leaves of Kitaake and the OsWRKY93VP64 3 and 4 days after inoculation with M. oryzae. Pathogen infection assays were performed on three biological replicates. (C) Schematic diagram for the CRISPR-edited mutant of OsWRKY93. Yellow boxes and black lines represent exons and introns, respectively. The sgRNA target is cyan. (D) Sequence of the oswrky93-1 mutant identified from transgenic plants of the OsWRKY93 sgRNA target. The reverse complementary sequence of the PAM sequence (5’-CGG-3’) of the sgRNA target is green. The red T represents a one-base insertion. (E) Representative leaves of Nipponbare and oswrky93-1 3 and 4 days after inoculation with M. oryzae. Pathogen infection assays were performed on three biological replicates.
In order to further confirm the role of OsWRKY93 in disease resistance, we generated oswrky93 mutants using CRISPR/Cas9 system in Nipponbare (Figure 4C). We found one mutant line oswrky93-1 that carries a one-base insertion in the first exon of the OsWRKY93 gene (Figure 4D). In contrast to Nipponbare plants, the CRISPR/Cas9-edited oswrky93 mutants are more susceptible to M. oryzae, showing more disease lesions and less healthy leaf area (Figure 4E), suggesting that oswrky93-1 plants exhibited elevated susceptibility to M. oryzae. Together with the results from the above analysis, these data imply the contribution of OsWRKY93 to rice defense against M. oryzae infection.
Detection of ROS Production in OsWRKY93 Transgenic Lines
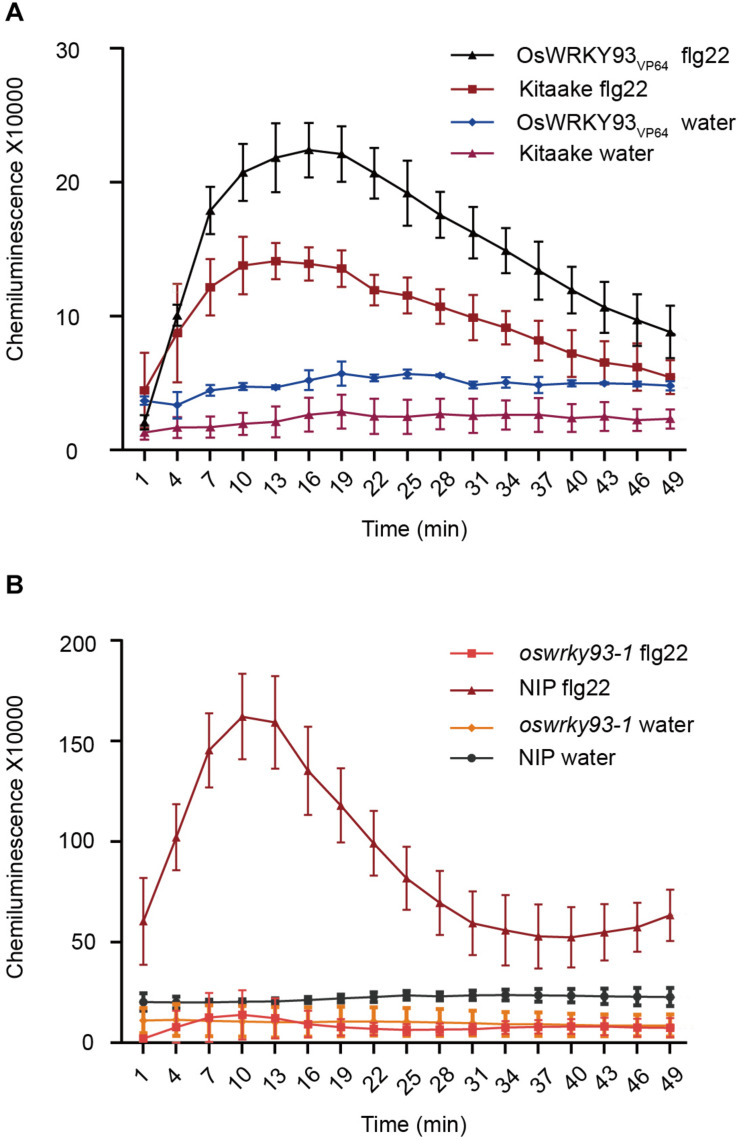
Reactive oxygen species (ROS) burst is a common feature in plant response to a number of biotic stresses, and flg22 has been shown to trigger ROS production in Arabidopsis (Mersmann et al., 2010). To examine whether enhanced-expression or knockout of OsWRKY93 affect ROS production after flg22 treatment, we collected leaves from the OsWRKY93VP64, oswrky93-1 and WT plants and measured immediately the ROS level after flg22 treatment. In our experiments, ROS production was increased in OsWRKY93VP64 activation plants after treatment with flg22, and the flg22-induced ROS generation was twofold higher, compared to the Kitaake plants control and water treatment (Figure 5A). As expected, no constitutive ROS production was observed in oswrky93-1 mutant plants (Figure 5B). Given these facts, we concluded that overexpressing OsWRKY93 enhances PAMP-triggered immune response in rice.
FIGURE 5.
ROS accumulation in rice leaves after flg22 treatment. (A) A flg22-induced ROS burst in the OsWRKY93VP64 and Kitaake plants. (B) A flg22-induced ROS burst in the oswrky93-1 and Nipponbare plants. Rice leaf disks were treated with 1 μM Flg22 or water. Error bars represents the SE (n = 3–5).
Detection of Darkness-Induced Leaf Senescence Phenotype in OsWRKY93 Transgenic Lines
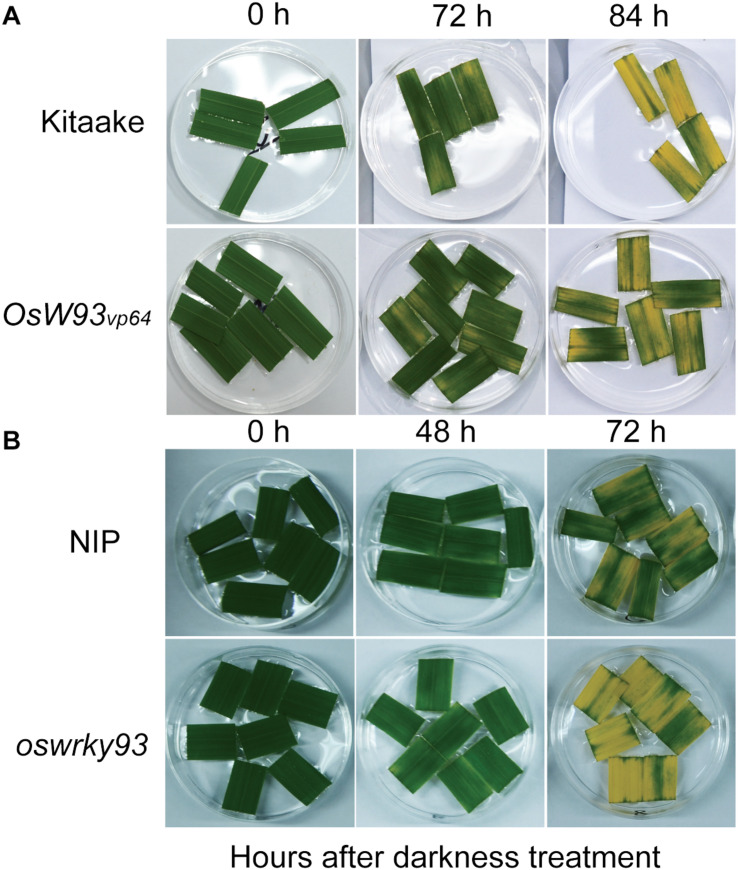
In order to further evaluate the potential role of OsWRKY93 in leaf senescence, the OsWRKY93vp64, oswrky93-1 mutant and two ecotypes of rice (Kitaake and NIP) plants were used for phenotype observation. The plants grown in the soil during the period of 39 days after germination did not show any visibly different phenotypes among enhanced-expression or knockout of OsWRKY93 and WT. However, the results of detached leaves after darkness treatment showed that the enhanced OsWRKY93 level clearly delayed leaf senescence after darkness treatment for 84 h in OsWRKY93vp64 line compared to Kitaake (Figure 6A), while knockout of OsWRKY93 apparently promoted leaf senescence after darkness treatment for 72 h in the oswrky93-1 line compared to NIP (Figure 6B). Therefore, OsWRKY93 plays function in darkness induced leaf senescence, although there is no visible senescence phenotype in the seedling stage of oswrky93 mutants.
FIGURE 6.
Phenotyping of detached leaves after darkness treatment. (A) A delaying leaf senescence shown in the OsWRKY93VP64 (OsW93vp64) compared to Kitaake plants. (B) An early leaf senescence shown in the oswrky93-1 compared to Nipponbare (NIP) plants. Detached leaf pieces of rice were incubated with 1 μM MES (pH8.5) buffer after darkness treatment for 0, 48, 72, and 84 h.
In view of these facts, OsWRKY93 is a new candidate protein for in-depth understanding of the regulatory mechanisms of pathogen-induced cell death and leaf senescence, helping in breeding high-yield and disease-resistant crops.
Discussion
Plant breeders are facing a serious challenge in rice production, that is, the premature senescence of leaves, in particular, flag leaves, which causes yield loss. There are, however, quite few studies that investigate the molecular mechanism of flag leaf senescence in rice. In this paper, we have identified 11 OsWRKYs that were differentially expressed during the senescence of flag leaves through RNA-Seq together with the RT-qPCR analysis. Importantly, we also surveyed the responses of 11 OsWRKY genes to M. oryzae to explore the correlation between leaf senescence and plant defense. Finally, we genetically identified OsWRKY93 as a new candidate protein for in-depth understanding of the regulatory mechanisms of pathogen-induced leaf senescence, helping in breeding high-yield and disease-resistant crops.
Our experimental results demonstrate that five senescence-inducible genes, OsWRKY2, OsWRKY14, OsWRKY26, OsWRKY69, and OsWRKY93, were induced in response to M. oryzae infection, implying that part of OsWRKY TFs connect leaf senescence and plant defense. In light of the fact that numerous studies have shown that the WRKY family plays a central role in leaf senescence as well as biotic stress tolerance (Bakshi and Oelmüller, 2014), it’s not surprising that some WRKY members might have dual functions between them, such as WRKY53, WRKY6, WRKY22, and WRKY70 in Arabidopsis (Robatzek and Somssich, 2002; Miao and Zentgraf, 2007; Rushton et al., 2010; Zhou et al., 2011; Hu et al., 2012; Chen J. et al., 2017; Zhou et al., 2018; Ramos et al., 2021). In this study, the transcript levels of OsWRKY93 increased as leaf senescence progressed, suggesting that OsWRKY93 is involved in the onset of flag leaf senescence. Gain-of OsWRKY93 delays a dark-induced leaf senescence, contrary to the loss-of OsWRKY93, and promotes a dark-induced leaf senescence (Figure 6). We further showed that rice transgenic plants overexpressing OsWRKY93 displayed an enhanced resistance to M. oryzae and the knockout oswrky93-1 mutants are more susceptible to M. oryzae. In addition, we also found that the OsWRKY93VP64 lines accumulated ROS highly in response to flg22 treatments (Figure 5A). In contrast, enhanced ROS production couldn’t be detected in the oswrky93-1 mutant plants (Figure 5B). These results clearly indicate that the senescence-inducible gene OsWRKY93 is also a positive regulator of the defense response in rice. These results also corroborate the findings of the previous study on OsWRKY23. As described in that paper, OsWRKY23 was strongly induced by dark-induced senescence and its overexpression in Arabidopsis increased tolerance to pathogen infection (Jing et al., 2009). In addition, as we knew, plant senescence is controlled by genetically materials and influenced by environmental cues. In this study our RT-qPCR profiles of a few of 11 candidate WRKYs are not matched well with RNA-seq data (Figure 2), an uncontrollable growth condition of different years might be one of reasons for a few OsWRKY members sensitively in response to unknown environmental factors.
Phylogenetic analyses of the WRKY domain sequences provide support for the hypothesis that gene duplication of single- and two-domain WRKY genes and loss of the WRKY domain occurred in the evolutionary history of this gene family in rice (Xie et al., 2005). Based on the number of WRKY domains and the characteristics of the zinc-finger-like motif, the WRKY family can be divided into three types. According to amino acid sequence similarity, 97 WRKY proteins in O. sativa were divided into three types and 13 groups, of which class II WRKYs were divided into 10 subclasses (IIa–IIj), and class III WRKYs were divided into two subclasses (IIIa and IIIb) (Qiu et al., 2004; Rushton et al., 2010). It has been reported that class II or III WRKY members are mostly involved in plant defense response (Dong et al., 2003; Cheng et al., 2019; Wang et al., 2020). Here, OsWRKY2, OsWRKY14, and OsWRKY26 belonged to class II of the WRKY family. OsWRKY69 and OsWRKY93 belonged to class III of the WRKY family. Interestingly, we found that the expression profiles of five OsWRKYs genes were altered in both after pathogen infection and during plant aging, which showed that OsWRKY2 was down-regulated: there was no resistance and no senescence; OsWRKY14 was down-regulated after infection but up-regulated during plant aging: there was no resistance and senescence; OsWRKY26 was up-regulated, with respect to both resistance and senescence; both OsWRKY69 and OsWRKY93 showed up-resistance after infection but were down-regulated during plant aging, with respect to resistance and no senescence (Table 1). Although the enhanced transgenic rice plants of OsWRKY2, OsWRKY14, and OsWRKY26 did not show significantly changing phenotypes of infection to M. oryzae at seedling stage, it is possible they rely on a specific kind of pathogen or developmentally dependent. OsWRKY69 and OsWRKY93, especially the latter, both are our favorite candidate genes for further in-depth understanding of their acting mechanism and the high yield and strong resistant genetically manipulation.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
YL, SL, PM, YP, YZ, and XZ performed the research. YM and YL designed the research and analyzed the data. YM and YX wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Wenxiong Lin and Zhixing Zhang, Key Laboratory of Crop Ecology and Molecular Physiology, Fujian Agriculture and Forestry University, Fuzhou, China, for kindly providing us with the OsWRKYs VP64 transgenic lines.
Footnotes
Funding. This work was financially supported by the China National Science Foundation (Grant Nos. 31801266 to YX; 32001437 to YZ), the Youth Project of Fujian Provincial Education Department (JAT190135 to YL), and the Key Project of Natural Science Foundation of Fujian Province (2015 N0019 to YM).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.643011/full#supplementary-material
Chlorophyll contents of rice flag leaves at six times during aging stages (0W, 1W, 2W, 3W, 4W, and 5W).
The transcript levels of five enhanced expression OsWRKYs VP64 transgenic lines compared to the Kitaake WT plants by RT-qPCR.
The infection phenotypes of five enhanced expression OsWRKYs VP64 transgenic lines to M. oryzae.
Primers used in this study.
Primers for genotyping CRISPR/Cas9 mutants.
The list of RPKM values and WRKY family DEGs.
References
- Bakshi M., Oelmüller R. (2014). WRKY transcription factors: jack of many trades in plants. Plant Signal. Behav. 9:e27700. 10.4161/psb.27700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Nolan T. M., Ye H., Zhang M., Tong H., Xin P., et al. (2017). Arabidopsis WRKY46, WRKY54, and WRKY70 transcription factors are involved in brassinosteroid-regulated plant growth and drought responses. Plant Cell 29 1425–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Xiang S., Chen Y., Li D., Yu D. (2017). Arabidopsis WRKY45 interacts with the DELLA protein RGL1 to positively regulate age-triggered leaf senescence. Mol. Plant 10 1174–1189. 10.1016/j.molp.2017.07.008 [DOI] [PubMed] [Google Scholar]
- Cheng H., Li H., Deng Y., Xiao J., Li X., Wang S. (2015). The WRKY45-2-WRKY13-WRKY42 transcriptional regulatory cascade is required for rice resistance to fungal pathogen. Plant Physiol. 167 1087–1099. 10.1104/pp.114.256016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X., Zhao Y., Jiang Q., Yang J., Zhao W., Taylor I. A., et al. (2019). Structural basis of dimerization and dual W-box DNA recognition by rice WRKY domain. Nucleic Acids Res. 47 4308–4318. 10.1093/nar/gkz113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chujo T., Miyamoto K., Shimogawa T., Shimizu T., Otake Y., Yokotani N., et al. (2013). OsWRKY28, a PAMP-responsive transrepressor, negatively regulates innate immune responses in rice against rice blast fungus. Plant Mol. Biol. 82 23–37. 10.1007/s11103-013-0032-5 [DOI] [PubMed] [Google Scholar]
- Chujo T., Takai R., Akimoto-Tomiyama C., Ando S., Minami E., Nagamura Y., et al. (2007). Involvement of the elicitor-induced gene OsWRKY53 in the expression of defense-related genes in rice. Biochim. Biophys. Acta 1769 497–505. 10.1016/j.bbaexp.2007.04.006 [DOI] [PubMed] [Google Scholar]
- Dong J., Chen C., Chen Z. (2003). Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol. Biol. 51 21–37. [DOI] [PubMed] [Google Scholar]
- Edwards K., Johnstone C., Thompson C. (1991). A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res. 19:1349. 10.1093/nar/19.6.1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulgem T., Rushton P. J., Robatzek S., Somssich I. E. (2000). The WRKY superfamily of plant transcription factors. Trends Plant Sci. 5 199–206. 10.1016/S1360-1385(00)01600-9 [DOI] [PubMed] [Google Scholar]
- Gan S., Amasino R. M. (1995). Inhibition of leaf senescence by autoregulated production of cytokinin. Science 270 1986–1988. 10.1126/science.270.5244.1986 [DOI] [PubMed] [Google Scholar]
- Ghosh S., Sahai V. N., Saran S. (1990). Role of flag leaf on grain yield and spikelet sterility in rice cultivar. Oryza 27 87–89. [Google Scholar]
- Guo P., Li Z., Huang P., Li B., Fang S., Chu J., et al. (2017). A tripartite amplification loop involving the transcription factor WRKY75, salicylic acid, and reactive oxygen species accelerates leaf senescence. Plant Cell 29 2854–2870. 10.1105/tpc.17.00438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Gan S. (2005). Leaf senescence: signals, execution, and regulation. Curr. Top. Dev. Biol. 71 83–112. 10.1016/S0070-2153(05)71003-6 [DOI] [PubMed] [Google Scholar]
- Han M., Kim C.-Y., Lee J., Lee S.-K., Jeon J.-S. (2014). OsWRKY42 represses OsMT1d and induces reactive oxygen species and leaf senescence in Rice. Mol. Cells 37 532–539. 10.14348/molcells.2014.0128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himelblau E., Amasino R. M. (2001). Nutrients mobilized from leavesof Arabidopsis thaliana during leaf senescence. J. Plant Physiol. 158 1317–1323. 10.1078/0176-1617-00608 [DOI] [Google Scholar]
- Hinderhofer K., Zentgraf U. (2001). Identification of a transcription factor specifically expressed at the onset of leaf senescence. Planta 213 469–473. 10.1007/s004250000512 [DOI] [PubMed] [Google Scholar]
- Hu Y., Dong Q., Yu D. (2012). Arabidopsis WRKY46 coordinates with WRKY70 and WRKY53 in basal resistance against pathogen Pseudomonas syringae. Plant Sci. 185–186 288–297. 10.1016/j.plantsci.2011.12.003 [DOI] [PubMed] [Google Scholar]
- Jing S., Zhou X., Song Y., Yu D. (2009). Heterologous expression of OsWRKY23 gene enhances pathogen defense and dark-induced leaf senescence in Arabidopsis. Plant Growth Regul. 58 181–190. 10.1007/s10725-009-9366-z [DOI] [Google Scholar]
- Kaneda T., Taga Y., Takai R., Iwano M., Matsui H., Takayama S., et al. (2009). The transcription factor OsNAC4 is a key positive regulator of plant hypersensitive cell death. EMBO J. 28 926–936. 10.1038/emboj.2009.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T., Kang K., Kim S.-H., An G., Paek N.-C. (2019). OsWRKY5 promotes rice leaf senescence via senescence-associated NAC and abscisic acid biosynthesis pathway. Int. J. Mol. Sci. 20:4437. 10.3390/ijms20184437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. K., Pinson S. R. M., Stansel J. W., Paterson A. H. (1998). Genetic dissection of the source-sink relationship affecting fecundity and yield in rice (Oryza sativa L.). Mol. Breed. 4 419–426. [Google Scholar]
- Lim P. O., Kim H. J., Nam H. G. (2007). Leaf senescence. Annu. Rev. Plant Biol. 58 115–136. [DOI] [PubMed] [Google Scholar]
- Ma Y. F., Lu D. Z. (1990). Effect of irrigation modes on the senescence and physiological activity in hybrid rice after heeding. Chin. J. Rice Sci. 4 56–62. [Google Scholar]
- Mersmann S., Bourdais G., Rietz S., Robatzek S. (2010). Ethylene signaling regulates accumulation of the FLS2 receptor and is required for the oxidative burst contributing to plant immunity. Plant Physiol. 154 391–400. 10.1104/pp.110.154567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y., Laun T., Zimmermann P., Zentgraf U. (2004). Targets of the WRKY53 transcription factor and its role during leaf senescence in Arabidopsis. Plant Mol. Biol. 55 853–867. 10.1007/s11103-005-2142-1 [DOI] [PubMed] [Google Scholar]
- Miao Y., Zentgraf U. (2007). The antagonist function of Arabidopsis WRKY53 and ESR/ESP in leaf senescence is modulated by the jasmonic and salicylic acid equilibrium. Plant Cell 19 819–830. 10.1105/tpc.106.042705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolayeva O., Robinson M. D. (2014). edgeR for Differential RNA-seq and ChIP-seq analysis: an application to stem cell biology. Methods Mol. Biol. 1150 45–79. 10.1007/978-1-4939-0512-6_3 [DOI] [PubMed] [Google Scholar]
- Pic E., de La Serve B. T., Tardieu F., Turc O. (2002). Leaf senescence induced by mild water deficit follows the same sequence of macroscopic, biochemical, and molecular events as monocarpic senescence in pea. Plant Physiol. 128 236–246. 10.1104/pp.128.1.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y. P., Jing S. J., Fu J., Li L., Yu D. Q. (2004). Cloning and analysis of expression profile of 13 WRKY genes in rice. Chin. Sci. Bull. 49 2159–2168. 10.1360/982004-183 [DOI] [Google Scholar]
- Ramos R. N., Martin G. B., Pombo M. A., Rosli H. G. (2021). WRKY22 and WRKY25 transcription factors are positive regulators of defense responses in Nicotiana benthamiana. Plant Mol. Biol. 105 65–82. 10.1007/s11103-020-01069-w [DOI] [PubMed] [Google Scholar]
- Riefler M., Novak O., Strnad M., Schmulling T. (2006). Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell 18 40–54. 10.1105/tpc.105.037796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robatzek S., Somssich I. E. (2001). A new member of the Arabidopsis WRKY transcription factor family, AtWRKY6, is associated with both senescence- and defence-related processes. Plant J. 28 123–133. 10.1046/j.1365-313X.2001.01131.x [DOI] [PubMed] [Google Scholar]
- Robatzek S., Somssich I. E. (2002). Targets of AtWRKY6 regulation during plant senescence and pathogen defense. Genes Dev. 16 1139–1149. 10.1101/gad.222702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton P. J., Somssich I. E., Ringler P., Shen Q. J. (2010). WRKY transcription factors. Trends Plant Sci. 15 247–258. [DOI] [PubMed] [Google Scholar]
- Ryu H. S., Han M., Lee S. K., Cho J. I., Ryoo N., Heu S., et al. (2006). A comprehensive expression analysis of the WRKY gene superfamily in rice plants during defense response. Plant Cell Rep. 25 836–847. 10.1007/s00299-006-0138-1 [DOI] [PubMed] [Google Scholar]
- Sadowski I., Ma J., Triezenberg S., Ptashne M. (1988). GAL4-VP16 is an unusually potent transcriptional activator. Nature 335 563–564. 10.1038/335563a0 [DOI] [PubMed] [Google Scholar]
- Shimono M., Sugano S., Nakayama A., Jiang C.-J., Ono K., Takatsuji H., et al. (2007). Rice WRKY45 plays a crucial role in benzothiadiazole-inducible blast resistance. Plant Cell 19 2064–2076. 10.1105/tpc.106.046250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo K. T., Kim C. Y., Hoang T. V., Lee S. K., Shirsekar G., Seo Y. S., et al. (2018). OsWRKY67 plays a positive role in basal and XA21-mediated resistance in rice. Front. Plant Sci. 8:2220. 10.3389/fpls.2017.02220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Wang L., Su W., Ren Y., You C., Zhang C., et al. (2020). A class III WRKY transcription factor in sugarcane was involved in biotic and abiotic stress responses. Sci. Rep. 10:20964. 10.1038/s41598-020-78007-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T., Ou B., Li J., Zhao Y., Guo D., Zhu Y., et al. (2013). Transcriptional profiling of rice early response to Magnaporthe oryzae identified OsWRKYs as important regulators in rice blast resistance. PLoS One 8:e59720. 10.1371/journal.pone.0059720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z., Zhang Z. L., Zou X., Huang J., Ruas P., Thompson D., et al. (2005). Annotations and functional analyses of the rice WRKY gene superfamily reveal positive and negative regulators of abscisic acid signaling in aleurone cells. Plant Physiol. 137 176–189. 10.1104/pp.104.054312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghmai R., Cutting G. R. (2002). Optimized regulation of gene expression using artificial transcription factors. Mol. Ther. 5 685–694. 10.1006/mthe.2002.0610 [DOI] [PubMed] [Google Scholar]
- Yokotani N., Sato Y., Tanabe S., Chujo T., Shimizu T., Okada K., et al. (2013). WRKY76 is a rice transcriptional repressor playing opposite roles in blast disease resistance and cold stress tolerance. J. Exp. Bot. 64 5085–5097. 10.1093/jxb/ert298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D., Chen C., Chen Z. (2001). Evidence for an important role of WRKY DNA binding proteins in the regulation of NPR1 gene expression. Plant Cell 13 1527–1539. 10.1105/TPC.010115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Peng Y., Guo Z. (2008). Constitutive expression of pathogen-inducible OsWRKY31 enhances disease resistance and affects root growth and auxin response in transgenic rice plants. Cell Res. 18 508–521. 10.1038/cr.2007.104 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang H.-L., Li Z., Guo H. (2020). Genetic network between leaf senescence and plant immunity: crucial regulatory nodes and new insights. Plants 9:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Lu Y., Bethke G., Harrison B. T., Hatsugai N., Katagiri F., et al. (2018). WRKY70 prevents axenic activation of plant immunity by direct repression of SARD1. New Phytol. 217 700–712. [DOI] [PubMed] [Google Scholar]
- Zhou X., Jiang Y., Yu D. (2011). WRKY22 transcription factor mediates dark-induced leaf senescence in Arabidopsis. Mol. Cells 31 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chlorophyll contents of rice flag leaves at six times during aging stages (0W, 1W, 2W, 3W, 4W, and 5W).
The transcript levels of five enhanced expression OsWRKYs VP64 transgenic lines compared to the Kitaake WT plants by RT-qPCR.
The infection phenotypes of five enhanced expression OsWRKYs VP64 transgenic lines to M. oryzae.
Primers used in this study.
Primers for genotyping CRISPR/Cas9 mutants.
The list of RPKM values and WRKY family DEGs.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.