Abstract
Aims
To analyze expression of members of the Toll-like receptor (TLR)4/myeloid differentiation primary response 88 (MyD88)/nuclear factor (NF)-κB signaling pathway in the heart and liver in a rat model of type 2 diabetes mellitus (T2DM). Our overall goal was to understand the underlying pathophysiological mechanisms.
Methods
We measured fasting blood glucose (FBG) and insulin (FINS) in a rat model of T2DM. Expression of members of the TLR4/MyD88/NF-κB signaling pathway as well as downstream cytokines was investigated. Levels of mRNA and protein were assessed using quantitative real-time polymerase chain reaction and western blotting, respectively. Protein content of tissue homogenates was assessed using enzyme-linked immunosorbent assays.
Results
Diabetic rats had lower body weights, higher FBG, higher FINS, and higher intraperitoneal glucose tolerance than normal rats. In addition, biochemical indicators related to heart and liver function were elevated in diabetic rats compared with normal rats. TLR4 and MyD88 were involved in the occurrence of T2DM as well as T2DM-related heart and liver complications. TLR4 caused T2DM-related heart and liver complications through activation of NF-κB.
Conclusions
TLR4/MyD88/NF-κB signaling induces production of tumor necrosis factor-α, interleukin-6, and monocyte chemoattractant protein-1, leading to the heart- and liver-related complications of T2DM.
Keywords: Type 2 diabetes mellitus, Toll-like receptor 4, nuclear factor-κB, tumor necrosis factor-α, insulin resistance, heart, liver
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic disease characterized by hyperglycemia and insulin deficiency. T2DM is accompanied by a variety of complications affecting the heart, kidney, liver, retina, and nervous system.1 The prevalence of T2DM in China is the highest in the world. Incidence is increasing year by year along with economic improvements and changing diets.2,3 T2DM has become a major public health issue of global concern.4 The pathogenesis of T2DM is complex and is influenced by genetic predisposition, inflammatory responses, insulin resistance, oxidative stress, and microcirculation abnormalities.5,6 Inflammation can lead to insulin resistance and T2DM, while long-term inflammation can also aggravate hyperglycemia and promote the occurrence of diabetic complications.7 Long-term chronic inflammation can cause abnormal adipocyte function, leading to changes in the insulin sensitivity of adipose tissue.8 Expression of Toll-like receptor (TLR) 2 and TLR4 in peripheral blood mononuclear cells, as well as expression of TLR2 and myeloid differentiation primary response 88 (MyD88) in abdominal subcutaneous fat, was significantly higher in patients with T2DM compared with normal subjects.9 TLRs may also contribute to dysfunction of islet β cells and development of T2DM.10–12 Increasing attention has been paid to the role of immune factors in the pathogenesis of T2DM.
TLR4 is a type I transmembrane protein and the first TLR to be well characterized. TLR4 can recognize the pathogen-associated molecular patterns of different pathogens including exogenous ligands such as lipopolysaccharide (LPS), peptidoglycan, unmethylated cytosine and guanine phosphate. Endogenous TLR4 ligands include heat shock protein and hyaluronic acid.13,14 TLR4 is widely expressed in various cells and tissues including the liver, adipose tissue, islet β cells, and vascular tissue.15 Following ligand binding to TLR4, various signaling transduction pathways are activated among which the TLR4/nuclear factor (NF)-κB axis is the most important.16 Activation of this pathway can lead to downstream production of inflammatory cytokines, such as tumor necrosis factor (TNF)-α and interleukin (IL)-6, which are involved in immune responses. NF-κB signaling can be activated by TLR4 via two pathways: the MyD88-dependent signaling pathway and the Toll/IL‐1R domain‐containing adaptor‐inducing interferon‐β (TRIF)‐dependent signaling pathway.17 Diets high in fat and sugar are associated with increased levels of LPS, TLR4, TNF-α, and oxidative stress and inflammation; all of these factors contribute to activation of the TLR4 signaling pathway.18 Expression of TLR4, MyD88, phosphorylated IL-1R-associated kinase 1 and other inflammatory factors (TNF-α, macrophage chemotactic protein [MCP]-1, IL-6 and IL-8) in monocytes from patients with T2DM was significantly higher compared with healthy individuals.19 These studies suggested that TLR4-associated inflammatory signaling may be closely related to the pathogenesis of T2DM. Cardiac, vascular, and kidney-associated diabetic complications are the most harmful.20,21 TLR4 signaling can activate the transcription and translation of TNF-α, IL-6, and IL-12, causing inflammatory responses and inducing liver and kidney injuries.19,22,23 Activation of NF-κB in the tissues and organs of diabetic rats resulted in up-regulation of TNF-α and IL-1 expression.24 Overactivation of NF-κB can cause abnormal expression of inflammatory response-related genes, promoting inflammatory responses and tissue damage.24 The above studies indicated that the TLR4 signaling pathway is involved in T2DM, although the specific mechanisms remain unknown.
In the present study, we investigated the role of TLR4 in heart- and liver-associated complications in a rat model of T2DM.
Materials and methods
Animals
Thirty-five male specific pathogen-free Sprague–Dawley rats (body weight 221 ± 9.34 g) were obtained from Vital River Laboratories (Beijing, China). The rats were raised in a well-ventilated laboratory with a 12-hour/12-hour light/dark cycle at 20°C to 25°C and 40% to 70% humidity. All rats had free access to water and food.
After 1 week of adaptive feeding, the animals were randomly divided into control and experimental groups (n = 25 rats each). The rats in the control group were fed regular feed, while the rats in the experimental group were fed a high-fat diet (feed supplemented with 20% sugar, 2.5% cholesterol and 15% lard). After 4 weeks of feeding, rats in the experimental group and the control group were intraperitoneally injected with 30 mg/kg streptozotocin (STZ; prepared in 0.1 M citric acid, pH 4.5) for 2 weeks to induce diabetes. Rats in the experimental group with fasting blood glucose (FBG) ≥12.8 mmol/L and decreased insulin sensitivity index were selected as the T2DM model group (n = 18). Over the entire duration of the experiment, urine volumes and body weights of all rats were recorded.
Prior to blood sample collection, rats were anesthetized with 10% chloral hydrate (3.5 mL/kg body weight). FBG and fasting insulin (FINS) levels were determined and an intraperitoneal glucose tolerance test (IPGTT) was administered. Biochemical indicators related to heart and liver function were also measured. Heart and liver tissues were collected, weighed, and stored at −80°C. All animal experiments were performed in accordance with the ethical guidelines of the PLA Rocket Force Characteristic Medical Center. Written informed consent for publication of any associated data and accompanying images were obtained from all patients or their parents, guardians or next of kin.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted with TRIzol and reverse-transcribed into cDNA using SuperScript™ III reverse transcriptase according to the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA, USA). qRT-PCR was performed to amplify target genes using an iQ5 instrument (Bio-Rad, Hercules, CA, USA). The amplification protocol was as follows: 94°C for 3 minutes; 40 cycles of 94°C for 30 s, 56°C for 30 s and 72°C for 45 s. The primer sequences were as follows: TLR4, forward 5′-TCAGTGTGCTTGTGGTAG-3′ and reverse 5′-TCTGCTAAGAAGGCGATAC-3′; NF-κB, forward 5′-AGAGGATTTCGATTCCGCTA-3′ and reverse 5′-CGTGAAGTATTCCCAGGTTTG-3′; β-actin, forward 5′-CCCATCTATGAGGGTTACGC-3′ and reverse 5ʹ-TITAATGTCACGCACGATTTC-3ʹ; and MyD88, forward 5′-GGCATCTCCACCCGAGTTAC-3′ and reverse 5′-TTGGCGATTTTAGGTGTCCG-3′. Relative expression levels of target genes were calculated using the 2-ΔΔCt method.16 β-actin was used as an internal reference.
Western blotting
Cells or tissues were lysed using radioimmunoprecipitation assay buffer. Protein concentrations were determined and then protein samples were separated by SDS-PAGE. Following electrophoresis, proteins were transferred to polyvinylidene difluoride membranes. After blocking with 5% skim milk, the membranes were incubated with primary antibodies (all from Cell Signaling Technology, Danvers, MA, USA) against TLR4 (1:1000 dilution; Cat. No. 14358), NF-κB (1:1000 dilution; Cat. No. 50010S) and β-actin (1:1000 dilution; Cat. No. 3700). After washing, the membranes were treated with a horseradish peroxidase-conjugated secondary antibody (1:1000; Cell Signaling Technology). Blots were developed using enhanced chemiluminescence (Abcam, Cambridge, UK). Band density was assessed using Image Lab v3.0 software (Bio-Rad). β-actin was used as an internal control.
Enzyme-linked immunosorbent assay (ELISA)
In a microplate, 50 µL of sample or standard was added to each well. After shaking, the plate was incubated at room temperature for 2.5 hours. After washing, the plate was incubated with biotin-labeled antibodies at room temperature for 1 hour. After another wash, horseradish peroxidase-conjugated streptavidin was added to the plate and incubated at room temperature for 45 minutes. After washing, tetramethylbenzidine substrate was added to each well and incubated at room temperature for 30 minutes, then 50 µL of stop solution was added to stop the reaction. Absorbance at 450 nm was measured within 15 minutes. Standard curves were plotted and the protein concentrations were calculated.
Hematoxylin and eosin (HE) staining
Islet tissues were removed and fixed in 10% formalin. The tissues were dehydrated in a series of graded alcohol solutions. After embedding in paraffin, tissues were sectioned to 3- to 4-µm thickness and then stained with HE for histopathological evaluation.
Statistical analysis
Data were expressed as means ± standard deviations. Statistical analysis was conducted using SPSS 22.0 (IBM, Armonk, NY, USA). Pairwise comparisons were performed using the paired Student’s t-test. Multiple comparisons were performed using one-way analysis of variance followed by a Student–Newman–Keuls post-hoc test. Values of P < 0.05 were considered statistically significant.
Results
Diabetic rats have lower body weights, higher FBG, higher FINS, and higher IPGTT results than normal rats
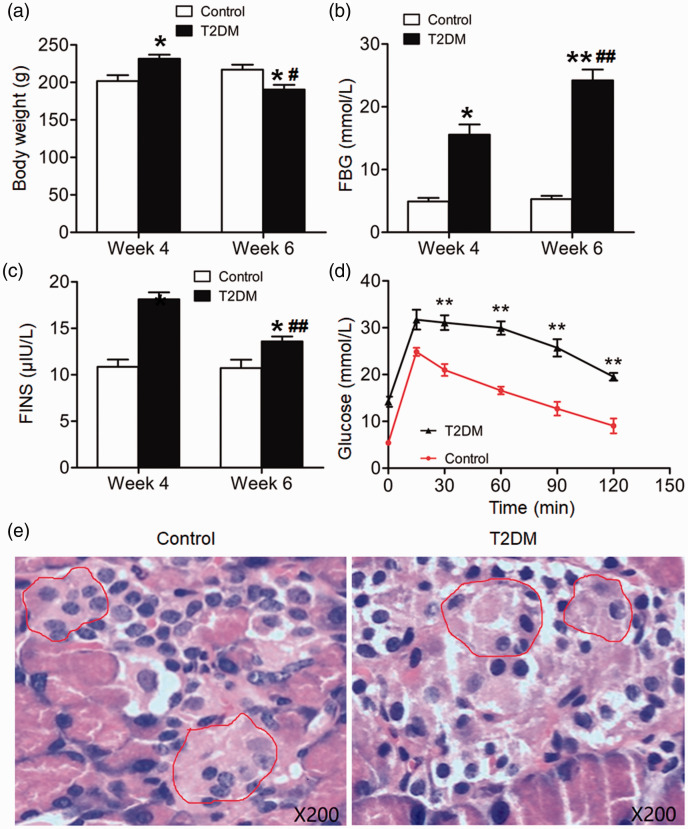
After 4 weeks of feeding a high-fat diet, rats in the experimental group were intraperitoneally injected with 30 mg/kg STZ for 2 weeks to induce diabetes (T2DM rats). After 4 weeks, the body weights of T2DM rats were significantly higher than those of control rats (P < 0.05). After 6 weeks, the body weights of T2DM rats were significantly lower than those of control rats (P < 0.05). The body weights of T2DM rats at week 6 were significantly lower than at week 4 (P < 0.05) (Figure 1a). Moreover, FBG and FINS in T2DM rats were significantly higher than those of control rats at week 4 (P < 0.05). FBG in T2DM rats at week 6 was significantly higher than at week 4 (P < 0.05), while FINS in T2DM rats at week 6 was significantly lower than at week 4 (P < 0.05) (Figure 1b and c). According to IPGTTs, glucose levels in T2DM rats peaked at 15 minutes and maintained elevated until 120 minutes, and were significantly higher than those of control rats from 30 minutes until 120 minutes (P < 0.01 for all time points) (Figure 1d). HE staining showed that in T2DM rats, only a small number of islet cells were observed in islet tissue; the islet structures were atrophic, scattered, and damaged, and the number of cells was reduced compared with control rats (Figure 1e). Thus, T2DM rats had lower body weights, higher FBG, higher FINS, and higher IPGTT results than normal rats.
Figure 1.
Body weights, fasting blood glucose (FBG), fasting insulin (FINS), intraperitoneal glucose tolerance test (IPGTT) results, and islet structure of normal rats and type 2 diabetes mellitus (T2DM) rats. (a) Body weight, (b) FBG, and (c) FINS of control and T2DM rats at the end of week 4 and week 6. *P < 0.05 and **P < 0.01 compared with control rats in the same week; #P < 0.05 and ##P < 0.01 compared with T2DM rats at week 4. (d) IPGTT results of control and T2DM rats at the end of week 6. **P < 0.01 compared with control rats at the same time point. (e) Islet structures of control and T2DM rats at the end of week 6 as shown by hematoxylin and eosin staining. Islets were circled using red lines. Magnification, ×200. N = 12.
T2DM rats have elevated biochemical indicators related to heart and liver function compared with normal rats
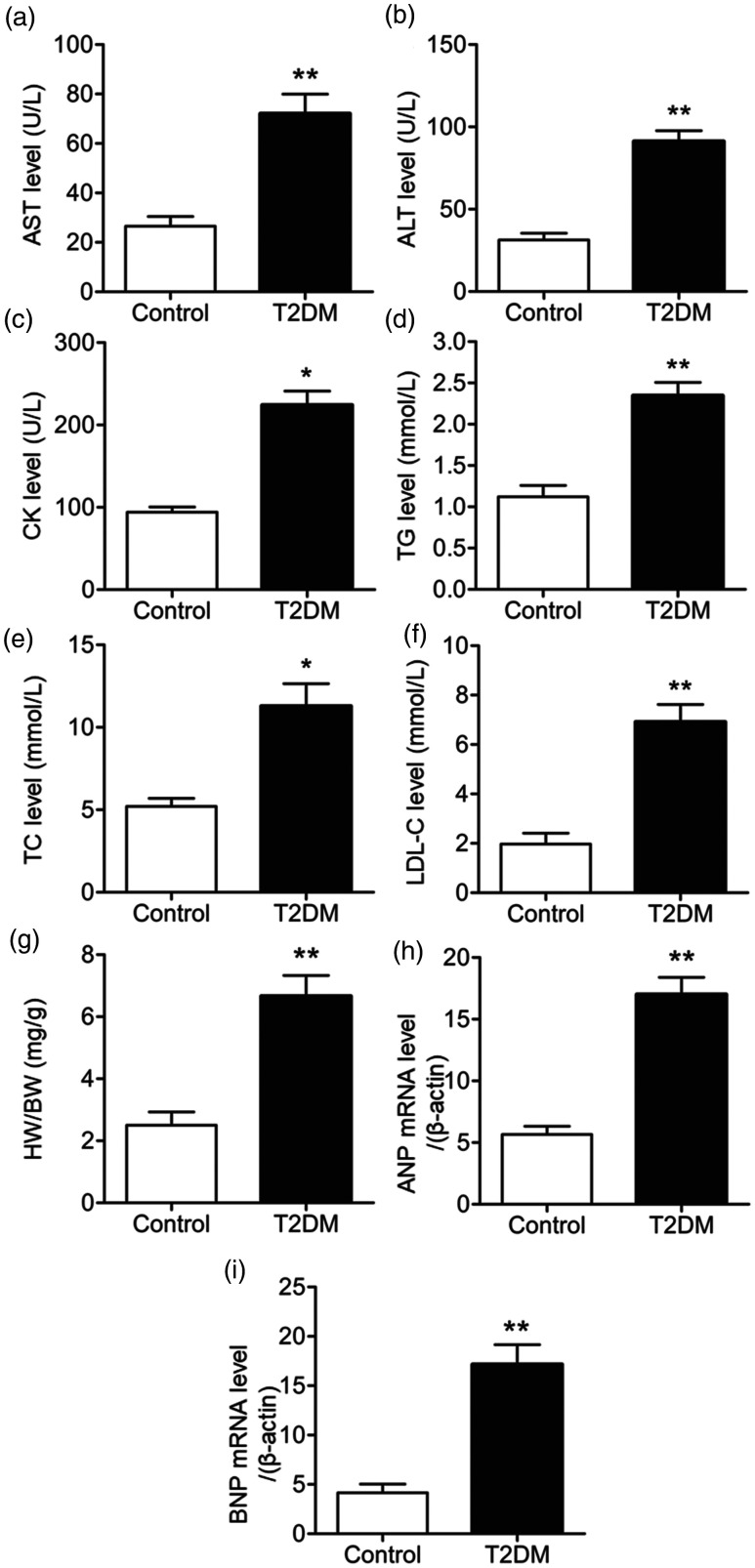
To evaluate the effects of T2DM on heart and liver function, sera were collected at the end of week 6 for biochemical analysis of indicators of heart and liver function. Serum levels of aspartate aminotransferase, alanine aminotransferase, triglycerides, creatinine kinase, total cholesterol, and low density lipoprotein-C in T2DM rats were significantly elevated compared with control rats (P < 0.05) (Figure 2a–f). These results indicated that T2DM rats had higher levels of biochemical indicators related to heart and liver function than normal rats.
Figure 2.
Biochemical indicators related to heart and liver function. Serum levels of (a) asparagine aminotransferase (AST), (b) alanine aminotransferase (ALT), (c) creatinine kinase (CK), (d) triglycerides (TG), (e) total cholesterol (TC), and (f) low density lipoprotein (LDL)-C in control and type 2 diabetes mellitus (T2DM) rats at the end of week 6. (g) Ratio of heart weight (HW) to body weight (HW). (h) Atrial natriuretic peptide (ANP) and (i) brain natriuretic peptide (BNP) mRNA levels normalized to mRNA levels of β-actin. *P < 0.05 and **P < 0.01 compared with control rats. N = 12.
TLR4 and MyD88 may be involved in regulating the occurrence of T2DM and its complications in the heart and liver
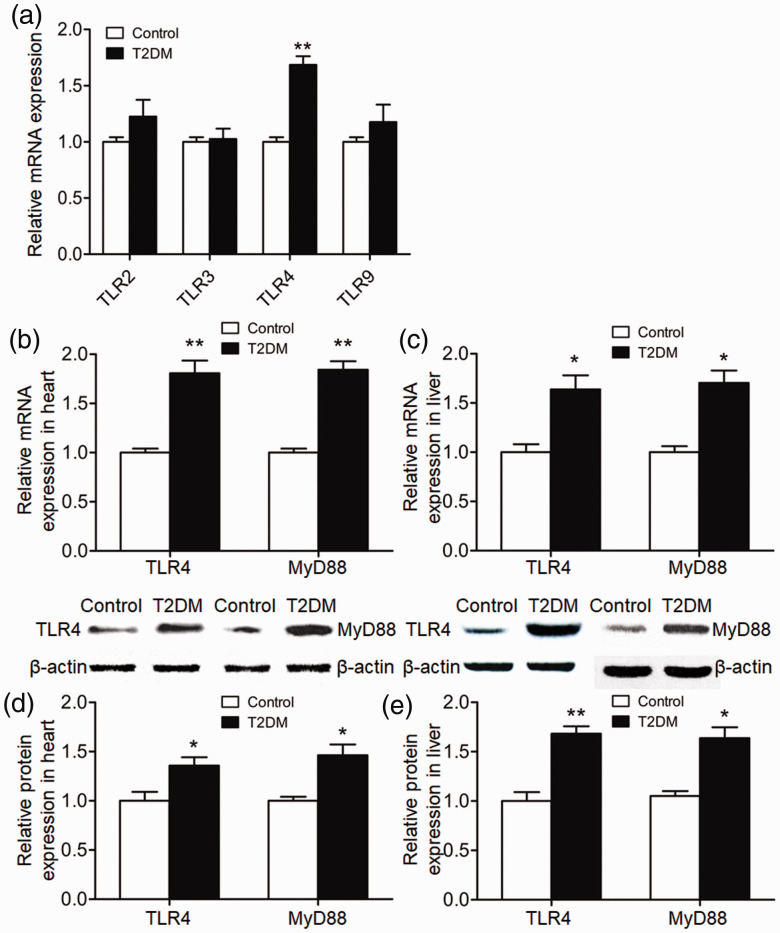
The heart and liver express other TLR family members in addition to TLR4. Following a literature search, we selected TLR2, TLR3 and TLR9 as potentially related to T2DM and evaluated the expression of these molecules in liver and heart by qRT-PCR. Levels of mRNA encoding these TLRs in T2DM rats did not differ from those of control rats. However, TLR4 mRNA levels in T2DM rats were significantly higher than those of control rats (P < 0.01) (Figure 3a). To investigate the expression levels of TLR4 and MyD88 in rat heart and liver tissue at week 6, qRT-PCR and western blotting was performed. Levels of TLR4 and MyD88 mRNA in the hearts and livers of T2DM rats were significantly elevated compared with those of control rats (P < 0.05) (Figure 3b and c). Similarly, levels of TLR4 and MyD88 protein in the hearts and livers of T2DM rats were significantly elevated compared with those of control rats (P < 0.05) (Figure 3d and e). Together, these results suggested that TLR4 and MyD88 may be involved in regulating the occurrence of T2DM and its complications in the heart and liver.
Figure 3.
Relative expression of Toll-like receptor (TLR) 4 and myeloid differentiation primary response 88 (MyD88) in rat heart and liver tissue. (a) Relative levels of TLR2, TLR3, TLR4 and TLR9 mRNA in heart and liver tissues of control and type 2 diabetes mellitus (T2DM) rats at the end of week 6. (b, c) Relative levels of TLR4 and MyD88 mRNA in (b) heart and (c) liver tissues of control and T2DM rats at the end of week 6. (d, e) Relative expression of TLR4 and MyD88 proteins in (d) heart and (e) liver tissues of control and T2DM rats at the end of week 6. *P < 0.05 and **P < 0.01 compared with control rats. N = 8.
TLR4 may cause heart and liver-related complications in T2DM rats through activation of NF-κB
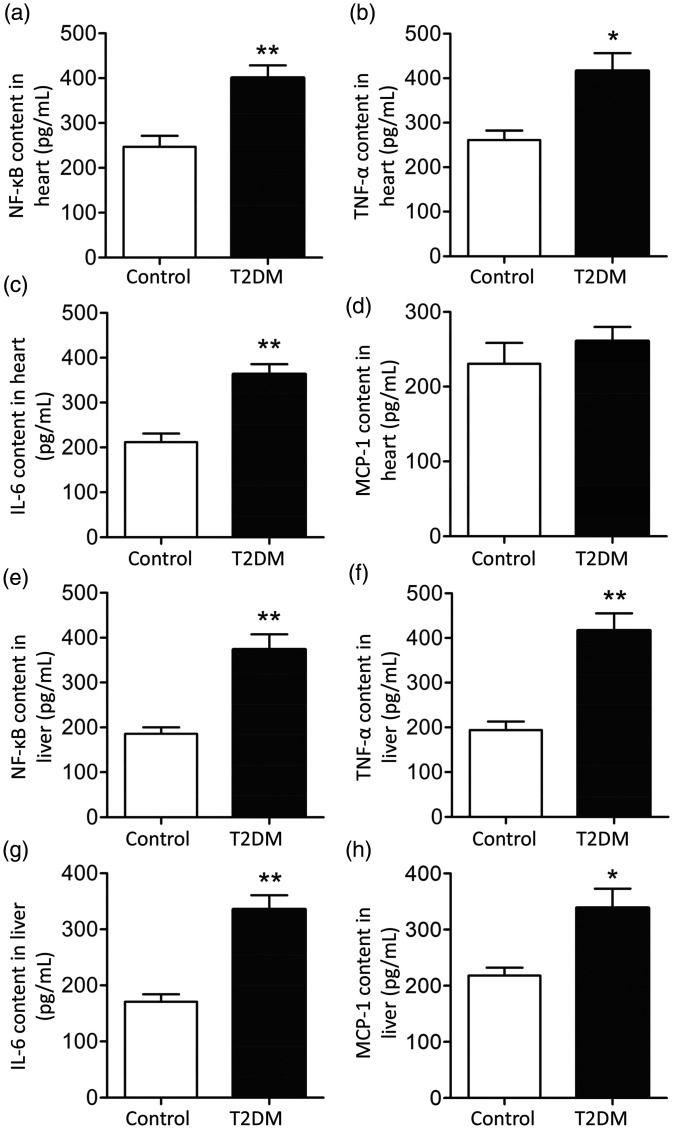
The expression of NF-κB and its downstream inflammatory factors (TNF-α, IL-6 and MCP-1) in heart and liver tissue homogenates was measured using ELISA. Levels of NF-κB, TNF-α and IL-6 in the heart tissues of T2DM rats were significantly elevated compared with those of control rats (P < 0.05) (Figure 4a–d). However, levels of MCP-1 were similar in the two groups. In addition, levels of NF-κB, TNF-α, IL-6 and MCP-1 in the liver tissues of T2DM rats were significantly elevated compared with those of control rats (P < 0.05) (Figure 4e–h). These results indicated that TLR4 may induce heart- and liver-related complications in T2DM rats through activation of NF-κB.
Figure 4.
Levels of nuclear factor (NF)-κB and downstream inflammatory factors in rat heart and liver homogenates. (a–d) levels of (a) NF-κB, (b) tumor necrosis factor (TNF)-α, (c) interleukin (IL)-6 and (d) macrophage chemotactic protein (MCP)-1 in heart tissue from control and type 2 diabetes mellitus (T2DM) rats at the end of week 6. (e-h) Levels of (e) NF-κB, (f) TNF-α, (g) IL-6 and (h) MCP-1 in liver tissue from control and T2DM rats at the end of week 6. *P < 0.05 and **P < 0.01 compared with control group. N = 8.
Discussion
Diabetes can affect many tissues and organs, leading to a series of complications.25 As disease progresses, complications can affect the heart, liver, kidneys, blood vessels, nervous system, and skin. Diabetic heart disease and liver injury can seriously threaten the lives and health of patients with diabetes.26 The pathogenesis of T2DM involves development of insulin resistance and decreased number or function of islet β cells.27 It is generally believed that heredity, diet, viral infections, and inflammation are key factors in development of T2DM.28,29
In patients with T2DM, levels of acute phase inflammatory markers such as IL-6, C-reactive protein, cortisol, and serum amyloid A are significantly higher compared with healthy individuals.30 Inflammation can lead to insulin resistance and T2DM. Long-term inflammation can also aggravate hyperglycemia and promote diabetic complications.31 Long-term chronic inflammation can alter the functions of adipocytes, leading to altered insulin sensitivity of adipose tissues.32
TLR4 plays important roles in orchestrating innate immune responses and in regulating insulin resistance and diabetic complications.33 Levels of TLR4 mRNA in the monocytes of patients with T2DM were higher than those of healthy individuals.30 Animal experiments showed that LPS-induced insulin resistance was mediated by TLR4 and could induce diabetes.34 MyD88 is one of the most important adaptor proteins in TLR signal transduction, activating downstream molecules such as NF-κB that trigger inflammatory pathways.35 The risk of diabetes in MyD88-knockout mice was significantly higher than that in wild-type mice.36 When MyD88-knockout mice were fed a high-fat diet, levels of insulin and cholesterol increased and liver dysfunction developed.36 These factors are significantly correlated with the occurrence of diabetes.36 NF-κB is a key factor in inflammatory signaling, and both TLR4 and NF-κB are generally recognized as inflammatory markers.37 Increased activation and expression of NF-κB leads to the secretion and release of IL-1β and promotes apoptosis of islet β cells.38 In this study, expression levels of TLR4 and NF-κB in T2DM rats were higher than those of normal rats, leading to heart- and liver-related complications. Adding palmitic acid to human microvascular endothelial cells activated NF-κB signaling, suppressed activation of protein kinase β, and led to insulin resistance. These data suggested that TLR4/NF-κB signaling might be related to T2DM.39
In conclusion, the present study demonstrated that TLR4/MyD88/NF-κB signaling triggered upregulation of TNF-α, IL-6 and MCP-1, subsequently leading to heart- and liver-related complications of T2DM.
Acknowledgements
The authors wish to thank their departments and research teams for their efforts and dedication.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author contributions: JT and LL designed the study. JT, YZ and LW performed the experiments. JT and LW analyzed the data. JT, YZ and LL interpreted results and prepared the manuscript. All authors read and approved the final version of the manuscript.
ORCID iD: Jiajia Tian https://orcid.org/0000-0002-7847-1721
References
- 1.Gitelman SE, Gottlieb PA, Felner EI, et al. Antithymocyte globulin therapy for patients with recent-onset type 1 diabetes: 2 year results of a randomised trial. Diabetologia 2016; 59: 1153–1161. doi: 10.1007/s00125-016-3917-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloomgarden Z, Ning G. Self-monitoring of blood glucose for type 2 diabetes 2. J Diabetes 2015; 7: 593–594. doi: 10.1111/1753-0407.12307. [DOI] [PubMed] [Google Scholar]
- 3.Koppes LL, Dekker JM, Hendriks HF, et al. Moderate alcohol consumption lowers the risk of type 2 diabetes: A meta-analysis of prospective observational studies. Diabetes Care 2005; 28: 719–725. doi: 10.2337/diacare.28.3.719. [DOI] [PubMed] [Google Scholar]
- 4.Das UN. Beneficial role of bioactive lipids in the pathobiology, prevention, and management of HBV, HCV and alcoholic hepatitis, NAFLD, and liver cirrhosis: A review. J Adv Res 2019; 17: 17–29. doi: 10.1016/j.jare.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madhumitha H, Mohan V, Babu S, et al. TLR-induced secretion of novel cytokine IL-27 is defective in newly diagnosed type-2 diabetic subjects. Cytokine 2018; 104: 65–71. doi: 10.1016/j.cyto.2017.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Rimm EB, Stampfer MJ, et al. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr 2005; 81: 555–563. doi: 10.1093/ajcn/81.3.555. [DOI] [PubMed] [Google Scholar]
- 7.Ye S, Zheng M, Hu Y, et al. Hydrochloride pioglitazone decreases urinary monocyte chemoattractant protein-1 excretion in type 2 diabetics. Diabetes Res Clin Pract 2010; 88: 247–251. doi: 10.1016/j.diabres.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Katavetin P, Eiam-Ong S, Suwanwalaikorn S. Pioglitazone reduces urinary protein and urinary transforming growth factor-beta excretion in patients with type 2 diabetes and overt nephropathy. J Med Assoc Thai 2006; 89: 170–177. [PubMed] [Google Scholar]
- 9.Gupta S, Maratha A, Siednienko J, et al. Analysis of inflammatory cytokine and TLR expression levels in type 2 diabetes with complications. Sci Rep 2017; 7: 7633. doi: 10.1038/s41598-017-07230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding T, Chen W, Li J, et al. High glucose induces mouse mesangial cell overproliferation via inhibition of hydrogen sulfide synthesis in a TLR-4-dependent manner. Cell Physiol Biochem 2017; 41: 1035–1043. doi: 10.1159/000461483. [DOI] [PubMed] [Google Scholar]
- 11.Rhoads JP, Lukens JR, Wilhelm AJ, et al. Oxidized low-density lipoprotein immune complex priming of the NLRP3 inflammasome involves TLR and FcgammaR cooperation and is dependent on CARD9. J Immunol 2017; 198: 2105–2114. doi: 10.4049/jimmunol.1601563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosseini H, Li Y, Kanellakis P, et al. Toll-like receptor (TLR)4 and MyD88 are essential for atheroprotection by peritoneal B1a B cells. J Am Heart Assoc 2016; 5: e002947. doi: 10.1161/jaha.115.002947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao M, Li CH, Liu YL. Toll-like receptor (TLR)-2/4 expression in retinal ganglion cells in a high-glucose environment and its implications. Genet Mol Res 2016; 15. doi: 10.4238/gmr.15026998. [DOI] [PubMed] [Google Scholar]
- 14.Larraufie P, Dore J, Lapaque N, et al. TLR ligands and butyrate increase Pyy expression through two distinct but inter-regulated pathways. Cell Microbiol 2017; 19. doi: 10.1111/cmi.12648. [DOI] [PubMed] [Google Scholar]
- 15.Abdul Y, Abdelsaid M, Li W, et al. Inhibition of Toll-like receptor-4 (TLR-4) improves neurobehavioral outcomes after acute ischemic stroke in diabetic rats: Possible role of vascular endothelial TLR-4. Mol Neurobiol 2019; 56: 1607–1617. doi: 10.1007/s12035-018-1184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh K, Kant S, Singh VK, et al. Toll-like receptor 4 polymorphisms and their haplotypes modulate the risk of developing diabetic retinopathy in type 2 diabetes patients. Mol Vis 2014; 20: 704–713. [PMC free article] [PubMed] [Google Scholar]
- 17.Keogh B, Parker AE. Toll-like receptors as targets for immune disorders. Trends Pharmacol Sci 2011; 32: 435–442. doi: 10.1016/j.tips.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Gong DJ, Wang L, Yang YY, et al. Diabetes aggravates renal ischemia and reperfusion injury in rats by exacerbating oxidative stress, inflammation, and apoptosis. Ren Fail 2019; 41: 750–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Degirmenci I, Ozbayer C, Kebapci MN, et al. Common variants of genes encoding TLR4 and TLR4 pathway members TIRAP and IRAK1 are effective on MCP1, IL6, IL1beta, and TNFalpha levels in type 2 diabetes and insulin resistance. Inflamm Res 2019; 68: 801–814. doi: 10.1007/s00011-019-01263-7. [DOI] [PubMed] [Google Scholar]
- 20.Lima ARR. Exercise and garlic modulate microRNAs involved in diabetic cardiopathy. Arq Bras Cardiol 2019; 112: 163–164. doi: 10.5935/abc.20180259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giri SR, Bhoi B, Jain MR, et al. Cardioprotective role of peroxisome proliferator-activated receptor-gamma agonist, rosiglitazone in a unique murine model of diabetic cardiopathy. Life Sci 2016; 162: 1–13. doi: 10.1016/j.lfs.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Szasz T, Wenceslau CF, Burgess B, et al. Toll-like receptor 4 activation contributes to diabetic bladder dysfunction in a murine model of type 1 diabetes. Diabetes 2016; 65: 3754–3764. doi: 10.2337/db16-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang NQ, Jin H, Zhou SY, et al. TLR4 is a link between diabetes and Alzheimer's disease. Behav Brain Res 2017; 316: 234–244. doi: 10.1016/j.bbr.2016.08.047. [DOI] [PubMed] [Google Scholar]
- 24.Guo X, Shi Y, Du P, et al. HMGB1/TLR4 promotes apoptosis and reduces autophagy of hippocampal neurons in diabetes combined with OSA. Life Sci 2019; 239: 117020. doi: 10.1016/j.lfs.2019.117020. [DOI] [PubMed] [Google Scholar]
- 25.Scibilia R. The diabetes 2-month turnaround. Clin Diabetes 2017; 35: 358–359. doi: 10.2337/cd17-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong X, Ma MZ, Huang K, et al. Increased plasma levels of the methylglyoxal in patients with newly diagnosed type 2 diabetes 2. J Diabetes 2014; 6: 535–540. doi: 10.1111/1753-0407.12160. [DOI] [PubMed] [Google Scholar]
- 27.Aamir K, Khan HU, Sethi G, et al. Wnt signaling mediates TLR pathway and promote unrestrained adipogenesis and metaflammation: Therapeutic targets for obesity and type 2 diabetes. Pharmacol Res 2020; 152: 104602. doi: 10.1016/j.phrs.2019.104602. [DOI] [PubMed] [Google Scholar]
- 28.Sadeghabadi ZA, Ziamajidi N, Abbasalipourkabir R, et al. Palmitate-induced IL6 expression ameliorated by chicoric acid through AMPK and SIRT1-mediated pathway in the PBMCs of newly diagnosed type 2 diabetes patients and healthy subjects. Cytokine 2019; 116: 106–114. doi: 10.1016/j.cyto.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Chinniah R, Vijayan M, Sivanadham R, et al. Diversity and association of HLA/KIR receptors with type 2 diabetes in South India. Int J Immunogenet 2019; 46: 166–178. doi: 10.1111/iji.12417. [DOI] [PubMed] [Google Scholar]
- 30.Feng H, Su R, Song Y, et al. Positive correlation between enhanced expression of TLR4/MyD88/NF-kappaB with insulin resistance in placentae of gestational diabetes mellitus. PLoS One 2016; 11: e0157185. doi: 10.1371/journal.pone.0157185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khondkaryan L, Margaryan S, Poghosyan D, et al. Impaired inflammatory response to LPS in type 2 diabetes mellitus. Int J Inflam 2018; 2018: 2157434. doi: 10.1155/2018/2157434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henschel AM, Cabrera SM, Kaldunski ML, et al. Modulation of the diet and gastrointestinal microbiota normalizes systemic inflammation and beta-cell chemokine expression associated with autoimmune diabetes susceptibility. PLoS One 2018; 13: e0190351. doi: 10.1371/journal.pone.0190351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gond DP, Singh S, Agrawal NK. Testing an association between TLR4 and CXCR1 gene polymorphisms with susceptibility to urinary tract infection in type 2 diabetes in north Indian population. Gene 2018; 641: 196–202. doi: 10.1016/j.gene.2017.10.060. [DOI] [PubMed] [Google Scholar]
- 34.Alibashe-Ahmed M, Brioudes E, Reith W, et al. Toll-like receptor 4 inhibition prevents autoimmune diabetes in NOD mice. Sci Rep 2019; 9: 19350. doi: 10.1038/s41598-019-55521-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuwabara WMT, Yokota CNF, Curi R, et al. Obesity and type 2 diabetes mellitus induce lipopolysaccharide tolerance in rat neutrophils. Sci Rep 2018; 8: 17534. doi: 10.1038/s41598-018-35809-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taha IM, Abdu Allah AM, Abd El Gayed EM. Expression of Toll-like receptor 4 and its connection with type 2 diabetes mellitus. Cell Mol Biol (Noisy-le-grand) 2018; 64: 15–20. [PubMed] [Google Scholar]
- 37.Salil G, Nithya R, Nevin KG, et al. Dietary coconut kernel protein beneficially modulates NFkappaB and RAGE expression in streptozotocin induced diabetes in rats. J Food Sci Technol 2014; 51: 2141–2147. doi: 10.1007/s13197-012-0729-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bagul PK, Deepthi N, Sultana R, et al. Resveratrol ameliorates cardiac oxidative stress in diabetes through deacetylation of NFkB-p65 and histone 3. J Nutr Biochem 2015; 26: 1298–1307. doi: 10.1016/j.jnutbio.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Dasu MR, Jialal I. Free fatty acids in the presence of high glucose amplify monocyte inflammation via Toll-like receptors. Am J Physiol Endocrinol Metab 2011; 300: E145–E154. doi: 10.1152/ajpendo.00490.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]