Abstract
Forward propulsion during the push-off phase of walking is largely governed at the ankle by differential neuromechanical contributions from the biarticular medial (MG) and lateral gastrocnemii (LG) and the uniarticular soleus (SOL). However, the relative contribution of these individual muscles to forward propulsion is equivocal, with important implications for the design and control of wearable assistive devices and for targeted therapeutics. The aim of this study was to evaluate the agreement between empirical and model-predicted triceps surae contributions to forward propulsion during walking using conditions that systematically manipulated both walking speed and the mechanical demand for forward propulsion at a fixed speed – through the use of aiding and impeding forces. Ten young adults (age: 24.1±3.6 years, 6M/4F) participated. We found that muscle-specific responses derived from experimental measurements (i.e., activation and fascicle behavior) were consistent with those derived from musculoskeletal simulations (i.e., muscle force and positive mechanical work) within the same subjects. In vivo, compared to walking normally, only LG muscle activation was affected by both aiding and impeding forces. Similarly, increased propulsive demand elicited greater relative fascicle shortening in the MG but not the SOL. In silico, only MG and LG force and positive mechanical work increased significantly to meet the increased demands for forward propulsion. By combining electromyography, ultrasound imaging, and musculoskeletal modeling in the same subjects, our cumulative findings suggest that the biarticular gastrocnemius muscles play a more significant role than the uniarticular soleus in governing changes in forward propulsion during the mid to late stance phase of walking.
Keywords: Triceps Surae, Neuromechanics, Ultrasound, OpenSim, Forward Propulsion
Introduction
Forward propulsion during the push-off phase of walking is largely governed by differential neuromechanical contributions from the biarticular medial (MG) and lateral gastrocnemius (LG) and the uniarticular soleus (SOL) spanning the ankle.30 Indeed, although they share a common Achilles tendon, some evidence suggests these muscles can act as relatively independent actuators while producing a net plantarflexion moment during late stance.7, 31, 61 However, the relative contribution of these individual muscles to forward propulsion remains equivocal. Characterizing these contributions has far reaching implications for the design and control of wearable assistive devices and for targeted therapeutics that attempt to overcome deficits in forward propulsion - for example, those due to age or gait pathology.
Historically, contributions from MG, LG, and SOL to forward propulsion in walking have been estimated by combining in vivo measurements (e.g., electromyography [EMG] and ultrasound imaging) with experimental manipulations. For example, one may consider changing the mechanical demand for forward propulsion by altering walking speed alone. However, walking speed affects both forward propulsion and vertical support, confounding conclusions made regarding inter-muscular differences.24, 38 Many studies have overcome this challenge by more directly manipulating the mechanical demand for forward propulsion at a fixed walking speed. Building off a paradigm introduced by Chang & Kram,5 Gottschall & Kram (2003) measured MG vs. SOL differences in muscle activation in subjects responding to horizontal aiding and impeding forces applied to the body’s center of mass.30 Their findings revealed that MG activation exhibited larger changes than the SOL in response to those targeted changes in the demand for forward propulsion. Similarly, Miyoshi et al. (2006) recorded triceps surae activation in a reduced gravity environment and suggested that MG activation was more dependent on speed-related changes to forward propulsion, while SOL activation was more dependent on changes to vertical support.47 In a third experimental manipulation, Francis et al. (2013) prescribed muscle activation during the stance phase at a fixed walking speed using electrical surface stimulation of the MG and SOL.24 Consistent with the prevailing empirical data, their results showed that MG stimulation elicited larger anterior shifts in stance phase center of pressure than that elicited by SOL stimulation. However, not all experimental studies yield a consistent interpretation; McGowen et al. (2008) manipulated trunk load and weight support and determined from EMG measurements that the SOL was the primary contributor to forward propulsion.46
EMG provides important information regarding the timing and magnitude of triceps surae muscle activity. However, EMG alone can yield inconsistent or incorrect interpretations of underlying muscle force and work – themselves highly dependent on muscle fascicle length and length change.17, 58 Advances in ultrasound imaging have catalyzed efforts to quantify triceps surae contractile behavior during functional activities such as walking, including the characterization of inter-muscular differences in fascicle length change. Despite considerable improvements in image collection and processing, there is wide variation regarding triceps surae contractile behavior in terms of absolute length change and type of contraction (i.e., shortening, isometric, lengthening) at different phases of the gait cycle.11, 39 Moreover, studies that have compared MG, LG, and/or SOL fascicle behavior during walking use methodological paradigms that manipulate speed (References12, 22, 38), walking duration (References11, 13), or slope (References36, 41). Although these studies are appropriately designed to answer their respective research questions, none have directly manipulated the mechanical demand for forward propulsion in isolation. Moreover, similar to those drawn from patterns of muscle activation, conclusions about inter-muscular differences in function drawn from estimates of muscle fascicle length change are incomplete.16, 30
Thus, to reconcile the prevailing literature on this scientifically and translationally important question, a combined empirical and computational simulation framework is warranted. Advances in musculoskeletal modeling provide the opportunity to complement patterns of muscle activation and fascicle length changes with estimated differences between MG, LG, and SOL force and positive mechanical work in silico. Of course, although empirically driven, biological complexity of musculoskeletal modeling is often exchanged for computational performance, potentially yielding reduced specificity to fully characterize inter-muscular differences.34 Despite these limitations, several studies have directly or indirectly compared the biomechanical function of the MG, LG, and SOL during walking. Some modeling studies suggest that the SOL is the primary contributor to forward propulsion. Neptune et al. (2001) used a musculoskeletal modeling and optimization framework and concluded that the SOL accelerates the trunk, whereas the MG and LG initiate leg swing.49 Those authors later added (2008) that the SOL contributes more than the MG and LG to forward propulsion in order to meet the demands of increased walking speeds.51 In a different modeling study, Lenhart et al. concluded that SOL activity induced greater anterior pelvic tilt and ankle plantarflexion than that of the MG during the push-off phase of walking.40 Nonetheless, other modeling studies have concluded that the gastrocnemius muscles are more responsible for forward propulsion. For example, using an induced acceleration analysis, Liu et al. (2006) suggested that the MG induced greater forward center of mass acceleration, while the SOL induced greater vertical acceleration.42
Electromyography, ultrasound imaging, and musculoskeletal modeling, when used alone, provide a valuable but incomplete representation of the relative contributions of the MG, LG, and SOL to forward propulsion during walking. Moreover, no study to date has combined these measurements while systematically manipulating both walking speed and the mechanical demand for forward propulsion at a fixed speed – namely, through the use of horizontal aiding and impeding forces. The aim of this study was to quantify inter-muscular differences in the response of the gastrocnemius and soleus muscles to changes in forward propulsion by assessing muscle activation, contractile behavior, and model-predicted estimates of force and positive mechanical work. We tested the null hypothesis that conclusions based on muscle-specific responses derived from empirical measurements would be consistent with those derived from musculoskeletal simulations over a range of tasks that alter the demand for forward propulsion.
Materials and Methods
Subjects and protocol
An a priori power analysis revealed that we would need n=6 and n=5 subjects to detect (p<0.05) speed effects52 and horizontal force effects30, respectively, on peak anterior force – a limb-level measure of forward propulsion we use in this study to establish task efficacy. We increase our sample size to n=10 to better approximate that used in those aforementioned studies. We recruited healthy young adults between the ages of 18 and 35. We excluded subjects based on the following criteria: lower extremity injury in the last 6 months, medication that causes dizziness, having a leg prosthesis, or needing an assistive walking device. Ten young adults (age: 24.1±3.6 years, height: 1.77±0.08 m, mass: 76.7 ± 10.0 kg, 6M/4F) participated. All subjects provided written informed consent in compliance with the UNC Biomedical Sciences Institutional Review Board.
Figure 1 summarizes our experimental protocol, measurements, and in vivo and in silico analyses. Based on a previous study in our lab, young adults have an average overground preferred walking speed of 1.3 m/s.8 Malatesta et al. (2017) revealed that the overground preferred walking speed in younger adults averages 0.12 m/s faster than the treadmill preferred walking speed.44 As such, we chose 1.2 m/s to represent our “normal” treadmill walking speed. Prior to data collection, subjects walked on an instrumented treadmill (Bertec Corp., Columbus, Ohio, USA) for 6 minutes at 1.2 m/s to precondition their triceps surae muscle-tendon units and to acclimate to treadmill walking.32 Subjects then walked for 1 min each at a range of speeds (0.8, 1.2, and 1.6 m/s) and again at 1.2 m/s with: (i) a 5% body weight (BW) horizontal aiding force (designed to decrease the mechanical demand for forward propulsion) and (ii) a 5% BW horizontal impeding force (designed to increase the mechanical demand for forward propulsion). We applied horizontal aiding and impeding forces using a custom, feedback-controlled, motor-driven horizontal force system described in detail previously (Fig. 1A).8–10 Briefly, in real-time, a LabVIEW interface (cRIO-9064, National Instruments, Austin, TX, USA) controlled a servo motor (Kollmorgen, Radford, VA, USA) in series with a horizontal cable affixed to a waist belt worn by the subject. During trials with horizontal forces, subjects received verbal encouragement to maintain upright posture (i.e., to avoid excess trunk lean and lumbar bending). All data was collected as part of a larger study that incorporated a second ultrasound probe, secured distal to the right soleus muscle-tendon junction. We removed the second probe at speeds greater than 1.2 m/s to ensure subject comfort and range of motion. As such, trials were block randomized. The first block contained 5 walking conditions (speed ≤ 1.2 m/s), 4 reported in this study (0.8 m/s, 1.2 m/s, 5% BW aiding force at 1.2 m/s, and 5% BW impeding force at 1.2 m/s). The second block contained 4 walking conditions (speed ≥ 1.2 m/s), 1 reported in this study (1.6 m/s). All trials were 1 min long and were separated by 2 min of rest.
Figure 1.
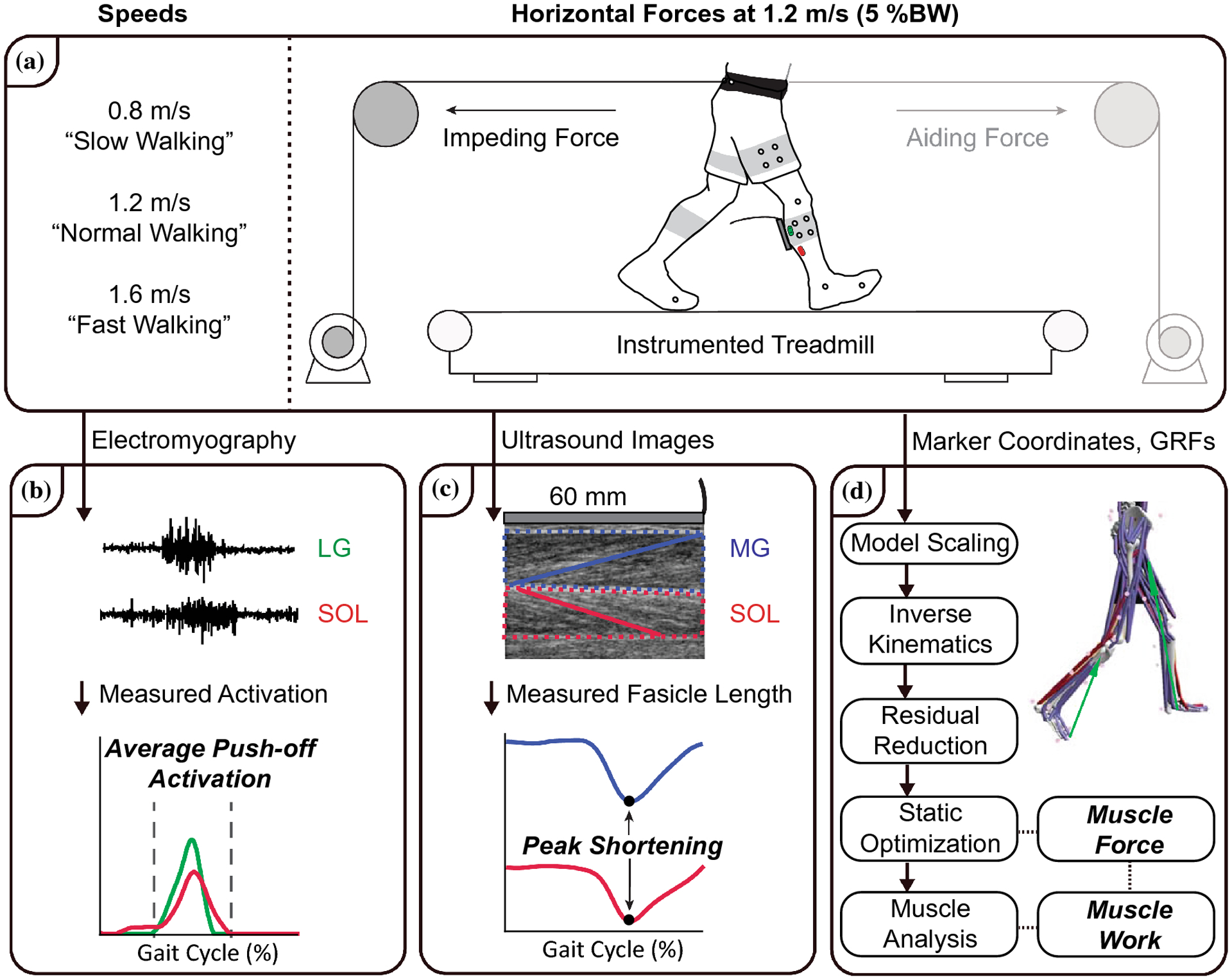
Summary of our experimental protocol, measurements, and in vivo and in silico analyses. A) Subjects walked for 1 minute on an instrumented treadmill at a range of speeds (0.8, 1.2, and 1.6 m/s) and again at 1.2 m/s with a 5% body weight (BW) horizontal aiding force and a 5% BW horizontal impeding force. B) We recorded EMG activity of the lateral gastrocnemius (LG) and soleus (SOL) and determined the average activation during the push-off phase of walking. C) We collected ultrasound data of the medial gastrocnemius (MG) and SOL and quantified time series of fascicle lengths from 2 strides per condition. D) Subject-specific, scaled musculoskeletal models, driven by marker coordinates and ground reaction forces (GRFs), estimated MG, LG and SOL excitation, force, and positive mechanical work using static optimization.
Experimental measurements and analyses
For all trials, we collected ground reaction forces from the instrumented treadmill at 1000 Hz. Simultaneously, twelve cameras (Motion Analysis Corporation, Santa Rosa, CA, USA) operating at 100 Hz recorded the three-dimensional positions of 34 retroreflective markers. Specifically, we attached anatomical markers to subject’s sacrum and bilateral anterior and posterior superior iliac spines, lateral femoral condyles, lateral malleoli, 1st and 5th metatarsal heads, and calcanei. We also secured right and left leg thigh and shank clusters to improve segment tracking. We filtered ground reaction force and marker data using a 4th order low-pass Butterworth filter with cutoff frequencies of 100 Hz and 6 Hz, respectively.62 For electromyographic (EMG) measurements, prior to sensor placement, we prepped the skin by shaving and using an alcohol wipe. We then placed differential wireless recording electrodes (Trigno, Delsys Inc., Natick, MA, USA) with 10 mm inter-electrode distance over the right leg soleus (SOL) and lateral gastrocnemius (LG) using published recommendations.33 Specifically, we placed the SOL recording electrode approximately two-thirds distal to the lateral femoral condyles along a line projecting to the lateral malleolus. We placed the LG recording electrode approximately one-third distal to the head of the fibula along a line projecting to the calcaneus. Post collection, we demeaned, full-wave rectified, bandpass filtered (4th order Butterworth, 20–450 Hz), and normalized each EMG signal to its mean value during the 1.2 m/s condition (Fig. 1B). We then averaged GRF and EMG measurements over 10 strides, with the first stride corresponding to the first heel strike captured by ultrasound data, detailed below.
For individual muscle fascicle measurements, a 60 mm ultrasound transducer (LV7.5/60/128Z-2, UAB Telemed, Vilnius, Lithuania) recorded cine B-mode images through the right medial gastrocnemius (MG) and SOL at 76 frames per second using an image depth of 50 mm (Fig. 1C). A 1000 Hz binary analog synchronization signal indicated the start and stop of each ultrasound video using a wave form generator (SDG1025, SIGLENT, Shenzhen). We co-registered ultrasound signals with ground reaction forces and determined the frames corresponding to each heel strike for strides coincident with the B-mode collection. The same investigator processed all ultrasound data to minimize inter-investigator variability. Ultrasound data was collected after the subject reached their prescribed walking speed and their movement pattern had stabilized. At the first co-registered heel strike, we defined a static region of interest surrounding the MG and SOL muscle bellies and aponeuroses. We then defined one MG and one SOL muscle fascicle for each muscle. An affine extension to an optic flow algorithm (UltraTrack) quantified time series of MG and SOL fascicle lengths from 2 strides per condition.19 We visually confirmed tracking results and manually corrected fascicle endpoints when necessary. Fascicle length change measurements were normalized by their length at heel strike. We filtered the manually corrected tracking results using a 4th order low-pass Butterworth filter with a cutoff frequency of 6 Hz and then averaged the results over 2 strides.
Musculoskeletal modeling and simulation
For each subject, we scaled a three-dimensional, 23-degree-of-freedom musculoskeletal model with 92 Hill-type musculotendon units (Gait 2392, OpenSim 4.0)14 to a standing calibration trial (Fig. 1D). For all subsequent processing, and consistent with published approaches,56 we locked the subtalar and metatarsophalangeal joints and assessed model-predicted outcomes from the first stride analyzed for ultrasound and EMG outcomes. For those, we also locked the lumbar joints to overcome our lack of trunk kinematic data. As recommended by Arnold et al., we set tendon strain at maximum isometric force (i.e., “FmaxTendonStrain”) for each muscle to 4%, except the MG, LG, and SOL, which we set to 10% to account for Achilles tendon compliance.3, 21 Likewise, we increased the maximum isometric force of all muscles to 2.0 times default.3 The Inverse Kinematics (IK) Tool determined generalized joint angles for each time step (marker root mean square [RMS] < 1.0 cm).14, 34 Driven by IK results and measured ground reaction forces, the Residual Reduction Algorithm (RRA) Tool updated segment masses, marker trajectories, and center of mass (CoM) location to minimize required compensatory forces. We continued reducing residuals (average uses of RRA per trial: 2.08±0.27) until recommended thresholds were satisfied in all cases (total mass adjustment < 0.5 kg, ΔCoM < 2.0 cm, RMS residual force < 5 N, RMS Residual Moment < 30 Nm).14, 34 Using RRA-adjusted kinematics and segmental mass properties, the Static Optimization Tool resolved net joint moments into individual muscle forces by minimizing the sum of cubed muscle activations.48, 64 Finally, the Analysis Tool estimated MG, LG, and SOL positive mechanical work (i.e., positive area under the respective force-velocity curves) and, to provide context for muscle fascicle length measurements, muscle-tendon unit (MTU) lengths. Similar to fascicle length measurements, MTU length change results were normalized by their length at heel strike.
Statistical analysis
For each muscle, we defined the “push-off” phase of the gait cycle from midstance to the offset of respective model-predicted muscle force generation (threshold = 10% peak force).30 For experimental outcomes, two repeated-measures ANOVAs tested for significant main effects of speed (0.8, 1.2, 1.6 m/s) or horizontal forces (5% BW aiding force, 1.2 m/s, 5% BW impeding force) on MG and SOL average fascicle length and peak fascicle shortening and, during the push-off phase of walking, average LG and SOL activation. Similarly, for model-predicted outcomes, two repeated-measures ANOVAs tested for significant main effects of speed and horizontal forces on peak MG, LG and SOL MTU shortening and, during the push-off phase of walking, average muscle force and positive mechanical work. Mauchly’s test of sphericity evaluated the homogeneity of variance; when the assumption was violated, Greenhouse-Geisser adjustments were applied. When significant main effects were identified, post-hoc Tukey’s t-tests identified significant differences vs. “normal” walking (i.e., 1.2 m/s). All statistical tests used an alpha level of 0.05. Effect sizes are reported as and Cohen’s d for main effects and pairwise comparisons, respectively.
Results
Experimental outcomes
Throughout our results narrative, we report pairwise comparisons that support the presence of inter-muscular differences in response to task demand. Where indicated, main effects were driven by increases in said outcome with faster walking speeds, with pairwise comparisons disclosed in their respective figures. Our experimental manipulations successfully altered the mechanical demand for forward propulsion; peak anterior ground reaction force systematically changed in response to changes in speed (main effect, p<0.001, ) and applied horizontal forces (main effect, p<0.001, ). LG and SOL muscle activations during push-off were significantly affected by speed (main effect, p-values<0.001, , Fig. 2A) and horizontal forces (main effect, p-values<0.001, , Fig. 2B). However, pairwise comparisons revealed that only LG activation was affected by both aiding (decreased) and impeding (increased) forces (p-values≤0.001, d≥1.171). Similarly, MG and SOL peak fascicle shortening and average lengths were significantly affected by speed (main effect, p-values≤0.003, , Fig. 3A) and horizontal forces (main effect, p-values≤0.004, , Fig. 3B). However, compared to walking normally, increased push-off demand (i.e., walking with impeding forces) elicited greater peak shortening in MG (p=0.006, d=0.874) but not SOL (p=0.15, d=0.714). Moreover, this effect on MG was not explained by changes in joint posture. Indeed, only SOL peak MTU shortening was significantly affected by horizontal forces (main effect, p-values=0.004, ); MG peak MTU shortening was unaffected (main effect, p-values=0.637, ). Compared to walking normally, both aiding and impeding forces resulted in significant differences in average fascicle length for MG (p-values≤0.021, d≥0.362, Fig. 4B but not SOL (p-values≥0.078, d≤0.33). Neither MG nor SOL average MTU length was affected by horizontal forces (main effect, p-values≥0.139, ).
Figure 2.

A) Lateral gastrocnemius (LG-green) and soleus (SOL-red) average muscle activation during push-off were similarly affected by changes in speed. B) In response to horizontal forces only LG activation was affected by both aiding and impeding forces. Shaded rectangular area represents the push-off phase of walking. Shaded (LG-green, SOL-red) represents standard error during normal walking (1.2 m/s). Boxplots show average muscle activation during push-off. Pairwise comparison vs. normal walking (1.2 m/s) represented by asterisks.
Figure 3.

Time series of medial gastrocnemius (MG-blue) and soleus (SOL-red) fascicle shortening during changes in speed (A) and horizontal forces (B). Fascicle length change measurements were normalized by their length at heel strike. Shaded area represents standard error during normal walking (1.2 m/s). Boxplots show peak fascicle shortening. Significant (p<0.05) pairwise comparisons vs. normal walking (1.2 m/s) represented by asterisks.
Figure 4.

Time series of medial gastrocnemius (MG-blue) and soleus (SOL-red) fascicle length during changes in speed (A) and horizontal forces (B). Shaded area represents standard error during normal walking (1.2 m/s). Boxplots show average fascicle length over the entire gait cycle. Significant (p<0.05) pairwise comparisons vs. normal walking (1.2 m/s) represented by asterisks.
Musculoskeletal modeling outcomes
Model-predicted MG, LG, and SOL muscle forces averaged during push-off were similarly affected by walking speed (main effect, p-values≤0.042, , Fig. 5A). Conversely, only average MG and LG muscle forces were significantly affected by horizontal forces (main effect, p-values<0.001, , Fig. 5B). Specifically, compared to walking normally, average MG and LG muscles forces decreased in response to aiding forces (p-values≤0.005, d≥1.085). Changes in walking speed did not affect model-predicted MG, LG, and SOL positive mechanical work (main effect, p-values≥0.331, , Fig. 6A). However, only MG and LG positive mechanical work were significantly affected by horizontal forces (main effect, p-values<0.001, , Fig. 6B); SOL positive mechanical work was unaffected (main effect, p=0.236, ). Specifically, compared to walking normally, MG and LG positive mechanical work decreased in response to aiding forces (p-values≤0.045, d≥0.357) and increased in response to impeding forces (p-values≤0.014, d≥0.287). Finally, model-predicted estimates of MG, LG, and SOL activation averaged during push-off were significantly affected by changes in speed (main effect, p-values≤0.001, , Fig. 7A) and horizontal forces (main effect, p-values≤0.008, Fig. 7B). However, compared to walking normally, only MG and LG activation significantly decreased in response to aiding forces (p-values≤0.001, d≥1.156).
Figure 5.

A) Model-predicted LG (green), MG (blue), and SOL (red) muscle forces averaged during push-off were similarly affected by walking speed. B) Conversely, only average LG and MG muscle forces were significantly affected by horizontal forces. Shaded grey rectangular area represents the push-off phase of walking. Shaded (LG-green, MG-blue, SOL-red) represents standard error during normal walking (1.2 m/s). Boxplots show average muscle force during push-off. Significant (p<0.05) pairwise comparisons vs. normal walking (1.2 m/s) represented by asterisks.
Figure 6.

A) Model-predicted LG (green), MG (blue), and SOL (red) positive work during push-off were unaffected by changes in walking speed. B) Conversely, only LG and MG positive work were significantly affected by horizontal forces. Boxplots show positive work performed during push-off. Significant (p<0.05) pairwise comparisons vs. normal walking (1.2 m/s) represented by asterisks.
Figure 7.

A) Model-predicted LG (green), MG (blue), and SOL (red) muscle forces averaged during push-off were similarly affected by walking speed. B) Compared to walking normally, only LG and MG activation significantly decreased in response to aiding forces (−19% and −19.3%, respectively). Shaded grey rectangular area represents the push-off phase of walking. Shaded (LG-green, MG-blue, SOL-red) represents standard error during normal walking (1.2 m/s). Shaded purple area represents the standard deviation of all associated experimental trials for the LG and SOL, normalized to peak model-predicted activation during normal walking. Boxplots show average muscle activation during push-off. Significant (p<0.05) pairwise comparisons vs. normal walking (1.2 m/s) represented by asterisks.
Discussion
The overarching goal of this study was to quantify inter-muscular differences between the medial gastrocnemius (MG), lateral gastrocnemius (LG), and soleus (SOL) in response to changes in forward propulsion during walking. Although many studies have added significant value to this ongoing scientific discussion, the wide array of in vivo measurements, in silico estimates, and experimental manipulations have led to considerable differences in inter-muscular conclusions. Here, toward a more complete understanding of triceps surae behavior, we combined electromyography, ultrasound imaging, and musculoskeletal modeling during conditions that systematically manipulated either walking speed or the mechanical demand for forward propulsion at a fixed speed. We interpreted our results in the context that the muscle that was more sensitive to altered demands for forward propulsion would therefore contribute more. In support of our null hypothesis, we found that muscle-specific responses derived from experimental measurements (i.e., activation and fascicle behavior) were consistent with those derived from musculoskeletal simulations (i.e., muscle force and positive mechanical work) within the same subjects. As we discuss in more detail below, our cumulative findings suggest that the biarticular gastrocnemius muscles play a more significant role than the uniarticular soleus in governing changes in forward propulsion during the mid to late stance phase of walking. Reconciling these inter-muscular differences, and the relatively disparate literature on this topic to date, is critical for the design and control of wearable assistive devices and for targeted therapeutics that attempt to mitigate deficits in forward propulsion.
Our in vivo and in silico results are within the range of values shown in prior literature. First, the amplitude and timing of our measured LG and SOL muscle activations agree well with previous studies, both in response to changes in walking speed38 and the application of horizontal forces.30 Moreover, our model-predicted estimates of LG and SOL activation generally well-replicated our in vivo measurements, with one notable difference. Specifically, similar to other studies,42 model-predicted activations were delayed compared to their respective in vivo measurement; however, both model-predicted and in vivo activations exhibited characteristic bursts within our analyzed region of interest (i.e., push-off). This comparison is shown for each main effect (i.e., speed [Fig. 7A] and horizontal forces [Fig. 7B]), with the purple shaded region representing the standard deviation of all associated experimental trials for the LG and SOL, normalized by peak model-predicted activation during normal walking. Second, our ultrasound measurements for the MG and SOL were generally consistent with the majority of previous studies in terms of length at heel-strike, average length, and absolute length change.11, 21, 38, 55 Similar those studies, in all conditions except those with aiding forces, both muscles maintained nearly isometric behavior during early to mid-stance before undergoing characteristic shortening that peaked just after toe-off (Fig. 3 and Fig. 4). During the aiding force condition, both muscles shortened during early stance, likely due to exaggerated dorsiflexion at heel-strike giving rise to increased plantarflexion to achieve foot flat. Although not completely analogous in terms experimental manipulations, this behavior is comparable to that shown for the MG during downhill walking.41 Finally, our model-predicted estimates of muscle force and positive work generally agree with prior studies.2, 4, 50, 51
Compared to walking at 1.2 m/s, MG, LG, and SOL experimental and model-predicted results were almost uniformly affected by changes in walking speed. During push-off, each muscle’s activation significantly increased with increasing walking speed. This phenomenon has been widely reported since it was first proposed in the seminal works by Hill (1953) and Winter (1983), who suggested the triceps surae contributes to forward propulsion.35, 63 Here, in agreement with Hill and Winters, LG and SOL average force produced during push-off significantly increased (+13.4% and +21.1%, respectively) from slow walking (i.e., 0.8 m/s) to normal walking, but not from normal walking to fast walking (i.e., 1.6 m/s). Riley et al. (2001) found nearly identical results when measuring propulsive adaptations to changes in walking speed.57 Their findings suggest that the contribution from muscles spanning the ankle to forward propulsion was more sensitive to changes at slower walking speeds due to an increased utilization of hip flexors and extensors at higher speeds. Increased use of hip flexors and extensors could also explain why our model-predictions for positive work performed by the MG, LG, and SOL during push-off was invariant between walking speeds. Conversely, Neptune et al. (2009) found both the MG and SOL increase fiber positive work in response to increasing walking speeds.51 However, their work estimates were not isolated to the mid to late stance phase of walking and only incorporated sagittal plane motion. Although our findings for activation, force, and work were similar for each muscle tested across changes in walking speed, inter-muscular differences existed at the fascicle level. Compared to walking normally, in our study only MG peak fascicle shortening decreased with slower speed (−13.5%). This behavior is very similar to the findings of Farris & Sawicki (2012), who found a reduction in MG peak shortening at walking speeds slower than normal, but consistent peak shortening at speeds faster than normal.21 Their results suggest that average positive power produced by the biarticular MG MTU increases with walking speed until reaching self-selected speeds (~1.2 m/s). On the other hand, the uniarticular SOL may have consistent fascicle behavior over the range of speeds due to a large demand for vertical support that spans both slow and fast walking.54 Indeed, some studies have suggested the SOL is primarily responsible for vertical support and secondarily responsible for forward propulsion.42, 47
Unfortunately, changes in walking speed influence the demand for both forward propulsion and vertical support, thereby likely confounding our interpretations of inter-muscular differences in activation, fascicle behavior, force, and mechanical work.24, 38 Thus, results derived from conditions using horizontal forces provide more clear estimates of inter-muscular contributions to forward propulsion alone. In agreement with our findings for activation, although they measured a different head of the gastrocnemius, Gottschall & Kram (2003) also found that while both gastrocnemius and soleus activation during push-off increased significantly in response to impeding forces, only gastrocnemius activation decreased significantly in response to aiding forces (−30.4%).30 As noted by those authors, these findings suggest that the gastrocnemius muscles play a more important role in modulating forward propulsion, but the SOL may act synergistically when the demand for forward propulsion is great. Similar to our findings for activation, MG peak fascicle shortening and average fascicle length were much more sensitive to altered demands for forward propulsion than those for the SOL. Despite the architectural differences between these muscles (i.e., uniarticular SOL vs. biarticular MG), we saw no evidence that changes in MTU length could explain muscle-level differences in the response to our experimental manipulations. Compared to walking normally, greater MG fascicle shortening during the impeding force condition (+17.4%) could be elicited by greater muscle activation in order to meet the demand for greater force transmission. Indeed, compared to the SOL, the MG has a greater percentage of Type II fiber physiological cross sectional area (PCSA), which produces more force than Type I fibers, more commonly represented in the SOL.29
MG muscle activation and fascicle shortening were both more sensitive to the application of horizontal forces than those for SOL, consistency that we interpret in support of the gastrocnemius’ role in governing changes in forward propulsion. However, some studies would suggest that the preservation of muscle contractile behavior in the presence of horizontal forces would be more indicative of that muscle’s functional role during walking. Indeed, greater fascicle shortening could lead to a force deficit due to a leftward shift on the length-tension curve and faster shortening velocities.26 Cronin et al. (2013) found that SOL fascicle behavior, not MG fascicle behavior, was preserved over the course of a 60 min prolong walk.11 They argued that the preservation of SOL behavior is due to an energetically favorable recruitment of shorter fascicles with slower shortening velocities and greater relative Type I fiber PCSA. In our study, compared to walking normally, greater MG fascicle shortening during the impeding force condition, driven more by greater activation than by effects on joint posture, may be energetically unfavorable but necessary to meet the demand for greater push-off. The average fascicle length of the MG increased with aiding forces (+2.5%) and decreased with impeding forces (−3.5%), with potential consequences for the economy of force generation (i.e., force per unit activation). On the other hand, the average operating length of the SOL remained unchanged across horizontal force conditions. In agreement, Rubenson et al. (2012) reported that SOL operating length is mostly gait-independent and consistently operates on the ascending limb of the force-length curve, thereby providing stable but suboptimal force production.59
Despite some disagreement in the interpretation of fascicle behavior, increased MG fascicle shortening and muscle activation were accompanied by greater MG force and positive work during mid to late stance - complimentary in silico evidence that the gastrocnemius muscles are more responsible for governing changes in forward propulsion than the SOL. Due to its larger size and thus force-generating capacity,60 the SOL accounted for the greatest percentage of average force and positive work among the triceps surae muscles. However, compared to walking normally, SOL kinetics were invariable in response to horizontal forces. Indeed, only MG and LG average force and positive work were significantly affected by the application of horizontal forces. Specifically, MG and LG force (−15.5% and −15.6%, respectively) and positive work (−21.9% and −21.3%, respectively) decreased in response to aiding forces. Additionally, MG and LG positive work increased (+19.5% and +19.9%, respectively) in response to impeding forces. One interpretation of these findings is that the SOL provides a large, but invariant contribution to forward propulsion while the gastrocnemius muscles provide a smaller, but task-dependent contribution that increases to meet the demand for forward propulsion. Similarly, though in response to changes in walking speed, Liu et al. suggested the SOL provides a task-dependent contribution to vertical support, while the gastrocnemius muscles’ contribution to vertical support was invariant.43
There is considerable evidence that forces produced and work performed by the MG, LG, and SOL contribute significantly to forward propulsion. This is translationally important, as those forces and that work is reduced by aging and gait pathology.15, 25, 28 Accordingly, many training programs have been designed to overcome reductions in triceps surae muscle strength or power capacity, albeit with limited success.15, 23 Our results suggest that rehabilitation techniques that preferentially strengthen the gastrocnemius muscles, perhaps through targeted biofeedback, may result in more beneficial neuromechanical strategies during push-off that could translate into improvements in gait performance. As an additional translational implication, many studies have disproportionately targeted uniarticular SOL function through the design and prescription of ankle exoskeletons (e.g.,20, 53, 65). On the other hand, at least one study has shown that exoskeleton configurations that mimic the biarticular gastrocnemius muscles yield greater reductions in metabolic cost during walking than those that mimic the uniarticular soleus.45
There are several limitations and assumptions in this study. First, the three-dimensional architecture of the MG, LG, and SOL is complex and contains multiple regions that may vary in activation, fascicle length, and force development. For example, we only imaged the posteromedial aspect of the SOL, which may differ substantially from other compartments.6 Second, we cannot be certain that the same representative fascicle was selected for all conditions for each participant. To mitigate variation in fascicle selection, for each subject, we overlaid the fascicle identified at initial contact for the 1.2 m/s condition and updated the endpoints to the correct positions before continuing the tracking procedure. Third, using automated tracking routines, several studies report standard errors between 5% and 10% of absolute length.1, 27, 37 More recently, using UltraTrack, Drazan et al. (2019) revealed MG fascicle length root mean square error values were near 7% when the ankle is rotating at 30 °/s and surpassed 10% when the ankle is rotating ≥ 120 °/s.18 To mitigate these errors and improve our scientific rigor, we recorded our ultrasound images at a higher frame rate (76 fps) to account for faster shortening velocities and we performed frame-by-frame manual corrections of the automated tracking results. Fourth, our study used three fixed walking speeds which themselves were not prescribed as a function of each subject’s preferred walking speed. However, this is a common methodological approach in the biomechanics literature and is unlikely to influence of overarching conclusions. Fifth, the horizontal forces were applied to the subject’s waist, and thus subjects necessarily recruited proximal muscle groups (e.g., muscles spanning the hip) in addition to the triceps surae.8 Sixth, although we consistently encouraged subjects to maintain upright posture, we did not measure trunk kinematics and therefore locked lumbar coordinates in our musculoskeletal simulations. Finally, despite using the Residual Reduction Algorithm tool, lower limb reserve torque actuators were needed to account for ignored passive structures and insufficient muscle force generating capacity, but were controlled to recommended thresholds.34
By combining electromyography, ultrasound imaging, and musculoskeletal modeling in the same subjects, this study provides the most complete report to date of the relative contributions of the gastrocnemius and soleus muscles to forward propulsion during walking. Based on consistent evidence from empirical measurements and musculoskeletal simulations, we conclude that the biarticular gastrocnemius muscles play a more significant role than the uniarticular soleus in governing changes in forward propulsion during the push-off phase of walking. Our results may have important implications for the prescription of targeted therapeutics and for the design and control of wearable assistive devices.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Nathan Lehr and Seth Thomas for their help with data collection. This study was funded by NIH (R01AG051748, F31AG060675) and the National Center for Simulation in Rehabilitation Research (NIH P2C HD065690).
REFERENCES
- 1.Aeles J, Lichtwark GA, Lenchant S, Vanlommel L, Delabastita T and Vanwanseele B. Information from dynamic length changes improves reliability of static ultrasound fascicle length measurements. PeerJ 5: e4164, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson FC and Pandy MG. Individual muscle contributions to support in normal walking. Gait Posture 17: 159–169, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Arnold EM, Hamner SR, Seth A, Millard M and Delp SL. How muscle fiber lengths and velocities affect muscle force generation as humans walk and run at different speeds. J Exp Biol 216: 2150–2160, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blazkiewicz M, Wiszomirska I, Kaczmarczyk K, Naemi R and Wit A. Inter-individual similarities and variations in muscle forces acting on the ankle joint during gait. Gait Posture 58: 166–170, 2017. [DOI] [PubMed] [Google Scholar]
- 5.Chang YH and Kram R. Metabolic cost of generating horizontal forces during human running. J Appl Physiol (1985) 86: 1657–1662, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Chow RS, Medri MK, Martin DC, Leekam RN, Agur AM and McKee NH. Sonographic studies of human soleus and gastrocnemius muscle architecture: gender variability. Eur J Appl Physiol 82: 236–244, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Clark WH and Franz JR. Do triceps surae muscle dynamics govern non-uniform Achilles tendon deformations? PeerJ 6: e5182, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conway KA, Bissette RG and Franz JR. The Functional Utilization of Propulsive Capacity During Human Walking. J Appl Biomech 1–31, 2018. [DOI] [PubMed] [Google Scholar]
- 9.Conway KA and Franz JR. Increasing the Propulsive Demands of Walking to their Maximum Elucidates Functionally Limiting Impairments in Older Adult Gait. J Aging Phys Act 1–28, 2019. [DOI] [PubMed] [Google Scholar]
- 10.Conway KA and Franz JR. Shorter gastrocnemius fascicle lengths in older adults associate with worse capacity to enhance push-off intensity in walking. Gait Posture 77: 89–94, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cronin NJ, Avela J, Finni T and Peltonen J. Differences in contractile behaviour between the soleus and medial gastrocnemius muscles during human walking. J Exp Biol 216: 909–914, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Cronin NJ and Finni T. Treadmill versus overground and barefoot versus shod comparisons of triceps surae fascicle behaviour in human walking and running. Gait Posture 38: 528–533, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Cronin NJ, Peltonen J, Sinkjaer T and Avela J. Neural compensation within the human triceps surae during prolonged walking. J Neurophysiol 105: 548–553, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Delp SL, Anderson FC, Arnold AS, Loan P, Habib A, John CT, Guendelman E and Thelen DG. OpenSim: open-source software to create and analyze dynamic simulations of movement. IEEE Trans Biomed Eng 54: 1940–1950, 2007. [DOI] [PubMed] [Google Scholar]
- 15.DeVita P and Hortobagyi T. Age causes a redistribution of joint torques and powers during gait. J Appl Physiol (1985) 88: 1804–1811, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Dick TJM, Biewener AA and Wakeling JM. Comparison of human gastrocnemius forces predicted by Hill-type muscle models and estimated from ultrasound images. J Exp Biol 220: 1643–1653, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Disselhorst-Klug C, Schmitz-Rode T and Rau G. Surface electromyography and muscle force: limits in sEMG-force relationship and new approaches for applications. Clin Biomech (Bristol, Avon) 24: 225–235, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Drazan JF, Hullfish TJ and Baxter JR. An automatic fascicle tracking algorithm quantifying gastrocnemius architecture during maximal effort contractions. PeerJ 7: e7120, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farris DJ and Lichtwark GA. UltraTrack: Software for semi-automated tracking of muscle fascicles in sequences of B-mode ultrasound images. Comput Methods Programs Biomed 128: 111–118, 2016. [DOI] [PubMed] [Google Scholar]
- 20.Farris DJ, Robertson BD and Sawicki GS. Elastic ankle exoskeletons reduce soleus muscle force but not work in human hopping. J Appl Physiol (1985) 115: 579–585, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Farris DJ and Sawicki GS. Human medial gastrocnemius force-velocity behavior shifts with locomotion speed and gait. Proc Natl Acad Sci U S A 109: 977–982, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farris DJ and Sawicki GS. The mechanics and energetics of human walking and running: a joint level perspective. J R Soc Interface 9: 110–118, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foure A, Nordez A, McNair P and Cornu C. Effects of plyometric training on both active and passive parts of the plantarflexors series elastic component stiffness of muscle-tendon complex. Eur J Appl Physiol 111: 539–548, 2011. [DOI] [PubMed] [Google Scholar]
- 24.Francis CA, Lenz AL, Lenhart RL and Thelen DG. The modulation of forward propulsion, vertical support, and center of pressure by the plantarflexors during human walking. Gait Posture 38: 993–997, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukashiro S, Hay DC and Nagano A. Biomechanical behavior of muscle-tendon complex during dynamic human movements. J Appl Biomech 22: 131–147, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Fukunaga T, Kubo K, Kawakami Y, Fukashiro S, Kanehisa H and Maganaris CN. In vivo behaviour of human muscle tendon during walking. Proc Biol Sci 268: 229–233, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillett JG, Barrett RS and Lichtwark GA. Reliability and accuracy of an automated tracking algorithm to measure controlled passive and active muscle fascicle length changes from ultrasound. Comput Methods Biomech Biomed Engin 16: 678–687, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Goldberg SR and Stanhope SJ. Sensitivity of joint moments to changes in walking speed and body-weight-support are interdependent and vary across joints. J Biomech 46: 1176–1183, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gollnick PD, Sjodin B, Karlsson J, Jansson E and Saltin B. Human soleus muscle: a comparison of fiber composition and enzyme activities with other leg muscles. Pflugers Arch 348: 247–255, 1974. [DOI] [PubMed] [Google Scholar]
- 30.Gottschall JS and Kram R. Energy cost and muscular activity required for propulsion during walking. J Appl Physiol (1985) 94: 1766–1772, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Handsfield GG, Inouye JM, Slane LC, Thelen DG, Miller GW and Blemker SS. A 3D model of the Achilles tendon to determine the mechanisms underlying nonuniform tendon displacements. J Biomech 51: 17–25, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hawkins D, Lum C, Gaydos D and Dunning R. Dynamic creep and pre-conditioning of the Achilles tendon in-vivo. J Biomech 42: 2813–2817, 2009. [DOI] [PubMed] [Google Scholar]
- 33.Hermens HJ, Freriks B, Disselhorst-Klug C and Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10: 361–374, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Hicks JL, Uchida TK, Seth A, Rajagopal A and Delp SL. Is my model good enough? Best practices for verification and validation of musculoskeletal models and simulations of movement. J Biomech Eng 137: 020905, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill AV The mechanics of active muscle. Proc R Soc Lond B Biol Sci 141: 104–117, 1953. [DOI] [PubMed] [Google Scholar]
- 36.Hoffman BW, Cresswell AG, Carroll TJ and Lichtwark GA. Muscle fascicle strains in human gastrocnemius during backward downhill walking. J Appl Physiol (1985) 116: 1455–1462, 2014. [DOI] [PubMed] [Google Scholar]
- 37.Kwah LK, Pinto RZ, Diong J and Herbert RD. Reliability and validity of ultrasound measurements of muscle fascicle length and pennation in humans: a systematic review. J Appl Physiol (1985) 114: 761–769, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Lai A, Lichtwark GA, Schache AG, Lin YC, Brown NA and Pandy MG. In vivo behavior of the human soleus muscle with increasing walking and running speeds. J Appl Physiol (1985) 118: 1266–1275, 2015. [DOI] [PubMed] [Google Scholar]
- 39.Leitner C, Hager PA, Penasso H, Tilp M, Benini L, Peham C and Baumgartner C. Ultrasound as a Tool to Study Muscle-Tendon Functions during Locomotion: A Systematic Review of Applications. Sensors (Basel) 19: 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lenhart RL, Francis CA, Lenz AL and Thelen DG. Empirical evaluation of gastrocnemius and soleus function during walking. J Biomech 47: 2969–2974, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lichtwark GA and Wilson AM. Interactions between the human gastrocnemius muscle and the Achilles tendon during incline, level and decline locomotion. J Exp Biol 209: 4379–4388, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Liu MQ, Anderson FC, Pandy MG and Delp SL. Muscles that support the body also modulate forward progression during walking. J Biomech 39: 2623–2630, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Liu MQ, Anderson FC, Schwartz MH and Delp SL. Muscle contributions to support and progression over a range of walking speeds. J Biomech 41: 3243–3252, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malatesta D, Canepa M and Menendez Fernandez A. The effect of treadmill and overground walking on preferred walking speed and gait kinematics in healthy, physically active older adults. Eur J Appl Physiol 117: 1833–1843, 2017. [DOI] [PubMed] [Google Scholar]
- 45.Malcolm P, Galle S, Derave W and De Clercq D. Bi-articular Knee-Ankle-Foot Exoskeleton Produces Higher Metabolic Cost Reduction than Weight-Matched Mono-articular Exoskeleton. Front Neurosci 12: 69, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGowan CP, Neptune RR and Kram R. Independent effects of weight and mass on plantar flexor activity during walking: implications for their contributions to body support and forward propulsion. J Appl Physiol (1985) 105: 486–494, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miyoshi T, Nakazawa K, Tanizaki M, Sato T and Akai M. Altered activation pattern in synergistic ankle plantarflexor muscles in a reduced-gravity environment. Gait Posture 24: 94–99, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Monaco V, Coscia M and Micera S. Cost function tuning improves muscle force estimation computed by static optimization during walking. Conf Proc IEEE Eng Med Biol Soc 2011: 8263–8266, 2011. [DOI] [PubMed] [Google Scholar]
- 49.Neptune RR, Kautz SA and Zajac FE. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. J Biomech 34: 1387–1398, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Neptune RR and Sasaki K. Ankle plantar flexor force production is an important determinant of the preferred walk-to-run transition speed. J Exp Biol 208: 799–808, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Neptune RR, Sasaki K and Kautz SA. The effect of walking speed on muscle function and mechanical energetics. Gait Posture 28: 135–143, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nilsson J and Thorstensson A. Ground reaction forces at different speeds of human walking and running. Acta Physiol Scand 136: 217–227, 1989. [DOI] [PubMed] [Google Scholar]
- 53.Nuckols RW, Dick TJM, Beck ON and Sawicki GS. Ultrasound imaging links soleus muscle neuromechanics and energetics during human walking with elastic ankle exoskeletons. Sci Rep 10: 3604, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orendurff MS, Segal AD, Klute GK, Berge JS, Rohr ES and Kadel NJ. The effect of walking speed on center of mass displacement. J Rehabil Res Dev 41: 829–834, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Panizzolo FA, Green DJ, Lloyd DG, Maiorana AJ and Rubenson J. Soleus fascicle length changes are conserved between young and old adults at their preferred walking speed. Gait Posture 38: 764–769, 2013. [DOI] [PubMed] [Google Scholar]
- 56.Rajagopal A, Dembia CL, DeMers MS, Delp DD, Hicks JL and Delp SL. Full-Body Musculoskeletal Model for Muscle-Driven Simulation of Human Gait. IEEE Trans Biomed Eng 63: 2068–2079, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riley PO, Della Croce U and Kerrigan DC. Propulsive adaptation to changing gait speed. J Biomech 34: 197–202, 2001. [DOI] [PubMed] [Google Scholar]
- 58.Roberts TJ and Gabaldon AM. Interpreting muscle function from EMG: lessons learned from direct measurements of muscle force. Integr Comp Biol 48: 312–320, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rubenson J, Pires NJ, Loi HO, Pinniger GJ and Shannon DG. On the ascent: the soleus operating length is conserved to the ascending limb of the force-length curve across gait mechanics in humans. J Exp Biol 215: 3539–3551, 2012. [DOI] [PubMed] [Google Scholar]
- 60.Sutherland DH, Cooper L and Daniel D. The role of the ankle plantar flexors in normal walking. J Bone Joint Surg Am 62: 354–363, 1980. [PubMed] [Google Scholar]
- 61.Szaro P, Witkowski G, Smigielski R, Krajewski P and Ciszek B. Fascicles of the adult human Achilles tendon - an anatomical study. Ann Anat 191: 586–593, 2009. [DOI] [PubMed] [Google Scholar]
- 62.Winter DA Biomechanics and Motor Control of Human Movement. New York: Wiley, 1990. [Google Scholar]
- 63.Winter DA Energy generation and absorption at the ankle and knee during fast, natural, and slow cadences. Clin Orthop Relat Res 147–154, 1983. [PubMed] [Google Scholar]
- 64.Zargham A, Afschrift M, De Schutter J, Jonkers I and De Groote F. Inverse dynamic estimates of muscle recruitment and joint contact forces are more realistic when minimizing muscle activity rather than metabolic energy or contact forces. Gait Posture 74: 223–230, 2019. [DOI] [PubMed] [Google Scholar]
- 65.Zhang J, Fiers P, Witte KA, Jackson RW, Poggensee KL, Atkeson CG and Collins SH. Human-in-the-loop optimization of exoskeleton assistance during walking. Science 356: 1280–1284, 2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


