Abstract
Objectives:
To explore the efficacy of glucosamine sulfate (GS) combined with loxoprofen sodium (LS) in rats with knee osteoarthritis (KOA) and its effect on chondrocytes.
Methods:
We randomly assigned 40 SPF SD rats to normal group (NG), control group (CG), treatment group (TG), and model group (MG). CG and TG were processed with continuous irrigation of LS and GS. NG and MG were given normal saline. We collected 3 mL of venous blood from the rat’s lower limb for the detection of serum IL-1β, IL-6, IL-8, and TNF-α by ELISA. Four weeks after irrigation, 5 rats in each group were randomly selected for anesthesia. The water content was detected, and the chondrocytes were collected. MTT assay was used to detect apoptosis, and Western blot (WB) to measure concentrations of Bax, Bcl-2, Caspase3, Caspase9, TLR4, and NF-kB.
Results:
The levels of IL-1 β, IL-6, IL-8, and TNF-α decreased in CG and TG, but increased in MG (P<0.05). After treatment, the expression of inflammatory factors was highest in MG (P<0.05).
Conclusions:
GS combined with LS showed good efficacy in rats with knee osteoarthritis, which may be achieved by inhibiting the expression of inflammatory factors and knee chondrocyte apoptosis via the TLR4-NF-kB pathway.
Keywords: Chondrocytes, Glucosamine Sulfate, Inflammatory Factors, Loxoprofen Sodium
Introduction
Osteoarthritis (OA) is the most common form of arthritis and the knee is the most commonly affected joint. Knee osteoarthritis (KOA) is a progressive and chronic inflammatory joint disease that causes pain, disability and functional limitations, and mainly affects the middle-aged and older adults[1,2]. KOA worldwide incidence is estimated at 3.1-6.6% and is increasing year by year[3,4]. KOA may cause severe joint pain, joint stiffness, joint function loss, and even paralysis in severe cases[5]. Patients with early KOA may show a good response to conservative treatment, but patients with advanced KOA require artificial joint replacement[6]. The goal of conservative treatment is to prevent further wear and tear of the cartilage. Glucosamine sulfate (GS) is the most common cartilage protector, which can facilitate cartilage synthesis, inhibits articular cartilage degeneration, and inflammation[7]. GS is a normal component of glycosaminoglycans in the cartilage matrix and synovial fluid. This salt may have a role in slowing cartilage breakdown. GS efficacy has been clinically established[8]. However, with the wide application of GS, we found that GS has quite a long response time[9]. A study pointed out that GS may aggravate the patient’s condition and seriously compromise the treatment effect[10].
Non-steroidal anti-inflammatory drugs (NSAIDs) are effective in relieving the symptoms of KOA[11]. Loxoprofen sodium (LS) is a propionic acid derivative non-steroidal anti-inflammatory drug that exerts good analgesic and anti-inflammatory effects safely, with only a few side effects[12]. LS is not only effective in the treatment of ankylosing spondylitis[13] but also has a marked analgesic effect in KOA rats[14]. GS combined with NSAIDs in the treatment of hand arthritis can remarkably enhance the efficacy and shorten the response time[15]. Therefore, we hypothesize that GS combined with LS improves the treatment of KOA. Thus, we established rat models of KOA to explore the effects of GS combined with LS on the inflammatory factors in cartilages, cartilage water content, and cellular changes in rats, aiming to introduce a new approach for the management of KOA.
Experimental preparation
General data of animals
We bought 40 SPF SD rats from Youcare Pharmaceutical Group Co., Ltd. (Beijing, China, animal license number: SYXK (Beijing) 2018-0027). Rats were in a 1:1 sex ratio, aged 8 weeks, weighing 180±20 g. Rats were regularly raised at 29±2°C (5 rats per cage), with normal access to food and light exposure. In line with the regulations on animal protection and use, this experiment has been approved by Animal Ethics Committee of Ningbo Hangzhou Bay Hospital (WYLS2021-1).
Methods
Methods of modeling
The modeling in this study was carried out with reference to Fan et al.[16] study. After adaptive feeding for 1 week, we randomly and equally assigned 40 SPF SD rats to the normal group (NG), the control group (CG), the treatment group (TG), and the model group (MG). Rats from NG and CG were not modeled, while rats from TG and MG were selected for KOA modeling. We anesthetized rats through intraperitoneal injection of 10% chloral hydrate (3 mg/kg). After the anesthesia took effect, the rats were fixed on the operating table. All operations were performed in a sterile environment. Following routine disinfection, we shaved the right knee joint of the rat and carefully cut the skin along the medial tibia with the tibial plateau of the knee as the center without causing injury on the articular cartilage. Then we cut off the medial collateral ligament, exposed the knee joint cavity, detached the medial meniscus, and cut it off from the middle position. Finally, we cut off the anterior cruciate ligament in the joint cavity depth, closed the articular cavity, and sutured the incision. After the operation, we gave rats an appropriate amount of penicillin for 3 days to prevent infection.
Drug administration
Five days after the successful modeling, we gave rats in CG and TG an LS (0.3 mg/kg) irrigation mixed with 5 mL normal saline for 7 days. One hour after each LS irrigation, we gave rats 2 mL of normal saline containing GS (100 mg/kg), which lasted for 28 days. Rats in NG and MG were given normal saline at the same dosage for irrigation.
Detection of inflammatory factors
Before the treatment (after successful modeling but before the irrigation) and after the treatment (28 days after the irrigation), we collected 3 mL of venous blood from the lower limb of the rat and placed the sample at room temperature for 30 minutes before centrifugation at 400 × g for 10 minutes to obtain the serum. The enzyme-linked immunosorbent assay (ELISA) was performed to detect serum interleukin-1 β (IL-1 β, Shanghai Lengton Bioscience Co., Ltd., item number: BP-E30419), interleukin-6 (IL-6, Shanghai Kemin Biotechnology Co., Ltd., item number: DXT-EK0412), interleukin-8 (IL-8, Shanghai Hengfei Biotechnology Co., Ltd., item number: E-EL-R0560km-1), and tumor necrosis factor-α (TNF- α, Shanghai Hengfei Biotechnology Co., Ltd., item number: 438204-1). All operations were carried out in strict accordance with the kit instructions.
Evaluation of walking gait
The walking gait of rats in each group was scored before treatment (T0), 2 weeks after irrigation (T1), and 4 weeks after irrigation (T2). In this study, 0 point represents normal gait; 1 point represents normal gait, with a certain degree of bending of lower limbs; 2 points represents evident abnormalities of lower limbs, with contacts to the ground; 3 points represents limping, with lower limbs not touching the ground. A lower score indicates better gait performance.
Detection of water content of articular cartilage
Four weeks after irrigation, we randomly chose 5 rats in each group for anesthesia and sacrificed them by cervical dislocation. Then we stripped the tibial plateau cartilage of the right knee and weighed the wet weight on the analytical balance. We placed the cartilage in a blast heating machine at 60°C for 12 hours and then measured the dry weight. The water content of cartilage = the difference between the dry weight and the wet weight of cartilage/wet weight of cartilage.
Extraction of chondrocytes
The remaining rats in each group were also anesthetized and sacrificed by cervical dislocation. Then we collected the cartilage tissue of the right knee joint, washed it repeatedly in PBS (Gibco, Carlsbad, CA, the USA), and cut it into pieces (1 mm3). Tissue pieces were first digested by trypsin (0.25%) (Amersco, Framingham, MA, the USA) at 37°C for 30 minutes and then digested with type II collagenase (0.1%) (Sigma, St. Louis, MO, the USA) for 60 minutes, proceeding with centrifugation at 200 × g for 10 minutes to collect chondrocytes. Chondrocytes were cultured in a DMEM medium supplemented by 10% fetal bovine serum (FBS, HyClone, Logan, UT, the USA). Then we inoculated cell suspension (1×106 cells/mL) in a petri dish and placed it in an incubator at 37°C, with 5% CO2. After 48 hours of incubation, we examined the adherence of cells. The culture medium was changed every 2-3 days. When chondrocytes’ adherent growth reached 80%, cells were mixed with trypsin (0.25%) for passage.
Detection of cell viability
We plated cell suspension (5×104 cells/mL) in 96-well plates and cultured for 24 hours, 48 hours, 72 hours, and 96 hours. After each culture, we introduced 20 μL of the MTT solution (5 mg/mL) (Gibco, Carlsbad, CA, the USA) to each well and incubated the plate for 4 hours. Then we discarded the culture medium, added 200 μL DMSO to each well, and tested each well’s absorbance at 570 nm by a microplate reader (Tecan, Switzerland). The cell growth curve was drawn.
Detection of apoptosis
Cell suspension (1×106 cells/mL) was inoculated in a petri dish and pre-cultured in an incubator for 24 hours. We digested cells with trypsin and washed them with PBS 3 times. The cell content was adjusted to 1×106 cells/mL. According to the instructions of the apoptosis kit (Dojindo, Kumamoto, Japan), we mixed cells with Annexin V-FITC (5 μL) and PI (5 μL) and incubated them in a dark place for 15 minutes. Then we measured the apoptosis by the flow cytometry (FCM, BD, Franklin Lakes, NJ, the USA) at 4°C.
Western Blot (WB) detection
Chondrocytes were lysed with lysate (Beyotime Biotechnology, Shanghai, China) to extract the total protein. All proteins were quantified by the BCA kit (Beyotime Biotechnology, Shanghai, China) and adjusted to the same content. Proteins were separated by electrophoresis using 10% SDS-PAGE gel (Beyotime Biotechnology, Shanghai, China). Then proteins were transferred to the PVDF membrane by the semi-dry method and sealed in 5% skimmed milk powder at room temperature for 1 hour. Next, proteins were cultured with Bax, Caspase3, Caspase9, TLR4, NF-kB, p-NF-kB, and β-actin primary antibodies (Abcam, Cambridge, UK) overnight at 4°C. The PVDF membrane was washed by TBST and incubated with goat anti-rabbit IgG second antibody for 1 hour. Finally, we added ECL reagent (Beyotime Biotechnology, Shanghai, China) for color development. The bands were scanned, and the WB images were collected. The relative expression of the protein = the gray value of the target protein/the internal reference’s gray value (β-actin).
Statistical analysis
Statistical analysis was performed with SPSS22.0. All tests were performed three times to determine the average value, which was represented by the `c±s. The comparison of measurement data between multiple groups was analyzed by the one-way analysis of variance (ANOVA); the pairwise comparison of measurement data between any two groups was analyzed by the LSD test; the comparison of measurement data between multiple time points was analyzed by the repeated measures ANOVA and bonferroni post-test. P<0.05 represents a statistically significant difference.
Results
Comparison of inflammatory factors
Before treatment, CG and NG were not different in the levels of IL-1 β, IL-6, IL-8, and TNF-α; MG and TG were not different in the levels of such inflammatory factors (P>0.05). The levels of such inflammatory factors were higher in MG and TG than in CG and NG before treatment (P<0.05). After treatment, the levels of inflammatory factors were not changed in NG (P>0.05). The levels of inflammatory factors in CG and TG were lower after treatment than those before treatment, while the levels of those factors in MG were higher after treatment than those before treatment (P<0.05). After treatment, the expression of inflammatory factors was the highest in MG, followed by TG, NG, and CG (P<0.05) (Figure 1).
Figure 1.
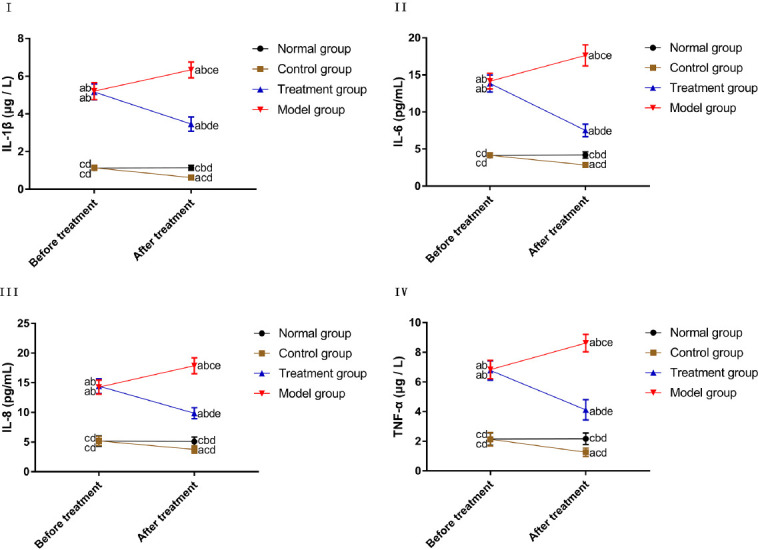
Comparison of inflammatory factors. I. Comparison of the IL-1 β level. II. Comparison of the IL-6 level. III. Comparison of the IL-8 level. IV. Comparison of the TNF- α level. “a” represents aP<0.05 when compared with NG at the same time point. “b” represents bP<0.05 when compared with CG at the same time point. “c” represents cP<0.05 when compared with TG at the same time point. “d” represents dP<0.05 when compared with MG at the same time point. “e” represents eP<0.05 when compared with data before treatment within the group.
Comparison of the gait score
In NG, CG, and MG, there were no marked differences in gait scores between T0, T1, and T2 (P>0.05). In TG, the gait score was the highest at T0, decreased at T1, and the lowest at T2 (P<0.05). At T0, NG and CG were not different in the gait score, and MG and TG were also not different in the gait score (P>0.05). The gait score was lower in NG and CG than in MG and TG at T0 (P<0.05). At T1 and T2, there was no difference in the gait score between CG and NG (P>0.05). The gait score was lower in CG and NG than in MG and TG T1 and T2, with a lower gait score in MG than in TG (P<0.05) (Figure 2).
Figure 2.
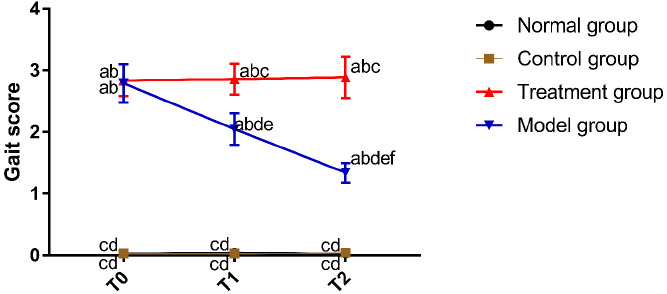
Comparison of the gait score. “a” represents aP<0.05 when compared with NG at the same time point. “b” represents bP<0.05 when compared with CG at the same time point. “c” represents cP<0.05 when compared with TG at the same time point. “d” represents dP<0.05 when compared with MG at the same time point. “e” represents eP<0.05 when compared with T0 within the group. “f” represents fP<0.05 when compared with T1 within the group.
Comparison of the water content of articular cartilage
Four weeks after irrigation, the water contents of knee cartilages in NG and TG were not different (P>0.05), and both were higher than that in CG and lower than that in MG (P<0.05) (Figure 3).
Figure 3.
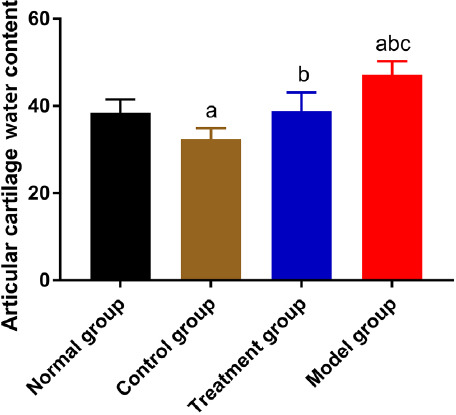
Comparison of the water content of articular cartilage. “a” represents aP<0.05 when compared with NG. “b” represents bP<0.05 when compared with CG. “c” represents cP<0.05 when compared with TG.
Comparison of the chondrocyte viability
According to the MTT assay results, there was no difference in chondrocyte proliferation between CG and NG (P>0.05). Chondrocyte proliferation was stronger in CG and NG than in MG and TG (P<0.05), with a higher proliferation rate in TG than in MG (P<0.05) (Figure 4).
Figure 4.
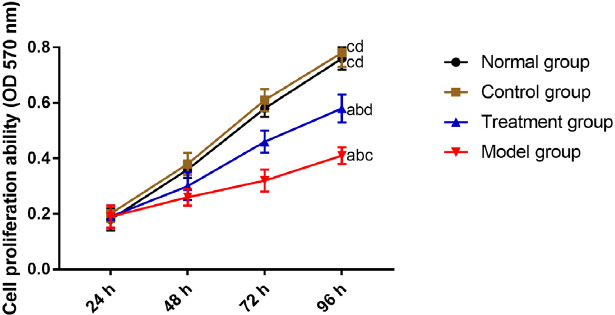
Comparison of the chondrocyte activity. “a” represents aP<0.05 when compared with NG. “b” represents bP<0.05 when compared with CG. “c” represents cP<0.05 when compared with TG. “d” represents dP<0.05 when compared with MG.
Comparison of the chondrocyte apoptosis
The results of the FCM revealed that the apoptosis rate of chondrocytes was the highest in MG, followed by TG, NG, and CG (P<0.05) (Figure 5).
Figure 5.
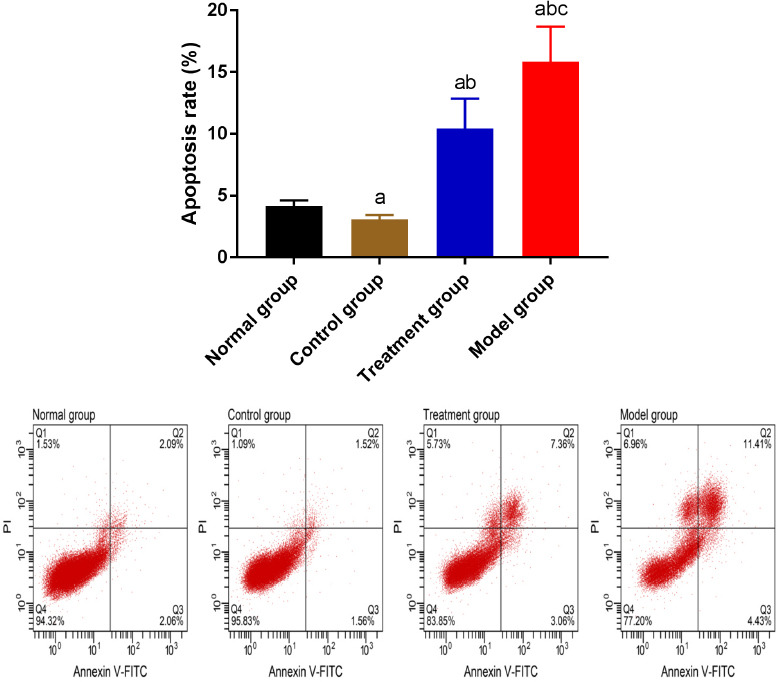
Comparison of the chondrocyte apoptosis. “a” represents aP<0.05 when compared with NG. “b” represents bP<0.05 when compared with CG. “c” represents cP<0.05 when compared with TG.
Comparison of the expression of proteins in chondrocytes
According to the western blot results, the Bcl-2 concentration was higher in TG, NG, and CG than in MG (P<0.05), and TG, NG, and CG were not different in the Bcl-2 concentration. The concentrations of pro-apoptotic proteins (Bax, Caspase3, and Caspase9) and TLR4/NF-kB signal pathway-related proteins (TLR4, NF-kB, and pNF-kB) were the lowest in CG, followed by NG, TG, and MG. More details are shown in Figure 6.
Figure 6.
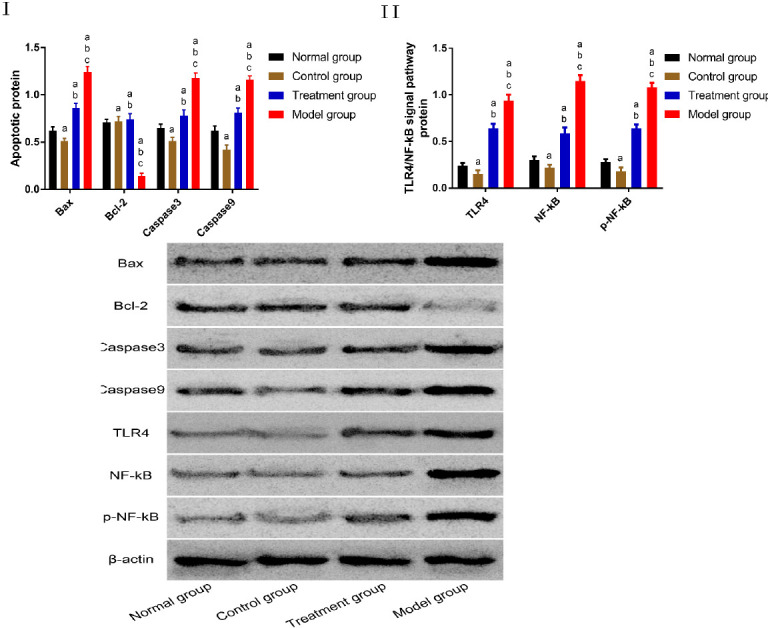
Comparison of the proteins expression in chondrocytes. I. Comparison of apoptosis-related proteins. II. Comparison of TLR4/NF-kB signal pathway-related proteins “a” represents aP<0.05 when compared with NG. “b” represents bP<0.05 when compared with CG. “c” represents cP<0.05 when compared with TG.
Discussion
The pathological features of KOA include deformation and destruction of articular cartilage, the formation of osteophytes at the edge of the joint, synovial hyperplasia, and so on[17]. During the pathogenesis of KOA, the biosynthesis and decomposition of proteoglycan in the articular cartilage matrix were impaired, and inflammatory factors were massively activated and released, causing a series of injuries to the surrounding tissues[18]. Therefore, improving the structure of knee cartilage is one of the keys to the treatment of KOA. GS is a frequently studied structure-modifying drug that has a recognized therapeutic effect on KOA[19]. LS, as a NSAID, is also widely used for anti-inflammation and analgesia in patients with rheumatic arthritis, lumbago, and omarthritis[20]. This study aims to explore the effects of GS combined with LS on the chondrocytes of KOA rats, broadening the understanding of the clinical therapeutic value of GS combined with LS.
No peritonitis was reported in rats during the modeling. We processed rat models, and normal rats with GS combined with LS and then detected the levels of inflammatory factors in the blood. Before treatment, TG and MG were not different in the inflammatory factors, and CG and NG were not different in the inflammatory factors. Still, the expression levels of inflammatory factors were higher in TG and MG than in CG and NG. These results suggest that the increased expression of inflammatory factors is a major cause of knee joints’ inflammatory injury during the pathogenesis of KOA. After treatment, the expression levels of inflammatory factors were decreased in both TG and CG groups, indicating that the joint therapy can inhibit the release of inflammatory factors, which was mainly achieved by LS. A previous study suggested that LS can inhibit cyclooxygenase, reduce interleukin and prostaglandin synthesis, and thus exert an anti-inflammatory effect[21]. The results of this study also support this view. Here we noted a gradual decrease in the gait score in TG, suggesting that rats in TG were gradually recovering from KOA. This may be a reasonable consequence of GS. As a necessary component for the synthesis of proteoglycans in articular cartilage matrix, GS can produce polymer structural glycoproteins to inhibit the production of superoxide free radicals and reduce the oxidative stress damage of cells, and hence cure osteoarthritis[22]. In our study, the MG group, showed an increase in the expression of inflammatory factors which was accompanied by an increase in the gait score, suggesting that with the progression of KOA, the damage to the knee joints caused by inflammatory factors gets more severe. Articular cartilage is a kind of non-vascular tissue, which gets its nutritional support mainly from the exchange between chondrocytes and the surrounding nutrient-rich synovial fluid[23]. We detected the highest water content of the knee cartilage in MG, suggesting that synovium secreted a large amount of synovial fluid during the occurrence of osteoarthritis, which leads to decreased osmotic pressure in the joint cavity. Proteoglycans in the cartilage matrix, with negative electricity, have a strong water-absorbing capacity and hence increase the water content. Such findings are consistent with the results of a previous study[24] that explored the water content of knee cartilage. In our study, the water content of knee cartilage was lower in TG than in MG and lower in CG than in NG, suggesting that GS combined with LS reduced the secretion of substances such as joint effusion to secure a stable environment of the knee cartilage, which facilitates the recovery from the disease. All the above results indicate that GS combined with LS is effective and safe and may protect the knee joints of healthy people. Nevertheless, further studies are required to confirm this finding.
We also performed the MTT, FCM, and WB assays to explore the effects of GS combined with LS on knee cartilage. In this study, TG had a higher chondrocyte proliferation rate and a lower apoptosis rate than MG, and CG had a lower chondrocyte apoptosis rate than NG. Thus, we hypothesize that GS combined with LS may also affect the progression of KOA by inhibiting the apoptosis of chondrocytes. Chondrocytes can synthesize and secrete matrix and fiber. A previous study revealed that a large number of chondrocytes would be subjected to apoptosis in the process of KOA[25]. Inhibited apoptosis of chondrocytes can strengthen the knee cartilage’s structural integrity and minimize the injuries caused by pathological changes of KOA. Ding et al.[26] demonstrated that the TLR4-NF-kB signaling pathway plays a critical part in osteoarthritis. We also detected the protein expression of TLR4 and NF-kB in chondrocytes in the 4 groups. The concentrations of TLR4 and NF-kB were lower in TG than in MG and lower in CG than in NG. Therefore, we hypothesize that GS’s inhibition of chondrocyte apoptosis combined with LS may be achieved through the TLR4-NF-kB pathway.
In summary, GS combined with LS has good efficacy for KOA rats, which may be achieved by inhibiting the expression of inflammatory factors and knee chondrocyte apoptosis via the TLR4-NF-kB pathway. Given the differences between the human body structure and the rat body structure, the effects of GS combined with LS on human knee chondrocytes need to be investigated by human experiments. In addition, whether GS combined with LS inhibits the expression of inflammatory indicators and the apoptosis of knee chondrocytes through the TLR4-NF-kB pathway needs to be verified by more experiments. Here we did not detect markers for oxidative stress reaction such as superoxide dismutase and malondialdehyde in rats, so the effects of GS combined with LS on the oxidative stress response of KOA are not clear.
Funding
Screening of P2X/P2Y key subtypes regulated by Mg2+ in the process of extracellular matrix calcification was funded by Ningbo Natural Science Foundation Project, No.2019A610240; Development of 3D printing percutaneous guide template assisted lag screw fixation and optimized structural design of femoral neck fracture were funded by Ningbo Medical Science and Technology Project, No. 2019Y27.
Authors’ contributions
ML and WL conceived and designed the study, and drafted the manuscript. ML, FX and WL collected, analyzed and interpreted the experimental data. WL revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Gu YT, Chen J, Meng ZL, Ge WY, Bian YY, Cheng SW, Xing CK, Yao JL, Fu J, Peng L. Research progress on osteoarthritis treatment mechanisms. Biomed Pharmacother. 2017;93:1246–1252. doi: 10.1016/j.biopha.2017.07.034. [DOI] [PubMed] [Google Scholar]
- 2.Ranmuthu CDS, Ranmuthu CKI, Khan WS. Evaluating the Current Literature on Treatments Containing Adipose-Derived Stem Cells for Osteoarthritis:a Progress Update. Curr Rheumatol Rep. 2018;20:67. doi: 10.1007/s11926-018-0776-7. [DOI] [PubMed] [Google Scholar]
- 3.Lankhorst NE, Damen J, Oei EH, Verhaar JAN, Kloppenburg M, Bierma-Zeinstra SMA, van Middelkoop M. Incidence, prevalence, natural course and prognosis of patellofemoral osteoarthritis:the Cohort Hip and Cohort Knee study. Osteoarthritis Cartilage. 2017;25:647–653. doi: 10.1016/j.joca.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Gungor S, Fields K, Aiyer R, Valle AGD, Su EP. Incidence and risk factors for development of persistent postsurgical pain following total knee arthroplasty:A retrospective cohort study. Medicine (Baltimore) 2019;98:e16450. doi: 10.1097/MD.0000000000016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzales T, Hernandez HJ, Seamon BA, Pennington D, McIntosh V, Blackman MR, Harris-Love MO. Alterations in stepping kinetics and leg strength are associated with knee arthritis asymmetries. Innovation in Aging. 2017;1:453. [Google Scholar]
- 6.Vina ER, Ran D, Ashbeck EL, Kaur M, Kwoh CK. Relationship Between Knee Pain and Patient Preferences for Joint Replacement:Health Care Access Matters. Arthritis Care Res (Hoboken) 2017;69:95–103. doi: 10.1002/acr.23084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reginster JL, Bruyere O, Cooper C. Different glucosamine sulfate products generate different outcomes on osteoarthritis symptoms. Ann Rheum Dis. 2018;77:e39. doi: 10.1136/annrheumdis-2017-212251. [DOI] [PubMed] [Google Scholar]
- 8.Roman-Blas JA, Castañeda S, Sánchez-Pernaute O, Largo R, Herrero-Beaumont G. Combined Treatment With Chondroitin Sulfate and Glucosamine Sulfate Shows No Superiority Over Placebo for Reduction of Joint Pain and Functional Impairment in Patients With Knee Osteoarthritis:A Six-Month Multicenter, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Arthritis Rheumatol. 2017;69:77–85. doi: 10.1002/art.39819. [DOI] [PubMed] [Google Scholar]
- 9.Lomonte ABV, Mendonça JA, de Castro Brandão G, Castro ML. Multicenter, randomized, double-blind clinical trial to evaluate efficacy and safety of combined glucosamine sulfate and chondroitin sulfate capsules for treating knee osteoarthritis. Adv Rheumatol. 2018;58:41. doi: 10.1186/s42358-018-0041-9. [DOI] [PubMed] [Google Scholar]
- 10.Roman-Blas JA, Mediero A, Tardío L, Portal-Nuñez S, Gratal P, Herrero-Beaumont G, Largo R. The combined therapy with chondroitin sulfate plus glucosamine sulfate or chondroitin sulfate plus glucosamine hydrochloride does not improve joint damage in an experimental model of knee osteoarthritis in rabbits. Eur J Pharmacol. 2017;794:8–14. doi: 10.1016/j.ejphar.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Liu Q, Niu J, Li H, Ke Y, Li R, Zhang Y, Lin J. Knee Symptomatic Osteoarthritis, Walking Disability, NSAIDs Use and All-cause Mortality:Population-based Wuchuan Osteoarthritis Study. Sci Rep. 2017;7:3309. doi: 10.1038/s41598-017-03110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hara M, Hayashi K, Kitamura T, Honda M, Tamaki M. A nationwide randomized, double-blind, placebo-controlled physicians'trial of loxoprofen for the treatment of fatigue, headache, and nausea after hangovers. Alcohol. 2020;84:21–25. doi: 10.1016/j.alcohol.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Fan M, Cao S, Tu L, Wei Q, Yuan R, Li X, Gu J. Efficacy and safety of loxoprofen hydrogel patch versus loxoprofen tablet in patients with ankylosing spondylitis:A 4-week randomized, open-label study. Biomed Rep. 2019;10:331–336. doi: 10.3892/br.2019.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukumoto A, Tajima K, Hori M, Toda Y, Kaku S, Matsumoto H. Analgesic effect of S (+)-flurbiprofen plaster in a rat model of knee arthritis:analysis of gait and synovial fluid prostaglandin E2 levels. J Pharm Pharmacol. 2018;70:929–936. doi: 10.1111/jphp.12914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tenti S, Giordano N, Mondanelli N, Giannotti S, Maheu E, Fioravanti A. A retrospective observational study of glucosamine sulfate in addition to conventional therapy in hand osteoarthritis patients compared to conventional treatment alone. Aging Clin Exp Res. 2020;32:1161–1172. doi: 10.1007/s40520-019-01305-4. [DOI] [PubMed] [Google Scholar]
- 16.Fan MP, Si M, Li BJ, Hu GH, Hou Y, Yang W, Liu L, Tang B, Nie L. Cell therapy of a knee osteoarthritis rat model using precartilaginous stem cells. Eur Rev Med Pharmacol Sci. 2018;22:2119–2125. doi: 10.26355/eurrev_201804_14745. [DOI] [PubMed] [Google Scholar]
- 17.Kaneguchi A, Ozawa J, Kawamata S, Yamaoka K. Development of arthrogenic joint contracture as a result of pathological changes in remobilized rat knees. J Orthop Res. 2017;35:1414–1423. doi: 10.1002/jor.23419. [DOI] [PubMed] [Google Scholar]
- 18.Boeth H, Raffalt PC, MacMahon A, Poole AR, Eckstein F, Wirth W, Buttgereit F, Önnerfjord P, Lorenzo P, Klint C, et al. Association between changes in molecular biomarkers of cartilage matrix turnover and changes in knee articular cartilage:a longitudinal pilot study. J Exp Orthop. 2019;6:19. doi: 10.1186/s40634-019-0179-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuenyongviwat V, Kiddee W, Tangtrakulwanich B. Effect of glucosamine sulfate on intraocular pressure in patients with knee osteoarthritis:A prospective randomized controlled trial. J Fr Ophtalmol. 2019;42:711–715. doi: 10.1016/j.jfo.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Cho C H, Bae K C, Kim D H. Treatment strategy for frozen shoulder. Clin Orthop Surg. 2019;11:249–257. doi: 10.4055/cios.2019.11.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto T, Kaku M, Sumi H, Yashima Y, Izumino J, Tanimoto K. Effects of loxoprofen on the apical root resorption during orthodontic tooth movement in rats. PLoS One. 2018;13:e0194453. doi: 10.1371/journal.pone.0194453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mousavi SH, Bakhtiari E, Hosseini A, Jamialahmadi K. Protective effects of glucosamine and its acetylated derivative on serum/glucose deprivation-induced PC12 cells death:Role of reactive oxygen species. Res Pharm Sci. 2018;13:121–129. doi: 10.4103/1735-5362.223794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graham BT, Moore AC, Burris DL, Price C. Mapping the spatiotemporal evolution of solute transport in articular cartilage explants reveals how cartilage recovers fluid within the contact area during sliding. J Biomech. 2018;71:271–276. doi: 10.1016/j.jbiomech.2018.01.041. [DOI] [PubMed] [Google Scholar]
- 24.Stärke F, Awiszus F, Lohmann CH, Stärke C. The effect of irrigation time and type of irrigation fluid on cartilage surface friction. J Mech Behav Biomed Mater. 2018;77:187–191. doi: 10.1016/j.jmbbm.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Sun Y, Kang S, Pei S, Sang C, Huang Y. MiR93-5p inhibits chondrocyte apoptosis in osteoarthritis by targeting lncRNA CASC2. BMC Musculoskelet Disord. 2020;21:26. doi: 10.1186/s12891-019-3025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding Y, Wang L, Zhao Q, Wu Z, Kong L. MicroRNA-93 inhibits chondrocyte apoptosis and inflammation in osteoarthritis by targeting the TLR4/NF-κB signaling pathway. Int J Mol Med. 2019;43:779–790. doi: 10.3892/ijmm.2018.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]


