Abstract
Objective
There are limited data regarding the typical characteristics of coronavirus disease 2019 (COVID-19) patients requiring interfacility transport or the clinical capabilities of the out-of-hospital transport clinicians required to provide safe transport. The objective of this study is to provide epidemiologic data and highlight the clinical skill set and decision making needed to transport critically ill COVID-19 patients.
Methods
A retrospective chart review of persons under investigation for COVID-19 transported during the first 6 months of the pandemic by Johns Hopkins Lifeline was performed. Patients who required interfacility transport and tested positive for severe acute respiratory syndrome coronavirus 2 by polymerase chain reaction assay were included in the analysis.
Results
Sixty-eight patients (25.4%) required vasopressor support, 35 patients (13.1%) were pharmacologically paralyzed, 15 (5.60%) were prone, and 1 (0.75%) received an inhaled pulmonary vasodilator. At least 1 ventilator setting change occurred for 59 patients (22.0%), and ventilation mode was changed for 11 patients (4.10%) during transport.
Conclusion
The safe transport of critically ill patients with COVID-19 requires experience with vasopressors, paralytic medications, inhaled vasodilators, prone positioning, and ventilator management. The frequency of initiated critical interventions and ventilator adjustments underscores the tenuous nature of these patients and highlights the importance of transport clinician reassessment, critical thinking, and decision making.
The coronavirus disease 2019 (COVID-19) pandemic has threatened to overwhelm hospitals and health care systems across the globe.1 Interfacility transport programs have been tasked with the movement of these critically ill patients to tertiary and quaternary care centers to receive additional clinical expertise and resources. These transport programs also help facilitate the regionalization of care to mitigate hospitals becoming disproportionally overwhelmed by the number and/or acuity of patients. Although data are emerging regarding the characteristics of COVID-19 patients transported by emergency medical services systems,2 there is only limited information describing the typical illness severity of patients cared for during critical care transport.3, 4, 5 Furthermore, there is a paucity of literature describing the interventions and decision making by critical care transport clinicians during ground and air transport.
Johns Hopkins Lifeline (Lifeline) is a high-volume, high-acuity critical care transport program responsible for the movement and care of approximately 16,000 intrafacility transports, 5,500 interfacility ground transports, and 800 air transports per year predominantly throughout Maryland and Washington DC with occasional transports between Delaware, Pennsylvania, and Virginia. Created in response to the Ebola pandemic of 2014 to 2015, the Lifeline Special Operations Response Team (SORT) is a subset of Lifeline team members specially trained in the movement of patients with high-consequence infectious diseases.6 Given the specific training and knowledge of the SORT members, this team was dedicated to the movement of patients under investigation (PUI) for COVID-19 and patients with confirmed COVID-19 infections into and throughout the Johns Hopkins Health System. A SORT mission included the addition of a safety officer along with the patient care team.7 Lifeline performed its first COVID-19 interfacility transport on February 29, 2020, approximately 11 days before the disease was declared a pandemic by the World Health Organization.8 This article is a novel observational study that describes the characteristics of COVID-19 patients and the clinical management by the Lifeline transport nurses and paramedics during the first 6 months of the pandemic.
Methods
This study was a retrospective chart review of patients with confirmed COVID-19 and PUIs for COVID-19 transported by Lifeline from February 29, 2020, to August 31, 2020. Patients who required interfacility transport and tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA by polymerase chain reaction (PCR) assay were included in the analysis. Patients were excluded if they required intrafacility transport or if they did not have a positive PCR assay for SARS-CoV-2 RNA. Patient characteristics before transport and clinical management during transport were captured via chart review (Zoll Data Systems, Broomfield, CO). All patients with confirmed COVID-19 and PUIs were transported by Lifeline SORT.
The pretransport data included patient demographics, vital signs, end-tidal carbon dioxide levels, and supplemental oxygen requirements. Patient temperature was inconsistently reported and was excluded. In addition, critical care interventions implemented before transport were recorded and included vasopressor administration, pharmacologic paralysis, prone position, and inhaled pulmonary vasodilator administration.
Clinical management decisions during transport were recorded. Variables of interest included ventilator changes, vasopressor initiation, pharmacologic paralysis initiation, initiation of prone position, administration of inhaled pulmonary vasodilators, and endotracheal intubation.
Descriptive statistics were used to report the findings of the study. Means were reported for continuous variables, and proportions were reported for dichotomous variables. Ninety-five percent confidence intervals (CIs) were reported for the calculated means and proportions. STATA 15.1 software (StataCorp LLC, College Station, TX) was used for data analysis.
The study was approved by the Johns Hopkins Institutional Review Board and conducted according to the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines.
Results
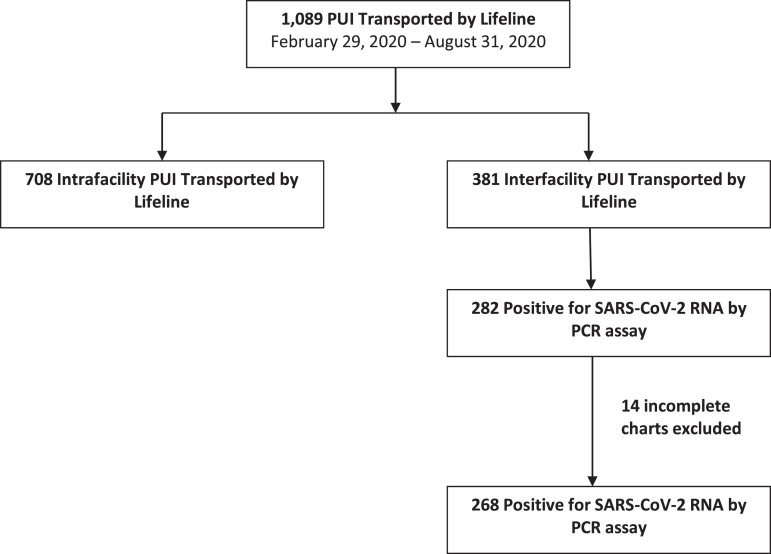
Between February 29, 2020, and August 31, 2020, Lifeline completed 1,089 PUI transports. There were 381 (35.0%) interfacility transports and 708 (65.0%) intrahospital transports. Two hundred eighty-two (74.0%) interfacility transport patients were COVID-19 positive, and 11 (3.9%) were transported by air. There were 14 (5.0%) incomplete charts that were excluded from analysis. A total of 268 patients were included in the final analysis (Fig. 1 ).
Figure 1.
A flowchart of the patient selection for inclusion in the analysis.
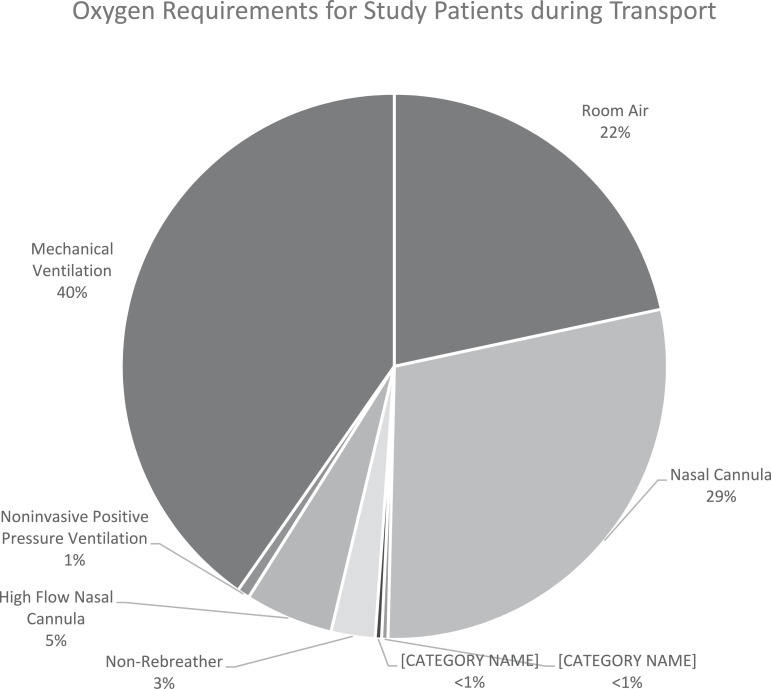
Table 1 describes patient demographics and pretransport clinical data for COVID-19 patients requiring interfacility transport. The mean age of patients and the number of comorbidities were 57.8 years (95% CI, 54.9-60.7) and 1.53 (95% CI, 1.37-1.70), respectively. Before transport, 210 patients (78.4%; 95% CI, 73.0%-82.9%) required supplemental oxygen, and 108 (40.3%; 95% CI, 34.6%-46.3%) were intubated for mechanical ventilatory support (Fig. 2 ). Sixty-eight patients (25.4%; 95% CI, 20.5%-31.0%) received vasopressor support, 35 (13.1%; 95% CI, 9.51%-17.7%) were pharmacologically paralyzed, 15 (5.60%; 95% CI, 3.39%-9.09%) were in the prone position, and 1 (0.75%; 95% CI, 0.19%-2.95%) received an inhaled pulmonary vasodilator. No patients received extracorporeal membrane oxygenation (ECMO) support.
Table 1.
Clinical Characteristics of Patients Requiring Interfacility Transport by Lifeline Special Operations Response Team Who Were Positive for Severe Acute Respiratory Syndrome Coronavirus 2 RNA by Polymerase Chain Reaction (N = 268)
| Clinical Characteristics | Mean (95% CI) | Number of Patients (%, 95% CI) |
|---|---|---|
| Age, y | 57.8 (54.9-60.7) | |
| Sex | ||
| Male | 149 (55.6, 49.6-61.5) | |
| Female | 119 (44.4, 38.5-50.4) | |
| Vital signs before transport | ||
| Blood pressure, mm Hg | ||
| Systolic | 124 (121-127) | |
| Diastolic | 70.7 (69.1-72.3) | |
| Heart rate, beats/min | 89.5 (87.3-91.7) | |
| Respiratory rate, breaths/min | 23.2 (22.3-24.1) | |
| Oxygen saturation by pulse oximetry, % | 95.7 (95.2-96.1) | |
| End-tidal carbon dioxide before transporta | 39.6 (37.4-41.9) | |
| Mode of oxygenation | ||
| Room air | 58 (21.6, 17.1-27.0) | |
| Nasal cannula | 77 (28.7, 23.6-34.5) | |
| Air entrainment mask | 1 (0.37, 0.05-2.62) | |
| Tracheostomy mask | 1 (0.37, 0.05-2.62) | |
| Nonrebreather mask | 7 (2.61, 1.25-5.39) | |
| High flow nasal cannula | 14 (5.22, 3.11-8.65) | |
| Noninvasive positive-pressure ventilation | 2 (0.75, 0.19-2.95) | |
| Invasive positive-pressure ventilation | 108 (40.3, 34.6-46.3) | |
| Interventions before transport | ||
| Vasopressor support | 68 (25.4, 20.5-30.9) | |
| Neuromuscular paralysis | 35 (13.1, 9.51-17.7) | |
| Prone positioning | 15 (5.60, 3.39-9.09) | |
| Inhaled nitric oxide | 2 (0.75, 0.19-2.95) |
End-tidal carbon dioxide levels were available for 116 patients.
Figure 2.
Pretransport oxygen requirements for patients meeting inclusion criteria.
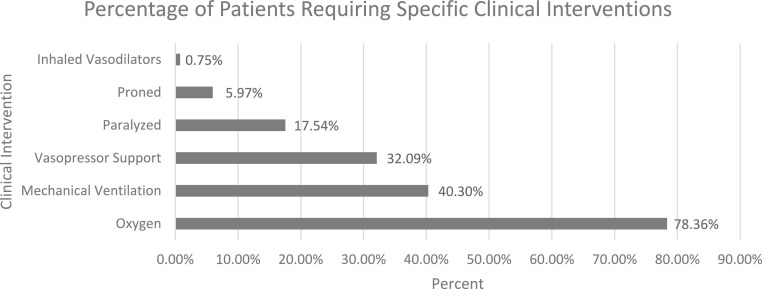
Clinical decisions and interventions initiated by the Lifeline transport team are described in Table 2 . Lifeline clinicians initiated vasopressor support for 18 patients (6.71%; 95% CI, 4.26%-10.4%), pharmacologic paralysis on 12 patients (4.48%; 95% CI, 2.55%-7.73%), and prone positioning for 1 patient (0.37%; 95 CI%, 0.05%-2.62%). Among the 108 patients who were intubated, Lifeline made at least 1 ventilator setting change for 59 patients (22.0%; 95% CI, 17.4%-27.3%) and changed the mode of ventilation for 11 patients (4.10%; 95% CI, 2.28%-7.27%). No patients required intubation during transport. Figure 3 shows the cumulative percentage of patients requiring the studied clinical interventions.
Table 2.
Clinical Interventions for Patients Requiring Interfacility Transport by Lifeline Special Operations Response Team Who Were Positive for Severe Acute Respiratory Syndrome Coronavirus 2 RNA by Polymerase Chain Reaction (N = 268)
| Transport Characteristics | Number of Patients (%, 95% CI) |
|---|---|
| Interventions by transport team | |
| Vasopressor initiation | 18 (6.7, 4.26-10.4) |
| Chemical paralysis initiation | 12 (4.48, 2.56-7.73) |
| Pronation of patient | 1 (0.37, 0.05-2.62) |
| At least 1 ventilator setting change | 59 (22.0, 17.4-27.4) |
| Ventilation mode change | 11 (4.10, 2.28-7.28) |
CI = confidence interval.
Figure 3.
The cumulative percentage of patients requiring critical clinical interventions.
Discussion
The COVID-19 pandemic has resulted in the frequent transfer of patients due to capacity limitations and illness severity. This retrospective analysis is the largest study to date describing the acuity and management required for COVID-19 patients undergoing interfacility transport. We have found these patients were frequently high acuity and required changes in management during transport to optimize care. Nearly 80% of patients received supplemental oxygen meeting the criteria for severe COVID-19 infection. Over half of these patients required invasive mechanical ventilation for respiratory failure meeting the definition for critical COVD-19 infection.9
Invasive mechanical ventilation was the most common intervention that a Lifeline clinician was required to manage. Greater than 11% of the intubated patients were paralyzed to optimize respiratory support, which emphasizes the critical illness of the study population. This is also reflected by the ventilator changes required during transport, including adjusting settings for more than half of the patients and changing the mode of ventilation for 10% of the patients transported. Ventilator changes were guided by adhering to lung-protective strategies and targeting tidal volumes of 6 to 8 mL/kg ideal body weight as well as the most recent sending facility's arterial blood gas, oxygen saturation by pulse oximeter, and end-tidal carbon dioxide level. Lifeline crews were encouraged to decrease supplemental fraction of inspired oxygen to maintain SpO2 between 92% and 96%.
In addition to ventilator management, critical care transport clinicians were required to provide hemodynamic support. One quarter of all transports received vasoactive infusions before transport to maintain adequate blood pressure, and another 7% had vasopressors initiated by the transport team.
Before the COVID-19 pandemic, transporting patients prone was rare as evidenced by the limited number of case reports.10, 11, 12 As proning became important for the management of COVID-19 patients,13, 14, 15, 16, 17, 18 it became necessary to have this available during transport for the most critically ill with prolonged out-of-hospital time. To meet this need, Lifeline created a protocol for nurses and paramedics to transport patients in the prone position. Didactic and hands-on education was required for all clinicians to ensure proficiency placing patients in the prone position and rapidly supinating if necessary. The successful transport of 15 patients in the prone position without adverse effects suggests that this can be performed safely and effectively, but further research is warranted.
Data from the first 6 months demonstrated a low number of patients had inhaled nitric oxide or ECMO initiated before transport. Inhaled nitric oxide was the only pulmonary vasodilator used during the study period given the risk of aerosolization with other inhaled pulmonary vasodilators. The reason for the low ECMO transports was due to the lack of ECMO capability at many of the referring hospitals, and often patients were being transferred for the consideration of ECMO. Inhaled nitric oxide remains controversial as a therapy for refractory hypoxemia due to the cost and lack of clear mortality benefit.19, 20, 21 Often ventilator optimization and pharmacologic paralysis achieved the respiratory stability necessary for transport.
Conclusions
The COVID-19 pandemic has resulted in the need for hospitals and health care systems to continuously evaluate their capacity and ability to provide optimal care for COVID-19 patients. The ability to regionalize care requires the use of highly trained critical care transport teams. Given the current and anticipated surge of COVID-19, there will likely be a high demand for critical care transport services.
The safe transport of these patients is paramount and requires competency and comfort with the titration and initiation of vasopressors, paralytic medications, and ventilator management. Although less common in the first 6 months, knowledge about how to transport patients requiring inhaled nitric oxide is also necessary. The frequency of Lifeline initiated vasopressors, pharmacologic paralysis, and ventilator adjustments underscores the tenuous nature of these patients and highlights the importance of transport clinician reassessment and critical thinking.
This report provides valuable insight to the skills needed by these out-of-hospital clinicians as well as the complexity of patients who require transport. Notable limitations include the use of data from a single system, absence of scene transports, and the use of paramedic/nurse crew configuration.
Acknowledgments
Chad Bowman, Heidi Hubble, Ben Quintanilla, and all of the Lifeline Special Operations Response Team members were vital in developing and implementing the training, education, management protocols, and transport systems used by Lifeline Special Operations Response Team SORT during the coronavirus disease 2019 pandemic and offered invaluable insight toward the completion of this study. We would also like to acknowledge STAT MedEvac for their fantastic partnership, clinical expertise, and years of collaboration taking care of critically ill patients.
References
- 1.Mareiniss DP. The impending storm: COVID-19, pandemics and our overwhelmed emergency departments. Am J Emerg Med. 2020;38:1293–1294. doi: 10.1016/j.ajem.2020.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang BY, Barnard LM, Emert JM. Clinical characteristics of patients with coronavirus disease 2019 (COVID-19) receiving emergency medical services in King County, Washington. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.14549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen R, Wanersdorfer K, Zebley J, Shapiro G, Coullahan T, Sarani B. Interhospital transfer of critically ill patients because of coronavirus disease 19–related respiratory failure. Air Med J. 2020;39:498–501. doi: 10.1016/j.amj.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hilbert-Carius P, Braun J, Abu-Zidan F. Pre-hospital care & interfacility transport of 385 COVID-19 emergency patients: an air ambulance perspective. Scand J Trauma Resusc Emerg Med. 2020;28:94. doi: 10.1186/s13049-020-00789-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tien H, Sawadsky B, Lewell M, Peddle M, Durham W. Critical care transport in the time of COVID-19. CJEM. 2020;22:S84–S88. doi: 10.1017/cem.2020.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garibaldi BT, Kelen GD, Brower RG. The creation of a biocontainment unit at a tertiary care hospital. The Johns Hopkins Medicine experience. Ann Am Thorac Soc. 2016;13:600–608. doi: 10.1513/AnnalsATS.201509-587PS. [DOI] [PubMed] [Google Scholar]
- 7.Garfinkel E, Lopez S, Troncoso R., Jr A critical care transport program's innovative approach to safety during the coronavirus disease 2019 pandemic. Air Med J. 2021;40:112–114. doi: 10.1016/j.amj.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghebreyesus TA. World Health Organization; 2020. WHO Director-General's opening remarks at the media briefing on COVID-19.https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19–11-march-2020 Available at: Accessed December 20, 2020. [Google Scholar]
- 9.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 10.DellaVolpe JD, Lovett J, Martin-Gill C, Guyette FX. Transport of mechanically ventilated patients in the prone position. Prehosp Emerg Care. 2016;20:643–647. doi: 10.3109/10903127.2016.1162888. [DOI] [PubMed] [Google Scholar]
- 11.Flabouris A, Schoettker P, Garner A. ARDS with severe hypoxia - aeromedical transportation during prone ventilation. Anaesth Intensive Care. 2003;31:675–678. doi: 10.1177/0310057X0303100613. [DOI] [PubMed] [Google Scholar]
- 12.Hersey D, Witter T, Kovacs G. Transport of a prone position acute respiratory distress syndrome patient. Air Med J. 2018;37:206–210. doi: 10.1016/j.amj.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Sartini C, Tresoldi M, Scarpellini P. Respiratory parameters in patients with COVID-19 after using noninvasive ventilation in the prone position outside the intensive care unit. JAMA. 2020;323:2338–2340. doi: 10.1001/jama.2020.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED's experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27:375–378. doi: 10.1111/acem.13994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elharrar X, Trigui Y, Dols A-M. Use of prone positioning in nonintubated patients with COVID-19 and hypoxemic acute respiratory failure. JAMA. 2020;323:2336–2338. doi: 10.1001/jama.2020.8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solverson K, Weatherald J, Parhar KKS. Tolerability and safety of awake prone positioning COVID-19 patients with severe hypoxemic respiratory failure. Can J Anaesth. 2021;68:64–70. doi: 10.1007/s12630-020-01787-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zang X, Wang Q, Zhou H, Liu S, Xue X. Efficacy of early prone position for COVID-19 patients with severe hypoxia: a single-center prospective cohort study. Intensive Care Med. 2020;46:1927–1929. doi: 10.1007/s00134-020-06182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seethala RR, Frakes MA, Cocchi MN. Feasibility and safety of prone position transport for severe hypoxemic respiratory failure due to coronavirus disease 2019. Crit Care Explor. 2020;2:e0293. doi: 10.1097/CCE.0000000000000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alvarez RA, Berra L, Gladwin MT. Home nitric oxide therapy for COVID-19. Am J Respir Crit Care Med. 2020;202:16–20. doi: 10.1164/rccm.202005-1906ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrari M, Santini A, Protti A. Inhaled nitric oxide in mechanically ventilated patients with COVID-19. J Crit Care. 2020;60:159–160. doi: 10.1016/j.jcrc.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Longobardo A, Montanari C, Shulman R, Benhalim S, Singer M, Arulkumaran N. Inhaled nitric oxide minimally improves oxygenation in COVID-19 related acute respiratory distress syndrome. Br J Anaesth. 2020;126:e44–e46. doi: 10.1016/j.bja.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]