Abstract
Objectives:
The study reports longitudinal changes in grip strength, muscle mass and muscle power of lower extremities. The aim is to identify early muscular changes to improve the diagnosis and treatment of sarcopenia.
Methods:
Grip strength was measured by hand dynamometer, muscle mass by dual-energy X-ray absorptiometry and muscle power by performing a chair rise test and two-leg jumps (2LJP) on the Leonardo Mechanograph®. Longitudinal changes were analysed using paired t-tests by age group and sex. Differences between groups in terms of the annual change were tested by Analysis of Variance and the Dunnett’s test. Comparisons between the variables were performed using one sample t-tests.
Results:
Six-year changes were determined in 318 randomly selected healthy participants aged 20-90 years from Berlin. 2LJP declined significantly earlier in 20-39 years old women (-3.70 W/kg) and men (-5.97 W/kg, both p<0.001). This is an absolute annual decline of -0.46 W/kg in females and -0.75 W/kg in males. In the oldest age group, 2LJP showed the highest absolute annual loss with -0.99 W/kg in women and -0.88 W/kg in men. 2LJP was significantly different compared to all variables of muscle mass and strength (p<0.01).
Conclusions:
The results underline the importance of assessing muscle power using 2LJP during aging.
Keywords: Aging, Longitudinal, Mechanography, Muscle Power, Sarcopenia
Introduction
Numerous studies describe the age-related decline in skeletal muscle mass and strength[1,2]. As the European Working Group on Sarcopenia in Older People (EWGSOP) stated in their revised European consensus on the definition of sarcopenia, sarcopenia is not only defined as a reduction in muscle mass and muscle strength with increasing age, but also with low physical performance[3]. The concept of physical performance includes multidimensional, neuromuscular functions during movement. Therefore, muscle power is a crucial parameter of physical performance because it is defined as the product of force and velocity and is derived from how quickly and powerfully the body can move[4]. Muscle power has been recently discussed as playing an important role in predicting immobilisation, the risk of falls, and sarcopenia[5-7]. Various studies on the relationship of muscle and age emphasise the role of muscle power, as it decreases earlier over the lifespan and to a greater extent than muscle mass or strength, and has greater association with functional status and impairments in functional daily activities than other muscle parameters[1,7-11]. Further, numerous studies reported that the loss of muscle strength and mass over time is greater in longitudinal studies than in cross-sectional studies, which remains to be investigated for muscle power[12-17]. In addition, longitudinal studies showed a relationship between muscle power and clinical parameters such as frailty or mobility decline in older adults[18,19]. Therefore, a longitudinal study design appears more appropriate than a cross-sectional design for examining the development and deterioration process of muscle power over age.
A suitable instrument for measuring muscle power over a wide range of ages is muscle mechanography. Computerised force platforms record the movements of the centre of gravity and the applied force vectors during locomotor tasks like two-leg jumps or a chair rise test[4]. Integrated software calculates muscle function parameters, such as force, velocity or power[4]. Reproducibility and excellent retest reliability have been confirmed for jumping mechanography[20-22] and moderate reliability has been reported for the chair rise test[20,21,23]. Muscle mechanography is also considered to be a safe assessment instrument[4,20,22,24,25] with a high acceptability[25] even in older populations. Age-related and sex-related reference values for muscle power measured by muscle mechanography have been published for use in diagnostics and therapy[26-29].
The EWGSOP identified the need to provide parameters and assessment instruments to detect sarcopenia more effectively and predict health related outcomes as being a gap in the research[3]. In addition, data on body composition shows strong regional and ethnic differences and such data is not sufficiently available from Germany[30]. Therefore, the purpose of this longitudinal study is to examine and to compare the changes over time in body composition, muscle strength and muscle power in a randomly selected sample of 20 to 90-year-old subjects in Berlin, Germany. This is the first study to provide longitudinal data for jumping mechanography and the chair rise test with Leonardo Mechanograph®. The results of this study should contribute to a better recognition and quantification of diseases in connection with body composition and functional muscle parameters in order to develop suitable diagnostic and preventive measures.
Methods
Study sample
The data of the present study was collected as part of the German project “muscle survey 2”. This is the six-year follow-up of a population-based, cross-sectional study focussing on muscle and bone health. The subjects recruited for the baseline evaluation were randomly-selected and age- and sex-stratified from the resident registration office in Berlin, Germany. The exclusion criteria for both evaluations were: 1) metal implants or artificial prostheses; 2) oedema; 3) medication affecting water-mineral homeostasis; 4) a walking aid; 5) contraindications for X-ray exposure; 6) pregnancy; 7) cognitive impairment that prevented informed consent[26,31]. Details regarding the recruitment process, screening criteria and results of the baseline muscle survey were provided elsewhere[26,31].
All examinations were performed at the Centre for Muscle and Bone Research at Charité-Universitätsmedizin Berlin. The study design and procedures were approved by the ethics committee of Charité-Universitätsmedizin Berlin (EA4/021/14; EA4/095/05) and by the German Radiation Protection Ordinance (Z5-22462/2-2005-063; Z5-22462/2-2014-025). Written informed consent was obtained from all participants.
The subjects of the baseline examination were contacted via postal letters again and asked to return for a follow-up examination. If the invitation letter was sent back due to the address of the participant being unknown, an extensive search at the resident registration office in Berlin was conducted to find out the new address or whether the person had died. Subjects who did not respond received a second invitation letter after 6 months.
For the current study, data of the participants who attended both investigations were used. Depending on the assessment instrument and the test, different numbers of data sets could be analysed, i.e. different n. This was caused, for example, by technical errors, test failures or exclusion due to extreme values. Some subjects were also not able to perform all tests due to medical conditions.
Anthropometry
Body weight and body height were measured to the nearest 0.1 kg and 0.1 cm, using an electronic measuring and weighing station (Seca 764). Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2).
Assessment of muscle power
The muscle power assessment was performed on the Leonardo Mechanograph® Ground Reaction Force Plate (Novotec Medical GmbH, Pforzheim, Germany software package 4.2). Sampling frequency was 800 Hz. The examiner showed and explained all tests in advance, but according to the protocol the participants were not allowed to practice the tests. The tests were performed with the participants’ own comfortable flat shoes and clothes. The same test order was used at baseline and follow-up.
Jumping mechanography
First, the participants performed three two-leg jumps (2LJ) as a countermovement jump on the platform. They were asked to jump as high as possible “to reach the bananas”. They were allowed to move their arms freely. There was a one-minute break between each jump. The jump with the highest jumping power was selected for further analysis. The main measurement outcome variable was the maximum total relative power per body weight in W/kg during lift off (2LJP).
Chair Rise Test (CRT)
Following the jumping mechanography, the chair rise test was performed. A bench with a height of 45 cm was anchored in the Leonardo force plate. During the CRT, the participants were asked to move 5 times, as quickly as possible, from a seated to standing position. They kept their arms crossed in front of their chest. The test was performed only once. CRT assesses the hip surrounding muscle function which is essential for preventing fall-related injuries[11]. The main measurement outcome variable was the maximum total relative power per body weight in W/kg during the rise phases (CRTP).
Assessment of muscle strength
Finally, hand grip strength was assessed using a hand dynamometer (Takei Scientific Instruments Co. Ltd., Tokyo, Japan). The subject was standing upright, and the arms were hanging down at the side of their body. The test person was asked to squeeze the dynamometer as tightly as possible. Their arm remained in an extended position. Both hands were measured three times each and the highest value out of six trials was used for analysis. As a variable of interest, the “maximum grip strength” was used as recommended by standardised approaches[32] and recorded to the nearest 0.1 kg.
Assessment of body composition
Body composition was evaluated by dual-energy X-ray absorptiometry (DXA). Measurements were conducted by Lunar Prodigy Advance, GE Healthcare (Software enCORE v13.6) or Lunar iDXA, GE Healthcare (Software enCORE v13.6).
Initially, the investigation began with the Prodigy device. The enhanced Lunar iDXA was used later during the baseline investigation. All participants of the follow-up were measured with the same device that they had used during the baseline survey (n=44 prodigy and n=274 iDXA). Therefore, the longitudinal changes do not depend on the distinction between the two devices.
Lean mass in the arms and legs in kg were used as indicators of muscle quantity, i.e. muscle mass, and thus integrated into the analysis. To obtain an estimate of appendicular skeletal muscle mass (ASM) the sum of the muscle mass of the arms and legs was used, as Baumgartner et al. suggested[33]. Due to the correlation between muscle mass and body size, there are different possibilities of adjustment: we integrated the appendicular skeletal muscle mass to height ratio (ASM/height2] in kg/m2) and to body mass index (ASM/BMI in m2) into the analysis[3].
Statistical analysis
Descriptive statistics and the comparison of baseline anthropometric characteristics are presented as mean ± standard deviation (SD). Differences between dropouts and subjects returning for testing at the follow-up were evaluated by unpaired students’ t-test.
Changes in body composition, muscle strength and muscle power over time were tested using paired t-tests by age group (20-39, 40-49, 50-59, 60-69, and over 70 years at baseline) and sex, and are reported as the absolute change with the mean ± standard deviation. To analyse the variation of all variables between subjects of an age group, the coefficient of variation in percentages (CV%) is reported for the absolute change. It was calculated from the SD divided by the mean of variable x multiplicated by 100. To determine the effect size, Cohen’s d was computed.
Absolute annual change in each measure was calculated as follow-up value minus baseline value divided by follow-up time.
The annual change is expressed as a percentage and determined in the following way:
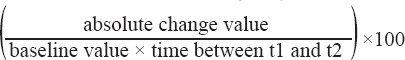
Two-way analysis of variance (ANOVA) was used to assess the absolute and percentage annual change by age group and sex. Because the age group*sex interaction term was never significant, only main effects were studied. Results are presented as mean ± standard deviation. Differences between subject groups were determined with Dunnett’s tests using the youngest age group (20-39 years) as a reference group.
The mean annual change (%) of each age group of 2LJP was compared with the mean of the same age group of CRTP, all variables of body composition and grip strength using one sample t-tests. Men and women were compared separately.
Statistical significance was set at p<0.05 for all analyses. All statistical analyses were performed with SPSS, software package version 25.
Results
318 subjects were integrated in the follow-up analysis. The average follow-up interval was 6.1±0.9 years. Of the original cohort (n=766), 46.5% returned for testing, 4.7% died (n=36), 4.3% moved away (n=33), 3.1% were not found (n=24) and 16.2% did not respond (n=124). 25.2% refused testing (n=193). Reasons for refusal included health problems (34.2%), lack of interest (15.4%), lack of time (27.5%) and other reasons (21.8%) (Figure 1). The lowest rates of return were in the youngest and oldest age groups, with 15.6% and 24.6%, respectively. Figure 1 shows a flowchart of the retention process of the study population.
Figure 1.
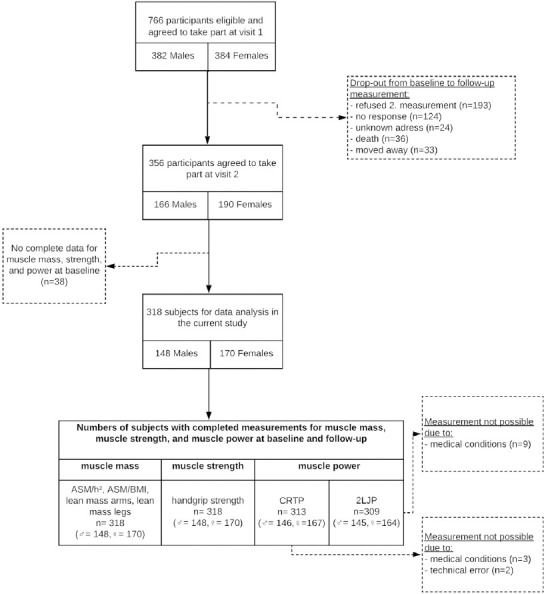
Flowchart.
Subject characteristics from the baseline evaluation are presented in Table 1. The mean age for follow-up was 54.7±14.0 years for females and 57.6±15.2 years for males. A comparison of baseline characteristics indicates that women who returned for testing were similar in their baseline age, weight, height and BMI to those who had dropped out. The males returned for testing were significantly older (p<0.01) and had significantly higher weight and BMI, with both p<0.05, than those who had dropped out.
Table 1.
Dropout comparison of baseline characteristics.
| Women | Men | |||
|---|---|---|---|---|
| Dropout | Follow-up | Dropout | Follow-up | |
| n | 214 | 170 | 234 | 148 |
| Age (years) | 52.6±20.9 | 54.7±14.0 | 51.1±20.6 | 57.6±15.2** |
| Weight (kg) | 67.7±11.8 | 68.1±10.8 | 81.1±12.9 | 83.7±11.6* |
| Height (cm) | 164.5±7.0 | 163.8±6.7 | 176.3±8.0 | 176.3±6.8 |
| BMI (kg/m2) | 25.1±4.4 | 25.4±4.0 | 26.11±3.8 | 26.9±3.4* |
Data is mean ± SD. Dropouts did not return for the follow-up.
Significant differences between dropouts and follow-ups for p<0.001 evaluated by unpaired t-test.
Significant differences between dropouts and follow-ups for p<0.05 evaluated by unpaired t-test.
Tables 2 and 3 show the longitudinal changes in muscle mass, muscle strength and muscle power by age group in women (2) and men (3).
Table 2.
Changes by age group during the follow-up period in women.
| Age group | n | Baseline | Absolute change | P-value absolute change | Cohen’s d absolute change | Absolute annual change | Annual change (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Muscle mass | mean±SD | mean±SD | CV (%) | mean±SD | mean±SD | ||||
| Lean mass arms (kg) | 20-39 | 28 | 4.21±0.60 | 0.13±0.28 | 215.4 | 0.022 | 0.5 | 0.02±0.04 | 0.37±0.87 |
| 40-49 | 37 | 4.30±0.45 | 0.06±0.24 | 400.0 | 0.136 | 0.3 | 0.01±0.04 | 0.29±1.04 | |
| 50-59 | 40 | 4.10±0.64 | -0.02±0.26 | 1452.1 | 0.647 | 0.1 | -0.003±0.05 | -0.05±1.13 | |
| 60-69 | 35 | 4.19±0.61 | -0.14±0.24 | 165.0 | 0.002 | 0.6 | -0.02±0.04* | -0.54±1.00** | |
| 70+ | 30 | 4.08±0.54 | -0.15±0.60 | 400.0 | 0.181 | 0.3 | -0.03±0.09* | -0.55±2.33** | |
| Lean mass legs (kg) | 20-39 | 28 | 14.26±1.99 | 0.24±0.84 | 350.0 | 0.139 | 0.3 | 0.03±0.11 | 0.19±0.72 |
| 40-49 | 37 | 14.18±1.68 | -0.14±0.65 | 467.7 | 0.195 | 0.2 | -0.03±0.12 | -0.17±0.85 | |
| 50-59 | 40 | 13.26±2.05 | -0.32±0.76 | 239.6 | 0.013 | 0.4 | -0.05±0.13* | -0.38±0.93** | |
| 60-69 | 35 | 13.10±1.93 | -0.44±0.65 | 147.7 | 0.000 | 0.7 | -0.08±0.11** | -0.53±0.88** | |
| 70+ | 30 | 12.74±1.35 | -0.31±0.56 | 180.6 | 0.005 | 0.6 | -0.05±0.09* | -0.37±0.71** | |
| ASM/h2 (kg/m2) | 20-39 | 28 | 6.57±0.81 | 0.13±0.36 | 276.9 | 0.066 | 0.4 | 0.02±0.05 | 0.23±0.68 |
| 40-49 | 37 | 6.69±0.64 | 0.03±0.28 | 860.1 | 0.527 | 0.1 | -0.01±0.05 | -0.07±0.78 | |
| 50-59 | 40 | 6.48±0.82 | -0.12±0.34 | 283.3 | 0.028 | 0.4 | -0.02±0.06* | -0.31±0.88** | |
| 60-69 | 35 | 6.65±0.77 | -0.21±0.30 | 144.3 | 0.000 | 0.7 | -0.04±0.05*** | -0.54±0.80** | |
| 70+ | 30 | 6.52±0.68 | -0.18±0.34 | 188.9 | 0.008 | 0.5 | -0.03±0.05** | -0.42±0.81** | |
| ASM/BMI (m2) | 20-39 | 28 | 0.75±0.12 | -0.02±0.04 | 200.0 | 0.039 | 0.5 | -0.002±0.006 | -0.27±0.81 |
| 40-49 | 37 | 0.72±0.11 | -0.02±0.03 | 150.0 | 0.000 | 0.7 | -0.005±0.006 | -0.66±0.85 | |
| 50-59 | 40 | 0.69±0.08 | -0.02±0.03 | 150.0 | 0.000 | 0.7 | -0.004±0.005 | -0.48±0.73 | |
| 60-69 | 35 | 0.65±0.07 | -0.01±0.04 | 400.0 | 0.029 | 0.3 | -0.002±0.006 | -0.31±0.87 | |
| 70+ | 30 | 0.63±0.08 | -0.02±0.04 | 200.0 | 0.014 | 0.5 | -0.003±0.007 | -0.47±1.11 | |
| Muscle strength | |||||||||
| Grip strength maximum (kg) | 20-39 | 28 | 31.53±5.35 | -0.19±3.32 | 1747.4 | 0.761 | 0.1 | -0.01±0.42 | 0.06±1.32 |
| 40-49 | 37 | 29.65±3.29 | 0.52±2.22 | 426.3 | 0.160 | 0.2 | 0.10±0.40 | 0.34±1.43 | |
| 50-59 | 40 | 28.93±4.61 | -1.36±2.38 | 175.0 | 0.001 | 0.6 | -0.24±0.41 | -0.79±1.37 | |
| 60-69 | 35 | 28.11±3.60 | -1.12±2.57 | 229.5 | 0.015 | 0.4 | -0.19±0.45 | -0.61±1.61 | |
| 70+ | 30 | 25.75±2.91 | -2.63±3.13 | 119.0 | 0.000 | 0.8 | -0.43±0.54** | -1.69±2.20*** | |
| Muscle power | |||||||||
| CRTP (W/kg) | 20-39 | 28 | 12.37±2.24 | -0.62±1.57 | 253.2 | 0.046 | 0.4 | -0.09±0.24 | -0.58±1.76 |
| 40-49 | 37 | 12.23±1.96 | -1.14±1.16 | 101.3 | 0.000 | 1.0 | -0.21±0.21 | -1.73±1.85 | |
| 50-59 | 39 | 10.90±1.74 | -0.89±1.24 | 139.8 | 0.000 | 0.7 | -0.16±0.22 | -1.42±1.99 | |
| 60-69 | 35 | 10.07±1.44 | -0.99±1.19 | 120.6 | 0.000 | 0.8 | -0.17±0.20 | -1.59±2.07 | |
| 70+ | 28 | 9.02±1.61 | -1.00±0.97 | 97.0 | 0.000 | 1.0 | -0.17±0.17 | -1.84±1.82* | |
| 2LJP (W/kg) | 20-39 | 28 | 37.41±5.61 | -3.70±2.80 | 75.7 | 0.000 | 1.3 | -0.46±0.34 | -1.22±0.86 |
| 40-49 | 37 | 34.02±5.13 | -2.99±3.07 | 102.7 | 0.000 | 1.0 | -0.55±0.56 | -1.64±1.78 | |
| 50-59 | 39 | 31.68±5.63 | -3.93±2.78 | 70.8 | 0.000 | 1.4 | -0.69±0.48 | -2.13±1.34* | |
| 60-69 | 34 | 28.52±4.65 | -4.53±2.62 | 57.8 | 0.000 | 1.7 | -0.78±0.45* | -2.68±1.37*** | |
| 70+ | 26 | 24.37±4.46 | -5.24±2.10 | 40.1 | 0.000 | 2.5 | -0.88±0.34** | -3.50±1.20*** | |
Notes. Data are means ± SD. CV%= coefficient of variation in %. Absolute change= follow-up value - baseline value. Absolute annual change= absolute change/follow-up period. P-value of paired t-test changes from Baseline, bold values = significant p<0.05. Annual change (%) = ((follow-up value - baseline value)/(baseline-value * follow-up period))*100.
significant differences compared to the youngest adults (20-39 years) (p<0.05).
significant differences compared to the youngest adults (20-39 years) (p<0.01).
significant differences compared to the youngest adults (20-39 years) (p<0.001).
Table 3.
Changes by age group during the follow-up period in men.
| Age group | n | Baseline | Absolute change | P-value absolute change | Cohen’s d absolute change | Absolute annual change | Annual change (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Muscle mass | mean±SD | mean±SD | CV (%) | mean±SD | mean±SD | ||||
| Lean mass arms (kg) | 20-39 | 25 | 7.57±1.23 | -0.07±0.36 | 514.3 | 0.322 | 0.2 | -0.01±0.05 | -0.12±0.59 |
| 40-49 | 17 | 7.67±1.41 | -0.11±0.45 | 424.1 | 0.345 | 0.2 | -0.02±0.08 | -0.16±1.07 | |
| 50-59 | 36 | 7.07±0.95 | -0.28±0.52 | 185.7 | 0.003 | 0.5 | -0.05±0.09 | -0.67±1.27 | |
| 60-69 | 37 | 6.72±0.91 | -0.32±0.39 | 121.9 | 0.000 | 0.8 | -0.06±0.07 | -0.78±1.02 | |
| 70+ | 33 | 6.55±0.84 | -0.48±0.44 | 91.7 | 0.000 | 1.1 | -0.08±0.07** | -1.19±1.07*** | |
| Lean mass legs (kg) | 20-39 | 25 | 20.78±2.45 | -0.06±0.90 | 1500.0 | 0.738 | 0.1 | -0.01±0.12 | -0.05±0.59 |
| 40-49 | 17 | 21.02±2.99 | -0.10±0.87 | 828.9 | 0.626 | 0.1 | -0.02±0.16 | -0.06±0.73 | |
| 50-59 | 36 | 19.12±1.87 | -0.35±1.13 | 322.9 | 0.071 | 0.3 | -0.06±0.20 | -0.33±1.07 | |
| 60-69 | 37 | 17.63±2.61 | -0.53±0.90 | 169.8 | 0.001 | 0.6 | -0.09±0.16 | -0.46±0.86 | |
| 70+ | 33 | 18.12±2.13 | -0.51±1.21 | 237.3 | 0.022 | 0.4 | -0.08±0.19 | -0.43±1.06 | |
| ASM/h2 (kg/m2) | 20-39 | 25 | 8.71±0.98 | -0.04±0.35 | 882.0 | 0.536 | 0.1 | -0.01±0.05 | -0.08±0.53 |
| 40-49 | 17 | 8.63±1.07 | -0.07±0.38 | 547.7 | 0.456 | 0.2 | -0.01±0.07 | -0.10±0.76 | |
| 50-59 | 36 | 8.26±0.73 | -0.20±0.48 | 240.0 | 0.019 | 0.4 | -0.04±0.09 | -0.42±1.04 | |
| 60-69 | 37 | 8.18±0.86 | -0.28±0.38 | 135.7 | 0.000 | 0.7 | -0.05±0.07 | -0.55±0.82 | |
| 70+ | 33 | 8.24±0.82 | -0.33±0.53 | 160.6 | 0.001 | 0.6 | -0.05±0.08 | -0.63±0.10 | |
| ASM/BMI (m2) | 20-39 | 25 | 1.06±0.11 | -0.05±0.07 | 140.0 | 0.001 | 0.7 | -0.006±0.009 | -0.55±0.84 |
| 40-49 | 17 | 1.06±0.08 | -0.03±0.05 | 166.7 | 0.023 | 0.6 | -0.005±0.008 | -0.47±0.77 | |
| 50-59 | 36 | 0.98±0.10 | -0.05±0.05 | 100.0 | 0.000 | 1.0 | -0.009±0.008 | -0.87±0.83 | |
| 60-69 | 37 | 0.88±0.11 | -0.02±0.03 | 150.0 | 0.000 | 0.7 | -0.004±0.005 | -0.40±0.59 | |
| 70+ | 33 | 0.86±0.07 | -0.03±0.05 | 166.7 | 0.002 | 0.6 | -0.005±0.009 | -0.58±0.99 | |
| Muscle strength | |||||||||
| Grip strength maximum (kg) | 20-39 | 25 | 46.83±6.14 | 0.29±5.50 | 1896.6 | 0.796 | 0.1 | 0.02±0.71 | 0.15±1.54 |
| 40-49 | 17 | 48.70±4.17 | -0.98±2.89 | 294.9 | 0.181 | 0.3 | -0.18±0.54 | -0.35±1.10 | |
| 50-59 | 36 | 45.82±7.51 | 0.29±5.08 | 1752.2 | 0.733 | 0.1 | 0.04±0.89 | 0.25±2.32 | |
| 60-69 | 37 | 43.47±7.60 | -1.71±5.42 | 317.0 | 0.063 | 0.3 | -0.29±0.94 | -0.38±2.72 | |
| 70+ | 33 | 40.87±6.03 | -3.99±4.99 | 125.1 | 0.000 | 0.8 | -0.62±0.78* | -1.48±1.84** | |
| Muscle power | |||||||||
| CRTP (W/kg) | 20-39 | 25 | 14.74±2.43 | -0.59±2.21 | 374.6 | 0.192 | 0.3 | -0.08±0.27 | -0.32±1.96 |
| 40-49 | 17 | 15.67±1.99 | -1.62±1.20 | 74.1 | 0.000 | 1.4 | -0.30±0.22* | -1.84±1.36 | |
| 50-59 | 35 | 13.89±1.86 | -1.68±2.02 | 120.7 | 0.000 | 0.8 | -0.30±0.37** | -2.13±2.70** | |
| 60-69 | 37 | 12.29±1.95 | -1.71±1.19 | 69.7 | 0.000 | 1.4 | -0.30±0.20* | -2.46±1.63** | |
| 70+ | 32 | 10.91±2.13 | -1.93±2.06 | 107.0 | 0.000 | 0.9 | -0.31±0.31** | -2.76±2.88*** | |
| 2LJP (W/kg) | 20-39 | 25 | 49.25±6.59 | -5.97±3.90 | 65.3 | 0.000 | 1.5 | -0.75±0.46 | -1.51±0.86 |
| 40-49 | 17 | 45.03±4.52 | -3.96±3.68 | 92.9 | 0.000 | 1.1 | -0.73±0.67 | -1.59±1.39 | |
| 50-59 | 36 | 38.94±6.08 | -3.94±3.47 | 88.1 | 0.000 | 1.1 | -0.72±0.64 | -1.78±1.56 | |
| 60-69 | 37 | 35.17±5.89 | -4.54±3.50 | 77.0 | 0.000 | 1.3 | -0.78±0.59 | -2.18±1.61 | |
| 70+ | 30 | 28.87±5.00 | -6.20±4.12 | 66.4 | 0.000 | 1.5 | -0.99±0.65 | -3.34±1.90*** | |
Notes. Data are means ± SD. CV%= coefficient of variation in %. Absolute change= follow-up value - baseline value. Absolute annual change= absolute change/follow-up period. P-value of paired t-test changes from Baseline, bold values = significant p<0.05. Annual change (%) = ((follow-up value - baseline value)/(baseline-value * follow-up period))*100.
significant differences compared to the youngest adults (20-39 years) (p<0.05).
significant differences compared to the youngest adults (20-39 years) (p<0.01).
significant differences compared to the youngest adults (20-39 years) (p<0.001).
Absolute longitudinal change of muscle power, strength and mass
CRTP and 2LJP decreased for both sexes from the age group 20-39 years. This absolute change is distinctly significant in all age groups for women (p<0.001), and also for men (p<0.001), except for the youngest age group of men for CRTP (p=0.192) (Tables 2, 3).
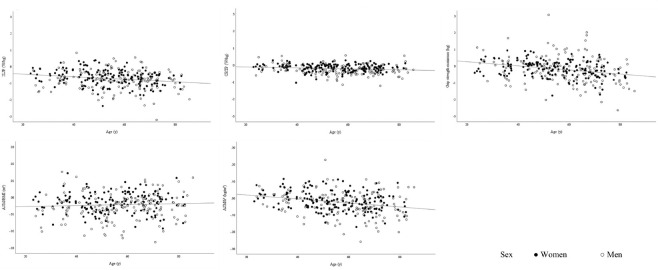
The highest annualized absolute decline can be seen in the oldest age group of 2LJP with -0.88 W/kg for women and -0.99 W/kg for men. The youngest age group shows an annual decline of -0.46 W/kg and -0.75 W/kg, respectively (Tables 2, 3). As Figure 2 shows, the evolution of 2LJP was quite variable at any age.
Figure 2.
Individual absolute annual change in 2LJP, CRTP, grip strength, ASM/BMI and ASM/h2 according to age and sex.
Maximum grip strength shows significant losses by the age of 70+ years in men (p<0.001) (Table 3). In women the decrease is noted significantly earlier from the age of 50 years (p=0.001) (Table 2).
A significant decrease of ASM/BMI can be seen in all age groups and for both sexes (p=0.001-0.039). For ASM/h2, a significant absolute decline can be observed in women and men in the age groups 50-59 (p=0.028 vs. 0.019), 60-69 (both p<0.001) and over 70 years (p=0.008 vs. 0.001) (Table 2, 3).
The absolute annual change of variables of strength and mass shows a highly variable trend over age and sex (Figure 2).
Annual percentage change of muscle power compared to strength and mass
Figures 3a and 3b present the mean annual changes in percentages. In both sexes, a pronounced annual decline of muscle power in CRT and 2LJ is evident in all age groups, beginning in the 20-39 years of age group (Figure 3a, b). The decline of 2LJP in this age group is much more pronounced and begins significant earlier compared to CRTP, muscle strength and mass in both sexes (-1.22% per year in females, -1.51% per year in males, p<0.001). The most obvious decrease in women is visible for 2LJP at the ages of 70 years and older (-3.5% per year). This is significantly different to all other variables (p<0.001 and p<0.01 for CRTP) in the same age group. In men, the oldest age group of 2LJP (-3.34% per year) is significantly different compared to all other variables in the same age group (p<0.001), except for CRTP (p=0.102). A significant difference between 2LJP and CRTP in men is only visible in the youngest age group (p<0.001).
Figure 3a, b.
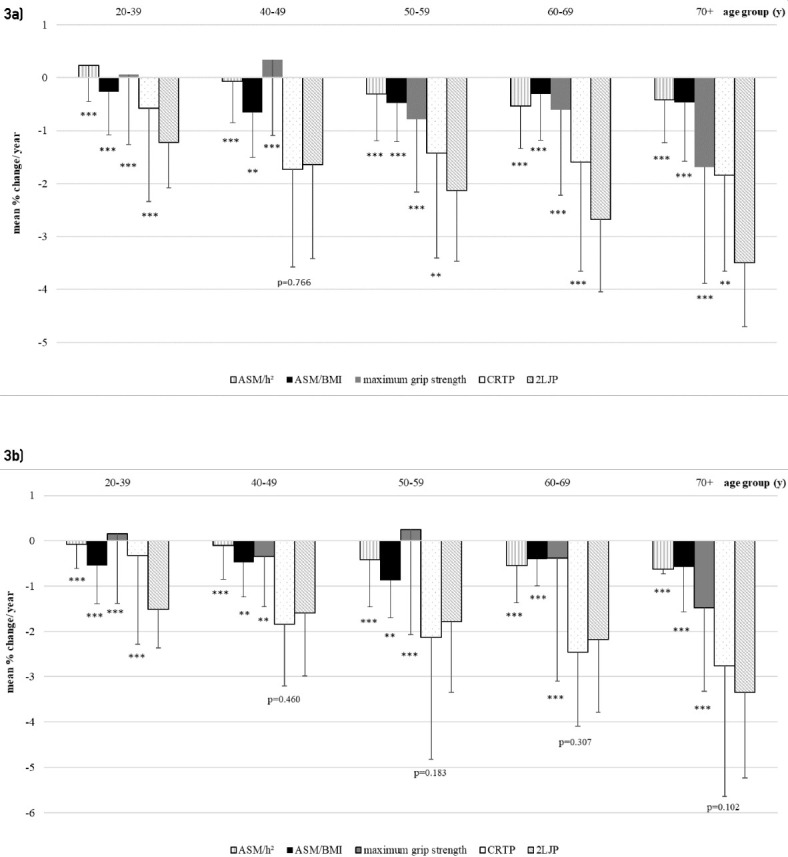
Annual percentage change in females (a) and males (b). Values of p, one sample t-test between the mean of each age group of 2LJP with the same age group of each other variable: *** significant difference with p<0.001, compared to the same age group of 2LJP, ** significant difference with p<0.01 compared to the same age group of 2LJP.
Discussion
The present study showed the longitudinal changes in body composition, muscle strength and muscle power measured by DXA, hand dynamometer and muscle mechanography in a healthy sample of Berlin inhabitants aged between 20 and 90 years. This is the first study to report longitudinal age- and sex-related data on muscle power measured by Leonardo Mechanograph®. Findings of this analysis underline the importance of the assessment of muscle power in the clinical diagnosis of sarcopenia.
One of the most obvious results of this study is the early age-related decline of muscle power assessed by 2LJ compared to CRTP, muscle mass and strength. Relative power of 2LJ seems to be an important indicator of the beginning of deterioration processes. Whereby the decline in 2LJP becomes apparent even earlier at the age of 20-39 years than in CRTP, which shows at the age of 40-49 years. This is in line with the few studies using jumping mechanography for the analysis of muscle power. Due to the lack of longitudinal studies using jumping mechanography, the results can only be compared with cross-sectional studies or with studies using other power assessment methods. Runge et al. identified, in a cross-sectional analysis with a wide range of ages of subjects between 18-88 years, that 2LJP showed an earlier and stronger decline with age compared to CRTP[5]. They concluded that jumping mechanography and the evaluation of muscle power are more sensitive for age-related decline than the evaluation of CRTP, muscle mass or muscle strength. Siglinsky et al. showed in their cross-sectional study that muscle power during countermovement jumps on a Leonardo Mechanograph® is more strongly correlated with age than grip strength or time for the chair rise test[34]. Dietzel et al. reported the cross-sectional results of the baseline evaluation of this study[26]. The cross-sectional as well as the current longitudinal analysis showed that 2LJP declines earlier than CRTP in both women and men[26]. In the cross-sectional analysis the group differences compared to a reference group evaluated by Dunnett’s test became significant earlier than in the longitudinal analysis. One main difference was the definition of the reference groups. The reference group of the baseline study by Dietzel et al. consists of people aged 20-29 years and thus includes one decade less than the current study. This was caused by the large drop-out rate in the youngest age group at the follow-up. To include homogeneously balanced sample sizes by age group in the analysis, the two youngest age groups have been collated. This may have led to different results in the comparisons between the groups.
There are further cross-sectional[35-37] and longitudinal studies[1,38], which focused on the differences between muscle power and muscle strength or mass with age, but used different assessment instruments compared to this study. They all support the observation of this study that muscle power declined earlier and more extensively than other muscle parameters in women and in men. However, they need to be interpreted with caution due to the different study designs.
In this study, both, 2LJP and CRTP, showed in the oldest age group a significantly higher reduction compared to variables of body composition and muscle strength. The decline of muscle power for the age group of 70+ in the present study ranges between -1.84% and -3.5% per year, depending on the assessment and sex. Similar rates of decline were also reported by Skelton et al[37]. They observed in a cross-sectional study for a sample of 65 to 89 years old an age-related decline of 3% per year for men and 1.7% per year for women for muscle power of leg extention[37]. A longitudinal study of Reid et al. revealed a decline of muscle power of 2.9% per year for subjects aged 70-85 years[1]. While the annual decline of the cross-sectional study by Skelton et al. should be carefully compared with the results of the present longitudinal study, the results of Reid et al. are comparable in its extent with the results of this study. The research team of Reid investigated muscle power in a 3-year longitudinal analysis using a bilateral leg press apparatus[1]. They identified that the healthy subjects maintained their muscle strength at follow-up while muscle power showed significant reductions. Additionally, Reid et al. measured neuromuscular activation of vastus lateralis by surface electromyography (EMG), which also showed a significant reduction in healthy subjects. They concluded that early decline of neuromuscular activation is associated with impairments in contraction velocity and thus in muscle power[1].
Further, a study of van Roie and team found out that the velocity component of peak power tended to decline more in women than in men[35]. They concluded that the decline in the velocity component of muscle power may be more relevant in women[35], which is in line with present findings that the deterioration process of muscle power tested by 2LJ, having a higher explosive power component than CRT, begins earlier in women than men and the differences in the age groups are much clearer and significant.
The influence of the velocity component of muscle power, notably during two-leg jumps, as a leading factor in the deterioration process was investigated by several biomechanical studies. As muscle power is the product of force and velocity, the large decrease seems to be due to an early loss of the largest and fastest contracting type II muscle fibres[39]. Due to a general demyelination of the central and peripheral nervous system and the dominance of type I muscle fibres, the axonal conduction velocity slows down with age. This observation was made for both men and women[40,41]. The only sex-specific difference was the smaller and decreasing size of type II muscle fibres in women than in men with increasing age[41,42]. Further intrinsic changes in actin-myosin structures, motor units and as well in hormones and metabolism contribute to reduced muscle power, which might explain the earlier loss in women than in men in this study[9,43,44]. Further this can be one of the reasons, why women have a higher risk of falls and fall-related injuries[45].
As a larger eccentric load is expected during the lift off in 2LJ than in CRT, one reason for the earlier reduction seems to be due to changes in tendon stiffness and composition as Narici et al. stated[46]. Although the effect of ageing on tendon structures has not been fully and systematically investigated, they concluded that more flexible tendons need more time to transmit fast forces from muscles to bones. They reported that older tendons were 15% more flexible than younger tendons[46]. Therefore, fast eccentric activation of the elastic elements of older people occurs with greater difficulty and thus is a crucial indicator for motor disorders in older adults[11]. In women, the influence of estrogen on tendons seems to play a crucial role in muscle performance. There is conflicting evidence on how the hormone estrogen affects tendon stiffness in postmenopausal women, but it should be considered as a factor for the earlier loss of muscle power compared to men in this study[47].
The present study revealed promising results for the assessment of muscle power using muscle mechanography. The results of the current study imply that not muscle strength or mass, but rather muscle power, shows earlier and pronounced decline and might be a more relevant parameter for the identification of sarcopenic changes. In a clinical pathway published by the EWGSOP, they recommend first assessing muscle strength by handgrip strength or CRT to decide if sarcopenia is possible followed by the evaluation of muscle mass to confirm sarcopenia, and finally the evaluation of the severity by physical performance tests[3]. In agreement with other studies, the evaluation of muscle power as a parameter of physical performance should not be the last step in the clinical pathway to assess the severity of sarcopenia, but the first to identify early changes[8,20,48]. The question remains as to whether, if muscle power is such a valuable parameter in the diagnostic process, it has the same importance for the prevention and treatment of sarcopenia. Many activities in daily life depend on powerful movements such as climbing stairs, getting up off chairs or reacting to perturbations to prevent falling. As mentioned above, the extent of muscle power might predict the risk of falls and impairments more effectively than traditional measurements[1,8,10,11]. To develop a full picture of muscle power for the diagnosis of sarcopenia, additional longitudinal studies will be needed to investigate its predictive value for clinically relevant outcomes.
We consider the longitudinal design as a strength of our study. However, there have also been several limitations. One weakness is that we were only able to include two time points of assessments. This could have affected the assumption of linear changes for which contradictory evidence exists. There are studies reporting linear patterns[5,29] and others curvilinear patterns[24,35]. Most of them confirm that power increases in young years, has a stable development between the ages of 20 and 40 years and then declines continuously over age[26,35]. Unfortunately, there is a lack of prospective studies which examine this age-related decline of muscle power with mechanography. Therefore, caution must be applied when comparing the current results with studies using a cross-sectional design or reporting rates of decline over the entire range of age, because this design can result in an overestimation of the decline in young years or an underestimation in the oldest age groups.
Particularly in longitudinal studies with more than one test session and different raters, an uncontrolled factor is the possibility of low inter-rater and intersession reliability of the measurement method. Several studies confirmed the reproducibility of jumping mechanography and the chair rise test[20-23,49]. To increase the reliability of this study, a standardised test protocol was used, and the examinations were carried out by the same evaluator of the baseline investigation.
We have only focused on healthy subjects and thus the normal aging process. If muscle power is to be included in the assessment of sarcopenia, cut-off values are required. It would have been of great interest to examine a large cohort with healthy and mobility-limited subjects to define cut-off points for sarcopenia diagnosis and to analyse the predictive value for clinical endpoints. An initial data exploration with a subset of the baseline cohort by Dietzel et al.[8] showed an association between muscular power assessed by mechanography and sarcopenia, falls and impairments in the activities of daily living. However, as the focus in sampling was put on healthy subjects, there were too few subjects in the cohort who were classified as sarcopenic according to the current definitions to provide generalised conclusions. So, defining cut-off points was not included in the scope of this study and should be explored in further research.
There are few studies that investigate muscle function using muscle mechanography, notably longitudinal studies[18,20,25,34,50,51]. For this reason, our results have to be compared with cross-sectional or longitudinal studies measuring muscle power by other assessment methods. This limitation must be taken into account when interpreting the results. To develop a full picture and comparable data of the assessment of muscle power by muscle mechanography, additional studies are required.
A limitation in the scope of the sample is the loss to follow-up rate of 53.5%. This high proportion of subjects lost to follow-up has certainly resulted in a bias in the results. In Table 1 the participants who dropped out are compared with those who returned to follow-up. One point to consider in the analyses is that men participating in the follow-up were significantly older at baseline (p<0.01) and had a higher weight and BMI. As mentioned above, this might have affected all measurements where the influence of anthropometric values has been demonstrated (i.e. ASM/BMI, ASM/h2, grip strength)[3,52]. However, for the power assessments it is important to know that the Leonardo Mechanograph® assesses the body-weight corrected power [W/kg], so that the results for CRTP and 2LJP cannot be affected by that difference[4].
Further the high drop-out rate can cause an overestimation or an underestimation of the outcome variables. As mentioned above the drop out resulted in an under-sampling of the youngest and oldest age group. We have managed this by structuring the baseline and follow-up sample size per age-group relatively homogeneously and used only complete data sets.
The use of two different devices for dual-energy X-Ray absorptiometer can be problematic. A study by Watson et al.[53] has shown significant differences in the measurement of iDXA and Prodigy, in particular for bone mineral content and fat mass when comparing absolute values. Minor differences were found for lean mass estimates of arms and legs[53]. In the current study, the same device was used for each subject at both timepoints and only the change between these two points in time were analysed. Therefore, sampling bias did not influence the results.
In conclusion, the results of this longitudinal study support the observation that the three indicators of sarcopenia muscle mass, muscle strength and physical performance, i.e. muscle power, show different reduction rates depending on age and sex. Muscle power assessed by two-leg jumps is the one with the earliest and highest rate of loss. Changes in muscle strength becomes obvious later in women from the age of 50 years and in men at the age of 70+ years. This indicates that the assessment of handgrip strength which is commonly and primarily used as a pre-screening tool regarding early sarcopenia should be supported by the evaluation of muscle power.
Taken together, the findings of our study support strong recommendations for measuring muscle power in the diagnostic process of sarcopenia. Muscle mechanography can be established as a useful tool in clinical practice, is easy to use and is associated with moderate equipment costs compared to iDXA or other technical devices.
Acknowledgement
We would like to express our thanks to all the participants who took part in this study. Our greatest honour goes to Dieter Felsenberg, co-author and initiator of this study, who passed away while working on this paper.
Footnotes
The authors of the study received grants from The Danish Research Foundation during the conduct of the study.
Edited by: G. Lyritis
Authors’ contributions
Sabine Wiegmann contributed to data analysis, interpretation and writing the manuscript. Dieter Felsenberg contributed to the conceptualisation, study design and revision of drafts for submission. Gabriele Armbrecht contributed to the conceptualisation, study design, data collection and revision of drafts for submission. Roswitha Dietzel contributed to data collection, data analysis, writing of the manuscript and revision of drafts for submission. All authors read and approved the final version.
Funding
The baseline evaluation was financed using the Institute’s own financial resources. The follow-up received a grant from The Danish Research Foundation. The article processing charges were covered by the German Research Foundation (DFG) and the Open Access Publication Fund of Charité - Universitätsmedizin Berlin.
References
- 1.Reid KF, Pasha E, Doros G, Clark DJ, Patten C, Phillips EM, et al. Longitudinal decline of lower extremity muscle power in healthy and mobility-limited older adults:influence of muscle mass, strength, composition, neuromuscular activation and single fiber contractile properties. Eur J Appl Physiol. 2014;114(1):29–39. doi: 10.1007/s00421-013-2728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doherty TJ. The influence of aging and sex on skeletal muscle mass and strength. Current opinion in clinical nutrition and metabolic care. 2001;4(6):503–8. doi: 10.1097/00075197-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age and ageing. 2018;48(1):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taani MH, Kovach CR, Buehring B. Muscle Mechanography: A Novel Method to Measure Muscle Function in Older Adults. Res Gerontol Nurs. 2017;10(1):17–24. doi: 10.3928/19404921-20161209-03. [DOI] [PubMed] [Google Scholar]
- 5.Runge M, Rittweger J, Russo CR, Schiessl H, Felsenberg D. Is muscle power output a key factor in the age-related decline in physical performance?A comparison of muscle cross section, chair-rising test and jumping power. Clin Physiol Funct Imaging. 2004;24(6):335–40. doi: 10.1111/j.1475-097X.2004.00567.x. [DOI] [PubMed] [Google Scholar]
- 6.Orr R, de Vos NJ, Singh NA, Ross DA, Stavrinos TM, Fiatarone-Singh MA. Power training improves balance in healthy older adults. The journals of gerontology Series A, Biological sciences and medical sciences. 2006;61(1):78–85. doi: 10.1093/gerona/61.1.78. [DOI] [PubMed] [Google Scholar]
- 7.Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci L. A comparison of leg power and leg strength within the InCHIANTI study:which influences mobility more? The journals of gerontology Series A, Biological sciences and medical sciences. 2003;58(8):728–33. doi: 10.1093/gerona/58.8.m728. [DOI] [PubMed] [Google Scholar]
- 8.Dietzel R, Felsenberg D, Armbrecht G. Mechanography performance tests and their association with sarcopenia, falls and impairment in the activities of daily living - a pilot cross-sectional study in 293 older adults. J Musculoskelet Neuronal Interact. 2015;15(3):249–56. [PMC free article] [PubMed] [Google Scholar]
- 9.McKinnon NB, Connelly DM, Rice CL, Hunter SW, Doherty TJ. Neuromuscular contributions to the age-related reduction in muscle power:Mechanisms and potential role of high velocity power training. Ageing research reviews. 2017;35:147–54. doi: 10.1016/j.arr.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Reid KF, Fielding RA. Skeletal muscle power:a critical determinant of physical functioning in older adults. Exercise and sport sciences reviews. 2012;40(1):4–12. doi: 10.1097/JES.0b013e31823b5f13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Runge M, Hunter G. Determinants of musculoskeletal frailty and the risk of falls in old age. J Musculoskelet Neuronal Interact. 2006;6(2):167–73. [PubMed] [Google Scholar]
- 12.Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, et al. Longitudinal muscle strength changes in older adults:influence of muscle mass, physical activity, and health. The journals of gerontology Series A, Biological sciences and medical sciences. 2001;56(5):B209–17. doi: 10.1093/gerona/56.5.b209. [DOI] [PubMed] [Google Scholar]
- 13.Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle:a 12-yr longitudinal study. J Appl Physiol (1985) 2000;88(4):1321–6. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- 14.Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol (1985) 1991;71(2):644–50. doi: 10.1152/jappl.1991.71.2.644. [DOI] [PubMed] [Google Scholar]
- 15.Frontera WR, Reid KF, Phillips EM, Krivickas LS, Hughes VA, Roubenoff R, et al. Muscle fiber size and function in elderly humans: a longitudinal study. J Appl Physiol (1985) 2008;105(2):637–42. doi: 10.1152/japplphysiol.90332.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clement FJ. Longitudinal and cross-sectional assessments of age changes in physical strength as related to sex, social class, and mental ability. Journal of gerontology. 1974;29(4):423–9. doi: 10.1093/geronj/29.4.423. [DOI] [PubMed] [Google Scholar]
- 17.Metter EJ, Lynch N, Conwit R, Lindle R, Tobin J, Hurley B. Muscle quality and age:cross-sectional and longitudinal comparisons. The journals of gerontology Series A, Biological sciences and medical sciences. 1999;54(5):B207–18. doi: 10.1093/gerona/54.5.b207. [DOI] [PubMed] [Google Scholar]
- 18.Min TJ, Cho J, Ha Y-C, Lim J-Y, Kang SH, Kim D-K, et al. Correlation Between Mechanography and Clinical Parameters at Six Months After Hip Fracture Surgery. Ann Rehabil Med. 2019;43(6):642–9. doi: 10.5535/arm.2019.43.6.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hicks GE, Shardell M, Alley DE, Miller RR, Bandinelli S, Guralnik J, et al. Absolute strength and loss of strength as predictors of mobility decline in older adults:the InCHIANTI study. The journals of gerontology Series A, Biological sciences and medical sciences. 2012;67(1):66–73. doi: 10.1093/gerona/glr055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buehring B, Krueger D, Fidler E, Gangnon R, Heiderscheit B, Binkley N. Reproducibility of jumping mechanography and traditional measures of physical and muscle function in older adults. Osteoporos Int. 2015;26(2):819–25. doi: 10.1007/s00198-014-2983-z. [DOI] [PubMed] [Google Scholar]
- 21.Veilleux LN, Rauch F. Reproducibility of jumping mechanography in healthy children and adults. J Musculoskelet Neuronal Interact. 2010;10(4):256–66. [PubMed] [Google Scholar]
- 22.Matheson LA, Duffy S, Maroof A, Gibbons R, Duffy C, Roth J. Intra- and inter-rater reliability of jumping mechanography muscle function assessments. J Musculoskelet Neuronal Interact. 2013;13(4):480–6. [PubMed] [Google Scholar]
- 23.Drey M, Ferrari U, Schraml M, Kemmler W, Schoene D, Franke A, et al. German Version of SARC-F: Translation, Adaption, and Validation. J Am Med Dir Assoc. 2020 doi: 10.1016/j.jamda.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Buehring B, Krueger D, Binkley N. Jumping mechanography:a potential tool for sarcopenia evaluation in older individuals. J Clin Densitom. 2010;13(3):283–91. doi: 10.1016/j.jocd.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Hannam K, Hartley A, Clark EM, Aihie Saye A, Tobias JH, Gregson CL. Feasibility and acceptability of using jumping mechanography to detect early components of sarcopenia in community-dwelling older women. J Musculoskelet Neuronal Interact. 2017;17(3):246–57. [PMC free article] [PubMed] [Google Scholar]
- 26.Dietzel R, Gast U, Heine T, Felsenberg D, Armbrecht G. Cross-sectional assessment of neuromuscular function using mechanography in women and men aged 20-85 years. J Musculoskelet Neuronal Interact. 2013;13(3):312–9. [PubMed] [Google Scholar]
- 27.Sumnik Z, Matyskova J, Hlavka Z, Durdilova L, Soucek O, Zemkova D. Reference data for jumping mechanography in healthy children and adolescents aged 6-18 years. J Musculoskelet Neuronal Interact. 2013;13(3):297–311. [PubMed] [Google Scholar]
- 28.Busche P, Rawer R, Rakhimi N, Lang I, Martin DD. Mechanography in childhood:references for force and power in counter movement jumps and chair rising tests. J Musculoskelet Neuronal Interact. 2013;13(2):213–26. [PubMed] [Google Scholar]
- 29.Dionyssiotis Y, Galanos A, Michas G, Trovas G, Lyritis GP. Assessment of musculoskeletal system in women with jumping mechanography. International journal of women's health. 2010;1:113–8. doi: 10.2147/ijwh.s5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walowski CO, Braun W, Maisch MJ, Jensen B, Peine S, Norman K, et al. Reference Values for Skeletal Muscle Mass - Current Concepts and Methodological Considerations. Nutrients. 2020;12(3) doi: 10.3390/nu12030755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiegmann S, Felsenberg D, Gast U, Börst H, Armbrecht G, Dietzel R. Balance Performance across the Lifespan Assessed by the Leonardo Mechanograph®:A Cross-Sectional Study. Journal of Functional Morphology and Kinesiology. 2020;5(1):1. doi: 10.3390/jfmk5010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, et al. A review of the measurement of grip strength in clinical and epidemiological studies:towards a standardised approach. Age and ageing. 2011;40(4):423–9. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 33.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–63. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 34.Siglinsky E, Krueger D, Ward RE, Caserotti P, Strotmeyer ES, Harris TB, et al. Effect of age and sex on jumping mechanography and other measures of muscle mass and function. J Musculoskelet Neuronal Interact. 2015;15(4):301–8. [PMC free article] [PubMed] [Google Scholar]
- 35.Van Roie E, Van Driessche S, Inglis AJ, Thomis M, Delecluse C. Rate of power development of the knee extensors across the adult life span: A cross-sectional study in 1387 Flemish Caucasians. Experimental gerontology. 2018;110:260–6. doi: 10.1016/j.exger.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 36.Alcazar J, Aagaard P, Haddock B, Kamper RS, Hansen SK, Prescott E, et al. Age- and sex-specific changes in lower-limb muscle power throughout the lifespan. The journals of gerontology Series A, Biological sciences and medical sciences. 2020 doi: 10.1093/gerona/glaa013. [DOI] [PubMed] [Google Scholar]
- 37.Skelton DA, Greig CA, Davies JM, Young A. Strength, power and related functional ability of healthy people aged 65-89 years. Age and ageing. 1994;23(5):371–7. doi: 10.1093/ageing/23.5.371. [DOI] [PubMed] [Google Scholar]
- 38.Metter EJ, Conwit R, Tobin J, Fozard JL. Age-associated loss of power and strength in the upper extremities in women and men. The journals of gerontology Series A, Biological sciences and medical sciences. 1997;52(5):B267–76. doi: 10.1093/gerona/52a.5.b267. [DOI] [PubMed] [Google Scholar]
- 39.Mosole S, Carraro U, Kern H, Loefler S, Fruhmann H, Vogelauer M, et al. Long-term high-level exercise promotes muscle reinnervation with age. Journal of neuropathology and experimental neurology. 2014;73(4):284–94. doi: 10.1097/NEN.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 40.Deschenes MR. Effects of aging on muscle fibre type and size. Sports medicine (Auckland, NZ) 2004;34(12):809–24. doi: 10.2165/00007256-200434120-00002. [DOI] [PubMed] [Google Scholar]
- 41.Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, et al. Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. Journal of gerontology. 1992;47(3):B71–6. doi: 10.1093/geronj/47.3.b71. [DOI] [PubMed] [Google Scholar]
- 42.Fayet G, Rouche A, Hogrel JY, Tomé FM, Fardeau M. Age-related morphological changes of the deltoid muscle from 50 to 79 years of age. Acta neuropathologica. 2001;101(4):358–66. doi: 10.1007/s004010000294. [DOI] [PubMed] [Google Scholar]
- 43.Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, et al. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiological reviews. 2019;99(1):427–511. doi: 10.1152/physrev.00061.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brunner F, Schmid A, Sheikhzadeh A, Nordin M, Yoon J, Frankel V. Effects of aging on Type II muscle fibers: a systematic review of the literature. Journal of aging and physical activity. 2007;15(3):336–48. doi: 10.1123/japa.15.3.336. [DOI] [PubMed] [Google Scholar]
- 45.Kramer IF, Snijders T, Smeets JSJ, Leenders M, van Kranenburg J, den Hoed M, et al. Extensive Type II Muscle Fiber Atrophy in Elderly Female Hip Fracture Patients. The journals of gerontology Series A, Biological sciences and medical sciences. 2017;72(10):1369–75. doi: 10.1093/gerona/glw253. [DOI] [PubMed] [Google Scholar]
- 46.Narici MV, Maffulli N, Maganaris CN. Ageing of human muscles and tendons. Disability and rehabilitation. 2008;30(20-22):1548–54. doi: 10.1080/09638280701831058. [DOI] [PubMed] [Google Scholar]
- 47.Chidi-Ogbolu N, Baar K. Effect of Estrogen on Musculoskeletal Performance and Injury Risk. Frontiers in physiology. 2019;9(1834) doi: 10.3389/fphys.2018.01834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh H, Kim D, Kim E, Bemben MG, Anderson M, Seo DI, et al. Jump test performance and sarcopenia status in men and women, 55 to 75 years of age. J Geriatr Phys Ther. 2014;37(2):76–82. doi: 10.1519/JPT.0b013e3182a51b11. [DOI] [PubMed] [Google Scholar]
- 49.Rittweger J, Schiessl H, Felsenberg D, Runge M. Reproducibility of the jumping mechanography as a test of mechanical power output in physically competent adult and elderly subjects. Journal of the American Geriatrics Society. 2004;52(1):128–31. doi: 10.1111/j.1532-5415.2004.52022.x. [DOI] [PubMed] [Google Scholar]
- 50.Houghton KM, Macdonald HM, McKay HA, Guzman J, Duffy C, Tucker L. Feasibility and safety of a 6-month exercise program to increase bone and muscle strength in children with juvenile idiopathic arthritis. Pediatric rheumatology online journal. 2018;16(1):67. doi: 10.1186/s12969-018-0283-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grimm A, Nickel MD, Chaudry O, Uder M, Jakob F, Kemmler W, et al. Feasibility of Dixon magnetic resonance imaging to quantify effects of physical training on muscle composition-A pilot study in young and healthy men. Eur J Radiol. 2019;114:160–6. doi: 10.1016/j.ejrad.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 52.Steiber N. Strong or Weak Handgrip?Normative Reference Values for the German Population across the Life Course Stratified by Sex, Age, and Body Height. PLoS One. 2016;11(10):e0163917. doi: 10.1371/journal.pone.0163917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watson LPE, Venables MC, Murgatroyd PR. An Investigation Into the Differences in Bone Density and Body Composition Measurements Between 2 GE Lunar Densitometers and Their Comparison With a 4-Component Model. J Clin Densitom. 2018;21(1):154. doi: 10.1016/j.jocd.2017.11.002. [DOI] [PubMed] [Google Scholar]