Abstract
Background
Oral squamous cell carcinoma (OSCC) is a common oral cancer. The current study aims to elucidate the potential roles of long noncoding RNA (lncRNA) LHFPL3-AS1 in OSCC development.
Methods
Gene expression was measured by qRT-PCR in tumor tissues and cell lines. Loss-of-function assays were performed to analyze the effects of LHFPL3-AS1 on malignant behaviors. Bioinformatics analysis was conducted to explore the downstream signaling pathway of LHFPL3-AS1 in OSCC.
Results
LHFPL3-AS1 was highly expressed in OSCC tissues and cell lines. LHFPL3-AS1 was upregulated in cisplatin-resistant tumor cell lines. LHFPL3-AS1 level was correlated with survival rate. LHFPL3-AS1 knockdown suppressed OSCC proliferation, migration and invasion. LHFPL3-AS1 downregulation reduced cisplatin resistance of OSCC cells. LHFPL3-AS1 was the competing endogenous RNA (ceRNA) for miR-194-5p to enhance CHSY1 expression.
Conclusion
LHFPL3-AS1/miR-362-5p/CHSY1 signaling pathway plays essential roles in regulating OSCC development and cisplatin resistance.
Keywords: oral squamous cell carcinoma, LHFPL3-AS1, miR-362-5p, CHSY1
Introduction
Oral squamous cell carcinoma (OSCC) is the 19th most common cancer in men in the world.1 There were over 300,000 new cases diagnosed with OSCC and 170,00 mortalities worldwide in 2018.2 This cancer is characterized by high mortality.1 Although some progress made in recent years, the prognosis of OSCC patients still remains unfavorable.3 Its five-year overall survival rate remains less than 50%.4 Chemo-resistance decreases the curative effect of OSCC patients.5 Cisplatin is one of the most commonly used chemotherapy drugs for oral cancer.6,7 However, some patients are resistant to Cisplatin treatment.8,9 Therefore, it is pivot to explore the molecular basis underlying OSCC development and chemo-resistance.
Long noncoding RNA (lncRNA) is a kind of noncoding RNAs that have over 200 nucleotides in length and lack protein-coding ability.10 Recently, lncRNA has been verified to work was oncogene or cancer suppressor through modulating biological behaviors of tumor cells.11 Dysregulation of lncRNA may affect apoptosis, growth, metabolism or metastasis in cancer.12 For example, lncRNA ELFN1-AS1 promotes growth and metastasis of ovarian cancer through targeting miR-497/CLDN4 pathway.13 LncRNA H19 affects breast cancer proliferation through regulating PARP1 expression.14 In addition, upregulation of lncRNA HOXA-AS2 contributes to prostate cancer development by regulating miR-509-3p/PBX3 signaling.15 Nevertheless, how lncRNA regulates OSCC development requires in-depth investigation. The molecular mechanism of lncRNA involved in cancer is also largely unclear.
LHFPL3-AS1 is an undefined lncRNA. A recent study reports that LHFPL3-AS1 promotes melanoma development.16 However, the detailed roles of LHFPL3-AS1 in OSCC is unclear. Thus, our study was conducted to illustrate the physiological roles of LHFPL3-AS1 in OSCC development and elucidate its functional mechanism. We confirmed that LHFPL3-AS1 was highly expressed in OSCC tissues by qRT-PCR. LHFPL3-AS1 knockdown suppressed proliferation, migration and invasion according to CCK8, colony formation and transwell assays. LHFPL3-AS1 silencing also increase the sensitivity to Cisplatin in OSCC cells. Our study suggests a critical role of LHFPL3-AS1 in OSCC progression.
Materials and Methods
Human Samples
Fifty-one pairs of OSCC tissues and adjacent normal tissues (within 3 cm around tumors) were collected from Shenzhen Longhua District Central Hospital. All tissues were stored in liquid nitrogen. Tissues were not treated with chemotherapy or radiotherapy before collection. Cancer tissues and adjacent normal tissues were confirmed by at least two pathologists. All patients provided written informed consents. This study was approved by the Ethics Committee of Shenzhen Longhua District Central Hospital (No. 20180921031). All experiments were conducted in accordance with the Declaration of Helsinki.
Cell Culture and Transfection
OSCC cell lines (SSC9, CAL27, SSC25 and HSC3) were purchased from the Chinese Academy of Sciences Cell Bank (Shanghai, China) and culture using DMEM medium containing 10% FBS. Stable cisplatin-resistant cell lines were obtained by treatment with gradually increasing concentrations of cisplatin and maintained at the concentration of 5 μM for SCC9-R and 7.5 μM for CAL27-R. miR-362-5p mimics, miR-362-5p inhibitors, siRNAs and negative controls were purchased from GenePharma (GenePharma, Shanghai, China) and transfected into cells at the concentration of 50 nM using Lipofectamine 2000.
RT-qPCR
Total RNA was extracted from tissues and cells through Trizol reagent. Complementary DNA (cDNA) was obtained through the PrimeScript RT reagent kit (TaKaRa, Dalian, China). qPCR was performed through a SYBR Green PCR Kit (TaKaRa). Relative expression was calculated based on the 2−ΔΔCt method. GAPDH or U6 was the normalized control.
Cell Viability Analysis
Cells were plated into the 96-well plates and cultured for indicated hours. Then, 20 μL CCK8 solution (Dojindo, Kyushu, Japan) was added and incubated for 2 h. The absorbance at 450 nm was determined using a microplate reader (Biotek, Winooski, VT, USA).
Colony Formation Assay
Five hundred cells per well was seeded into 6-well plates and cultured for 14 days. Then the colonies were fixed with methanol and stained with 0.1% crystal violet. Total visible colonies were counted.
Transwell Assay
Cells were seeded into the uncoated (for migration) or Matrigel-coated (for invasion) upper chamber (8 μm pore size; Corning Inc., Corning, NY, USA) with 200 μL serum-free medium. The lower chamber was fixed with 600 μL serum-containing medium. After cultured for 24 h, the cells on the lower membrane was fixed with methanol and stained with 0.1% crystal violet. Cell number was finally counted.
Bioinformatics Analysis
The prediction between LHFPL3-AS1 and miR-362-5p was carried out using miRDB tool. The prediction between miR-362-5p and CHSY1 was performed through TargetScan tool.
Dual-Luciferase Reporter Assay
The sequence of LHFPL3-AS1 or CHSY1 containing predicted binding sites for miR-362-5p was inserted into pMIR-REPORT vector (Promega, Madison, WI, USA). For luciferase reporter assay, the luciferase reporter and miR-362-5p mimics or control were transfected into cells. Forty-eight-hour later, the relative luciferase activity was measured through a dual luciferase assay kit (Promega).
Statistical Analysis
Data were analyzed using SPSS 22.0 software (IBM Corp., Armonk, NY, USA) and displayed as the mean ± standard deviation. Student’s t-test or one-way ANOVA was used for comparison. Survival curves were analyzed via Kaplan–Meier analysis and compared using log-rank test. P < 0.05 was considered as statistically significant.
Results
LHFPL3-AS1 is Upregulated in OSCC and Associated with Cisplatin Resistance
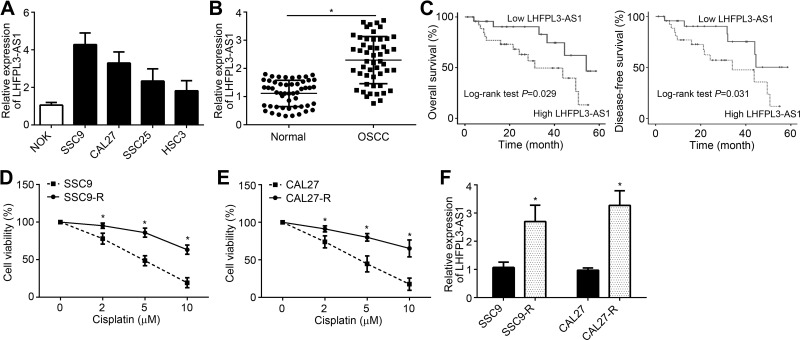
Through qRT-PCR analysis, we found that LHFPL3-AS1 was upregulated in OSCC cell lines, including SSC9, CAL27, SSC25 and HSC3 cells (Figure 1A). Besides, LHFPL3-AS1 level was remarkably increased in OSCC tissues compared to adjacent normal tissues (Figure 1B). Next, 51 OSCC tissues were divided into two groups based on LHFPL3-AS1 level (LHFPL3-AS1 median value as the cut-off). It is found that LHFPL3-AS1 high expression was associated with low survival rate (Figure 1C). To explore whether LHFPL3-AS1 regulates cisplatin resistance, we constructed cisplatin-resistant SSC9 and CAL27 cells. Through CCK8 assay, we verified the cisplatin-resistance in SSC9-R and CAL27-R cells (Figure 1D and E). Interestingly, we observed that LHFPL3-AS1 expression was higher in SSC9-R and CAL27-R cells than that in control cells (Figure 1F).
Figure 1.
LHFPL3-AS1 is upregulated in OSCC and associated with cisplatin resistance. (A) Levels of LHFPL3-AS1 in OSCC cell lines. (B) LHFPL3-AS1 expression in OSCC tissues by qRT-PCR. (C) Overall and disease-free survival rates based on LHFPL3-AS1 median value in OSCC tissues. (D) Analysis of cisplatin-resistance in SSC9 and SCC9-R cells by CCK8 assay. (E) Analysis of cisplatin-resistance in CAL27 and CAL27-R cells by CCK8 assay. (F) Relative expression of LHFPL3-AS1 in indicated cell lines. *P<0.05.
LHFPL3-AS1 Knockdown Inhibits OSCC Development and Cisplatin Resistance
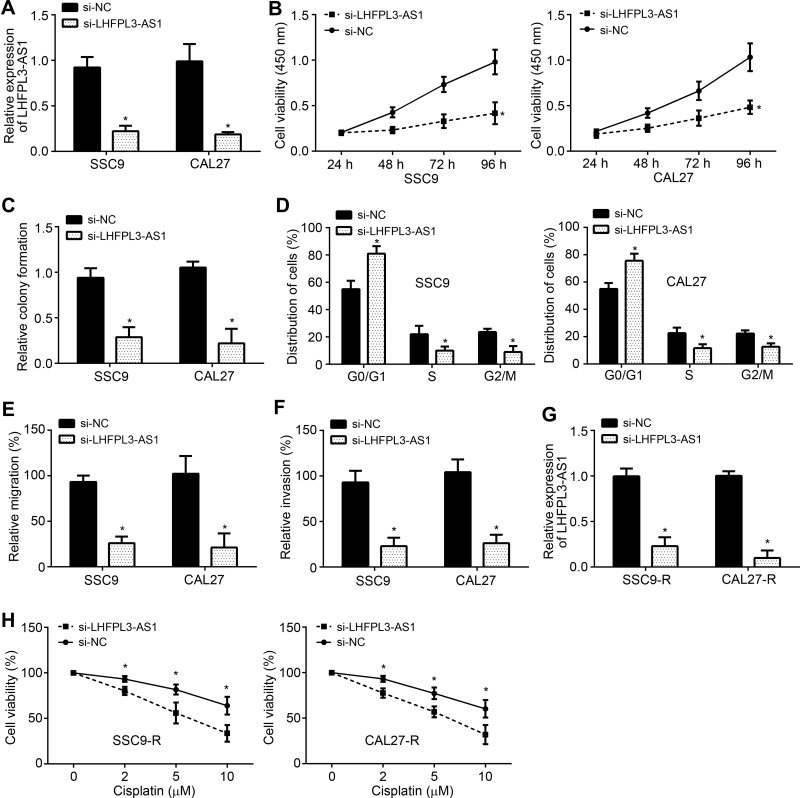
To explore the function of LHFPL3-AS1, its expression was knocked down in SSC9 and CAL27 cells by siRNA (Figure 2A). Via CCK8 assay, it is obvious that LHFPL3-AS1 knockdown suppressed cell proliferation (Figure 2B). The effect of LHFPL3-AS1 knockdown on proliferation was further validated by colony formation assay (Figure 2C). Importantly, we observed that LHFPL3-AS1 knockdown caused more cells arrested in G0/G1 phase (Figure 2D), suggesting that LHFPL3-AS1 knockdown inhibits cell cycle progression. Transwell assay results indicated that LHFPL3-AS1 knockdown repressed migration and invasion (Figure 2E and F). We then knocked down LHFPL3-AS1 in SSC9-R and CAL27-R cells (Figure 2G). LHFPL3-AS1 silencing significantly enhanced susceptibility to cisplatin in SCC9-R and CAL27-R cells (Figure 2H).
Figure 2.
LHFPL3-AS1 knockdown inhibits OSCC development and cisplatin resistance. (A) Relative expression of LHFPL3-AS1 after transfection with siRNAs. (B) CCK8 assay for cell proliferation analysis. (C) Relative colony formation was determined to analyze cell proliferation. (D) Cell cycle distribution was analyzed. (E and F) Cell migration and invasion analyses by Transwell assay. (G) Relative expression of LHFPL3-AS1 in cisplatin-resistant cell lines after transfection of siRNAs. (H) Cell proliferation potential of SCC9-R and CAL27-R cells was assessed in the presence of cisplatin after transfection with siRNAs. *P<0.05.
LHFPL3-AS1 Works as the ceRNA for miR-362-5p
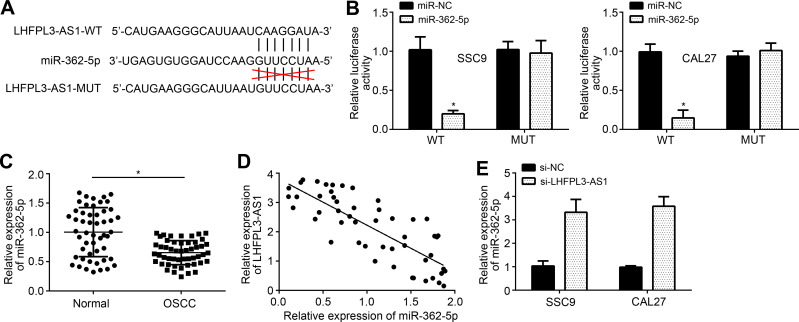
Through bioinformatics analysis, we predicted the potential targets of LHFPL3-AS1. MiR-362-5p achieved the highest score and luciferase reporter vectors were constructed (Figure 3A). The luciferase reporter assay showed that miR-362-5p mimics significantly inhibited the activity of LHFPL3-AS1-WT vector in SSC9 and CAL27 cells (Figure 3B). We observed that miR-362-5p level was downregulated in OSCC tissues (Figure 3C). Interestingly, miR-362-5p level was negatively correlated with LHFPL3-AS1 in OSCC tissues (Figure 3D). Moreover, miR-362-5p level was increased in SSC9 and CAL27 cells after LHFPL3-AS1 silencing (Figure 3E), confirming that LHFPL3-AS1 was the ceRNA for miR-362-5p.
Figure 3.
LHFPL3-AS1 works as the ceRNA for miR-362-5p. (A) Predicted binding site between LHFPL3-AS1 and miR-362-5p by miRDB software. (B) Luciferase reporter assay. (C) Relative expression of miR-362-5p in OSCC tissues. (D) Expression correlation between LHFPL3-AS1 and miR-362-5p in OSCC tissues. (E) RT-qPCR analysis of miR-362-5p levels after siRNA transfection. *P<0.05.
MiR-362-5p Targets CHSY1
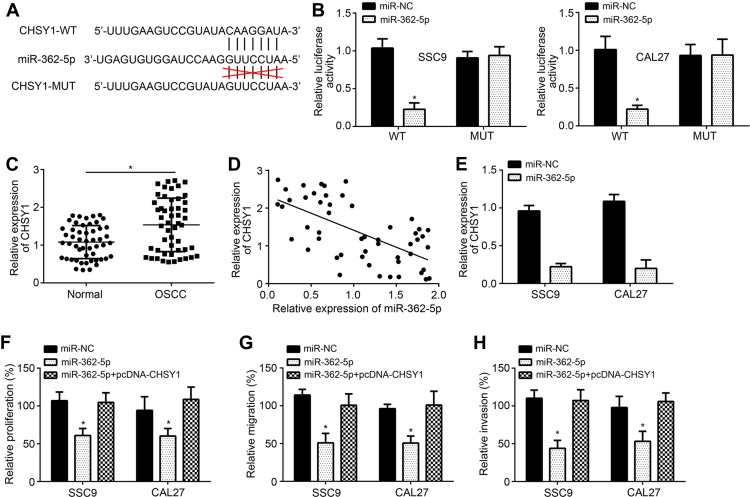
By bioinformatics analysis, we further predicted CHSY1 as the most potential target of miR-362-5p because it achieved the highest score. Similarly, the luciferase reporter vectors were constructed (Figure 4A). Luciferase reporter assay proved that miR-362-5p mimics only inhibited the activity of CHSY1-WT vector (Figure 4B). We found that CHSY1 expression was raised in OSCC tissues compared to normal tissues (Figure 4C). And CHSY1 level was inversely correlated with miR-362-5p in OSCC tissues (Figure 4D). Moreover, miR-362-5p mimics remarkably reduced the levels of CHSY1 (Figure 4E). Notably, miR-362-5p mimics inhibited the proliferation, migration and invasion of OSCC cells while ectopic expression of CHSY1 abolished this trend (Figure 4F–H).
Figure 4.
miR-362-5p targets CHSY1. (A) Predicted binding site between miR-362-5p and CHSY1 by TargetScan software. (B) Luciferase reporter assay. (C) Relative expression of CHSY1 in OSCC tissues. (D) Expression correlation between miR-362-5p and CHSY1 in OSCC tissues. (E) RT-qPCR analysis of CHSY1 after miR-362-5p mimic transfection. (F) CCK8 assay for proliferation analysis. (G and H) Transwell assay for analysis of migration and invasion. *P<0.05.
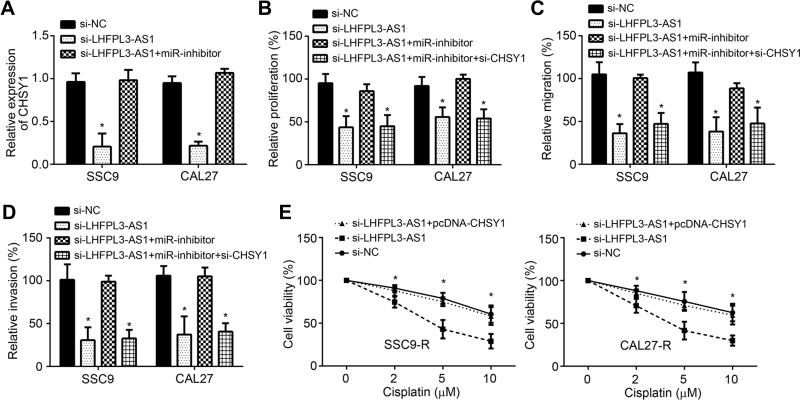
LHFPL3-AS1/miR-362-5p/CHSY1 Cascade Promotes OSCC Development
It was observed that LHFPL3-AS1 knockdown suppressed the expression of CHSY1 by restraining miR-362-5p (Figure 5A). To explore the roles of LHFPL3-AS1/miR-362-5p/CHSY1 cascade, CCK8 and Transwell assays were performed. LHFPL3-AS1 knockdown suppressed proliferation, migration and invasion of OSCC cells (Figure 5B–D). The ability of proliferation, migration and invasion was recovered in si-LHFPL3-AS1+miR-inhibitor group and suppressed again in si-LHFPL3-AS1+miR-inhibitor+si- CHSY1 group (Figure 5B–D). Moreover, CHSY1 overexpression rescued the cisplatin-resistance in si-LHFPL3-AS-transfected SSC9-R and CAL27-R cells (Figure 5E). Therefore, LHFPL3-AS1 promotes OSCC development and chemo-resistance through miR-362-5p/CHSY1 axis.
Figure 5.
LHFPL3-AS1/miR-362-5p/CHSY1 cascade promotes OSCC development. (A) qRT-PCR analysis of CHSY1 expression. (B) CCK8 assay for cell proliferation analysis. (C and D) Transwell assay for migration and invasion assessment. (E) Cell proliferation assay by CCK8 using SCC9-R and CAL27-R cells. *P<0.05.
Discussion
OSCC pathogenesis remains undiscovered. In this study, we explored the correlation between LHFPL3-AS1 expression and OSCC development. We found that LHFPL3-AS1 was upregulated in OSCC tissues and correlated with cisplatin resistance. LHFPL3-AS1 knockdown suppressed proliferation, migration, invasion and cisplatin resistance in OSCC cells. Mechanistically, LHFPL3-AS1 was the sponge for miR-362-5p to facilitate CHSY1 expression.
LncRNA has been reported to play essential roles in cancer and regulate OSCC development.17 For example, lncRNA RBM5-AS1 contributes to proliferation, migration and invasion of OSCC through regulating miR-1285-3p/YAP1 cascade.18 LINC01234 promotes tumor growth and invasion in OSCC by targeting miR-647/NUPR1 axis.19 LncRNA CCAT1 acts as the biomarker for OSCC prognosis and promotes tumor development through activation of Wnt/β-catenin pathway.20 LHFPL3-AS1 was only demonstrated to promote melanoma progression.16 In our study, we first observed that LHFPL3-AS1 was upregulated in OSCC tissues and correlated with advanced stages and chemo-resistance. Next, we carried out CCK8, colony formation and transwell assays and validated that LHFPL3-AS1 promoted OSCC proliferation, migration, invasion and chemo-resistance. In this study, we for the first time demonstrated the oncogenic roles of LHFPL3-AS1 in OSCC.
LncRNA may exert roles through acting as ceRNA for miRNAs in cancer.21 For instance, lncRNA NEAT1 sponges miR-129 to enhance nasopharyngeal cancer development.22 LncRNA PVT1 targets miRNAs to promote prostate cancer growth and invasion.23 LHFPL3-AS1 is the sponge for miR-580-3p in melanoma.16 However, we did not observe that LHFPL3-AS1 regulates miR-580-3p in OSCC (data not shown). Otherwise, we found that LHFPL3-AS1 sponges miR-362-5p through bioinformatics analysis. The interaction between LHFPL3-AS1 and miR-362-5p was validated by luciferase reporter assay. Moreover, LHFPL3-AS1 expression was negatively correlated with miR-362-5p in OSCC tissues. MiR-362-5p level was also increased after LHFPL3-AS1 silencing. MiR-362-5p has important functions in various cancers, such as bladder cancer, acute myeloid leukemia and hepatocellular carcinoma.24–26 MiR-362-5p may serve as an oncogene or tumor suppressor.24–26 However, the role of miR-362-5p in OSCC is unknown. In our study, we showed that miR-362-5p expression was downregulated in OSCC tissues. Moreover, miR-362-5p inhibited OSCC proliferation, migration and invasion.
Afterwards, we performed bioinformatics analysis to search the target of miR-362-5p. We identified CHSY1 and demonstrated the interaction between miR-362-5p and CHSY1 via luciferase reporter assay. Besides, we validated that LHFPL3-AS1 facilitates CHSY1 expression through restraining miR-362-5p. CHSY1 contributes to hepatocellular carcinoma progression.27 CHSY1 also promotes colorectal cancer development.28 Recently, CHSY1 was reported to participate in clear cell renal cell carcinoma aggravation.29 Obviously, the current study, for the first time, discovered that CHSY1 promotes OSCC development and cisplatin resistance, indicating CHSY1 is also an oncogene in OSCC.
In summary, our data elucidated that LHFPL3-AS1 works as on oncogene to promote OSCC cell proliferation, migration and invasion through regulating miR-362-5p/CHSY1 axis at least partially. Our study has some limitations. It is better to analyze the correlation between LHFPL3-AS1 and clinicopathological characteristics in the future. And the upstream regulator of LHFPL3-AS1 expression in OSCC is also required to be explored.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma–an update. CA Cancer J Clin. 2015;65(5):401–421. doi: 10.3322/caac.21293 [DOI] [PubMed] [Google Scholar]
- 2.Thomson PJ. Perspectives on oral squamous cell carcinoma prevention-proliferation, position, progression and prediction. J Oral Pathol Med. 2018;47(9):803–807. doi: 10.1111/jop.12733 [DOI] [PubMed] [Google Scholar]
- 3.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45(4–5):309–316. doi: 10.1016/j.oraloncology.2008.06.002 [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 5.Yamauchi K, Yang M, Hayashi K, et al. Induction of cancer metastasis by cyclophosphamide pretreatment of host mice: an opposite effect of chemotherapy. Cancer Res. 2008;68(2):516–520. doi: 10.1158/0008-5472.CAN-07-3063 [DOI] [PubMed] [Google Scholar]
- 6.Sun CY, Zhang QY, Zheng GJ, Feng B. Phytochemicals: current strategy to sensitize cancer cells to cisplatin. Biomed Pharmacother. 2019;110:518–527. doi: 10.1016/j.biopha.2018.12.010 [DOI] [PubMed] [Google Scholar]
- 7.Ma SQ, Wang YC, Li Y, Li XY, Yang J, Sheng YM. LncRNA XIST promotes proliferation and cisplatin resistance of oral squamous cell carcinoma by downregulating miR-27b-3p. J Biol Regul Homeost Agents. 2020;34(6):1993–2001. doi: 10.23812/20-222-A [DOI] [PubMed] [Google Scholar]
- 8.Wang F, Ji X, Wang J, et al. LncRNA PVT1 enhances proliferation and cisplatin resistance via regulating miR-194-5p/HIF1a axis in oral squamous cell carcinoma. Onco Targets Ther. 2020;13:243–252. doi: 10.2147/OTT.S232405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang R, Lu X, Yu R. lncRNA MALAT1 promotes EMT process and cisplatin resistance of oral squamous cell carcinoma via PI3K/AKT/m-TOR signal pathway. Onco Targets Ther. 2020;13:4049–4061. doi: 10.2147/OTT.S251518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao J, Bai X, Feng C, Shang X, Xi Y. Long non-coding RNA HCP5 facilitates cell invasion and epithelial-mesenchymal transition in oral squamous cell carcinoma by miR-140-5p/SOX4 axis. Cancer Manag Res. 2019;11:10455–10462. doi: 10.2147/CMAR.S230324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai J, Mu JW, Mu H. Long non-coding RNA CRNDE regulates cell proliferation, migration, invasion, epithelial-mesenchymal transition and apoptosis in oral squamous cell carcinoma. Oncol Lett. 2019;17(3):3330–3340. doi: 10.3892/ol.2019.9978 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Gomes CC, de Sousa SF, Calin GA, Gomez RS. The emerging role of long noncoding RNAs in oral cancer. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123(2):235–241. doi: 10.1016/j.oooo.2016.10.006 [DOI] [PubMed] [Google Scholar]
- 13.Jie Y, Ye L, Chen H, et al. ELFN1-AS1 accelerates cell proliferation, invasion and migration via regulating miR-497-3p/CLDN4 axis in ovarian cancer. Bioengineered. 2020;11(1):872–882. doi: 10.1080/21655979.2020.1797281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Zhou P, Li P, Yang F, Gao XQ. Long non-coding RNA H19 regulates proliferation and doxorubicin resistance in MCF-7 cells by targeting PARP1. Bioengineered. 2020;11(1):536–546. doi: 10.1080/21655979.2020.1761512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao S, Song B. LncRNA HOXA-AS2 promotes the progression of prostate cancer via targeting miR-509-3p/PBX3 axis. Biosci Rep. 2020;40:8. doi: 10.1042/BSR20193287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng Q, Liu L, Pei H, Zhang J, Chen M, Zhai X. A LHFPL3-AS1/miR-580-3p/STAT3 feedback loop promotes the malignancy in melanoma via activation of JAK2/STAT3 signaling. Mol Cancer Res. 2020;18(11):1724–1734. doi: 10.1158/1541-7786.MCR-19-1046 [DOI] [PubMed] [Google Scholar]
- 17.Gao L, Wang S, Meng J, Sun Y. LncRNA LUADT1 promotes oral squamous cell carcinoma cell proliferation by regulating miR-34a/GAS1 axis. Cancer Manag Res. 2020;12:3401–3407. doi: 10.2147/CMAR.S238830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li C, Ye J, Zhang Z, Gong Z, Lin Z, Ding M. Long non-coding RNA RBM5-AS1 promotes the aggressive behaviors of oral squamous cell carcinoma by regulation of miR-1285-3p/YAP1 axis. Biomed Pharmacother. 2020;123:109723. doi: 10.1016/j.biopha.2019.109723 [DOI] [PubMed] [Google Scholar]
- 19.Huang W, Cao J, Peng X. LINC01234 facilitates growth and invasiveness of oral squamous cell carcinoma through regulating the miR-637/NUPR1 axis. Biomed Pharmacother. 2019;120:109507. doi: 10.1016/j.biopha.2019.109507 [DOI] [PubMed] [Google Scholar]
- 20.Li GH, Ma ZH, Wang X. Long non-coding RNA CCAT1 is a prognostic biomarker for the progression of oral squamous cell carcinoma via miR-181a-mediated Wnt/beta-catenin signaling pathway. Cell Cycle. 2019;18(21):2902–2913. doi: 10.1080/15384101.2019.1662257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He H, Wang Y, Ye P, et al. Long noncoding RNA ZFPM2-AS1 acts as a miRNA sponge and promotes cell invasion through regulation of miR-139/GDF10 in hepatocellular carcinoma. J Exp Clin Cancer Res. 2020;39(1):159. doi: 10.1186/s13046-020-01664-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xue F, Cheng Y, Xu L, et al. LncRNA NEAT1/miR-129/Bcl-2 signaling axis contributes to HDAC inhibitor tolerance in nasopharyngeal cancer. Aging. 2020;12(14):14174–14188. doi: 10.18632/aging.103427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun F, Wu K, Yao Z, et al. Long noncoding RNA PVT1 promotes prostate cancer metastasis by increasing NOP2 expression via targeting tumor suppressor microRNAs. Onco Targets Ther. 2020;13:6755–6765. doi: 10.2147/OTT.S242441 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Wei X, Wang B, Wang Q, et al. MiR-362-5p, which is regulated by long non-coding RNA MBNL1-AS1, promotes the cell proliferation and tumor growth of bladder cancer by targeting QKI. Front Pharmacol. 2020;11:164. doi: 10.3389/fphar.2020.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu F, Yin C, Qi J, et al. miR-362-5p promotes cell proliferation and cell cycle progression by targeting GAS7 in acute myeloid leukemia. Hum Cell. 2020;33(2):405–415. doi: 10.1007/s13577-019-00319-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao L, Zhang Y, Zhang Y. Long noncoding RNA CASC2 regulates hepatocellular carcinoma cell oncogenesis through miR-362-5p/Nf-kappaB axis. J Cell Physiol. 2018;233(10):6661–6670. doi: 10.1002/jcp.26446 [DOI] [PubMed] [Google Scholar]
- 27.Liu CH, Lan CT, Chou JF, Tseng TJ, Liao WC. CHSY1 promotes aggressive phenotypes of hepatocellular carcinoma cells via activation of the hedgehog signaling pathway. Cancer Lett. 2017;403:280–288. doi: 10.1016/j.canlet.2017.06.023 [DOI] [PubMed] [Google Scholar]
- 28.Zeng L, Qian J, Luo X, Zhou A, Zhang Z, Fang Q. CHSY1 promoted proliferation and suppressed apoptosis in colorectal cancer through regulation of the NFkappaB and/or caspase-3/7 signaling pathway. Oncol Lett. 2018;16(5):6140–6146. doi: 10.3892/ol.2018.9385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang Y, Zhang H, Li W, Yan Y, Yao X, Gu W. FOXM1-activated LINC01094 promotes clear cell renal cell carcinoma development via microRNA 224-5p/CHSY1. Mol Cell Biol. 2020;40(3). doi: 10.1128/MCB.00357-19 [DOI] [PMC free article] [PubMed] [Google Scholar]