Abstract
This study was aimed to investigate the effects of miR-218-5p on the proliferation, apoptosis, autophagy, and oxidative stress of rheumatoid arthritis synovial fibroblasts (RASFs), and the related mechanisms. Quantitative reverse transcription–PCR showed that the expression of miR-218-5p in rheumatoid arthritis synovial tissue was significantly higher than that in healthy synovial tissue. Compared with healthy synovial fibroblasts, miR-218-5p expression was obviously upregulated in RASFs, while KLF9 protein expression was markedly downregulated. Mechanistically, miR-218-5p could directly bind to the 3′ untranslated region of KLF9 to inhibit the expression of KLF9. Additionally, transfection of miR-218-5p small interfering RNA (siRNA) inhibited the proliferation but promoted apoptosis and autophagy of RASFs. Simultaneously, miR-218-5p silencing reduced reactive oxygen species and malondialdehyde levels and increased superoxide dismutase and glutathione peroxidase activity to improve oxidative stress in RASFs. More importantly, the introduction of KLF9 siRNA reversed the effects of miR-218-5p siRNA transfection on RASF proliferation, apoptosis, autophagy, and oxidative stress. What is more, silencing miR-218-5p inhibited the activation of JAK2/STAT3 signaling pathway by targeting KLF9. Collectively, knockdown of miR-218-5p could regulate the proliferation, apoptosis, autophagy and oxidative stress of RASFs by increasing the expression of KLF9 and inhibiting the activation of the JAK2/STAT3 signaling pathway, which may provide a potential target for the mechanism research of RA.
Keywords: apoptosis
Significance of this study.
What is already known about this subject?
The pathological changes of rheumatoid arthritis (RA) are characterized by abnormal proliferation of rheumatoid arthritis synovial fibroblast (RASFs).
A large number of dysregulated microRNAs have been found to be involved in the pathogenesis of RA.
Autophagy has recently been shown to be involved in the apoptosis of RASFs.
What are the new findings?
The expression of miR-218-5p in RA synovial tissue was upregulated.
miR-218-5p regulated the proliferation, apoptosis, autophagy, and oxidative stress of RASFs.
miR-218-5p could directly bind to the 3′ untranslated region of KLF9.
How might these results change the focus of research or clinical practice?
miR-218-5p could directly target KLF9 to regulate proliferation, apoptosis, autophagy and oxidative stress of RASFs. The inhibitory effect of miR-218-5p may provide new ideas for the treatment of RA.
Introduction
Rheumatoid arthritis (RA), a complex, chronic and progressive autoimmune disease, is characterized by synovial proliferation, inflammation, and bone destruction.1 2 With the progress of the disease, RA can also cause multiple organ involvement, such as atherosclerosis, pulmonary fibrosis, and eye diseases.3 The treatment of RA is mainly to control the disease, alleviate the disease state, and improve joint function.4 However, there is no specific treatment for RA as yet.5 The pathogenesis of RA is very complex and has not yet been clarified. Current research suggests that RA is related to factors such as heredity, infection, immunity, hormones, and environment, involving a variety of cells, cytokines, genetic materials and signaling pathways.6 Finding specific targets for RA treatment is expected to greatly improve the current treatment situation. Recent research suggests that as the main synovial cell type in synovial tissue, rheumatoid arthritis synovial fibroblast (RASF) is involved in maintaining the homeostasis of the synovial environment.7 Studies have reported that the transformation of biological characteristics of RASFs in the progression of RA is an early pathological change.8 9 Research based on RASFs has become a direction for RA treatment.
With the study of epigenetics, new breakthroughs have been made in the research of non-coding RNAs (ncRNAs) in the mechanism of RA.10 MicroRNAs (miRNAs) are a class of small ncRNAs with a length of 18–25 nucleotides.11 By downregulating the translation of downstream target gene mRNA, miRNAs act as negative regulators of gene expression. Studies have confirmed that miRNAs are essential in cell proliferation, apoptosis, oxidative stress and immune response by regulating target genes. A large number of dysregulated miRNAs have been found to be involved in the pathogenesis of RA by regulating RASFs, such as miR-19,12 miR-21,13 miR-27a,14 and miR-29a.15 These studies suggest that miRNAs may be potential targets for RA treatment.
In the present study, we are interested in studying the role of miR-218-5p in the development of RA. In view of the role of RASFs in the pathogenesis of RA, we isolated RASFs from synovial tissues of patients with RA. Additionally, miR-218-5p in RASFs was silenced by small RNA interference technology to investigate its effect on apoptosis, autophagy and oxidative stress of RASFs, and the pathogenesis of RA was also explored from the level of miRNA/mRNA axis.
Materials and methods
Object of study
Thirty synovial tissue samples (19 women and 11 men, aged 30–75 years, mean 56±6 years) were collected from Tianjin Medical University General Hospital. All patients with RA were based on the American College of Rheumatology Disease Analysis Standards. Thirty healthy control samples (18 women and 12 men, aged 30–73 years, mean 53±8 years) were collected from patients with joint trauma who underwent joint replacement surgery in Tianjin Medical University General Hospital. Written informed consent was obtained from all subjects.
Cells isolated and cell culture
As described previously,16 human healthy synovial fibroblasts (HSFs) and RASFs were isolated and cultured. Briefly, the synovial tissues were washed with sterile PBS and then digested with 1 mg/mL type I collagenase at 37°C for 4 houra. Cells were collected and placed in Roswell Park Memorial Institute medium 1640 (Solarbio, Beijing, China) containing 10% fetal bovine serum (FBS; Hyclone, USA) for 12 hours followed by sterile PBS. The primary HSFs and RASFs were obtained and incubated in PRMI medium 1640 with 10% FBS, 100 U/mL penicillin (Sigma-Aldrich, USA), and 100 U/mL streptomycin (Sigma-Aldrich) in a 37°C, 5% CO2 incubator. HSFs and RASFs were used from passages 3–7 for experimentation.
Quantitative reverse transcription–PCR (qRT-PCR)
Total RNA was extracted from cells (1×104) using TRIzol reagent (Invitrogen, USA). The concentration of total RNA was measured using an ND-1000 spectrophotometer (NanoDrop Technologies, Thermo Fisher Scientific, USA). To determine the expression of miR-218-5p, the TaqMan miRNA Reverse Transcription Kit (Applied Biosystems, Thermo Fisher Scientific) was used to reverse transcribe RNA into complementary DNA (cDNA). Real-Time PCR was performed on an Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems) using Hairpinit miRNAs Real-Time PCR Quantitation Kit (Gene-Pharma, China). U6 served as an endogenous reference for miR-218-5p expression. For the expression of KLF9 mRNA, total RNA was synthesized into cDNA using M-MLV reverse transcriptase (Promega, USA) and amplified using SYBR Premix Ex Taq Kit (Takara Bio, Japan). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal reference for KLF9 mRNA expression. The calculation was performed using the 2−ΔΔCT method.
Western blot
The cells were lysed in RIPA buffer (Beyotime, China) and then centrifuged at 12,000 g for 15 min. Eighty micrograms of protein was separated on a 10% sodium dodecyl sulfate–polyacrylamide gel and transferred to a nitrocellulose membrane (EMD Millipore, USA). The membrane was blocked with 5% skim milk containing 0.1% Tween-20 (Phosphate Buffered Saline and Tween) for 1 hour at room temperature, and then the membrane was blocked with anit-KLF9, anti-JAK2, anti-p-JAK2, anti-STAT3, anti-p-STAT3 or anti-GAPDH and incubated at 4°C overnight. Subsequently, the membrane was incubated with goat anti-rabbit horseradish peroxidaxse-conjugated secondary antibody (sc-2004, 1: 2000; Santa Cruz Biotechnology) at 37°C for 1 hour and was detected using a Western Bright Enhanced Chemiluminescence detection system (Advansta, USA). Quantification was performed using Image Lab V.3.0 software (Bio-Rad Laboratories, USA).
Dual luciferase assay
The potential target of miR-218-5p was predicted using bioinformatics, and it was identified that miR-218-5p was able to bind to the 3′ untranslated region (UTR) of KLF9 mRNA. The wild type (Wt) and mutant (Mut) seed regions of miR-218-5p in KLF9 mRNA 3′ UTR were chemically synthesized in vitro, and Spe I and HindIII restriction sites were added. Subsequently, the sequence was cloned into a Pmir-report luciferase reporter plasmid (Beyotime). Lipofectamine 2000 was used to co-transfect KLF9-Wt or KLF9-Mut plasmid vector with miR-218-5p mimic into RASFs. After 24 hours of incubation, the fluorescence intensity was determined using the dual luciferase reporter system (Promega Corporation) according to the manufacturer’s instructions. Renal fluorescence activity was used as an internal reference.
Transfection
miRNA mimics (miR-218-5p mimic and miR-NC), as well as small interfering RNA (siRNA) targeting miR-218-5p (si-miR-218-5p and si-NC) and KLF9 (si-KLF9 and si-Ctrl) were purchased from GenePharma (Shanghai, China). RASFs were seeded into 6-well plates at 2×105 cells / well and incubated at 37°Cfor 24 hours. When the cells reached 80% confluence, the medium was discarded and washed three times with PBS. Lipofectamine 2000 (Invitrogen, USA) was used to transfect miR-218-5pmimic or si-miR-218-5p into cells. For rescue experiments, cells were cotransfected with si-miR-218-5p and si-KLF9, simultaneously. For inhibition of JAK2/STAT3, RASFs were pretreated with AG490 for 24 hours prior to miR-218-5p mimic transfection.17 The expression of miR-218-5p and KLF9 in transfected cells was detected by qRT-PCR and western blot to confirm the transfection efficiency.
MTT assay
RASFs (5×103) were inoculated into 96-well plates, and 20 µl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (5 mg/mL; Sigma-Aldrich) was added to each well and incubated for 4 hours at room temperature. Subsequently, 150 µL of dimethyl sulfoxide (Sigma-Aldrich) was added to each well and stirred at room temperature for 20 min. The optical density at 490 nm was measured using a microplate reader (Bio-Rad Laboratories).
Flow cytometry
RASFs (1×106) were seeded into six-well plates, and Annexin V-Alexa Fluor 647/PI apoptosis detection kit (Fcmacs, Jiangsu, China) was used to evaluate apoptosis. The cells were incubated with Annexin V-fluorescein isothiocyanate and propidium iodide for 30 min in the dark, and then the cells were analyzed by flow cytometry (Thermo Fisher Scientific).
Caspase-3 activity
A caspase-3 viability assay kit (Beyotime) was used to evaluate caspase-3 activity in cells. In brief, after transfection, RASFs cells were lysed with RIPA buffer (Beyotime), and the protein concentration was determined using the BCA protein assay kit (Beyotime). Thirty micrograms of the cell extract was placed in a 96-well plate loaded with 20 µg Ac-DEVD-pNA and incubated at 37°C for 2 hours. The caspase-3 activity was subsequently assessed by measuring the cleavage of the Ac-DEVD-pNA substrate to pNA, and the absorbance at 405 nm was measured. Relative caspase-3 activity was measured by the ratio of the emission of treated cells to untreated cells.
Detection of oxidative stress indicators
Fluorescent probe dichloro-dihydro-fluorescein diacetate (DCFH-DA) was used to measure intracellular reactive oxygen species (ROS) levels.18 RASFs were placed in a dark incubator, and 10 µM DCFH-DA was added to the medium and incubated at 37°C for 30 min. Subsequently, after washing three times with PBS, the fluorescence intensity was measured in a fluorescence spectrophotometer at excitation and emission wavelengths of 488 and 535 nm, respectively. MDA levels, superoxide dismutase (SOD) activity, and GPx activity were measured as previously described.19 Related kits were purchased from Nanjing Jiangcheng Bioengineering Institute (Nanjing, China).
Statistical analysis
All statistical analyses were performed using SPSS software V.16.0. Data are expressed as the mean±SD. Two groups of data were compared using t-test. Comparisons among three or more groups were conducted using a one-way analysis of variance followed by Tukey’s post hoc test. A p value of <0.05 was considered to indicate a statistically significant difference.
Results
The expression of miR-218-5p in synovial tissues of patients with RA and RASFs was upregulated.
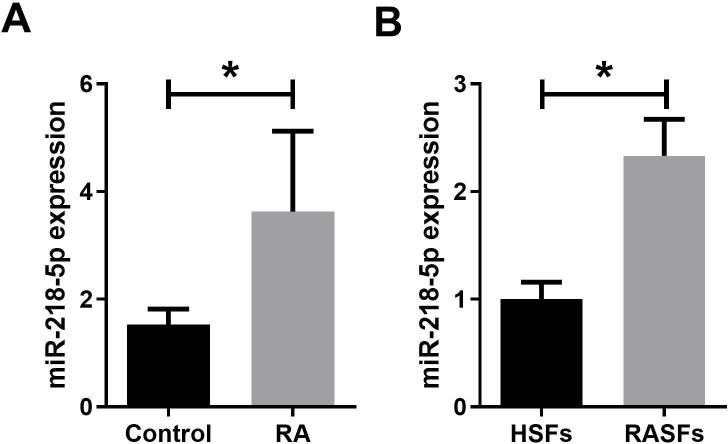
To investigate the role of miR-218-5p in RA, we examined miR-218-5p expression in synovial tissue samples from 30 patients with RA and 30 healthy controls. qRT-PCR analysis showed that miR-218-5p expression was significantly higher in RA synovial tissues than in healthy synovial tissue (figure 1A). In addition, HSFs and RASFs were isolated from healthy synovial tissues and RA synovial tissues, respectively. It was found that miR-218-5p expression was markedly increased in RASFs compared with HSFs (figure 1B). These results indicated that upregulation of miR-218-5p expression may be involved in the development of RA.
Figure 1.
Expression of miR-218-5p in synovial tissues of patients with RA and RASFs was upregulated. (A) Expression of miR-218-5p in healthy controls (n=30) and RA synovial tissues (n=30) was analyzed by qRT-PCR. (B) Expression of miR-218-5p in in HSFs and RASFs was analyzed by qRT-PCR. *P<0.05. HSF, healthy synovial fibroblast; qRT-PCR, quantitative reverse transcription–PCR; RA, rheumatoid arthritis; RASF, rheumatoid arthritis synovial fibroblast.
KLF9 was a target of miR-218-5p
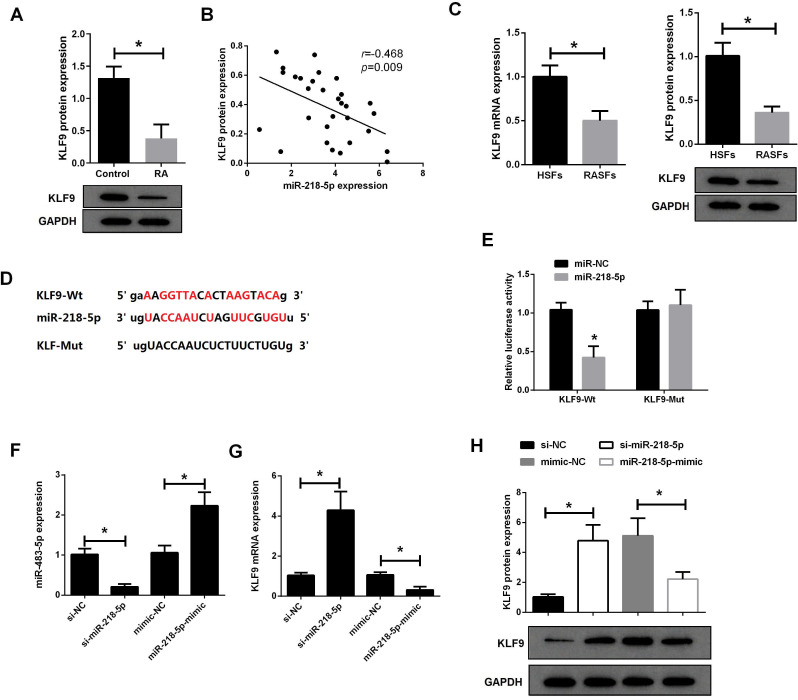
Next, we used western blot assay to analyze the expression of KLF9 in RA synovial tissues and RASFs. As shown in figure 2A, the expression of KLF9 protein in RA synovial tissue was markedly lower than that in healthy controls. Correlation analysis found that miR-218-5p was negatively correlated with KLF9 protein expression in RA synovial tissues (figure 2B). qRT-PCR and western blot analysis revealed that the expression of KLF9 mRNA and protein in RASFs was significantly lower than that in HSFs (figure 2C). Additionally, Targetscan was used to predict potential targets for miR-218-5p, and it was shown that there is a miR-218-5p binding site in the 3′ UTR of KLF9 mRNA (figure 2D). The luciferase reporter vector containing KLF9-Wt and KLF9-Mut was constructed, and co-transfected with miR-218-5p or miR-NC into RASFs, respectively. The results of the dual luciferase assay showed that miR-218-5p significantly reduced the luciferase activity of the KLF9-Wt luciferase reporter vector (figure 2E), which indicated that KLF9 is the target of miR-218-5p.
Figure 2.
KLF9 was a target of miR-218-5p. (A) The expression of KLF9 protein in synovial tissue from patients with RA (n=30) and healthy controls (n=30) was detected by western blot. (B) There was a negative correlation between miR-218-5p and KLF9 protein expression in RA synovial tissues (n=30). (C) The expression of KLF9 mRNA and protein in RASFs and HSFs was analyzed by qRT-PCR and western blot. (D) TargetScan showed that miR-218-5p binds to 3′ UTR of KLF9. (E) Luciferase reporter vectors containing KLF9-Wt and KLF9-Mut were constructed and then cotransfect with miR-218-5p or miR-NC into 293 T cells to detect luciferase activity. (F) The expression of miR-218-5 in RASFs after transfection with si-miR-218-5p or miR-218-5p mimic was determined by qRT-PCR. qRT-PCR (G) and western blot assay (H) were used to analyze the expression of KLF9 mRNA and protein in RASFs after transfection with si-miR-218-5p or miR-218-5p mimic. *P<0.05. HSF, healthy synovial fibroblast; qRT-PCR, quantitative reverse transcription–PCR; RA, rheumatoid arthritis; RASF, rheumatoid arthritis synovial fibroblast; UTR, untranslated region; Wt, wild type.
To investigate the effect of miR-218-5p on the expression of KLF9 in RASFs, RASFs were transfected with miR-218-5p siRNA or miR-218-5p mimic (figure 2F). Compared with si-NC, si-miR-218-5p transfection resulted in a significant decrease in miR-218-5p expression, whereas compared with miR-NC, miR-218-5p mimic transfection resulted in an obvious increase in miR-218-5p expression. Moreover, qRT-PCR (figure 2G) and western blot (figure 2H) showed that downregulation of miR-218-5p significantly increased the expression of KLF9 mRNA and protein in RASFs. In contrast, miR-218-5p mimic significantly reduced the expression of KLF9 in RASFs. These results indicated that miR-218-5p could directly bind to the 3′ UTR of KLF9 to inhibit the expression of KLF9.
Knockdown of miR-218-5p regulated proliferation, apoptosis and autophagy of RASFs by targeting KLF9
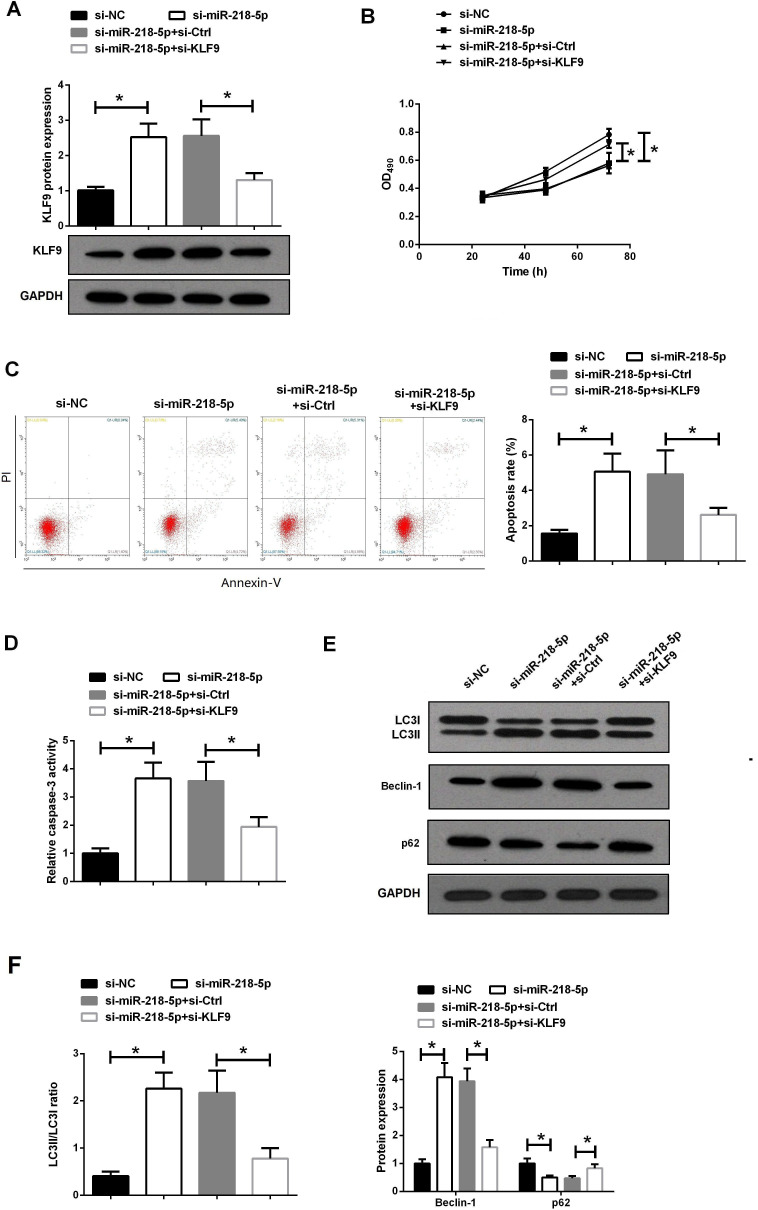
To further study the role of miR-218-5p in RA progression, RASFs were cotransfected with miR-218-5p siRNA and KLF9 siRNA. Western blot assay showed that si-miR-218-5p significantly increased KLF9 protein expression in RASFs, whereas si-KLF9 reversed this effect (figure 3A). Subsequently, MTT assay was used to evaluate the effect of miR-218-5p on the proliferation of RASFs. It was found that downregulation of miR-218-5p markedly reduced the cell viability of RASFs, which was obviously reversed by si-KLF9 (figure 3B).
Figure 3.
Knockdown of miR-218-5p regulated proliferation, apoptosis and autophagy of RASFs by targeting KLF9. (A) The expression of KLF9 protein in RASFs transfected with si-miR-218-5p and si-KLF9 was detected by western blot. (B) Proliferation of RASFs transfected with si-miR-218-5p and si-KLF9 was detected by MTT assay. (C) Apoptosis of RASFs transfected with si-miR-218-5p and si-KLF9 was detected by flow cytometry. (D) The activity of caspase-3 in RASFs transfected with si-miR-218-5p and si-KLF9 was detected by caspase-3 activity detection kit. (E–F) The protein expression of LC3, Beclin-1 and p62 in RASFs transfected with si-miR-218-5p and si-KLF9 was analyzed by western blot assay. *P<0.05. MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; OD490, optical density at 490 nm; RASF, rheumatoid arthritis synovial fibroblast.
Abnormal cell autophagy and apoptosis are closely related to the persistent proliferation of RASFs.20 Moreover, induction of autophagy can cause programmed cell death.21 We used flow cytometry to analyze the effect of miR-218-5p on apoptosis (figure 3C). The results showed that si-miR-218-5p promoted the apoptosis of RASFs, and si-KLF9 eliminated the effect of si-miR-218-5p on apoptosis. Additionally, caspase-3 activity test also proved that si-KLF9 removed the enhancement effect of si-miR-218-5p on caspase-3 activity (figure 3D). We further evaluated the effect of miR-218-5p on autophagy in RASFs cells by detecting the expression of autophagy marker proteins LC3, Beclin-1 and p62. As shown in figure 3E, F, knockdown of miR-218-5p in RASFs observed higher levels of the conversion of LC3I to LC3II and the expression of Beclin-1, and inhibited the expression of p62, suggesting that knockdown of miR-218-5p could promote autophagy of RASFs. As expected, the introduction of si-KLF9 was able to reverse this effect. These results revealed that the inhibitory effect of knockdown miR-218-5p on RASFs proliferation may be related to its promotion of apoptosis and autophagy.
Knockdown of miR-218-5p reduced oxidative stress in RASFs by targeting KLF9
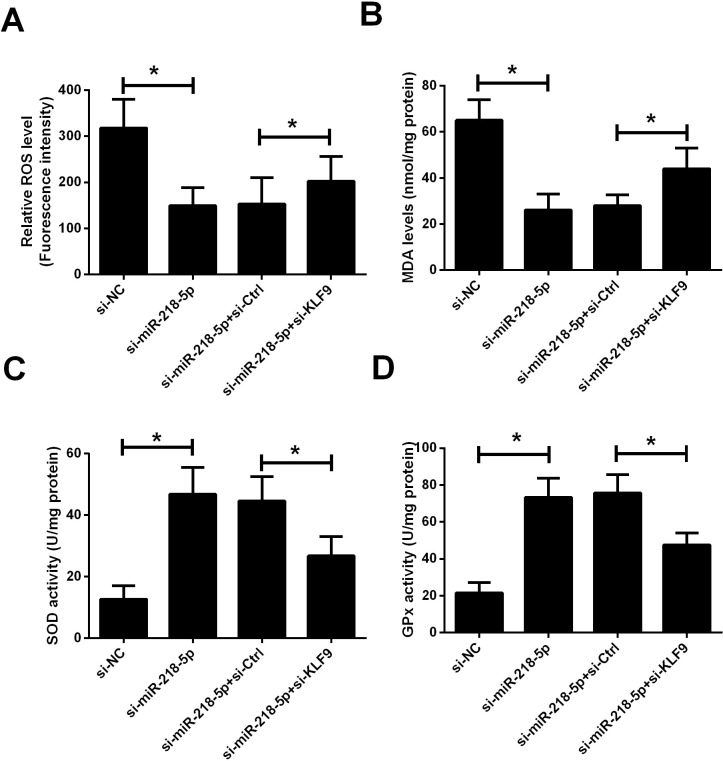
To assess the effect of miR-218-5p on RASFs oxidative stress, markers related to oxidative stress were measured, including levels of ROS and MDA, and activities of SOD and GPx. As shown in figure 4A–D, knockdown of miR-218-5p significantly reduced the levels of ROS and MDA in RASFs cells and increased the activity of SOD and GPx, suggesting that inhibition of miR-218-5p expression can improve oxidative stress in RASFs. In addition, silencing of KLF9 was able to reverse the antioxidant stress effect of si-miR-218-5p.
Figure 4.
Knockdown of miR-218-5p reduced oxidative stress in RASFs by targeting KLF9. The effect of cotransfection of si-miR-218-5p and si-KLF9 on oxidative stress in RASFs was evaluated by evaluating ROS level (A), MDA content (B), SOD activity (C), and GPx activity (D). *P<0.05. RASF, rheumatoid arthritis synovial fibroblast; ROS, reactive oxygen species; SOD, superoxide dismutase.
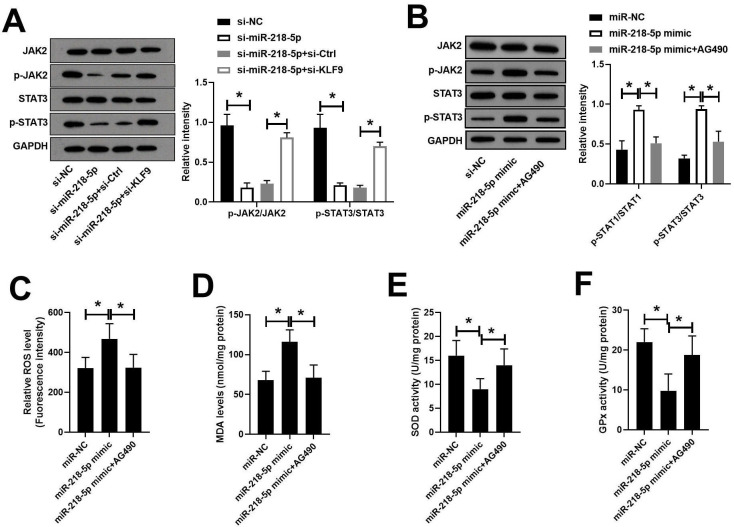
Knockdown of miR-218-5p inhibited JAK2/STAT3 signaling pathway by targeting KLF9
The JAK2/STAT3 pathway has been shown to participate in the onset of RA. We subsequently analyzed the effect of miR-218-5p on the JAK2/STAT3 pathway. Western blot analysis showed that transfection of si-miR-218-5p significantly inhibited the phosphorylation of JAK2 and STAT3 in RASFs, while the introduction of si-KLF9 reversed this effect (figure 5A). To confirm the role of JAK2/STAT3, RASFs were pretreated with AG490, the inhibitor of JAK2/STAT3, followed by miR-218-5p mimic transfection. As expected, the phosphorylation of JAK2 and STAT3 induced by miR-218-5p mimic could be reversed by AG490 (figure 5B). Moreover, the oxidative stress enhanced by miR-218-5p also could be abolished by AG490 treatment (figure 5C–F).
Figure 5.
Knockdown of miR-218-5p inhibitedJAK2/STAT3 signaling pathway by targeting KLF9. The protein expression of Jak2, p-JAK2, STAT3, p-STAT3 in RASFs transfected with si-miR-218-5p and si-KLF9 was analyzed by western blot assay. *P<0.05. RASF, rheumatoid arthritis synovial fibroblast; ROS, reactive oxygen species; SOD, superoxide dismutase.
Discussion
Although the pathogenesis of RA is not clear, a large amount of data indicates that miRNAs can promote the pathogenesis of RA by regulating related genes in RASFs.22 23 Previous studies have shown that miR-218-5p is involved in inflammation and immune regulation.24–26 Additionally, the expression of miR-218-5p is significantly upregulated in moderate and severe osteoarthritis, which may be an inducer of cartilage destruction.27 Interestingly, Iwamoto et al 28 confirmed that miR-218 is upregulated in RASF through miRNA array analysis and is involved in regulating the osteogenic differentiation of RASFs. Based on these data, we tried to explore the influence of miR-218-5p on the characteristics of RASFs. As a results, the present study confirmed that miR-218-5p regulated the proliferation, apoptosis, autophagy, and oxidative stress of RASFs by targeting KLF9, which may involve the JAK2/STAT3 pathway. Our findings suggested that miR-218-5p may be a potential biomarker or therapeutic target for RA, which may help explore new therapies for RA.
Generally, miRNAs regulate the expression of a target gene by directly binding to the 3′ UTR of the target gene mRNA in a manner that degrades or inhibits translation. By binding to various target genes, various miRNAs are involved in the pathogenesis of RA. For example, Liu et al 29 found that miR-146a inhibits the proliferation and inflammation of RASFs by inhibiting the TLR4/nuclear factor-kappa B (NF-κB) pathway. Yang et al 30 showed that miR-338-5p promotes the proliferation, migration and invasion of RASFs by targeting SPRY1. Liu et al 31 demonstrated that miR-212–3 p inhibits the proliferation of RASFs and promotes apoptosis by targeting SOX5, suggesting that miR-212-3p may become one of the biological targets for RA treatment. Therefore, we speculated that miR-218-5p may participate in the pathological process of RA by directly binding its target genes. We found through online prediction software that miR-218-5p may target the 3′ UTR of KLF9 mRNA. The results of the luciferase reporter assay also confirmed that KLF9 was a direct target of miR-218-5p. Therefore, we believed that miR-218-5p could target KLF2 to participate in the regulation of a series of cellular activities in RASFs.
The pathological changes of RA are characterized by abnormal proliferation of RASFs.8 The abnormal proliferation of RASFs mainly involves two aspects: active proliferation and inhibition of apoptosis.32 Autophagy, as a protective mechanism in the evolution of eukaryotic cells, has also recently been shown to be involved in the apoptosis of RASFs.33 Kim et al 20 found that initiation of autophagy helps relieve bone damage in RA. Chatzikyriakidou et al 34 also found that RA susceptible persons lack autophagy-related gene polymorphisms, suggesting that autophagy defects may be related to the pathogenesis of RA. In addition, Kato et al 35 proposed that autophagy plays a dual role in cell death that autophagy not only helps RASFs resist apoptosis induced by endoplasmic reticulum stress, but also induces autophagic death of RASFs.
The role of miRNAs in proliferation, apoptosis and autophagy has been widely confirmed. For example, miR-381 promotes apoptosis and autophagy of prostate cancer cells through inhibiting the reelin-mediated PI3K/AKT/mTOR pathway.36 miR-142 directly targets ULK1, ATG4A and ATG5 to regulate autophagy in osteosarcoma cells.37 Ran et al 38 showed that HMGB1-mediated autophagy can be inhibited by miR-218 overexpression. Besides, as a Krüppel-like factor (KLF) family member, KLF9 has also been shown to be involved in the regulation of apoptosis and autophagy.39 Ma et al 39 reported that KLF9 knockdown can significantly inhibit CRL-7566 cell proliferation and autophagy, and induce apoptosis, and this process is regulated by miR-142-3p. In the present study, we found that knockdown of miR-218-5p inhibited the proliferation of RASFs and promoted apoptosis through targeting KLF9. Similarly, we also observed in RASFs that silencing of miR-218-5p increased the expression of autophagy marker protein LC3II and Beclin-1 and promoted the degradation of autophagy substrate protein p62, while KLF9 silencing reversed this effect. Therefore, the mechanism of miR-218-5p/KLF9 axis-mediated RASFs proliferation may lie in its inhibitory effect on autophagy.
It is well known that oxidative stress is closely related to cell proliferation and apoptosis.40 In the mitochondrial apoptosis pathway, mitochondria mainly increase the production of ROS. Excess ROS mediates apoptosis through multiple pathways, including enhanced lipid peroxidation, affecting cytokine expression.41 Zuo et al 42 found that ROS plays an important role in inhibiting the proliferation of RASFs by mediating the NF-κB/MAPK feedback loop and apoptosis. Interestingly, miR-218-5p not only regulates the proliferation and apoptosis of many types of cells43 but also has been reported to be involved in regulating immune responses, autophagy, and oxidative stress.38 44 We also found that knockdown of miR-218-5p improved oxidative stress in RASFs in the present study. Study has confirmed that ROS plays an important role in inducing autophagy, regulating autophagy proteins by regulating autophagy transcription factors.45 Alsousi et al 46 found that ROS may promote the pathogenesis of RA in RASFs by inducing autophagy. However, the mechanisms by which miR-218-5p/KLF9 axis regulates autophagy and oxidative stress in RASFs, as well as crosstalk between autophagy and oxidative stress, remain to be explored.
The role of the JAK2/STAT3 pathway in the pathogenesis of RA has been widely confirmed and is considered to be a key pathway for activating complex signaling mechanisms in RA.47 The activation of JAK/STAT signaling pathway is one of the aggressive inflammation mechanisms of joint injury in RA, and several JAK inhibitors are currently being developed for therapy of RA.48 In addition to inflammation, the JAK/STAT pathway is also involved in regulating the proliferation, apoptosis and autophagy of RASFs. For example, STAT1 and STAT3 have been shown to participate in promoting the proliferation of RASFs.49 Blocking the JAK/STAT3 pathway can promote RASFs apoptosis and inhibit proliferation.50 Chang et al 51 found that inhibiting STAT3 activation can reduce autophagy in interleukin-7-treated RASFs. Yang et al 52 showed that miR-218 can regulate the proliferation and invasion of lung cancer cells in vitro by regulating the JAK2/STAT3 pathway. In the present study, we found that knockdown of miR-218-5p could inhibit the phosphorylation of JAK2 and STAT3, while knockdown of KLF9 could eliminate the inhibitory effect of miR-218-5p silencing on the JAK2/STAT3 pathway. Additionally, the oxidative stress induced by miR-218-5p also could be abolished by AG490, the inhibitor of the JAK2/STAT3 pathway. Collectively, the JAK2/STAT3 pathway may be involved in the mechanism of miR-218-5p/KLF9 axis in RA.
In summary, we confirmed that miR-218-5p was upregulated in RA synovial tissue and RASFs. We also confirmed that miR-218-5p could directly target KLF9 to regulate proliferation, apoptosis, autophagy and oxidative stress of RASFs, and this process also involved the JAK2/STAT3 pathway. Therefore, the inhibitory effect of miR-218-5p may provide new ideas for the treatment of RA.
Footnotes
Contributors: MC was responsible for the conception and design, contributed to the acquisition of data and took part in drafting the article. MHL, NZ, WWS and HW conducted the analysis and interpretation of data, revising it critically for important intellectual content; WW gave the final revision and approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The present study was approved by the ethics committee of Tianjin Medical University General Hospital.
References
- 1. Nygaard G, Di Paolo JA, Hammaker D, et al. Regulation and function of apoptosis signal-regulating kinase 1 in rheumatoid arthritis. Biochem Pharmacol 2018;151:282–90. 10.1016/j.bcp.2018.01.041 [DOI] [PubMed] [Google Scholar]
- 2. Wu B, Goronzy JJ, Weyand CM. Metabolic fitness of T cells in autoimmune disease. Immunometabolism 2020;2:e200017. 10.20900/immunometab20200017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wilson A, Yu H-T, Goodnough LT, et al. Prevalence and outcomes of anemia in rheumatoid arthritis: a systematic review of the literature. Am J Med 2004;116 Suppl 7A:50–7. 10.1016/j.amjmed.2003.12.012 [DOI] [PubMed] [Google Scholar]
- 4. Mehta N, Schneider LK, McCardell E. Rheumatoid arthritis: selecting monotherapy versus combination therapy. J Clin Rheumatol 2017. 10.1097/RHU.0000000000000410. [Epub ahead of print: 18 Jan 2017]. [DOI] [PubMed] [Google Scholar]
- 5. Piranavan P, Bhamra M, Perl A. Metabolic targets for treatment of autoimmune diseases. Immunometabolism 2020;2:e200012. 10.20900/immunometab20200012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van Raemdonck K, Umar S, Palasiewicz K, et al. CCL21/CCR7 signaling in macrophages promotes joint inflammation and TH17-mediated osteoclast formation in rheumatoid arthritis. Cell Mol Life Sci 2020;77:1387–99. 10.1007/s00018-019-03235-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kontny E, Grabowska A, Kowalczewski J, et al. Taurine chloramine inhibition of cell proliferation and cytokine production by rheumatoid arthritis fibroblast-like synoviocytes. Arthritis Rheum 1999;42:2552–60. [DOI] [PubMed] [Google Scholar]
- 8. Ma J, Wang X, Lv T, et al. Effects of ghrelin on the apoptosis of rheumatoid arthritis fibroblast-like synoviocyte MH7A cells. Biol Pharm Bull 2019;42:158–63. 10.1248/bpb.b18-00285 [DOI] [PubMed] [Google Scholar]
- 9. Yan Z-F, Zhao X-Y, Liu W, et al. Uca1 impacts progress of rheumatoid arthritis by inducing the apoptosis of fibroblast-like synoviocyte. Eur Rev Med Pharmacol Sci 2018;22:914–20. 10.26355/eurrev_201802_14370 [DOI] [PubMed] [Google Scholar]
- 10. Mousavi MJ, Jamshidi A, Chopra A, et al. Implications of the noncoding RNAs in rheumatoid arthritis pathogenesis. J Cell Physiol 2019;234:335–47. 10.1002/jcp.26911 [DOI] [PubMed] [Google Scholar]
- 11. Rupaimoole R, Slack FJ. Microrna therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov 2017;16:203–22. 10.1038/nrd.2016.246 [DOI] [PubMed] [Google Scholar]
- 12. Li Z, Cai J, Cao X. Mir-19 suppresses fibroblast-like synoviocytes cytokine release by targeting toll like receptor 2 in rheumatoid arthritis. Am J Transl Res 2016;8:5512–8. [PMC free article] [PubMed] [Google Scholar]
- 13. Xiong G, Huang Z, Jiang H, et al. Inhibition of microRNA-21 decreases the invasiveness of fibroblast-like synoviocytes in rheumatoid arthritis via TGFβ/Smads signaling pathway. Iran J Basic Med Sci 2016;19:787–93. [PMC free article] [PubMed] [Google Scholar]
- 14. Shi D-L, Shi G-R, Xie J, et al. Microrna-27A inhibits cell migration and invasion of fibroblast-like synoviocytes by targeting follistatin-like protein 1 in rheumatoid arthritis. Mol Cells 2016;39:611–8. 10.14348/molcells.2016.0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu J, Fei D, Xing J, et al. Microrna-29A inhibits proliferation and induces apoptosis in rheumatoid arthritis fibroblast-like synoviocytes by repressing STAT3. Biomed Pharmacother 2017;96:173–81. 10.1016/j.biopha.2017.09.120 [DOI] [PubMed] [Google Scholar]
- 16. Li H, Guan S-B, Lu Y, et al. Mir-140-5P inhibits synovial fibroblasts proliferation and inflammatory cytokines secretion through targeting TLR4. Biomed Pharmacother 2017;96:208–14. 10.1016/j.biopha.2017.09.079 [DOI] [PubMed] [Google Scholar]
- 17. Jiang L, Zhao X-H, Mao Y-L, et al. Long non-coding RNA RP11-468E2.5 curtails colorectal cancer cell proliferation and stimulates apoptosis via the JAK/STAT signaling pathway by targeting STAT5 and STAT6. J Exp Clin Cancer Res 2019;38:465. 10.1186/s13046-019-1428-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qi X, Zhou R, Liu Y, et al. Trans-cinnamaldehyde protected PC12 cells against oxygen and glucose deprivation/reperfusion (OGD/R)-induced injury via anti-apoptosis and anti-oxidative stress. Mol Cell Biochem 2016;421:67–74. 10.1007/s11010-016-2785-z [DOI] [PubMed] [Google Scholar]
- 19. Wu J, Li Q, Wang X, et al. Neuroprotection by curcumin in ischemic brain injury involves the Akt/Nrf2 pathway. PLoS One 2013;8:e59843. 10.1371/journal.pone.0059843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim T-H, Choi SJ, Lee YH, et al. Combined therapeutic application of mTOR inhibitor and vitamin D3 for inflammatory bone destruction of rheumatoid arthritis. Med Hypotheses 2012;79:757–60. 10.1016/j.mehy.2012.08.022 [DOI] [PubMed] [Google Scholar]
- 21. Chi EY, Viriyapak B, Kwack HS, et al. Regulation of paclitaxel-induced programmed cell death by autophagic induction: a model for cervical cancer. Obstet Gynecol Sci 2013;56:84–92. 10.5468/OGS.2013.56.2.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Niederer F, Trenkmann M, Ospelt C, et al. Down-Regulation of microRNA-34a* in rheumatoid arthritis synovial fibroblasts promotes apoptosis resistance. Arthritis Rheum 2012;64:1771–9. 10.1002/art.34334 [DOI] [PubMed] [Google Scholar]
- 23. Senousy MA, Helmy HS, Fathy N, et al. Association of MTMR3 rs12537 at miR-181a binding site with rheumatoid arthritis and systemic lupus erythematosus risk in Egyptian patients. Sci Rep 2019;9:e12299. 10.1038/s41598-019-48770-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang T, Xiang L. Honokiol alleviates sepsis-induced acute kidney injury in mice by targeting the miR-218-5p/heme oxygenase-1 signaling pathway. Cell Mol Biol Lett 2019;24:15. 10.1186/s11658-019-0142-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yamada K, Takizawa S, Ohgaku Y, et al. Microrna 16-5p is upregulated in calorie-restricted mice and modulates inflammatory cytokines of macrophages. Gene 2020;725:e144191. 10.1016/j.gene.2019.144191 [DOI] [PubMed] [Google Scholar]
- 26. Conickx G, Mestdagh P, Avila Cobos F, et al. Microrna profiling reveals a role for MicroRNA-218-5p in the pathogenesis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2017;195:43–56. 10.1164/rccm.201506-1182OC [DOI] [PubMed] [Google Scholar]
- 27. Lu J, Ji M-L, Zhang X-J, et al. MicroRNA-218-5p as a potential target for the treatment of human osteoarthritis. Mol Ther 2017;25:2676–88. 10.1016/j.ymthe.2017.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iwamoto N, Fukui S, Takatani A, et al. Osteogenic differentiation of fibroblast-like synovial cells in rheumatoid arthritis is induced by microRNA-218 through a ROBO/Slit pathway. Arthritis Res Ther 2018;20:189. 10.1186/s13075-018-1703-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu W, Wu Y-H, Zhang L, et al. Microrna-146A suppresses rheumatoid arthritis fibroblast-like synoviocytes proliferation and inflammatory responses by inhibiting the TLR4/NF-kB signaling. Oncotarget 2018;9:23944–59. 10.18632/oncotarget.24050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang Y, Wang Y, Liang Q, et al. MiR-338-5p Promotes Inflammatory Response of Fibroblast-Like Synoviocytes in Rheumatoid Arthritis via Targeting SPRY1. J Cell Biochem 2017;118:2295–301. 10.1002/jcb.25883 [DOI] [PubMed] [Google Scholar]
- 31. Liu Y, Zhang X-L, Li X-F, et al. miR-212-3p reduced proliferation, and promoted apoptosis of fibroblast-like synoviocytes via down-regulating SOX5 in rheumatoid arthritis. Eur Rev Med Pharmacol Sci 2018;22:461–71. 10.26355/eurrev_201801_14196 [DOI] [PubMed] [Google Scholar]
- 32. Jia S, Zhang S, Yuan H, et al. Lunasin inhibits cell proliferation via apoptosis and reduces the production of proinflammatory cytokines in cultured rheumatoid arthritis synovial fibroblasts. Biomed Res Int 2015;2015:1–9. 10.1155/2015/346839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Connor AM, Mahomed N, Gandhi R, et al. Tnfα modulates protein degradation pathways in rheumatoid arthritis synovial fibroblasts. Arthritis Res Ther 2012;14:R62. 10.1186/ar3778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chatzikyriakidou A, Voulgari PV, Drosos AA. Lack of association of the autophagy-related gene polymorphism Atg16L1 rs2241880 in RA predisposition. Rheumatol Int 2014;34:477–9. 10.1007/s00296-013-2726-z [DOI] [PubMed] [Google Scholar]
- 35. Kato M, Ospelt C, Gay RE, et al. Dual role of autophagy in stress-induced cell death in rheumatoid arthritis synovial fibroblasts. Arthritis Rheumatol 2014;66:40–8. 10.1002/art.38190 [DOI] [PubMed] [Google Scholar]
- 36. Liao W, Zhang Y. MicroRNA-381 facilitates autophagy and apoptosis in prostate cancer cells via inhibiting the RELN-mediated PI3K/Akt/mTOR signaling pathway. Life Sci 2020;254:117672. 10.1016/j.lfs.2020.117672 [DOI] [PubMed] [Google Scholar]
- 37. Zhu K, Yuan Y, Wen J, et al. Lncrna Sox2OT-V7 promotes doxorubicin-induced autophagy and chemoresistance in osteosarcoma via tumor-suppressive miR-142/miR-22. Aging 2020;12:6644–66. 10.18632/aging.103004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ran X, Yang J, Liu C, et al. Mir-218 inhibits HMGB1-mediated autophagy in endometrial carcinoma cells during chemotherapy. Int J Clin Exp Pathol 2015;8:6617–26. [PMC free article] [PubMed] [Google Scholar]
- 39. Ma L, Li Z, Li W, et al. MicroRNA-142-3p suppresses endometriosis by regulating KLF9-mediated autophagy in vitro and in vivo. RNA Biol 2019;16:1733–48. 10.1080/15476286.2019.1657352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Luo R, Liao Z, Song Y, et al. Berberine ameliorates oxidative stress-induced apoptosis by modulating ER stress and autophagy in human nucleus pulposus cells. Life Sci 2019;228:85–97. 10.1016/j.lfs.2019.04.064 [DOI] [PubMed] [Google Scholar]
- 41. Dai Y-L, Jiang Y-F, Lu Y-A, et al. Fucoidan from acid-processed Hizikia fusiforme attenuates oxidative damage and regulate apoptosis. Int J Biol Macromol 2020;160:390–7. 10.1016/j.ijbiomac.2020.05.143 [DOI] [PubMed] [Google Scholar]
- 42. Zuo J, Dou D-Y, Wang H-F, et al. Reactive oxygen species mediated NF-κB/p38 feedback loop implicated in proliferation inhibition of HFLS-RA cells induced by 1,7-dihydroxy-3,4-dimethoxyxanthone. Biomed Pharmacother 2017;94:1002–9. 10.1016/j.biopha.2017.07.164 [DOI] [PubMed] [Google Scholar]
- 43. Yu S-F, Feng W-Y, Chai S-Q, et al. Down-Regulation of miR-218-5p promotes apoptosis of human umbilical vein endothelial cells through regulating high-mobility group box-1 in Henoch-Schonlein purpura. Am J Med Sci 2018;356:64–71. 10.1016/j.amjms.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 44. Wu Z, Han Y, Li Y, et al. miR-218-5p inhibits the stem cell properties and invasive ability of the A2B5+CD133− subgroup of human glioma stem cells. Oncol Rep 2016;35:869–77. 10.3892/or.2015.4418 [DOI] [PubMed] [Google Scholar]
- 45. Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J 2012;441:523–40. 10.1042/BJ20111451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Alsousi AA, Igwe OJ. Autophagy protects against redox-active trace metal-induced cell death in rabbit synovial fibroblasts through Toll-like receptor 4 activation. Exp Cell Res 2019;374:19–28. 10.1016/j.yexcr.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 47. El-Ghafar OAMA, Helal GK, Abo-Youssef AM. Apixaban exhibits anti-arthritic effects by inhibiting activated factor X-mediated JAK2/STAT3 and MAPK phosphorylation pathways. Inflammopharmacology 2020;28:1253–67. 10.1007/s10787-020-00693-8 [DOI] [PubMed] [Google Scholar]
- 48. Bonilla-Hernán MG, Miranda-Carús ME, Martin-Mola E. New drugs beyond biologics in rheumatoid arthritis: the kinase inhibitors. Rheumatology 2011;50:1542–50. 10.1093/rheumatology/ker192 [DOI] [PubMed] [Google Scholar]
- 49. Krause A, Scaletta N, Ji J-D, et al. Rheumatoid arthritis synoviocyte survival is dependent on STAT3. J Immunol 2002;169:6610–6. 10.4049/jimmunol.169.11.6610 [DOI] [PubMed] [Google Scholar]
- 50. Zhang X, Feng H, Du J, et al. Aspirin promotes apoptosis and inhibits proliferation by blocking G0/G1 into S phase in rheumatoid arthritis fibroblast-like synoviocytes via downregulation of JAK/STAT3 and NF-κB signaling pathway. Int J Mol Med 2018;42:3135–48. 10.3892/ijmm.2018.3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chang L, Feng X, Gao W. Proliferation of rheumatoid arthritis fibroblast-like synoviocytes is enhanced by IL-17-mediated autophagy through STAT3 activation. Connect Tissue Res 2019;60:358–66. 10.1080/03008207.2018.1552266 [DOI] [PubMed] [Google Scholar]
- 52. Yang Y, Ding L, Hu Q, et al. Microrna-218 functions as a tumor suppressor in lung cancer by targeting IL-6/STAT3 and negatively correlates with poor prognosis. Mol Cancer 2017;16:141. 10.1186/s12943-017-0710-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.